Abstract
Globally, wound infections are considered as one of the major healthcare problems owing to the delayed healing process in diabetic patients and microbial contamination. Thus, the development of advanced materials for wound skin repair is of great research interest. Even though several biomaterials were identified as wound healing agents, gel-based scaffolds derived from either polymer or small molecules have displayed promising wound closure mechanism. Herein, for the first time, we report an injectable and self-healing self-assembled anesthetic oleogel derived from glycolipid, which exhibits antibiofilm and wound closure performance in diabetic rat. Glycolipid derived by the reaction of hydrophobic vinyl ester with α-chloralose in the presence of novozyme 435 undergoes spontaneous self-assembly in paraffin oil furnished an oleogel displaying self-healing behavior. In addition, we have prepared composite gel by encapsulating curcumin in the 3D fibrous network of oleogel. More interestingly, glycolipid in its native form demoed potential in disassembling methicillin-resistant Staphylococcus aureus, methicillin-susceptible Staphylococcus aureus, and Pseudomonas aeruginosa biofilms. Both oleogel and composite gel enhanced the wound skin repair in diabetic induced Wistar rats by promoting collagen synthesis, controlling free radical generation and further regulating tissue remodeling phases. Altogether, the reported supramolecular self-assembled anesthetic glycolipid could be potentially used for diabetic skin wound repair and to treat bacterial biofilm related infections.
Subject terms: Health care, Chemistry, Materials science, Nanoscience and technology, Antimicrobials, Bacteria, Biofilms, Molecular medicine
Introduction
An external injury to any of the skin or muscle tissues in the body is generally referred as wound, which ensue in a critical damage to membrane, extracellular matrices, blood vessels, cells, and organ structures. In order to prevent bacterial infections of wounds caused by both traumatic and surgical injury, special medical attention is required because of the involvement of various dynamic process involving coagulation, inflammation, proliferation, and tissue remodeling1. Generally, sutures, staples and adhesive tapes are common wound closure techniques adopted by the medical practitioners despite its drawbacks such as high rate of infection, inflammatory reaction, nerve damage, fluid leakage, granuloma and scar tissue formation. It is worth mentioning that the delayed closure of chronic wounds in diabetic patients encompasses improper angiogenesis, enormous production of free radicals, lack of cell to cell communication, suppressed cell migration, substandard production of extracellular matrix (ECM) and growth factors in the wound environment2. Besides these factors, acute infection, poor perfusion and nutrition and repetitive pressure also act as barriers to wound healing process3. Further, microbial infection on both acute and chronic wounds would result in the delay of healing process and impart severe infliction and associated secondary health problems4.
In order to eradicate the bacterial infection over the wound, a large variety of antibiotics and biocides are used. Even though planktonic bacteria can be effectively treated with antibiotics, the prolonged use of antibiotics substantially reduces the effectiveness of treatment, wherein the pathogens develop resistivity by forming a biofilm. In biofilm formation, in order to protect themselves, planktonic bacteria adhere strongly to the wound site and slowly develop into microcolonies through interaction with neighbouring microcolonies that emerge into a mature biofilm covered with extracellular polymeric substance (EPS)5 A matured biofilm protects bacteria from antibiotics by developing the tolerance to antimicrobials, environmental stress, inhibitors and chemical attack6. Hence, the bacterial biofilms on wound bed impairs the healing of both acute and chronic wounds.
Though a variety of strategies like mechanical debridement, application of extensive topical antimicrobials, wound cleansing, systemic application of antibiotics and antiseptics are usually employed to eliminate the impact of biofilm on the wound healing process, they are ineffective and have limitations7. For example, in case of frequent wound debridement, patients feel discomfort because of the pain and delayed healing due to the continuous disturbances in the wound bed8. This necessitates the need for an advanced strategy to treat bacterial biofilm-based infections. Recently, several antibiofilm agents such as lactoferrin, xylitol, gallium, dispersin B, acetylsalicylic acid, and numerous gel-based materials and silver alginate wound dressings have been developed to eliminate biofilms9–13. However, none of the above strategies proved to be efficient in suppressing or eliminating the biofilms, when used alone. Hence the development of multifunctional materials displaying effective wound closure and antibiofilm properties are required. In particular, biofilm formation is generally observed in diabetes patients undergoing wound closure treatment14,15 because of delayed improper hemostasis, extended inflammation, poor angiogenesis, fibroblasts, keratinocytes impaired migration and proliferation, faint vascularization, cellular infiltration, difficulty in re-epithelialization and connective tissue remodelling14–17. For diabetic wound closure, several clinical strategies such as topical application of growth factors, cellular therapies and biomaterial based scaffolds were used to regenerate skin and muscle18–21, which has several limitations such as uncontrolled release, short half-life, diffusion into other parts from the targeted site, high costs, and multiple dosages leading to undesirable side effects22,23. Therefore, a probable solution for the management of diabetic wound is to develop a multifunctional wound dressing material displaying anesthetic, antibiofilm and wound closure properties.
In the last few decades several commercial polymer-based gels such as Gelrin C, Mebiol Gel, Hystem hydrogel and Biogelx products, to name a few, have been developed for biomedical applications24–26. Various cross linking and conductive hydrogels for effective wound healing along with antibacterial activity have also been reported27–31. Researchers have been actively involved in the development of self-assembled low molecular weight gels for the effective tissue regeneration and wound closure applications, which could overcome the existing limitations associated with the polymer gels such as thermo-reversibility, processability, bio-compatibility, critical gelation concentration, sol–gel transition temperature (Tgel) and high molecular weight32–36. In this line, there is a great demand in the construction of multifunctional self-assembled gel-based materials for biomedical application, especially diabetic wound closure and infection management. In our previous report, we have developed an oleogel and composite gel from α-chloralose, a carbohydrate based molecule displaying long lasting anesthetic property37, which displayed good viscoelastic properties by molecular self-assembly of glycolipidand excellent wound healing activity in normal Wistar rat models38. In continuation of our previous work with promising results, we were curious to examine the wound closure behaviour of our self-assembled low molecular weight gels towards diabetic wounds and explore the antibiofilm property of sugar derived self-assembled gels39–41. In this regards, we report a multifunctional self-assembled glycolipid-based oleogel from FDA approved molecules, α-chloralose fatty acid and paraffin oil. The multifunctional anesthetic gel display potential diabetic wound closure and antibiofilm characteristics.
Experimental section
Materials and general methods
All essential chemicals, reagents and solvents used for the synthesis of glycolipids were purchased from Sigma Aldrich, Merck, TCI chemicals, Alfa aesar, SRL, and Avra chemicals. LR grade solvents were used for the compounds purification and AR grade solvents were used for synthesis and gelation studies. Distilled solvents were used, when necessary. Novozyme 435 was obtained from NOVOZYMES A/S, Denmark as a gift sample for our research purpose. The progress of the reactions was monitored by thin-layer chromatography (TLC) on pre-coated silica gel plates purchased from Merck and visualized by UV detection or using sulfuric acid spray or molecular iodine. Column chromatography was performed on Silica Gel (100–200 mesh) purchased from Avra chemicals, India38.
Bacterial strains and culture conditions
Strains used in this work are Methicillin-resistant Staphylococcus aureus ATCC 43866, Methicillin-susceptible Staphylococcus aureus ATCC 25923, and Pseudomonas aeruginosa ATCC 27853. Lysogeny broth (LB) was used to maintain and grow the bacteria for most of the bacteriological experiments. However, biofilm experiments were performed with Tryptic Soy broth (TSB) for S. aureus and LB medium for P. aeruginosa. The absorbance for measuring the planktonic and biofilm growth was monitored in Tecan Sunrise™ Microplate Reader38,40.
Planktonic and biofilm assay
Quantitative planktonic growth and biofilm development were measured by the method described by O’Toole and Kolter42. Briefly, an overnight grown culture was diluted to 1:100 and inoculated in 200 μL of the medium containing different concentrations of the glycolipids in the microtiter well and was incubated for 24 h at 37 °C40. The absorbance of each well was measured at 595 nm using the microplate reader for the planktonic growth. Then the grown culture was aspirated, and the wells were washed three times with 200 μL of sterile phosphate-buffered saline (PBS) to remove the non-adherent cells. Wells were then dried, and the adherent biofilm was stained with 200 μL of 0.1% crystal violet (CV) for 15 min. The unbound CV was removed by rinsing thrice with 200 μL of PBS. Further, 70% ethanol was added and incubated for 15 min to de-stain the CV and the absorbance of each well was measured at 595 nm using the microplate reader as a proxy for the biomass38.
For fluorescent microscopic analysis of the biofilms, cells were grown on glass slides in a petri plate containing broth (LB/TSB) with DMSO/Compounds of concentration 400 μg mL−1 at 37 °C for 24 h. Then the slides were washed thrice with PBS and biofilm was stained with SYTO9 in dark condition. Slides were then observed under the fluorescence microscope (Nikon Eclipse Ni–U). Multiple images (minimum 20 images) were taken and processed with auto-thresholding technique and the intensity was measured using the ImageJ software.
In vivo wound closure studies and ethical issues
Healthy Wistar strain female rats (200–230 g) of 8–9 weeks old were procured from Central Animal Facility, SASTRA Deemed University, Thanjavur, Tamil Nadu, India. The animals were housed individually in the standard laboratory environment for 7 days. During the experiment, the rats were fed with standard pellet diet and water ad libitum. The experimental protocols used in this study were approved by the Institutional Animal Ethics Committee (IAEC), SASTRA Deemed University and experiments were carried out as per the guidelines of Committee for Control and Supervision of Experimental Animal (CPCSEA), New Delhi (Reg. No. 817/04/ac/CPCSEA), SASTRA Deemed University, Thanjavur, India and its approval number is 442/SASTRA/IAEC/RPP38.
Diabetic induction
Rats were fasted overnight and then induced by intraperitoneal injections of streptozotocin (STZ) dissolved in sodium citrate buffer (0.1 M, pH 4.5) at a dosage of 65 mg/kg body weight. Blood glucose levels were monitored by a glucose meter (On-call plus, USA) one week after induction of diabetes. Blood was drawn from the tail vein and glucose levels were determined. Rats with blood glucose levels > 250 mg/dL were considered as diabetic rats43.
Experimentally inflicted wounds
Diabetes-induced Wistar strain female rats were randomly segregated into five groups comprising six rats each and caged individually. The animals were anesthetized by thiopentone sodium (60 mg/Kg, i.p) before the wound creation. The rats were inflicted with excision wounds. The dorsal fur of the animals was shaved, and the anticipated area of the wound created was marked. A full-thickness excision wound was created by existing 200 mm2 areas of skin in length and 0.2 cm depth from the dorsal region using a sharp surgical blade and pointed scissors38.
Topical application of gels
The wound of Group 1 rats was kept as such without any treatment, and this group served as the control, Group 2 rats were treated with 0.1 mL of paraffin oil and this is served as vehicle control. The wound of Group 3 and Group 4 was treated with 0.1 mL of oleogel 1 (2% w/v) and oleogel 2 (4% w/v) respectively. Group 5 was treated with Composite gel (2% w/v). For the studies, 0.1 mL of the gel was applied on the wound surface once a day and monitored the wound closure every day38.
Estimation of the rate of wound contraction
Progress of wound closure was monitored by tracking the wound area on a transparent graph sheet on alternate days up to 21 days for all six animals in each group. The transparent graph sheet was laid over the wound and traced it using a permanent marker. The area of wound size was studied by using ImageJ software. The diameter was recorded and the percentage of closure of the wound was calculated until the day of complete epithelialization. The standard and mean deviations were given in sq.mm. Percentage of wound contraction was calculated using the initial area and the area under investigation:
where W1 and W0 represent the initial wound size and wound size of the specific day respectively38.
On the 21st post wounding day, the animals were sacrificed, blood samples and granulation tissue samples were collected for the estimation of free radicals44, ascorbic acids45, hexosamine46, and collagen tissue parameters47.
Statistical analysis
All values were reported as the mean ± error bars that either indicate SEM or SD or CI at 95%, which is mentioned in the legends accordingly Significance was measured by either t tests for comparison of two samples and one-way ANOVA with appropriate post hoc tests for multiple samples, which is also mentioned in the legends. A value of P < 0.05 was considered significant. Statistical analysis was performed using GraphPad Prism for Windows software38.
Histopathological examination
Wound tissue specimens from control and test groups were taken after complete healing of excision wound and after usual processing skin samples were cut a thickness of 6 µm and stained with hematoxylin and eosin (H&E). Sections were qualitatively assessed under the light microscope and the results were recorded38.
Results and discussion
Synthesis, gelation, morphological and rheological studies
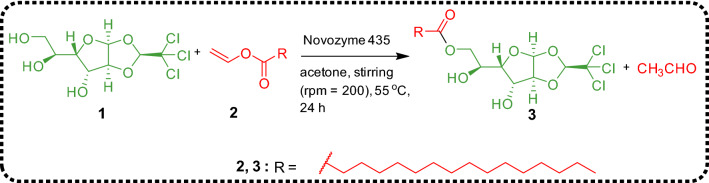
Glycolipids are a type of biological lipids found widely in plant and animal systems, performing various brilliant tasks. Generally, glycolipids consist of hydrophilic carbohydrate moiety covalently attached with hydrophobic fatty acids or any other source via glycosidic bond. In the present studies, glycolipid 3 was synthesized by the enzymatic transesterification of α-chloralose with vinyl palmitate using Novozyme 435 by following the procedure described in our previous report (Scheme 1)38. The synthesized glycolipid was characterized by NMR and mass spectral techniques38. The glycolipid 3 displayed gelation in eucalyptus oil (1% w/v), 1: 4 DMSO + Water (2.5% w/v) and paraffin oil (1% w/v). In the present investigation, we have chosen an oleogel formed by 3 in paraffin oil via molecular self-assembly guided by intermolecular non-covalent interactions because of broad range of applications displayed by paraffin oil in cosmetics, food and pharmaceutical industries38. In addition to the oleogel, we have prepared composite gel by encapsulating the natural drug, curcumin within the oleogel matrix.
Scheme 1.
Synthesis of anesthetic glycolipid 3.
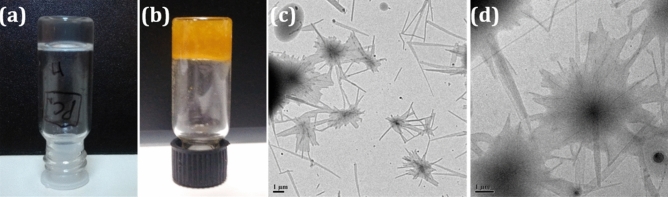
Morphological analysis of both oleogel and composite gel was investigated by HRTEM analysis. In both the cases the formation of entangled fibrillar network with 200–500 nm thickness is observed (Fig. 1), which implies that after incorporation of curcumin, there is no drastic change in the morphology of gel. However, in composite gel, the influence of curcumin in the gel network could be identified by rheological measurements. Rheological studies of the oleogel and the composite gel confirmed their thermoreversible, thermal stability and thixotropic nature (Figs. S1–S3)38. Both oleogel and composite gel were stable under physiological conditions.
Figure 1.
(a,b) Images of oleogel and composite gel in paraffin oil; (c,d) HRTEM images of oleogel and composite gel.
Rheological studies clearly establish the injectable nature of both oleogel and composite gel derived from glycolipid 3, which would help in wound closure by regulating the overlapping phases. However, in both traumatic or surgical injury, the healing of wound is delayed due to the microbial contamination, particularly in diabetics. This phenomenon motivated us to search for new materials with the antimicrobial, antibiofilm and tissue regenerating properties.
Antibacterial studies
In vitro antibacterial properties of glycolipid 3 were investigated against various wound infection-causing pathogenic bacteria namely Methicillin-resistant Staphylococcus aureus (MRSA), Methicillin-susceptible Staphylococcus aureus (MSSA) and Pseudomonas aeruginosa (PA). The influence of various concentrations of the glycolipid 3 on bacterial growth were studied. It is observed that the glycolipid 3 inhibited the growth of MRSA and MSSA at concentrations 200 μg mL−1, 300 μg mL−1 and 400 μg mL−1 (P < 0.001). In the case of PA, growth was not reduced, however, a mild inhibition of 20 and 25% was observed at 300 μg mL−1 and 400 μg mL−1 (P < 0.01) respectively, while the standard control drug, cipladine inhibited more than 75% growth of all organisms at 400 μg mL−1 (P < 0.001). Among these three pathogens tested, the glycolipid 3 displayed a substantial antibacterial effect on both MRSA and MSSA (Fig. 2a). The MIC of the glycolipid 3 on planktonic growth is shown in Table 1.
Figure 2.
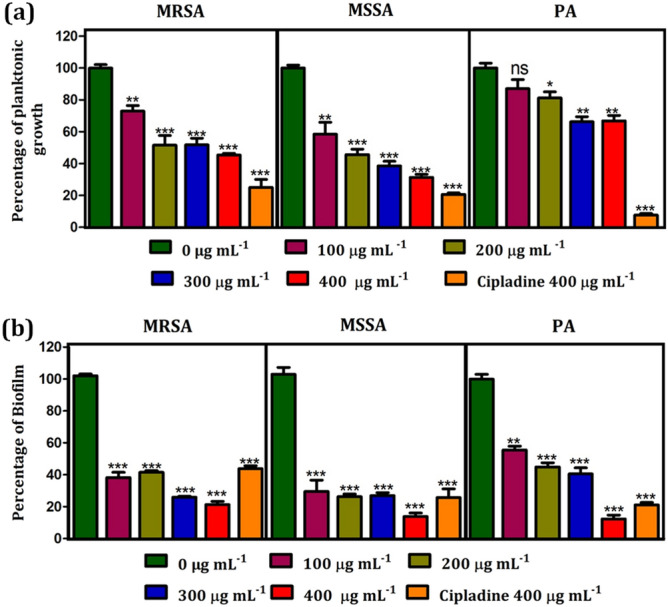
Influence of the different concentrations of glycolipid 3 on (a) bacterial cell viability and (b) biofilm formation. The bars represent the bacterial growth and biofilm biomass at an absorbance of 595 nm. Error bars represent 95% CI, n = 4. One-way ANOVA was performed to analyze the data. Significance between 0 μg mL−1 with other concentrations is depicted (*P < 0.05, **P < 0.01, ***P < 0.001).
Table 1.
The minimum inhibitory concentration of glycolipid 3 on planktonic growth and biofilm formation.
| Bacterial species | MICa (µg mL−1) | MBICb (µg mL−1) |
|---|---|---|
| Methicillin-resistant S. aureus | 400 | 300 |
| Methicillin-susceptible S. aureus | 300 | 200 |
| Pseudomonas aeruginosa | ND | 300 |
ND not determined.
aMinimum inhibitory concentration.
bMinimum biofilm inhibitory concentration.
Anti-biofilm studies
After exploring the antibacterial activity of the glycolipid 3 against various wound infection-causing pathogenic bacteria, the anti-biofilm activity of 3 towards different pathogenic bacteria was investigated by varying the concentrations (Fig. 2b). The glycolipid 3 inhibited MRSA, MSSA, and PA at all concentrations (P < 0.001). Surprisingly, at 400 µg mL−1, glycolipid 3 displayed more antibiofilm activity than the standard drug cipladine. These results clearly showed that the mode of action of the drugs was different in planktonic and biofilm form of microbes. The minimum inhibitory concentration required to inhibit the biofilm formation by glycolipid 3 is 200 µg mL−1 for MSSA and 300 µg mL−1 for both MRSA and PA (Table 1).
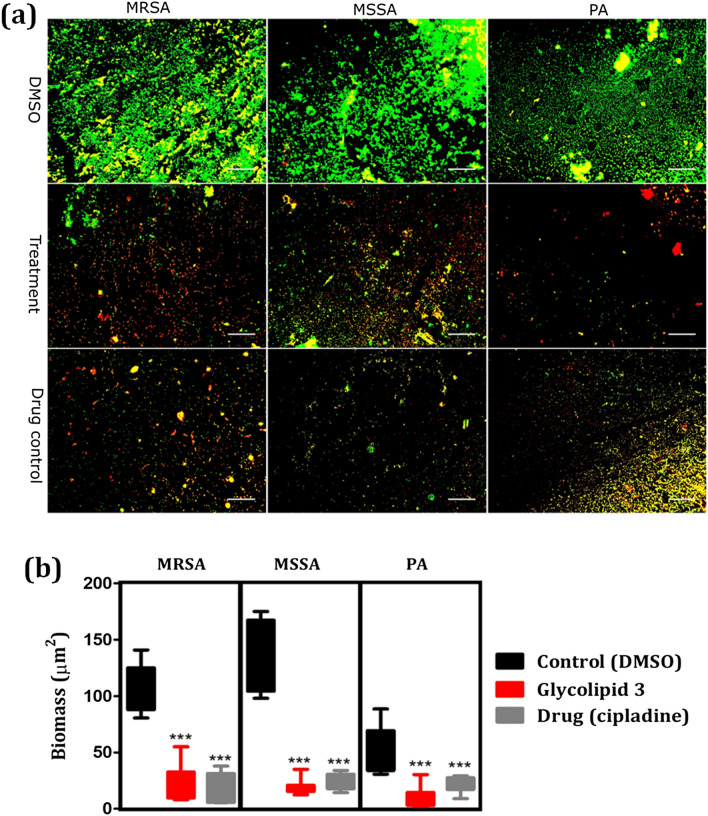
Further, the influence of glycolipid 3 on biofilm formation was confirmed by using fluorescence microscopy. Biofilm formation was monitored on glass slides that were grown on broth (LB or TSB) containing DMSO and 400 μg mL−1 of glycolipid 3 or standard drug, cipladine as a drug control. The dense matured biofilm formation with several microcolonies fused with voids was observed in control slides for all three pathogens (Fig. 3a). Compared to DMSO and drug control, glycolipid 3 displayed the inhibition of the formation of MRSA, MSSA, and PA biofilms to a greater extent (Fig. 3a). The quantification of the images also revealed the significant reduction of the biofilm biomass by the glycolipid 3 (Fig. 3b).
Figure 3.
(a) Representative fluorescence images of the biofilms treated with DMSO (control), glycolipid 3 (treatment) and drug control (cipladine). Biofilm formed by MRSA, MSSA, and PA was stained with SYTO9 and PI. Green colour represents live cells and red represents the dead cells. Scale bar is 250 µm; (b) Quantification data obtained from fluorescent microscopic images of biofilms formed from MRSA, MSSA and PA. N = > 20. Mann–Whitney U test was performed to analyze the data (***P < 0.001).
The chronic wound infections are usually caused by bacteria in its biofilm lifestyle48. The microbial clusters enmeshed in matrix components are defined as biofilms. The cells lose the typical biofilm characteristics devoid of matrix. Therefore, we hypothesized that the glycolipid 3 might repress the matrix production in the cultures thereby inhibiting its biofilm. As higher levels of intracellular c-di-GMP determines matrix production, we used the fluorescent biomarker of P.aeruginosa to identify the effect of glycolipid 3 on the levels of c-di-GMP49 Sodium nitroprusside that produces nitric oxide, which acts as a signalling molecule to reduce c-di-GMP levels was used as a positive control50. The glycolipid 3 did not influence the intracellular c-di-GMP levels in P. aeruginosa indicating that the matrix production may possibly not be intervened by it through this signalling pathway (Fig. S4). We tested the effect of glycolipid 3 on matrix production by Congo red (CR) plate assay, where the matrix components bind to CR and turns the Staphylococcus colony colour into black51. The MSSA and the MRSA colonies did not show any change in the colony colour or the morphology indicating that the glycolipid 3 did not impair the matrix production to inhibit biofilm formation (Fig. S5). We further hypothesised that the glycolipid 3 possibly inhibited biofilm by altering the surface similar to other surfactants. Biosurfactant production by the bacteria is a strategy to disperse from the biofilm cells and it aids in the emergence of typical biofilm structure52. Surfactants are also well known to display antibiofilm activity. We determined the equivalent concentration of cationic surfactant, CTAB, an anionic surfactant, the SDS, and chitosan (a known cell permeabilizing agent) to the permeablizing activity of glycolipid 3 (Fig. S6). We then used these compounds in appropriate concentrations with more or less similar permeabilization index (Fig. S6) to test the biofilm inhibitiory activity of the organisms. We observed that the planktonic growth as well as the biofilm formation was significantly inhibited by the compounds used (Fig. 4). This indicates that cell permeabilization is the possible mechanism for inhibiting planktonic growth, and the alteration of the cell surface or biofilm matrix could be the mechanism for biofilm inhibition. However, the glycolipid 3 was more effective in biofilm inhibition in all the three organisms than the planktonic growth inhibition (Fig. 4). Surfactant-based polymer dressings have also been reported to disrupt biofilm matrix in P. aeruginosa and S. aureus53.
Figure 4.
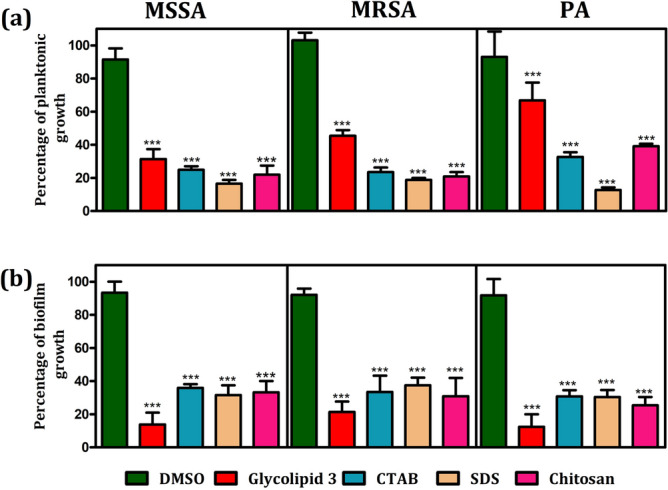
Influence of glycolipid 3 on (a) planktonic growth and (b) biofilm formation in comparison with CTAB (a cationic surfactant), SDS (an anionic surfactant), chitosan, and DMSO (vehicle control) on MSSA, MRSA, and PA. Error bar indicates 95% CI, n = 4, one-way ANOVA with Tukey’s test was used to determine significance (***P < 0.001).
In vivo wound closure studies
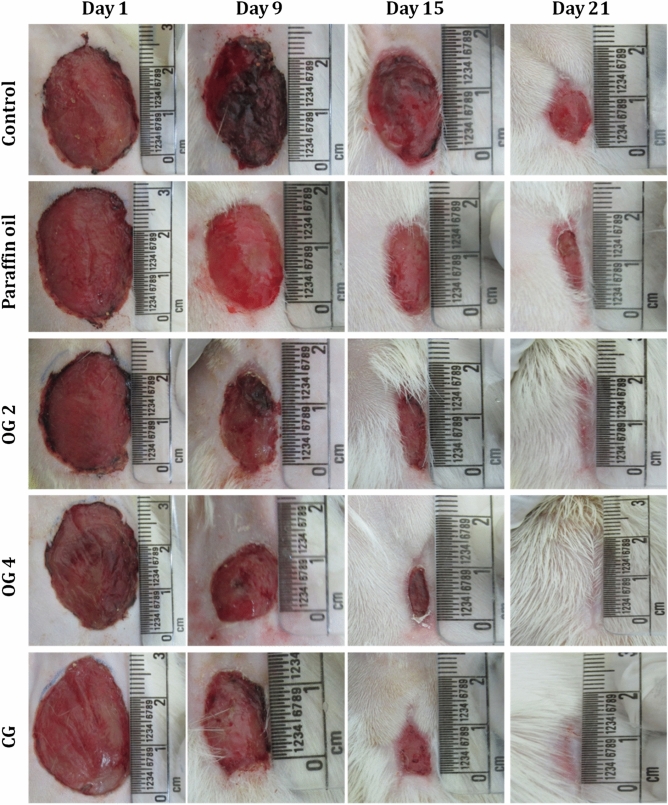
In our early work, we have demonstrated the wound closure ability of oleogel and composite gel in normal Wistar rat models. Since antibacterial and antibiofilm results were highly promising, we were curious to investigate the wound closure behavior of oleogel and composite gel in diabetic rats. In this investigation, we have used Oleogels and composite gel derived from glycolipid 3 and paraffin oil as vehicle control, and one group is left without any treatment, which is considered as a control group. Figure 5 clearly reveals that on the 21st day, 98% and 97% of wound healing were observed in diabetic rats treated with oleogels and composite gel respectively. Oleogel treated animals displayed dose-dependent wound closure from day 3 onwards indicating progressive wound healing. On the 15th day, more than 85% of the wound contraction was seen in oleogel and composite gel treated rats (Fig. S7, Table 2).
Figure 5.
The comparative assessment of the gross appearance of wound healing on different treated groups for different experimental days 1, 9, 15 and 21 in diabetes induced Wistar rats. OG 2 (oleogel 1) and OG 4 (oleogel 2) represents oleogel of 2% w/v and 4% w/v respectively. CG denotes composite gel of 2% w/v.
Table 2.
Effect of oleogel and composite gel on the percentage (%) of wound healing in experimental rats.
| Group | Percentage of wound healing (mean + SEM) | |||
|---|---|---|---|---|
| Day 3 | Day 9 | Day 15 | Day 21 | |
| Control | 5.62 ± 1.27 | 39.402 ± 0.772 | 68.208 ± 0.544 | 85.97 ± 0.46 |
| Paraffin oil | 6.34 ± 1.55 | 54.56 ± 0.62** | 76.89 ± 0.625* | 90.91 ± 0.135* |
| Oleogel 1 | 10.04 ± 1.30 | 64.26 ± 0.62*** | 86.60 ± 0.44** | 96.05 ± 0.19** |
| Oleogel 2 | 12.94 ± 0.88* | 72.91 ± 0.33*** | 90.60 ± 0.32*** | 98.92 ± 0.14** |
| Composite gel | 12.83 ± 0.50* | 68.21 ± 1.07*** | 87.39 ± 0.35** | 97.06 ± 0.069** |
aPercentage value of mean ± SEM of each group. Significance was P < 0.05 (*), P < 0.01 (**), P < 0.001 (***). Comparison of treated groups with the control group. The results were analyzed statistically using one-way ANOVA followed by Dunnett’s post-hoc test for multiple comparisons.
Generally, wound healing is a delayed process in diabetic patients due to poor angiogenesis, lack of oxygen and nutrient supply32. In this context, improving revascularization in the wound area is essential to improve the wound closure. Currently, various scaffolds combined with growth factors are used to promote angiogenesis in wound healing and tissue engineering applications34,54,55. In this study, oleogel and composite gel displaying 3-dimensional fibrous network formed via various intermolecular interactions could have served as a scaffold for wounds, which in turn possibly enhanced cell–cell communication, increased collagen synthesis, cell migration and promoted angiogenesis.
Further, the wound healing property of the gels was investigated in the aspect of expressing the biochemical profiles like collagen expression and stabilization, the proliferation of fibroblasts and lipid peroxidation. Generally, wound closure involves the migration and proliferation of endothelial cells44,56. We have discussed the importance of collagen in hemostasis and epithelization at the later phase of the wound healing in our earlier work38. Compared to control group (10.20 ± 1.03 mg/100 mg), the oleogel 1, 2 and composite gel treated groups displayed an increased amount in hydroxyproline of 20.59 ± 1.56, 24.95 ± 1.97 and 21.88 ± 1.50 mg/100 mg respectively. Rapid formation of granulation tissues and increased synthesis of collagen around the wound area can be attributed to the enhancement of the wound healing rate with oleogel 2 and composite gel treated group (Fig. S8, Table 3). Vehicle group also showed increased amount of hydroxyproline compared to control group this is due to the medicinal properties of paraffin oil. From long time people has been using paraffin oil for various health issues57–59.
Table 3.
Biochemical profile of granulation tissue obtained from the skin-excised wound of different experimental groups.
| Group | Hydroxyproline (mg/100 mg) | Hexosamine (mg/100 mg) | Vitamin C (mg/100 mL) | Lipid peroxide (mg/100 mg) |
|---|---|---|---|---|
| Control | 10.20 ± 1.03 | 0.55 ± 0.03 | 2.44 ± 0.07 | 4.86 ± 0.42 |
| Paraffin oil | 14.23 ± 1.03 | 0.72 ± 0.04* | 2.53 ± 0.05 | 4.12 ± 0.20 |
| Oleogel 1 | 20.59 ± 1.56*** | 1.27 ± 0.05*** | 3.28 ± 0.09*** | 2.16 ± 0.08*** |
| Oleogel 2 | 24.95 ± 1.97*** | 1.41 ± 0.04*** | 3.93 ± 0.12*** | 1.26 ± 0.05 *** |
| Composite gel | 21.88 ± 1.50*** | 1.38 ± 0.04*** | 3.98 ± 0.14*** | 1.29 ± 0 04*** |
Values of mean ± SEM of each group. P < 0.05 (*), P < 0.01 (**), P < 0.001 (***). Comparison of treated groups with a control group. The results were analyzed statistically using one-way ANOVA followed by Dunnett’s post-hoc test for multiple comparisons.
Glycosaminoglycans are one of the major components of the extracellular matrix of the skin, which exhibits viscoelastic and hygroscopic properties that are responsible for normal dermal tissue function. Hexosamine is a component of glycosaminoglycans, , which strengthens the collagen fibers by molecular interactions that manifests in its assembly. In oleogel 2 (1.41 ± 0.04) and composite gel (1.38 ± 0.04), treated groups display higher level of hexosamine, which further stabilized collagen via assembly process and facilitated the production of new extracellular matrix (Fig. S8, Table 3). Ascorbic acid commonly known as Vitamin C plays a major role in all phases of wound healing, it is an essential constituent that displayed antioxidant property, facilitate the blood vessel repair and activation of dermal fibroblasts in the tissue regeneration process. In the present study, increased levels of ascorbic acid have been observed in the groups treated with the oleogel 2 and composite gel (3.93 ± 0.12 and 3.98 ± 0.14 respectively) compared to the other groups (Fig. S8, Table 3). Increased levels of ascorbic acid in this groups are evidence of effective wound healing. Hydroxyl radical produced in the wound area stimulates the lipid peroxidation that affects the healing process by causing damage to proteins, lipids, DNA, cellular membranes, and fibroblast metabolism60. However, the topical application of oleogel 2 and composite gel treated groups decreased the levels of lipid peroxide 1.26 ± 0.05 and 1.29 ± 0.04 respectively (Fig. S8, Table 3). Lipid peroxidation result revealed the formation of collagen fibrils and the activation of various enzymes that prevents cell damage. Inhibition of lipid peroxidation levels also induced the Vascular Endothelial Growth Factor (VEGF) expressions61 favoring the wound contraction in diabetic wounds.
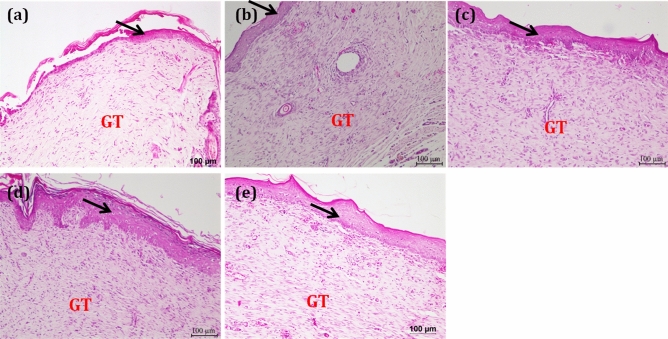
Further, histopathological studies have been performed to investigate the wound healing rates of various treated groups. The hematoxylin and eosin-stained skin sections of various treatment groups displayed different wound healing rates (Fig. 6). Control groups shows moderate epithelialization with early and mid wound contraction phase respectively, where as in oleogel and composite gel treated groups shows complete epithelialization with late wound contraction phase. Thus histological data also reveals the high degree of wound healing in oleogel and composite gel treated groups compare to control groups The topical application of oleogel 2 and composite gel displayed effective tissue regeneration when compared to other groups without any edema and congestion.
Figure 6.
Haematoxylin and Eosin (H&E) stained sections of healed skin of experimental groups (a) Control group shows moderate epithelialization with early wound contraction phase, (b) vehicle control paraffin oil-treated skin section shows moderate epithelialization with mid wound contraction phase, (c) oleogel 1- treated group showing granulation tissue with moderate epithelialization with late fibroblast phase, (d) oleogel 2—treated group showing granulation tissue with complete epithelialization, (e) composite gel displaying complete epithelialization at 10X magnifications. Arrow—Epidermis; GT—Granulation tissue.
Conclusions
We have synthesized anesthetic glycolipid from FDA approved starting materials, α-chloralose and palmitic acid using Novozyme 435 as a biocatalyst. Supramolecular self-assembly of glycolipid in paraffin oil using various intermolecular interactions furnished oleogel, which form 3-dimensional fibrous architecture identified using HRTEM analysis. In medicine, paraffin oil is commonly used as an additive in pediatric laxative, cosmetic products, and to treat arthritis, fibromyalgia and dry skin, hence, we have explored the application of oleogel and composite gel prepared by encapsulating curcumin into the gel matrix in skin wound closure. In this report, we present a multifunctional gel material displaying anesthetic, antibacterial, antibiofilm and skin wound closure for traumatic and surgical injury. The antibiofilm property of anesthetic glycolipid was investigated over common dermal infection-causing bacteria, Methicillin-resistant Staphylococcus aureus, Methicillin-susceptible Staphylococcus aureus, and Pseudomonas aeruginosa and found to be better than standard drug cipladene at 400 μg mL−1. Both oleogel and composite gel displayed enhanced skin wound closure in diabetic Wistar rats. The reported multifunctional gel-based material will surely open up a new avenue in biomedical field, especially for diabetic patients suffering from traumatic and surgical injury.
Supporting information
Supplemenary methods, rheology, wound healing studies, biochemical profile, C-di-GMP study, CRA assay, and cell permeability studies.
Supplementary information
Acknowledgements
Financial support from the Science and Engineering Research Board (SERB), Department of Science and Technology, India (Sanction Order No. CRG/2018/001386) and SPARC, Ministry of Human Resource Development, India (SPARC/2018-2019/P263/SL) is gratefully acknowledged. CSS thanks the SERB for the Core Research Grant (CRG/2018/001827). We thank the DST-FIST funded fluorescence microscopy facility (SR/FST/ETI-331/2013). We thank the National Institute of Technology, Warangal and SASTRA Deemed University, Thanjavur for the infrastructure facilities. Authors also thank Dr C. Davidraj, Central Animal Facility, SASTRA Deemed University for his help in in vivo studies.
Abbreviations
- ECM
Extracellular matrix
- EPS
Extracellular polymeric substance
- FDA
US food and drug administration
- TLC
Thin-layer chromatography
- LB
Lysogeny broth
- TSB
Tryptic soy broth
- PBS
Phosphate-buffered saline
- MRSA
Methicillin-resistant Staphylococcus aureus
- MSSA
Methicillin-susceptible Staphylococcus aureus
- PA
Pseudomonas aeruginosa
- MIC
Minimum inhibitory concentration
- MBIC
Minimum biofilm inhibitory concentration
- VEGF
Vascular endothelial growth factor
Authors’ contributions
S.N., Y.S. and C.S.S. conceptualized the work and devised the protocol. S.N., Y.S., B.S., K.L. and S.M. executed the research. V.S. and C.U. provided suggestions for the improvement of this work. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
C. S. Srinandan, Email: srinandan@gmail.com
Subbiah Nagarajan, Email: snagarajan@nitw.ac.in.
Supplementary information
is available for this paper at 10.1038/s41598-020-73708-7.
References
- 1.Mutsaers SE, Bishop JE, McGrouther G, Laurent GJ. Mechanisms of tissue repair: from wound healing to fibrosis. Int. J. Biochem. Cell Biol. 1997;29:5–17. doi: 10.1016/S1357-2725(96)00115-X. [DOI] [PubMed] [Google Scholar]
- 2.Singer AJ, Clark RAF. Cutaneous wound healing. N. Engl. J. Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 3.Akopian G, Nunnery SP, Piangenti J, Rankin P, Rinoie C, Lee E, Alexander M. Outcomes of conventional wound treatment in a comprehensive wound center. Am. Surg. 2006;72:314–317. doi: 10.1177/000313480607200407. [DOI] [PubMed] [Google Scholar]
- 4.Percival SL, Emanuel C, Cutting KF, Williams DW. Microbiology of the skin and the role of biofilms in infection. Int. Wound J. 2012;9:14–32. doi: 10.1111/j.1742-481X.2011.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolcott RD, Rhoads DD. A study of biofilm-based wound management in subjects with critical limb Ischaemia. J. Wound Care. 2008;17:145–155. doi: 10.12968/jowc.2008.17.4.28835. [DOI] [PubMed] [Google Scholar]
- 6.Sauer K, Camper AK, Ehrlich GD, Costerton JW, Davies DG. Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. J. Bacteriol. 2002;184:1140–1154. doi: 10.1128/jb.184.4.1140-1154.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Black CE, Costerton JW. Current concepts regarding the effect of wound microbial ecology and biofilms on wound healing. Surg. Clin. 2010;90:1147–1160. doi: 10.1016/j.suc.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Malone M, Swanson T. Biofilm-based wound care: the importance of debridement in biofilm treatment strategies. Br. J. Commun. Nurs. 2017;22:S20–S25. doi: 10.12968/bjcn.2017.22.Sup6.S20. [DOI] [PubMed] [Google Scholar]
- 9.McCarty, S., Jones, E.M., Finnegan, S., Woods, E., Cochrane, C.A. & Percival, S.L. Chapter Eighteen - Wound Infection and Biofilms. In Biofilms in Infection Prevention and Control; Percival, S. L., Williams, D. W., Randle, J., Cooper, T., Eds.; Academic Press: Boston, pp. 339–358 (2014).
- 10.Percival SL, McCarty SM. Silver and alginates: role in wound healing and biofilm control. Adv. Wound Care. 2014;4:407–414. doi: 10.1089/wound.2014.0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finnegan S, Percival SL. Clinical and antibiofilm efficacy of antimicrobial hydrogels. Adv. Wound Care. 2014;4:398–406. doi: 10.1089/wound.2014.0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pérez-Díaz M, Alvarado-Gomez E, Magaña-Aquino M, Sánchez-Sánchez R, Velasquillo C, Gonzalez C, Ganem-Rondero A, Martínez-Castañon G, Zavala-Alonso N, Martinez-Gutierrez F. Anti-biofilm activity of chitosan gels formulated with silver nanoparticles and their cytotoxic effect on human fibroblasts. Mater. Sci. Eng. C. 2016;60:317–323. doi: 10.1016/j.msec.2015.11.036. [DOI] [PubMed] [Google Scholar]
- 13.Alvarado-Gomez E, Martínez-Castañon G, Sanchez-Sanchez R, Ganem-Rondero A, Yacaman MJ, Martinez-Gutierrez F. Evaluation of anti-biofilm and cytotoxic effect of a gel formulation with pluronic F-127 and silver nanoparticles as a potential treatment for skin wounds. Mater. Sci. Eng. C. 2018;92:621–630. doi: 10.1016/j.msec.2018.07.023. [DOI] [PubMed] [Google Scholar]
- 14.Goodson WH, Hunt TK. Studies of wound healing in experimental diabetes mellitus. J. Surg. Res. 1977;22:221–227. doi: 10.1016/0022-4804(77)90137-8. [DOI] [PubMed] [Google Scholar]
- 15.Frykberg RG, Banks J. Challenges in the treatment of chronic wounds. Adv. Wound Care. 2015;4:560–582. doi: 10.1089/wound.2015.0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:1736–1743. doi: 10.1016/S0140-6736(05)67700-8. [DOI] [PubMed] [Google Scholar]
- 17.Sharp A, Clark J. Diabetes and its effects on wound healing. Nurs. Stand. R. Coll. Nurs. G. B. 2011;25:41–47. doi: 10.7748/ns.25.45.41.s48. [DOI] [PubMed] [Google Scholar]
- 18.Moura LIF, Dias AMA, Carvalho E, de Sousa HC. Recent advances on the development of wound dressings for diabetic foot ulcer treatment—a review. Acta Biomater. 2013;9:7093–7114. doi: 10.1016/j.actbio.2013.03.033. [DOI] [PubMed] [Google Scholar]
- 19.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 20.Futrega K, King M, Lott WB, Doran MR. Treating the whole not the hole: necessary coupling of technologies for diabetic foot ulcer treatment. Trends Mol. Med. 2014;20:137–142. doi: 10.1016/j.molmed.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Barrientos S, Brem H, Stojadinovic O, Tomic-Canic M. Clinical application of growth factors and cytokines in wound healing. Wound Repair Regen. 2014;22:569–578. doi: 10.1111/wrr.12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balmayor ER. Targeted delivery as key for the success of small osteoinductive molecules. Adv. Drug Deliv. Rev. 2015;94:13–27. doi: 10.1016/j.addr.2015.04.022. [DOI] [PubMed] [Google Scholar]
- 23.Lee K, Silva EA, Mooney DJ. Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J. R. Soc. Interface. 2011;8:153–170. doi: 10.1098/rsif.2010.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Truong, W.T., Su, Y., Meijer, J.T., Thordarson, P. & Braet, F. Self-assembled gels for biomedical applications. Chem. Asian J. 6, 30–42 (2011). [DOI] [PubMed]
- 25.Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chem. Rev. 2001;101:1869–1880. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 26.Ko DY, Shinde UP, Yeon B, Jeong B. Recent progress of in situ formed gels for biomedical applications. Prog. Polym. Sci. 2013;38:672–701. doi: 10.1016/j.progpolymsci.2012.08.002. [DOI] [Google Scholar]
- 27.Zhao X, Liang Y, Huang Y, He J, Han Y, Guo B. Physical double-network hydrogel adhesives with rapid shape adaptability, fast self-healing, antioxidant and NIR/PH stimulus-responsiveness for multidrug-resistant bacterial infection and removable wound dressing. Adv. Funct. Mater. 2020;30:1910748. doi: 10.1002/adfm.201910748. [DOI] [Google Scholar]
- 28.Qu J, Zhao X, Liang Y, Zhang T, Ma PX, Guo B. Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials. 2018;183:185–199. doi: 10.1016/j.biomaterials.2018.08.044. [DOI] [PubMed] [Google Scholar]
- 29.He J, Shi M, Liang Y, Guo B. Conductive adhesive self-healing nanocomposite hydrogel wound dressing for photothermal therapy of infected full-thickness skin wounds. Chem. Eng. J. 2020;394:124888. doi: 10.1016/j.cej.2020.124888. [DOI] [Google Scholar]
- 30.Liang Y, Zhao X, Hu T, Chen B, Yin Z, Ma PX, Guo B. Adhesive hemostatic conducting injectable composite hydrogels with sustained drug release and photothermal antibacterial activity to promote full-thickness skin regeneration during wound healing. Small. 2019;15:1900046. doi: 10.1002/smll.201900046. [DOI] [PubMed] [Google Scholar]
- 31.Liang Y, Chen B, Li M, He J, Yin Z, Guo B. Injectable antimicrobial conductive hydrogels for wound disinfection and infectious wound healing. Biomacromol. 2020;21:1841–1852. doi: 10.1021/acs.biomac.9b01732. [DOI] [PubMed] [Google Scholar]
- 32.Zhao L, Niu L, Liang H, Tan H, Liu C, Zhu F. pH and glucose dual-responsive injectable hydrogels with insulin and fibroblasts as bioactive dressings for diabetic wound healing. ACS Appl. Mater. Interfaces. 2017;9:37563–37574. doi: 10.1021/acsami.7b09395. [DOI] [PubMed] [Google Scholar]
- 33.Carrejo, N.C., Moore, A.N., Lopez Silva, T.L., Leach, D.G., Li, I.-C., Walker, D.R. & Hartgerink, J.D. Multidomain peptide hydrogel accelerates healing of full-thickness wounds in diabetic mice. ACS Biomater. Sci. Eng.4, 1386–1396 (2018). [DOI] [PMC free article] [PubMed]
- 34.Kong L, Wu Z, Zhao H, Cui H, Shen J, Chang J, Li H, He Y. Bioactive injectable hydrogels containing desferrioxamine and bioglass for diabetic wound healing. ACS Appl. Mater. Interfaces. 2018;10:30103–30114. doi: 10.1021/acsami.8b09191. [DOI] [PubMed] [Google Scholar]
- 35.Lee, C.-H., Hsieh, M.-J., Chang, S.-H., Lin, Y.-H., Liu, S.-J., Lin, T.-Y., Hung, K.-C., Pang, J.-H.S. & Juang, J.-H. Enhancement of diabetic wound repair using biodegradable nanofibrous metformin-eluting membranes: in vitro and in vivo. ACS Appl. Mater. Interfaces6, 3979–3986 (2014). [DOI] [PubMed]
- 36.Liu J, Chen Z, Wang J, Li R, Li T, Chang M, Yan F, Wang Y. Encapsulation of curcumin nanoparticles with MMP9-responsive and thermos-sensitive hydrogel improves diabetic wound healing. ACS Appl. Mater. Interfaces. 2018;10:16315–16326. doi: 10.1021/acsami.8b03868. [DOI] [PubMed] [Google Scholar]
- 37.Gaertner, D.J., Hallman, T.M., Hankenson, F.C. & Batchelder, M.A. Anesthesia and Analgesia for Laboratory Rodents. In Anesthesia and Analgesia in Laboratory Animals; Elsevier, pp. 239–297 (2008).
- 38.Prasad, Y.S, Saritha, B., Tamizhanban, A., Lalitha, K., Kabilan, S., Uma Maheswari, C., Sridharan, V. & Nagarajan, S. Enzymatic synthesis and self-assembly of glycolipids: robust self-healing and wound closure performance of assembled soft materials. RSC Adv.8, 37136–37145 (2018). [DOI] [PMC free article] [PubMed]
- 39.Miraftab M, Masood R, Edward-Jones V. A New carbohydrate-based wound dressing fibre with superior absorption and antimicrobial potency. Carbohydr. Polym. 2014;101:1184–1190. doi: 10.1016/j.carbpol.2013.10.058. [DOI] [PubMed] [Google Scholar]
- 40.Prasad YS, Miryala S, Lalitha K, Ranjitha K, Barbhaiwala S, Sridharan V, Maheswari CU, Srinandan CS, Nagarajan S. Disassembly of bacterial biofilms by the self-assembled glycolipids derived from renewable resources. ACS Appl. Mater. Interfaces. 2017;9:40047–40058. doi: 10.1021/acsami.7b12225. [DOI] [PubMed] [Google Scholar]
- 41.Bowler PG, Duerden BI, Armstrong DG. Wound microbiology and associated approaches to wound management. Clin. Microbiol. Rev. 2001;14:244–269. doi: 10.1128/CMR.14.2.244-269.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O'Toole GA, Kolter R. Initiation of biofilm formation in Pseudomonas Fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol. Microbiol. 1998;28:449–461. doi: 10.1046/j.1365-2958.1998.00797.x. [DOI] [PubMed] [Google Scholar]
- 43.Wu, K.K. & Huan, Y. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. Pharmacol.40, 5.47.1–5.47.14. (2008) [DOI] [PubMed]
- 44.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 45.Omaye, S.T., David Turnbull, J. & Sauberlich, H.E. Selected Methods for the Determination of Ascorbic Acid in Animal Cells, Tissues, and Fluids. In Methods in Enzymology; Vitamins and Coenzymes Part D; Academic Press,62, 3–11 (1979). [DOI] [PubMed]
- 46.Wagner, W.D.A More Sensitive Assay Discriminating Galactosamine and Glucosamine in Mixtures. Anal. Biochem.94, 394–396 (1979). [DOI] [PubMed]
- 47.Neuman RE, Logan MA. The determination of hydroxyproline. J. Biol. Chem. 1950;184:299–306. [PubMed] [Google Scholar]
- 48.Wolcott RD, Rhoads DD, Bennett ME, Wolcott BM, Gogokhia L, Costerton JW, Dowd SE. Chronic wounds and the medical biofilm paradigm. J Wound Care. 2010;19:45–53. doi: 10.12968/jowc.2010.19.2.46966. [DOI] [PubMed] [Google Scholar]
- 49.Rybtke MT, Borlee BR, Murakami K, Irie Y, Hentzer M, Nielsen TE, Givskov M, Parsek MR, Tolker-Nielsen T. Fluorescence-based reporter for gauging cyclic Di-GMP levels in pseudomonas aeruginosa. Appl. Environ. Microbiol. 2012;78:5060–5069. doi: 10.1128/AEM.00414-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barraud N, Schleheck D, Klebensberger J, Webb JS, Hassett DJ, Rice SA, Kjelleberg S. Nitric oxide signaling in pseudomonas aeruginosa biofilms mediates phosphodiesterase activity, decreased cyclic Di-GMP levels, and enhanced dispersal. J. Bacteriol. 2009;191:7333–7342. doi: 10.1128/JB.00975-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwartbeck B, Birtel J, Treffon J, Langhanki L, Mellmann A, Kale D, Kahl J, Hirschhausen N, Neumann C, Lee JC, Götz F, Rohde H, Henke H, Küster P, Peters G, Kahl BC. Dynamic in vivo mutations within the Ica operon during persistence of staphylococcus aureus in the airways of cystic fibrosis patients. PLoS Pathog. 2016;12:e1006024. doi: 10.1371/journal.ppat.1006024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Banat IM, De Rienzo MAD, Quinn GA. Microbial biofilms: biosurfactants as antibiofilm agents. Appl. Microbiol. Biotechnol. 2014;98:9915–9929. doi: 10.1007/s00253-014-6169-6. [DOI] [PubMed] [Google Scholar]
- 53.Das Ghatak, P., Mathew-Steiner, S.S., Pandey, P., Roy, S. & Sen, C.K. A surfactant polymer dressing potentiates antimicrobial efficacy in biofilm disruption. Sci. Rep.8, 873 (2018) [DOI] [PMC free article] [PubMed]
- 54.Mandriota SJ, Pepper MS. Vascular endothelial growth factor-induced in vitro angiogenesis and plasminogen activator expression are dependent on endogenous basic fibroblast growth factor. J. Cell Sci. 1997;110:2293–2302. doi: 10.1242/jcs.110.18.2293. [DOI] [PubMed] [Google Scholar]
- 55.Rocha FG, Sundback CA, Krebs NJ, Leach JK, Mooney DJ, Ashley SW, Vacanti JP, Whang EE. The effect of sustained delivery of vascular endothelial growth factor on angiogenesis in tissue-engineered intestine. Biomaterials. 2008;29:2884–2890. doi: 10.1016/j.biomaterials.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Broughton, G., Janis, J.E. & Attinger, C.E. Wound Healing: An Overview: Plast. Reconstr. Surg.117, 1e-S–32e-S (2006). [DOI] [PubMed]
- 57.Gray HMW. The use of liquid paraffin in the treatment of war wounds. Br Med J. 1917;2:509. doi: 10.1136/bmj.2.2964.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Speight, J.G. Chapter 13—Pharmaceuticals. In Handbook of Industrial Hydrocarbon Processes; Speight, J. G., Ed.; Gulf Professional Publishing: Boston, 467–497 (2011)
- 59.Gurd FB, McKim LH. The use of BIPP and liquid paraffin in the treatment of wounds. Am. J. Surg. 1941;51:584–600. doi: 10.1016/S0002-9610(41)90197-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Loo AEK, Wong YT, Ho R, Wasser M, Du T, Ng WT, Halliwell B. Effects of hydrogen peroxide on wound healing in mice in relation to oxidative damage. PLoS ONE. 2012;7:e49215. doi: 10.1371/journal.pone.0049215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sen CK, Khanna S, Babior BM, Hunt TK, Ellison EC, Roy S. Oxidant-induced vascular endothelial growth factor expression in human keratinocytes and cutaneous wound healing. J. Biol. Chem. 2002;277:33284–33290. doi: 10.1074/jbc.M203391200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.