Abstract
Nogo-B is an endoplasmic reticulum-residential protein with distinctive functions in different diseases. However, it remains unclear the role of Nogo-B in liver sterile inflammatory injury. This study aims to elucidate the functions and mechanisms in liver ischemia and reperfusion injury (IRI). The Nogo-B expression and liver function were analyzed in biopsy/serum specimens from 36 patients undergoing ischemia-related hepatectomy and in a mouse model of liver IRI. Human specimens were harvested prior to ischemia and post-reperfusion. The Nogo-B knockout (Nogo-BKO) and myeloid-specific Nogo-B knockout (Nogo-BMKO) mice were used to analyze the function and mechanism of Nogo-B in a mouse model of liver IRI. In human specimens, the Nogo-B expression was positively correlated with higher levels of serum transaminase (sALT) and severe histopathological injury at one day post-hepatectomy. Moreover, Nogo-B is mainly expressed on macrophages in normal and ischemic liver tissues from human and mice. Unlike in controls, the Nogo-BKO or Nogo-BMKO livers was protected against IRI, with reduced reactive oxygen species (ROS) production and liver inflammation in ischemic livers. In parallel in vitro studies, Nogo-B deficiency reduced M1 macrophage polarization and inhibited proinflammatory cytokines (TNF-α, IL-6, MCP-1 and iNOS) in response to LPS or HMGB-1 stimulation. Mechanistic studies showed that Nogo-B bound to MST1/2, increased MST1/2, LAST1, and YAP phosphorylation, leading to reduced YAP activity. Interestingly, disruption of macrophage YAP abolished Nogo-B deficiency-mediated cytoprotective effects in vitro and in vivo. Thus, YAP is crucial for the regulation of macrophage Nogo-B-triggered liver inflammation. Nogo-B promotes macrophage-related innate inflammation and contributes to IR-induced liver injury by activating the MST-mediated Hippo/YAP pathway, which provides a potential therapeutic target for clinical management in liver IRI.
Keywords: Nogo-B, Hepatic ischemia/reperfusion injury, Macrophage, Inflammation, MST1/2, Hippo/YAP signaling
Lay summary
Liver ischemia and reperfusion injury (IRI), characterized by acute sterile inflammation and hepatocellular damage, is a major factor in initiating liver dysfunction and failure after liver transplantation and hepatectomy. This study first documented the roles and mechanisms of Nogo-B in hepatic IRI. Nogo-B promotes macrophage-related innate inflammation and contributes to IR-induced liver injury by activating the MST-mediated Hippo/YAP pathway. Our findings revealed that Nogo-B is a key mediator of hepatic IRI.
1. Introduction
Ischemia and reperfusion injury (IRI) is a major factor in initiating liver dysfunction and failure after hemorrhagic shock, liver transplantation, and hepatectomy [[1], [2], [3]]. The multiple molecular mechanisms have been identified during the process of liver IRI, which includes oxidation-reduction imbalance, calcium overload, and activation of inflammatory cells [[3], [4], [5]]. Innate Toll-like receptor 4 (TLR4) plays a key role in IR-triggered liver inflammation [6,7]. In the early stage of liver IRI, Danger-associated molecular patterns (DAMPs) such as high mobility group box 1 (HMGB1), which releases from damaged or dying cells, activates TLR4 in liver macrophages (Kupffer cells, KCs) and produces reactive oxygen species (ROS) and proinflammatory cytokines, including TNF-α and IL-6 [8]. During the reperfusion stage, inflammatory cytokines may further activate the intracellular signaling cascades to drive adaptive immune responses leading to hepatocellular damage [9]. Thus, inhibition of macrophage TLR4-mediated inflammatory responses is crucial in alleviating clinical or experimental organ IRI.
Nogo-B, also known as Reticulon 4B, is a member of the reticulon (Rtn) family of proteins that are primarily localized to the endoplasmic reticulum. In mammalian cells, there are four Rtn genes, Rtn-1, Rtn-2, Rtn-3, and Rtn-4, and each gene encodes multiple isoforms. Rtn-4 can encode three isoform proteins in different tissues: Nogo-A, Nogo-B, and Nogo-C. Nogo-A and Nogo-C are highly expressed in the central nervous system, and Nogo-C is also present in skeletal muscle [10,11]. Nogo-A is well known to inhibit axonal growth and repair, whereas the function of Nogo-C remains unclear [10,11]. Nogo-B is found in endothelial and smooth muscle cells, and regulates vascular remodeling by enhancing migration of endothelial cells; in pathological vascular conditions, Nogo-B inhibits migration and proliferation of smooth muscle cells [10,12,13]. In mice and rabbits, neointimal expansion of injured blood vessels is associated with a marked reduction in endogenous Nogo-B levels, suggesting that Nogo-B negatively regulates the extent of vascular injury. Moreover, loss of Nogo-B strongly correlates with stenotic lesions and plaque rupture in humans [12,14,15]. Indeed, Nogo-B is highly expressed on nonparenchymal cells (NPCs) while minimally expressing in hepatocytes of normal human liver tissues [16]. However, Nogo-B can be detected in hepatocytes in mouse livers after partial hepatectomy, and has been shown to facilitate hepatocyte proliferation and liver regeneration [17]. Furthermore, Nogo-B is also highly expressed in other tissues, especially in the immune system. A large and growing number of studies have illustrated that Nogo-B is essential for macrophage infiltration and polarization [18,19], suggesting that Nogo-B may be key in the modulation of macrophage-mediated inflammatory response in acute liver inflammatory injury.
As hepatic IRI is characterized by acute sterile inflammation and hepatocellular damage [3], we hypothesized that Nogo-B may be involved in liver IRI. First, the Nogo-B expression was assessed in normal and IRI human/mice livers. Second, the normal or IR-induced Nogo-B distribution was determined in human/mice livers. Third, the functional roles and molecular mechanisms of Nogo-B were tested in liver IRI using Nogo-B knockout (Nogo-BKO) and myeloid-specific Nogo-B knockout (Nogo-BMKO) mice.
2. Materials and methods
2.1. Patients and clinical study
The study was approved by the Research Ethics Committee of the First Affiliated Hospital of Nanjing Medical University, Nanjing, China (Institutional Review Board approval number 2018-SRFA-197). Biopsy specimens were obtained from 36 patients (Supporting Table 1) with benign liver disease undergoing hepatectomy with pringle maneuver (January 2018–December 2018, Hepatobiliary Center, the First Affiliated Hospital of Nanjing Medical University). Pre-hepatectomy hepatic biopsies were harvested after laparotomy (prior to hepatic portal occlusion) and post-hepatectomy hepatic biopsies were obtained after reperfusion (prior to abdominal closure). Ischemic time was from 15 to 30 min (min). The serum alanine aminotransferase (sALT and sAST) levels were analyzed at 1 day after resection. Informed consent was obtained from all participants.
2.2. Mouse model of warm liver IRI
Male, 6–8 week old, wild-type (WT), Nogo-B knockout (Nogo-BKO), FloxP-Nogo-B (Nogo-BFL/FL), Lyz2-Cre or myeloid-specific Nogo-B knockout (Nogo-BMKO) mice on C57BL/6 background (Nanjing Biomedical Research Institute of Nanjing University) were used in the experiments. Nogo-BFL/FL mice were bred with myeloid-specific Cre (Lyz2-Cre) mice to create Nogo-BMKO mice. Briefly, homozygous Nogo-BFL/FL mice were first bred with homozygous Lyz2-Cre mice, and the heterozygous offspring (for both Nogo-B and Cre) were back-crossed with Nogo-BFL/FL mice. We used a well-established mouse model of warm hepatic ischemia (90 min) followed by reperfusion (6 h), as previously described [20,21]. Briefly, an atraumatic clip was used to interrupt the arterial and portal venous blood supply to the cephalad lobes of the liver. After 90 min of ischemia, the clip was removed, initiating hepatic reperfusion. Samples (liver tissues and serum) were harvested after 6 h of reperfusion. Sham controls underwent the same procedure, but without vascular occlusion. Some mice were injected through the tail vein with bone marrow-derived macrophages (BMMs) (1 × 106 cells in phosphate-buffered saline/mouse) 24 h prior to ischemia, YAP siRNA mixed with mannose-conjugated polymers (Polyplus transfection™, Illkirch, France) 4 h prior to ischemia, or YAP inhibitor (verteporfin, VP, 0.8 μmol/kg, MedChemExpress, USA) 1 h prior to ischemia [21,22]. Levels of sALT and sAST were measured with an automated chemical analyzer (Olympus Automated Chemistry Analyzer AU5400, Tokyo, Japan). Some liver specimens were fixed in 10% buffered formalin and embedded in paraffin. Liver sections (4 μm) were stained with hematoxylin and eosin (HE). The severity of pathological injury was graded blindly with a Suzuki's criteria on a scale from 0 to 4 [23]. All animal experiments were approved by the Nanjing Medical University (NJMU) Animal Research Committee.
2.3. Statistical analysis
The significance of differences was determined by an unpaired Student's t-test using Prism software (GraphPad 8.0). Linear regression (R2) was used to evaluate the strength of linear relationship between variables. P values < 0.05 were considered statistically significant.
For further details regarding the methods and materials, please refer to supplementary information.
3. Results
3.1. Nogo-B expression is positively correlated with IR-induced liver injury
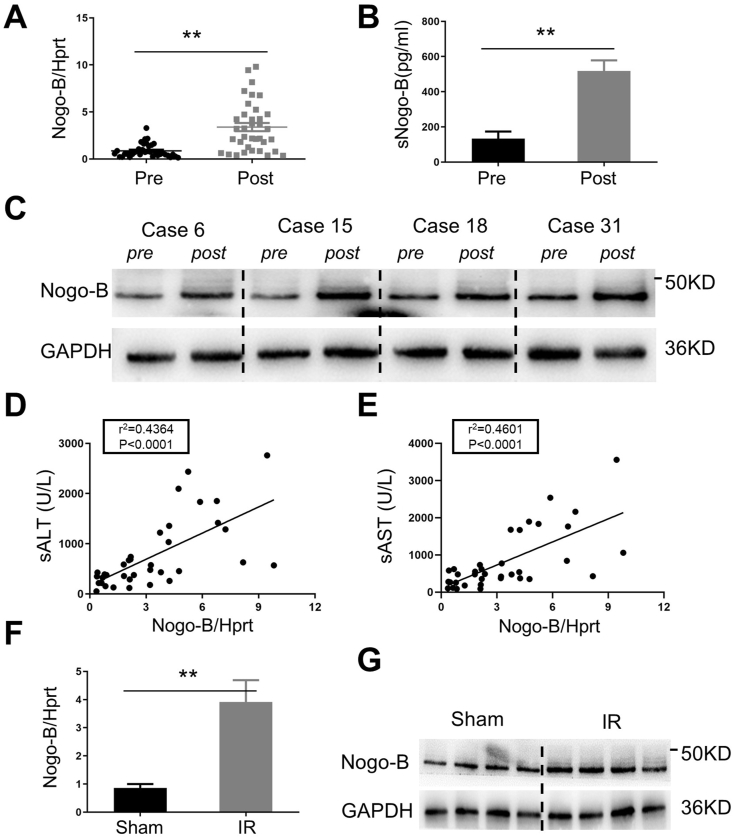
To determine the role of Nogo-B in the pathogenesis of hepatic IRI, we first examined Nogo-B expression levels in liver/serum specimens from 36 patients (Table S1) at pre-/post-hepatectomy. Pre-hepatectomy hepatic biopsies were harvested after laparotomy (prior to hepatic portal occlusion) and post-hepatectomy hepatic biopsies were obtained at 1.5–2 h after reperfusion (prior to abdominal closure). Ischemic (hepatic portal occlusion) time ranged from 15 to 30 min. IR significantly increased the expression of Nogo-B mRNA in ischemic livers (Fig. 1A, 0.87 ± 0.12 and 3.39 ± 0.44, respectively; p < 0.001). This was consistent with ELISA data, which showed that serum Nogo-B was significantly increased post-hepatectomy compared to the pre-hepatectomy (Figs. 1B and 517.7 ± 242.9 and 133.2 ± 161.4 pg/mL, respectively, p < 0.001). Unlike the pre-hepatectomy controls, the protein expression of Nogo-B was augmented in the post-hepatectomy groups (Fig.1C and Fig. S1A, representative of 4 cases). Interestingly, post-hepatectomy Nogo-B levels were correlated positively with sALT (Fig. 1D: r2 = 0.4364, p < 0.001) and sAST (Fig.1E; r2 = 0.4601, p < 0.001) levels at POD1, suggesting that IR-induced Nogo-B expression promoted liver injury. This was further supported by pathological evaluation (data not shown). Using a mouse model of liver IRI, we found that IR enhanced Nogo-B expression levels of mRNA (Fig. 1F) and protein (Fig. 1G and Figs. S1B–C) in ischemic livers. Consistent with human results, Nogo-B levels were correlated positively with sALT (Fig. S1D; r2 = 0.7497, p = 0.003) and sAST (Fig. S1E; r2 = 0.7668, p = 0.002) levels in the sham and IR groups.
Fig. 1.
IR-triggered Nogo-B expression is positively correlated with liver injury in human/mice ischemic liver. Human biopsies (N = 36) were harvested after laparotomy (prior to hepatic portal occlusion) and post-hepatectomy (prior to abdominal closure): ischemic time, 15–30 min; reperfusion time, 1.5–2 h. Mice samples (N = 6) were harvested after 90 min of ischemia and 6 h of reperfusion. (A) qRT-PCR analysis of Nogo-B gene expression levels of in human livers; (B) ELISA analysis of Nogo-B in human serum; (C) Western blotting analysis of Nogo-B protein expression in human livers (representative 4 cases); (D) and (E) The ratio of post-hepatectomy Nogo-B/Hprt correlated positively with sALT and sAST at POD1; (F) Nogo-B gene expression levels were determined by qRT-PCR in livers from sham and IR mice; (G) Western blotting analysis of Nogo-B protein expression in mice livers. **p < 0.01 by Student's t-test.
3.2. Nogo-B is mainly expressed on macrophages in liver tissue
Previous studies found that Nogo-B is mainly expressed on NPCs but not on hepatocytes. However, they did not identify cell type-specific expression of Nogo-B [16]. We then isolated KCs, HSCs, and hepatocytes from WT mouse livers. Western blotting revealed a high expression of Nogo-B on KCs and a low expression of Nogo-B on HSCs and hepatocytes in WT untreated mice (Fig. 2A). Using immunofluorescence staining, we found that more strong expression of Nogo-B on KCs than Nogo-B expression on hepatocytes in mouse liver sections (Fig.2B and Fig. S2A). In human liver, dual-immunofluorescence staining also showed that Nogo-B is mainly expressed on KCs (Fig.2C and Fig. S2B). Furthermore, immunofluorescence staining revealed an increase of macrophage Nogo-B expression and macrophage infiltration in mouse ischemic livers (Fig. 2D).
Fig. 2.
Nogo-B is primarily expressed on macrophages in liver tissue. (A) Cell type-specific expression of Nogo-B in livers of wild-type (WT) mice. Protein lysates were prepared from hepatocytes, KCs, or HSCs and subjected to western blot analysis of Nogo-B and GAPDH. (B) Dual immunofluorescence staining of CD68 (green), Nogo-B (red), and DAPI (blue) in mice livers. (C) Dual immunofluorescence staining of CD68 (green), Nogo-B (red), and DAPI (blue) in human livers. (D) Dual immunofluorescence staining of CD68 (green), Nogo-B (red), and DAPI (blue) in sham livers and ischemic reperfusion livers. Scale is 100 μm. Representative of three experiments. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.3. Nogo-B promotes liver injury in IR-stressed livers
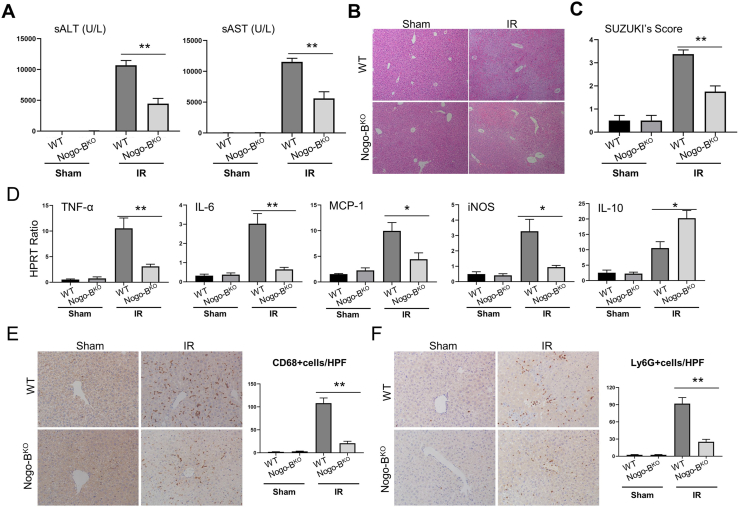
Next, we investigated the functional role of Nogo-B in hepatic IRI using Nogo-BKO mice. Unlike in the WT group, Nogo-B-deficient mice were effectively resistant to liver IRI, as evidenced by reduced sALT and sAST levels (Fig. 3A) and well-preserved hepatic architecture, including less sinusoidal congestion, edema, vacuolization or necrosis (Fig. 3B) in Nogo-BKO mice, which correlated with Suzuki's histological grading of liver IRI (Fig. 3C). To determine whether Nogo-B may affect cell apoptosis during liver reperfusion, TUNEL staining was used to analyze hepatocellular apoptosis in ischemic livers. Nogo-B deletion significantly reduced frequency of TUNEL positive cells compared to the WT controls (Figs. S3A–B). Western blot analysis indicated that Nogo-B deficiency upregulated the expression of p-AKT, Bcl-2, and Bcl-xl, but downregulated cleaved caspase-3 expression in ischemic livers compared to the WT controls (Figs. S3C–D).
Fig. 3.
Nogo-B promotes liver injury in IR-stressed livers. WT and Nogo-BKO mice were used to establish liver IRI. Samples were harvested after 90 min ischemia and 6 h of reperfusion. (A) sALT and sAST levels were measured from sham and IRI groups. (B) Representative histological staining (HE) of ischemic liver tissue. Scale is 100 μm. (C) Liver damage was evaluated by Suzuki's histological score. (D) Detection of cytokines TNF-α, IL-6, MCP-1, iNOS, and IL-10 by qRT-PCR in ischemic livers. (E and F) Macrophage and neutrophil infiltration were analyzed by immunohistological staining with antibodies against CD68 and Ly6G, respectively. CD68+ and Ly6G + cells were quantitated by counting numbers of positive cells/area. Scale is 100 μm. N = 6, *p < 0.05, **p < 0.01 by Student's t-test.
As IR activates liver macrophages and promotes production of ROS and proinflammatory cytokines to trigger sterile inflammatory responses and hepatocellular damage [7,8,21], We then determined the roles of Nogo-B on IR-mediated oxidative stress. The intracellular ROS production was measured with the ROS-sensing dye dihydroethidium (DHE). We found a reduced level of ROS in the Nogo-BKO groups compared to the WT controls (Fig. S3E). Consistent with this result, Nogo-B deficiency decreased the level of MDA and increased GSH activity in ischemic livers (Figs. S3F–G). Moreover, Nogo-B deficiency increased the expression of antioxidant genes (MnSOD and HO-1) in ischemic tissues (data not shown). MPO activity, which reflects neutrophil activity, was significantly decreased after reperfusion in Nogo-BKO livers as compared to the WT controls (Fig. S3H). Moreover, disruption of Nogo-B inhibited the expression of TNF-α, IL-6, MCP-1 and iNOS while increasing IL-10 expression in ischemic livers (Fig. 3D). Nogo-B deficiency reduced CD68+ (Fig. 3E) macrophage and Ly6G+ (Fig. 4F) neutrophil accumulation in ischemic livers, as compared with WT controls.
Fig. 4.
Myeloid Nogo-B deficiency attenuates ischemic reperfusion-induced liver inflammation and injury. Nogo-BFL/FL and Nogo-BMKO mice were used to establish liver IRI. Samples were harvested after 90 min ischemia and 6 h of reperfusion. (A) Protein lysates were prepared from isolated KCs (untreated mice) and subjected to western blot analysis of Nogo-B and GAPDH. (B) Quantitation of ROS-sensing dye DHE staining, MDA, and GSH activities were assessed in ischemic liver tissues. (C) MPO activities were assessed in ischemic liver tissues. (D) Detection of cytokines TNF-α, IL-6, MCP-1, iNOS, and IL-10 by qRT-PCR in ischemic livers. (E and F) Macrophage and neutrophil infiltration were analyzed by immunohistological staining with antibodies against CD68 and Ly6G, respectively. CD68+ and Ly6G + cells were quantitated by counting numbers of positive cells/area. Scale is 100 μm. (G) sALT levels were measured from the IRI groups. (H) Representative histological staining (HE) of ischemic liver tissue; liver damage was evaluated by Suzuki's histological score. N = 6, *p < 0.05, **p < 0.01, ***p < 0.001 by Student's t-test.
3.4. Myeloid Nogo-B deficiency attenuates IR-induced liver inflammation
To test functional role of macrophage Nogo-B in IR-induced liver inflammation and injury, we used the Cre-Loxp system to create a myeloid-specific Nogo-B-deficient (Nogo-BMKO) strain. Indeed, high expression of Nogo-B was found in liver macrophages from the Nogo-BFL/FL mice (Fig.4A and Fig. S6C). However, the Nogo-B protein expression was lacking in liver macrophages from the Nogo-BMKO mice (Fig.4A and Fig. S6C). Consist with the results from the Nogo-BKO mice, myeloid Nogo-B deficiency significantly reduced IR-induced ROS and MDA production and enhanced GSH activity in ischemic livers (Fig. 4B). Unlike the Nogo-BFL/FL controls, Nogo-BMKO exhibited reduced IR-induced liver MPO activity (Fig. 4C) and proinflammatory TNF-α, IL-6, MCP-1, and iNOS, but increased anti-inflammatory IL-10 expression in ischemic livers (Fig. 4D). Disruption of myeloid Nogo-B clearly reduced CD68+ macrophage (Fig. 4E) and Ly6G + neutrophil (Fig. 4F) infiltration in ischemic livers compared to the Nogo-BFL/FL controls. Most importantly, Nogo-BMKO protected mice against IR-induced liver injury, as evidenced by lower sALT (Fig. 4G) and sAST (Fig. S4A) levels and well-preserved hepatic architecture (lower Suzuki scores in HE staining) compared to the Nogo-BFL/FL controls (Fig. 4H). This was further supported by TUNEL staining, which showed that Nogo-BMKO mice exhibited reduced hepatocellular apoptosis in ischemic livers (Fig. S4B). The expression of anti-apoptotic proteins p-AKT, Bcl-2, and Bcl-xl were increased, and the levels of pro-apoptotic protein cleaved-caspase-3 were decreased in Nogo-BMKO livers compared to the Nogo-BFL/FL controls (Figs. S4C–D). These results suggest that myeloid Nogo-B deficiency reduces IR-induced liver oxidative stress and inflammatory responses and attenuates hepatocellular damage after liver IRI.
3.5. Synergistic action of liver resident and infiltrating macrophages is required for Nogo-B-mediated liver inflammatory injury
To determine the role of Nogo-B on infiltrating cells versus resident KCs in contributing to IR-induced liver injury, we treated lethally irradiated Nogo-BFL/FL or Nogo-BMKO mice with Nogo-BFL/FL or Nogo-BMKO BMMs to generate four groups of chimeric mice: Nogo-BFL/FL mice with Nogo-BFL/FL BMMs, Nogo-BFL/FL mice with Nogo-BMKO BMMs, Nogo-BMKO mice with Nogo-BFL/FL BMMs, and Nogo-BMKO mice with Nogo-BMKO BMMs. Liver dual staining of CD68 and Nogo-B demonstrated chimeric mice were successfully established (Fig. 5A). These chimeric mice were subjected to 90 min of ischemia followed by 6 h of reperfusion. Corroborating results showed that macrophage Nogo-B promotes IR-induced liver injury, as evidenced by lower levels of sALT and sAST in Nogo-BMKO mice reconstituted with Nogo-BMKO BMMs but not Nogo-BFL/FL BMMs (Fig. 5B and C). Interestingly, Nogo-BMKO mice transplanted with Nogo-BFL/FL BMMs (or Nogo-BFL/FL mice transplanted with Nogo-BMKO BMMs) showed less liver damage compared to the Nogo-BFL/FL mice reconstituted with Nogo-BFL/FL BMMs (Fig. 5B and C). Moreover, much less liver injury was found when Nogo-B was deleted in infiltrating and resident macrophages. These results imply that Nogo-B aggravates liver inflammation and injury via its synergetic action in both liver resident KCs and infiltrating macrophages. These results were further confirmed by histology data (Fig. 5D). We next analyzed inflammatory cytokine gene expression in ischemic livers. Fig. 5E showed Nogo-BMKO mice with Nogo-BMKO BMMs had reduced levels of proinflammatory cytokines (TNF-α and iNOS) and increased levels of the anti-inflammatory cytokine IL-10 as compared with the other three groups. Thus, our findings support the hypothesis that Nogo-B has an adverse effect on the liver, which is mainly dependent on both resident KCs and infiltrating macrophages during IR-induced liver injury.
Fig. 5.
Synergetic action of liver resident and infiltrating cells are required for Nogo-B to IR-induced liver injury. We treated lethally irradiated Nogo-BMFL/FL or Nogo-BMKO mice with Nogo-BFL/FL or Nogo-BMKO BMMs to generate four groups of chimeric mice: Nogo-BFL/FL mice with Nogo-BFL/FL BMMs, Nogo-BFL/FL mice with Nogo-BMKO BMMs, Nogo-BMKO mice with Nogo-BFL/FL BMMs, and Nogo-BMKO mice with Nogo-BMKO BMMs. These mice were established liver IRI. Samples were harvested after 90min ischemia and 6h of reperfusion. (A) Dual immunofluorescence staining of CD68 (green), Nogo-B (red), and DAPI (blue) in chimeric mice livers. Scale is 100 μm. (B) sALT and (C) sAST levels were measured from sham and IRI groups. (D) Representative histological staining (H&E) of ischemic liver tissue; Liver damage was evaluated by Suzuki's histological score. (E) Detection of cytokines TNF-α, iNOS and IL-10 by qRT-PCR in ischemic livers. N = 6, *P < 0.05, **P < 0.01, ***P < 0.001 by Student's t-test. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.6. Macrophage Nogo-B deficiency diminishes sterile inflammation
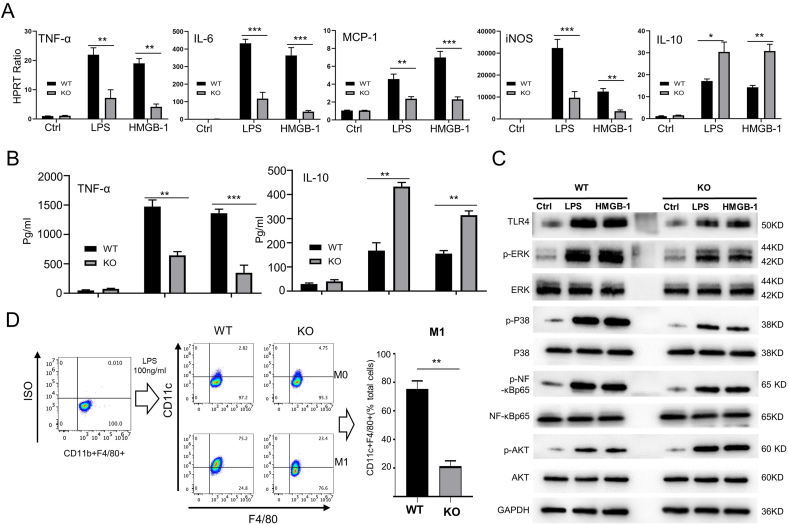
It is known that TLR4-mediated innate immunity initiates IR-triggered liver inflammation, activates NF-κB and proinflammatory mediators, resulting in local sterile inflammation [3,4,20,21,24]. We examined the effects of Nogo-B deficiency on the inflammatory program in vitro. Indeed, Nogo-BKO macrophages displayed decreased expression of TNF-α, IL-6, MCP-1, and iNOS and increased expression of IL-10 in response to LPS or HMGB1 stimulation (Fig. 6A). ELISA data confirmed that Nogo-B ablation inhibited TNF-α while enhancing IL-10 expression followed by LPS or HMGB-1 (Fig. 6B). Moreover, Nogo-BKO appeared reduced phosphorylation of ERK and p38 in LPS- or HMGB-1-stimulated macrophages. However, increased AKT phosphorylation was found in the Nogo-BKO but not the WT macrophages (Fig.6C and Fig. S5A). Nogo-B deletion decreased TLR4 expression and inhibited NF-κB activation following LPS or HMGB-1 challenge (Fig.6C and Fig. S5A). Interestingly, the M1 macrophage polarization was significantly decreased in Nogo-BKO macrophages after LPS stimulation compared to the WT macrophages (Fig. 6D). However, the M2 macrophage polarization was no change between Nogo-BKO and WT groups after IL-4 treatment (Figs. S5B–C).
Fig. 6.
Macrophage Nogo-B deficiency diminishes sterile inflammation. BMMs were isolated from WT and Nogo-BKO mice. (A) Gene expression levels of TNF-α, IL-6, MCP-1, iNOS and IL-10 by qRT-PCR in BMMs cultured with LPS (100 ng/mL, 3 h) and rHMGB-1(1 μg/mL, 3 h) medium. (B) Protein levels of TNF-α and IL-10 were measured by ELISA in culture supernatants after LPS (100 ng/mL, 24 h) or rHMGB-1 (1 μg/mL, 24 h) stimulation. (C) p-ERK, ERK, p-P38, P38, p–NF–κBp65, NF-κBp65, p-AKT, and AKT protein expression levels measured by western blot. Representative of three experiments. BMMs were treated with LPS (100 ng/mL) or rHMGB-1 (1 μg/mL) stimulation for 6 h; untreated BMMs were used as controls. (D) BMMs were differentiated into M1 macrophages (CD11b + F4/80+CD11c+) with LPS treatment (100 ng/mL, 24 h); Flow cytometry-assisted detection of M1 representative of three experiments. N = 6, *p < 0.05, **p < 0.01, ***p < 0.001 by Student's t-test.
3.7. MST-mediated Hippo/YAP signaling is critical in regulating macrophage Nogo-B-induced inflammatory response
Immunoprecipitation (IP) assays were performed to explore the potential binding proteins of Nogo-B (Fig. S6A). Mass spectrometry and co-immunoprecipitation showed the MST1/2 protein was captured by Nogo-B (Fig. 7A). Confocal immunofluorescence analysis further demonstrated that Nogo-B and MST1/2 were co-located in the cytoplasm of BMMs (Fig. 7B). Western blotting revealed that Nogo-B deletion markedly inhibited phosphorylation of MST1/2 and decreased the p-MST1/2:MST1/2 ratio (Fig. 7C and D). Moreover, Nogo-B deficiency inhibited phosphorylation of LATS1 and YAP and reduced the p-LAST1:LATS1 and p-YAP: YAP ratios (Fig. 7C and D). Double staining showed that Nogo-B deficiency promoted nuclear import of YAP (Fig. 7E and F). This was further confirmed by western blots, which indicated that nuclear YAP expression was increased in Nogo-BKO BMMs compared to the WT controls after LPS or HMGB-1 treatment (Fig.7G and Fig. S6B). We then isolated KCs from ischemic livers in the Nogo-BMKO and Nogo-BFL/FL mice. Consistently, reduced p-MST1/2, p-LATS1, p-YAP, and augmented YAP expression were observed in the Nogo-BMKO but not the Nogo-BFL/FL KCs (Figs. S6C–D). Nogo-B deficiency significantly decreased phosphorylation of NF-κBp65 in LPS- or HMGB-1-stimulated BMMs or IR-stressed KCs, as compared with the controls (Fig. 7C and Figs. S6C–D). To test whether Nogo-B-induced inflammatory response is dependent on MST-mediated Hippo/YAP signaling, the Nogo-BKO BMMs were transfected with YAP siRNA and stimulated with LPS or HMGB-1. YAP siRNA effectively inhibited Nogo-B deletion-induced YAP expression (Fig. 7E and F), which was confirmed by western blots (Fig.7G and Fig. S6E). YAP siRNA treatment increased TNF-α, IL-6, MCP-1, and iNOS but reduced IL-10 expression in Nogo-BKO BMMs compared to the NS siRNA controls after LPS stimulation (Fig. 7G), suggesting that disruption of macrophage Hippo/YAP signaling restores Nogo-B-induced inflammatory response.
Fig. 7.
MST-mediated Hippo/YAP signaling is critical in regulating macrophage Nogo-B-induced inflammatory response. (A) Lysates of BMMs were immunoprecipitated with anti-MST1/2 or Nogo-B antibody, and Nogo-B or MST1/2 protein was examined using western blotting analysis. MST1/2 was detected in the immunoprecipitated Nogo-B complex, but not in the IgG control sample. Representative of three experiments. (B) Immunofluorescence staining for macrophage Nogo-B (red) and MST1/2 (green) colocalization in the cytoplasm after coculture with LPS (100 ng/mL, 6 h). DAPI was used to visualize nuclei (blue). (C–D) p-MST1, MST1, p-LATS1, LATS1, p-YAP, YAP, p–NF–κB p65, and GAPDH protein expression levels measured by western blot in BMMs treated LPS (100 ng/mL, 6 h) or rHMGB-1 (1 μg/mL, 6 h). Representative of three experiments. (E–F) Immunofluorescence staining for macrophage Nogo-B (green) and MST1/2 (red) after coculture with LPS (100 ng/mL, 6 h) or rHMGB-1 (1 μg/mL, 6 h). Scale is 100 μm. Representative of three experiments. (G) The BMMs (from WT and Nogo-BKO mice) were harvested to extract the nucleoprotein after LPS treatment (100 ng/mL, 6h) or rHMGB-1 (1 μg/mL, 6h), YAP and TBP protein expression levels measured by western blot. Representative of three experiments. (H) Gene expression levels of TNF-α, IL-6, MCP-1, iNOS and IL-10 by qRT-PCR in BMMs cultured with LPS (100 ng/mL, 3 h) and rHMGB-1 (1 μg/mL, 3 h) medium. N = 6, *p < 0.05, **p < 0.01, ***p < 0.001 by Student's t-test. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
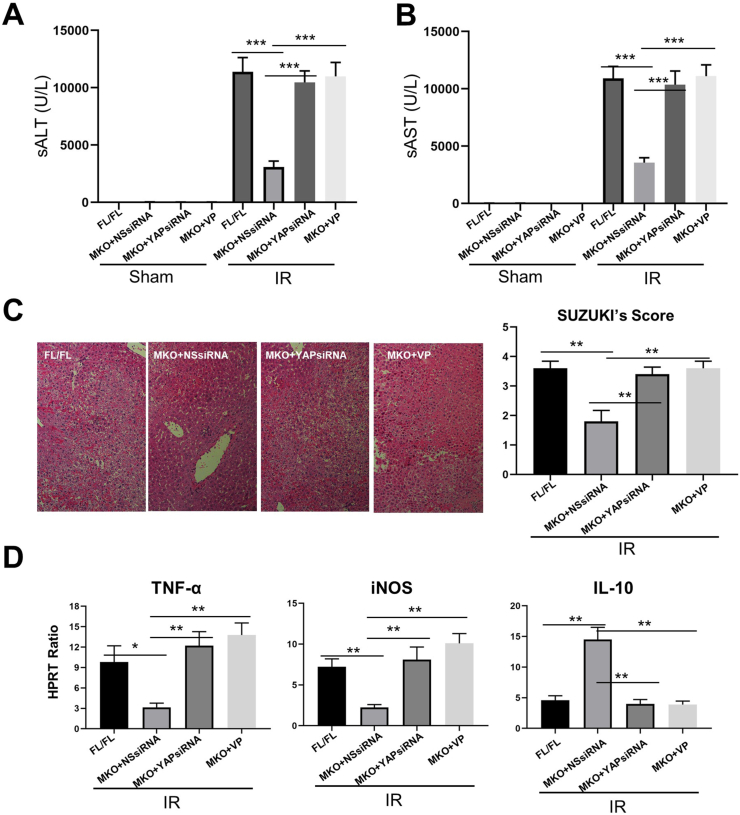
3.8. Inhibition of YAP abolishes the beneficial effects of macrophage Nogo-B deficiency in IR-induced liver injury
To further elucidate whether Nogo-B-mediated adverse effects was dependent on MST-mediated Hippo/YAP signaling in IR-stressed liver injury, Nogo-BMKO mice were injected via the tail vein with YAP siRNA mixed with mannose-conjugated polymers or YAP inhibitor. YAP siRNA and Verteporfin (VP) effectively inhibited YAP expression (Figs. S6E–F). In the liver IRI model, the sALT and sAST levels were significantly increased in Nogo-BMKO mice after YAP siRNA or VP treatment, as compared with the control groups (Fig. 8A and B). These results were correlated with Suzuki's histological grading of IR-induced liver injury (Fig. 8C), which showed that the livers of Nogo-BMKO mice treated with NS siRNA displayed mild to moderate edema without significant necrosis. In contrast, livers in the mice with YAP siRNA or VP treatment showed significant edema, severe sinusoidal congestion/cytoplasmic vacuolization, and extensive necrosis, similar to the Nogo-BMKO mice (Fig. 8C). We then analyzed cytokine gene expression in the ischemic livers. YAP siRNA or VP treatment in the Nogo-BMKO mice augmented the expression of proinflammatory cytokines (TNF-α and iNOS) and reduced expression of the anti-inflammatory cytokine IL-10 compared to the NS siRNA-treated controls (Fig. 8D). Moreover, YAP siRNA or VP treatment significantly increased the expression of M1 macrophages markers (iNOS, NOS2, CD80, and CD86) (Fig. 8D and Fig. S7). Taken together, our results suggest that Nogo-B-related adverse effects may be dependent on the activation of MST-mediated hippo/YAP signaling during liver IRI.
Fig. 8.
Inhibition of YAP abolishes the beneficial effects of macrophage Nogo-B deficiency in liver IRI. Nogo-BFL/FL and Nogo-BMKO mice were established in a liver IRI model. Some Nogo-BMKO mice were injected via tail vein with YAP siRNA mixed with mannose-conjugated polymers or YAP inhibitor (verteporfin, VP) prior to liver IRI model. (A) sALT and (B) sAST levels were measured from sham and IRI groups. (C) Representative histological staining (HE) of ischemic liver tissue; liver damage was evaluated by Suzuki's histological score. (D) Detection of cytokines TNF-α, iNOS and IL-10 by qRT-PCR in ischemic livers. N = 6, *p < 0.05, **p < 0.01, ***p < 0.001 by Student's t-test.
4. Discussion
This study first documents that Nogo-B expression is correlated with clinical outcomes in human livers after IR. High Nogo-B expression is associated with worse preserved liver histopathology and hepatocellular function in hepatectomy patients. In a murine liver IRI model, Nogo-B is mainly expressed on KCs and plays a critical role in TLR4-mediated inflammatory responses during liver IRI. IR stress activates KC Nogo-B, which promotes IR-triggered liver inflammation. Indeed, macrophage Nogo-B deficiency decreases sALT and sAST levels, preserves hepatic architecture, upregulates anti-oxidative gene program, limits TLR4-mediated inflammatory responses, and ameliorates hepatocellular necrosis/apoptosis. Using primary mouse macrophages treated with TLR4 ligands (LPS or HMGB-1), disruption of Nogo-B reduces ROS production, diminishes TLR4-mediated inflammation, and inhibits M1 macrophage polarization. Moreover, activation of Nogo-B augmented MST1/2, LAST1, and YAP phosphorylation, reduces nuclear YAP, and induces TLR4-driven inflammation in macrophages. Taken together, macrophage Nogo-B promotes IR-induced liver inflammatory injury by activating the MST-mediated Hippo-YAP pathway.
Nogo-B is known to play important roles in pathological vascular conditions in response to vascular injuries, such as ischemia and atherosclerosis [12,18,25]. In addition, overexpression of the soluble Nogo-B, which binds to Nogo-B receptor (NgBR), improved diabetic glomerulopathy as evidenced by a reduction in albuminuria [26]. The Nogo-B has been also studied in some chronic liver diseases, including the roles of myofibroblast Nogo-B in liver fibrosis [16], Nogo-B in hepatocyte proliferation and liver regeneration [17], and Nogo-B in alcoholic liver disease [19]. Although studies have shown that Nogo-B is necessary for macrophage-mediated inflammatory response and acute organ injury, little is known the functional roles and molecular mechanisms of Nogo-B in acute inflammatory liver injury, such as liver IRI. Our current study demonstrates novel roles and potential mechanisms of Nogo-B in acute inflammatory liver injury.
As Nogo-B plays an important role in human liver IRI, we then used a mouse model of liver IRI to determine the function of Nogo-B in ischemic livers. Consistent with human data, IR stress enhanced hepatic endogenous Nogo-B expression in mouse livers, which was positively correlated with liver injury. It is plausible that Nogo-B induction progresses in parallel with IR-mediated hepatocellular damage. Previous studies reported that Nogo-B was highly expressed in NPCs and minimally expressed in hepatocytes in human and rat livers. However, it is unclear for the cell type-specific Nogo-B expression in injured livers [16]. Using Nogo-B/CD68 double immunofluorescence staining, we demonstrated that Nogo-B is mainly expressed on macrophages in normal or ischemic liver tissues.
To analyze the function of Nogo-B, we determined the degree of damage of ischemic livers in the WT and Nogo-BKO mice. Strikingly, Nogo-B deficiency markedly attenuated liver IRI, evidenced by reduced levels of serum transaminase, well-preserved hepatic architecture, and diminished hepatocellular necrosis/apoptosis, whereas Nogo-B deletion decreased ROS production, inhibited pro-inflammatory cytokines, and dampened CD68+ macrophage/Ly6G + neutrophil infiltration. As Nogo-B is mainly expressed on KCs, we used the Cre-Loxp system to create a myeloid-specific Nogo-B-deficient strain to analyze the functional role of macrophage Nogo-B in liver IRI. Expectedly, macrophage Nogo-B ablation attenuated IR-induced liver oxidative stress, inflammatory responses, and hepatocellular damage. It is known that hepatic macrophages include resident/innate macrophages and monocyte derived/infiltrating macrophages, which have different functions in various diseases process. In N-acetyl-p-aminophenol (APAP)-induced acute liver injury, infiltrating monocyte-derived macrophages and resident KCs display different ontogeny and functions [27]. In acute and chronic liver injury, the intrahepatic macrophage count is massively expanded following the influx of peripheral monocytes rather than augmentation of tissue-resident macrophages [28,29]. In addition, tissue macrophages are notoriously difficult to eliminate from tissues, including nervous tissues, heart tissues, and liver tissues [30,31]. Consistent with these results, we demonstrated that Nogo-BMKO mice transplanted with Nogo-BFL/FL BMMs (or Nogo-BFL/FL mice transplanted with Nogo-BMKO BMMs) showed less liver damage compared to the Nogo-BFL/FL mice reconstituted with Nogo-BFL/FL BMMs. Thus, our data suggests that synergetic action of liver resident and infiltrating macrophages is required for Nogo-B-mediated liver inflammatory injury after IR.
Hepatic macrophages are crucial in triggering liver inflammation. Activation of hepatic macrophages results in the release of several inflammatory mediators, which contributes directly and indirectly to hepatocyte killing. Moreover, the release of intracellular constituents from stressed/necrotic cells due to whatever cause (e.g., hypoxia, chemicals) precludes hepatic macrophage activation [3,5,32]. In liver IRI, the liberation of “danger” signals from inflamed, necrotic or hypoxic parenchyma cells directly contributes to KC activation. These DAMPs bind to pattern recognition receptors (PRRs). HMGB-1 is a main ligand of TLR4 during IR of most organs, such as liver, heart, and kidney [8,[33], [34], [35]]. Exogenous DAMPs (e.g., HMGB-1) accumulating in the anhepatic phase of liver transplantation through translocation of intestinal microbes have been shown to play a role in TLR4-mediated acute liver injury (ALI) [36,37]. To mimic the function of macrophages in liver IRI, we used LPS or HMGB-1 stressed primary macrophage cultures. Our results demonstrated that either LPS or HMGB-1 stimulation triggered Nogo-B expression, whereas macrophage Nogo-B deficiency significantly reduced TNF-α, IL-6, MCP-1, and iNOS expression and increased IL-10 expression. Moreover, Nogo-B ablation inhibited TLR-4-driven phosphorylation of ERK, P38, and NF-κB, and upregulated AKT phosphorylation. It has been reported that an ethanol diet or LPS injection increased the level of M1 type KCs in WT mice and M2 type KCs in Nogo-BKO mice [20]. Interestingly, we found that LPS treatment increased the level of M1 type macrophages in WT BMMs, whereas IL-4 treatment did not affect M2 type macrophages in Nogo-BKO BMMs in vitro. Although it is unclear why IL-4 treatment did not promote M2 polarization in Nogo-BKO BMMs, we speculate that Nogo-B deficiency restricted the phosphorylation of kinases MST1/2 and LATS1 and increased YAP nuclear translocation, leading to inhibition of the M1 polarization.
One striking finding was that macrophage Nogo-B and MST1/2 colocalized in the cytoplasm, and that Nogo-B promoted liver IRI through regulation of the MST1/2-mediated Hippo-YAP pathway. The Hippo-YAP pathway was initially identified as a regulator of organ size by the management of cell proliferation and cell death [38,39]. Activation of Hippo signaling results in the phosphorylation and inactivation of the transcriptional co-activator YAP [40]. YAP exerts its transcriptional activity mostly by interacting with the TEAD family of transcription factors and activating target gene expression [41]. Recently, a growing number of studies have shown the immunoregulatory functions of Hippo-YAP signaling, including its antimicrobial role in flies, its pro- or anti-inflammatory roles in mammals, as well as its relevance to cancer immunity [42]. In ischemic heart disease, epicardial YAP/TAZ orchestrates an immunosuppressive response and attenuates post-myocardial infarction pericardial inflammation and myocardial fibrosis [43]. In addition, IR stress activated the Hippo core kinase cascade and phosphorylated YAP, sequestering YAP to the cytoplasm which in turn triggered liver inflammation [22]. YAP activation attenuated hepatocellular oxidative stress and diminished the innate immune response in mouse livers following IRI [21]. In the current study, we found that Nogo-B deficiency reduced kinase MST1/2 activity, inhibited phosphorylation of LATS1 and YAP, increased YAP nuclear translocation leading to decreased TLR4-driven inflammation. However, Nogo-B deficiency-mediated protection was eliminated with YAP siRNA intervention in vivo and in vitro, suggesting the Hippo-YAP pathway is crucial for the mechanism of Nogo-B-triggered liver inflammation. Taken together, these results reveal that Nogo-B colocalizes with MST1/2, promotes phosphorylation of MST1/2, activates Hippo signaling, and enhances TLR4-mediated inflammation. Although the exact mechanism is still unclear, such as how Nogo-B regulates kinase MST1/2 activity, and how Nogo-B may regulate Rac activation and STAT3 phosphorylation during the inflammatory response [17,18], our results suggest that Nogo-B could be an attractive target for promoting the resolution of inflammation during liver injury and for the prevention of parenchymal cell death.
In conclusion, we demonstrate the functional roles of myeloid Nogo-B in liver IRI. Nogo-B induces TLR4-driven inflammatory response by activating the Hippo-YAP signaling in the ischemic reperfusion-stressed liver. By identifying the molecular pathways by which Nogo-B-mediated liver inflammation, our findings provide the rationale for novel therapeutic approaches to ameliorate ischemic reperfusion-triggered liver inflammation and injury.
Financial support
The project was supported by the National Natural Science Foundation of China (81871259, 81530048, 81971495, and 81570562), the Foundation of Jiangsu Collaborative Innovation Center of Biomedical Functional Materials, the Priority Academic Program Development of Jiangsu Higher Education Institutions, The Six talent peaks project in Jiangsu Province (2017-WSW-019).
Author contributions
JR, FC, HZ and XW were involved in study design, conducted the experiments and drafted the paper; JR, WY, JQ, CY, XN, SK, and XP performed the experiments; JR, FC, and LL provided material support; FC, YX, XP and FZ commented the manuscript; JR, LL and XW designed, supervised the study and wrote the paper.
Declaration of competing interest
The authors disclose no conflicts of interest.
Acknowledgments
We are grateful to Bibo Ke at UCLA for instructive comments and revising the English text.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2020.101745.
Contributor Information
Ling Lu, Email: Lvling@njmu.edu.cn.
Xuehao Wang, Email: Wangxh@njmu.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
figs2.
figs3.
figs4.
figs5.
figs6.
figs7.
References
- 1.Howard T.K., Klintmalm G.B., Cofer J.B. The influence of preservation injury on rejection in the hepatic transplant recipient. Transplantation. 1990;49:103–107. doi: 10.1097/00007890-199001000-00023. [DOI] [PubMed] [Google Scholar]
- 2.He S., Atkinson C., Qiao F. A complement-dependent balance between hepatic ischemia/reperfusion injury and liver regeneration in mice. J. Clin. Invest. 2009;119:2304–2316. doi: 10.1172/JCI38289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhai Y., Petrowsky H., Hong J.C. Ischaemia-reperfusion injury in liver transplantation-from bench to bedside. Nat. Rev. Gastroenterol. Hepatol. 2013;10:79–89. doi: 10.1038/nrgastro.2012.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ke B., Shen X.D., Zhang Y. KEAP1-NRF2 complex in ischemia-induced hepatocellular damage of mouse liver transplants. J. Hepatol. 2013;59:1200–1207. doi: 10.1016/j.jhep.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Panel M., Ruiz I., Brillet R. Small-Molecule inhibitors of cyclophilins block opening of the mitochondrial permeability transition pore and protect mice from hepatic ischemia/reperfusion injury. Gastroenterology. 2019;157:1368–1382. doi: 10.1053/j.gastro.2019.07.026. [DOI] [PubMed] [Google Scholar]
- 6.Lu L., Zhou H., Ni M. Innate immune regulations and liver ischemia-reperfusion injury. Transplantation. 2016;100:2601–2610. doi: 10.1097/TP.0000000000001411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhai Y., Busuttil R.W., Kupiec-Weglinski J.W. Liver ischemia and reperfusion injury: new insights into mechanisms of innate-adaptive immune-mediated tissue inflammation. Am. J. Transplant. 2011;11:1563–1569. doi: 10.1111/j.1600-6143.2011.03579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamo N., Ke B., Ghaffari A.A. ASC/caspase-1/IL-1beta signaling triggers inflammatory responses by promoting HMGB1 induction in liver ischemia/reperfusion injury. Hepatology. 2013;58:351–362. doi: 10.1002/hep.26320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwabe R.F., Brenner D.A. Mechanisms of Liver Injury. I. TNF-alpha-induced liver injury: role of IKK, JNKand ROS pathways. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;290:G583–G589. doi: 10.1152/ajpgi.00422.2005. [DOI] [PubMed] [Google Scholar]
- 10.Oertle T., Schwab M.E. Nogo and its paRTNers. Trends Cell Biol. 2003;13:187–194. doi: 10.1016/s0962-8924(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 11.Serruys P.W., Rutsch W., Heyndrickx G.R. Prevention of restenosis after percutaneous transluminal coronary angioplasty with thromboxane A2-receptor blockade. A randomized, double-blind, placebo-controlled trial. Coronary Artery Restenosis Prevention on Repeated Thromboxane-Antagonism Study (CARPORT) Circulation. 1991;84:1568–1580. doi: 10.1161/01.cir.84.4.1568. [DOI] [PubMed] [Google Scholar]
- 12.Acevedo L., Yu J., Erdjument-Bromage H. A new role for Nogo as a regulator of vascular remodeling. Nat. Med. 2004;10:382–388. doi: 10.1038/nm1020. [DOI] [PubMed] [Google Scholar]
- 13.Yang Y.S., Strittmatter S.M. The reticulons: a family of proteins with diverse functions. Genome Biol. 2007;8:234. doi: 10.1186/gb-2007-8-12-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paszkowiak J.J., Maloney S.P., Kudo F.A. Evidence supporting changes in Nogo-B levels as a marker of neointimal expansion but not adaptive arterial remodeling. Vasc. Pharmacol. 2007;46:293–301. doi: 10.1016/j.vph.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodriguez-Feo J.A., Hellings W.E., Verhoeven B.A. Low levels of Nogo-B in human carotid atherosclerotic plaques are associated with an atheromatous phenotype, restenosis, and stenosis severity. Arterioscler. Thromb. Vasc. Biol. 2007;27:1354–1360. doi: 10.1161/ATVBAHA.107.140913. [DOI] [PubMed] [Google Scholar]
- 16.Zhang D., Utsumi T., Huang H.C. Reticulon 4B (Nogo-B) is a novel regulator of hepatic fibrosis. Hepatology. 2011;53:1306–1315. doi: 10.1002/hep.24200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao L., Utsumi T., Tashiro K. Reticulon 4B (Nogo-B) facilitates hepatocyte proliferation and liver regeneration in mice. Hepatology. 2013;57:1992–2003. doi: 10.1002/hep.26235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu J., Fernandez-Hernando C., Suarez Y. Reticulon 4B (Nogo-B) is necessary for macrophage infiltration and tissue repair. Proc. Natl. Acad. Sci. U.S.A. 2009;106:17511–17516. doi: 10.1073/pnas.0907359106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ParkJK, ShaoM, KimMY An endoplasmic reticulum protein, Nogo-B, facilitates alcoholic liver disease through regulation of kupffer cell polarization. Hepatology. 2017;65:1720–1734. doi: 10.1002/hep.29051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rao J., Yue S., Fu Y. ATF6 mediates a pro-inflammatory synergy between ER stress and TLR activation in the pathogenesis of liver ischemia-reperfusion injury. Am. J. Transplant. 2014;14:1552–1561. doi: 10.1111/ajt.12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LiuY, LuT, ZhangC Activation of YAP attenuates hepatic damage and fibrosis in liver ischemia-reperfusion injury. J. Hepatol. 2019;71:719–730. doi: 10.1016/j.jhep.2019.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li C., Jin Y., Wei S. Hippo signaling controls NLR family pyrin domain containing 3 activation and governs immunoregulation of mesenchymal stem cells in mouse liver injury. Hepatology. 2019;70:1714–1731. doi: 10.1002/hep.30700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suzuki S., Toledo-Pereyra L.H., Rodriguez F.J. Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury. Modulating effects of FK506 and cyclosporine. Transplantation. 1993;55:1265–1272. doi: 10.1097/00007890-199306000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Rao J., Qian X., Li G. ATF3-mediated NRF2/HO-1 signaling regulates TLR4 innate immune responses in mouse liver ischemia/reperfusion injury. Am. J. Transplant. 2015;15:76–87. doi: 10.1111/ajt.12954. [DOI] [PubMed] [Google Scholar]
- 25.Bullard T.A., Protack T.L., Aguilar F. Identification of Nogo as a novel indicator of heart failure. Physiol. Genom. 2008;32:182–189. doi: 10.1152/physiolgenomics.00200.2007. [DOI] [PubMed] [Google Scholar]
- 26.Hernandez-Diaz I., Pan J., Ricciardi C.A. Overexpression of circulating soluble Nogo-B improves diabetic kidney disease by protecting the vasculature. Diabetes. 2019;68(9):1841–1852. doi: 10.2337/db19-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zigmond E., Samia-Grinberg S., Pasmanik-Chor M. Infiltrating monocyte-derived macrophages and resident kupffer cells display different ontogeny and functions in acute liver injury. J. Immunol. 2014;193:344–353. doi: 10.4049/jimmunol.1400574. [DOI] [PubMed] [Google Scholar]
- 28.Karlmark K.R., Weiskirchen R., Zimmermann H.W. Hepatic recruitment of the inflammatory Gr1+ monocyte subset upon liver injury promotes hepatic fibrosis. Hepatology. 2009;50:261–274. doi: 10.1002/hep.22950. [DOI] [PubMed] [Google Scholar]
- 29.Holt M.P., Cheng L., Ju C. Identification and characterization of infiltrating macrophages in acetaminophen-induced liver injury. J. Leukoc. Biol. 2008;84:1410–1421. doi: 10.1189/jlb.0308173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prinz M., Priller J. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat. Rev. Neurosci. 2014;15:300–312. doi: 10.1038/nrn3722. [DOI] [PubMed] [Google Scholar]
- 31.Khmelewski E., Becke rA., Meinertz T. Tissue resident cells play a dominant role in arteriogenesis and concomitant macrophage accumulation. Circ. Res. 2004;95:E56–E64. doi: 10.1161/01.RES.0000143013.04985.E7. [DOI] [PubMed] [Google Scholar]
- 32.Perugorria M.J., Esparza-Baquer A., Oakley F. Non-parenchymal TREM-2 protects the liver from immune-mediated hepatocellular damage. Gut. 2018:1–14. doi: 10.1136/gutjnl-2017-314107. 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeng S., Dun H., Ippagunta N. Receptor for advanced glycation end product (RAGE)-dependent modulation of early growth response-1 in hepatic ischemia/reperfusion injury. J. Hepatol. 2009;50:929–936. doi: 10.1016/j.jhep.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 34.Andrassy M., Volz H.C., Igwe J.C. High-mobility group box-1 in ischemia-reperfusion injury of the heart. Circulation. 2008;117:3216–3226. doi: 10.1161/CIRCULATIONAHA.108.769331. [DOI] [PubMed] [Google Scholar]
- 35.Lee J.Y., Ismail O.Z., Zhang X. Donor kidney injury molecule-1 promotes graft recovery by regulating systemic necroinflammation. Am. J. Transplant. 2018;18:2021–2028. doi: 10.1111/ajt.14745. [DOI] [PubMed] [Google Scholar]
- 36.Fiorini R.N., Shafizadeh S.F., Polito C. Anti-endotoxin monoclonal antibodies are protective against hepatic ischemia/reperfusion injury in steatotic mice. Am. J. Transplant. 2004;4:1567–1573. doi: 10.1111/j.1600-6143.2004.00549.x. [DOI] [PubMed] [Google Scholar]
- 37.Nakamura K., Kageyama S., Ito T. Antibiotic pretreatment alleviates liver transplant damage in mice and humans. J. Clin. Invest. 2019;129:3420–3434. doi: 10.1172/JCI127550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yimlamai D., Christodoulou C., Galli G.G. Hippo pathway activity influences liver cell fate. Cell. 2014;157:1324–1338. doi: 10.1016/j.cell.2014.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dong J., Feldmann G., Huang J. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramos A., Camargo F.D. The Hippo signaling pathway and stem cell biology. Trends Cell Biol. 2012;22:339–346. doi: 10.1016/j.tcb.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu S., Liu Y., Zheng Y. The TEAD/TEF family protein Scalloped mediates transcriptional output of the Hippo growth-regulatory pathway. Dev. Cell. 2008;14:388–398. doi: 10.1016/j.devcel.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 42.Zhou Y., Huang T., Zhang J. Emerging roles of Hippo signaling in inflammation and YAP-driven tumor immunity. Canc. Lett. 2018;426:73–79. doi: 10.1016/j.canlet.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 43.Ramjee V., Li D., Manderfield L.J. Epicardial YAP/TAZ orchestrate an immunosuppressive response following myocardial infarction. J. Clin. Invest. 2017;127:899–911. doi: 10.1172/JCI88759. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.