Abstract
This review summarizes the anti-depressant mechanisms of repetitive transcranial magnetic stimulation in preclinical studies, including anti-inflammatory effects mediated by activation of nuclear factor-E2-related factor 2 signaling pathway, anti-oxidative stress effects, enhancement of synaptic plasticity and neurogenesis via activation of the endocannabinoid system and brain derived neurotrophic factor signaling pathway, increasing the content of monoamine neurotransmitters via inhibition of Sirtuin 1/monoamine oxidase A signaling pathway, and reducing the activity of the hypothalamic-pituitary-adrenocortical axis. We also discuss the shortcomings of transcranial magnetic stimulation in preclinical studies such as inaccurate positioning, shallow depth of stimulation, and difficulty in elucidating the neural circuit mechanism up- and down-stream of the stimulation target brain region.
Keywords: Repetitive transcranial magnetic stimulation, Anti-depressant mechanisms, Nuclear factor-e2-related factor 2, Endocannabinoid system, Monoamine oxidase, Hypothalamic-pituitary-adrenocortical axis, Brain derived neurotrophic factor
Core Tip: Repetitive transcranial magnetic stimulation (rTMS) is an effective treatment for major depressive disorder. This paper reviews the anti-depressant mechanisms of rTMS that have been found in preclinical studies in recent years and discusses the shortcomings of TMS in preclinical studies.
INTRODUCTION
Repetitive transcranial magnetic stimulation (rTMS), electroconvulsive therapy (ECT), and vagus nerve stimulation are three physical strategies that have been approved by the United States Food and Drug Administration for the treatment of major depressive disorder (MDD)[1]. Compared with ECT and vagus nerve stimulation, rTMS is more readily accepted by patients with MDD because it does not require anesthesia and minimally invasive surgery with fewer side effects.
Since rTMS is an effective treatment for MDD[2-6], understanding its anti-depressant mechanisms may help to deepen the understanding of depression. This paper focuses on preclinical studies, reviews the underlying mechanisms of rTMS in animal models of depressive-like behavior, and discusses the shortcomings and deficiencies of rTMS in preclinical studies. Preclinical studies have shown that rTMS plays an anti-depressant role through a variety of mechanisms with diverse signaling pathways (Table 1).
Table 1.
Main preclinical findings on antidepressant mechanisms of repetitive transcranial magnetic stimulation
| Ref. | Model |
rTMS
|
Main findings | |||
|
Frequency (Hz)
|
Intensity (T)
|
Duration (d)
|
Coil size
|
|||
| Tian et al[21], 2020 | CUMS | 15 | 1.26 | 7 | Inner diameter 2.5 cm; outer diameter 5 cm (SD rats) | Regulation of Nrf2-induced anti-inflammatory effect in the hippocampus |
| Yang et al[42], 2019 | CUS | 1 | 20 mT | 14 | Not mentioned | Enhancement of synaptic plasticity in the hippocampus |
| Xue et al[45], 2019 | CUS | 5 | 1.26 | 7 | Inner diameter 2.5 cm; outer diameter 5 cm (SD rats) | Activation of the ECS in the hippocampus |
| Zhao et al[49], 2018 | CUMS | 10 | 50% of the resting motor threshold | 15 | 5 cm in diameter (SD rats) | Decreasing the activity of the HPA axis |
| Heath et al[48], 2018 | OB | 10 | 50 mT | 20 | 8 mm in diameter (C57BL/6J mice) | Activation of the BDNF signaling pathway and pro-proliferative in the hippocampus |
| Fang et al[43], 2018 | CUMS | 15 | rTMS device maximum power | 7 | Not mentioned | Activation of the ECS in the hippocampus |
| Peng et al[68], 2017 | CUS | 5/10 | 0.84/1.26 | 7 | Inner diameter 2.5 cm; outer diameter 5 cm (SD rats) | Suppression of Sirt1/MAO-A signaling in the PFC |
| Chen et al[47], 2015 | CUS | 15 | 1.26 | 7 | Inner diameter 2.5 cm; outer diameter 5 cm (SD rats) | Activation of the BDNF signaling pathway and pro-proliferative in the hippocampus |
| Wang et al[44], 2014 | CUMS | 15 | rTMS device maximum power | 7 | Inner diameter 2.5 cm; outer diameter 5 cm (SD rats) | Activation of the ECS and BDNF signaling pathway in the hippocampus |
| Kim et al[78], 2014 | CUMS | 10 | 1.4 | 14 | 7 cm in diameter (SD rats) | Changing metabolic patterns in the brain |
| Feng et al[46], 2012 | CUMS | 15 | rTMS device maximum power | 21 | Inner diameter 5 cm; outer diameter 7 cm (SD rats) | Decreasing the activity of the HPA axis, and activation of BDNF signaling pathway in the hippocampus |
| Tasset et al[29], 2010 | OB | 60 | 0.7 mT | 14 | Not mentioned | Anti-oxidative stress effects |
BDNF: Brain derived neurotrophic factor; CUS: Chronic unpredictable stress; CUMS: Chronic unpredictable mild stress; ECS: Endocannabinoid system; HPA axis: Hypothalamic-pituitary-adrenocortical axis; MAO-A: Monoamine oxidase A; Nrf2: Nuclear factor-E2-related factor 2; OB: Olfactory bulbectomy; PFC: Prefrontal cortex; rTMS: Repetitive transcranial magnetic stimulation; SD rats: Sprague Dawley rats; Sirt1: Sirtuin 1.
REPETITIVE TRANSCRANIAL MAGNETIC STIMULATION ACTIVATES THE ANTI-INFLAMMATORY EFFECTS MEDIATED VIA THE NUCLEAR FACTOR-E2-RELATED FACTOR 2 SIGNALING PATHWAY
Inflammation is strongly associated with depression[7-10]. Patients with autoimmune and infectious diseases are more likely to develop depression[11]. Patients with aseptic inflammation in the brain (such as stroke) are more likely to be accompanied by depression[12]. There are few clinical studies on the effects of rTMS on immune inflammation in MDD patients. Langguth et al[13] reported the recurrence of rheumatoid arthritis in an elderly female patient with treatment-resistant depression (TRD) comorbid with rheumatoid arthritis after receiving 20 Hz (intensity, 90% motor threshold) rTMS. After the patient received rTMS, the peripheral C-reactive protein and interleukin-6 (IL-6) contents increased (from 6.7 mg/L to 25 mg/L and from 3.4 ng/L to 15 ng/L, respectively), indicating that rTMS enhanced the inflammatory response of the patient. In contrast, Zhao et al[14] recruited 58 elderly patients with TRD and 30 healthy controls. The levels of peripheral IL-1β and tumor necrosis factor α (TNFα) in the patients were higher than those in healthy controls. Compared with the non-rTMS treated group, peripheral IL-1β and TNFα levels were markedly reduced in patients who were continuously treated with 10 Hz (intensity, 80 % motor threshold) rTMS for 4 wk. In addition, the levels of peripheral pro-inflammatory cytokines had no observable change in healthy controls after treatment by rTMS, which was consistent with the results of animal studies[15].
Nuclear factor-E2-related factor 2 (Nrf2) is a transcription factor that binds to antioxidant response elements and has anti-inflammatory effects in addition to anti-oxidative stress[16-18]. There was significantly reduced expression of Nrf2 both in the hippocampus and prefrontal cortex (PFC) of depressive-like mice induced by chronic social defeat stress or stress susceptible rats induced by learned helplessness[18,19]. Moreover, autopsy revealed that Nrf2 expression was decreased in the PFC region of MDD patients[19,20]. Tian et al[21] found that depressive-like behavior induced by chronic unpredictable mild stress (CUMS) in rats were improved by rTMS (15 Hz/1.26 T) via increasing Nrf2 translocation into the nucleus and decreasing the expression of TNFα, inducible nitric oxide synthase, IL-1β, and IL-6 in the hippocampus. When the Nrf2 gene was silenced, the anti-depressant effect of rTMS disappeared simultaneously with a decrease of inflammatory factors. The results suggested that rTMS plays an anti-depressant role via enhancement of an anti-inflammatory action mediated by the Nrf2 signaling pathway. However, the mechanism by which rTMS exerts its anti-depressant effect through an anti-inflammatory effect has not been fully elucidated. Therefore, more preclinical studies are needed in this respect.
ANTI-OXIDATIVE STRESS EFFECTS
Humans produce reactive oxygen species (ROS) in the process of using O2 to oxidize glucose to generate adenosine triphosphate. Excessive ROS act on lipids, proteins, and deoxyribonucleic acid, and produce a large number of peroxidation products such as lipid peroxidation products malondialdehyde and 4-hydroxyalkenals, resulting in oxidative stress, which is considered an important factor for disease development. There are two types of anti-oxidative stress systems in the body: One is the enzymatic anti-oxidative stress system including glutathione peroxidase (GSH-Px) and superoxide dismutase; The other is the non-enzymatic anti-oxidative stress system including glutathione (GSH). Several meta-studies have shown increased peroxidation products and decreased anti-oxidative stress agents in MDD patients[22-25]. Durmaz et al[26] found that, compared with the value before treatment, the serum content of thiol, an anti-oxidant organic sulfur, was reduced in TRD patients after treatment with 20 Hz (intensity, 110 % motor threshold) rTMS. However, there are different views on the anti-depressant effect of rTMS through anti-oxidative stress. Aydın et al[27] found that there was no difference in serum thioredoxin, a protein with anti-oxidant function, between healthy and TRD patients, or between TRD patients before and after rTMS treatment.
The review of Medina-Fernández et al[28] detailed the anti-oxidative stress effects of rTMS in a variety of diseases. In preclinical studies of depression, the mechanism of anti-oxidative stress in rTMS has been poorly studied. Compared with sham rTMS group, rTMS at 60 Hz/0.7 mT increased the decreased contents of GSH, GSH-Px, and superoxide dismutase, and decreased the contents of malondialdehyde, 4-hydroxyalkenals, and caspase-3, a key terminal shear enzyme in apoptosis, in brain tissue homogenate in a rat depression model induced by olfactory bulbectomy[29]. The idea that oxidative stress is one of the causes or characteristics of MDD has not been widely accepted by the academic community. In addition, the anti-depressive role of rTMS through the mechanism of anti-oxidative stress has been controversial in clinical studies, in which the measurement of oxidative stress markers in peripheral blood may not reflect the real situation in the brain truthfully and completely. Unfortunately, only one preclinical study was found to clarify the mechanism of anti-oxidative stress in the anti-depressive effect of rTMS.
ACTIVATION OF ENDOCANNABINOID SYSTEM AND BRAIN DERIVED NEUROTROPHIC FACTOR SIGNALING PATHWAY ENHANCES SYNAPTIC PLASTICITY AND NEUROGENESIS
Cumulative evidence suggests that the endocannabinoid system (ECS) is involved in the physiopathologic mechanism of depression[30-32]. ECS receptors include cannabinoid type 1 receptor (CB1R) and CB2R. ECS ligands include arachidonoyl ethanolamide (AEA), 2-arachidonyloglycerol (2-AG), noladine ether, virodhamine, and N-arachidonoyldopamine. Other endogenous compounds such as palmitoylethanolamide and oleylethanolamide also have affinity for the ECS receptors. AEA and 2-AG are the most important ligands in the ECS. The key proteins in AEA biosynthesis and decomposition are N-acyl phosphatidyl ethanolamine-phospholipase D and fatty acid amide hydrolase, respectively. The key proteins in 2-AG biosynthesis and decomposition are diacylglycerol lipase α (DAGLα) and monoacylglycerol lipase (MAGL), respectively. Endocannabinoids produced in the postsynaptic element activate endocannabinoid receptors located in the presynaptic membrane and perform a number of anti-depressant biological functions including: (1) Reducing the activity of the hypothalamic-pituitary-adrenocortical (HPA) axis; (2) Enhancing hippocampal synaptic plasticity; (3) Promoting the neurogenesis of the hippocampus; and (4) Increasing the expression of brain derived neurotrophic factor (BDNF) in hippocampal tissue[33-36]. BDNF, a key factor affecting multiple signaling pathways in the brain, binds to the receptor TrkB on the membrane and activates the Ras/MAPK, PI3K/Akt, PLCγ, and GTPase signaling pathways to promote neurogenesis and enhance synaptic plasticity[37-41].
One Hz/20 mT rTMS increased the expression of synaptic proteins PSD95 and NR2B in the hippocampus of Wista rats with a depressive-like behavior induced by chronic unpredictable stress (CUS)[42]. rTMS at 15 Hz (intensity, device maximum power) reduced the expression of MAGL and Bax, increased the contents of 2-AG, CB1R, BDNF, and Bcl-2, and promoted neurogenesis in the hippocampus, improving depressive-like behavior induced by CUMS. CB1R antagonist (AM251) counteracts the biological function of the above mentioned rTMS[43,44]. rTMS at 5 Hz/1.26 T improved depressive-like behavior induced by CUS in rats via increasing the expression of CB1R, DAGLα, N-acyl phosphatidyl ethanolamine-phospholipase D, PSD95, and synaptophysin, and decreasing the expression of MAGL and fatty acid amide hydrolase in the hippocampus. The anti-depressant effect of rTMS was offset by short hairpin RNA targeting DAGL or CB1R[45]. The above preclinical studies suggested that rTMS plays an anti-depressant role by activating the ECS, which may provide evidence for the development of new anti-depressants targeting ECS.
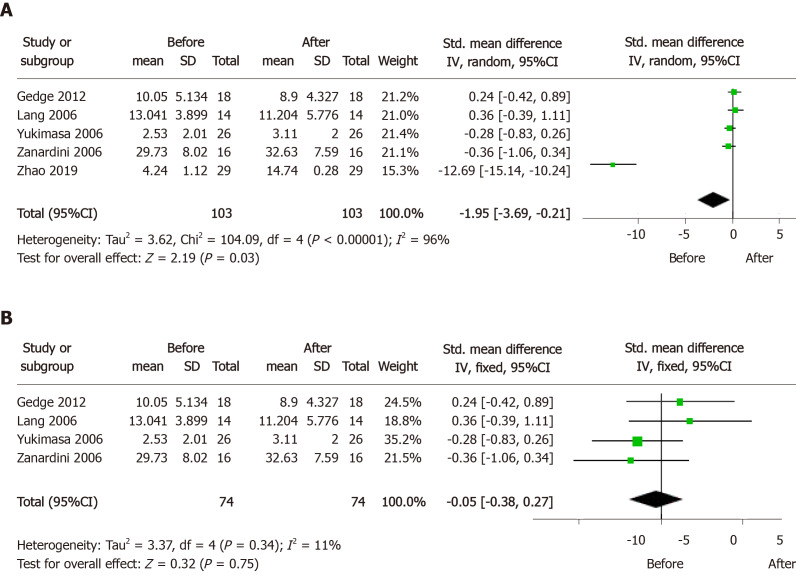
Moreover, several preclinical studies have observed that rTMS increased the content of BDNF in the hippocampus and PFC, and promoted hippocampal neurogenesis in rodent CUMS, CUS, and olfactory bulbectomy depression models[44,46-49]. However, there are inconsistencies between the results of preclinical and clinical studies. BDNF may be involved in hippocampal neurogenesis and synaptic plasticity as a neurotrophic factor, but the existence of a neuro-regenerative disorder hypothesis for depression is controversial. As compared with normal controls, the serum BDNF level markedly decreased in MDD patients, and significantly negatively correlated with the severity of depressive symptoms. In addition, in TRD patients carrying a homozygous BDNF Val/Val allele, the BDNF levels increased after treatment by rTMS[50,51]. However, the results of treatment effect were controversial in TRD patients, and it can be confirmed to be a predictor of treatment response in TRD patients. We included five relevant articles and conducted a mini meta-analysis using Review Manager 5.3 software[14,52-55]. It was found that serum BDNF levels in TRD patients increased after rTMS treatment (Figure 1A). Considering the issue of high heterogeneity, we applied the leave-one-out method to analyze the results[56], and found that after removing the study of Zhao et al[14], the heterogeneity decreased from 96% to 11%, and the conclusion that rTMS treatment had no effect on serum BDNF content in TRD patients was reached, which was consistent with the study results of Brunoni et al[57] (Figure 1B). BDNF is a macromolecular protein that does not easily cross the blood-brain barrier, which may be responsible for the inconsistency between the results of the above preclinical and clinical studies.
Figure 1.
Forest plots of serum brain derived neurotrophic factor levels in treatment-resistant depression patients before and after repetitive transcranial magnetic stimulation treatment.
SUPPRESSION OF SIRTUIN 1/MONOAMINE OXIDASE A SIGNALING PATHWAY
Certain monoamine oxidase (MAO) inhibitors are used to treat patients with MDD, which include phenelzine, brofaromine, toloxatone, isocarboxazide, tranylcypromine, and moclobemide. MAO includes MAO-A and MAO-B subtypes in the brain, where MAO-A is involved in the metabolism of 5-hydroxytryptamine, norepinephrine, and dopamine, and MAO-B metabolizes dopamine, benzylamine, and phenethylamine[58-60]. Moreover, MAO-A produces ROS during the process of metabolizing monoamine neurotransmitters, which promotes neuronal apoptosis[61,62]. The expression of MAO-A is regulated by the transcription factors NHLH2, KLF11, R1, and FOXO1. Among them, NHLH2 and FOXO1 need to be deacetylated by Sirtuin 1 (Sirt1) to play the role of transcription factors[63-67]. Injection of Sirt1 inhibitor EX527 into the PFC of CUS rats improved depressive-like behavior. Further, the 5-hydroxytryptamine concentration in the PFC was increased by reducing the expression of Sirt1 and MAO-A and the activity of MAO, and the depressive-like behavior in CUS rats was improved after treatment by rTMS at 5 Hz/0.84 T, 5 Hz/1.26 T, 10 Hz/0.84 T, and 10 Hz/1.26 T[68]. MAO inhibitors take several weeks to demonstrate anti-depressant efficacy[69,70], whereas rTMS is not only effective in patients with TRD but also works faster than anti-depressants. Therefore, the Sirt1/MAO-A signaling pathway may not be a key mechanism for anti-depressant therapy in rTMS, especially for patients with TRD.
DECREASING THE ACTIVITY OF THE HYPOTHALAMIC-PITUITARY-ADRENOCORTICAL AXIS
Hyperactivity of the HPA axis may be one of the pathophysiological mechanisms of depression[71-73]. Both preclinical and clinical studies have found increased activity of the HPA axis either in depressive-like animal models or in MDD patients. However, the results were contradictory, especially in clinical studies. There are many interfering factors for hormone levels, such as changes in circadian rhythm[74]. Drugs targeting the HPA axis have not been successful in clinical trials[70]. As a result, it has not been possible to use HPA related measurements in the diagnosis of MDD, and there may be many mechanisms that have not been clarified. rTMS at 10 Hz (intensity, 50 % of the resting motor threshold) and 15 Hz (intensity, device maximum power) reduced the levels of adrenocorticotropic hormone and cortisol in peripheral blood of depressive-like rats induced by CUMS[46,49]. There are few preclinical and clinical studies supporting that rTMS exerts an anti-depressant effect by reducing the activity of the HPA axis, and the current evidence is still insufficient.
FUTURE PERSPECTIVES
This review summarizes several newly discovered anti-depressant mechanisms of rTMS in preclinical studies, including: (1) Anti-inflammatory effects mediated by activation of the Nrf2 signaling pathway; (2) Anti-oxidative stress effects; (3) Enhancement of synaptic plasticity and neurogenesis via activation of the ECS and BDNF signaling pathway; (4) Increasing the content of monoamine neurotransmitters via inhibition of the Sirt1/MAO-A signaling pathway; and (5) reducing the activity of the HPA axis.
Preclinical research has an incomparable huge advantage in obtaining brain specimens compared to clinical research. In the future preclinical research on rTMS mechanism, the following issues can be appropriately considered:
(1) The efficacy of rTMS is closely related to parameters, treatment time, and experimental protocol[75,76], and there is no unified treatment protocol in preclinical studies although it is available in clinical practice;
(2) Epidemiological studies show that the morbidity of females is higher than that of males for MDD[70,77]. However, all the studies in Table 1 used male rodents, which may be inappropriate or incomplete to elucidate the anti-depressant mechanisms of rTMS in animals;
(3) Different intensity of 10 Hz rTMS affects the metabolic patterns in the brain and periphery of the depression models[48,78]. rTMS at 1.4 T reduced the level of γ-amino butyric acid in the hippocampus and PFC regions of CUMS rats[78]. rTMS at 1 T increased the content of α-amino butyric acid and reduced the content of 3-methylhistidine in peripheral blood of mice suffering from olfactory bulbectomy[48]. Recently, proteomics and metabolomics techniques are gradually used to explore the anti-depressant mechanism of rTMS to focus on screening candidate biomolecule or biomarkers, which may provide a clue for further elucidating the relevant mechanisms;
(4) Preclinical studies have mostly focused on the hippocampus in animal models of depressive-like behavior. However, an increasing amount of evidence indicates that, other core brain regions are also closely associated with the development and progression of depression, such as the PFC, amygdala, cingulate gyrus, habenula, and visual cortex[79-81], which should be considered in future studies. In addition, deep brain stimulation plays an important anti-depressant role in patients with MDD[82-85], suggesting that neural circuit dysfunction may be one of the mechanisms of depression[86]; however, there is no animal research to probe the regulation of neural circuits related to the anti-depressant effect of rTMS, which may be one of the future research directions;
(5) The shortcomings of TMS include inaccurate positioning and shallow depth of stimulation, which cause the difficulty to elucidate the neural circuit mechanism of up- and down-stream of the stimulation target region in animal models.
The left and right dorsolateral PFC of MDD patients have asymmetric low and high activity, respectively[87]. In clinical practice, high and/or low frequency rTMS are commonly used to stimulate the left and/or right side, respectively, to obtain anti-depressant effects. However, in preclinical studies, as shown in Table 1, due to the relatively large diameter of the coils of the rTMS device, it is not possible to accurately stimulate a specific brain region in rodents, and instead a large range of a hemisphere or even the whole brain is stimulated. This may be detrimental to the elucidation of the potential anti-depressant mechanism of rTMS. The development of rTMS devices for rats and mice with appropriate size and precise focus may be more conducive to exploring the mechanism of the anti-depressant effect of rTMS.
Another unavoidable defect of TMS is the problem of depth of stimulation, that is, TMS can only stimulate the superficial cerebral cortex, but has no stimulation effect on the subcortical brain regions[88]. Deep TMS can stimulate relatively deep brain regions compared to other TMS, but it may cause the superficial regions to be strongly stimulated[89]. In addition, TMS has energy consumption in non-target tissues such as the scalp, skull, and cerebrospinal fluid, which can reduce the energy occupancy rate of target tissues.
The application of a smaller size rTMS coil can achieve precise positioning, but the efficacy of the stimulation is insufficient and the therapeutic effect is affected. Exhilaratingly, enhancing the therapeutic effect by using magnetic nanoparticles may be in a creative way. Kong et al[90] found that magnetic nanoparticles could pass through the blood-brain barrier in mice. Furthermore, Li et al[91] intravenously injected magnetic nanoparticles with a core of Fe3O4 in rats, which can enhance the therapeutic effect of TMS. Our research team constructed a novel superparamagnetic Fe2O3 nanoparticle, accurately injected it into the left prelimbic cortex of CUMS mice treated by rTMS at10 Hz/0.1 T for 5 d, and significantly improving the depressive symptoms of these mice[92].
CONCLUSION
In conclusion, fully elucidating the anti-depressant mechanisms of rTMS will increase the chances of discovering new therapeutic strategies.
Footnotes
Conflict-of-interest statement: The authors deny any conflict of interest.
Manuscript source: Unsolicited manuscript
Peer-review started: June 16, 2020
First decision: July 30, 2020
Article in press: September 2, 2020
Specialty type: Psychiatry
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kotzalidis GD S-Editor: Zhang L L-Editor: Wang TQ P-Editor: Li JH
Contributor Information
Di Luan, Department of Neurology, Affiliated Zhongda Hospital, Research Institution of Neuropsychiatry, School of Medicine, Southeast University, Nanjing 210009, Jiangsu Province, China.
Ming-Ge Zhao, Department of Nursing, School of Medicine, Southeast University, Nanjing 210009, Jiangsu Province, China.
Ya-Chen Shi, Department of Neurology, Affiliated Zhongda Hospital, Research Institution of Neuropsychiatry, School of Medicine, Southeast University, Nanjing 210009, Jiangsu Province, China.
Ling Li, Department of Neurology, Affiliated Zhongda Hospital, Research Institution of Neuropsychiatry, School of Medicine, Southeast University, Nanjing 210009, Jiangsu Province, China.
Yu-Jia Cao, Department of Neurology, Affiliated Zhongda Hospital, Research Institution of Neuropsychiatry, School of Medicine, Southeast University, Nanjing 210009, Jiangsu Province, China.
Hai-Xia Feng, Department of Nursing, School of Medicine, Southeast University, Nanjing 210009, Jiangsu Province, China.
Zhi-Jun Zhang, Department of Neurology, Affiliated Zhongda Hospital, Research Institution of Neuropsychiatry, School of Medicine, Southeast University, Nanjing 210009, Jiangsu Province, China; Department of Psychology, Xinxiang Medical University, Xinxiang 453003, Henan Province, China; Mental Health Center, Zhejiang University School of Medicine, Hangzhou 310013, Zhejiang province, China. janemengzhang@vip.163.com.
References
- 1.Trapp NT, Xiong W, Conway CR. Neurostimulation Therapies. Handb Exp Pharmacol. 2019;250:181–224. doi: 10.1007/164_2018_157. [DOI] [PubMed] [Google Scholar]
- 2.Iglesias AH. Transcranial Magnetic Stimulation as Treatment in Multiple Neurologic Conditions. Curr Neurol Neurosci Rep. 2020;20:1. doi: 10.1007/s11910-020-1021-0. [DOI] [PubMed] [Google Scholar]
- 3.Lefaucheur JP, Aleman A, Baeken C, Benninger DH, Brunelin J, Di Lazzaro V, Filipović SR, Grefkes C, Hasan A, Hummel FC, Jääskeläinen SK, Langguth B, Leocani L, Londero A, Nardone R, Nguyen JP, Nyffeler T, Oliveira-Maia AJ, Oliviero A, Padberg F, Palm U, Paulus W, Poulet E, Quartarone A, Rachid F, Rektorová I, Rossi S, Sahlsten H, Schecklmann M, Szekely D, Ziemann U. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014-2018) Clin Neurophysiol. 2020;131:474–528. doi: 10.1016/j.clinph.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Somani A, Kar SK. Efficacy of repetitive transcranial magnetic stimulation in treatment-resistant depression: the evidence thus far. Gen Psychiatr. 2019;32:e100074. doi: 10.1136/gpsych-2019-100074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trevizol AP, Blumberger DM. An Update on Repetitive Transcranial Magnetic Stimulation for the Treatment of Major Depressive Disorder. Clin Pharmacol Ther. 2019;106:747–762. doi: 10.1002/cpt.1550. [DOI] [PubMed] [Google Scholar]
- 6.Rizvi S, Khan AM. Use of Transcranial Magnetic Stimulation for Depression. Cureus. 2019;11:e4736. doi: 10.7759/cureus.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roman M, Irwin MR. Novel neuroimmunologic therapeutics in depression: A clinical perspective on what we know so far. Brain Behav Immun. 2020;83:7–21. doi: 10.1016/j.bbi.2019.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su WJ, Cao ZY, Jiang CL. Blocking the trigger: An integrative view on the anti-inflammatory therapy of depression. Brain Behav Immun. 2019;82:10–12. doi: 10.1016/j.bbi.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Lee CH, Giuliani F. The Role of Inflammation in Depression and Fatigue. Front Immunol. 2019;10:1696. doi: 10.3389/fimmu.2019.01696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maydych V. The Interplay Between Stress, Inflammation, and Emotional Attention: Relevance for Depression. Front Neurosci. 2019;13:384. doi: 10.3389/fnins.2019.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benros ME, Waltoft BL, Nordentoft M, Ostergaard SD, Eaton WW, Krogh J, Mortensen PB. Autoimmune diseases and severe infections as risk factors for mood disorders: a nationwide study. JAMA Psychiatry. 2013;70:812–820. doi: 10.1001/jamapsychiatry.2013.1111. [DOI] [PubMed] [Google Scholar]
- 12.Robinson RG, Jorge RE. Post-Stroke Depression: A Review. Am J Psychiatry. 2016;173:221–231. doi: 10.1176/appi.ajp.2015.15030363. [DOI] [PubMed] [Google Scholar]
- 13.Langguth B, Braun S, Aigner JM, Landgrebe M, Weinerth J, Hajak G, Eichhammer P. Repetitive transcranial magnetic stimulation in a patient suffering from depression and rheumatoid arthritis: evidence for immunomodulatory effects. Neuro Endocrinol Lett. 2005;26:314–316. [PubMed] [Google Scholar]
- 14.Zhao X, Li Y, Tian Q, Zhu B, Zhao Z. Repetitive transcranial magnetic stimulation increases serum brain-derived neurotrophic factor and decreases interleukin-1β and tumor necrosis factor-α in elderly patients with refractory depression. J Int Med Res. 2019;47:1848–1855. doi: 10.1177/0300060518817417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okada K, Matsunaga K, Yuhi T, Kuroda E, Yamashita U, Tsuji S. The long-term high-frequency repetitive transcranial magnetic stimulation does not induce mRNA expression of inflammatory mediators in the rat central nervous system. Brain Res. 2002;957:37–41. doi: 10.1016/s0006-8993(02)03582-5. [DOI] [PubMed] [Google Scholar]
- 16.Hashimoto K. Essential Role of Keap1-Nrf2 Signaling in Mood Disorders: Overview and Future Perspective. Front Pharmacol. 2018;9:1182. doi: 10.3389/fphar.2018.01182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 18.Yao W, Zhang JC, Ishima T, Dong C, Yang C, Ren Q, Ma M, Han M, Wu J, Suganuma H, Ushida Y, Yamamoto M, Hashimoto K. Role of Keap1-Nrf2 signaling in depression and dietary intake of glucoraphanin confers stress resilience in mice. Sci Rep. 2016;6:30659. doi: 10.1038/srep30659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang JC, Yao W, Dong C, Han M, Shirayama Y, Hashimoto K. Keap1-Nrf2 signaling pathway confers resilience versus susceptibility to inescapable electric stress. Eur Arch Psychiatry Clin Neurosci. 2018;268:865–870. doi: 10.1007/s00406-017-0848-0. [DOI] [PubMed] [Google Scholar]
- 20.Martín-Hernández D, Caso JR, Javier Meana J, Callado LF, Madrigal JLM, García-Bueno B, Leza JC. Intracellular inflammatory and antioxidant pathways in postmortem frontal cortex of subjects with major depression: effect of antidepressants. J Neuroinflammation. 2018;15:251. doi: 10.1186/s12974-018-1294-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tian L, Sun SS, Cui LB, Wang SQ, Peng ZW, Tan QR, Hou WG, Cai M. Repetitive Transcranial Magnetic Stimulation Elicits Antidepressant- and Anxiolytic-like Effect via Nuclear Factor-E2-related Factor 2-mediated Anti-inflammation Mechanism in Rats. Neuroscience. 2020;429:119–133. doi: 10.1016/j.neuroscience.2019.12.025. [DOI] [PubMed] [Google Scholar]
- 22.Black CN, Bot M, Scheffer PG, Cuijpers P, Penninx BW. Is depression associated with increased oxidative stress? Psychoneuroendocrinology. 2015;51:164–175. doi: 10.1016/j.psyneuen.2014.09.025. [DOI] [PubMed] [Google Scholar]
- 23.Liu T, Zhong S, Liao X, Chen J, He T, Lai S, Jia Y. A Meta-Analysis of Oxidative Stress Markers in Depression. PLoS One. 2015;10:e0138904. doi: 10.1371/journal.pone.0138904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mazereeuw G, Herrmann N, Andreazza AC, Khan MM, Lanctôt KL. A meta-analysis of lipid peroxidation markers in major depression. Neuropsychiatr Dis Treat. 2015;11:2479–2491. doi: 10.2147/NDT.S89922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palta P, Samuel LJ, Miller ER 3rd, Szanton SL. Depression and oxidative stress: results from a meta-analysis of observational studies. Psychosom Med. 2014;76:12–19. doi: 10.1097/PSY.0000000000000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Durmaz O, İspir E, Baykan H, Alişik M, Erel Ö. The Impact of Repetitive Transcranial Magnetic Stimulation on Oxidative Stress in Subjects With Medication-Resistant Depression. J ECT. 2018;34:127–131. doi: 10.1097/YCT.0000000000000467. [DOI] [PubMed] [Google Scholar]
- 27.Aydın EP, Genç A, Dalkıran M, Uyar ET, Deniz İ, Özer ÖA, Karamustafalıoğlu KO. Thioredoxin is not a marker for treatment-resistance depression but associated with cognitive function: An rTMS study. Prog Neuropsychopharmacol Biol Psychiatry. 2018;80:322–328. doi: 10.1016/j.pnpbp.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 28.Medina-Fernández FJ, Escribano BM, Padilla-Del-Campo C, Drucker-Colín R, Pascual-Leone Á, Túnez I. Transcranial magnetic stimulation as an antioxidant. Free Radic Res. 2018;52:381–389. doi: 10.1080/10715762.2018.1434313. [DOI] [PubMed] [Google Scholar]
- 29.Tasset I, Drucker-Colín R, Peña J, Jimena I, Montilla P, Medina FJ, Túnez I. Antioxidant-like effects and protective action of transcranial magnetic stimulation in depression caused by olfactory bulbectomy. Neurochem Res. 2010;35:1182–1187. doi: 10.1007/s11064-010-0172-9. [DOI] [PubMed] [Google Scholar]
- 30.Chadwick VL, Rohleder C, Koethe D, Leweke FM. Cannabinoids and the endocannabinoid system in anxiety, depression, and dysregulation of emotion in humans. Curr Opin Psychiatry. 2020;33:20–42. doi: 10.1097/YCO.0000000000000562. [DOI] [PubMed] [Google Scholar]
- 31.Mechoulam R, Parker LA. The endocannabinoid system and the brain. Annu Rev Psychol. 2013;64:21–47. doi: 10.1146/annurev-psych-113011-143739. [DOI] [PubMed] [Google Scholar]
- 32.Augustin SM, Lovinger DM. Functional Relevance of Endocannabinoid-Dependent Synaptic Plasticity in the Central Nervous System. ACS Chem Neurosci. 2018;9:2146–2161. doi: 10.1021/acschemneuro.7b00508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang WJ, Chen WW, Zhang X. Endocannabinoid system: Role in depression, reward and pain control (Review) Mol Med Rep. 2016;14:2899–2903. doi: 10.3892/mmr.2016.5585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poleszak E, Wośko S, Sławińska K, Szopa A, Wróbel A, Serefko A. Cannabinoids in depressive disorders. Life Sci. 2018;213:18–24. doi: 10.1016/j.lfs.2018.09.058. [DOI] [PubMed] [Google Scholar]
- 35.Zhou D, Li Y, Tian T, Quan W, Wang L, Shao Q, Fu LQ, Zhang XH, Wang XY, Zhang H, Ma YM. Role of the endocannabinoid system in the formation and development of depression. Pharmazie. 2017;72:435–439. doi: 10.1691/ph.2017.7474. [DOI] [PubMed] [Google Scholar]
- 36.Estrada JA, Contreras I. Endocannabinoid receptors in the CNS: potential drug targets for the prevention and treatment of neurologic and psychiatric disorders. Curr Neuropharmacol. :2020. doi: 10.2174/1570159X18666200217140255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Björkholm C, Monteggia LM. BDNF - a key transducer of antidepressant effects. Neuropharmacology. 2016;102:72–79. doi: 10.1016/j.neuropharm.2015.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caviedes A, Lafourcade C, Soto C, Wyneken U. BDNF/NF-κB Signaling in the Neurobiology of Depression. Curr Pharm Des. 2017;23:3154–3163. doi: 10.2174/1381612823666170111141915. [DOI] [PubMed] [Google Scholar]
- 39.Hing B, Sathyaputri L, Potash JB. A comprehensive review of genetic and epigenetic mechanisms that regulate BDNF expression and function with relevance to major depressive disorder. Am J Med Genet B Neuropsychiatr Genet. 2018;177:143–167. doi: 10.1002/ajmg.b.32616. [DOI] [PubMed] [Google Scholar]
- 40.Kowiański P, Lietzau G, Czuba E, Waśkow M, Steliga A, Moryś J. BDNF: A Key Factor with Multipotent Impact on Brain Signaling and Synaptic Plasticity. Cell Mol Neurobiol. 2018;38:579–593. doi: 10.1007/s10571-017-0510-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leal G, Bramham CR, Duarte CB. BDNF and Hippocampal Synaptic Plasticity. Vitam Horm. 2017;104:153–195. doi: 10.1016/bs.vh.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 42.Yang J, Wang L, Wang F, Tang X, Zhou P, Liang R, Zheng C, Ming D. Low-Frequency Pulsed Magnetic Field Improves Depression-Like Behaviors and Cognitive Impairments in Depressive Rats Mainly via Modulating Synaptic Function. Front Neurosci. 2019;13:820. doi: 10.3389/fnins.2019.00820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fang G, Wang Y. Effects of rTMS on Hippocampal Endocannabinoids and Depressive-like Behaviors in Adolescent Rats. Neurochem Res. 2018;43:1756–1765. doi: 10.1007/s11064-018-2591-y. [DOI] [PubMed] [Google Scholar]
- 44.Wang HN, Wang L, Zhang RG, Chen YC, Liu L, Gao F, Nie H, Hou WG, Peng ZW, Tan Q. Anti-depressive mechanism of repetitive transcranial magnetic stimulation in rat: the role of the endocannabinoid system. J Psychiatr Res. 2014;51:79–87. doi: 10.1016/j.jpsychires.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 45.Xue SS, Xue F, Ma QR, Wang SQ, Wang Y, Tan QR, Wang HN, Zhou CH, Peng ZW. Repetitive high-frequency transcranial magnetic stimulation reverses depressive-like behaviors and protein expression at hippocampal synapses in chronic unpredictable stress-treated rats by enhancing endocannabinoid signaling. Pharmacol Biochem Behav. 2019;184:172738. doi: 10.1016/j.pbb.2019.172738. [DOI] [PubMed] [Google Scholar]
- 46.Feng SF, Shi TY, Fan-Yang , Wang WN, Chen YC, Tan QR. Long-lasting effects of chronic rTMS to treat chronic rodent model of depression. Behav Brain Res. 2012;232:245–251. doi: 10.1016/j.bbr.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 47.Chen YH, Zhang RG, Xue F, Wang HN, Chen YC, Hu GT, Peng Y, Peng ZW, Tan QR. Quetiapine and repetitive transcranial magnetic stimulation ameliorate depression-like behaviors and up-regulate the proliferation of hippocampal-derived neural stem cells in a rat model of depression: The involvement of the BDNF/ERK signal pathway. Pharmacol Biochem Behav. 2015;136:39–46. doi: 10.1016/j.pbb.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 48.Heath A, Lindberg DR, Makowiecki K, Gray A, Asp AJ, Rodger J, Choi DS, Croarkin PE. Medium- and high-intensity rTMS reduces psychomotor agitation with distinct neurobiologic mechanisms. Transl Psychiatry. 2018;8:126. doi: 10.1038/s41398-018-0129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao L, Ren H, Gu S, Li X, Jiang C, Li J, Zhang M, Mu J, Li W, Wang W, Zhang Z, Song J. rTMS ameliorated depressive-like behaviors by restoring HPA axis balance and prohibiting hippocampal neuron apoptosis in a rat model of depression. Psychiatry Res. 2018;269:126–133. doi: 10.1016/j.psychres.2018.08.017. [DOI] [PubMed] [Google Scholar]
- 50.Beuzon G, Timour Q, Saoud M. Predictors of response to repetitive transcranial magnetic stimulation (rTMS) in the treatment of major depressive disorder. Encephale. 2017;43:3–9. doi: 10.1016/j.encep.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 51.Silverstein WK, Noda Y, Barr MS, Vila-Rodriguez F, Rajji TK, Fitzgerald PB, Downar J, Mulsant BH, Vigod S, Daskalakis ZJ, Blumberger DM. NEUROBIOLOGICAL PREDICTORS OF RESPONSE TO DORSOLATERAL PREFRONTAL CORTEX REPETITIVE TRANSCRANIAL MAGNETIC STIMULATION IN DEPRESSION: A SYSTEMATIC REVIEW. Depress Anxiety. 2015;32:871–891. doi: 10.1002/da.22424. [DOI] [PubMed] [Google Scholar]
- 52.Gedge L, Beaudoin A, Lazowski L, du Toit R, Jokic R, Milev R. Effects of electroconvulsive therapy and repetitive transcranial magnetic stimulation on serum brain-derived neurotrophic factor levels in patients with depression. Front Psychiatry. 2012;3:12. doi: 10.3389/fpsyt.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lang UE, Bajbouj M, Gallinat J, Hellweg R. Brain-derived neurotrophic factor serum concentrations in depressive patients during vagus nerve stimulation and repetitive transcranial magnetic stimulation. Psychopharmacology (Berl) 2006;187:56–59. doi: 10.1007/s00213-006-0399-y. [DOI] [PubMed] [Google Scholar]
- 54.Yukimasa T, Yoshimura R, Tamagawa A, Uozumi T, Shinkai K, Ueda N, Tsuji S, Nakamura J. High-frequency repetitive transcranial magnetic stimulation improves refractory depression by influencing catecholamine and brain-derived neurotrophic factors. Pharmacopsychiatry. 2006;39:52–59. doi: 10.1055/s-2006-931542. [DOI] [PubMed] [Google Scholar]
- 55.Zanardini R, Gazzoli A, Ventriglia M, Perez J, Bignotti S, Rossini PM, Gennarelli M, Bocchio-Chiavetto L. Effect of repetitive transcranial magnetic stimulation on serum brain derived neurotrophic factor in drug resistant depressed patients. J Affect Disord. 2006;91:83–86. doi: 10.1016/j.jad.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 56.Luan D, Zhang Y, Yang Q, Zhou Z, Huang X, Zhao S, Yuan L. Efficacy and Safety of Intravenous Thrombolysis in Patients with Unknown Onset Stroke: A Meta-Analysis. Behav Neurol. 2019;2019:5406923. doi: 10.1155/2019/5406923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brunoni AR, Baeken C, Machado-Vieira R, Gattaz WF, Vanderhasselt MA. BDNF blood levels after non-invasive brain stimulation interventions in major depressive disorder: a systematic review and meta-analysis. World J Biol Psychiatry. 2015;16:114–122. doi: 10.3109/15622975.2014.958101. [DOI] [PubMed] [Google Scholar]
- 58.Higuchi Y, Soga T, Parhar IS. Regulatory Pathways of Monoamine Oxidase A during Social Stress. Front Neurosci. 2017;11:604. doi: 10.3389/fnins.2017.00604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Higuchi Y, Soga T, Parhar IS. Potential Roles of microRNAs in the Regulation of Monoamine Oxidase A in the Brain. Front Mol Neurosci. 2018;11:339. doi: 10.3389/fnmol.2018.00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Naoi M, Riederer P, Maruyama W. Modulation of monoamine oxidase (MAO) expression in neuropsychiatric disorders: genetic and environmental factors involved in type A MAO expression. J Neural Transm (Vienna) 2016;123:91–106. doi: 10.1007/s00702-014-1362-4. [DOI] [PubMed] [Google Scholar]
- 61.Ou XM, Stockmeier CA, Meltzer HY, Overholser JC, Jurjus GJ, Dieter L, Chen K, Lu D, Johnson C, Youdim MB, Austin MC, Luo J, Sawa A, May W, Shih JC. A novel role for glyceraldehyde-3-phosphate dehydrogenase and monoamine oxidase B cascade in ethanol-induced cellular damage. Biol Psychiatry. 2010;67:855–863. doi: 10.1016/j.biopsych.2009.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Youdim MB, Bakhle YS. Monoamine oxidase: isoforms and inhibitors in Parkinson's disease and depressive illness. Br J Pharmacol. 2006;147 Suppl 1:S287–S296. doi: 10.1038/sj.bjp.0706464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grunewald M, Johnson S, Lu D, Wang Z, Lomberk G, Albert PR, Stockmeier CA, Meyer JH, Urrutia R, Miczek KA, Austin MC, Wang J, Paul IA, Woolverton WL, Seo S, Sittman DB, Ou XM. Mechanistic role for a novel glucocorticoid-KLF11 (TIEG2) protein pathway in stress-induced monoamine oxidase A expression. J Biol Chem. 2012;287:24195–24206. doi: 10.1074/jbc.M112.373936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harris S, Johnson S, Duncan JW, Udemgba C, Meyer JH, Albert PR, Lomberk G, Urrutia R, Ou XM, Stockmeier CA, Wang JM. Evidence revealing deregulation of the KLF11-MAO A pathway in association with chronic stress and depressive disorders. Neuropsychopharmacology. 2015;40:1373–1382. doi: 10.1038/npp.2014.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Johnson S, Stockmeier CA, Meyer JH, Austin MC, Albert PR, Wang J, May WL, Rajkowska G, Overholser JC, Jurjus G, Dieter L, Johnson C, Sittman DB, Ou XM. The reduction of R1, a novel repressor protein for monoamine oxidase A, in major depressive disorder. Neuropsychopharmacology. 2011;36:2139–2148. doi: 10.1038/npp.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Libert S, Pointer K, Bell EL, Das A, Cohen DE, Asara JM, Kapur K, Bergmann S, Preisig M, Otowa T, Kendler KS, Chen X, Hettema JM, van den Oord EJ, Rubio JP, Guarente L. SIRT1 activates MAO-A in the brain to mediate anxiety and exploratory drive. Cell. 2011;147:1459–1472. doi: 10.1016/j.cell.2011.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu JB, Shih JC. Valproic acid induces monoamine oxidase A via Akt/forkhead box O1 activation. Mol Pharmacol. 2011;80:714–723. doi: 10.1124/mol.111.072744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Peng ZW, Xue F, Zhou CH, Zhang RG, Wang Y, Liu L, Sang HF, Wang HN, Tan QR. Repetitive transcranial magnetic stimulation inhibits Sirt1/MAO-A signaling in the prefrontal cortex in a rat model of depression and cortex-derived astrocytes. Mol Cell Biochem. 2018;442:59–72. doi: 10.1007/s11010-017-3193-8. [DOI] [PubMed] [Google Scholar]
- 69.Willner P, Scheel-Krüger J, Belzung C. The neurobiology of depression and antidepressant action. Neurosci Biobehav Rev. 2013;37:2331–2371. doi: 10.1016/j.neubiorev.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 70.Malhi GS, Mann JJ. Depression. Lancet. 2018;392:2299–2312. doi: 10.1016/S0140-6736(18)31948-2. [DOI] [PubMed] [Google Scholar]
- 71.Juruena MF, Bocharova M, Agustini B, Young AH. Atypical depression and non-atypical depression: Is HPA axis function a biomarker? J Affect Disord. 2018;233:45–67. doi: 10.1016/j.jad.2017.09.052. [DOI] [PubMed] [Google Scholar]
- 72.Menke A. Is the HPA Axis as Target for Depression Outdated, or Is There a New Hope? Front Psychiatry. 2019;10:101. doi: 10.3389/fpsyt.2019.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 2008;31:464–468. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 74.Liyanarachchi K, Ross R, Debono M. Human studies on hypothalamo-pituitary-adrenal (HPA) axis. Best Pract Res Clin Endocrinol Metab. 2017;31:459–473. doi: 10.1016/j.beem.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 75.Galletly C, Gill S, Clarke P, Burton C, Fitzgerald PB. A randomized trial comparing repetitive transcranial magnetic stimulation given 3 days/week and 5 days/week for the treatment of major depression: is efficacy related to the duration of treatment or the number of treatments? Psychol Med. 2012;42:981–988. doi: 10.1017/S0033291711001760. [DOI] [PubMed] [Google Scholar]
- 76.Herrmann LL, Ebmeier KP. Factors modifying the efficacy of transcranial magnetic stimulation in the treatment of depression: a review. J Clin Psychiatry. 2006;67:1870–1876. doi: 10.4088/jcp.v67n1206. [DOI] [PubMed] [Google Scholar]
- 77.Kessler RC, Bromet EJ. The epidemiology of depression across cultures. Annu Rev Public Health. 2013;34:119–138. doi: 10.1146/annurev-publhealth-031912-114409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim SY, Lee DW, -Kim H, Bang E, Chae JH, Choe BY. Chronic repetitive transcranial magnetic stimulation enhances GABAergic and cholinergic metabolism in chronic unpredictable mild stress rat model: ¹H-NMR spectroscopy study at 11. Neurosci Lett. 2014;572:32–37. doi: 10.1016/j.neulet.2014.04.033. [DOI] [PubMed] [Google Scholar]
- 79.Koenigs M, Grafman J. The functional neuroanatomy of depression: distinct roles for ventromedial and dorsolateral prefrontal cortex. Behav Brain Res. 2009;201:239–243. doi: 10.1016/j.bbr.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Williams LM. Amygdala-Guided Neurofeedback for Major Depression. Am J Psychiatry. 2017;174:717–718. doi: 10.1176/appi.ajp.2017.17050561. [DOI] [PubMed] [Google Scholar]
- 81.Zhang Z, Zhang H, Xie CM, Zhang M, Shi Y, Song R, Lu X, Zhang H, Li K, Wang B, Yang Y, Li X, Zhu J, Zhao Y, Yuan TF, Northoff G. Task-related functional magnetic resonance imaging-based neuronavigation for the treatment of depression by individualized repetitive transcranial magnetic stimulation of the visual cortex. Sci China Life Sci. :2020. doi: 10.1007/s11427-020-1730-5. [DOI] [PubMed] [Google Scholar]
- 82.Kisely S, Li A, Warren N, Siskind D. A systematic review and meta-analysis of deep brain stimulation for depression. Depress Anxiety. 2018;35:468–480. doi: 10.1002/da.22746. [DOI] [PubMed] [Google Scholar]
- 83.Dandekar MP, Fenoy AJ, Carvalho AF, Soares JC, Quevedo J. Deep brain stimulation for treatment-resistant depression: an integrative review of preclinical and clinical findings and translational implications. Mol Psychiatry. 2018;23:1094–1112. doi: 10.1038/mp.2018.2. [DOI] [PubMed] [Google Scholar]
- 84.Zhou C, Zhang H, Qin Y, Tian T, Xu B, Chen J, Zhou X, Zeng L, Fang L, Qi X, Lian B, Wang H, Hu Z, Xie P. A systematic review and meta-analysis of deep brain stimulation in treatment-resistant depression. Prog Neuropsychopharmacol Biol Psychiatry. 2018;82:224–232. doi: 10.1016/j.pnpbp.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 85.Drobisz D, Damborská A. Deep brain stimulation targets for treating depression. Behav Brain Res. 2019;359:266–273. doi: 10.1016/j.bbr.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 86.Chaudhury D, Liu H, Han MH. Neuronal correlates of depression. Cell Mol Life Sci. 2015;72:4825–4848. doi: 10.1007/s00018-015-2044-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brunoni AR, Chaimani A, Moffa AH, Razza LB, Gattaz WF, Daskalakis ZJ, Carvalho AF. Repetitive Transcranial Magnetic Stimulation for the Acute Treatment of Major Depressive Episodes: A Systematic Review With Network Meta-analysis. JAMA Psychiatry. 2017;74:143–152. doi: 10.1001/jamapsychiatry.2016.3644. [DOI] [PubMed] [Google Scholar]
- 88.Wagner T, Gangitano M, Romero R, Théoret H, Kobayashi M, Anschel D, Ives J, Cuffin N, Schomer D, Pascual-Leone A. Intracranial measurement of current densities induced by transcranial magnetic stimulation in the human brain. Neurosci Lett. 2004;354:91–94. doi: 10.1016/s0304-3940(03)00861-9. [DOI] [PubMed] [Google Scholar]
- 89.Deng ZD, Peterchev AV, Lisanby SH. Coil design considerations for deep-brain transcranial magnetic stimulation (dTMS) Conf Proc IEEE Eng Med Biol Soc. 2008;2008:5675–5679. doi: 10.1109/IEMBS.2008.4650502. [DOI] [PubMed] [Google Scholar]
- 90.Kong SD, Lee J, Ramachandran S, Eliceiri BP, Shubayev VI, Lal R, Jin S. Magnetic targeting of nanoparticles across the intact blood-brain barrier. J Control Release. 2012;164:49–57. doi: 10.1016/j.jconrel.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li R, Wang J, Yu X, Xu P, Zhang S, Xu J, Bai Y, Dai Z, Sun Y, Ye R, Liu X, Ruan G, Xu G. Enhancing the effects of transcranial magnetic stimulation with intravenously injected magnetic nanoparticles. Biomater Sci. 2019;7:2297–2307. doi: 10.1039/c9bm00178f. [DOI] [PubMed] [Google Scholar]
- 92.Lu QB, Sun JF, Yang QY, Cai WW, Xia MQ, Wu FF, Gu N, Zhang ZJ. Magnetic brain stimulation using iron oxide nanoparticle-mediated selective treatment of the left prelimbic cortex as a novel strategy to rapidly improve depressive-like symptoms in mice. Zool Res. 2020;41:381–394. doi: 10.24272/j.issn.2095-8137.2020.076. [DOI] [PMC free article] [PubMed] [Google Scholar]