Abstract
Prospection (mentally simulating future events) generates emotionally-charged mental images that guide social decision-making. Positive and negative social expectancies—imagining new social interactions to be rewarding versus threatening—are core components of social approach and avoidance motivation, respectively. Interindividual differences in such positive and negative future-related cognitions may be underpinned by distinct neuroanatomical substrates. Here, we asked 100 healthy adults to vividly imagine themselves in a novel self-relevant event that was ambiguous with regards to possible social acceptance or rejection. During this task we measured participants’ expectancies for social reward (anticipated feelings of social connection) or threat (anticipated feelings of rejection). On a separate day they underwent structural MRI; voxel-based morphometry was used to explore the relation between social reward and threat expectancies and regional grey matter volumes (rGMV). Increased rGMV in key default-network regions involved in prospection, socio-emotional cognition, and subjective valuation, including ventromedial prefrontal cortex, correlated with both higher social reward and lower social threat expectancies. In contrast, social threat expectancies uniquely correlated with rGMV of regions involved in social attention (posterior superior temporal sulcus, pSTS) and interoception (somatosensory cortex). These findings provide novel insight into the neurobiology of future-oriented cognitive-affective processes critical to adaptive social functioning.
Subject terms: Reward, Cognitive control, Motivation, Perception, Personality, Risk factors, Human behaviour
Introduction
Making friends—and/or forming romantic partnerships—is of critical importance for adults' adjustment to new environments, for instance, starting university1. Humans are therefore intrinsically motivated to actively seek out and affiliate with others, with the aim of fostering new social connections2. By their nature, however, social interactions with unfamiliar others simultaneously offer the prospect of both rewards (e.g. having a pleasant conversation, feeling a sense of belonging)2 and threats (e.g., feeling embarrassed, being socially rejected)3,4.
Neurobehavioral motivation frameworks posit two basic systems that mediate actions geared towards desirable and undesirable outcomes—an approach (or behavioural activation) system (BAS) and an avoidance (or behavioural inhibition) system (BIS), respectively5–7. These are suggested to be independent neurobehavioral systems, which may compete to drive behaviour5,8. Models of social motivation connect these basic approach/avoidance motivational processes with social cognition, including attentional focus and beliefs about other people's behaviour in social interactions4,9,10.
People differ in their sensitivity to social reward and threat and such inter-individual differences are relatively stable11, although such sensitivities be heightened during adolescence12,13. These stable traits are associated with the likelihood of being socially connected or, conversely, isolated4,14. It seems plausible that individual differences in these neurocognitive systems might exist on continua of shyness and sociability, respectively, with the extreme ends of these continua being clinically relevant15,16.
An emerging literature details neural responses to rejection or connection experiences and visual cues of social reward or threat4,17–19. An abundance of research implicates the amygdala in threat processing, including social threat20, and notably increased amygdala volume has been linked to behavioural inhibition and social anxiety21,22. The amygdala works in concert with pSTS in mediating sustained vigilance for signs of social threat23. The pSTS and amygdala are also active when recalling and reliving social evaluative threat situations24. By contrast, vmPFC is considered a “hub” of reward processing, including social reward17,25 and interacts with core social cognition regions including dorsomedial PFC (dmPFC) to mediate the experienced and remembered reward (pleasure) of social belonging or approval4,17. vmPFC-amygdala functional connectivity is also implicated in the cognitive regulation of negative affect, including in the face of social rejection26,27. Previous work has found that greater wellbeing and more successful emotion regulation are associated with greater rGMV in vmPFC28,29.
There is reason, however, to think that prospective cognitive-affect representations are at the heart of these putatively distinct social reward and threat motivational systems. BAS or BIS have been theorized to be primarily future-oriented (e.g., mediating hopes and fears about future desirable or undesirable outcomes7). MacLeod30,31 argued that affect is directly related to cognition and that positive and negative future-related cognitions may best be perceived as two separate dimensions of experience. Such future-oriented emotion systems depend on the capacity for “mental time travel” (MTT) inherent in episodic memory32,33. MTT enables vivid, detail-rich simulations of future events based on the flexible re-combination of episodic memories and newly generated images constructed by drawing on episodic memories combined with components of semantic memory, such as beliefs and schemas. Through the vivid imagination of future events, humans generate embodied predictions of events’ emotional impacts before their occurrence, which act as powerful motivators of behaviour33,34.
The capacity for MTT is mediated by a ‘core’ network, largely overlapping with the default-network, notably including vmPFC as a core node32,35. vmPFC is implicated, alongside medial temporal lobe regions, both in the construction of episodic memories and imagined future events (in part by accessing relevant schematic knowledge), as well as in their affective valuation based on current needs and goals25,32,35.
While recent research has studied individual differences in anticipated social reward and threat separately (e.g.19,36,37.), to our knowledge, no neuroimaging research has directly examined both individual differences in future-oriented social reward and threat expectancies in the context of fostering new social connections. Building on work in the domain of close relationships9,38,39 we developed a new instrument to examine inter-individual differences in reward and threat expectancies in the context of an imagined self-relevant social interaction with unfamiliar peers. This novel measure, the levels of dispositional expectancies for social threat and reward scale (LODESTARS), asks participants to vividly imagine that they have joined a new group, club, or society, then make predictions about the probable emotional consequences of these novel interactions and report their anticipatory and anticipated cognitions and emotions.
Individuals’ social reward and threat expectancies as measured by the LODESTARS are stable over time40, are associated with other stable affective traits such as self-esteem, and may be grounded in temperament and attachment experiences41. Given this trait-like stability, we predicted that individual differences in expectancies for social threat and reward would be associated with stable, structural aspects of the brain. Recent structural magnetic resonance imaging (sMRI) studies indicate that several aspects of real-world social behaviour are reflected in brain macrostructure (regional grey matter volume, rGMV) as assessed by voxel-based morphometry (VBM)42.
Here, we used VBM and an unbiased, whole-brain analysis to investigate the unique and overlapping rGMV correlates of inter-individual differences in social threat and reward expectancies (STE and SRE, respectively) using a combination of raw LODESTARS and LODESTARS scores that were orthogonalised (residualised) with respect to one another (see Methods for details). Further, to facilitate interpretation of the results, we examined (a) functional associations of rGMV peak voxels revealed by our analysis using the large-scale meta-analytic platform Neurosynth (https://neurosynth.org/) and (for key regions) (b) their structural covariance, which may reflect the extent to which brain regions belong to the same (or antagonistic) functional system(s)43. This was primarily an exploratory study. However, we made two tentative predictions, based on the close alignment of regional brain macrostructure and function43. First, given vmPFC involvement in the construction and valuation of events, including imagined personal future events, as well the more general finding that vmPFC activity scales with experienced and anticipated positive value44, we expected that vmPFC rGMV would correlate positively with SRE. In addition, given vmPFC involvement in emotion regulation, we also expected increased vmPFC volume to relate to lower STE.
Secondly, given their involvement in the processing of social threat and links to anxiety, we predicted that rGMV of the amygdala and posterior superior temporal sulcus (pSTS) would be positively correlated with STE.
Methods
This study was approved by the Cardiff University Psychology Research Ethics Committee and was carried out in accordance with the relevant guidelines and regulations. All participants provided written informed consent in accordance with the Declaration of Helsinki.
Participants and procedure
A power analysis45 indicated a sample size of n = 82 was required to detect a medium sized correlation (r = 0.3, alpha = 0.05, power = 0.8). One hundred right-handed healthy volunteers participated (74 female, 26 male, mean age 24 years, range 18–54). Participants completed a battery of measures including the LODESTARS, administered using Qualtrics (Provo, UT, https://www.qualtrics.com). Participants attended the imaging centre on a separate occasion for MRI.
Measuring dispositional social expectancies: The LODESTARS
The LODESTARS is a 10-item inventory examining the extent to which respondents expect to experience social reward and threat during an imminent vividly imagined social encounter with a group of unfamiliar peers. Participants are asked to imagine that they have joined a new group, club or society and that this evening they will be going to a social event organized by this group/club/society. Participants imagine that this will be the first time they will meet other people who are in the group/club/society. After noting down the name of the group/club/society they have chosen, participants indicate their anticipated and anticipatory cognitions and emotions about the upcoming imagined event, by responding to 5 threat items and 5 reward items on a 5-point Likert scale. These items include “I will probably meet one or more people who I will like a lot” and “I am a bit worried about feeling embarrassed during these interactions” (see https://osf.io/hq5sg/ for the full measure). Approaching unfamiliar others and establishing initial social connections are core tasks when transitioning into novel social environments (e.g. entering university), and a prerequisite for integrating new people into one’s social network1,11.
Expectancies about social interactions are partly situation-specific46; however, there is a component of them that is influenced by individuals' temperament and stable working models (schemas) of self and others47. The LODESTARS was designed to tap the stable component, by probing participants’ expectancies for interactions with peers (with whom the participant is motivated to interact) in a generic social event context. The scenario described in the LODESTARS is nuanced (it simultaneously holds the possibility for social reward and threat), and thus in line with existing measures in which participants imagine themselves in an emotionally ambiguous (future) scenario48. These measures are sensitive to individual differences in affective style30. We used an imminent imaginary scenario, since short-term predictions enhance the tendency to rely on episodic emotional information, relative to personal semantic knowledge (beliefs etc.)30,49,50.
Data from more than 1300 participants demonstrate that the LODESTARS has a two-factor (reward, threat) structure and excellent psychometric properties, including high test–retest reliability and measurement invariance across gender40. The LODESTARS yields two scores for each participant: a social reward expectancy (SRE) score and a social threat expectancy (STE) score, both of which can range from 1 (low) to 5 (high). The LODESTARS has excellent construct validity and appears to be sensitive in distinguishing different social cognitive-affective processing styles. For example, attachment anxiety is associated with heightened STE, while avoidant attachment is associated with reduced social SRE41. Qualitative data from a community sample confirmed that people find the LODESTARS to be highly naturalistic40, consistent with findings that people devote considerable time in daily life to imagining and evaluating social encounters51.
Image acquisition
T1-weighted anatomical images for each participant were acquired using a 3-T GE HDx MRI scanner at Cardiff University Brain Research Imaging Centre (CUBRIC). The 3-D T1-weighted whole-brain images were acquired using a fast-spoiled gradient echo sequence (FSPGR) with 1 × 1 × 1 mm voxel size and between 168 and 182 contiguous slices. Image acquisition parameters were as follows: repetition time (TR) = 7.8 ms echo time (TE) = 2.984 ms; inversion time = 450 ms; flip angle = 15°; data matrix = 256 × 192. These data were usually acquired within one week of the participant completing the LODESTARS (mode = 3 days).
Image analysis
Voxel-based morphometry (VBM) was performed using SPM12 (Wellcome Trust Centre for Neuroimaging, https://www.fil.ion.ucl.ac.uk/spm/software/spm12) implemented in MATLAB v. R2012b (The MathWorks). First each participant’s structural image was segmented into grey matter (GM), white matter (WM) and cerebrospinal fluid (CSF) using the ‘unified segmentation’ set of algorithms in SPM12. The image segments of interest (the GM segments) were then normalised to MNI space using the diffeomorphic anatomical registration through exponentiated lie-algebra (DARTEL) registration method in SPM1252. The GM images were smoothed using a Gaussian kernel of 8 mm full width at half maximum. An 8 mm smoothing kernel is optimal for detecting morpho-metric differences in both large and small neural structures53.
Statistical analysis 1: LODESTARS VBM
We examined correlations between regional grey matter volume (rGMV) and social reward expectancy and social threat expectancies from the LODESTARS. We accounted for the potentially confounding variables of age and gender54 by entering them into the general linear models as ‘regressors of no interest’. Participants’ overall brain volumes were also accounted for, by means of proportional scaling in SPM1255. A binary MNI brain mask (SPM8 brainmask.nii) was used to restrict the analysed volume to voxels within the brain.
Model specification
Inference as to whether regional rGMV significantly correlates with one or both regressors of interest requires that both LODESTARS-reward and threat scores be included within the same model56.
There is debate as to the extent to which reward and threat expectancies are independent, both in terms of underlying brain substrates and as they manifest in behaviour/self-report5,7. It is informative, therefore, to clarify the effects on rGMV that are uniquely attributable to each of these two regressors. Entering both into a GLM will automatically achieve this: an essential property of the GLM is that only the variability unique to each regressor drives the parameter estimate for it, so that each effect is adjusted for all others57. Only assessing the rGMV associations of variance that is unique to threat and to reward carries its own problems however. These are due to the fact that the standard process of GLM parameter estimation removes the effects of shared variability57. When two regressors are highly correlated, their shared variability is large and the unique component for each is correspondingly small. This results in a loss of statistical power. Further, in this case, it is interesting to explore not only the regional rGMV differences uniquely associated with social threat or reward expectancies, but also those present when the shared variance is included within the model.
The correlation between LODESTARS-STE and -SRE scores in the present study was − 0.36, p = 0.0002 (95% CI = − 0.56 to − 0.137), indicating significant shared variance between these two regressors. To explore the shared as well unique variance, while maintaining the same degrees of freedom across models, we used orthogonalised LODESTARS scores in combination with raw scores. Orthogonalised scores are the residuals that result from regressing STE on SRE scores and vice versa. By definition, these constitute the portions of each LODESTARS score that are not predicted by the other LODESTARS score.
We conducted two GLMs, which between them allowed assessment of individual differences in rGMV uniquely attributable to variance in LODESTARS-STE or SRE, as well as rGMV associations present when the shared variance was included but attributed exclusively to social threat or reward. The two models are specified below. See Fig. 1 for a diagrammatic representation of the assignation of (shared) variance that results from orthogonalisation.
Figure 1.
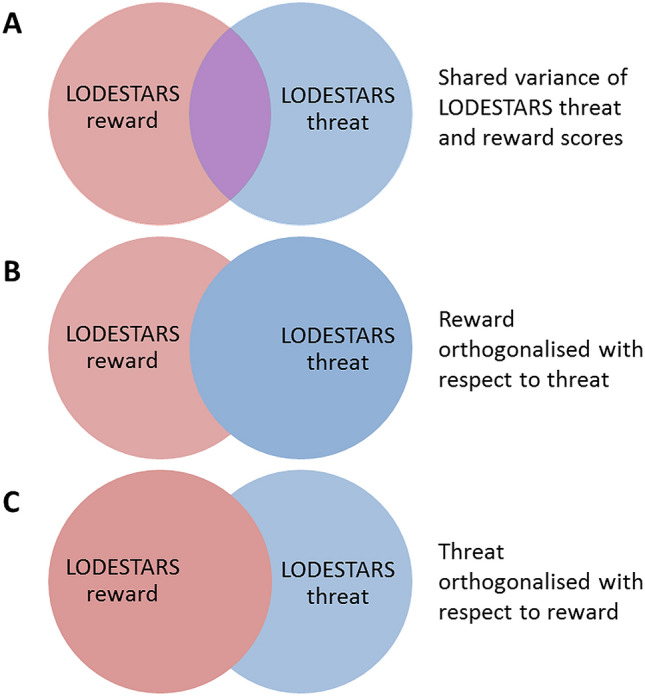
Venn diagrams illustrating how the variability is distributed across the 2 LODESTARS regressors where red is unique to SRE, blue is unique to STE and purple is shared. (A) depicts ‘raw’ LODESTARS-STE and SRE scores, which exhibit some overlapping variance. (B, C) depict the two regression models run, demonstrating the effects of variable orthogonalisation. In (B), all the shared variance is assigned to LODESTARS-STE while in C, all shared variance is assigned to LODESTARS-SRE.
Model 1: All shared variance assigned to social threat expectancies.
Model 2: All shared variance assigned to social reward expectancies.
where LODESTARS_threat_orth = LODESTARS-STE orthogonalised with respect to SRE scores and LODESTARS_reward_orth = LODESTARS-SRE orthogonalised with respect to STE scores.
Correction for multiple comparisons
To correct for multiple comparisons across the whole brain, we applied non-stationary cluster extent correction as implemented in the VBM8 toolbox (https://dbm.neuro.uni-jena.de/vbm/) running in SPM12. We used 3DClustSim (AFNI) to calculate the overall expected voxels-per-cluster threshold for our data, for α = 0.05, p ≤ 0.001, based on the brain mask we used (SPM8 brainmask.nii). This gave an expected cluster size of ≥ 86 voxels.
Neurosynth meta-analytic decoding
We sought to gain insight into what aspects of functional paradigms are most frequently associated with the regions identified in our VBM analysis via functional decoding. Functional decoding is a quantitative, data-driven method by which researchers can infer which mental processes may be related to activation in a specific brain region (or set of brain regions) across published fMRI studies. We conducted meta-analytic decoding using Neurosynth, a platform for large-scale, automated synthesis of functional magnetic resonance imaging (fMRI) data (https://neurosynth.org), to find the functional terms most frequently associated with the peak voxels identified in the VBM analysis. To facilitate interpretation, the top 10 terms with the highest correlation values for each peak voxel were selected. Non-content terms (such as “MRI,” “statistical”) and anatomical terms (such as “prefrontal,” “MTL”) were excluded. Terms that were near-duplicates of terms already included in the list were removed, such as “emotional” and “emotions” if “emotion” was higher on the list. This left two or three functional terms for each peak voxel; these are reported in Tables 1 and 2.
Table 1.
Clusters that survived nonstationary cluster extent correction: shared variance between threat and reward included.
| LODESTARS variable | Direction of correlation | Anatomical region | Cluster size (voxels) | MNI coordinates | T-score | Reflects unique variance? | Neurosynth decoding results | ||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
| Social reward expectancy | Positive |
 left dorsomedial PFC
left dorsomedial PFC |
171 | − 1.5 | 48 | 48 | 4.03 | No. Does not survive when shared variance allocated to threat | “imagine” “social” |
| Social reward expectancy | Negative | [no clusters survive threshold] | – | – | – | – | |||
| Social threat expectancy | Positive |
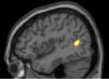 right posterior superior temporal sulcus (pSTS)
right posterior superior temporal sulcus (pSTS) |
212 | 45 | − 58.5 | 10.5 | 4.24 | Yes. Survives when shared variance allocated to reward | “eye gaze” “action observation” |
| Social threat expectancy | Negative |
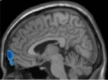 right ventromedial PFC
right ventromedial PFC |
282 | 3 | 69 | − 4.5 | 4.01 | No. Does not survive when shared variance allocated to reward | “Theory of mind” “resting state” |
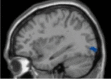 left lateral inferior occipital lobe
left lateral inferior occipital lobe |
90 | − 28.5 | – 90 | − 6 | 3.80 | Yes. Survives when shared variance allocated to reward | “face” “visual” | ||
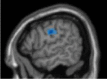 right postcentral gyrus (somatosensory cortex)
right postcentral gyrus (somatosensory cortex) |
187 | 60 | − 12 | 30 | 3.58 | No. Does not survive when shared variance allocated to threat | “somatosensory” “action observation” | ||
Table 2.
Overlap of clusters reflecting bipolar valence that survived p < 0.005, 86-voxel extent threshold.
| Anatomical region | Extent of overlap (voxels) | MNI coordinates | T-value | Neurosynth decoding results | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Right ventromedial prefrontal cortex | 68 | 9 | 48 | − 21 | 3.41 | “social”, “reward”, “autobiographical” |
| Right lateral inferior temporal gyrus | 90 | 61.5 | − 13.5 | − 36 | 3.39 | “Theory of mind”, “social” |
| Right parahippocampal gyrus | 19 | 27 | − 25.5 | − 31.5 | 3.04 | “episodic”, “semantic”, “recollection” |
Statistical analysis 2: overlap analysis
To test for brain voxels in which rGMV is significantly correlated (positively or negatively) with social threat and reward expectancies, two further GLMs were applied. These models each contained only one LODESTARS variable as the regressor of interest. The same thresholding was applied as in statistical analysis 1: p < 0.005, with an 86-voxel cluster extent threshold.
These models yielded statistical parametric maps (SPMs) of brain regions in which rGMV correlated positively with SRE, positively with STE, and negatively with STE. (No clusters survive threshold for negative correlation with SRE). These gave rise to two overlap analyses: 1, {social reward-positive and social threat-negative} (henceforth ‘bipolar valence’) and 2, {social reward-positive and social threat-positive} (henceforth ‘salience’).
The combinations of SPMs were inspected for overlap by means of masking in SPM12.
Statistical analysis 3: structural covariance analyses
Inter-individual differences in the macrostructure of a brain region often co-vary with inter-individual differences in macrostructure of anatomically connected regions—so-called structural covariance43. Like other forms of connectivity, inter-regional SC may reflect shared functional specialization43. Thus, to further characterize the network affinities of regions linked with SRE and STE, we examined potential grey matter SC between dmPFC and vmPFC, between vmPFC and amygdala, and between pSTS and amygdala.
We extracted GMVs for the peak voxels of the dmPFC, vmPFC and pSTS clusters that survived cluster-extent correction in the LODESTARS VBM. These voxels were used as seeds in the subsequent analysis.
Our target regions of interest (ROIs) were specified by masks: a bilateral amygdala mask created from the Caltech atlas of the human amygdala58 and a bilateral vmPFC mask created from the Neuromorphometrics atlas (Neuromorphometrics labels, Right and Left MFC medial frontal cortex)59. We used seed-based SC analyses60, conducted in SPM12, to identify voxels within our target ROIs in which GMV covaried with GMV in the seed voxel. Our analyses identified voxels in which target region GMV covaried positively with seed GMV, and (separately) voxels in which target region GMV covaried negatively with seed GMV. The effects of gender, age, and total brain volume were accounted for in these models. As this was a hypothesis-driven, rather than exploratory analysis, we employed more stringent correction for multiple comparisons than in analyses 1 and 2. Specifically, threshold-free cluster enhancement (TFCE), which controls the family-wise error rate at p < 0.0561.
Results
The mean LODESTARS-SRE score in this sample was 3.7 (from a max. possible score of 5; range = 2.0–4.8); std. dev. = 0.49) and the mean LODESTARS-STE score was 3.3 (range = 1.0–5.0, std. dev. = 0.92). Cronbach’s alpha was 0.65 for LODESTARS-SRE and 0.87 for LODESTARS-STE. There were no significant gender differences in the LODESTARS scores. LODESTARS-SRE scores did not correlate with age, however LODESTARS-STE scores decreased with increasing age (r = − 0.30, p = 0.003, 95% CI = − 0.49 to − 0.103).
A paired-samples t-test indicated that the mean LODESTARS-SRE score was significantly higher than mean LODESTARS-STE score, t = 3.05, p = 0.003, dav = 0.5.
Statistical analysis 1: LODESTARS VBM results
First, correlations between rGMV and LODESTARS-STE/SRE were examined in the SPM T-maps in which shared variance was included. That is, the outputs of the STE orthogonalised with respect to SRE model were inspected for correlations between rGMV and LODESTARS-SRE scores. The outputs of the SRE orthogonalised with respect to STE model were inspected for correlations between rGMV and LODESTARS-STE scores. Details of the clusters that survived non-stationary cluster extent correction are given in Table 1. The extent to which the correlations within each cluster reflect unique variance of STE or SRE was then assessed by checking whether the clusters survived cluster-extent correction thresholding for the equivalent contrasts in the complementary model (i.e. SRE correlation contrasts in the SRE orthogonalised with respect to STE model). These results are reported in the penultimate column (‘Reflects unique variance?’) of Table 1.
A positive correlation between SRE and rGMV was found in a dorsomedial region of left prefrontal cortex (dmPFC, see Fig. 2). This result was significant only in the model in which the shared variance was allocated to SRE however; it did not remain significant (at the cluster-size-corrected level) in the model in which the shared variance is allocated to LODESTARS-STE, indicating that this rGMV-expectancy association is partially attributable to shared variance between social reward and threat expectancies. No other correlations (positive or negative) of rGMV with LODESTARS-SRE survived cluster extent correction.
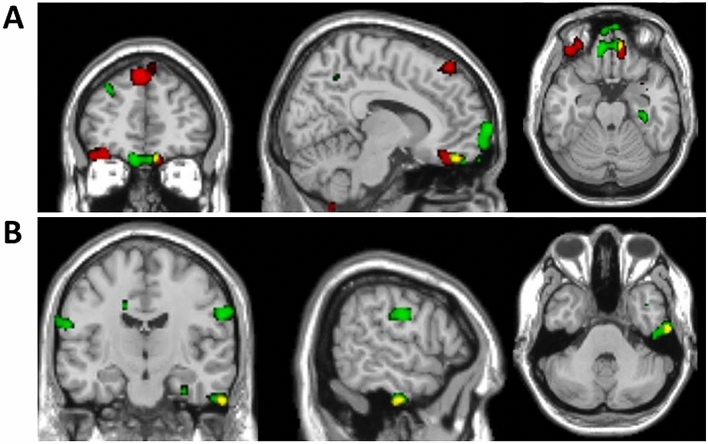
Figure 2.
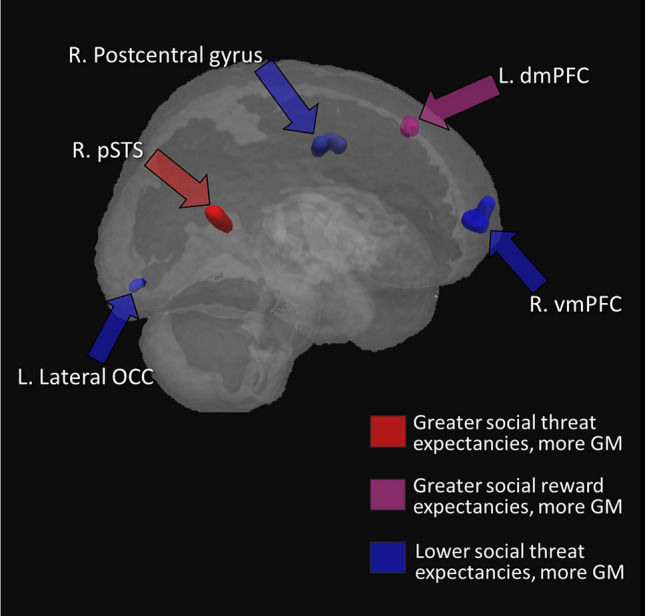
Brain regions in which there were significant associations between self-reported social expectancies and rGMV. For display purposes the clusters are shown at a threshold of p < .001, uncorrected.
Greater rGMV in right posterior superior temporal sulcus (pSTS) was associated with higher STE (Fig. 2), whereas individuals who reported lower expectancies of social threat had greater GM volumes in right ventromedial PFC (vmPFC, see Fig. 2), left lateral occipital lobe (lOCC, see Fig. 2), and right postcentral gyrus (somatosensory cortex, Fig. 2).
The extent and location of each of the clusters that survived non-stationary extent correction are summarised in Fig. 2.
Statistical analysis 2: brain regions in which rGMV is correlated with both reward and threat expectancies
The results of these overlap analyses are given in Table 2 and Fig. 3. The only pairing for which there were overlapping clusters (at p < 0.005, with 86 voxel extent threshold) was {SRE-positive and STE-negative} (‘bipolar valence’). There was overlap between clusters in the vmPFC (Fig. 3A), in the right lateral inferior temporal gyrus (Fig. 3B) and in right parahippocampal gyrus.
Figure 3.
Overlay of regions in which rGMV correlates positively with social reward expectancy and negatively with social threat expectancy. Red = SRE_positive; green = STE_negative; yellow = overlap. The SPMs were thresholded at p < 0.005 with 10 voxel minimum cluster extent. (A) (upper panel) shows the extent of overlap in right orbitofrontal/ventromedial prefrontal cortex. (B) (lower panel) shows the overlap in right lateral inferior temporal gyrus.
Statistical analysis 3: Structural covariance analyses
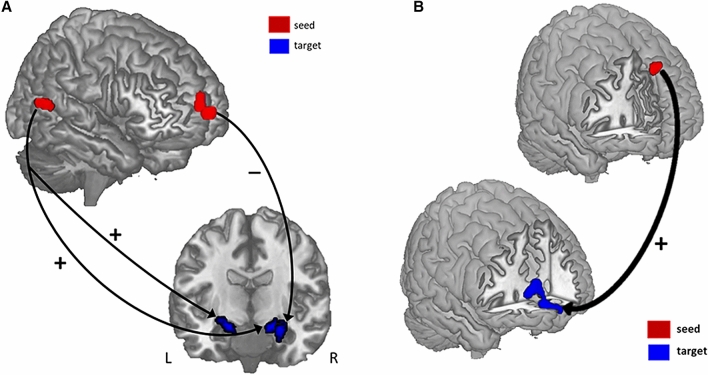
Seed-based SC revealed that pSTS rGMV covaried positively with right and left amygdala rGMV (16.5, − 4.5, − 15 and − 22.5, − 12, − 0.5, respectively), while vmPFC rGMV covaried negatively with right amygdala rGMV (27, − 6, − 15; see Fig. 4A). rGMV in the dmPFC seed covaried positively with rGMV in vmPFC (3, 63, − 7.5; see Fig. 4B).
Figure 4.
Structural covariance results. (A) rGMV in the vmPFC and pSTS seed regions covaried with rGMV in the amygdala. vmPFC and amygdala rGMV were negatively correlated, while pSTS and amygdala rGMV were positively correlated. (B) rGMV in the dmPFC seed region covaried positively with rGMV in the vmPFC.
Discussion
We report a set of focal brain regions in which regional grey matter volume (rGMV) is associated with individual differences in dispositional expectancies of social reward or threat. The results extend previous functional studies revealing that individual differences in future-oriented emotions are underpinned by a network centred on vmPFC62, and additionally point to involvement of other key structures including dmPFC, pSTS and SRC, that have been argued to be important ‘hub’ or ‘nexus’ regions within networks supporting social cognition and interoception, respectively. Further, seed-based structural covariance analyses, showing that vmPFC and pSTS volumes covaried (negatively vs. positively) with those of the amygdala, suggest that these regions may functionally interact with broader networks anchored in the amygdala thought to support unique dispositions for fostering and maintaining social relationships63.
Our novel scenario-based measure generated considerable individual differences in both reward and threat expectancies for the imagined social event. Social reward expectancies (SRE) were significantly higher than social threat expectancies (STE). This finding is robust (n > 1,30040) and in line with previous research showing that healthy young adults typically anticipate social acceptance and positive social evaluation from novel interpersonal interactions (e.g.36,64,65) as part of a more general optimistic view of their personal future30. This optimism bias is considered to be adaptive33, beneficial for physical health and vital for mental health30. SRE were largely independent of STE, although the two were modestly inversely correlated (see also38,39,66).
Several prominent models posit that two neurobehavioral systems underlie individual differences in affect and motivation7,9. Prospection is at the heart of these models. The appetitive (or approach) system underlies reward pursuit, in part by generating anticipatory and/or anticipated positive emotions. The aversive system underlies anxiety, vigilance, and withdrawal (behavioural inhibition) at the prospect of threat. Our findings align with these models and are broadly consistent with other research showing that social approach and avoidance motives—characterized as the ‘hope for affiliation’ and ‘fear of rejection’ respectively—are distinct dispositions10,67. Further, our work and others’ indicates that positive and negative future-related cognitions are best conceived as separate dimensions of experience, differentially associated with anhedonia and anxiety, respectively30.
VBM findings—correlations with social reward expectancies
Previous research shows that the anticipated pleasure from imagined social interactions correlates with the vividness of imagined people and places in such scenes68, and complementary, that the spatio-temporal clarity of imagined events is greater for events evoking anticipated positive versus negative affect69. Further, dispositional optimism is associated with the tendency to vividly imagine positive events in one's future (e.g.70.); whereas anhedonia is associated with reduced capacity to simulate detailed future positive events (e.g.71,72.) as well as reduced accessibility of such images30. Positive episodic expectancy (‘anticipatory savouring’) in summary requires a vivid, contextually detailed mental representation of future reward.
Here, overlap analysis revealed that rGMVs in vmPFC, parahippocampal cortex (PHC) and ventral anterior temporal lobe (vATL) were positively correlated with social reward expectancies and negatively correlated with social threat expectancies. These are all regions of the core remembering-imagining network underpinning MTT (e.g.32). Consistent with our VBM results, these regions are more activated during the simulation of positive versus negative events73. As part of this network, vmPFC tracks the anticipated positive affective quality of future scenarios62,74–76, consistent with a broader role in subjective valuation77.
vmPFC tracks subjective value of imagined events as a function of one’s needs78 and chronic goals79 and is sensitive to individuals’ optimism bias in their expectancies about the hedonic rewards (or other benefits) that the participant hopes to obtain from such events79. For example, the level of vmPFC activity when imagining positive vs. negative future scenarios is positively correlated with trait optimism76. Of particular relevance to our findings, vmPFC activity to anticipated social feedback is enhanced when participants have positive expectancies about social outcomes37,80,81 (see also73).
Functionally, vmPFC interacts with PHC and vATL to produce structured positively-valenced mental representations replete with detailed spatiotemporal context and rich (personal) semantic and sensory details62. Our findings that rGMV not only in vmPFC, but also PHC and ATL, is higher in individuals with higher social reward expectancies is congruent with the behavioural work cited above, linking the vividness of future simulations to their reward value, and further relates to the finding of reduced engagement of these regions during prospection in patients with depression and anhedonia82.
Positively biased simulations are partly grounded in biased encoding, consolidation and/or retrieval of autobiographical memories32,83. Speer et al.84 found increased dmPFC activity linked to recall of positive autobiographical memories (‘savouring’). Our finding of greater dmPFC rGMV in people with more positive expectancies further corroborates the neural entwining of autobiographical expectancies and memories32. Our finding of positive structural covariance between dmPFC and vmPFC—which potentially reflects long term increased functional connectivity43,60—may be because the social context inherent in positive mental constructions enhances their value62,68. It is also possible that the reward value of a simulated event may motivate the degree to which participants engage in mentalizing processes subserved by dmPFC62.
rGMV in vmPFC, PHC, and vATL were also correlated with lower STE. Reduced vividness of positive future thinking is characteristic of anxious as well as depressed individuals, in addition to anxious expectancies about future social interactions unique to anxiety72. Social anxiety can be regarded as a position along a continuum ranging from a lack of anxiety, to mild shyness and then social anxiety disorder (SAD)15,16,85, so our findings can meaningfully be compared with studies of SAD, which show reduced vmPFC volume86,87.
The correlation of vmPFC rGMV with lower STE and greater SRE concurs with the well-established role of vmPFC in emotion regulation. A large-scale neuroimaging meta-analysis of affect regulation across 3 distinct domains (fear extinction, placebo effects, cognitive reappraisal) identified vmPFC activation as the only ‘common neural regulator’ dampening current and anticipated negative affect27.
These results support the hypothesis that vmPFC plays a ubiquitous role in dampening current and anticipated negative affect27. Our data extend previous work by indicating that the minimisation of STE—and/or the maintenance low threat expectancies—may be implemented in the brain by similar means as the reduction of fear or negative affect in other emotion regulation scenarios, possibly by self-generating positive emotion in negative situations88.
In healthy adults, successful down-regulation of negative affect is consistently associated not only with increased BOLD activity in the vmPFC, but also with concordant reduction of activity in the amygdala27. Further, vmPFC damage engenders disinhibited activity of the amygdala and, consequently, elevated levels of negative affect89. Our structural covariance findings add further convergent evidence of the regulatory link between these regions by demonstrating a negative correlation between amygdala rGMV and vmPFC rGMV. This negative coupling may reflect the finding that during typical development, amygdala-vmPFC functional connectivity becomes more strongly negative, and in adults, such negative coupling is linked to both reduced amygdala reactivity to social threat and lower anxiety90.
VBM findings—correlations with social threat expectancies
There were several unique rGMV correlates of individual differences in STE. Heightened threat expectancies (fears of potential embarrassment and social rejection) were associated with increased rGMV in right pSTS, alongside decreased rGMV in somatosensory-related cortex (SRC) and lateral occipital cortex (OCC).
Cognitive theories posit that heightened social anxiety results from biased information processing91. Alongside regulatory deficits, a processing style marked by hypervigilance and an attentional bias to the social environment for signals of social evaluation is considered a causal and maintaining factor in social anxiety91.
Our results are in line with studies suggesting that pSTS serves as an interface between perception of social information and social cognition92. pSTS plays a role in analysing socially relevant perceptual information (eye gaze, tone of voice, facial and bodily threat signals), evaluating its implications and orienting and sustaining attention accordingly, in line with the individual’s present affective state and social goals93. pSTS rGMV is increased in SAD and shyness (e.g.87,94), and increased pSTS activity to social perceptual cues (eye gaze etc.) has been consistently demonstrated in individuals who are social inhibited, shy, and socially anxious95–98. Further, resting amygdala–pSTS functional connectivity has been linked to biased social attention and perception in social anxiety92,99. Collectively, this work suggests that chronic hypervigilance for threat may result from, or result in, increased rGMV in right pSTS. Increased expectancies of threat when anticipating future situations may be fundamentally underpinned by these attentional biases91,100,101.
Heightened attention to threat may lead to enhanced encoding, elaboration, consolidation and retrieval of negatively biased memories100,102, resulting in an increased tendency to construct negatively biased expectancies100. Further, increased internal attention to threat may maintain attention to negatively constructed future simulations in spontaneous thought, leading to heightened subjective expectancies of their occurrence and increased anticipatory worry100,103. In turn, this may lead to repercussive effects with increased expectancies further increasing biased attention101.
Our findings thus support “combined cognitive bias” models of anxiety91 as we show that the neural structures underpinning attentional biases also underpin prospective ones. Other research has found that pSTS activity is related to remembering and imagining socially threatening situations24; and is increased during such simulations in individuals with SAD104.
Surprisingly, we did not find that amygdala volume directly correlates with individuals’ STE, despite its established role in threat processing, including anticipation of social evaluation105 and a proposed role in mediating temperamental shyness23,106. However, we did find positive structural co-variation of pSTS with amygdala (alongside, as discussed above, negative structural covariance of vmPFC and amygdala) consistent with bidirectional anatomical connectivity between pSTS and amygdala107. The reasons for this null result are unclear and may reflect type II (false negative) error. Alternatively, the influence of the amygdala may primarily be modulatory, influencing structural development in connected cortical regions90.
We also found reduced rGMV in left lateral OCC, a region that, together with fusiform gyrus, pSTS and amygdala, forms a face perception network108. This may link to fMRI work showing increased pSTS activity to face emotion, but decreased OCC activity (alongside poor face identity recognition) in socially inhibited individuals109,110.
Somatosensory-related cortex (SRC), in contrast plays a key role in both interoception111 and social aversion63. Our finding of greater SRC rGMV associated with lower STE thus align closely with findings that individuals with reduced interoceptive sensitivity report significantly greater uncertainty and worry in anticipation of public speaking112. Increased uncertainty in social situations may arise not just because of reduced ability to represent/regulate one’s own interoceptive signals, but also because SRC plays a role in automatic affective empathy via simulation of others’ bodily states. Personal distress (a dysfunctional form of empathy linked with maladaptive emotion regulation and social avoidance) has been shown to be linked to lower rGMV in SRC113.
Together, the rGMV correlates of STE we find concur with cognitive models of anxiety91, which contend that socially anxious persons simultaneously exhibit altered processing of internal (distress) cues and external stimuli potentially indicative of negative social evaluation.
Limitations
There are some limitations that should be considered when interpreting our results.
Our study was cross-sectional and so cannot determine whether the relationships between rGMV, SRE and STE arise over time through experience-dependent brain plasticity, or alternatively whether individuals with a specific brain structure are predisposed to acquire different expectancies13. Most likely, our findings reflect complex brain-body-environment interactions over development114,115. In future, longitudinal or training studies could address this.
The cellular basis of rGMV differences identified by VBM is still poorly understood60. Any tissue property (e.g. cell density, cell size, myelination) that affects relaxation times, and hence voxel images on T1-weighted MRI, will influence VBM measures.
Finally, the generalizability of our results is unknown. We deliberately chose to study a population of university students, because of the ecological relevance of joining new social groups1. Additionally, participants imagined just one scenario. The scenario was designed, however, to be both sufficiently specific to allow episodic simulation whilst sufficiently generic, such that generalized expectancies (e.g. beliefs, schemas) could be tapped. Previous studies (e.g.66), reassuringly, suggest a marked degree of consistency across social situations in reward/threat expectancies.
Conclusions
We found that inter-individual differences in future-oriented thinking in the social domain are reflected in brain macrostructure. In particular, the extent to which individuals hold optimistic vs. pessimistic expectancies for the hedonic outcomes of an imagined social interaction is reflected in rGMV of key brain regions, most notably vmPFC. Our findings concur with the suggestion that vmPFC may integrate various sources of information to conceive the meaning of events for one’s well-being and future prospects25. Our results may reflect a neural embedding of such self-related affective valuation, perhaps accounting for the link between vmPFC macrostructure and adaptive social functioning and well-being28.
Acknowledgements
This work was funded by an ESRC PhD studentship and Wellcome Trust Institutional Strategic Support Fund Reconnect with Science Fellowship to B.C; by a Wellcome Trust Institutional Strategic Support Fund Cross-disciplinary award to A.D.L and N.M.; by a Wellcome Strategic Award ((104943/Z/14/Z) to A.D.L.; and the Welsh Government (via the Wales Institute of Cognitive Neuroscience). We thank our participants, CUBRIC core staff for scanning support, and Prof. Yu-Chen Chan for advice on creating the structural covariance figures.
Author contributions
B.C., G.M. and A.D.L. developed the study concept. B.C. and A.D.L. designed the study and wrote the paper, B.C. and N.M. collected the data, B.C. analysed the data with help from N.M. and A.D.L. N.M., G.M. and A.D.L. provided critical feedback on manuscript drafts and all authors approved the final version of manuscript submission.
Data availability
The LODESTARS is available to download from https://osf.io/hq5sg/. All unthresholded SPMs produced in these analyses are freely available on Neurovault (https://neurovault.org/collections/5897/). Ethical approval conditions do not permit public sharing of raw MRI data as participants have not provided consent for this.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Stadtfeld C, Vörös A, Elmer T, Boda Z, Raabe IJ. Integration in emerging social networks explains academic failure and success. Proc. Natl. Acad. Sci. 2019;116(3):792–797. doi: 10.1073/pnas.1811388115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leary MR. Affiliation, acceptance, and belonging: The pursuit of interpersonal connection. In: Fiske ST, Gilbert DT, Lindzey G, editors. Handbook of Social Psychology. 2. Hoboken: Wiley; 2010. pp. 864–897. [Google Scholar]
- 3.Dickerson SS. Emotional and physiological responses to social-evaluative threat. Soc. Personal. Psychol. Compass. 2008;2(3):1362–1378. doi: 10.1111/j.1751-9004.2008.00095.x. [DOI] [Google Scholar]
- 4.Eisenberger NI, Cole SW. Social neuroscience and health: neurophysiological mechanisms linking social ties with physical health. Nat. Neurosci. 2012;15(5):669–674. doi: 10.1038/nn.3086. [DOI] [PubMed] [Google Scholar]
- 5.Carver CS. Two distinct bases of inhibition of behaviour: Viewing biological phenomena through the lens of psychological theory. Eur. J. Personal. 2008;22(5):388–390. [Google Scholar]
- 6.Gray JA, McNaughton N. The Neuropsychology of Anxiety: An Enquiry into the Functions of the Septo-Hippocampal System. Oxford: Oxford University Press; 2000. [Google Scholar]
- 7.Corr PJJA. Gray’s reinforcement sensitivity theory and frustrative nonreward: a theoretical note on expectancies in reactions to rewarding stimuli. Personal. Individ. Differ. 2002;32(7):1247–1253. doi: 10.1016/S0191-8869(01)00115-5. [DOI] [Google Scholar]
- 8.Vrtička P. Interpersonal closeness and social reward processing. J. Neurosci. 2012;32(37):12649–12650. doi: 10.1523/JNEUROSCI.3157-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gable SL. Balancing rewards and cost in relationships: an approach-avoidance motivational perspective. In: Elliot AJ, editor. Advances in Motivation Science, pp 1–32. Cambridge: Academic Press; 2015. pp. 1–32. [Google Scholar]
- 10.Nikitin J, Schoch S. Social approach and avoidance motivations. In: Coplan RJ, Bowker JC, editors. The Handbook of Solitude, pp 202–223. Hoboken: Wiley; 2013. pp. 202–223. [Google Scholar]
- 11.Nikitin J, Burgermeister LC, Freund AM. The role of age and social motivation in developmental transitions in young and old adulthood. Front. Psychol. 2012;3:1–14. doi: 10.3389/fpsyg.2012.00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foulkes L, Blakemore S-J. Is there heightened sensitivity to social reward in adolescence? Curr. Opin. Neurobiol. 2016;40:81–85. doi: 10.1016/j.conb.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 13.Orben A, Tomova L, Blakemore S-J. The effects of social deprivation on adolescent development and mental health. Lancet Child Adolesc. Health. 2020;4(8):634–640. doi: 10.1016/S2352-4642(20)30186-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holt-Lunstad J. Why social relationships are important for physical health: A systems approach to understanding and modifying risk and protection. Annu. Rev. Psychol. 2018;69(1):437–458. doi: 10.1146/annurev-psych-122216-011902. [DOI] [PubMed] [Google Scholar]
- 15.Miskovic V, Schmidt LA. Social fearfulness in the human brain. Neurosci. Biobehav. Rev. 2012;36(1):459–478. doi: 10.1016/j.neubiorev.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Barkus E, Badcock JC. A transdiagnostic perspective on social anhedonia. Front. Psychiatry. 2019;10:1–15. doi: 10.3389/fpsyt.2019.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Redcay E, Schilbach L. Using second-person neuroscience to elucidate the mechanisms of social interaction. Nat. Rev. Neurosci. 2019;20(8):495–505. doi: 10.1038/s41583-019-0179-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Redcay E, Warnell KR. A social-interactive neuroscience approach to understanding the developing brain. Adv. Child Dev. Behav. 2018;54:1–44. doi: 10.1016/bs.acdb.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Silk JS, Davis S, McMakin DL, Dahl RE, Forbes EE. Why do anxious children become depressed teenagers? The role of social evaluative threat and reward processing. Psychol. Med. 2012;42(10):2095–2107. doi: 10.1017/S0033291712000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calder AJ, Lawrence AD, Young AW. Neuropsychology of fear and loathing. Nat. Rev. Neurosci. 2001;2(5):352–363. doi: 10.1038/35072584. [DOI] [PubMed] [Google Scholar]
- 21.Machado-de-Sousa JP, et al. Increased amygdalar and hippocampal volumes in young adults with social anxiety. PLoS ONE. 2014;9(2):e88523. doi: 10.1371/journal.pone.0088523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van der Plas EAA, Boes AD, Wemmie JA, Tranel D, Nopoulos P. Amygdala volume correlates positively with fearfulness in normal healthy girls. Soc. Cogn. Affect. Neurosci. 2010;5(4):424–431. doi: 10.1093/scan/nsq009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyahara M, Harada T, Ruffman T, Sadato N, Iidaka T. Functional connectivity between amygdala and facial regions involved in recognition of facial threat. Soc. Cogn. Affect. Neurosci. 2013;8(2):181–189. doi: 10.1093/scan/nsr085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson-Mendenhall CD, Barrett LF, Barsalou LW. Situating emotional experience. Front. Hum. Neurosci. 2013;7(Article 764):1–16. doi: 10.3389/fnhum.2013.00764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roy M, Shohamy D, Wager TD. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends Cogn. Sci. 2012;16(3):147–156. doi: 10.1016/j.tics.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burklund LJ, Eisenberger NI, Lieberman MD. The face of rejection: Rejection sensitivity moderates dorsal anterior cingulate activity to disapproving facial expressions. Soc. Neurosci. 2007;2(3–4):238–253. doi: 10.1080/17470910701391711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diekhof EK, Geier K, Falkai P, Gruber O. Fear is only as deep as the mind allows: A coordinate-based meta-analysis of neuroimaging studies on the regulation of negative affect. NeuroImage. 2011;58(1):275–285. doi: 10.1016/j.neuroimage.2011.05.073. [DOI] [PubMed] [Google Scholar]
- 28.Holmes AJ, et al. Individual differences in amygdala-medial prefrontal anatomy link negative affect, impaired social functioning, and polygenic depression risk. J. Neurosci. 2012;32(50):18087–18100. doi: 10.1523/JNEUROSCI.2531-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hermann A, Bieber A, Keck T, Vaitl D, Stark R. Brain structural basis of cognitive reappraisal and expressive suppression. Soc. Cogn. Affect. Neurosci. 2014;9(9):1435–1442. doi: 10.1093/scan/nst130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacLeod AK. Prospection, Well-being, and Mental Health. Oxford: Oxford University Press; 2017. [Google Scholar]
- 31.MacLeod AK. Affect, emotional disorder, and future-directed thinking. Cogn. Emot. 1996;10(1):69–86. doi: 10.1080/026999396380394. [DOI] [Google Scholar]
- 32.Schacter DL, Benoit RG, Szpunar KK. Episodic future thinking: mechanisms and functions. Curr. Opin. Behav. Sci. 2017;17:41–50. doi: 10.1016/j.cobeha.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miloyan B, Suddendorf T. Feelings of the future. Trends Cogn. Sci. 2015;19(4):196–200. doi: 10.1016/j.tics.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 34.Gilbert DT, Wilson TD. Why the brain talks to itself: sources of error in emotional prediction. Philos. Trans. R. Soc. B Biol. Sci. 2009;364(1521):1335–1341. doi: 10.1098/rstb.2008.0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams AN, et al. The role of the pre-commissural fornix in episodic autobiographical memory and simulation. Neuropsychologia. 2020;142:107457. doi: 10.1016/j.neuropsychologia.2020.107457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van der Molen MJW, et al. Fear of negative evaluation modulates electrocortical and behavioral responses when anticipating social evaluative feedback. Front. Hum. Neurosci. 2014;7(Article 936):1–12. doi: 10.3389/fnhum.2013.00936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gunther Moor B, van Leijenhorst L, Rombouts SARB, Crone EA, Van der Molen MW. Do you like me? Neural correlates of social evaluation and developmental trajectories. Soc. Neurosci. 2010;5(5–6):461–482. doi: 10.1080/17470910903526155. [DOI] [PubMed] [Google Scholar]
- 38.Spielmann SS, Macdonald G, Tackett JL. Social threat, social reward, and regulation of investment in romantic relationships. Pers. Relatsh. 2011 doi: 10.1111/j.1475-6811.2011.01377.x. [DOI] [Google Scholar]
- 39.Spielmann SS, Maxwell JA, Macdonald G, Baratta PL. Don’t get your hopes up: avoidantly attached individuals perceive lower social reward when there is potential for intimacy. Pers. Soc. Psychol. Bull. 2013;39(2):219–236. doi: 10.1177/0146167212472541. [DOI] [PubMed] [Google Scholar]
- 40.Crawford, B. & Lawrence, A. D. (unpublished data).
- 41.Crawford B. Social reward and threat processing. Cardiff: Cardiff University; 2015. [Google Scholar]
- 42.Kiesow H, et al. 10,000 social brains: Sex differentiation in human brain anatomy. Sci. Adv. 2020;6(12):eaaz1170. doi: 10.1126/sciadv.aaz1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clos M, Rottschy C, Laird AR, Fox PT, Eickhoff SB. Comparison of structural covariance with functional connectivity approaches exemplified by an investigation of the left anterior insula. NeuroImage. 2014;99:269–280. doi: 10.1016/j.neuroimage.2014.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murray EA, Rudebeck PH. Specializations for reward-guided decision-making in the primate ventral prefrontal cortex. Nat. Rev. Neurosci. 2018;19(7):404–417. doi: 10.1038/s41583-018-0013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Faul F, Erdfelder E, Buchner A, Lang A-G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods. 2009;41(4):1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- 46.Kube T, et al. Integrating situation-specific dysfunctional expectations and dispositional optimism into the cognitive model of depression: a path-analytic approach. J. Affect. Disord. 2018;229:199–205. doi: 10.1016/j.jad.2017.12.082. [DOI] [PubMed] [Google Scholar]
- 47.Cao X, Madore KP, Wang D, Schacter DL. Remembering the past and imagining the future: attachment effects on production of episodic details in close relationships. Memory. 2018;26(8):1140–1150. doi: 10.1080/09658211.2018.1434800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berna C, Lang TJ, Goodwin GM, Holmes EA. Developing a measure of interpretation bias for depressed mood: an ambiguous scenarios test. Personal. Individ. Differ. 2011;51(3):349–354. doi: 10.1016/j.paid.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weems CF, Watts SE. Cognitive models of childhood anxiety. In: Velotis CM, editor. Anxiety Disorder Research. Hauppauge: Nova Science Publishers; 2005. pp. 205–232. [Google Scholar]
- 50.Robinson MD, Clore GL. Belief and feeling: evidence for an accessibility model of emotional self-report. Psychol. Bull. 2002;128(6):934–960. doi: 10.1037/0033-2909.128.6.934. [DOI] [PubMed] [Google Scholar]
- 51.D’Argembeau A, Lardi C, der Linden MV. Self-defining future projections: Exploring the identity function of thinking about the future. Memory. 2012;20(2):110–120. doi: 10.1080/09658211.2011.647697. [DOI] [PubMed] [Google Scholar]
- 52.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 53.Honea R, Crow TJ, Passingham D, Mackay CE. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am. J. Psychiatry. 2005;162(12):2233–2245. doi: 10.1176/appi.ajp.162.12.2233. [DOI] [PubMed] [Google Scholar]
- 54.Barnes J, et al. Head size, age and gender adjustment in MRI studies: a necessary nuisance? NeuroImage. 2010;53(4):1244–1255. doi: 10.1016/j.neuroimage.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 55.Ashburner J. VBM Tutorial. London: The Wellcome Centre for Human Neuroimaging, UCL; 2010. [Google Scholar]
- 56.Bartra O, McGuire JT, Kable JW. The valuation system: A coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. NeuroImage. 2013;76:412–427. doi: 10.1016/j.neuroimage.2013.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mumford JA, Poline J-B, Poldrack RA. Orthogonalization of regressors in fMRI models. PLoS ONE. 2015;10(4):e0126255. doi: 10.1371/journal.pone.0126255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tyszka JM, Pauli WM. In vivo delineation of subdivisions of the human amygdaloid complex in a high-resolution group template. Hum. Brain Mapp. 2016;37(11):3979–3998. doi: 10.1002/hbm.23289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Neuromorphometrics, Inc. | Building a Model of the Living Human Brain. https://www.neuromorphometrics.com/.
- 60.Alexander-Bloch A, Giedd JN, Bullmore E. Imaging structural co-variance between human brain regions. Nat. Rev. Neurosci. 2013;14(5):322–336. doi: 10.1038/nrn3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage. 2009;44(1):83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 62.Benoit RG, Szpunar KK, Schacter DL. Ventromedial prefrontal cortex supports affective future simulation by integrating distributed knowledge. Proc. Natl. Acad. Sci. 2014;111(46):16550–16555. doi: 10.1073/pnas.1419274111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bickart KC, Dickerson BC, Feldman Barrett L. The amygdala as a hub in brain networks that support social life. Neuropsychologia. 2014;63:235–248. doi: 10.1016/j.neuropsychologia.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Duffy KA, Helzer EG, Hoyle RH, Helzer JF, Chartrand TL. Pessimistic expectations and poorer experiences: The role of (low) extraversion in anticipated and experienced enjoyment of social interaction. PLoS ONE. 2018;13(7):e0199146. doi: 10.1371/journal.pone.0199146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hepper EG, Hart CM, Gregg AP, Sedikides C. Motivated expectations of positive feedback in social interactions. J. Soc. Psychol. 2011;151(4):455–477. doi: 10.1080/00224545.2010.503722. [DOI] [PubMed] [Google Scholar]
- 66.Glass CR, Merluzzi TV, Biever JL, Larsen KH. Cognitive assessment of social anxiety: Development and validation of a self-statement questionnaire. Cogn. Ther. Res. 1982;6(1):37–55. doi: 10.1007/BF01185725. [DOI] [Google Scholar]
- 67.Gable SL, Gosnell CL. Approach and avoidance behavior in interpersonal relationships. Emot. Rev. 2013;5(3):269–274. doi: 10.1177/1754073913477513. [DOI] [Google Scholar]
- 68.Zagacki KS, Edwards R, Honeycutt JM. The role of mental imagery and emotion in imagined interaction. Commun. Q. 1992;40(1):56–68. doi: 10.1080/01463379209369820. [DOI] [Google Scholar]
- 69.de Vito S, Neroni MA, Gamboz N, Della Sala S, Brandimonte MA. Desirable and undesirable future thoughts call for different scene construction processes. Q. J. Exp. Psychol. 2015;68(1):75–82. doi: 10.1080/17470218.2014.937448. [DOI] [PubMed] [Google Scholar]
- 70.Beaty RE, Seli P, Schacter DL. Thinking about the past and future in daily life: an experience sampling study of individual differences in mental time travel. Psychol. Res. 2019;83(4):805–816. doi: 10.1007/s00426-018-1075-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hallford DJ, et al. Impairments in episodic future thinking for positive events and anticipatory pleasure in major depression. J. Affect. Disord. 2020;260:536–543. doi: 10.1016/j.jad.2019.09.039. [DOI] [PubMed] [Google Scholar]
- 72.Morina N, Deeprose C, Pusowski C, Schmid M, Holmes EA. Prospective mental imagery in patients with major depressive disorder or anxiety disorders. J. Anxiety Disord. 2011;25(8):1032–1037. doi: 10.1016/j.janxdis.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.D’Argembeau A, Xue G, Lu Z-L, Van der Linden M, Bechara A. Neural correlates of envisioning emotional events in the near and far future. NeuroImage. 2008;40(1):398–407. doi: 10.1016/j.neuroimage.2007.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bray S, Shimojo S, O’Doherty JP. Human medial orbitofrontal cortex is recruited during experience of imagined and real rewards. J. Neurophysiol. 2010;103(5):2506–2512. doi: 10.1152/jn.01030.2009. [DOI] [PubMed] [Google Scholar]
- 75.Murphy SE, et al. Increased rostral anterior cingulate activity following positive mental imagery training in healthy older adults. Soc. Cogn. Affect. Neurosci. 2017;12(12):1950–1958. doi: 10.1093/scan/nsx120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sharot T, Riccardi AM, Raio CM, Phelps EA. Neural mechanisms mediating optimism bias. Nature. 2007;450(7166):102–105. doi: 10.1038/nature06280. [DOI] [PubMed] [Google Scholar]
- 77.Peters J, Büchel C. Neural representations of subjective reward value. Behav. Brain Res. 2010;213(2):135–141. doi: 10.1016/j.bbr.2010.04.031. [DOI] [PubMed] [Google Scholar]
- 78.Lin W-J, Horner AJ, Bisby JA, Burgess N. Medial prefrontal cortex: adding value to imagined scenarios. J. Cogn. Neurosci. 2015 doi: 10.1162/jocn_a_00836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.D’Argembeau A. On the role of the ventromedial prefrontal cortex in self-processing: The valuation hypothesis. Front. Hum. Neurosci. 2013;7(Article 372):1–13. doi: 10.3389/fnhum.2013.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jones RM, et al. Adolescent-specific patterns of behavior and neural activity during social reinforcement learning. Cogn. Affect. Behav. Neurosci. 2014;14(2):683–697. doi: 10.3758/s13415-014-0257-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Scholz C, et al. A neural model of valuation and information virality. Proc. Natl. Acad. Sci. 2017;114(11):2881–2886. doi: 10.1073/pnas.1615259114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hach S, Tippett LJ, Addis DR. Neural changes associated with the generation of specific past and future events in depression. Neuropsychologia. 2014;65:41–55. doi: 10.1016/j.neuropsychologia.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 83.Painter JM, Kring AM. Back to the future: Similarities and differences in emotional memories and prospections. Appl. Cogn. Psychol. 2015;29(2):271–279. doi: 10.1002/acp.3105. [DOI] [Google Scholar]
- 84.Speer ME, Bhanji JP, Delgado MR. Savoring the past: Positive memories evoke value representations in the striatum. Neuron. 2014;84(4):847–856. doi: 10.1016/j.neuron.2014.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Knappe S, Beesdo K, Fehm L, Lieb R, Wittchen H-U. Associations of familial risk factors with social fears and social phobia: evidence for the continuum hypothesis in social anxiety disorder? J. Neural Trans. 2009;116(6):639–648. doi: 10.1007/s00702-008-0118-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cha J, et al. Circuit-wide structural and functional measures predict ventromedial prefrontal cortex fear generalization: Implications for generalized anxiety disorder. J. Neurosci. 2014;34(11):4043–4053. doi: 10.1523/JNEUROSCI.3372-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang X, Cheng B, Luo Q, Qiu L, Wang S. Gray matter structural alterations in social anxiety disorder: a voxel-based meta-analysis. Front. Psychiatry. 2018;9:449. doi: 10.3389/fpsyt.2018.00449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nawa NE, Ando H. Effective connectivity within the ventromedial prefrontal cortex-hippocampus-amygdala network during the elaboration of emotional autobiographical memories. NeuroImage. 2019;189:316–328. doi: 10.1016/j.neuroimage.2019.01.042. [DOI] [PubMed] [Google Scholar]
- 89.Motzkin JC, Philippi CL, Wolf RC, Baskaya MK, Koenigs M. Ventromedial prefrontal cortex is critical for the regulation of amygdala activity in humans. Biol. Psychiatry. 2015;77(3):276–284. doi: 10.1016/j.biopsych.2014.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tottenham N, Gabard-Durnam LJ. The developing amygdala: a student of the world and a teacher of the cortex. Curr. Opin. Psychol. 2017;17:55–60. doi: 10.1016/j.copsyc.2017.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wong QJJ, Rapee RM. The aetiology and maintenance of social anxiety disorder: A synthesis of complimentary theoretical models and formulation of a new integrated model. J. Affect. Disord. 2016;203:84–100. doi: 10.1016/j.jad.2016.05.069. [DOI] [PubMed] [Google Scholar]
- 92.Kreifelts B, et al. Tuned to voices and faces: Cerebral responses linked to social anxiety. NeuroImage. 2019;197:450–456. doi: 10.1016/j.neuroimage.2019.05.018. [DOI] [PubMed] [Google Scholar]
- 93.Patel GH, Sestieri C, Corbetta M. The evolution of the temporoparietal junction and posterior superior temporal sulcus. Cortex. J. Devoted Study Nerv. Syst. Behav. 2019;118:38–50. doi: 10.1016/j.cortex.2019.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yang X, et al. Structural and functional connectivity changes in the brain associated with shyness but not with social anxiety. PLoS ONE. 2013;8(5: e63151):1–13. doi: 10.1371/journal.pone.0063151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Frick A, Howner K, Fischer H, Kristiansson M, Furmark T. Altered fusiform connectivity during processing of fearful faces in social anxiety disorder. Transl. Psychiatry. 2013;3(10):e312–e312. doi: 10.1038/tp.2013.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kret ME, Denollet J, Grèzes J, de Gelder B. The role of negative affectivity and social inhibition in perceiving social threat: an fMRI study. Neuropsychologia. 2011;49(5):1187–1193. doi: 10.1016/j.neuropsychologia.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 97.Gentili C, et al. Beyond emotions: A meta-analysis of neural response within face processing system in social anxiety. Exp. Biol. Med. Maywood NJ. 2016;241(3):225–237. doi: 10.1177/1535370215603514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Evans TC, Bar-Haim Y, Fox NA, Pine DS, Britton JC. Neural mechanisms underlying heterogeneous expression of threat-related attention in social anxiety. Behav. Res. Ther. 2020;132(Article 103657):1–13. doi: 10.1016/j.brat.2020.103657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kreifelts B, et al. Cerebral resting state markers of biased perception in social anxiety. Brain Struct. Funct. 2019;224(2):759–777. doi: 10.1007/s00429-018-1803-1. [DOI] [PubMed] [Google Scholar]
- 100.Miloyan B, Pachana NA, Suddendorf T. The future is here: a review of foresight systems in anxiety and depression. Cognit. Emot. 2014;28(5):795–810. doi: 10.1080/02699931.2013.863179. [DOI] [PubMed] [Google Scholar]
- 101.Aue T, Okon-Singer H. Expectancy biases in fear and anxiety and their link to biases in attention. Clin. Psychol. Rev. 2015;42:83–95. doi: 10.1016/j.cpr.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 102.Krans J, de Bree J, Bryant RA. Autobiographical memory bias in social anxiety. Memory. 2014;22(8):890–897. doi: 10.1080/09658211.2013.844261. [DOI] [PubMed] [Google Scholar]
- 103.Szpunar KK, Schacter DL. Get real: Effects of repeated simulation and emotion on the perceived plausibility of future experiences. J. Exp. Psychol. Gen. 2013;142(2):323–327. doi: 10.1037/a0028877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bas-Hoogendam JM, van Steenbergen H, Tissier RLM, van der Wee NJA, Westenberg PM. Altered neurobiological processing of unintentional social norm violations: A multiplex, multigenerational functional magnetic resonance imaging study on social anxiety endophenotypes. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2019 doi: 10.1016/j.bpsc.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 105.Guyer AE, et al. Amygdala and ventrolateral prefrontal cortex function during anticipated peer evaluation in pediatric social anxiety. Arch. Gen. Psychiatry. 2008;65(11):1303–1312. doi: 10.1001/archpsyc.65.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Clauss JA, et al. Structural and functional bases of inhibited temperament. Soc. Cogn. Affect. Neurosci. 2014;9(12):2049–2058. doi: 10.1093/scan/nsu019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Aggleton JP. The contribution of the amygdala to normal and abnormal emotional states. Trends Neurosci. 1993;16(8):328–333. doi: 10.1016/0166-2236(93)90110-8. [DOI] [PubMed] [Google Scholar]
- 108.Behrmann M, Scherf KS, Avidan G. Neural mechanisms of face perception, their emergence over development, and their breakdown. WIREs Cogn. Sci. 2016;7(4):247–263. doi: 10.1002/wcs.1388. [DOI] [PubMed] [Google Scholar]
- 109.Avery SN, VanDerKlok RM, Heckers S, Blackford JU. Impaired face recognition is associated with social inhibition. Psychiatry Res. 2016;236:53–57. doi: 10.1016/j.psychres.2015.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gentili C, et al. Differential modulation of neural activity throughout the distributed neural system for face perception in patients with social phobia and healthy subjects. Brain Res. Bull. 2008;77(5):286–292. doi: 10.1016/j.brainresbull.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 111.Khalsa SS, Rudrauf D, Feinstein JS, Tranel D. The pathways of interoceptive awareness. Nat. Neurosci. 2009;12(12):1494–1496. doi: 10.1038/nn.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Werner NS, Duschek S, Mattern M, Schandry R. Interoceptive sensitivity modulates anxiety during public speaking. J. Psychophysiol. 2009;23(2):85–94. doi: 10.1027/0269-8803.23.2.85. [DOI] [Google Scholar]
- 113.Banissy MJ, Kanai R, Walsh V, Rees G. Inter-individual differences in empathy are reflected in human brain structure. NeuroImage. 2012;62(3):2034–2039. doi: 10.1016/j.neuroimage.2012.05.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bzdok D, Dunbar RIM. The neurobiology of social distance. Trends Cogn. Sci. 2020;24:717–733. doi: 10.1016/j.tics.2020.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sjåstad H, Zhang M, Masvie AE, Baumeister R. Social exclusion reduces happiness by creating expectations of future rejection. Self Identity. 2020 doi: 10.1080/15298868.2020.1779119. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The LODESTARS is available to download from https://osf.io/hq5sg/. All unthresholded SPMs produced in these analyses are freely available on Neurovault (https://neurovault.org/collections/5897/). Ethical approval conditions do not permit public sharing of raw MRI data as participants have not provided consent for this.