Key Points
Question
What is the most effective systemic treatment option in advanced hepatocellular carcinoma (HCC)?
Findings
In this network meta-analysis of 14 relevant phase 3 clinical trials, the combination of atezolizumab and bevacizumab was associated with superior results compared with other first-line agents including sorafenib and lenvatinib. In the refractory setting, both regorafenib and cabozantinib ranked highest compared with other agents including ramucirumab and pembrolizumab.
Meaning
The treatment landscape of advanced HCC is changing with the combination atezolizumab and bevacizumab now considered the standard of care in the first-line setting; regorafenib and cabozantinib are the preferred options in refractory patients.
This systematic review with meta-analysis compares the effectiveness of different vascular endothelial growth factor inhibitors, checkpoint inhibitors, or their combinations in patients with advanced hepatocellular carcinoma in the first-line or refractory settings.
Abstract
Importance
The treatment landscape for advanced hepatocellular carcinoma (HCC) has recently changed and become relatively confusing. Head-to-head comparisons between most of the available agents have not been performed and are less likely to be examined in a prospective fashion in the future. Therefore, a network meta-analysis (NMA) is helpful to compare different agents from across different trials.
Objective
To evaluate comparative effectiveness of different systemic treatments in advanced patients with HCC across lines of therapy.
Data Sources
We searched various databases for abstracts and full-text articles published from database inception through March 2020.
Study Selection
We included phase 3 trials evaluating different vascular endothelial growth factor inhibitors (VEGFis), checkpoint inhibitors (CPIs), or their combinations in advanced HCC, in the first-line or refractory setting.
Data Extraction and Synthesis
The reporting of this systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline. The overall effect was pooled using the random effects model.
Main Outcomes and Measures
Outcomes of interest included overall (OS) and progression-free survival (PFS).
Findings
Fourteen trials (8 in the first-line setting and 6 in the second-line setting) at low risk of bias were included. The 8 trials in the first-line setting encompassed a total of 6290 patients, with an age range of 18 to 89 years. The 5 trials included in the second-line analysis encompassed a total of 2653 patients, with an age range of 18 to 91 years. Network meta-analysis showed the combination of atezolizumab and bevacizumab was superior in patients with HCC treated in the first-line setting compared with lenvatinib (HR, 0.63; 95% CI, 0.44-0.89), sorafenib (HR, 0.58; 95% CI, 0.42-0.80), and nivolumab (HR, 0.68; 95% CI, 0.48-0.98). In the refractory setting, NMA showed that all studied drugs had PFS benefit compared with placebo. However, this only translated into OS benefit with regorafenib (HR, 0.62; 95% CI, 0.51-0.75) and cabozantinib (HR, 0.76; 95% CI, 0.63-0.92) compared with placebo. In the NMA of patients with α-fetoprotein (AFP) levels of 400 ng/mL or greater, regorafenib, cabozantinib, and ramucirumab showed PFS and OS benefit compared with placebo with no superiority of an active drug compared with any others.
Conclusions and Relevance
This systematic review and NMA of 14 trials found that atezolizumab and bevacizumab in combination is now considered the standard of care in the first-line setting in patients with advanced HCC. Regorafenib and cabozantinib are preferred options in refractory patients, with ramucirumab as an additional option in those with levels of AFP of 400 ng/mL or higher. Future trials should focus on other potential combinations and best treatment strategy in patients with prior VEGFi/CPI exposure.
Introduction
The mainstay of treatment for advanced hepatocellular carcinoma (HCC) was the tyrosine kinase inhibitor (TKI) sorafenib, which targets vascular endothelial growth factor (VEGF) receptors, among other kinases.1 Sorafenib showed overall survival (OS) benefit compared with placebo in the SHARP trial and, since then, multiple studies evaluating different agents have failed to show survival benefit until recently, when multiple VEGF TKIs showed survival benefit compared with placebo in the second-line setting2,3 and noninferiority compared with sorafenib in the untreated population.4 On the other hand, although checkpoint inhibitor monotherapy showed promising results in early phase 2 studies, subsequent phase 3 trials showed no improved efficacy compared with sorafenib and placebo in the first- and second-line setting, respectively.5,6 Most recently, the combination of atezolizumab, a checkpoint inhibitor, and bevacizumab showed—for the first time—an OS benefit compared with sorafenib in advanced treatment-naive patients with HCC.7 Therefore, systemic treatment for advanced HCC currently incorporates VEGF inhibitors (VEGFis), checkpoint inhibitors (CPIs), or the combination of both. Because these studies show variable efficacy across lines of therapy, a network meta-analysis (NMA) is helpful to compare different agents across randomized clinical trials (RCTs). This is especially important because current guidelines list the available treatments with no guidance regarding which regimen to be considered first.8 In this systematic review and network meta-analysis, we aim to compare the efficacy of different treatment agents for patients with advanced HCC across the lines of therapy.
Methods
The reporting of this systematic review follows the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) statement.9 Institutional review board approval and informed consent was waived as this was not an individual patient–level meta-analysis.
Study Objective
The current study aimed to compare the efficacy of different VEGFis and CPIs in patients with unresectable/metastatic HCC in the first-line and refractory settings.
Eligibility Criteria
Randomized phase 3 clinical trials were included if they were published in English. Trials of interest were comparing VEGFis, CPIs, or their combination in patients with advanced (unresectable or metastatic) HCC. Treatment with VEGFis included TKIs and/or monoclonal antibodies. Studies were grouped into first-line studies and second-line studies. Studies including combination therapies other than the above were excluded.
Data Sources and Search Strategies
A comprehensive literature search was performed for abstracts and full-text articles published in print or online from database inception up through March 2020 from electronic databases, MEDLINE, Embase, Scopus, Web of Science, and the Cochrane Central Register of Controlled Trials (CENTRAL).
Detailed search strategy is as described in (eMethods in the Supplement). The search strategy was designed and conducted by an experienced librarian with input from the study investigators. Outcomes of interests included: OS, and progression-free survival (PFS) for all patients in first- and second-line treatment. Moreover, additional outcomes included OS and PFS for patients with levels of 400 α fetoprotein (AFP) or higher in the second-line treatment group.
Study Selection
Two individual reviewers (M.B.S. and D.R.A.) identified and reviewed full-text articles and abstracts that were deemed relevant by screening the list of titles. Disagreements between the 2 reviewers were resolved with consensus.
Data Extraction
Prespecified data elements were extracted from each trial using a structured data abstraction form, including baseline characteristics, sample size, and interventions used. Two reviewers extracted the data from the included trials (S.A.A.M. and D.R.A.), and disagreements were resolved by referring to a third reviewer (M.B.S.).
Risk of Bias and Certainty of Evidence
The Cochrane Collaboration’s tool for assessing the risk of bias in the trial was used,10 which includes the following domains: random sequence generation, allocation concealment, blinding, incomplete outcome data, and selective outcome reporting. Two reviewers independently assessed trial quality (S.S. and S.A.A.M.) and disagreements were resolved by referring to a third reviewer (M.B.S.).The certainty of evidence (ie, certainty in the estimates) was evaluated using the GRADE approach (Grading of Recommendations Assessment, Development, and Evaluation).11
Statistical Analysis
The statistical analysis was conducted using R statistical software (version 3.6.3, R Project for Statistical Computing). Precalculated hazard ratios (HRs) with corresponding 95% confidence intervals (CIs), abstracted from the included trials, were log transformed and used to express outcomes. Mixed treatment comparisons were made using a random-effects network meta-analysis within the frequentist framework.12 League tables and forest plots were generated for back-transformed network estimates. Heterogeneity between and within designs was assessed using Cochran’s Q and quantified using I2 statistics. I2 values less than 25%, 25% to75%, and greater than 75% represented low, moderate, and high degrees of heterogeneity, respectively. P scores, which are frequentist analogues to surface under the cumulative ranking curve (SUCRA),13 were computed and used to rank treatments. Rankograms were made to visualize treatment ranking based on P scores. A higher P score indicated a better efficacy. The NMA was carried out using R package “netmeta” (version 1.2.0, R Foundation).
Results
Study Selection
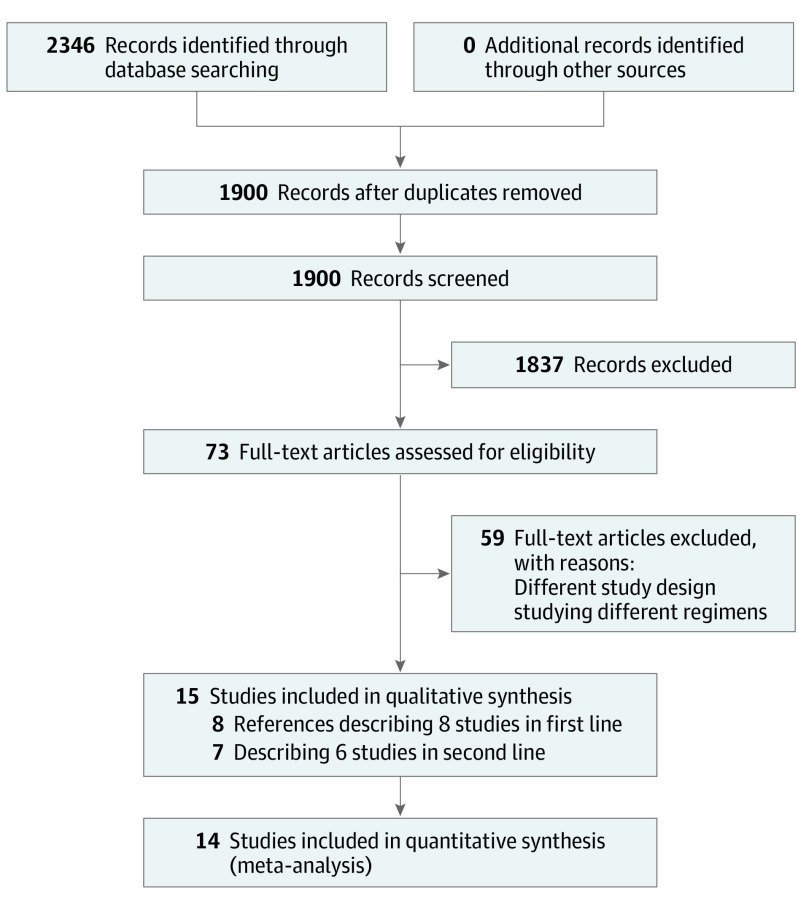
A total of 1900 titles and abstracts were identified by the screening electronic search strategy, of which 63 met the eligibility for assessment (Figure 1). Fifteen references were identified reporting 8 studies in the first-line setting and 6 in the second-line setting.1,2,3,4,5,6,7,14,15,16,17,18,19,20,21
Figure 1. PRISMA Flow Diagram Showing Screening and Selection Process.
First-Line Treatments
Study Characteristics
The 8 trials in the first-line setting encompassed a total of 6290 patients.1,4,6,7,14,15,16,17 Two trials were comparing sorafenib with placebo1,14 and 6 comparing either VEGF TKIs (linifanib, sunitinib, brivanib, and lenvatinib), nivolumab, or atezolizumab and bevacizumab (AteBev), with sorafenib.4,6,7,15,16,17 (eTable 1, eFigure 1 in the Supplement). All trials were designed for testing superiority except Kudo et al4 (noninferiority), Johnson et al,17 and Cainap et al16 (noninferiority and superiority). Age in these trials ranged from 18 to 89 years. Most trials included patients with ECOG PS of less than 2, and Child Pugh A disease. The characteristics of the included RCTs are outlined in eTable 1 in the Supplement.
Network Meta-analysis
In the network meta-analysis, we found that all treatments showed OS benefit over placebo except for sunitinib (HR, 0.89; 95% CI, 0.74-1.08) (Table 1). The combination of AteBev showed OS benefit compared with all other treatments including lenvatinib (HR, 0.63; 95% CI, 0.44-0.89), nivolumab (HR, 0.68; 95% CI, 0.48-0.98), and sorafenib (HR, 0.58; 95% CI, 0.42-0.80). Both lenvatinib (HR, 0.66; 95% CI, 0.57-0.77) and AteBev (HR, 0.59; 95% CI, 0.46-0.75) showed PFS benefit compared with sorafenib (Table 1). These results were reflected by AteBev ranking the highest in terms of PFS (P score = 94.16%) and OS (P score = 99.65%) (eFigure 2 and 3, eTable 3 in the Supplement).
Table 1. League Table Showing Indirect Comparisons Among First-Line Treatmentsa.
| Treatment | Progression-free survival | |||||||
|---|---|---|---|---|---|---|---|---|
| Overall Survival | AteBev | 0.89 (0.67-1.19) | 0.59 (0.46-0.75) | 0.73 (0.55-0.97) | 0.52 (0.40-0.69) | |||
| 0.68 (0.48-0.98) | Nivolumab | |||||||
| 0.63 (0.44-0.89) | 0.92 (0.74-1.16) | Lenvatinib | 0.66 (0.57-0.77) | 0.81 (0.66-1.01) | 0.58 (0.48-0.72) | |||
| 0.58 (0.42-0.80) | 0.85 (0.71-1.01) | 0.92 (0.79-1.07) | Sorafenib | 1.23 (1.06-1.42) | 0.88 (0.77-1.01) | |||
| 0.55 (0.39-0.78) | 0.81 (0.64-1.02) | 0.88 (0.71-1.08) | 0.95 (0.82-1.11) | Linifanib | 0.72 (0.58-0.88) | |||
| 0.54 (0.38-0.76) | 0.79 (0.64-0.99) | 0.86 (0.70-1.05) | 0.93 (0.82-1.07) | 0.98 (0.80-1.20) | Brivanib | |||
| 0.45 (0.32-0.63) | 0.65 (0.52-0.82) | 0.71 (0.58-0.87) | 0.77 (0.67-0.89) | 0.81 (0.66-0.99) | 0.82 (0.68-1.00) | Sunitinib | ||
| 0.40 (0.28-0.56) | 0.59 (0.47-0.73) | 0.63 (0.52-0.77) | 0.69 (0.61-0.78) | 0.72 (0.59-0.88) | 0.74 (0.61-0.89) | 0.89 (0.74-1.08) | Placebo | |
Abbreviation: AteBev, atezolizumab and bevacizumab.
Hazard ratios (HRs) and 95% CIs for the pairwise comparisons of the network meta-analysis from indirect comparisons. Comparisons should be read from left to right. The HRs for comparisons are in the cell in common between the column-defining and row-defining treatment. For progression-free survival, an HR of less than 1 favors row-defining treatment. For overall survival, an HR of less than 1 favors column-defining treatment.
Risk of Bias
A qualitative assessment was performed by assessing various indicators for each individual study using the Cochrane tool for risk of bias. Overall, the trials were deemed to be at low risk of bias except for detection bias for the outcome of PFS where only 1 trial had blinded assessment of outcome assessors, whereas the others were either not blinded or unclear. (eFigure 4 in the Supplement).
Certainty of Evidence
The certainty of the evidence for the indirect comparisons was overall moderate (due to imprecision) to high (eTable 4 in the Supplement).
Second-Line (Refractory) Treatments
Study Characteristics
Seven references reporting 6 trials were identified in the second-line setting.2,3,5,18,19,20,21 One of the studies (REACH-2) only included pretreated patients with HCC with AFP levels of 400 or higher, therefore, was only included in our subgroup analysis.19 The 5 trials included in the second-line analysis encompassed a total of 2653 patients. All trials were compared with placebo with either VEGF TKIs (cabozantinib, regorafenib, or brivanib), ramucirumab, or pembrolizumab (eTable 2, eFigure 5 in the Supplement). Age in these trials ranged from 18 to 91 years. Most trials included patients with ECOG PS of less than 2, and Child Pugh A disease. The characteristics of the included RCTs are outlined in eTable 2 in the Supplement.
Network Meta-analysis
Overall Population
In the network meta-analysis, all studied drugs showed PFS benefit compared with placebo. However, this only translated into OS benefit with regorafenib (HR, 0.62; 95% CI, 0.51-0.75), and cabozantinib (HR, 0.76; 95% CI, 0.63-0.92) compared with placebo (Table 2). In addition, both cabozanitinib and regorafenib showed PFS advantage compared with ramucirumab and pembrolizumab. However, only regorafenib compared with ramucirumab translated into statistically significant OS benefit (HR, 0.71; 95% CI, 0.54-0.93). These results were reflected by both regorafenib and cabozantinib ranking the highest in PFS (P score of 90.45% and 83.93% for cabozantinib and regorafenib, respectively) and OS (P score of 96.73% and 66.2% for regorafenib and cabozantinib, respectively) (eTable 5, eFigures 6 and 7 in the Supplement).
Table 2. League Table Showing Indirect Comparisons Among Second-Line Treatmentsa.
| Treatment | Progression-free survival | |||||
|---|---|---|---|---|---|---|
| Overall survival | Regorafenib | 1.04 (0.79-1.36) | 0.64 (0.47-0.87) | 0.74 (0.56-0.98) | 0.46 (0.37-0.57) | |
| 0.82 (0.62-1.07) | Cabozantinib | 0.61 (0.46-0.82) | 0.71 (0.55-0.92) | 0.44 (0.37-0.53) | ||
| 0.79 (0.58-1.08) | 0.97 (0.71-1.33) | Pembrolizumab | 1.16 (0.86-1.56) | 0.72 (0.57-0.90) | ||
| 0.71 (0.54-0.93) | 0.87 (0.67-1.14) | 0.90 (0.66-1.22) | Ramucirumab | 0.62 (0.52-0.74) | ||
| 0.70 (0.51-0.96) | 0.85 (0.62-1.17) | 0.88 (0.62-1.25) | 0.98 (0.71-1.34) | Brivanib | ||
| 0.62 (0.51-0.75) | 0.76 (0.63-0.92) | 0.78 (0.61-1.00) | 0.87 (0.72-1.05) | 0.89 (0.69-1.15) | Placebo | |
Hazard ratios (HRs) and 95% CIs for the pairwise comparisons of the network meta-analysis from indirect comparisons. Comparisons should be read from left to right. The HRs for comparisons are in the cell in common between the column-defining and row-defining treatment. For progression-free survival, an HR of less than 1 favors row-defining treatment. For overall survival, an HR of less than 1 favors column-defining treatment.
Subgroup Analysis
The REACH-2 trial only included patients with pretreated HCC with AFP levels of 400 or higher comparing ramucirumab with placebo. In addition, 3 other trials reported the outcomes for the subgroup of patients with AFP levels of 400 or higher with ramucirumab, regorafenib, and cabozantinib, compared with placebo.2,3,19,20,21 Therefore, 4 trials were included with 1084 patients (eFigure 8 in the Supplement). In the network meta-analysis, all study drugs showed PFS and OS benefit compared with placebo (eTable 6 in the Supplement). There was no significant difference seen between the active drugs (regorafenib, cabozantinib, or ramucirumab) in terms of PFS or OS (eFigure 9 and 10 in the Supplement).
Risk of Bias
A qualitative assessment was performed by assessing various indicators for each individual study using the Cochrane tool for risk of bias. Overall, the trials were deemed to be at low risk for bias (eFigure 11 in the Supplement).
Certainty of Evidence
The certainty of the evidence for the indirect comparisons was overall moderate (due to imprecision) to high (eTable 8 in the Supplement).
Discussion
In this systematic review and network meta-analysis of patients with advanced HCC, we found that the combination of atezolizumab and bevacizumab shows OS and PFS benefit compared with placebo, sorafenib, lenvatinib, and nivolumab. On the other hand, in the refractory HCC population, both regorafenib and cabozantinib seem to have comparable efficacy with a slight OS advantage for regorafenib compared with ramucirumab in the overall population. In the population with AFP levels of 400 or higher, regorafenib, cabozantinib, and ramucirumab appear to have similar efficacy.
Because sorafenib was previously the sole systemic treatment option for patients with advanced HCC,1 most of the second-line trials were designed with patients who had progressed or were intolerant to prior sorafenib. Regorafenib and cabozantinib have been studied in phase 3 trials showing level 1 evidence of efficacy compared with placebo, and currently approved by the US Food and Drug Administration (FDA). Both agents ranked the highest in our analysis with regorafenib scoring the highest in terms of OS. Therefore, regorafenib may be a preferred option in patients with refractory HCC (Figure 2).3,21 However, the absolute advantage of regorafenib compared with cabozantinib is very modest (see the absolute risk reduction in eTable 8A in the Supplement) especially when taking into consideration that the CELESTIAL trial was less selective in its inclusion criteria without mandating prior tolerance to sorafenib and it allowed for 2 or more prior lines of treatments. It is currently unclear whether sequencing these agents derives any clinical benefit. The CELESTIAL trial did allow more than 1 prior line of therapy, with only 2% of patients receiving prior regorafenib.2 In addition to these factors, cost-effectiveness is considered another important component in selecting the most appropriate regimen in refractory HCC because the absolute benefit of regorafenib and cabozantinib is considered marginal compared with the cost.22,23
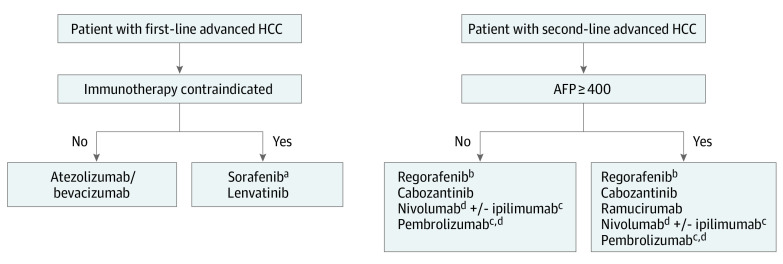
Figure 2. Suggested Treatment Algorithms for Patients With Advanced Hepatocellular Carcinoma (HCC).
AFP indicates α-fetoprotein.
aConsider lower starting dose of 200 mg and escalate as tolerated.
bConsider starting dose-escalation strategy starting with 80-mg dose.
cNot supported by level 1 evidence.
dIf no prior programmed cell death-1 or programmed cell death ligand-1 failure.
Similar to nivolumab monotherapy or the combination with ipilimumab, pembrolizumab monotherapy was approved by the FDA based on early phase 1/2 data showing promising response rates in mostly pretreated patients with HCC.24,25,26 However, the phase 3 KEYNOTE-240 trial failed to support level 1 evidence of OS or PFS benefit of pembrolizumab compared with placebo.5 Similarly, the VEGF monoclonal antibody, ramucirumab, did not show any benefit in the phase 3 REACH trial, and only a modest benefit compared with placebo in the subsequent REACH-2 trial, which included pretreated patients with HCC with AFP levels of 400 or higher. In our analysis and consistent with the level 1 evidence, regorafenib, cabozanitinib, or ramucirumab (only in patients with AFP levels ≥400) are all considered reasonable options for patients with advanced second-line HCC.
Although nivolumab was ranked second, the NMA comparison did not show a significant advantage of nivolumab compared lenvatinib or sorafenib and there is no level 1 evidence to support its use as monotherapy in the first-line setting because nivolumab failed to show superiority compared with sorafenib in a phase 3 trial.6 This, along with pembrolizumab’s limited efficacy to only small subset of patients compared with placebo in the second-line setting,5 further supports that checkpoint inhibitors’ role is mostly in combination rather as monotherapy. Multiple other combinations of programmed death-1 (PD-1) ligand 1 (PD-L1) inhibitors with VEGF inhibitors or with CTLA-4 inhibitors have shown promising activity and will further delineate the role of checkpoint inhibitors in this disease.27,28,29 Moreover, combination of VEGF TKIs and checkpoint inhibitors are being evaluated in multiple trials.
The combination of atezolizumab with bevacizumab in first-line advanced HCC have recently shown a superiority advantage compared with sorafenib in a recently published phase 3 trial.7 This was confirmed in our NMA with atezolizumab with bevacizumab ranking the highest with significant advantage compared with all other first-line agents including sorafenib, lenvatinib, and nivolumab. Therefore, the combination is currently considered the standard of care in the first-line treatment for most patients with advanced HCC. Sorafenib and lenvatinib remain as options when immunotherapy is contraindicated or associated with a higher toxic effects risk, such as in patients with autoimmune diseases or organ transplantation (Figure 2). Similarly, nivolumab is considered a reasonable option in patients with absolute contraindication to VEGF inhibitors. For patients where atezolizumab with bevacizumab is used as first-line treatment, the question remains regarding which regimen to be used as second-line on progression. Indeed, this has yet to be studied with the only refractory trial that included a very limited number of patients (3%) with prior checkpoint inhibitors is CELESTIAL.2 In addition, the activity of TKIs with predominant anti-VEGF activity (such as lenvantinib) and monoclonal antibodies (such as ramucirumab) following exposure to bevacizumab, or the use of CPI following exposure to atezolizumab, remain questionable. Although some clinicians might consider using sorafenib or lenvatinib in such settings, others might prefer to use either regorafenib or cabozanitinib, especially because both of these agents were studied in patients with prior VEGFi exposure. In contrast, sorafenib and lenvatinib were studied in VEGFi-naive patients.
It is possible that a strategy that includes another CPI (anti—CTLA-4) or a wide ranging multikinase inhibitor (such as regorafenib or cabozantinib) added to a PD-1 or PD-L1 inhibitors following exposure to atezolizumab with bevacizumab may yield benefit. This strategy has been successfully studied more extensively in other malignant diseases such as renal cell carcinoma (RCC). For example, the addition of ipilimumab to nivolumab in patients with RCC who did not respond to prior nivolumab monotherapy showed 12% to 15% response rate, including in patients who were refractory to prior VEGF TKI.30,31,32,33 Of course, extrapolation and cross-comparison from these studies to patients with HCC is not possible owing to differences in biology and future studies are needed.
The applicability of all of the studies herein to patients with Child-Pugh B score (especially B7) is limited because these patients were either excluded or only contributed to less than 10% of the patients in the trials. Nonetheless, these agents are used in clinical practice with less stringent inclusion criteria than the corresponding phase 3 studies.34 For example, in the prospective observational study (REFINE study) of regorafenib in patients with HCC, 11% of the treated patients had Child-Pugh B disease and 28% of the overall patients were started on 80 mg of regorafenib instead of the recommended 160-mg dose (58%).34 Such dose modifications to mitigate TKI-associated adverse effects without compromising efficacy have been studied in a prospective fashion in metastatic colorectal cancer where a dose-escalation strategy of regorafenib showed favorable toxic effects and efficacy outcomes compared with the full dose.35 Similarly, sorafenib at 200 mg has been shown to be more tolerable compared with the 400 mg dosing with similar efficacy in a large retrospective study.36
Limitations
The limitations to this study are related to the nature of this network analysis because most of the evidence was driven from indirect comparisons. In addition, this analysis was performed with study-level data rather than individual patient data, which would limit the power of the analysis. Despite these limitations, we believe that this study allows a better understanding of the current changing landscape of HCC treatment.
Conclusions
The treatment landscape of advanced HCC has significantly changed over the past few years. Atezolizumab/bevacizumab combination is now considered the standard of care in first-line setting in patients with advanced HCC. Regorafenib and cabozantinib are preferred options in patients with refractory HCC, with ramucirumab as an additional (but less preferred in prior bevacizumab exposure) option in those with AFP levels of 400 ng/mL or higher. Future trials should focus on other potential combinations, sequencing and the best treatment strategy in patients with prior VEGF/CPI exposure.
eMethods
References
- 1.Llovet JM, Ricci S, Mazzaferro V, et al. ; SHARP Investigators Study Group . Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378-390. doi: 10.1056/NEJMoa0708857 [DOI] [PubMed] [Google Scholar]
- 2.Abou-Alfa GK, Meyer T, Cheng AL, et al. . Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379(1):54-63. doi: 10.1056/NEJMoa1717002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruix J, Qin S, Merle P, et al. ; RESORCE Investigators . Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56-66. doi: 10.1016/S0140-6736(16)32453-9 [DOI] [PubMed] [Google Scholar]
- 4.Kudo M, Finn RS, Qin S, et al. . Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163-1173. doi: 10.1016/S0140-6736(18)30207-1 [DOI] [PubMed] [Google Scholar]
- 5.Finn RS, Chan SL, Zhu AX, et al. . KEYNOTE-240: Phase 3, randomized study of pembrolizumab (pembro) vs best supportive care (BSC) for second-line advanced hepatocellular carcinoma (HCC). Annals of Oncology. 2017;28 (Supple_5):v266. doi: 10.1093/annonc/mdx369.157 [DOI] [Google Scholar]
- 6.Yau T, Park JW, Finn RS, et al. . CheckMate 459: A randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Annals of Oncology. 2019;30 (Supple_ 5):v874-v875. doi: 10.1093/annonc/mdz394.029 [DOI] [Google Scholar]
- 7.Finn RS, Qin S, Ikeda M, et al. ; IMbrave150 Investigators . Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382(20):1894-1905. doi: 10.1056/NEJMoa1915745 [DOI] [PubMed] [Google Scholar]
- 8.Network NCC. Hepatobiliary Cancers (Version 4.2020). NCCN website. https://www.nccn.org/professionals/physician_gls/default.aspx. Published 2020. Accessed July 21, 2020.
- 9.Liberati A, Altman DG, Tetzlaff J, et al. . The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1-e34. doi: 10.1016/j.jclinepi.2009.06.006 [DOI] [PubMed] [Google Scholar]
- 10.Higgins JP, Altman DG, Gøtzsche PC, et al. ; Cochrane Bias Methods Group; Cochrane Statistical Methods Group . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brignardello-Petersen R, Bonner A, Alexander PE, et al. ; GRADE Working Group . Advances in the GRADE approach to rate the certainty in estimates from a network meta-analysis. J Clin Epidemiol. 2018;93:36-44. doi: 10.1016/j.jclinepi.2017.10.005 [DOI] [PubMed] [Google Scholar]
- 12.White IR, Barrett JK, Jackson D, Higgins JP. Consistency and inconsistency in network meta-analysis: model estimation using multivariate meta-regression. Res Synth Methods. 2012;3(2):111-125. doi: 10.1002/jrsm.1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rücker G, Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Methodol. 2015;15:58. doi: 10.1186/s12874-015-0060-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng AL, Kang YK, Chen Z, et al. . Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25-34. doi: 10.1016/S1470-2045(08)70285-7 [DOI] [PubMed] [Google Scholar]
- 15.Cheng AL, Kang YK, Lin DY, et al. . Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J Clin Oncol. 2013;31(32):4067-4075. doi: 10.1200/JCO.2012.45.8372 [DOI] [PubMed] [Google Scholar]
- 16.Cainap C, Qin S, Huang WT, et al. . Linifanib versus Sorafenib in patients with advanced hepatocellular carcinoma: results of a randomized phase III trial. J Clin Oncol. 2015;33(2):172-179. doi: 10.1200/JCO.2013.54.3298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson PJ, Qin S, Park JW, et al. . Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study. J Clin Oncol. 2013;31(28):3517-3524. doi: 10.1200/JCO.2012.48.4410 [DOI] [PubMed] [Google Scholar]
- 18.Llovet JM, Decaens T, Raoul JL, et al. . Brivanib in patients with advanced hepatocellular carcinoma who were intolerant to sorafenib or for whom sorafenib failed: results from the randomized phase III BRISK-PS study. J Clin Oncol. 2013;31(28):3509-3516. doi: 10.1200/JCO.2012.47.3009 [DOI] [PubMed] [Google Scholar]
- 19.Zhu AX, Kang YK, Yen CJ, et al. ; REACH-2 study investigators . Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(2):282-296. doi: 10.1016/S1470-2045(18)30937-9 [DOI] [PubMed] [Google Scholar]
- 20.Zhu AX, Park JO, Ryoo BY, et al. ; REACH Trial Investigators . Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015;16(7):859-870. doi: 10.1016/S1470-2045(15)00050-9 [DOI] [PubMed] [Google Scholar]
- 21.Waldschmidt D, Granito A, Merle P, et al. . Overall Survival (OS) Update: 2-year follow-up from the phase-3 RESORCE trial of Regorafenib for patients with hepatocellular carcinoma (HCC) progressing on Sorafenib. Zeitschrift fur Gastroenterologie. 2019;57 (9):e265. doi: 10.1055/s-0039-1695314 [DOI] [Google Scholar]
- 22.Shlomai A, Leshno M, Goldstein DA. Cabozantinib for patients with advanced hepatocellular carcinoma: a cost-effectiveness analysis. Therap Adv Gastroenterol. 2019;12:1756284819878304. doi: 10.1177/1756284819878304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shlomai A, Leshno M, Goldstein DA. Regorafenib treatment for patients with hepatocellular carcinoma who progressed on sorafenib—a cost-effectiveness analysis. PLoS One. 2018;13(11):e0207132. doi: 10.1371/journal.pone.0207132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El-Khoueiry AB, Sangro B, Yau T, et al. . Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492-2502. doi: 10.1016/S0140-6736(17)31046-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yau T, Kang Y-K, Kim T-Y, et al. . Nivolumab (NIVO) + ipilimumab (IPI) combination therapy in patients (pts) with advanced hepatocellular carcinoma (aHCC): results from CheckMate 040. J Clin Oncol. 2019;37(Suppl_15):4012-4012. [Google Scholar]
- 26.Zhu AX, Finn RS, Edeline J, et al. ; KEYNOTE-224 investigators . Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19(7):940-952. doi: 10.1016/S1470-2045(18)30351-6 [DOI] [PubMed] [Google Scholar]
- 27.Yau T, Zagonel V, Santoro A, et al. . Nivolumab (NIVO) + ipilimumab (IPI) + cabozantinib (CABO) combination therapy in patients (pts) with advanced hepatocellular carcinoma (aHCC): results from CheckMate 040. Journal of Clinical Oncology Conference. 2020;38(Suppl_4). doi: 10.1200/JCO.2020.38.4_suppl.478 [DOI] [Google Scholar]
- 28.Kelley RK, Sangro B, Harris WP, et al. . Efficacy, tolerability, and biologic activity of a novel regimen of tremelimumab (T) in combination with durvalumab (D) for patients (pts) with advanced hepatocellular carcinoma (aHCC). J Clin Oncol. 2020;38(Suppl_15):4508-4508. [Google Scholar]
- 29.Zhu AX, Finn RS, Ikeda M, et al. . A phase Ib study of lenvatinib (LEN) plus pembrolizumab (PEMBRO) in unresectable hepatocellular carcinoma (uHCC). J Clin Oncol. 2020;38(Suppl_15):4519-4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKay RR, Xie W, McGregor BA, et al. . Optimized management of nivolumab (Nivo) and ipilimumab (Ipi) in advanced renal cell carcinoma (RCC): a response-based phase II study (OMNIVORE). J Clin Oncol. 2020;38(Suppl_15):5005-5005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Atkins MB, Jegede O, Haas NB, et al. . Phase II study of nivolumab and salvage nivolumab + ipilimumab in treatment-naïve patients (pts) with advanced renal cell carcinoma (RCC) (HCRN GU16-260). J Clin Oncol. 2020;38(Suppl_15):5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choueiri TK, Kluger HM, George S, et al. . FRACTION-RCC: Innovative, high-throughput assessment of nivolumab + ipilimumab for treatment-refractory advanced renal cell carcinoma (aRCC). J Clin Oncol. 2020;38(Suppl_15):5007. [Google Scholar]
- 33.Grimm MSMDMIea Tailored immunotherapy approach with nivolumab in advanced renal cell carcinoma (TITAN-RCC). Annals of Oncology. 2019;30. [Google Scholar]
- 34.Lim HY, Merle P, Finn RS, et al. . Regorafenib in patients with unresectable hepatocellular carcinoma (uHCC) in routine clinical practice: Interim analysis of the prospective, observational REFINE trial. Journal of Clinical Oncology Conference. 2020;38(4 Supplement). doi: 10.1200/JCO.2020.38.4_suppl.542 [DOI] [Google Scholar]
- 35.Bekaii-Saab TS, Ou FS, Ahn DH, et al. . Regorafenib dose-optimisation in patients with refractory metastatic colorectal cancer (ReDOS): a randomised, multicentre, open-label, phase 2 study. Lancet Oncol. 2019;20(8):1070-1082. doi: 10.1016/S1470-2045(19)30272-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reiss KA, Yu S, Mamtani R, et al. . Starting Dose of Sorafenib for the Treatment of Hepatocellular Carcinoma: A Retrospective, Multi-Institutional Study. J Clin Oncol. 2017;35(31):3575-3581. doi: 10.1200/JCO.2017.73.8245 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods