Abstract
The recent coronavirus disease 2019 outbreak around the world has had an enormous impact on the global health burden, threatening the lives of many individuals, and has had severe socio-economic consequences. Many pharmaceutical and biotechnology companies have commenced intensive research on different therapeutic strategies, from repurposed antiviral drugs to vaccines and monoclonal antibodies to prevent the spread of the disease and treat infected patients. Among the various strategies, advanced therapeutic approaches including cell- and gene-editing-based therapeutics are also being investigated, and initial results in in-vitro and early phase I studies have been promising. However, further assessments are required. This article reviews the underlying mechanisms for the pathogenesis of severe acute respiratory syndrome coronavirus-2, and discusses available therapeutic candidates and advanced modalities that are being evaluated in in-vitro/in-vivo models and are of note in clinical trials.
Keywords: SARS-CoV-2, COVID-19, Advanced therapeutic approaches, ATMP
1. SARS-CoV-2 background
The recent outbreak of coronavirus disease 2019 (COVID-19), caused by a novel betacoronavirus (CoV), is now a major worldwide medical (and economic) challenge. As such, specifying the therapeutic approaches and the mechanisms which lead to these strategies are of utmost importance. By reviewing the literature regarding the mechanisms of action and state-of-the-art medications, this article aims to draw an overall picture of the mechanisms involved and the related therapeutic approaches. This review included original articles, review articles and HTML documents from official websites (e.g. World Health Organization). The search terms used were: coronavirus, severe acute respiratory syndrome coronavirus-2, 2019-nCoV, and novel therapeutic approaches. Registered and active clinical trials were found on ClinicalTrials.gov and the index of studies of novel CoV pneumonia in the Chinese Clinical Trial Registry. The cut-off date for the data search was September 2020.
CoVs are enveloped viruses in which the 27–32-kb genomic RNA is capped and polyadenylated [1]. They are subdivided into four distinct groups; alpha, beta, delta and gamma [2]. The CoV species HKU1, NL63, OC43 and 229E cause common cold symptoms. Others, such as severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory coronavirus (MERS-CoV), result in fatal diseases. SARS-CoV-2 is the third virus in the CoV family with the potential to cause life-threatening disease in a wide range of individuals [3].
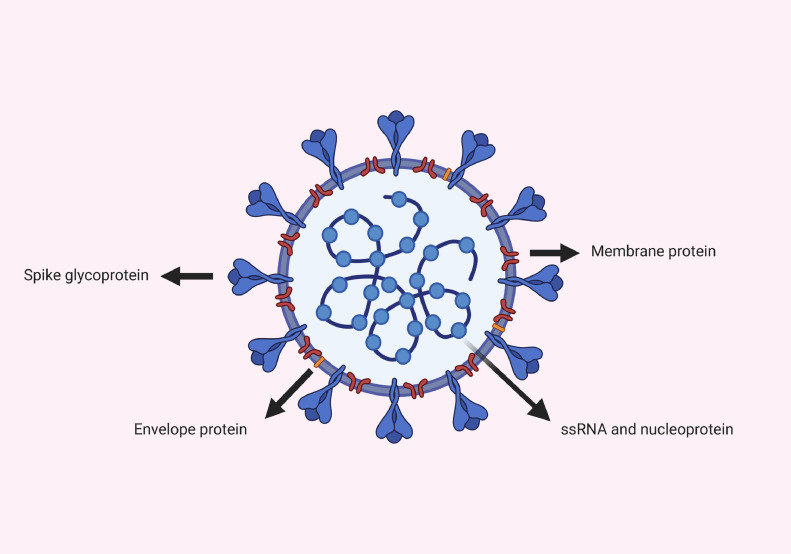
Approximately 30 kb of single‐strand positive‐sense RNA (+ssRNA) forms the genomic content of SARS-CoV-2, which makes it the largest known RNA virus [4]. The genome interacts with the nucleoprotein (N), which is bound to the membrane (M) protein. The M protein plays a crucial role in viral assembly and budding. The envelope (E) protein functions in viral morphogenesis, release and pathogenesis [5]. Finally, the spike (S) protein is a trimeric glycoprotein that includes two subunits: S1 and S2 (Fig. 1 ). Using the S1 and S2 subunits of the S protein, coronaviruses have acquired the ability to attach and fuse to the target cell membrane, respectively (Fig. 2 b) [6]. The M, E and S proteins comprise the virus envelope [5]. The genome and subgenome of a typical CoV contain at least six open reading frames (ORFs) [4]. Two-thirds of these are ORF1a and 1b, which are translated into polyproteins 1a (PP1a) and PP1ab, respectively (Fig. 3 a). The remaining ORFs encode for the main structural (S, E, M and N) and accessory proteins. After translation, the viral genome initiates replication. Most of the 16 non-structural proteins, produced from PP1a and PP1ab, form a very large protein complex that is responsible for viral genome replication and subgenomic mRNA synthesis [7]. The viral life cycle is completed by fusion of virus particles with the plasma membrane and release into the extracellular space. In MERS-CoV, the S protein binds to the dipeptidyl peptidase 4 receptor to gain entry into cells; however, both SARS-CoV and SARS-CoV-2 bind to the human angiotensin-converting enzyme-2 (hACE2) receptor [8].
Fig. 1.
Overview of the structure of severe acute respiratory syndrome coronavirus-2. ssRNA, single-strand RNA.
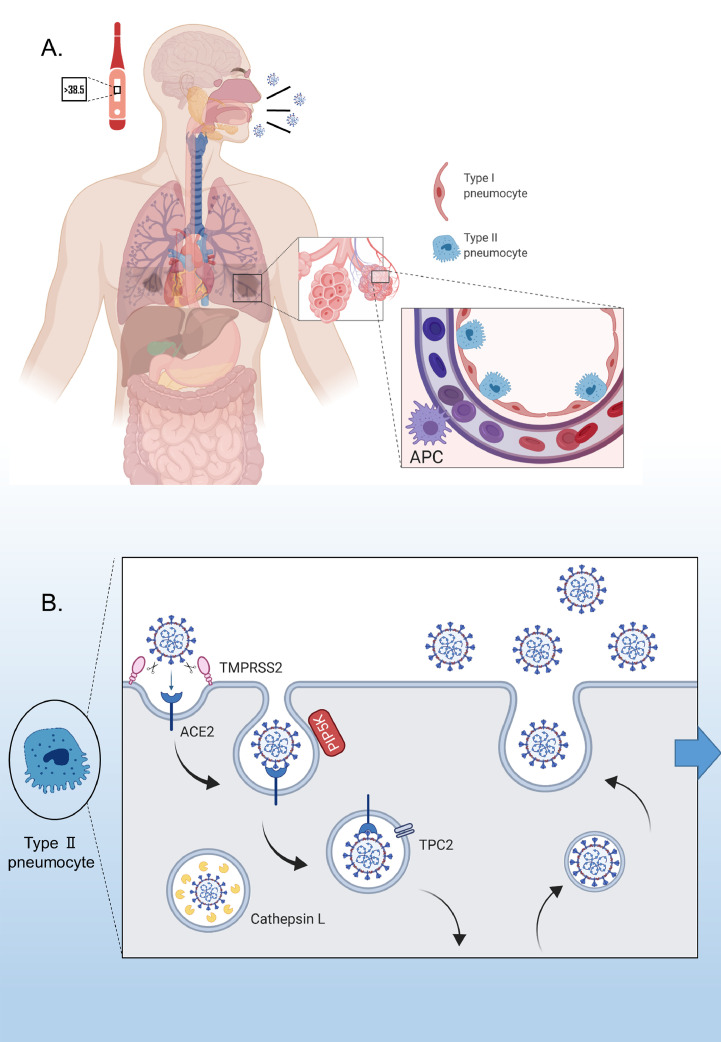
Fig. 2.
Pathogenesis of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). (A) Fever, cough and headache are common symptoms of coronavirus disease 2019. Acute respiratory distress syndrome and multi-organ failure account for the critical stage of the disease (left). Type II alveolar cells highly express angiotensin-converting enzyme-2 (ACE2), the receptor for SARS-CoV-2 (right). (B) Virus entry mechanism and enzymes involved in this process. APC, antigen-presenting cell (created using Biorender,com).
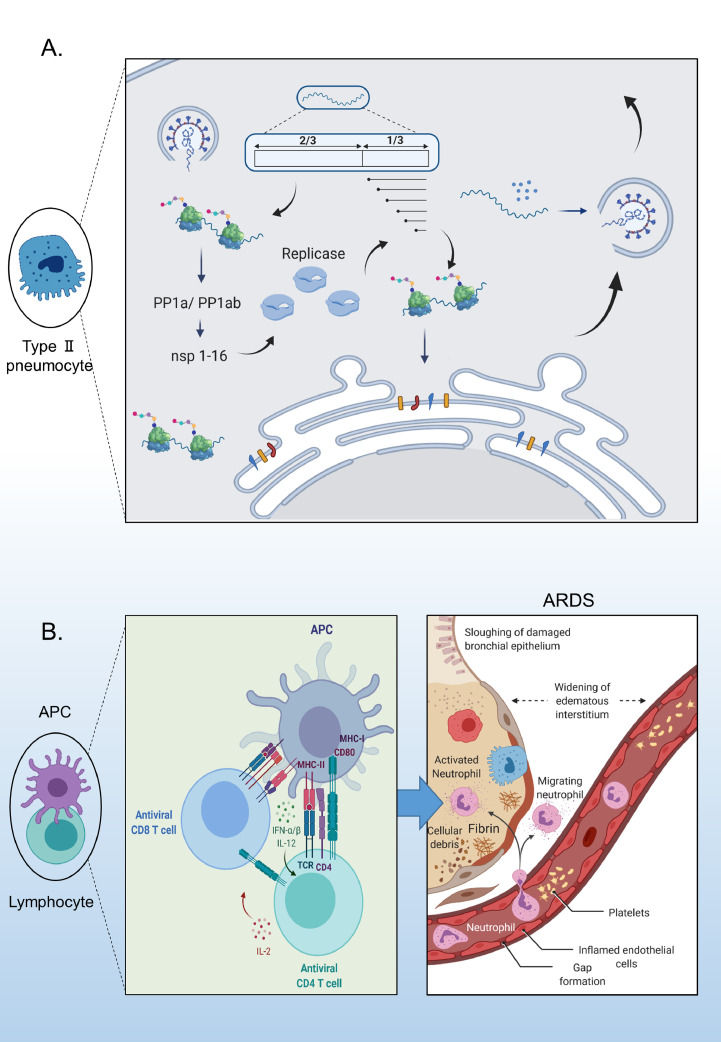
Fig. 3.
(A) Viral genome replication and subgenomic mRNA (sgmRNA) synthesis. (B) Interaction of antigen-presenting cells (APCs) with lymphocytes and cytokine activation (created using Biorender.com). ARDS, acute respiratory distress syndrome; IFN, interferon; IL, interleukin; TCR, T-cell receptor; MHC, major histocompatibility complex; PP1a, polyprotein 1a; PP1ab, polyprotein 1ab.
2. Pathogenesis
Aside from the oral mucosa, especially the tongue, high expression levels of the SARS-CoV-2 receptor, hACE2, have been reported in lung type II alveolar cells, upper and stratified oesophageal epithelial cells, absorptive enterocytes from ileum and colon, cholangiocytes, myocardial cells, kidney proximal tubule cells, podocytes, bladder urothelial cells, male reproductive cells, placental trophoblasts, eye and vascular endothelial cells [9], [10], [11]. Two pathophysiological patterns may be responsible for severe pulmonary injury in COVID-19: a direct cytopathic effect (CPE) and immunopathological pathogenesis. The CPE may be related to a high viral load, while immune-mediated pulmonary effects are more prevalent in late respiratory failure when the viral load has already reduced [12]. Both of these events are discussed below.
2.1. Direct cytopathic effect
The exact mechanism(s) underlying the CPE of SARS-CoV-2 is (are) not yet completely understood. However, as there are close similarities between SARS-CoV-2 and SARS-CoV (structure and entry-receptor specificity) and MERS-CoV (structure but not entry-receptor specificity), knowledge related to SARS-CoV and MERS-CoV will undoubtedly speed up understanding of the mechanism of SARS-CoV-2-mediated pulmonary cell death [13]. SARS-CoV causes cell death via both apoptosis and necrosis, and MERS-CoV has been shown to induce apoptosis in both immune and non-immune cells (e.g. lung and kidney cells) [14], [15], [135]. These findings can form the basis for possible underlying mechanisms through which SARS-CoV-2 may prompt its CPE, which has been demonstrated in human airway epithelial cells following virus inoculation along with the cessation of cilia movements [3]. Regarding kidney cells, there is evidence for a direct CPE of SARS-CoV-2 on various renal cells [16]. For other cell types with ACE2 receptors, further studies are needed to determine the probable direct CPE of SARS-CoV-2; for instance, one postmortem study found no apparent histological alterations in heart tissue from a patient with COVID-19, whereas other studies have suggested cardiac injury due to the direct effect of virus entry into myocardial tissue [17], [18], [19]. Also, very little is known regarding the CPE of SARS-CoV-2 in gastrointestinal cells [20]. Nevertheless, an increased rate of CPE has been reported following inoculation of human intestinal organoids and liver organoids with SARS-CoV-2 [21,22]. ACE2 is not expressed on haematopoietic cells; hence, direct infection of immune cells by SARS-CoV-2 may not be likely. However, it remains to be determined whether SARS-CoV-2 can infect immune cells directly [23].
2.2. Immunological effects
The precise mechanisms through which SARS-CoV-2 affects the immune system are not yet fully known. Similarities in the pathogenesis of CoVs, especially SARS-CoV and SARS-CoV-2, have, however, provided valuable insights into how SARS-CoV-2 might affect the immune system [24].
Both innate and adaptive immune responses are involved in the pathogenesis of COVID-19. Several components of the innate immune system have been reported to be overactivated or increased in number. In fact, macrophage activation syndrome has been suggested as a possible reason for COVID-19-related hyperinflammation [25], as SARS-CoV-2 has been shown to activate the NLRP3 inflammasome in macrophages which leads to increased production of pro-inflammatory cytokines [26]. Moreover, neutrophils have been found predominantly in lung infiltrates from patients with COVID-19. An elevated level of neutrophils and neutrophil-to-lymphocyte ratio usually predict poor clinical outcome [27]. In addition, it has been shown that necro-inflammation is one of the results of neutrophil infiltration and the formation of neutrophil extracellular traps in patients with COVID-19 [28]. On the other hand, recognition of the pathogen-associated molecular pattern of SARS-CoV (genomic RNA) by TLR3 and TLR7 and the cytosolic RNA sensor, RIG-I/MDA5, lead to pro-inflammatory cytokine induction, particularly type I interferon (IFN) [136]. However, both structural and non-structural proteins from CoVs interfere with the type-I-IFN-related signalling pathways [9,29]. The delayed type-I-IFN response along with the confirmed increase in neutrophils and monocyte/macrophage influx [30], followed by excessive production of type I IFN in the later phases, may, in part, explain the viral symptoms.
In addition to innate immunity, both humoral and cellular immune responses play significant roles in the clinical complications of CoVs. Many attempts to identify T- and B-cell epitopes for the viral structural proteins have been undertaken [31]. Cytotoxic CD8 T cells (cytotoxic T cells) and helper CD4 T cells (helper T cells), as key elements of antiviral immunity, require the presentation of viral antigens through human leukocyte antigen (HLA) I and II molecules on the surface of antigen-presenting cells (APCs). Genetic polymorphisms in components of the antigen presentation system appear to account, at least in part, for the risk of SARS-CoV infection. This can also provide valuable insights for predicting the susceptiblity to COVID-19 regarding the genetic background [29]. An in-silico study showed that HLA-B*46:01 expression may make individuals more susceptible to COVID-19 since it has the fewest predicted binding peptides for SARS-CoV-2, while HLA-B*15:03 expression may enable cross-protective T-cell-based immunity [32]. However, the correlation between different allele frequencies and susceptibility to SARS-CoV-2 infection needs more investigation. CD8+ T cells can destroy virally infected cells. CD4+ cells have a key role in promoting the activation of T‐dependent B cells and the production of pro-inflammatory cytokines, leading to recruitment of monocytes and macrophages, and overproduction of cytokines and chemokines [33]. Therefore, reduction of T helper cells may lead to strong immune‐mediated interstitial pneumonitis and delayed clearance of SARS‐CoV from lungs [34]. It is also noteworthy that all of the SARS‐CoV-related memory T cells found in SARS‐CoV convalescent patients, mediated an anti-SARS‐CoV structural protein response, where the S protein was mainly involved in these T-cell responses [35,36]. This implies a role for the structural proteins in the design of efficient SARS vaccines. Furthermore, clinical observations in patients with COVID-19 confirm the reduction of excessively activated CD4+ and CD8+ T cells [18]. Production of early-stage-related immunoglobulin (Ig) M, long-lasting specific IgG, and IgA forms the main B-cell immune response to SARS-CoV [29]. In this regards, the isolation of specific B-cell clones that produce neutralizing monoclonal antibodies (mAbs) was shown in a SARS-CoV convalescent patient [37]. Although neutralizing antibodies may have the potential to block the entry of viruses into human cells [38], anti-S protein neutralizing IgGs may also be associated with risk of fatal acute lung injury [39].
It is interesting to note that ACE2, through which SARS-CoV-2 enters the cells, was also reported to be downregulated in one mouse model of SARS-CoV infection and pulmonary disease, and that this downregulation may lead to more severe lung injury [40]. In the renin–angiotensin–aldosterone system, ACE converts angiotensin I to angiotensin II, while ACE2 has a role in the inactivation of angiotensin II [40]. Therefore, it is possible that SARS-CoV-2 may cause an increase in blood flow, not only by the acute inflammation response, but also via an increase in angiotensin II due to ACE2 downregulation. In addition, the circulatory fraction of immunosenescent T cells (i.e. CD8+CD28−CD57+ cells that accumulate with aging and in chronic inflammatory conditions [41]) has been found to be higher in patients with high blood pressure, which may increase the risk for severe COVID-19. This, together with elevated levels of C-X-C chemokine receptor type 3 (CXCR3) chemokines and serum granzyme B – hypothesized to be involved in T-cell-driven inflammation in human hypertension [42] – may explain why aging and/or hypertension worsen the incidence and prognosis of COVID-19.
2.2.1. Cytokine-storm-based effects
Acute respiratory distress syndrome (ARDS), a common immunopathological process, may be the leading cause of death in patients with COVID-19 (Fig. 3b) [43]. In addition, the failure of several other organs has been reported in patients with severe COVID-19 [18]. Uncontrolled production of anti-COVID-19 pro-inflammatory cytokines and chemokines, also termed the ‘cytokine storm’, causes ARDS [44].
However, it is important to note that there are doubts regarding the relevance of this event to COVID-19. Sinha et al. questioned the precise function of imbalanced cytokine responses in patients with COVID-19. They suggested that lung injury in these patients is not solely attributed to the cytokine storm. Of note, they mentioned that although the level of interleukin-6 (IL-6), a key cytokine in acute inflammation, in patients with COVID-19 is higher than the median value, it is 10- to 200-fold lower than the IL-6 level in patients with ARDS. Based on some available evidence, they noted that alveolar microthrombi may be the culprits for lung injury in COVID-19 [45]. Moreover, there is evidence indicating that the defect in both innate and adaptive immune responses is of greater importance than hypercytokinaemia-induced organ injury in terms of pathophysiological abnormalities in patients with COVID-19 [46].
2.3. Coagulation dysfunctions
High blood pressure, pulmonary embolism and thrombosis are among the symptoms seen in patients with COVID-19, leading to the hypothesis that COVID-19 is an endothelial dysfunction disease [47,48]. Significantly higher levels of D-dimer and fibrin degradation products, and a longer prothrombin time, have been confirmed in survivors compared with non-survivors upon admission to hospital [49]. Coagulation dysfunction is related to the imbalanced immune response and massive inflammatory reactions, leading to microvascular system damage and activation of coagulation processes [50]. This, in turn, leads to extensive microthrombosis [51]. Due to the widespread inflammation, negative control mechanisms by which thrombin production is monitored can be inhibited [51]. The inflammatory reactions caused by overproduction of pro-inflammatory cytokines also promote vascular permeability [51]. On the other hand, reduced activity of ACE2 in the lungs of animal models with CoV-induced severe ARDS may increase the risk of vascular hyperpermeability and pulmonary oedema, as ACE2 is a negative regulatory factor for the severity of lung oedema [52]. Pulmonary embolus is known to be frequent in patients with COVID-19 [53].
2.4. Circulating ACE2
ADAM17 and TMPRSS2 proteases are responsible for the cleavage of ACE2, which is mainly anchored at the apical surface of the cell (Fig. 2b). However, while ADAM17 may have a protective role against SARS-CoV-2, TMPRSS2 facilitates virus entry [54,55]. In fact, ADAM17 cleaves the N-terminal catalytic domain of ACE2, which is also the CoV-binding site, and releases it into the circulation. The exact role of cleaved ACE2 in the circulation of patients with COVID-19 remains unclear; however, it has been shown that serum ACE2 activity is elevated during hypertension and progression of cardiovascular disease [56,57].
3. Therapeutics
Currently, the main strategy for managing COVID-19 focuses on supportive treatments such as oxygen therapy, fluid management and ventilator support. It should be noted that despite controversy regarding the use of non-invasive ventilation (NIV) to manage ARDS in patients with COVID-19, there may be a selected subpopulation of patients who would benefit from NIV [58].
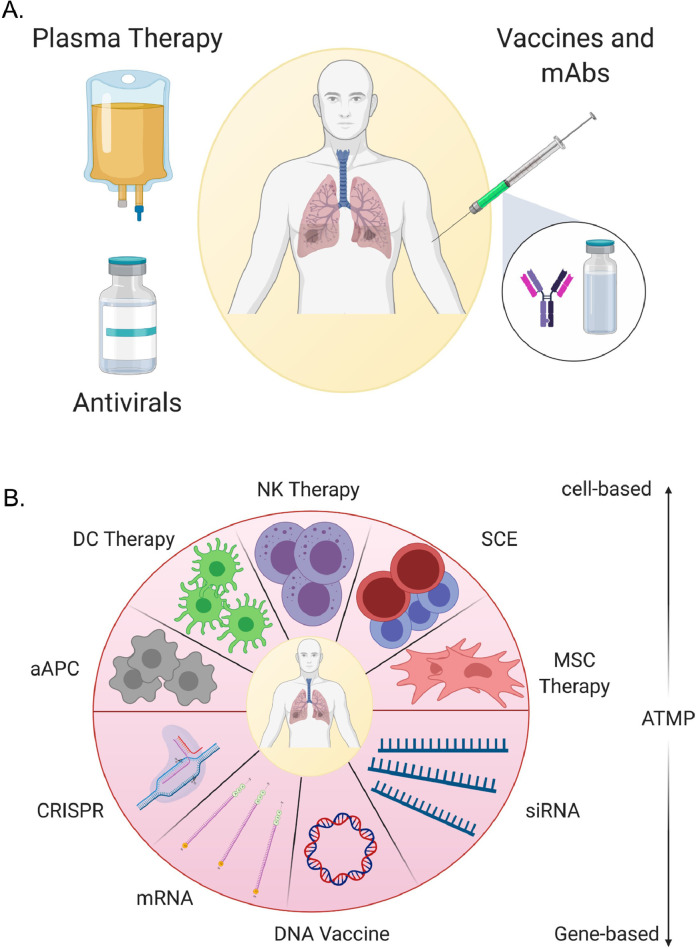
Disease-specific therapies may include available antiviral medications as well as advanced strategies, such as cell- and gene-based protocols. The following sections discuss some of the currently suggested and available therapeutics for the management of patients with COVID-19 (Fig. 4 ). These include repurposed antiviral agents, cell-/gene-based strategies, anticytokine modalities and anticoagulation therapies.
Fig. 4.
Therapeutics. (A) Current therapeutics used to treat patients with coronavirus disease 2019. (B) Advanced therapeutic medicinal products (ATMPs), with cell- and gene-based candidate therapies (created by Biorender.com). mAbs, monoclonal antibodies; DC, dendritic cell; aAPC, artificial antigen-presenting cell; NK, natural killer; SCE, stem cell educator; MSC, mesenchymal stem/stromal cell.
3.1. Prevention of direct cell death due to virus
3.1.1. Repurposed antiviral agents
Several existing antiviral drugs have been repurposed and used to treat patients with COVID-19. Remdesivir (RDV), an adenosine analogue, was originally developed for the treatment of Ebola virus disease. RDV may have antiviral activity against a number of other RNA viruses, including SARS-CoV and MERS-CoV [59,60]. The triphosphate form of RDV competes with adenosine triphosphate for incorporation into the genome, and inhibits the RNA-dependent RNA polymerase [59]. Following promising results of the drug in in-vitro and in-vivo studies against MERS-CoV and SARS-CoV [61], RDV is approved by the US Food and Drug Administration (FDA) for emergency use against COVID-19, and has been tested in multi-site clinical trials. Initial studies suggest that treating patients with COVID-19 with intravenous RDV improves their clinical condition, although side-effects such as temporary gastrointestinal upset have been noted [62]. Recently, Beigel et al. evaluated the beneficial effects of RDV in hospitalized patients with COVID-19. Based on their findings, it appears that RDV treatment can shorten the recovery time in patients [63].
Other nucleotide analogues, such as favipiravir (nucleoside analogue), ribavirin (guanosine analogue), galidesivir (adenosine analogue), sofosbuvir (pyrimidine nucleotide analogue), alovudine (thymidine dideoxynucleoside analogue) and zidovudine (thymidine analogue), are also under investigation for the treatment of COVID-19 [29,64]. Favipiravir is an RNA polymerase inhibitor for a number of RNA viruses, and is approved for the treatment of influenza in China and Japan. Several clinical trials are investigating the therapeutic effect of favipiravir for COVID-19 [65]. Favipiravir may have more potent antiviral action than some protease inhibitors such as kaletra (lopinavir/ritonavir) [65]. However, compared with arbidol (an inhibitor of membrane fusion between the virus and the plasma membrane, and also endocytic vesicle membranes), no significant improvement in the clinical recovery rate of patients was reported after 7 days of favipiravir therapy [66]. It is of note that a recent small phase 3 trial in India with 150 patients showed faster viral clearance in patients with mild-to-moderate COVID-19 who had received favipiravir [67]. Accordingly, favipiravir is approved for restricted emergency use in moderate cases of COVID-19 by the Drugs Controller General of India [68].
Other antiviral drugs, such as oral oseltamivir (a neuraminidase inhibitor), intravenous ganciclovir and chloroquine phosphate tablets, may reduce the symptoms of COVID-19 [69]. At the beginning of the pandemic, there were some reports indicating that chloroquine phosphate (an antimalarial agent), which has both antiviral and anti-inflammatory activity, might prevent worsening of pneumonia. However, due to the serious cardiac adverse events and other potential serious side-effects of chloroquine phosphate and hydroxychloroquine sulfate, and low efficacy against COVID-19, FDA has recently cancelled its emergency use authorization [69,70]. Also, the combination of hydroxychloroquine and azithromycin has been shown to have no significant effect on the rate of virologic clearance in patients with COVID-19 [71]. Cavalcanti et al. observed that the use of hydroxychloroquine, alone or in combination with azithromycin, has no effect on the clinical status of patients with COVID-19 [72].
Chymotrypsin-like proteases, such as cinanserin and flavonoids, along with papain-like proteases, such as diarylheptanoids, may prevent the replication of CoVs and are considered as candidates to battle the virus [29]. Finally, low-dose systemic administration of corticosteroids and IFN inhalation are other anti-COVID-19 strategies [69]. Recently, it has been shown that dexamethasone may reduce the mortality rate in critically ill cases of COVID-19 [73], and that dexamethasone 6 mg once daily leads to a lower mortality rate in patients hospitalized with COVID-19 [74].
As mentioned above, ACE2 is the receptor responsible for entry of SARS-CoV-2, and S protein and TMPRSS2 are among the molecules that are essential for viral entry. The use of neutralizing antibodies against S protein and TMPRSS2 inhibitors (camostate mesylate) has been shown to block viral entry [75]. Moreover, recombinant ACE2 (APN01) could reduce the levels of angiotensin ІІ and IL-6 in patients with ARDS. This agent is currently under investigation for patients with COVID-19 in China [76]. However, there is a challenge regarding the use of ACE inhibitors in the context of cardiovascular diseases. For example, high levels of urinary ACE2 have been detected in patients who received the angiotensin-receptor blocker olmesartan, but this was not seen in patients who received the ACE inhibitor, enalapril or another angiotensin-receptor blocker, losarthan [77]. Thus, the use of angiotensin-receptor blockers or ACE inhibitors in the treatment of COVID-19 needs further investigation [78].
3.1.2. Monoclonal antibodies
Researchers investigated whether SARS-CoV-2 could be neutralized with SARS-CoV receptor binding domain-specific mAbs. However, no significant binding to SARS-CoV-2 was seen [79]. On the other hand, SARS-specific human mAb CR3022 may have some cross-reactive binding between SARS-CoV-2 and SARS-CoV [80]. Therefore, investigating the cross-reactivity of other mAbs against SARS-CoV, including m396 and CR3014, may have potential for the treatment of patients with COVID-19 [29].
Moreover, neutralizing antibodies in the plasma of patients recovering from SARS, MERS or the 2009 H1N1 pandemic could modify the disease progression of other patients with these infections. Therefore, using convalescent plasma from patients with COVID-19 may hold promise to attenuate clinical symptoms, mitigate pulmonary damage, and eliminate the clearance of SARS-CoV-2 RNA [81,82]. In this regard, FDA has issued an emergency use authorization for COVID-19 convalescent plasma, although the significant efficacy of this approach for the treatment of patients with COVID-19 is yet to be demonstrated in placebo-controlled randomized controlled trials [83].
3.1.3. Gene-therapy-based strategies
In the context of development of novel treatments for viral infections, including SARS-CoV-2, nucleic-acid-based strategies and clustered regularly interspaced short palindromic repeat (CRISPR)-associated protein nuclease (Cas)-based approaches could be used.
3.1.3.1. siRNAs
The potential of siRNA technology for the treatment of viral infections has been shown previously [84]. Therefore, siRNAs may be considered as potential candidates for use against SARS-CoV-2. A number of studies in this regard will be discussed.
Several studies have targeted different structural and functional proteins of CoVs using siRNAs. Zhang et al. provided evidence that the RNAi tool can be a therapeutic approach for SARS-CoV infection. Using spike-specific siRNAs, they showed that the siRNAs inhibited the expression of S protein effectively in cultured cells [85]. In addition, He et al. also evaluated the efficacy of six distinct siRNAs designed for different regions of the SARS-CoV replicase gene. Although they did not report any synergistic effects of these siRNAs, inhibitory effects of each of the siRNAs were demonstrated in vitro [86]. However, they subsequently reported synergistic antiviral activities of siRNAs targeting structural and replicase genes of SARS-associated CoV [87]. It was also reported that targeting different regions of the SARS-CoV viral genome using siRNAs may inhibit viral infection and replication [88]. Li et al. demonstrated the potential prophylactic as well as therapeutic ability of SARS-CoV-specific siRNA therapy in Rhesus macaque, and reported decreased body temperature, lower levels of viral RNA and some decrease in lung damage [89]. Overall, there are already several siRNA-related patents regarding SARS-CoV [90,91]. However, to the best of the authors’ knowledge, no active clinical trials are underway using the potential siRNAs for COVID-19.
3.1.3.2. CRISPR/Cas system
The CRISPR/Cas system is an adaptive immune system in archaea and bacteria to protect against foreign DNA or, in some cases, RNA coming from viruses or mobile genetic elements. This immune response consists of three steps: (1) acquisition of the foreign DNA, during which foreign DNA segments (spacers) are inserted into the genome of the host in between the repeats in the CRISPR locus, therefore storing the memory of the previous invader; (2) transcription of the CRISPR locus long transcript and subsequent processing into short CRISPR RNAs (crRNAs) which guide Cas effector proteins to the complementary DNA or RNA sequence in the invading organism; and (3) interference, when the target region is cleaved by a single-protein, Cas effector protein (Class 2) or a large multi-subunit protein complex (Class 1) [92]. The CRISPR system comprises two classes and different types and subtypes. Class 1 consists of Types I, III and IV, and Class 2 consists of Types II (Cas9 effector), V and VI, each having different subtypes. The CRISPR system has mainly been used to perform genome editing and transcription modification. However, this system has evolved naturally in bacteria to defend against invading phages. This suggests that the system can be repurposed in mammalian cells to defend against RNA and DNA viruses. Types III and VI CRISPR systems have RNA-targeting activities, while the rest target DNA; therefore, they can target RNA and DNA viruses, respectively [93]. Successful examples of targeting DNA viruses with the type II CRISPR system (Cas9) have been provided in cell culture and animal models for human immunodeficiency virus, hepatitis B virus, herpesvirus, human papillomavirus and many other viruses [94]. Recent studies of type IV CRISPR/Cas (effector Cas13) have suggested that they may be able to target and degrade RNA [95], [96], [97], [98]. Therefore, this system provides a potential therapeutic approach for elimination of RNA viruses. Additionally, Cas13 can process the long transcript crRNA and therefore can be used for multiplex targeting. Another advantage of Cas13 is the minimal off-target activity on the host transcriptome, as shown in recent studies [96,99]. Freije et al. showed that 94.6% of the 396 ssRNA human-associated viruses have >10 putative Cas13a target sites [93]. Potent Cas13 activity against three different ssRNA viruses (lymphocytic choriomeningitis virus, influenza A virus and vesicular stomatitis virus) was described by Freije et al. The first evidence that Cas13 targets SARS-CoV-2 was reported by Abbott et al. [100]. They tested CRISPR/Cas13 as a prophylactic antiviral CRISPR in human cells (PAC-MAN), and showed efficient degradation of SARS-CoV-2 sequences as well as the influenza A genome in vitro in human lung epithelial cells [100]. This approach could constitute a pan-CoV inhibitory strategy, as a group of six crRNAs may target >90% of CoVs. Although this study provided the first evidence for the potential usefulness of CRISPR/Cas to target SARS-CoV-2, further studies using replication-incompetent viruses and validation in animal models are required.
3.2. Immune-based approaches
3.2.1. Modulating immune responses
3.2.1.1. Cell-based-therapy approaches
Mesenchymal stem/stromal cells (MSCs), which can be harvested relatively easily from fat tissue, bone marrow or placenta, among others, have potential for preventing the immune-mediated consequences of SARS-CoV-2 and restoring damaged tissues by production of trophic factors and anti-inflammatory molecules [101,102]. These include prostaglandin 2, indoleamine 2,3-dioxygenase, transforming growth factor-β, HLA-G5, IL-10, nitric oxide and tumour necrosis factor (TNF)-α-induced gene/protein 6 (TSG-6) [103]. The anti-inflammatory feature of MSCs is exploited for the treatment of steroid-resistant, severe, acute graft-versus-host disease [104,105], and is being tested in the setting of a number of lung inflammatory disorders [106,107]. Moreover, positive effects of stem cells in the setting of other viral diseases of the lung have been reported [108]. In addition, it has been suggested that MSCs may ameliorate ARDS, as seen in severe cases of COVID-19 [109]. The immunomodulatory role of MSCs in the context of COVID-19 is dual, including antiviral protection mediated by increased IFN-stimulated gene (ISG) expression and a secondary response to IFN, resulting in ISG stimulation and widespread viral resistance [110]. Leng et al. showed that infusion of MSCs may improve some laboratory and clinical parameters of patients with COVID-19, and may affect dysregulated inflammatory responses specifically. For instance, MSC therapy increased peripheral lymphocytes and regulatory dendritic cells, while reducing levels of TNF-α and the overactivated cytokine-secreting immune cells, CXCR3+CD4+ T cells, CXCR3+CD8+ T cells and CXCR3+ natural killer (NK) cells [111]. Liang et al. reported that the clinical manifestations of a 65-year-old woman with COVID-19 improved following treatment with human umbilical cord MSCs [112].
An alternative approach that might be considered as therapy for COVID-19-related lung disease is the use of stem cell educator (SCE) therapies. SCE cells are created by culturing patient lymphocytes with cord-blood-derived stem cells, after which the autologous lymphocytes can be re-infused into patients. SCE therapy has been demonstrated to potentially restore the balance of immune responses in a variety of autoimmune disease [113]. Considering the immunomodulatory properties of SCE cells, researchers are now addressing their efficacy in SARS-related pneumonia (Table 2).
Table 2.
An overview of cell-based clinical trials for coronavirus disease 2019
| Status | Cell- Based Interventions | Population | Phase | Location | NCT number | |
|---|---|---|---|---|---|---|
| 1. | Recruiting | Umbilical cord Wharton's jelly-derived human |
40 | 1,2 | Hôpital Pitié-Salpêtrière, APHP, Paris, France Hôpital Européen Georges Pompidou, APHP, Paris, France |
NCT04333368 |
| 2. | Recruiting | MSCs | 30 | 1,2 | Istinye University, Istanbul, Turkey |
NCT04392778 |
| 3. | Recruiting | MSC | 60 | 2,3 | Royan Institute, Tehran, Iran | NCT04366063 |
| 4. | Enrolling by invitation |
Adipose-derived MSCs | 56 | 2 | Hope Biosciences Stem Cell Research Foundation, Sugar Land, Texas, USA | NCT04349631 |
| 5. | Active, not recruiting |
Umbilical cord MSCs | 100 | 2 | General Hospital of Central Theatre Command, Wuhan, Hubei, China Maternal and Child Hospital of Hubei Province, Wuhan, Hubei, China Wuhan Huoshenshan Hospital, Wuhan, Hubei, China |
NCT04288102 |
| 6. | Recruiting | MSCs | 20 | 2 | Armed Forces Bone Marrow Transplant Centre, Rawalpindi, Punjab, Pakistan | NCT04444271 |
| 7. | Recruiting | Human cord tissue MSCs | 1,2 | Duke Hospital, Durham, North Carolina, USA | NCT04399889 | |
| 8. | Not yet recruiting | Wharton's jelly MSCs | 40 | 1,2 | Clinical Somer, Rionegro, Antioquia, Colombia | NCT04390152 |
| 9. | Enrolling by invitation |
Allogenic pooled olfactory mucosa MSCs | 40 | 1,2 | Institute of Biophysics and Cell Engineering of National Academy of Sciences of Belarus, Minsk, Belarus | NCT04382547 |
| 10. | Not yet recruiting | MSCs | 40 | 2 | University Hospital Tuebingen, Tuebingen, Germany | NCT04377334 |
| 11. | Not yet recruiting |
Adipose-derived MSCs | 100 | 2 | River Oaks Hospital and Clinics, Houston, Texas, USA | NCT04362189 |
| 12. | Recruiting | Umbilical cord MSCs |
24 | 1,2 | Diabetes Research Institute, University of Miami Miller School of Medicine, Miami, Florida, USA | NCT04355728 |
| 13. | Enrolling by invitation |
Adipose-derived MSCs | 100 | 2 | Hope Biosciences Stem Cell Research Foundation, Sugar Land, Texas, USA |
NCT04348435 |
| 14. | Not yet recruiting | Bone marrow MSCs | 20 | 1,2 | Guangzhou Institute of Respiratory Health, First Affiliated Hospital of Guangzhou Medical University, Guangzhou, Guangdong, China | NCT04346368 |
| 15. | Not yet recruiting |
Dental pulp MSCs | 24 | Early phase 1 | Not defined | NCT04302519 |
| 16. | Not yet recruiting |
Umbilical cord MSCs | 48 | Not applicable | Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China | NCT04273646 |
| 17. | Recruiting | Umbilical cord MSCs | 10 | 2 | Zhongnan Hospital of Wuhan University, Wuhan, Hubei, China | NCT04269525 |
| 18. | Recruiting | Wharton's jelly MSCs | 5 | 1 | Stem Cells Arabia Amman, Jordan | NCT04313322 |
| 19. | Recruiting | MSCs | 20 | 1 | Beijing 302 Military Hospital of China, Beijing, China | NCT04252118 |
| 20. | Recruiting | Umbilical cord MSCs | 30 | 1,2 | Puren Hospital Affiliated to Wuhan University of Science and Technology, Wuhan, Hubei, China | NCT04339660 |
| 21. | Recruiting | Wharton's jelly MSCs | 30 | 1,2 | Hospital del Mar, Barcelona, Spain | NCT04390139 |
| 22. | Recruiting | MSCs | 300 | 3 | USA | NCT04371393 |
| 23. | Recruiting | MSCs | 20 | 1,2 | CHU de Liège, Liège, Belgium | NCT04445454 |
| 24. | Recruiting | Human-umbilical-cord-derived CD362-enriched MSCs | 75 | 1,2 | Belfast Health and Social Care Trust, Royal Hospitals Belfast, UK | NCT03042143 |
| 25. | Recruiting | MSCs | 10 | 2 | Instituto Nacional de Ciencias Médicas y Nutrición, Salvador Zubirán, Mexico City, Mexico | NCT04416139 |
| 26. | Recruiting | MSCs | 24 | 2 | Hospital Universitario Rio Hortega, Valladolid, Spain | NCT04361942 |
| 27. | Not yet recruiting | Autologous adipose-derived stem cells |
200 | 2 | Armed Forces Bone Marrow Transplant Centre, Rawalpindi, Punjab, Pakistan | NCT04428801 |
| 28. | Recruiting | Intravenous infusions of stem cells |
20 | 2 | Jinnah Hospital, Lahore, Punjab, Pakistan | NCT04437823 |
| 29. | Active, not recruiting |
COVID-19-specific T-cell-derived exosomes (CSTC Exo) | 60 | 1 | GENKOK, Kayseri, Melikgazi, Turkey | NCT04389385 |
| 30. | Recruiting | CYNK-001 | 86 | 1,2 | Hackensack University Medical Center, Hackensack, New Jersey, USA Atlantic Health, Morristown, New Jersey, USA Atlantic Health, Summit, New Jersey, USA Multicare Health System, Tacoma, Washington, USA |
NCT04365101 |
| 31. | Recruiting | NK cells | 30 | 1 | First Affiliated Hospital of Xinxiang Medical University, Xinxiang, Henan, China | NCT04280224 |
| 32. | Not yet recruiting |
SCE-treated mononuclear cell apheresis |
20 | 2 | Not defined | NCT04299152 |
| 33. | Not yet recruiting |
MSC-derived exosomes |
30 | 1 | Not defined | NCT04276987 |
MSC, mesenchymal stem cell, SCE, stem cell educator; NK, natural killer.
NK cells are a subset of lymphocytes that exert cytotoxic effects against tumour cells and virus-infected cells. In fact, cytokine production (mainly IFNs) by dendritic cells stimulates activation of NK cells, and these cells perform their cytotoxic activity by secretion of perforin and limitation of viral replication [114]. Of particular interest is CYNK-001, a NK therapy developed from placenta-derived haematopoietic stem cells, that is introduced as a potential therapeutic option for a variety of malignancies from haematological to solid tumours (NCT04310592). Celularity Inc. are developing allogeneic cellular therapies from human placenta, and recently announced that FDA has cleared the company's investigational new drug application for the use of its proprietary CYNK-001 in individuals infected with COVID-19. They will undertake a Phase I/II clinical study including up to 86 patients with COVID-19 (not mentioned in Table 2) [115].
3.2.1.2. Anti-cytokine-storm therapies
Cytokine release syndrome, first described in patients who received chimeric antigen receptor T-cell therapy, is characterized by the release of a large body of cytokines including IL-6 and IL-1ß, as well as IL-2, IL-8, IL-17, G-CSF, GM-CSF, IP10, MCP1, MIP1a (also known as CCL3) and TNF [27]. TH17 responses, which lead to vascular permeability and leakage, are enhanced by both IL-1β and TNF-α. Moreover, Th-17 shows a wide inflammatory response through producing G-CSF, IL-1β and TNF-α. TH-17 cells are among the cells involved in the COVID-19 cytokine storm. There is evidence that circulating levels of TH-17 cells are increased in patients with COVID-19, suggesting the use of TH-17 blockers as a potential treatment. STAT-3 is a transcription factor that mediates the inflammatory response of TH-17 cells, while IL-6 activates STAT3 through the JAK2 pathway. In the context of COVID-19, Wu et al. proposed that the FDA-approved drug, fedratinib (a JAK2 pathway inhibitor) might be a promising candidate treatment [116]. Baricitinb, another JAK inhibitor, can improve the clinical status of patients with COVID-19 by exerting an inhibitory effect on numb-associated kinase [117].
Type I IFN is involved in intracellular pathogen defence in the context of both innate and adaptive immunity. The DNA sensor cyclic GMP–AMP synthase (cGAS) and its downstream effector STING (stimulator of IFN genes) regulate transcription of many inflammatory molecules, including type I and type III IFNs. However, dysregulation of IFNs production may lead to inflammatory diseases. Delayed IFN-I production in animals with SARS causes the accumulation of pathogenic monocyte macrophages, resulting in lung immunopathology, vascular leakage and suboptimal T-cell responses. Therefore, targeting the cGAS-STING pathway may be a suitable strategy for the treatment of severe lung diseases caused by SARS-CoV and SARS-CoV-2. In line with this, Deng et al. evaluated the efficacy of various FDA-approved drugs for targeting the STING pathway, and found that approved drugs, such as suramin and ALK inhibitors, might be efficient and therefore worth testing in clinical trials. Adalimumab (TNF-α) and CMAB806 (IL-6) are among the cytokine-directed antagonists that are in clinical trials for COVID-19 [118]. Indeed, anti-IL-6 receptor antibody (tocilizumab), a humanized mAb, was developed and showed prophylactic efficacy against a broad spectrum of autoimmune diseases. However, as mentioned, previously, it is possible that, as a result of inaccurate attribution of ‘cytokine storm’ to COVID-19, the related therapies would also need reconsideration [45].
Moreover, considering the profound lymphopenia in patients with COVID-19 which can lead to severe pathological symptoms, treatments regarding the restoration of lymphocyte numbers have gained attention. IL-7 has a key role in the survival and expansion of lymphocytes. In this regard, Laterre et al. have shown the safety of IL-7 administration in patients with COVID-19 [119].
Interestingly, restoration of Th17/Treg imbalance seems to be the mechanism through which tocilizumab confers protection over a range of diseases [120,121]. The therapeutic potential of tocilizumab for the treatment of patients with COVID-19 is therefore being evaluated. These initial studies suggest that tocilizumab might revert lymphopenia, reduce oxygen need and improve lung lesions, and therefore suggest that tocilizumab might be a promising therapeutic strategy for individuals with COVID-19 [122]. The results of a retrospective cohort study suggest that tocilizumab may mitigate the risk of mechanical ventilation or death in COVID-19 patients with severe pneumonia [123].
3.2.2. Preventive strategies
3.2.2.1. Vaccines
Researchers in biotechnology companies and academic laboratories are working hard to develop efficient vaccines against COVID-19 with unprecedented speed. The number of vaccines that are currently undergoing preclinical investigation confirms this endeavour. A number of approaches have shown sufficiently promising in-vitro and in-vivo animal results that they are already moving to early clinical phase studies [124]. Examples of such vaccines are discussed below. mRNA-1273 is a lipid nanoparticle encapsulated platform that encodes the S protein of SARS-CoV-2, and has the potential to elicit a robust immune response (NCT04283461) [125].
Recently, Zhang et al. developed a novel thermostable mRNA-based vaccine, ARCoV, which encodes the receptor binding domain of SARS-CoV-2 and elicited a protective response in animals challenged with SARS-CoV-2 (ChiCTR2000034112) [126].
Ad5-nCoV (NCT04313127) and INO-4800 (NCT04336410) vaccine candidates employ the same strategy but using different delivery platforms: adenovirus type 5 and DNA plasmid, respectively. LV-SMENP-DC (NCT04276896) and pathogen-specific artificial APCs (NCT04299724) are dendritic cells and artificial APCs which are manipulated with lentiviral vector to express mini-genes based on conserved and critical structural and protease protein domains of the virus, respectively. However, developing new vaccination strategies may take months to years in terms of getting approval for both safety and efficacy.
3.2.2.1.1. Recombinant nucleic acids
DNA vaccines
Vaccination is among the most efficient measures against a wide variety of communicable diseases. In DNA vaccine technology, the gene encoding an immunogen is inserted into a suitable vector which may lead to humoral and/or cellular immunity against this immunogen. Compared with traditional vaccination with an inactivated virus, DNA vaccination holds less risk for infection, is inexpensive, safe and more stable [127]. Yang et al. reported that a DNA vaccine encoding the S protein of SARS-CoV exerts a T-cell-mediated immune response that is accompanied by increased neutralizing antibodies, and protects mice from infection with SARS-CoV [128]. Wang et al. also demonstrated that a DNA vaccine encoding a combination of two immunogens, the S1 subunit and full-length S protein, confers protection against several strains of MERS in non-human primates and mice. Thus, in the context of MERS-CoV, this study is of interest as the S protein and S1 subunit DNA vaccine provides potent protection in animal models. Of note, yeilding a more diverse neutralizing antibodies along with the production of Th1 immune response are among the advantages of using S DNA prime-S1 protein over the protein alone [129]. mRNA-based strategies
An alternative to DNA vaccines is the RNA-based approach. The problems with instability of RNA have been overcome, in part, over the last decade [130]. Compared with DNA, RNA-based therapeutics do not suffer from the risk of insertional mutations, and are only present in the short term due to faster RNA degradation. In-vitro-transcribed (IVT) mRNA has received increasing attention. mRNA-based drugs are being developed as protein-replacement therapies, immunotherapeutics and in regenerative medicine. Preclinical and clinical applications of IVT-mRNA have been evaluated in a wide range of diseases, including viral infections. However, these molecules have some disadvantages, such as their stimulatory effect on immune responses through inducing IFN production. mRNA-1273, as an example of an IVT-mRNA, isa novel lipid-nanoparticle-encapsulated mRNA-based vaccine that encodes a full-length S protein of SARS-CoV-2 (Table 1 ) [131].
Table 1.
An overview of Phase 1 and 2 clinical trials regarding gene-based therapy for coronavirus disease 2019
| Status | Interventions | Population | Phase | Location | NCT number | |
|---|---|---|---|---|---|---|
| 1 | Recruiting | mRNA-1273 | 45 | 1 | •Emory Vaccine Center, The Hope Clinic, Decatur, Georgia, USA •National Institutes of Health Clinical Center – Vaccine Research Center Clinical Trials Program, Bethesda, Maryland, USA •Kaiser Permanente Washington Health Research Institute Vaccines and Infectious Diseases, Seattle, Washington, USA |
NCT04283461 |
| 2 | Recruiting | Injection and infusion of LV-SMENP-DC vaccine and antigen-specific cytotoxic T cells | 100 | 1,2 | •Shenzhen Geno-immune Medical Institute, Shenzhen, Guangdong, China •Shenzhen Second People's Hospital, Shenzhen, Guangdong, China •Shenzhen Third People's Hospital, Shenzhen, Guangdong, China |
NCT04276896 |
| 3 | Recruiting | Pathogen-specific artificial antigen-presenting cells | 100 | 1 | •Shenzhen Geno-immune Medical Institute, Shenzhen, Guangdong, China | NCT04299724 |
| 4 | Active, not recruiting |
Recombinant novel coronavirus vaccine (adenovirus type 5 vector) |
108 | 1 | •Hubei Provincial Centre for Disease Control and Prevention, Wuhan, Hubei, China |
NCT04313127 |
| 5 | Recruiting | INO-4800 | 40 | 1 | •Center for Pharmaceutical Research, Kansas City, Missouri, USA •University of Pennsylvania, Philadelphia, Pennsylvania, USA |
NCT04336410 |
NCT, National Clinical Trial.
3.3. Anticoagulation therapies
Coagulopathy, characterized by high D-dimer levels, increased fibrinogen and low antithrombin, is one of the hallmarks associated with death due to COVID-19. Fibrinolytic therapy using tissue plasminogen activator (t-PA) could improve survival in animal models and patients suffering from ARDS. A study by Wand et al. demonstrated that administration of alteplase (t-PA) was able to improve the P/F ratio (arterial pO2 divided by the fraction of inspired oxygen, expressed as a decimal, that the patient is receiving) in patients with COVID-19. However, as the improvement was transient, they suggested that redosing the antifibrotic drug might result in a more durable response [132]. Disseminated intravascular coagulation, caused by endothelial dysfunction due to excessive production of thrombin as well as decreased fibrinolysis, is also responsible for the lethality of COVID-19. This suggests that anticoagulant agents (e.g. heparin) may be considered as potential anti-COVID-19 candidates. Supporting this idea, Tang et al. showed that anticoagulation therapy using heparin is presumably associated with a better prognosis in patients infected with COVID-19 [133]. In addition, due to some critical features of unfractionated heparin (UFH), also called ‘nebulized heparin’, including anticoagulant, anti-inflammatory and mucolytic effects, UFH may be a potentially effective treatment for COVID-19 [134].
4. Conclusion
The global challenge of the COVID-19 pandemic, associated with high morbidity and mortality, highlights the urgent need for the development of efficient therapeutic strategies. Tremendous advances in understanding the molecular basis of disease pathogenesis in various CoV-based diseases, and rapid insights into the pathogenesis of COVID-19 offer opportunities to take a leap in introducing novel and efficient therapeutics as well as preventive measures against COVID-19. Assessing the efficiency of previously approved antiviral agents for inhibiting SARS-CoV-2 is one of the main areas that is being pursued clinically in the short term. Despite encouraging results with some of these agents, more research is needed regarding safety and efficiency, as exemplified by the initial adoption and later withdrawal of the use of chloroquine as a therapy for COVID-19. Different novel therapeutic strategies including cell- and gene-based therapies against SARS-CoV-2 are now being developed, some of which are already being tested in early-phase clinical trials worldwide. Dysregulation of inflammatory responses, a manifestation of individuals infected with SARS-CoV-2, can be modulated using MSCs. Likewise, SCE cells represent another cell-based strategy which will be assessed for its efficiency in relieving SARS-related inflammation. An alternative cell-based strategy is NK cell therapy to inhibit viral replication. Another approach is based on nucleic-acid-based therapies and includes CRISPR methods along with DNA vaccines and mRNA molecules; these are also being studied intensively, and some have shown encouraging effects in preclinical trials. Overall, all of the aforementioned studies inspire further investigation of advanced therapeutic strategies as novel treatment options for COVID-19.
Acknowledgments
Acknowledgements
The authors wish to thank the Royan Institute and the Katholieke Universiteit Leuven for their support throughout the course of this work.
Funding
None.
Competing interests
None declared.
Ethical approval
Not required.
References
- 1.van der Hoek L., Pyrc K., Jebbink M.F., Vermeulen-Oost W., Berkhout R.J.M., Wolthers K.C., et al. Identification of a new human coronavirus. Nat Med. 2004;10:368–373. doi: 10.1038/nm1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paules C.I., Marston H.D., Fauci A.S. Coronavirus infections – more than just the common cold. JAMA. 2020;323:707–708. doi: 10.1001/jama.2020.0757. [DOI] [PubMed] [Google Scholar]
- 3.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Y., Liu Q., Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol. 2020;92:418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang S., Peng W., Zhu Y., Lu S., Zhou M., Lin W., et al. Recent progress in understanding 2019 novel coronavirus (SARS-CoV-2) associated with human respiratory disease: detection, mechanisms and treatment. Int J Antimicrob Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 2020;11:1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sola I., Almazán F., Zúñiga S., Enjuanes L. Continuous and discontinuous RNA synthesis in coronaviruses. Annu Rev Virol. 2015;2:265–288. doi: 10.1146/annurev-virology-100114-055218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu H., Zhong L., Deng J., Peng J., Dan H., Zeng X., et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12:8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hikmet F., Méar L., Edvinsson Å., Micke P., Uhlén M., Lindskog C. The protein expression profile of ACE2 in human tissues. Molec Syst Biol. 2020;16:e9610. doi: 10.15252/msb.20209610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan X.-W., Xu D., Zhang H., Zhou W., Wang L.-H., Cui X.-G. Identification of a potential mechanism of acute kidney injury during the COVID-19 outbreak: a study based on single-cell transcriptome analysis. Intens Care Med. 2020;46:1114–1116. doi: 10.1007/s00134-020-06026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lescure F.-X., Bouadma L., Nguyen D., Parisey M., Wicky P.-H., Behillil S., et al. Clinical and virological data of the first cases of COVID-19 in Europe: a case series. Lancet Infect Dis. 2020;20:697–706. doi: 10.1016/S1473-3099(20)30200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu J., Zheng X., Tong Q., Li W., Wang B., Sutter K., et al. Overlapping and discrete aspects of the pathology and pathogenesis of the emerging human pathogenic coronaviruses SARS-CoV, MERS-CoV, and 2019-nCoV. J Med Virol. 2020;92:491–494. doi: 10.1002/jmv.25709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yeung M.-L., Yao Y., Jia L., Chan J.F.W., Chan K.-H., Cheung K.-F., et al. MERS coronavirus induces apoptosis in kidney and lung by upregulating Smad7 and FGF2. Nat Microbiol. 2016;1:16004. doi: 10.1038/nmicrobiol.2016.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chu H., Zhou J., Wong B.H., Li C., Chan J.F., Cheng Z.S., et al. Middle East respiratory syndrome coronavirus efficiently infects human primary T lymphocytes and activates the extrinsic and intrinsic apoptosis pathways. J Infect Dis. 2016;213:904–914. doi: 10.1093/infdis/jiv380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Durvasula R., Wellington T., McNamara E., Watnick S. COVID-19 and kidney failure in the acute care setting: our experience from Seattle. Am J Kidney Dis. 2020;76:4–6. doi: 10.1053/j.ajkd.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alsaad K., Arabi Y., Hajeer A. Spectrum of histopathological findings in coronavirus disease-19, Middle East respiratory syndrome and severe acute respiratory syndrome. Ann Thorac Med. 2020;15:52–53. doi: 10.4103/atm.ATM_105_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L., Zhang Y., Zhang S. Cardiovascular impairment in COVID-19: learning from current options for cardiovascular anti-inflammatory therapy. Front Cardiovasc Med. 2020;7:78. doi: 10.3389/fcvm.2020.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Musa S. Hepatic and gastrointestinal involvement in coronavirus disease 2019 (COVID-19): what do we know till now? Arab J Gastroenterol. 2020;21:3–8. doi: 10.1016/j.ajg.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou J., Li C., Liu X., Chiu M.C., Zhao X., Wang D., et al. Infection of bat and human intestinal organoids by SARS-CoV-2. Nat Med. 2020;26:1077–1083. doi: 10.1038/s41591-020-0912-6. [DOI] [PubMed] [Google Scholar]
- 22.Zhao B., Ni C., Gao R., Wang Y., Yang L., Wei J., et al. Recapitulation of SARS-CoV-2 infection and cholangiocyte damage with human liver ductal organoids. Protein Cell. 2020;11:771–775. doi: 10.1007/s13238-020-00718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prompetchara E., Ketloy C., Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020;38:1–9. doi: 10.12932/AP-200220-0772. [DOI] [PubMed] [Google Scholar]
- 24.Khan S., Siddique R., Shereen M.A., Ali A., Liu J., Bai Q., et al. Emergence of a novel coronavirus, severe acute respiratory syndrome coronavirus 2: biology and therapeutic options. J Clin Microbiol. 2020;58:e00187. doi: 10.1128/JCM.00187-20. e00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Otsuka R., Seino K.-I. Macrophage activation syndrome and COVID-19. Inflamm Regen. 2020;40:19. doi: 10.1186/s41232-020-00131-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sokolowska M., Lukasik Z., Agache I., Akdis C.A., Akdis D., Akdis M., et al. Immunology of COVID-19: mechanisms, clinical outcome, diagnostics and perspectives – a report of the European Academy of Allergy and Clinical Immunology (EAACI) Allergy. 2020;75:2445–2476. doi: 10.1111/all.14462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao X. COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020;20:269–270. doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomar B., Anders H.J., Desai J., Mulay S.R. Neutrophils and neutrophil extracellular traps drive necroinflammation in COVID-19. Cells. 2020;9:1383. doi: 10.3390/cells9061383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li X., Geng M., Peng Y., Meng L., Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharmaceut Anal. 2020;10:102–108. doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perlman S., Dandekar A.A. Immunopathogenesis of coronavirus infections: implications for SARS. Nat Rev Immunol. 2005;5:917–927. doi: 10.1038/nri1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahmed S.F., Quadeer A.A., McKay M.R. Preliminary identification of potential vaccine targets for the COVID-19 coronavirus (SARS-CoV-2) based on SARS-CoV immunological studies. Viruses. 2020;12:254. doi: 10.3390/v12030254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nguyen A., David J.K., Maden S.K., Wood M.A., Weeder B.R., Nellore A., et al. Human leukocyte antigen susceptibility map for severe acute respiratory syndrome coronavirus 2. J Virol. 2020;94:e00510–e00520. doi: 10.1128/JVI.00510-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bunte K., Beikler T. Th17 cells and the IL-23/IL-17 axis in the pathogenesis of periodontitis and immune-mediated inflammatory diseases. Int J Mol Sci. 2019;20:3394. doi: 10.3390/ijms20143394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li G., Fan Y., Lai Y., Han T., Li Z., Zhou P., et al. Coronavirus infections and immune responses. J Med Virol. 2020;92:424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ng O.W., Chia A., Tan A.T., Jadi R.S., Leong H.N., Bertoletti A., et al. Memory T cell responses targeting the SARS coronavirus persist up to 11 years post-infection. Vaccine. 2016;34:2008–2014. doi: 10.1016/j.vaccine.2016.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou G., Zhao Q. Perspectives on therapeutic neutralizing antibodies against the novel coronavirus SARS-CoV-2. Int J Biol Sci. 2020;16:1718–1723. doi: 10.7150/ijbs.45123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Traggiai E., Becker S., Subbarao K., Kolesnikova L., Uematsu Y., Gismondo M.R., et al. An efficient method to make human monoclonal antibodies from memory B cells: potent neutralization of SARS coronavirus. Nat Med. 2004;10:871–875. doi: 10.1038/nm1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fast E., Chen B. Potential T-cell and B-cell epitopes of 2019-nCoV. bioRxiv 2020:2020.02.19.955484.
- 39.Liu L., Wei Q., Lin Q., Fang J., Wang H., Kwok H., et al. Anti-spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI Insight. 2019;4 doi: 10.1172/jci.insight.123158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Imai Y., Kuba K., Rao S., Huan Y., Guo F., Guan B., et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Miguel C., Rudemiller N.P., Abais J.M., Mattson D.L. Inflammation and hypertension: new understandings and potential therapeutic targets. Curr Hypertens Rep. 2015;17:507. doi: 10.1007/s11906-014-0507-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Youn J.C., Yu H.T., Lim B.J., Koh M.J., Lee J., Chang D.Y., et al. Immunosenescent CD8+ T cells and C-X-C chemokine receptor type 3 chemokines are increased in human hypertension. Hypertension. 2013;62:126–133. doi: 10.1161/HYPERTENSIONAHA.113.00689. [DOI] [PubMed] [Google Scholar]
- 43.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Q., Zhou Y.H., Yang Z.Q. The cytokine storm of severe influenza and development of immunomodulatory therapy. Cell Mol Immunol. 2016;13:3–10. doi: 10.1038/cmi.2015.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sinha P., Matthay M.A., Calfee C.S. Is a “cytokine storm” relevant to COVID-19? JAMA Intern Med. 2020;180:1152–1154. doi: 10.1001/jamainternmed.2020.3313. [DOI] [PubMed] [Google Scholar]
- 46.Remy K.E., Mazer M., Striker D.A., Ellebedy A.H., Walton A.H., Unsinger J., et al. Severe immunosuppression and not a cytokine storm characterizes COVID-19 infections. JCI Insight. 2020;5 doi: 10.1172/jci.insight.140329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sardu C., Gambardella J., Morelli M., Wang X., Marfella R., Santulli G. Hypertension, thrombosis, kidney failure, and diabetes: is COVID-19 an endothelial disease? A comprehensive evaluation of clinical and basic evidence. J Clin Med. 2020;9:1417. doi: 10.3390/jcm9051417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li T., Lu H., Zhang W. Clinical observation and management of COVID-19 patients. Emerg Microbes Infect. 2020;9:687–690. doi: 10.1080/22221751.2020.1741327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Song J.-C., Wang G., Zhang W., Zhang Y., Li W.-Q., Zhou Z., et al. Chinese expert consensus on diagnosis and treatment of coagulation dysfunction in COVID-19. Mil Med Res. 2020;7:19. doi: 10.1186/s40779-020-00247-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jose R.J., Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med. 2020;8:e46–e47. doi: 10.1016/S2213-2600(20)30216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grillet F., Behr J., Calame P., Aubry S., Delabrousse E. Acute pulmonary embolism associated with COVID-19 pneumonia detected by pulmonary CT angiography. Radiology 2020:201544. [DOI] [PMC free article] [PubMed]
- 54.Xiao L., Sakagami H., Miwa N. ACE2: the key molecule for understanding the pathophysiology of severe and critical conditions of COVID-19: demon or angel? Viruses. 2020;12:491. doi: 10.3390/v12050491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heurich A., Hofmann-Winkler H., Gierer S., Liepold T., Jahn O., Pöhlmann S. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J Virol. 2014;88:1293–1307. doi: 10.1128/JVI.02202-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guo J., Huang Z., Lin L., Lv J. Coronavirus disease 2019 (COVID‐19) and cardiovascular disease: a viewpoint on the potential influence of angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers on onset and severity of severe acute respiratory syndrome coronavirus 2 infection. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.016219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Úri K., Fagyas M., Kertész A., Borbély A., Jenei C., Bene O., et al. Circulating ACE2 activity correlates with cardiovascular disease development. J Renin Angiotensin Aldosterone Syst. 2016;17 doi: 10.1177/1470320316668435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rahmanzade R., Rahmanzadeh R., Tabarsi P., Hashemian S.M. Non-invasive versus invasive ventilation in COVID-19: one size does not fit all! Anesth Analg. 2020;131:e114–e115. doi: 10.1213/ANE.0000000000004943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tchesnokov E.P., Feng J.Y., Porter D.P., Gotte M. Mechanism of inhibition of Ebola virus RNA-dependent RNA polymerase by remdesivir. Viruses. 2019;11:326. doi: 10.3390/v11040326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gordon C.J., Tchesnokov E.P., Feng J.Y., Porter D.P., Gotte M. The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J Biol Chem. 2020;295:4773–4779. doi: 10.1074/jbc.AC120.013056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Amirian E.S., Levy J.K. Current knowledge about the antivirals remdesivir (GS-5734) and GS-441524 as therapeutic options for coronaviruses. One Health. 2020;9 doi: 10.1016/j.onehlt.2020.100128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., et al. Remdesivir for the treatment of Covid-19 – preliminary report. N Engl J Med. 2020;NEJMoa2007764 doi: 10.1056/NEJMoa2007764. [DOI] [PubMed] [Google Scholar]
- 64.Ju J., Li X., Kumar S., Jockusch S., Chien M., Tao C., et al. Nucleotide analogues as inhibitors of SARS-CoV polymerase. bioRxiv 2020:2020.03.12.989186. [DOI] [PMC free article] [PubMed]
- 65.Dong L., Hu S., Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discov Ther. 2020;14:58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- 66.Chen C., Huang J., Yin P., Zhang Y., Cheng Z., Wu J., et al. Favipiravir versus arbidol for COVID-19: a randomized clinical trial. medRxiv 2020:2020.03.17.20037432.
- 67.GlenmarkPharmaceuticalsLtd. Glenmark Announces Top-Line Results From Phase 3 Clinical Trial of Favipiravir in Patients With Mild to Moderate COVID-19 Mumbai, India: Glenmark Pharmaceuticals Ltd; 2020 [Available from: https://www.glenmarkpharma.com/media/newsroom
- 68.Agrawal U, Raju R, UdwadiaFavipiravir ZF. A new and emerging antiviral option in COVID-19. Med J Armed Forces India. 2020;76(4):370–376. doi: 10.1016/j.mjafi.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cunningham A.C., Goh H.P., Koh D. Treatment of COVID-19: old tricks for new challenges. Crit Care. 2020;24:91. doi: 10.1186/s13054-020-2818-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.US Food and Drug Administration . FDA; Silver Spring, MD: 2020. Coronavirus (COVID-19) update: FDA revokes emergency use authorization for chloroquine and hydroxychloroquine.https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-chloroquine-and Available at: [Google Scholar]
- 71.Molina J.M., Delaugerre C., Le Goff J., Mela-Lima B., Ponscarme D., Goldwirt L., et al. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med Mal Infect. 2020;50:384. doi: 10.1016/j.medmal.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cavalcanti A.B., Zampieri F.G., Rosa R.G., Azevedo L.C.P., Veiga V.C., Avezum A., et al. Hydroxychloroquine with or without azithromycin in mild-to-moderate Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2019014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chaib F. WHO; Geneva: 2020. WHO welcomes preliminary results about dexamethasone use in treating critically ill COVID-19 patients.https://www.who.int/news-room/detail/16-06-2020-who-welcomes-preliminary-results-about-dexamethasone-use-in-treating-critically-ill-covid-19-patients Available at: accessed 29 October 2020. [Google Scholar]
- 74.RECOVERY Collaborative Group. Hornby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., et al. Dexamethasone in hospitalized patients with Covid-19 – preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Danser A.H.J., Epstein M., Batlle D. Renin–angiotensin system blockers and the COVID-19 pandemic. Hypertension. 2020;75:1382–1385. doi: 10.1161/HYPERTENSIONAHA.120.15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Clerkin K.J., Fried J.A., Raikhelkar J., Sayer G., Griffin J.M., Masoumi A., et al. Coronavirus disease 2019 (COVID-19) and cardiovascular disease. Circulation. 2020;141:1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 77.Furuhashi M., Moniwa N., Mita T., Fuseya T., Ishimura S., Ohno K., et al. Urinary angiotensin-converting enzyme 2 in hypertensive patients may be increased by olmesartan, an angiotensin II receptor blocker. Am J Hypertens. 2015;28:15–21. doi: 10.1093/ajh/hpu086. [DOI] [PubMed] [Google Scholar]
- 78.Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer M.A., Solomon S.D. Renin–angiotensin–aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382:1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.-L., Abiona O., et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yuan M., Wu N.C., Zhu X., Lee C.-C.D., So R.T.Y., Lv H., et al. A highly conserved cryptic epitope in the receptor-binding domains of SARS-CoV-2 and SARS-CoV. Science. 2020;368:630–633. doi: 10.1126/science.abb7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Duan K., Liu B., Li C., Zhang H., Yu T., Qu J., et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci USA. 2020;117:9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shen C., Wang Z., Zhao F., Yang Y., Li J., Yuan J., et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323:1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Joyner M.J., Senefeld J.W., Klassen S.A., Mills J.R., Johnson P.W., Theel E.S., et al. Effect of convalescent plasma on mortality among hospitalized patients with COVID-19: initial three-month experience. medRxiv 2020:2020.08.12.20169359.
- 84.Gitlin L., Karelsky S., Andino R. Short interfering RNA confers intracellular antiviral immunity in human cells. Nature. 2002;418:430–434. doi: 10.1038/nature00873. [DOI] [PubMed] [Google Scholar]
- 85.Zhang Y., Li T., Fu L., Yu C., Li Y., Xu X., et al. Silencing SARS-CoV spike protein expression in cultured cells by RNA interference. FEBS Lett. 2004;560:141–146. doi: 10.1016/S0014-5793(04)00087-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.He M.L., Zheng B., Peng Y., Peiris J.S., Poon L.L., Yuen K.Y., et al. Inhibition of SARS-associated coronavirus infection and replication by RNA interference. JAMA. 2003;290:2665–2666. doi: 10.1001/jama.290.20.2665. [DOI] [PubMed] [Google Scholar]
- 87.He M.L., Zheng B.J., Chen Y., Wong K.L., Huang J.D., Lin M.C., et al. Kinetics and synergistic effects of siRNAs targeting structural and replicase genes of SARS-associated coronavirus. FEBS Lett. 2006;580:2414–2420. doi: 10.1016/j.febslet.2006.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zheng B.J., Guan Y., Tang Q., Du C., Xie F.Y., He M.L., et al. Prophylactic and therapeutic effects of small interfering RNA targeting SARS-coronavirus. Antiviral Ther. 2004;9:365–374. [PubMed] [Google Scholar]
- 89.Li B.J., Tang Q., Cheng D., Qin C., Xie F.Y., Wei Q., et al. Using siRNA in prophylactic and therapeutic regimens against SARS coronavirus in Rhesus macaque. Nat Med. 2005;11:944–951. doi: 10.1038/nm1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu C., Zhou Q., Li Y., Garner L.V., Watkins S.P., Carter L.J., et al. Research and development on therapeutic agents and vaccines for COVID-19 and related human coronavirus diseases. ACS Cent Sci. 2020;6:315–331. doi: 10.1021/acscentsci.0c00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ghosh S., Firdous S.M., Nath A. siRNA could be a potential therapy for COVID-19. EXCLI J. 2020;19:528–531. doi: 10.17179/excli2020-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Amitai G., Sorek R. CRISPR-Cas adaptation: insights into the mechanism of action. Nat Rev Microbiol. 2016;14:67–76. doi: 10.1038/nrmicro.2015.14. [DOI] [PubMed] [Google Scholar]
- 93.Freije C.A., Myhrvold C., Boehm C.K., Lin A.E., Welch N.L., Carter A., et al. Programmable inhibition and detection of RNA viruses using Cas13. Mol Cell. 2019;76:826–837. doi: 10.1016/j.molcel.2019.09.013. e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee C. CRISPR/Cas9-based antiviral strategy: current status and the potential challenge. Molecules. 2019;24:1349. doi: 10.3390/molecules24071349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Abudayyeh O.O., Gootenberg J.S., Konermann S., Joung J., Slaymaker I.M., Cox D.B., et al. C2c2 is a single-component programmable RNA-guided RNA-targeting CRISPR effector. Science. 2016;353:aaf5573. doi: 10.1126/science.aaf5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Abudayyeh O.O., Gootenberg J.S., Essletzbichler P., Han S., Joung J., Belanto J.J., et al. RNA targeting with CRISPR–Cas13. Nature. 2017;550:280–284. doi: 10.1038/nature24049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Aman R., Mahas A., Butt H., Aljedaani F., Mahfouz M. Engineering RNA virus interference via the CRISPR/Cas13 machinery in arabidopsis. Viruses. 2018;10:732. doi: 10.3390/v10120732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhao X., Liu L., Lang J., Cheng K., Wang Y., Li X., et al. A CRISPR-Cas13a system for efficient and specific therapeutic targeting of mutant KRAS for pancreatic cancer treatment. Cancer Lett. 2018;431:171–181. doi: 10.1016/j.canlet.2018.05.042. [DOI] [PubMed] [Google Scholar]
- 99.Konermann S., Lotfy P., Brideau N.J., Oki J., Shokhirev M.N., Hsu P.D. Transcriptome engineering with RNA-targeting type VI-D CRISPR effectors. Cell. 2018;173:665–676. doi: 10.1016/j.cell.2018.02.033. e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Abbott T.R., Dhamdhere G., Liu Y., Lin X., Goudy L., Zeng L., et al. Development of CRISPR as a prophylactic strategy to combat novel coronavirus and influenza. bioRxiv 2020:2020.03.13.991307.
- 101.Ankrum J., Karp J.M. Mesenchymal stem cell therapy: two steps forward, one step back. Trends Mol Med. 2010;16:203–209. doi: 10.1016/j.molmed.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hossein-Khannazer N., Shokoohian B., Shpichka A., Aghdaei H.A., Timashev P., Vosough M. Novel therapeutic approaches for treatment of COVID-19. J Mol Med. 2020;98:789–803. doi: 10.1007/s00109-020-01927-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Elgaz S., Kuçi Z., Kuçi S., Bönig H., Bader P. Clinical use of mesenchymal stromal cells in the treatment of acute graft-versus-host disease. Transfus Med Hemother. 2019;46:27–34. doi: 10.1159/000496809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Le Blanc K., Frassoni F., Ball L., Locatelli F., Roelofs H., Lewis I., et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 105.Muroi K., Miyamura K., Okada M., Yamashita T., Murata M., Ishikawa T., et al. Bone marrow-derived mesenchymal stem cells (JR-031) for steroid-refractory grade III or IV acute graft-versus-host disease: a phase II/III study. Int J Hematol. 2016;103:243–250. doi: 10.1007/s12185-015-1915-9. [DOI] [PubMed] [Google Scholar]
- 106.Emukah C., Dittmar E., Naqvi R., Martinez J., Corral A., Moreira A., et al. Mesenchymal stromal cell conditioned media for lung disease: a systematic review and meta-analysis of preclinical studies. Respir Res. 2019;20:239. doi: 10.1186/s12931-019-1212-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Behnke J., Kremer S., Shahzad T., Chao C.-M., Böttcher-Friebertshäuser E., Morty R., et al. MSC based therapies – new perspectives for the injured lung. J Clin Med. 2020;9:682. doi: 10.3390/jcm9030682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen J., Hu C., Chen L., Tang L., Zhu Y., Xu X., et al. Clinical study of mesenchymal stem cell treating acute respiratory distress syndrome induced by epidemic influenza A (H7N9) infection, a hint for COVID-19 treatment. Engineering (Beijing) 2020 doi: 10.1016/j.eng.2020.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Horie S., Gonzalez H.E., Laffey J.G., Masterson C.H. Cell therapy in acute respiratory distress syndrome. J Thorac Dis. 2018;10:5607–5620. doi: 10.21037/jtd.2018.08.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Khoury M., Cuenca J., Cruz F.F., Figueroa F.E., Rocco P.R.M., Weiss D.J. Current status of cell-based therapies for respiratory virus infections: applicability to COVID-19. Eur Respir J. 2020;55 doi: 10.1183/13993003.00858-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Leng Z., Zhu R., Hou W., Feng Y., Yang Y., Han Q., et al. Transplantation of ACE2(-) mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;11:216–228. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liang B., Chen J., Li T., Wu H., Yang W., Li Y., et al. Clinical remission of a critically ill COVID-19 patient treated by human umbilical cord mesenchymal stem cells. Medicine (Baltimore) 2020;99:e21429. doi: 10.1097/MD.0000000000021429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhao Y. Stem cell educator therapy and induction of immune balance. Curr Diab Rep. 2012;12:517–523. doi: 10.1007/s11892-012-0308-1. [DOI] [PubMed] [Google Scholar]
- 114.Lang P.A., Lang K.S., Xu H.C., Grusdat M., Parish I.A., Recher M., et al. Natural killer cell activation enhances immune pathology and promotes chronic infection by limiting CD8+ T-cell immunity. Proc Natl Acad Sci. 2012;109:1210. doi: 10.1073/pnas.1118834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Celularity Inc. Celularity Inc.; Florham Park, NJ: 2020. Celularity announces FDA clearance of IND application for CYNK-001 in coronavirus, first in cellular therapy.https://www.prnewswire.com/news-releases/celularity-announces-fda-clearance-of-ind-application-for-cynk-001-in-coronavirus-first-in-cellular-therapy-301034141.html Available at: [Google Scholar]
- 116.Wu D., Yang X.O. TH17 responses in cytokine storm of COVID-19: an emerging target of JAK2 inhibitor fedratinib. J Microbiol Immunol Infect. 2020;53:368–370. doi: 10.1016/j.jmii.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stebbing J., Phelan A., Griffin I., Tucker C., Oechsle O., Smith D., et al. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis. 2020;20:400–402. doi: 10.1016/S1473-3099(20)30132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Deng X., Yu X., Pei J. Regulation of interferon production as a potential strategy for COVID-19 treatment. arXiv:2003.00751 [q-bio.MN] 2020.
- 119.Laterre P.F., François B., Collienne C., Hantson P., Jeannet R., Remy K.E., et al. Association of interleukin 7 immunotherapy with lymphocyte counts among patients with severe coronavirus disease 2019 (COVID-19) JAMA Network Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.16485. e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tanaka T., Narazaki M., Kishimoto T. Therapeutic targeting of the interleukin-6 receptor. Ann Rev Pharmacol Toxicol. 2012;52:199–219. doi: 10.1146/annurev-pharmtox-010611-134715. [DOI] [PubMed] [Google Scholar]
- 121.Kang S., Tanaka T., Kishimoto T. Therapeutic uses of anti-interleukin-6 receptor antibody. Int Immunol. 2015;27:21–29. doi: 10.1093/intimm/dxu081. [DOI] [PubMed] [Google Scholar]
- 122.Xu X., Han M., Li T., Sun W., Wang D., Fu B., et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci USA. 2020;117:10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Guaraldi G., Meschiari M., Cozzi-Lepri A., Milic J., Tonelli R., Menozzi M., et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2020;2:e474–e484. doi: 10.1016/S2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Le T.T., Andreadakis Z., Kumar A., Román R.G., Tollefsen S., Saville M., et al. The COVID-19 vaccine development landscape. Nat Rev Drug Discov. 2020;19:305–306. doi: 10.1038/d41573-020-00073-5. [DOI] [PubMed] [Google Scholar]
- 125.Jackson L.A., Anderson E.J., Rouphael N.G., Roberts P.C., Makhene M., Coler R.N., et al. An mRNA vaccine against SARS-CoV-2 – preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang N.-N., Li X.-F., Deng Y.-Q., Zhao H., Huang Y.-J., Yang G., et al. A Thermostable mRNA vaccine against COVID-19. Cell. 2020;182:1271–1283. doi: 10.1016/j.cell.2020.07.024. e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Khan K.H. DNA vaccines: roles against diseases. Germs. 2013;3:26–35. doi: 10.11599/germs.2013.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yang Z.Y., Kong W.P., Huang Y., Roberts A., Murphy B.R., Subbarao K., et al. A DNA vaccine induces SARS coronavirus neutralization and protective immunity in mice. Nature. 2004;428:561–564. doi: 10.1038/nature02463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang L., Shi W., Joyce M.G., Modjarrad K., Zhang Y., Leung K., et al. Evaluation of candidate vaccine approaches for MERS-CoV. Nat Commun. 2015;6:7712. doi: 10.1038/ncomms8712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Guo P., Haque F., Hallahan B., Reif R., Li H. Uniqueness, advantages, challenges, solutions, and perspectives in therapeutics applying RNA nanotechnology. Nucl Acid Ther. 2012;22:226–245. doi: 10.1089/nat.2012.0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sahin U., Karikó K., Türeci Ö. mRNA-based therapeutics – developing a new class of drugs. Nat Rev Drug Discov. 2014;13:759–780. doi: 10.1038/nrd4278. [DOI] [PubMed] [Google Scholar]
- 132.Wang J., Hajizadeh N., Moore E.E., McIntyre R.C., Moore P.K., Veress L.A., et al. Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): a case series. J Thromb Haemost. 2020;18:1752–1755. doi: 10.1111/jth.14828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.van Haren F.M.P., Page C., Laffey J.G., Artigas A., Camprubi-Rimblas M., Nunes Q., et al. Nebulised heparin as a treatment for COVID-19: scientific rationale and a call for randomised evidence. Crit Care. 2020;24:454. doi: 10.1186/s13054-020-03148-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tan Yee-Joo, Lim Seng Gee, Wanjin Hong. Regulation of cell death during infection by the severe acute respiratory syndrome coronavirus and other coronaviruses. Cell Microbiol. 2007;9(11):2552–2561. doi: 10.1111/j.1462-5822.2007.01034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Frieman M, Heise M, Baric R. SARS coronavirus and innate immunity. Virus research. 2007;133(1):101–112. doi: 10.1016/j.virusres.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]