Abstract
Coxiella burnetii is a ubiquitous zoonotic bacterium reported worldwide that causes Q-fever. Infections result in profound economic losses to livestock producers by causing abortions and low birth weights. Current information about the disease in the Caribbean region is scarce. With multiple small islands and territories, it is often considered that the bacterium is absent or circulates at a low prevalence. Our study aimed to determine whether sheep and cattle housed at a veterinary campus in St Kitts had previous exposure to C. burnetii. Blood samples were taken from cattle (n = 63; 72% of the herd) and sheep (n = 133; 71% of the flock). Antibodies to C. burnetii were detected by a commercial indirect enzyme-linked immunosorbent assay (IDvet® ELISA) test. The seroprevalence was estimated at 26.3% (95% CI: 19.1–34.7%) in sheep and 0% (95% CI: 0–5.7%) in cattle. Sheep importation to St. Kitts is very rare, thus, these results suggest that C. burnetii is present on the island. The seronegativity of all the cattle highlights the absence of the bacterium on the veterinary campus. The high seroprevalence in sheep, however, has potentially important implications for animal health and public health as well as for wildlife conservation. Further investigation about animal seroprevalence and human exposure are warranted in St. Kitts and in the Caribbean region.
Keywords: Cattle, Coxiella burnetii, Caribbean islands, Q fever, Sheep, Seroepidemiologic studies, Veterinary school
Highlights
-
•
Seroprevalence in sheep from a veterinary university was 26.3%
-
•
No cattle from the university were seropositive
-
•
Risk for human and animal health is likely to be important in St. Kitts
-
•
There is a need to investigate the prevalence of Coxiella burnetii in Caribbean region
1. Introduction
Coxiella burnetii is a ubiquitous obligate intracellular, Gram negative bacterium that can infect a broad range of invertebrate and vertebrate hosts [1]. This zoonotic infection causes Q fever or “query fever” in humans [2,3]. Whereas many cases are asymptomatic, the symptoms of Q fever vary from person to person ranging from an acute form characterized by flu-like illness and myalgias to a chronic ailment associated with serious complications such as endocarditis and hepatitis [4,5]. In livestock, non-gravid animals manifest mild flu-like symptoms or are simply asymptomatic [4]. Therefore, identification of infected animals is challenging and the incidence of the disease is often unknown. Economic losses are mainly observed in small ruminant farms as a result of reproductive disorders such as abortion, stillbirth, and mastitis [[6], [7], [8]].
The life cycle of C. burnetii is complex, involving long incubation periods and both vertebrate and invertebrate hosts. Natural reservoirs of C. burnetii are tick species and rodents [1,[9], [10], [11], [12], [13], [14]]. Sources of infection to humans are primarily ruminants (cattle, goats and sheep) [1]. In all species, transmission of the bacterium between two individuals can be direct (contact with infected placenta, birth products or through milk) or indirect (contaminated soil, dust or ticks). The portal of entry is predominantly the respiratory tract. To date, the bacterium is considered to be present worldwide, except in New Zealand [15,16]. However, the geographical distribution varies considerably. Europe and Australia are highly endemic areas with reported outbreaks in the Netherlands in Europe, as well as, in West Queensland and Northwest New South Wales in Australia [[17], [18], [19]]. Data from North America is scarce and C. burnetii infection is less commonly recognized as a disease of importance, though notably the pathogen is listed as a select agent by the United States Department of Health and Human Services (HHS) and as a Category B bioterrorism agent by the Centers for Disease Control and Prevention (CDC) [20].
In the Caribbean, a limited number of studies focusing on zoonotic pathogens did not shed light on the presence of the bacterium on the islands. Healthy pregnant women were found seropositive in Antigua, Jamaica, Montserrat and St. Kitts [21]. The same study did not detect seropositive cases in Belize, Bermuda, Dominica, Grenada, St. Lucia or St. Vincent-Grenadines [21]. From surveys in animals, some countries and territories such as Cuba, Curaçao, Dominica, French Guyana and Trinidad detected the bacterium or seropositive animals [[22], [23], [24], A. Dwarkasing, personal communication, November 2018; M. Vely, personal communication, November 2018]. Investigations in St. Lucia, Montserrat and Nevis concluded that the bacteria was not present in animals on those islands [23,25]. In St. Kitts, to our knowledge, only one study screened for C. burnetii in 2008 and found one goat and one cat seropositive [23]. These results could be interpreted as an extremely low prevalence or two false positive animals. The general belief in St. Kitts remains that the bacterium is absent on the island.
The present study aimed to determine whether sheep and cattle housed at a veterinary campus in St. Kitts had previous exposure to C. burnetii.
2. Materials and methods
Study location and study population.
Ross University School of Veterinary Medicine (RUSVM) is located on the island of St. Kitts (Federation of St. Christopher and Nevis) in the Caribbean (Fig. 1). RUSVM students are taught pre-clinical veterinary skills during their time on the island. The RUSVM cattle herd (Holstein dairy cattle; beef cross-breeds of local Creole, Red Angus, Simmental and Brangus) is a closed animal population where replacement animals are bred on site using artificial insemination, whereas the sheep herd is renewed every four months to accommodate laboratory surgery sessions. Male sheep are purchased through local island farmers and are predominantly a mixture of Barbados Black Belly, Pelibuey and St. Croix breeds. After their laboratory use, they are resold on island.
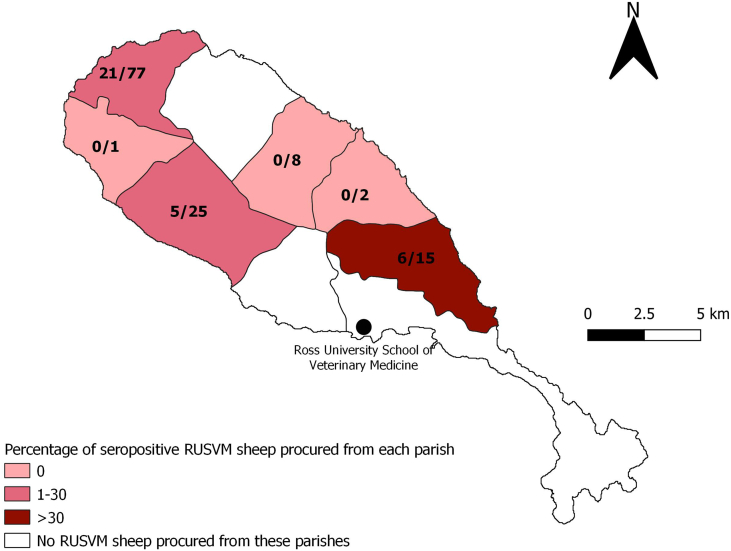
Fig. 1.
Percentage of seropositive RUSVM sheep procured from each parish. Seropositivity is defined as a strongly positive or a positive result to IDvet® ELISA. Number of seropositive and number of sheep by parish is indicated on the map. The origin of 5 sheep (including 3 seropositive) could not be retrieved. Ross University School of Veterinary Medicine is the location where the sheep were housed at the time of the sampling.
Sample collection.
Sampling of sheep and cattle occurred on the 31st of January and the 27th of February 2019 respectively. The objective of the sampling was to sample all sheep and all cattle present on the RUSVM campus. Due to academic and husbandry activity, access to both herds was limited and only animals which were not used that day were sampled. Peripheral blood samples were collected aseptically from the jugular vein of sheep (by syringe) and the coccygeal vein of cattle (by vacuum tubes) and placed in red top serum tubes.
Primary serological test method.
Samples were centrifuged to obtain sera and subsequently stored at −4 °C. Antibodies to C. burnetii were detected by a commercial indirect enzyme-linked immunosorbent assay (ELISA) test using microtiter plates pre-coated with the C. burnetii phase I and II strains (ID Screen® Q Fever Indirect Multi-Species, IDvet®). Positive and negative control sera were included in each plate. As recommended by the manufacturer, an animal was considered to be ELISA-strong positive if the optical density (OD) was over 80%. An OD between 50% and 80% was considered positive. A doubtful ELISA result was noted if the OD was between 40% and 50%, while an OD ≤ 40% was considered a negative animal. The sensitivity and specificity of the ELISA test kit as provided by the manufacturer was 99% and 98%, respectively.
Confirmatory testing of positive samples.
All positive samples were sent to the USDA National Veterinary Service Laboratory (NVSL) in Ames (Iowa, U.S.) for confirmatory testing. There, samples were tested using a complement-fixation test (CFT). Samples with a discrepancy of results between the first ELISA test run in-house (RUSVM) and the CFT were then tested in the NVSL laboratory using another ELISA kit (IDEXX Q Fever ab Test®, IDEXX®).
Statistical analyses.
Data was entered in Microsoft Excel™ and analyzed using R software [26]. Statistics were descriptive only by presentation of the apparent seroprevalence. Sensitivity and specificity of the tests were not included in these calculations. Positive Predictive Value (PPV) was calculated by dividing the number of samples positive to IDEXX® ELISA test by the number of samples positive to IDvet® ELISA test. Confidence intervals of 95% (95% CI) were computed using the function ‘ci.binomial’ in the package EpiDisplay [27]. Maps were built using QGIS software [28].
Ethical consideration
This investigation was part of the health monitoring program of ruminants within RUSVM's Animal Care and Use Program. RUSVM Institutional Animal Care and Use Committee (IACUC) regards this as animal disease surveillance and protocol approval was therefore waived.
3. Results
One hundred thirty three (133) sheep out of 191 present on campus (69.6%) were successfully sampled. Of the 87 cattle (4 to 16 years of age), 63 (72%) were sampled. The apparent seroprevalence using the IDvet® test was estimated at 26.3% (95% CI: 19.1–34.7%) in sheep and 0% (95% CI: 0–5.7%) in cattle. All results are summarized in Table 1. The sheep were bought from eleven farmers from 6 parishes out of 9 on the island: Christ Church (1 farm, number of sheep N = 8), St. Anne (1 farm, N = 1), St. Mary (2 farms, N = 1 and N = 1), St. Paul (3 farms, N = 65, N = 10 and N = 2), St. Peter (1 farmer, N = 15), St. Thomas (3 farmers, N = 15, N = 6 and N = 4). The origin location of 5 sheep could not be retrieved. No spatial pattern was observed regarding the origin of positive samples (Fig. 1).
Table 1.
Serology results of the 133 sheep sampled at Ross University School of Veterinary Medicine (RUSVM), January 2019.
| Number of samples | Positive samples | Negative samples | Seroprevalence (95% CI) |
|||
|---|---|---|---|---|---|---|
| ELISA test (IDvet®) | 133 | 351 | 982 | 26.3% (19.1–34.7%) | ||
| Strongly Positive (OD > 80%) |
Positive (OD = 50–80%) |
Doubtful (OD = 40–50%) |
Negative (OD ≤ 40%) |
|||
| 27 | 8 | 4 | 94 | |||
| CFT | 35 | 0 | 35 | – | ||
| ELISA test (IDEXX®) | 35 | 27 | 8 | – | ||
Positive samples from the ELISA test (IDvet®) were further analyzed with complement fixation test and ELISA test (IDEXX®).
Negative samples from the ELISA test (IDvet®) were not further analyzed.
All 35 positive and strongly positive samples on the IDvet® ELISA were sent for confirmatory testing to the NVSL laboratory and tested negative for the CFT. Therefore, they were all retested using IDEXX® ELISA test. Twenty-seven samples were then found positive to this latest test (Table 2). If we consider the IDEXX® ELISA to be the gold standard, calculated Positive Predictive Value (PPV) of the IDvet® ELISA test is then 77.1% (95%CI; 59.9–89.6).
Table 2.
Agreement between the IDvet® Elisa test and the IDEXX ELISA test results (35 positive samples at IDvet® ELISA).
|
IDEXX ELISA |
|||
|---|---|---|---|
| Negative | Positive | ||
| IDvet ELISA | Positive (N = 8) | 2 (25.0%) | 6 (75.0%) |
| Strongly positive (N = 27) | 6 (27.8%) | 21 (77.8%) | |
4. Discussion
The seroprevalence of 26.3% detected in RUSVM resident sheep can be considered the first proof of the presence of Coxiella burnetii on St. Kitts. Introduction of new animals through importation of sheep to the island is rare. Thus, the RUSVM sheep were most likely exposed to the bacterium at their farm of origin. On St. Kitts, to our knowledge, only one animal study was undertaken in 2008 where 55 cattle, 5 sheep, 18 goats and 34 cats were sampled. One goat and one cat were found seropositive [23]. Our study found a much higher seroprevalence in sheep, while the seroprevalence in cattle was the same (0%). While the seroprevalence was considered low in the aforementioned study, given the small sample size, it is not possible to definitely conclude that the pathogen was present or absent. It could be that the cat and the goat were false positive results. Moreover, IgG can be detected up to one year after exposure, so knowing when the bacterium was introduced in animal populations on St. Kitts is almost impossible. Seroprevalence of 13.6% in healthy pregnant women in 2009–2011 [21] tends to show a presence of C. burnetii in St. Kitts before 2010. Another hypothesis from this low prevalence in the 2008 study would be a spatial heterogeneity of the endemicity. This is supported by our map of farm origin (Fig. 1) and makes us hypothesize that only some areas of the island are endemic. On the other hand, sheep in St. Kitts are predominantly free-roaming flocks, and trade between farms is frequent. Such practices would naturally promote endemic bacterial spread across the island. This should be investigated further with an island-wide study to confirm or refute this hypothesis of spatial heterogeneity.
Coxiella burnetii has been previously reported in the Caribbean region. Curaçao and Trinidad had seropositive farms [22, A. Dwarkasing, personal communication, November 2018], a serosurvey in 2008 detected one seropositive sheep in Dominica [23], French Guyana reported clinical cases [M. Vely, personal communication, November 2018] and the bacterium was isolated from ticks in Cuba [24]. Investigations in St. Lucia, Montserrat and Nevis concluded that the bacteriun was not present in animals on those islands [23,25]. Other territories such as the Cayman Islands and the U.S. Virgin Islands have never officially reported any cases of C. burnetii, but to date no investigation has been conducted [K. Gikonyo, B. Bradford, personal communications, November 2018].
The seroprevalence we detected in the sheep is similar to findings in Curaçao where 30% of goats and sheep were seropositive using an ELISA test [A. Dwarkasing, Personal communication, November 2018]. This high seroprevalence in small ruminants can be found in countries extensively farming small ruminants, for example 33.3% of sheep in Thailand [29], 6.7–20% of sheep and 20–46% of goats in Kenya [30] or 54.2% of goats in Ethiopia [31]. Figures in the U.S. are much lower at a seroprevalence of 5% in Idaho sheep [32], but this low number doesn't decrease the risk of sheep being reservoirs and a source of human infection, as sheep have been implicated in C. burnetii outbreaks in the U.S. [33,34].
The absence of anti-C. burnetii antibodies in the RUSVM cattle herd leads us to hypothesize that the bacterium is not present on RUSVM campus. The herd is closed and no introduction of new animals has occurred during the last years. This may have protected the herd from the introduction of carrier animals. RUSVM's sheep and cattle herds are housed separately. However, distance between both is less than 100 m and trade winds (an important mode of transmission for Q fever) are often strong on St. Kitts. If the sheep were reservoirs of the bacterium on campus, they would have likely transmitted it to cattle through the inhalation of contaminated dust. While a number of abortion events have occurred in RUSVM cattle during the last few years, the number or frequency did not elicit an investigation for Q fever. On St. Kitts, there is no intensive cattle or sheep farming. In general, abortions in cattle and sheep, if reported, are not extensively investigated. An economic evaluation exercise to estimate the impact of the disease on livestock production in St. Kitts is warranted.
Because the modes of transmission of C. burnetii are varied including direct (contact with infected placenta, birth products or through milk) or indirect (contaminated soil, dust or ticks) [1], there are opportunities for the disease to disseminate widely between humans and animals on the island. Our results here suggest the presence, and maybe a high prevalence of C. burnetii in St. Kitts. The island of St. Kitts has unique characteristics that favor the transmission of the bacterium to humans such as predominantly extensive ruminant farming on a small island (174 km2). This increases the probability of contact between farm animals and the general population. If the disease is present in St. Kitts, then zoonotic transmission is potentially occurring to farmers, similar to what has been reported in other countries. For example, in South Africa, a seroprevalence of 61% was found in people working with cattle [35]. The same cattle population had a seroprevalence of 38% [36]. Risk to the general population should also be taken into account as the farming animals roam on roads and private properties. Most of the infections in humans are asymptomatic but also underreported [37,38]. However, complications such as endocarditis or hepatitis can be fatal [5]. Our results, in addition to the seroprevalence of 13.6% in healthy pregnant women [21], stress the need for Kittitian medical and public health services to test for C. burnetii in cases of associated symptoms. Pachira et al. (2012) recommends that all patients with blood culture-negative endocarditis be tested for Q fever [39]. To assess the risk for the different populations (farmers, veterinarians/students, general population), serology investigations and passive surveillance are warranted.
C. burnetii reservoirs in St. Kitts should be identified to adopt appropriate control measures, but also protect the endangered wildlife, such as the white-tailed deer. Free-living cervids and ovis were previously reported as exposed species [11,40]. Also some wild mammals of St. Kitts could be candidate reservoirs including rodents, white tail deer, African green monkeys, or small Indian mongooses. At the border of the Caribbean Sea, French Guyana identified the three-toed sloth to be a reservoir [41]. In Cuba, C. burnetii has been detected in ticks [24]. Dermacentor spp. is thought to be present in St. Kitts, and warrants further investigation. Reservoir hosts could include various animals living in the different Caribbean islands.
Finally, our results here confirm poor sensitivity of the complement-fixation test (CFT) because none of our ELISA positive samples were positive with CFT. This is lower than the parameters reported in the literature [[42], [43], [44], [45], [46], [47]]. Some previous studies reported discordant individual results between ELISA kits [45,46], similar to our findings here. It is generally accepted that CFT has a better sensitivity to detect IgM antibodies and therefore recent infection [46,48]. World Organisation for Animal Health (OIE) advises the use of the CFT to differentiate latent and evolving phases of infection, while ELISA is more reliable for seroprevalence [49]. ELISA assays can be used in various species (cattle, sheep, goat and others), however sensitivity and specificity of tests are variable between the different species. Based on our results, it may not be advisable to use the CFT, but instead to encourage the use of indirect immunofluorescent antibody test (IFAT) [50], or the enzyme-linked immunosorbent assay (ELISA) in areas where the presence of the bacterium is unknown (as the Caribbean islands).
In our study, we only collected data at the scale of a veterinary campus. While we can not extrapolate the C. burnetii seroprevalence to the entire island of St. Kitts or to other Caribbean islands, the detection of antibodies in the local sheep provides a need for further studies. Isolation of the pathogen or detection of the antigen by polymerase chain reaction (PCR) with genotyping in local animals is needed in the future to investigate the epidemiology and the pathogenicity of C. burnetii in St. Kitts. It was previously believed that Q fever was not present or at low prevalence in St. Kitts, and regionally only few studies were conducted to evaluate the importance of the disease. With our study, it is obvious that more interest should be given to this disease. Further investigations to evaluate the prevalence and potential risk of Q fever in the Caribbean are thus warranted as they could inform the development of prevention and control recommendations and mitigate consequences on animal health, public health and conservation in this region.
Acknowledgments
Acknowledgement
We thank Dr. Roger Hancock (Department of Clinical Sciences), the Department of Animal Resources and the Ross University American Association of Small Ruminant Practitioners, who helped us to take blood samples at Ross University School of Veterinary Medicine (RUSVM). We also would like to acknowledge the Caribbean Animal Health Network (CaribVET), its coordinator Dr. Jennifer Pradel and its members for their invaluable information about Q fever in the Caribbean region. We thank Dr. Albert van Geelen and the National Veterinary Services Laboratory (NVSL) for running and interpreting the confirmatory testing.
Funding: This work was supported by the RUSVM One Health Center for Zoonoses and Tropical and Veterinary Medicine and the department of Animal Resources.
Conflict of interest
The authors declare no conflicts of interest.
Author contributions
Conceptualization: ACo, AB, HA, CG; Data curation: ACo, AB, VA; Formal analysis: ACo, AB, VA; Funding acquisition: ACo, HA; Investigation: ACo, VA, ACh, JC, KR; Methodology: ACo, CG; Project administration: ACo; Roles/Writing - original draft: ACo, CG; Writing - review & editing: AB, VA, ACh, JC, KR, HA.
References
- 1.Quinn P.J., Markey B.K., Leonard F.C., Hartigan P., Fanning S., Fitzpatrick E.S. John Wiley & Sons; 2011. Veterinary Microbiology and Microbial Disease. [Google Scholar]
- 2.Grist N.R., Ross C.A., fever Q. Br. Med. J. 1968;2:119–120. doi: 10.1136/bmj.2.5597.119-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olivier-Gougenheim L., Freychet C., Collardeau-Frachon S., Roure-Sobas C., Di Filippo S., Riva R., Lega J.-C., Belot A. A quest for Q fever. Lancet. 2019;394:419. doi: 10.1016/S0140-6736(19)31675-7. [DOI] [PubMed] [Google Scholar]
- 4.R. Bauerfeind, A. von Graevenitz, P. Kimmig, H.G. Schiefer, T. Schwarz, W. Slenczka, H. Zahner, Zoonoses: Infectious diseases transmissible from animals and humans, fourth edition, American Society of Microbiology, 2016. doi: 10.1128/9781555819262. [DOI]
- 5.Straily A., Dahlgren F.S., Peterson A., Paddock C.D. Surveillance for Q fever endocarditis in the United States, 1999–2015. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2017;65:1872–1877. doi: 10.1093/cid/cix702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agerholm J.S. Coxiella burnetii associated reproductive disorders in domestic animals-a critical review. Acta Vet. Scand. 2013;55 doi: 10.1186/1751-0147-55-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canevari J.T., Firestone S.M., Vincent G., Campbell A., Tan T., Muleme M., Cameron A.W.N., Stevenson M.A. The prevalence of Coxiella burnetii shedding in dairy goats at the time of parturition in an endemically infected enterprise and associated milk yield losses. BMC Vet. Res. 2018;14 doi: 10.1186/s12917-018-1667-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plummer P.J., McClure J.T., Menzies P., Morley P.S., Van den Brom R., Van Metre D.C. Management of Coxiella burnetii infection in livestock populations and the associated zoonotic risk: a consensus statement. J. Vet. Intern. Med. 2018;32:1481–1494. doi: 10.1111/jvim.15229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdel-Moein K.A., Hamza D.A. Rat as an overlooked reservoir for Coxiella burnetii: a public health implication. Comp. Immunol. Microbiol. Infect. Dis. 2018;61:30–33. doi: 10.1016/j.cimid.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Meredith A.L., Cleaveland S.C., Denwood M.J., Brown J.K., Shaw D.J. Coxiella burnetii (Q-fever) seroprevalence in prey and predators in the United Kingdom: evaluation of infection in wild rodents, foxes and domestic cats using a modified ELISA. Transbound. Emerg. Dis. 2015;62:639–649. doi: 10.1111/tbed.12211. [DOI] [PubMed] [Google Scholar]
- 11.Psaroulaki A., Chochlakis D., Angelakis E., Ioannou I., Tselentis Y. Coxiella burnetii in wildlife and ticks in an endemic area. Trans. R. Soc. Trop. Med. Hyg. 2014;108:625–631. doi: 10.1093/trstmh/tru134. [DOI] [PubMed] [Google Scholar]
- 12.Cooper A., Stephens J., Ketheesan N., Govan B. Detection of Coxiella burnetii DNA in wildlife and ticks in northern Queensland, Australia. Vector-Borne Zoonotic Dis. 2013;13:12–16. doi: 10.1089/vbz.2011.0853. [DOI] [PubMed] [Google Scholar]
- 13.Široký P., Kubelová M., Modrý D., Erhart J., Literák I., Špitalská E., Kocianová E. Tortoise tick Hyalomma aegyptium as long term carrier of Q fever agent Coxiella burnetii—evidence from experimental infection. Parasitol. Res. 2010;107:1515–1520. doi: 10.1007/s00436-010-2037-1. [DOI] [PubMed] [Google Scholar]
- 14.Mediannikov O., Fenollar F., Socolovschi C., Diatta G., Bassene H., Molez J.-F., Sokhna C., Trape J.-F., Raoult D. Coxiella burnetii in humans and ticks in rural Senegal. PLoS Negl. Trop. Dis. 2010;4 doi: 10.1371/journal.pntd.0000654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fox-Lewis A., Isteed K., Austin P., Thompson-Faiva H., Wolfgang J., Ussher J.E. A case of imported Q fever in New Zealand. N. Z. Med. J. 2019;132:92–94. [PubMed] [Google Scholar]
- 16.Pexara A., Solomakos N., Govaris A. Coxiella burnetii in wildlife and ticks in an endemic area. Vet. Ital. 2018:265–279. doi: 10.12834/VetIt.1113.6046.3. [DOI] [PubMed] [Google Scholar]
- 17.Rahaman M.R., Milazzo A., Marshall H., Bi P. Spatial, temporal, and occupational risks of Q fever infection in South Australia, 2007–2017. J. Infect. Public Health. 2019 doi: 10.1016/j.jiph.2019.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Gidding H.F., Wallace C., Lawrence G.L., McIntyre P.B. Australia’s national Q fever vaccination program. Vaccine. 2009;27:2037–2041. doi: 10.1016/j.vaccine.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 19.Schneeberger P.M., Wintenberger C., van der Hoek W., Stahl J.P. Q fever in the Netherlands – 2007–2010: what we learned from the largest outbreak ever. Médecine Mal. Infect. 2014;44:339–353. doi: 10.1016/j.medmal.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Federal Select Agent Program 2019. https://www.selectagents.gov/ (accessed December 23, 2019)
- 21.Wood H., Drebot M.A., Dewailly E., Dillon L., Dimitrova K., Forde M., Grolla A., Lee E., Loftis A., Makowski K., Morrison K., Robertson L., Krecek R.C. Seroprevalence of seven zoonotic pathogens in pregnant women from the Caribbean. Am. J. Trop. Med. Hyg. 2014;91:642–644. doi: 10.4269/ajtmh.14-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adesiyun A.A., Cazabon E.P. Seroprevalences of brucellosis, Q-fever and toxoplasmosis in slaughter livestock in Trinidad. Rev. Elev. Med. Vet. Pays Trop. 1996;49:28–30. [PubMed] [Google Scholar]
- 23.Johnson J.W., Lucas H., King S., Caron T., Wang C., Kelly P.J. Serosurvey for Brucella spp. and Coxiella burnetii in animals on Caribbean islands. Vet. Med. Sci. 2019 doi: 10.1002/vms3.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noda A.A., Rodríguez I., Miranda J., Contreras V., Mattar S. First molecular evidence of Coxiella burnetii infecting ticks in Cuba. Ticks Tick-Borne Dis. 2016;7:68–70. doi: 10.1016/j.ttbdis.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 25.Stone D.M., Kumthekar S., Chikweto A., Thomas D., Tiwari K., Sharma R.N. Exposure to zoonotic abortifacients among sheep and goats in Grenada. International Journal of Animal and Veterinary Advances. 2012;4(2):113–118. http://agris.fao.org/agris-search/search.do?recordID=DJ2012072835 (accessed February 14, 2019) [Google Scholar]
- 26.R Core Team R: A Language and Environment for Statistical Computing. 2017. http://www.R-project.org/
- 27.Virasakdo Chongsuvivatwong, epiDispay: Epidemiological Data Display Package. R package version 3.5.0.1, (2018).
- 28.QGIS Development Team QGIS Geographic Information System. Open Source Geospatial Foundation Project. 2013. http://qgis.osgeo.org/
- 29.Colombe S., Watanapalachaigool E., Ekgatat M., Ko A.I., Hinjoy S. Cross-sectional study of brucellosis and Q fever in Thailand among livestock in two districts at the Thai-Cambodian border, Sa Kaeo province. One Health. 2018;6:37–40. doi: 10.1016/j.onehlt.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Njeru J., Henning K., Pletz M.W., Heller R., Neubauer H. Q fever is an old and neglected zoonotic disease in Kenya: a systematic review. BMC Public Health. 2016;16 doi: 10.1186/s12889-016-2929-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gumi B., Firdessa R., Yamuah L., Sori T., Tolosa T., Aseffa A., Zinsstag J., Schelling E. Seroprevalence of brucellosis and Q-fever in southeast Ethiopian pastoral livestock. J. Vet. Sci. Med. Diagn. 2013;2 doi: 10.4172/2325-9590.1000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oliveira R.D., Mousel M.R., Pabilonia K.L., Highland M.A., Taylor J.B., Knowles D.P., White S.N. Domestic sheep show average Coxiella burnetii seropositivity generations after a sheep-associated human Q fever outbreak and lack detectable shedding by placental, vaginal, and fecal routes. PLoS One. 2017;12 doi: 10.1371/journal.pone.0188054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McQuiston J.H., Childs J.E. Q fever in humans and animals in the United States. Vector-Borne Zoonotic Dis. 2002;2:179–191. doi: 10.1089/15303660260613747. [DOI] [PubMed] [Google Scholar]
- 34.Guo H.-R., Gilmore R., Waag D., Shireley L., Freund E. Prevalence of Coxiella burnetii infections among North Dakota sheep producers. J. Occup. Environ. Med. 1998;40:999–1006. doi: 10.1097/00043764-199811000-00011. [DOI] [PubMed] [Google Scholar]
- 35.Simpson G.J.G., Quan V., Frean J., Knobel D.L., Rossouw J., Weyer J., Marcotty T., Godfroid J., Blumberg L.H. Prevalence of selected zoonotic diseases and risk factors at a human-wildlife-livestock interface in Mpumalanga province, South Africa. Vector-Borne Zoonotic Dis. 2018;18:303–310. doi: 10.1089/vbz.2017.2158. [DOI] [PubMed] [Google Scholar]
- 36.Adesiyun A.A., Knobel D.L., Thompson P.N., Wentzel J., Kolo F.B., Kolo A.O., Conan A., Simpson G.J.G. Sero-epidemiological study of selected zoonotic and abortifacient pathogens in cattle at a wildlife-livestock interface in South Africa. Vector-Borne Zoonotic Dis. 2019 doi: 10.1089/vbz.2019.2519. vbz.2019.2519. [DOI] [PubMed] [Google Scholar]
- 37.Echeverría G., Reyna-Bello A., Minda-Aluisa E., Celi-Erazo M., Olmedo L., García H.A., Garcia-Bereguiain M.A., de Waard J.H. Serological evidence of Coxiella burnetii infection in cattle and farm workers: is Q fever an underreported zoonotic disease in Ecuador? Infect. Drug Resist. 2019;12:701–706. doi: 10.2147/IDR.S195940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaufman H.W., Chen Z., Radcliff J., Batterman H.J., Leake J. Q fever: an under-reported reportable communicable disease. Epidemiol. Infect. 2018;146:1240–1244. doi: 10.1017/S0950268818001395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pachirat O., Fournier P.-E., Pussadhamma B., Taksinachanekij S., Lulitanond V., Baggett H.C., Thamthitiwat S., Watt G., Raoult D., Maloney S.A. The first reported cases of Q fever endocarditis in Thailand. Infect. Dis. Rep. 2012;4:17–18. doi: 10.4081/idr.2012.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zanatto D.C. de S., Duarte J.M.B., Labruna M.B., Tasso J.B., Calchi A.C., Machado R.Z., André M.R. Evidence of exposure to Coxiella burnetii in neotropical free-living cervids in South America. Acta Trop. 2019;197 doi: 10.1016/j.actatropica.2019.05.028. [DOI] [PubMed] [Google Scholar]
- 41.Pommier de Santi V., Briolant S., Mahamat A., Ilcinkas C., Blanchet D., de Thoisy B., Reynaud Y., Hyvert G., Marié J.-L., Edouard S., Davoust B., Raoult D. Q fever epidemic in Cayenne, French Guiana, epidemiologically linked to three-toed sloth. Comp. Immunol. Microbiol. Infect. Dis. 2018;56:34–38. doi: 10.1016/j.cimid.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 42.Emery M.P., Ostlund E.N., Schmitt B.J. Comparison of Q fever serology methods in cattle, goats, and sheep. J. Vet. Diagn. Investig. 2012 doi: 10.1177/1040638711434943. [DOI] [PubMed] [Google Scholar]
- 43.Field P.R., Mitchell J.L., Santiago A., Dickeson D.J., Chan S.-W., Ho D.W.T., Murphy A.M., Cuzzubbo A.J., Devine P.L. Comparison of a commercial enzyme-linked immunosorbent assay with immunofluorescence and complement fixation tests for detection of Coxiella burnetii (Q fever) immunoglobulin M. J. Clin. Microbiol. 2000;38:1645–1647. doi: 10.1128/jcm.38.4.1645-1647.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gyuranecz M., Dénes B., Hornok S., Kovács P., Horváth G., Jurkovich V., Varga T., Hajtós I., Szabó R., Magyar T., Vass N., Hofmann-Lehmann R., Erdélyi K., Bhide M., Dán Á. Prevalence of Coxiella burnetii in Hungary: screening of dairy cows, sheep, commercial milk samples, and ticks. Vector-Borne Zoonotic Dis. 2012;12:650–653. doi: 10.1089/vbz.2011.0953. [DOI] [PubMed] [Google Scholar]
- 45.Horigan M.W., Bell M.M., Pollard T.R., Sayers A.R., Pritchard G.C. Q fever diagnosis in domestic ruminants: comparison between complement fixation and commercial enzyme-linked immunosorbent assays. J. Vet. Diagn. Investig. 2011;23:924–931. doi: 10.1177/1040638711416971. [DOI] [PubMed] [Google Scholar]
- 46.Kittelberger R., Mars J., Wibberley G., Sting R., Henning K., Horner G.W., Garnett K.M., Hannah M.J., Jenner J.A., Piggott C.J., O’Keefe J.S. Comparison of the Q-fever complement fixation test and two commercial enzyme-linked immunosorbent assays for the detection of serum antibodies against Coxiella burnetti (Q-fever) in ruminants : recommendations for use of serological tests on imported animals in New Zealand. N. Z. Vet. J. 2009;57:262–268. doi: 10.1080/00480169.2009.58619. [DOI] [PubMed] [Google Scholar]
- 47.Muleme M., Stenos J., Vincent G., Campbell A., Graves S., Warner S., Devlin J.M., Nguyen C., Stevenson M.A., Wilks C.R., Firestone S.M. Bayesian validation of the indirect immunofluorescence assay and its superiority to the enzyme-linked immunosorbent assay and the complement fixation test for detecting antibodies against Coxiella burnetii in goat serum. Clin. Vaccine Immunol. CVI. 2016;23:507–514. doi: 10.1128/CVI.00724-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Skevaki C.L., Papadopoulos N.G., Tsakris A., Johnston S.L. Microbiologic diagnosis of respiratory illness, Kendig Chernicks Disord. Respir. Tract Child. 2012:399–423. doi: 10.1016/B978-1-4377-1984-0.00024-3. [DOI] [Google Scholar]
- 49.OIE, Q Fever, in . 2019. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. [Google Scholar]
- 50.Wood C., Muleme M., Tan T., Bosward K., Gibson J., Alawneh J., McGowan M., Barnes T.S., Stenos J., Perkins N., Firestone S.M., Tozer S. Validation of an indirect immunofluorescence assay (IFA) for the detection of IgG antibodies against Coxiella burnetii in bovine serum. Prev. Vet. Med. 2019;169 doi: 10.1016/j.prevetmed.2019.104698. [DOI] [PubMed] [Google Scholar]