Abstract
Objective
The toxicity associated with the use of kanamycin includes irreversible hearing loss. There is limited data describing the relationship between hearing loss and kanamycin pharmacokinetics (PK). We explored the association of kanamycin PK with hearing loss in patients on MDR-TB treatment.
Design
We prospectively recruited patients on kanamycin-based MDR-TB treatment in Cape Town. Hearing thresholds from 0.25 to 16 kHz were tested at baseline and at 4, 8 and 12 weeks. We determined kanamycin concentrations at steady-state in serial plasma samples over 10 hours, and explored factors associated with hearing loss.
Study sample
One hundred and two participants including 58 (56.9%) men had analysable audiometric data; median age was 34.9 years, 65 (63.7%) were HIV-positive, and 24 (23.5%) had been treated for MDR-TB previously.
Results
Eighty-four participants (82.4%) developed hearing loss. We found a 3% (95% CI: 1 to 6%, p=0.028) increased risk of cochleotoxicity for each 10μg•hr/L increase in 0–10 hour AUC.
Conclusion
We describe a high incidence of hearing loss in MDR-TB patients treated with kanamycin, with higher AUC0–10 significantly associated with hearing loss.
Keywords: cochleotoxicity, AUC, aminoglycosides, kanamycin, hearing loss, pharmacokinetics
INTRODUCTION
Risk factors for aminoglycoside-induced hearing loss, which ranges from 18% to 90%, 1–7 include: older age, HIV, excessive noise, the presence of identified mitochondrial mutations, prior use of a drug known to cause hearing loss and high aminoglycoside plasma concentrations.5,8,9 The WHO currently recommends that an aminoglycoside be included in the shortened treatment regimen for MDR-TB, and be considered in longer regimens where there is toxicity or intolerability of one of the group A or group B drugs.10 Although kanamycin-induced hearing loss is well described,1,3,6 there is limited data describing the relationship between kanamycin pharmacokinetics (PK) including area under the concentration-time curve, and hearing loss. 11,12 Furthermore, few studies have included ultra-high frequency audiometry. One recent study showed a cumulative dose-response relationship between amikacin exposure and hearing loss in patients on treatment for MDR-TB.12 We measured ototoxicity prospectively including ultra-high frequency audiometry in a cohort of patients treated for MDR-TB, and determined the pharmacokinetic parameters of kanamycin. We then explored some of the risk factors for kanamycin-induced hearing loss including kanamycin PK.
MATERIALS AND METHODS
We performed a prospective observational cohort study in adult patients on treatment for pulmonary MDR-TB at two TB hospitals in Cape Town: Brooklyn Chest Hospital and DP Marais Hospital. We enrolled patients 18 years of age or older initiated on therapy for MDR-TB within the previous month, and explored the relationship between covariates including kanamycin exposure with hearing loss. Patients with middle ear pathology were excluded from the hearing analysis. During the study period, the standard regimen for MDR-TB consisted of pyrazinamide, moxifloxacin, kanamycin, terizidone and either ethionamide or isoniazid (depending on the presence of katG and inhA mutations identified by line-probe assay in the pre-treatment sputum culture, indicating high-level resistance to isoniazid or low-level resistance to isoniazid and resistance to ethionamide, respectively).13 Ethambutol was added if the risk of ethambutol resistance was considered to be low. Kanamycin was dosed intramuscularly daily, 6 times per week at 15mg/kg per dose according to the South African Department of Health guidelines during the study period,14 and adjusted for renal dysfunction at the discretion of the treating clinician. We assessed renal function at 4, 8 and 12 weeks post treatment initiation, using the Cockroft-Gault method to calculate creatinine clearance.
Audiological assessment
We performed pure tone audiometry including ultra-high frequencies (12.5–16 kHz), using an Interacoustic AC40–audiometer. Hearing assessments were performed at baseline and at 4, 8 and 12 weeks after starting treatment. American Speech Hearing Association criteria15 were used to define cochleotoxic hearing loss, comparing the baseline with follow-up audiograms as follows: a shift of 10 dB at any two contiguous frequencies with reference to the baseline audiogram, a 20 dB shift at any one test frequency, or loss of response at three contiguous frequencies where responses were previously obtained.15 A minimum of two audiograms were required to include participants in the hearing analysis. We used the University of Cape Town (UCT) cochleotoxic criteria16 to classify the grade of hearing loss as the currently used cochleotoxicity scales for adults (CTCAEV & TUNE)17–19 use conventional-frequency ranges and do not include ultra-high frequency testing (12.5–16 kHz), which we performed in our study.
Pharmacokinetic sampling
We performed pharmacokinetic sampling once patients were established on treatment between two and six weeks. Blood was drawn at the following time points: predose and at 2, 4, 6, 8 and 10 hours postdose. Dosing was strictly observed and performed under fasting conditions. Blood samples were immediately centrifuged and the plasma was stored at −70°C. Kanamycin concentrations were measured using liquid chromatography tandem mass spectrometry (LC-MS/MS) using methods validated according to US Food and Drug Administration20 and European Medicines Agency guidelines.21 The samples were processed with a solid phase extraction method using 50μl plasma. Five microliters of the extracted sample were injected onto the HPLC column. Isocratic chromatographic separation was achieved on a Discovery C18, 5μm, 50 mm x 4.6 mm analytical column using 4 mM HFBA in 0.1% formic acid in water / acetonitrile (80:20, v/v) at a flow-rate of 500 μl/min. The mobile phase flow was split (1:1) at the source of the mass spectrometer. An AB Sciex API 3000 mass spectrometer was operated at unit resolution in the multiple-reaction monitoring mode, monitoring the transition of the protonated molecular ions at m/z 485.2 to the product ions at m/z 163.2 for kanamycin A and the protonated molecular ions at m/z 494.3 to the product ions at m/z 165.3 for the Kanamycin-d9 internal standard. Electrospray ionization was used for ion production. The assay was validated over the concentration range of 0.625 to 40 μg/ml. The combined accuracy (%Nom) and precision (%CV) statistics of the lower limit of quantification (LLQ), low, medium, and high-quality controls (3 validation batches, n=18) were between 101.3% and 107.0%, and 3.0% and 14.3%, respectively.
Statistical analysis
We imputed predose kanamycin plasma concentrations below LLQ (0.625 μg/mL) as half the LLQ value and used STATA version 15.0 (Stata Corp, College Station, Texas, USA) to perform the non-compartmental and statistical analyses. Area under the concentration-time curve (AUC) from 0 to 10 hours after the dose (AUC0–10), AUC to infinity (AUC∞), half-life, peak concentration, and time to peak concentration were assessed. The trapezoidal rule was applied for computation of the AUC0–10 and the exponential extrapolation option was used to calculate AUC∞. We calculated the cumulative dose of kanamycin by multiplying the dose by the number of days a particular dose was administered before hearing loss developed. The average daily dose was calculated by dividing the cumulative dose of kanamycin by the number of days recorded from treatment initiation to first detection of hearing loss. Cumulative AUC was measured by multiplying the AUC0–10 on the PK sampling day by the number of days the same dose was administered before first detection of hearing loss. If the dose was changed during the treatment period, we predicted the change in AUC by increasing or decreasing the exposure proportionally to the change in dose, since AUC after parental administration equals dose divided by clearance. For example, if the dose of kanamycin was halved by the treating clinician, assuming linear kanamycin pharmacokinetics, we considered the AUC to be 50% lower for the time period that the lower dose was administered. We calculated the average daily AUC of kanamycin by dividing the cumulative AUC0–10 of kanamycin by the number of days from treatment initiation to first detection of hearing loss.
We explored factors associated with hearing loss using Cox proportional hazards regression, including the following factors in the univariate model: sex, age, previous exposure to second line anti-TB drugs, HIV status and AUC0–10. We included covariates with a p value of <0.2 in the multivariate model, and used Kaplan-Meier failure analyses to estimate the incidence of cochleotoxicity over time. We used the two-sample Wilcoxon rank-sum (Mann-Whitney) test to compare cumulative and average daily dose and AUC between participants with and without hearing loss.
Ethics approval
The study protocol was reviewed and approved by the Human Research Ethics Committee at the University of Cape Town (HREC 065/2015). Written informed consent was taken from each participant in a language of their choice (either English, Afrikaans or isiXhosa).
RESULTS
Participant characteristics are shown in Table 1. Of the 147 participants initially recruited into the study, 102 (69.4%) had analyzable hearing data. The reasons why 45 participants (30.6%) were unable to complete the two valid hearing tests required for the analysis were as follows: ten were discharged from hospital prior to study completion, five withdrew from the study, five died, five were too sick for the hearing tests to be completed timeously, two left hospital against medical advice, one participant was transferred out to another facility, six had comorbid medical conditions that made the hearing tests uninterpretable, and 11 participants had hearing test results, which were determined by the study audiologist to be unreliable.
Table 1:
Participant characteristics of 102 patients with analyzable hearing data on treatment with kanamycin for multidrug-resistant tuberculosis
| Variable | Value |
|---|---|
| No. (%) male | 58 (56.9%) |
| No. (%) HIV infected | 65 (63.7%) |
| No. (%) with previous MDR-TB treatment | 24 (23.5%) |
| Median age, yr | 34.9 (27.2–42.2) |
| Median BMI, kg/m2 | 17.3 (15.6–18.9) |
| Creatinine clearance, mL/min (n=95) | 79.7 (58.8 to 98.8) |
Where appropriate, the percentage/ interquartile range is shown in brackets
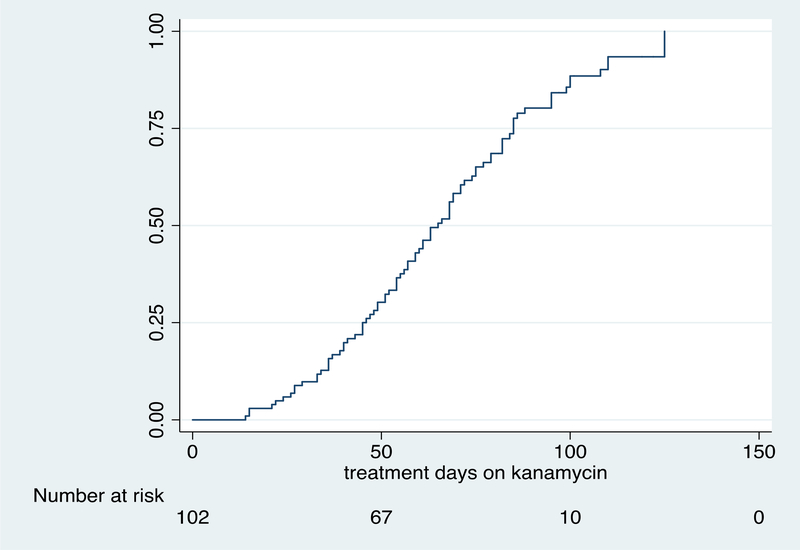
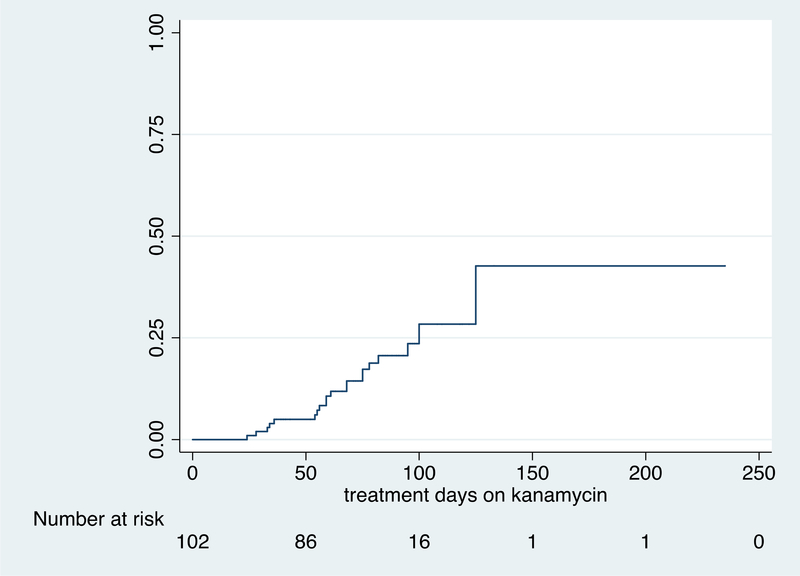
The median (IQR) duration of kanamycin therapy to first detection of hearing loss was 61 (43 to 81) days. Of the 102 participants with analyzable hearing data, 84 (82.4%) developed hearing loss on treatment with kanamycin including 20 participants (23.8%) who developed moderate-severe hearing loss. The grade of cochleotoxicity in those participants who developed hearing loss is shown in table 2.16 The key pharmacokinetics measures of kanamycin are shown in Table 3. Covariates associated with any degree of hearing loss and moderate to severe hearing loss are shown in tables 4 and 5 respectively. On multivariate analysis in those participants who developed any degree of cochleotoxicity, hearing loss was significantly associated with kanamycin AUC0–10 (aHR: 1.03, 95% CI: 1.00 to 1.06; p=0.028) – see table 4. We observed a stronger association between kanamycin AUC0–10 and moderate-severe hearing loss in a subgroup of 20 participants (HR: 1.05, 95% CI: 1.01 to 1.10; p=0.017) – see table 5. Table 6 compares cumulative kanamycin exposure between those who developed hearing loss and those who did not. Figures 1A and 1B show time to any grade of hearing loss and time to moderate-severe hearing loss respectively in the 102 participants with analysable hearing data. The follow up time period for figures 1A and 1B extends beyond the 12 week study period as the final hearing tests for some patients were either delayed or postponed for logistical reasons.
Table 2:
Grade of cochleotoxicity in 84 participants who developed hearing loss during the first 12 weeks of treatment with kanamycin for multidrug-resistant tuberculosis
| Grade of cochleotoxicity *(UCT criteria) | |||
|---|---|---|---|
| Grade of Impairment | Change in hearing thresholds [PTA: 0.5, 1, 2 & 4 kHz] (SANS 10154–1) | Description of activity limitation | n=84 |
| 0 (No impairment) | No significant change in hearing thresholds | None | 0 |
| Grade 1a (UHF impairment) | ≥10 dB threshold shift relative to baseline at ≥2 frequencies OR ≥ 20 dB threshold shift at ≥ 1 frequency; 9–16 kHz PTA: 10–15 dB HL |
None; able to hear a whisper | 10 |
| Grade 1b (Slight impairment) | ≥10 dB threshold shift relative to baseline at ≥2 frequencies OR ≥ 20 dB threshold shift at ≥ 1 frequency; 2–16 kHz PTA: 16–25 dB HL |
Slight hearing problems especially in the presence of background noise | 37 |
| Grade 2a (Mild Impairment) | ≥10 dB threshold shift relative to baseline at ≥2 frequencies OR ≥ 20 dB threshold shift at ≥ 1 frequency; 2–16 kHz PTA: 26–40 dB HL |
Able to hear and repeat words spoken in normal voice at 1 meter. Likely to experience difficulties listening in noisy environments | 17 |
| Grade 2b (Moderate Impairment) | ≥10 dB threshold shift relative to baseline at ≥2 frequencies OR ≥ 20 dB threshold shift at ≥ 1 frequency; 2–16 kHz PTA: 41–60 dB HL |
Able to hear some words when shouted into better ear | 10 |
| Grade 3 (severe Impairment) | ≥10 dB threshold shift relative to baseline at ≥2 frequencies OR ≥ 20 dB threshold shift at ≥ 1 frequency; 2–16 kHz PTA: 61–80 dB HL |
Able to hear some speech when shouted into better ear; more likely to have poor word discrimination scores | 10 |
| Grade 4 (Profound Impairment) | ≥10 dB threshold shift relative to baseline at ≥2 frequencies OR ≥ 20 dB threshold shift at ≥ 1 frequency; 2–16 kHz PTA ≥ 81 dB HL | Unable to hear speech even at a shouted voice. Less likely to benefit from conventional hearing aid | 0 |
UCT criteria for cochleotoxicity in adults (Ramma, 2016)
Grade of impairment is based on the most severely affected ear
PTA=pure tone average
UHF=Ultra high frequency
Table 3:
Pharmacokinetic measures of kanamycin exposure in participants on treatment for multidrug-resistant tuberculosis
| Pharmacokinetic measure | Hearing Loss (n=69) | No Hearing Loss(n=16) |
|---|---|---|
| Dose (mg/kg/day) | 15.9 (15.0 to 17.5) | 16.0 (14.5 to 17.2) |
| Peak concentration (μg/mL) | 36.4 (29.4 to 42.7) | 34.1 (29.5 to 38) |
| Trough concentration (μg/mL) | 0.3125 (0.3125 to 0.84) | 0.3125 (0.3125 to 0.3125) |
| AUC 0–10 (μg•hr/L) | 155.6 (127.3 to 212.1) | 152.5 (121.2 to 168.2) |
| AUC∞ (μg•hr/L) | 168.8 (134.6 to 244.0) | 160.1 (128.9 to 199.3) |
| Half- life (hours) | 2.5 (2.2 to 3.4) | 2.5 (2.2 to 2.9) |
Median is shown with interquartile range in brackets
Table 4.
Covariates associated with hearing loss in 102 participants on treatment with kanamycin for multidrug-resistant tuberculosis
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95%CI) | P value | aHR (95%CI) | P value | |
| Sex | 0.99 (0.64 to 1.53) | 0.968 | ||
| Age | 0.98 (0.96 to 1.00) | 0.058 | 0.97 (0.95 to 1.00) | 0.050 |
| HIV | 1.06 (0.67 to 1.67) | 0.813 | ||
| Previous MDR-TB treatment | 0.90 (0.56 to 1.45) | 0.670 | ||
| AUC0–10 (per 10 μg•hr/L increase) | 1.03 (1.00 to 1.05) | 0.051 | 1.03 (1.00 to 1.06) | 0.028 |
aHR: adjusted Hazard Ratio
HR: Hazard ratio
CI: Confidence Interval
Table 5.
Covariates associated with moderate-severe hearing loss in participants on treatment with kanamycin for multidrug-resistant tuberculosis
| Variable (n=20) | Univariate | |
|---|---|---|
| HR (95% CI) | P value | |
| Sex | 0.98 (0.40 to 2.36) | 0.959 |
| Age | 1.02 (0.98 to 1.06) | 0.302 |
| HIV | 1.71 (0.67 to 1.67) | 0.300 |
| Previous MDR-TB treatment | 0.61 (0.62 to 4.75) | 0.409 |
| AUC0–10 (per 10 μg•hr/L increase) | 1.05 (1.01 to 1.10) | 0.017 |
HR: Hazard ratio
CI: Confidence Interval
Table 6.
Comparison of key pharmacokinetic measures in participants with and without hearing loss, treated with kanamycin for multidrug-resistant tuberculosis
| Variable | Hearing loss (n=84)* | No hearing loss (n=18)* | P value |
|---|---|---|---|
| AUC0–10 | 155.6 (127.3 to 212.1) | 152.5 (121.2 to 168.2) | 0.425 |
| Cumulative AUC0–10 | 9450.9 (6541.3 to 12615.2) | 7226.8 (4794.7 to 9885.0) | 0.103 |
| Average daily AUC0–10 | 639.2 |(450.9 to 689) | 644.1 (477.6 to 666.7) | 0.567 |
| Cumulative dose (mg) | 46625 (33687.5 to 59375) | 41678 (27750 to 62500) | 0.390 |
| Average daily dose (mg) | 639.2 (450.9 to 689.0) | 644.1 (477.6 to 666.7) | 0.568 |
Median is shown with interquartile range in brackets
Figure 1A.
Time to any grade of hearing loss in 102 participants on treatment with kanamycin for multidrug-resistant tuberculosis
Figure 1B.
Time to moderate-severe grade of hearing loss in 102 participants on treatment with kanamycin for multidrug-resistant tuberculosis
DISCUSSION
We describe a nearly universal (>8 in 10 patients) incidence of hearing loss in patients treated with kanamycin for MDR-TB. Given that kanamycin-containing regimens have been associated with worse outcomes compared with regimens without it, this high level toxicity is not balanced by efficacy benefits.22 Our findings therefore support the WHO’s recent recommendation to remove kanamycin from MDR-TB treatment regimens.23 There are several possible reasons for the high incidence of hearing loss we observed. First, considering aminoglycosides initially affect the highest hearing frequencies, we tested ultra-high frequencies (up to 16 kHz) in all participants, which is higher than the maximum conventional frequency (8 kHz) tested in most studies.1,2,24 Thus, we diagnosed patients with hearing loss who would otherwise not be identified at conventional frequency thresholds. Second, HIV infection is a risk factor for hearing loss1 and there was a large proportion of HIV-infected patients in our cohort (63.7%). However, HIV-infection was not a significant risk factor in the univariate analysis in our study. Third, 24/102 participants (23.5%) had documented evidence of prior treatment for MDR-TB with aminoglycosides, which may have predisposed some participants to developing hearing loss. We did not find prior aminoglycoside use to be significantly associated with hearing loss, although we had limited information on previous MDR-TB treatment exposure at the time of recruitment.
We found kanamycin exposure to be significantly associated with hearing loss with a 3% increased risk of hearing loss for every 10 μg•hr/L increase in kanamycin AUC0–10 (see table 3). When we explored the association of kanamycin exposure in a subgroup of 20 participants who developed moderate-severe hearing loss, the effect of kanamycin exposure on hearing loss was enhanced (see table 5). Cumulative assessments of kanamycin exposure including AUC and dose as well as the average daily dose and AUC were not significantly higher in those participants who developed hearing loss compared with those who did not (see table 6). A possible reason for the lack of association between cumulative exposure and hearing loss, which has been described previously,12 is that the treating clinicians responded to the hearing test results in real time by either stopping or decreasing the dose of kanamycin, which may have attenuated the effect of cumulative exposure in those patients who developed hearing loss. A second possible reason could be statistical: the relationship between cumulative AUC and hearing loss described previously is non-linear while the regression method we used in our study follows a linear analytical approach. There was an unexpected trend toward younger patients being at higher risk of hearing loss, possibly due to patients with age-related hearing loss at baseline being excluded from the analysis. We also considered this may be due to a higher incidence of HIV in younger patients, which has previously been described as risk factor for hearing loss,1 although we did not find HIV to be associated with hearing loss in this study.
Aerosolized administration of kanamycin in the treatment of MDR-TB has been described as having the potential to reduce systemic exposure, and hence hearing loss, with enhanced kanamycin concentrations at the bronchi.25 Further research is required to determine the safety and efficacy of aerosolised kanamycin in the treatment of patients with MDR-TB. As aminoglycoside induced hearing loss is progressive from high to low frequencies, we used ultra-high frequency audiometry (up to 16 kHz) to detect early cochleotoxic hearing loss before damage to the speech frequency range occurs. In clinical practice, testing ultra-high frequencies may allow earlier detection of hearing loss thereby prompting clinicians to stop aminoglycosides before speech range hearing loss develops.
Our study has some limitations. First, a baseline audiogram could not be obtained before commencement of MDR-TB treatment in all participants, as some participants initiated treatment at local clinics prior to referral to the study sites. This may have led to under-reporting of hearing loss if only one audiogram was able to be performed at the TB hospitals. Second, because we measured the AUC to a maximum of 10 hours post dose, the cumulative AUC0–10 is likely an underestimation of the true cumulative AUC until the first detection of hearing loss. Third, we had limited information on previous aminoglycoside use and other risk factors such as genetic factors which are known to influence patients’ susceptibility to aminoglycoside-induced ototoxicity.9 Fourth, we were unable to include patients with only one hearing test or those with pre-existing hearing loss, which had a negative effect on our sample size.
CONCLUSION
Using ultra-high frequency audiometry, we report a high incidence of hearing loss in patients on treatment with kanamycin for MDR-TB, with approximately a quarter of patients with analysable data developing moderate to severe hearing loss. Higher Kanamycin AUC0–10 is strongly associated with an increased incidence of hearing loss, which adds to the growing body of evidence in support of the rollout of injectable-sparing treatment regimens for MDR-TB.
ACKNOWLEDGMENTS
This study was supported by a grant from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (R01AI116155 to Helen McIlleron and Tawanda Gumbo). The University of Cape Town (UCT) Clinical PK Laboratory is also supported by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health under award numbers UM1 AI068634, UM1 AI068636, and UM1 AI106701. Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) at UCT was provided by the National Institute of Allergy and Infectious Diseases (U01 AI068632), The Eunice Kennedy Shriver National Institute of Child Health and Human Development, and National Institute of Mental Health grant AI068632. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Helen McIlleron and Tawanda Gumbo are also supported by the National Research Foundation of South Africa (grant numbers 90729 and 85810 respectively). HM is also supported by the Wellcome Trust (206379/Z/17/Z). We would like to acknowledge the contributions of the patients who volunteered for the study.
Footnotes
Declaration of interest: there is no conflict of interest regarding the publication of this article.
REFERENCES
- 1.Harris A, Bardien S, Schaaf HS, Petersen L, de Jong G, Johannes JF. Aminoglycoside-induced hearing loss in HIV-positive and HIV-negative multidrug-resistant tuberculosis patients. South African Med J [Internet]. 2012. May 8 [cited 2018 Jul 12];102(6):363–6. Available from: http://www.samj.org.za/index.php/samj/article/view/4964/4128 [DOI] [PubMed] [Google Scholar]
- 2.Ghafari N, Rogers C, Petersen L, Singh SA. The occurrence of auditory dysfunction in children with TB receiving ototoxic medication at a TB hospital in South Africa. Int J Pediatr Otorhinolaryngol [Internet]. 2015. July [cited 2019 Feb 18];79(7):1101–5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26003627 [DOI] [PubMed] [Google Scholar]
- 3.Ramma L, Ibekwe TS. Cochleo-vestibular clinical findings among drug resistant Tuberculosis Patients on therapy-a pilot study. Int Arch Med [Internet]. 2012. January 31 [cited 2018 Dec 13];5(1):3 Available from: http://www.ncbi.nlm.nih.gov/pubmed/22293572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sturdy A, Goodman A, Jose RJ, Loyse A, O’Donoghue M, Kon OM, et al. Multidrug-resistant tuberculosis (MDR-TB) treatment in the UK: a study of injectable use and toxicity in practice. J Antimicrob Chemother [Internet]. 2011. August 1 [cited 2019 Mar 11];66(8):1815–20. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21642291 [DOI] [PubMed] [Google Scholar]
- 5.Brits J, Strauss S, Eloff Z, Becker PJ, Swanepoel DW. Hearing profile of gold miners with and without tuberculosis. Occup Environ Med [Internet]. 2012. April [cited 2018 Aug 30];69(4):243–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22094854 [DOI] [PubMed] [Google Scholar]
- 6.de Jager P, van Altena R. Hearing loss and nephrotoxicity in long-term aminoglycoside treatment in patients with tuberculosis. Int J Tuberc Lung Dis [Internet]. 2002. July [cited 2018 Dec 13];6(7):622–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12102302 [PubMed] [Google Scholar]
- 7.Heysell SK, Ahmed S, Rahman MT, Akhanda MW, Gleason AT, Ebers A, et al. Hearing loss with kanamycin treatment for multidrug-resistant tuberculosis in Bangladesh. Eur Respir J [Internet]. 2018. [cited 2019 Jul 10];51(3). Available from: http://www.ncbi.nlm.nih.gov/pubmed/29348152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Human H, Hagen CM, Jong G de, Harris T, Lombard D, Christiansen M, et al. Investigation of mitochondrial sequence variants associated with aminoglycoside-induced ototoxicity in South African TB patients on aminoglycosides. Biochem Biophys Res Commun [Internet]. 2010;393(4):751–6. Available from: 10.1016/j.bbrc.2010.02.075 [DOI] [PubMed] [Google Scholar]
- 9.Kokotas H, Petersen M, Willems P. Mitochondrial deafness. Clin Genet [Internet]. 2007. May 2 [cited 2018 Jul 12];71(5):379–91. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17489842 [DOI] [PubMed] [Google Scholar]
- 10.WHO. Rapid Communication: Key changes to treatment of multidrug- and rifampicin-resistant tuberculosis (MDR/RR-TB) [Internet]. 2018. [cited 2018 Dec 6]. Available from: https://www.who.int/tb/publications/2018/WHO_RapidCommunicationMDRTB.pdf?ua=1
- 11.van Altena R, Dijkstra JA, van der Meer ME, Borjas Howard JF, Kosterink JGW, van Soolingen D, et al. Reduced Chance of Hearing Loss Associated with Therapeutic Drug Monitoring of Aminoglycosides in the Treatment of Multidrug-Resistant Tuberculosis. Antimicrob Agents Chemother [Internet]. 2017. [cited 2019 Jan 8];61(3). Available from: http://www.ncbi.nlm.nih.gov/pubmed/28069654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Modongo C, Pasipanodya JG, Zetola NM, Williams SM, Sirugo G, Gumbo T. Amikacin concentrations predictive of ototoxicity in multidrug-resistant tuberculosis patients. Antimicrob Agents Chemother. 2015;59(10):6337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caminero JA, Sotgiu G, Zumla A, Migliori GB. Best drug treatment for multidrug-resistant and extensively drug-resistant tuberculosis. Lancet Infect Dis [Internet]. 2010. September [cited 2018 Dec 5];10(9):621–9. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1473309910701390 [DOI] [PubMed] [Google Scholar]
- 14.South African Department of Health. Management of drug-resistant tuberculosis. 2013;(January). Available from: https://www.health-e.org.za/wp-content/uploads/2014/06/MDR-TB-Clinical-Guidelines-Updated-Jan-2013.pdf
- 15.American Speech-Language Hearing Association. Audiologic Management of Individuals Receiving Cochleotoxic Drug Therapy [Internet]. 1994. March [cited 2018 Jul 12]. Available from: http://www.asha.org/policy/GL1994-00003/
- 16.Ramma L An alternative grading system for ototoxicity in adults: Towards a uniform international standard for grading ototoxicity. In: 3rd International Conference and Exhibition on Rhinology & Otology, Dubai, UAE 2016. [Google Scholar]
- 17.Theunissen EAR, Dreschler WA, Latenstein MN, Rasch CRN, van der Baan S, de Boer JP, et al. A New Grading System for Ototoxicity in Adults. Ann Otol Rhinol Laryngol [Internet]. 2014. October 12 [cited 2019 Feb 2];123(10):711–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24820112 [DOI] [PubMed] [Google Scholar]
- 18.Crundwell G, Gomersall P, Baguley DM. Ototoxicity (cochleotoxicity) classifications: A review. Int J Audiol [Internet]. 2016. February [cited 2019 Jul 10];55(2):65–74. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26618898 [DOI] [PubMed] [Google Scholar]
- 19.King KA, Brewer CC. Clinical trials, ototoxicity grading scales and the audiologist’s role in therapeutic decision making. Int J Audiol [Internet]. 2018. August 24 [cited 2019 Apr 19];57(sup4):S89–98. Available from: https://www.tandfonline.com/doi/full/10.1080/14992027.2017.1417644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.FDA center for drug evaluation and research. Guidance for industry: bioanalytical method validation. [Google Scholar]
- 21.European Medicines Agency. Guideline on bioanalytical method validation [Internet]. 2011. [cited 2018 Jul 13]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2011/08/WC500109686.pdf [DOI] [PubMed]
- 22.Ahmad N, Ahuja SD, Akkerman OW, Alffenaar JWC, Anderson LF, Baghaei P, et al. Treatment correlates of successful outcomes in pulmonary multidrug-resistant tuberculosis: an individual patient data meta-analysis. Lancet. 2018;392(10150):821–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.WHO. Rapid Communication: Key changes to treatment of multidrug-and rifampicin-resistant tuberculosis. 2018. [Google Scholar]
- 24.De Jager P, Van Altena R. Hearing loss and nephrotoxicity in long-term aminoglycoside treatment in patients with tuberculosis. INT J TUBERC LUNG DIS [Internet]. [cited 2018 Jul 12];6(7):622–7. Available from: https://www.ingentaconnect.com/contentone/iuatld/ijtld/2002/00000006/00000007/art00012?crawler=true [PubMed] [Google Scholar]
- 25.Momin MAM, Sinha S, Tucker IG, Doyle C, Das SC. Dry powder formulation of kanamycin with enhanced aerosolization efficiency for drug-resistant tuberculosis. Int J Pharm [Internet]. 2017. August 7 [cited 2019 Jul 10];528(1–2):107–17. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28583333 [DOI] [PubMed] [Google Scholar]