Abstract
Objectives
Acute appendicitis is the most common surgical condition in children. In the UK, appendicectomy is the most common treatment with non-operative management unusual. Due to concerns about the risk of SARS-CoV-2 transmission during surgical procedures, surgeons were advised to consider non-operative treatment and avoid laparoscopy where possible. This study aims to report management and outcomes, to date, of children with appendicitis in the UK and Ireland during the COVID-19 pandemic.
Design
Survey of consultant surgeons who treat children with appendicitis that informed a prospective multicentre observational cohort study.
Setting
Data were collected from centres in the UK and Ireland for cases admitted between 1 April and 31 May 2020 (first 2 months of the COVID-19 pandemic) at both general surgical and specialist paediatric surgical centres.
Participants
The study cohort includes 838 children with a clinical and/or radiological diagnosis of acute appendicitis of which 527 (63%) were male.
Main outcomes measured
Primary outcome was treatment strategy used for acute appendicitis. Other outcomes reported include change in treatment strategy over time, use of diagnostic imaging and important patient outcomes to 30 days following hospital admission.
Results
From very early in the pandemic surgeons experienced a change in their management of children with appendicitis and almost all surgeons who responded to the survey anticipated further changes during the pandemic. Overall, 326/838 (39%) were initially treated non-operatively of whom 81/326 (25%) proceeded to appendicectomy within the initial hospital admission. Of cases treated initially surgically 243/512 (48%) were performed laparoscopically. Diagnostic imaging was used in 445/838 (53%) children. Cases treated non-operatively had a shorter hospital stay than those treated surgically but hospital readmissions within 30 days were similar between groups. In cases treated surgically the negative appendicectomy rate was 4.5%. There was a trend towards increased use of surgical treatment and from open to laparoscopic appendicectomy as the pandemic progressed.
Conclusion
Non-operative treatment of appendicitis has been widely used for the first time in children in the UK and Ireland and is safe and effective in selected patients. Overall patient outcomes do not appear to have been adversely impacted by change in management during the pandemic thus far.
Keywords: gastroenterology
What is known about the subject?
Acute appendicitis is a common condition in children and in the UK is typically treated with emergency appendicectomy.
The SARS-CoV-2 pandemic has caused widespread disruption to healthcare delivery and surgeons were advised to alter their usual practice due to possible viral transmission during surgery.
How the pandemic would impact on the management and outcomes of children with appendicitis was unclear.
What this study adds?
During the first 2 months of the pandemic, nearly 40% of all cases of appendicitis were managed non-operatively.
Non-operative treatment appears a safe alternative to surgery for selected cases in this real-world setting and overall treatment outcomes are satisfactory.
Introduction
Acute appendicitis is the most common surgical condition in children and affects approximately 8% of all people throughout their lifetime. In the UK, treatment of children with appendicitis is shared between general surgeons in district general hospitals and specialist paediatric surgeons at specialist paediatric centres,1 but typically treatment is surgical with the majority of cases undergoing urgent appendicectomy. Although international guidelines do support the use of non-operative treatment for selected children with uncomplicated acute appendicitis,2 non-operative treatment is not widespread in the UK being used by only a small minority of surgeons or as part of a research study.3
The SARS-CoV-2 (COVID-19) pandemic has caused widespread disruption to hospitals worldwide. The disruption to the delivery of acute surgical services was anticipated to impact how children with appendicitis were managed for a wide variety of reasons including staff redeployment, operating theatre availability and concerns about transmission of SARS-CoV-2 from patients to healthcare staff during anaesthesia and surgical procedures, particularly during laparoscopic procedures.4 Early guidance from the Royal College of Surgeons England suggested that laparoscopy should only be used in procedures where the risk of an open procedure to the patient outweighed the potential risk to staff in theatre as laparoscopy was believed to be an aerosol generating procedure.4 It was also recommended that non-operative treatment should be used to avoid surgery for all conditions, including appendicitis, if it was considered an acceptable alternative treatment option.5 In addition, it has been shown that exposing a patient with COVID-19 to a surgical procedure has a significant adverse impact on outcome.6 This may also have influenced surgeons towards greater use of non-operative treatment for children with appendicitis, although data emerged through the first months of the pandemic that children are much less likely to become infected with COVID-19 than adults and the impact of COVID-19 on outcomes following surgery in children is less clear.7
The Children with AppendicitiS during the CoronAvirus panDEmic study was initiated in late March 2020 to capture data relating to the impact of this disruption on the management and outcomes of children with appendicitis during the pandemic. The study comprised a rapid survey of surgeons in the UK who treat children to understand the current or anticipated impact of the pandemic on management of children with appendicitis followed by an observational cohort study. This report details the findings of the survey and the management observed during the first 2 months of the pandemic in the UK and early (30 days) outcomes. It is provided to assist surgeons with clinical decision making throughout the pandemic and in the event that there is a second wave resulting in further disruption to acute surgical services.
Methods
Survey of surgeons who treat children
A survey was designed to understand the impact of the pandemic on treatment being offered to children with acute appendicitis at the start of the pandemic. Questions were developed, piloted on a convenience sample of surgeons and modified prior to survey distribution. Specialist paediatric and general surgeons who treat children with appendicitis were invited to complete the survey during the 2-week period leading up to 14 April 2020. Invitations were made via personal contacts, social media and mailshots from the British Association of Paediatric Surgeons and the survey was advertised repeatedly through these channels. The survey was administered online using REDCap data capture tool8 and is available in online supplemental material S1. Questions asked were focused around understanding the impact of the COVID-19 pandemic on the management of children with appendicitis experienced to date, the anticipated impact over the coming weeks and the rationale behind any change in management.
bmjpo-2020-000831supp001.pdf (38.9KB, pdf)
Cohort study design
This is a prospective multicentre observational cohort study of children aged <16 years at time of hospital admission diagnosed with and treated for acute appendicitis in the UK and Ireland. This includes children treated by general surgeons and specialist paediatric surgeons. Participating hospitals were not required to alter diagnostic or treatment pathways and no changes were made to patient care as part of this study. Data collection for the study commenced 1 April 2020.
Centre recruitment and patient identification
Hospitals providing acute surgical care to children were invited to participate in this study via a number of channels including targeted emails, newsletters, social media and websites of surgical and paediatric national organisations including the British Association of Paediatric Surgeons, the Royal College of Surgeons of England and the Royal College of Paediatrics and Child Health. Children were included in the study if they were diagnosed with and treated for acute appendicitis in hospital. Diagnosis was based on clinical and/or radiological criteria. Children who presented with abdominal pain but not felt to have appendicitis were excluded. This report includes all children in the study dataset with an initial admission date between 1 April 2020 and 31 May 2020 and for whom 30-day outcome data were provided to the coordinating team by the data cut-off date of 13 July 2020. Follow-up data were censored at 30 days posthospital discharge from initial admission.
Outcomes
The primary outcome was the initial treatment strategy for acute appendicitis, defined as surgical or non-operative. Secondary outcomes related to patient management included number and proportion of operative cases performed open and laparoscopically, use of diagnostic imaging and variation in patient management over time, as the pandemic progressed. Other clinical outcomes were failure rate of non-operative treatment (defined as appendicectomy within initial hospital inpatient episode in a case in whom the initial treatment strategy was non-operative), need for hospital readmission, wound infection, bowel obstruction, intra-abdominal collection, further surgery or interventional radiology procedure, length of hospital stay and mortality. These outcomes were all reported to 30 days following initial hospital admission and were selected as important outcomes from a core outcome set for paediatric appendicitis.9
Data collection and analysis
Anonymous data were collected by local study teams within each hospital and submitted to the study team monthly. Data were checked for duplication since we were aware that some cases were transferred from one hospital to another during the study period (typically from a district general hospital to a local specialist paediatric surgery centre) and we wished to avoid duplication. Duplicated data records were identified and excluded if all of age, sex, C reactive protein (CRP) and white cell count (WCC) at admission were identical.
Statistical analysis was performed using StataSE V.15 (StataCorp, Texas, USA) and the figures were produced using GraphPad Prism V.8 (GraphPad Software, La Jolla, California, USA). Data are presented as median (IQR or range) and/or number/total (%) as appropriate. Fisher’s exact test or χ2 test, as appropriate, were used for comparison of categorical data and the Mann-Whitney U test was used for continuous data. A p value of <0.05 was considered as statistically significant. The study was conducted according to Strengthening the Reporting of Observational studies in Epidemiology guidelines for observational studies.10
Patient and public involvement
Given the restrictions and need for rapid study commencement, there was no active patient and public involvement in this study at this stage. There is no relevant patient group to disseminate findings of this study.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. The corresponding author had full access to all study data and responsibility for publication.
Results
Survey of surgeons who treat children
One hundred and one complete responses (75% specialist paediatric surgeons, 25% general surgeons) were received from surgeons at 19 district general hospitals and 26 specialist children’s centres. One-fifth of respondents (representing 60% of hospitals) had already experienced some change in their usual clinical management of children with appendicitis. The most frequent changes experienced were the use of non-operative treatment for uncomplicated acute appendicitis (63%), open (instead of laparoscopic) appendicectomy for complicated appendicitis (37%), more frequent use of imaging to confirm diagnosis and greater use of oral rather than intravenous antibiotics (both 32%). In the majority of cases (95%), this change was an active individual surgeon or departmental decision.
Most respondents (87%) indicated they anticipated some change in the management pathway for these children, either in their diagnostic workup, the type of treatment offered or that they would cease treating children with appendicitis altogether during the coronavirus pandemic (table 1).
Table 1.
Anticipated future effect on management of children with appendicitis during coronavirus pandemic
| Anticipated change | GS, n=21 (%) | SPS, n=65 (%) |
| Simple appendicitis: I will actively offer non-operative treatment to all children with simple appendicitis | 15 (71) | 45 (69) |
| Simple appendicitis: I will consider non-operative treatment for children with simple appendicitis at parental request | 5 (24) | 14 (22) |
| Simple appendicitis: I will actively perform open (as opposed to laparoscopic) appendicectomy in children with simple appendicitis | 4 (19) | 32 (49) |
| Complicated appendicitis: I will actively offer non-operative treatment to children with complicated appendicitis | 3 (14) | 10 (15) |
| Complicated appendicitis: I will consider non-operative treatment to children with complicated appendicitis at parental request | 6 (29) | 11 (17) |
| Complicated appendicitis: I will actively perform open (as opposed to laparoscopic) appendicectomy in children with complicated appendicitis | 12 (57) | 44 (68) |
| Complicated appendicitis: I will actively pursue a shorter than usual course of intravenous antibiotics in children with complicated appendicitis | 5 (24) | 9 (14) |
| Appendix mass: I will actively offer non-operative treatment to children with appendix mass | 12 (57) | 26 (40) |
| Appendix mass: I will consider non-operative treatment to children with appendix mass at parental request | 4 (19) | 6 (9) |
| Appendix mass: I will not offer routine interval appendicectomy in children who have has successful non-operative treatment of appendix mass | 3 (14) | 17 (26) |
| Any appendicitis: routine imaging for all cases of suspected appendicitis to be certain of diagnosis | 6 (29) | 14 (22) |
| Any appendicitis: CT scan instead of US for diagnosis of appendicitis | 0 | 0 |
| Any appendicitis: more frequent use of imaging to guide management (eg, select cases for non-operative treatment/reduce negative appendicectomy rate) | 13 (62) | 31 (48) |
| Any appendicitis: consultant review for all cases prior to considering surgery | 15 (71) | 46 (71) |
| Any appendicitis: we will likely be sending children with appendicitis to another hospital for treatment | 3 (14) | 1 (2) |
| Any appendicitis: we will likely be treating children at my hospital who would usually be treated somewhere else | 0 | 33 (51) |
GS, general surgeons; SPS, specialist paediatric surgeons; US, ultrasound.
Observational cohort study: children included and radiological investigations
Data were submitted prior to the data cut-off date for this report for 838 children treated for appendicitis between 1 April and 31 May 2020 in 67 centres (approximately half of all centres who treat children with appendicitis in the UK). No duplicated records were identified so all are included. The median age was 10 (range 1–15) years and 527 (63%) children were male. General surgeons treated 343 (41%) of cases with the remaining 496 (59%) being treated by specialist paediatric surgeons. In this cohort of children treated for appendicitis, diagnostic imaging was used in 445 (53%) children with abdominal ultrasound (US) and abdominal CT scan undertaken in 420 (50%) and 46 (5.5%) cases, respectively. At the point of diagnosis, 600 children (72%) were suspected by the treating surgeon to have simple acute appendicitis, 201 (24%) complicated appendicitis and 35 (4%) an appendix mass (data missing in 3 cases).
At diagnosis of acute appendicitis, the COVID-19 status was known positive in 4 (0.5%) children, known negative in 171 (20%) children, tested awaiting result in 397 (47%) children and 266 (32%) children were untested.
Initial treatment strategy
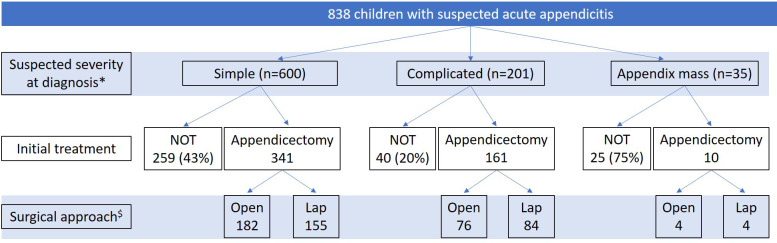
Initial treatment strategy was non-operative in 326 (39%) children. In the 512 (61%) children treated initially with surgery, 262 (52%) had an open procedure and 243 (48%) an initially laparoscopic procedure (data on surgical approach not available for 7 cases). Initial treatment stratified by suspected severity of appendicitis at diagnosis is shown in figure 1.
Figure 1.
Initial treatment stratified by suspected severity of appendicitis at diagnosis. *Data on severity at diagnosis missing for two cases; both had non-operative treatment (NOT). $ Data on surgical approach missing for seven cases. Lap, laparoscopic.
Comparative clinical, laboratory and radiological details for cases treated surgically and non-operatively are shown in table 2. Cases treated surgically typically had more advanced appendicitis with higher CRP and white cell count at diagnosis and were more likely to have suspected complicated (as opposed to simple) appendicitis. A higher proportion of cases treated non-operatively had an US scan than of cases treated surgically. Of cases suspected to be simple appendicitis, 43% (259/600) were initially treated non-operatively compared with 20% (40/201) of cases suspected to be complicated appendicitis.
Table 2.
Clinical, laboratory and radiological characteristics of cases treated initially non-operatively or operatively
| Non-operative (n=326) | Operative (n=512) | P value | ||
| Age (years) | 10.5 (8–13) | 10 (8–12) | 0.19 | |
| Male (n, %) | 195 (60) | 331 (65) | 0.16 | |
| Duration of symptoms (hours) | 36 (24–72) | 48 (24–72) | 0.58 | |
| Specialty (n, %) | GS | 140 (43) | 202 (39) | 0.35 |
| SPS | 186 (57) | 310 (61) | ||
| Admission bloods | WCC—×109/L | 14.4 (10.7–17.8) | 15.2 (12.0–18.5) | 0.01 |
| CRP—mg/L | 32 (7.1–81) | 52 (15–126) | <0.0001 | |
| US performed (n, %) | 193 (59) | 227 (44) | <0.0001 | |
| CT performed (n, %) | 16 (4.9) | 30 (5.9) | 0.76 | |
| Suspected severity at diagnosis (n, %)* | Simple | 259 (80) | 341 (67) | <0.0001 |
| Complicated | 40 (12) | 161 (31) | ||
| Appendix mass | 25 (7.7) | 10 2.0) | ||
*For two cases suspected severity was missing.
CRP, C reactive protein; GS, general surgeon; SPS, specialist paediatric surgeon; US, Ultrasound scan; WCC, white cell count.
For cases treated surgically with either open or laparoscopic appendicectomy, comparative clinical, laboratory and radiological details for are shown in table 3. Overall, 48% (n=243) of cases that were treated surgically underwent a laparoscopic procedure. There was no relationship identified between choice of procedure and suspected severity of appendicitis pre-operatively. Cases treated laparoscopically were older, more likely to be treated by a specialist paediatric surgeon and more likely to have had a diagnostic US than cases performed open. For both open and laparoscopic procedures surgeons tended to underestimate disease severity at diagnosis compared with the surgical findings (table 3). The overall negative appendicectomy rate was 4.5% (n=23) in cases treated surgically.
Table 3.
Clinical, laboratory and radiological characteristics of cases treated initially operatively stratified by open or laparoscopic procedure
| Open (n=262) |
Laparoscopic (n=243) | P value | ||
| Age (years) | 10 (7–12) | 11 (9–13) | 0.0004 | |
| Male (n,%) | 176 (67) | 149 (61) | 0.19 | |
| Specialty (n,%) | GS | 119 (60) | 81 (40) | 0.006 |
| SPS | 143 (47) | 162 (53) | ||
| Admission bloods | WCC—×109/L | 15.6 (12.3–18.6) | 14.9 (11.6–18.0) | 0.34 |
| CRP—mg/L | 52 (15–130) | 52 (15–124) | 0.91 | |
| US performed (n,%) | 101 (39) | 122 (50) | 0.009 | |
| CT performed (n,%) | 18 (6.8) | 12 (4.9) | 0.19 | |
| Suspected severity pre-operatively (n,%) | Simple | 182 (69) | 155 (64) | 0.40 |
| Complicated | 76 (29) | 84 (35) | ||
| Appendix mass | 4 (1.5) | 4 (1.7) | ||
| Operative findings (n,%) | Normal | 13 (5.0) | 10 (4.1) | 0.66 |
| Mass | 7 (2.7) | 8 (3.3) | ||
| Simple | 128 (49) | 108 (44) | ||
| Complicated | 113 (43) | 117 (48) | ||
Data missing for seven cases.
CRP, C reactive protein; GS, general surgeon; SPS, specialist paediatric surgeon; US, Ultrasound scan; WCC, white cell count.
Change in initial treatment method during the pandemic
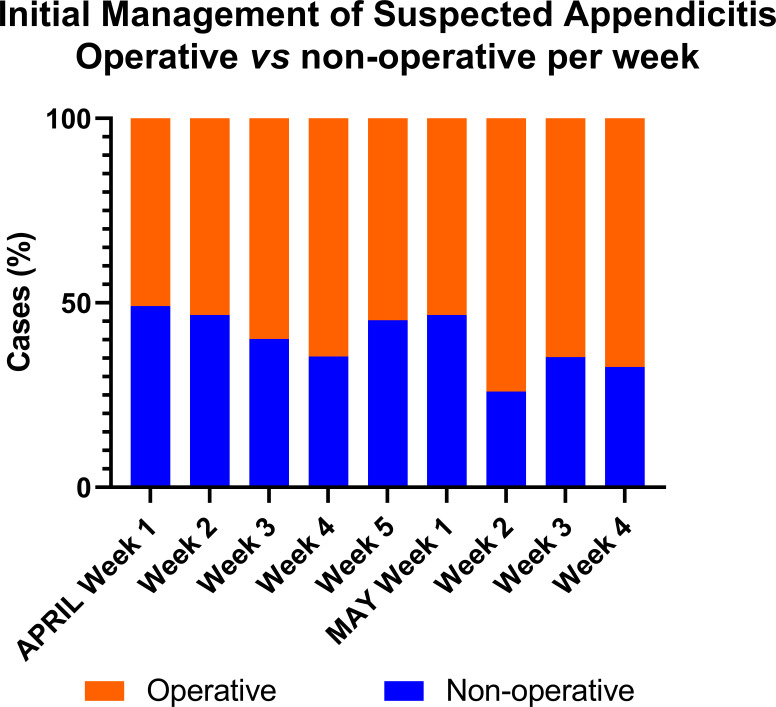
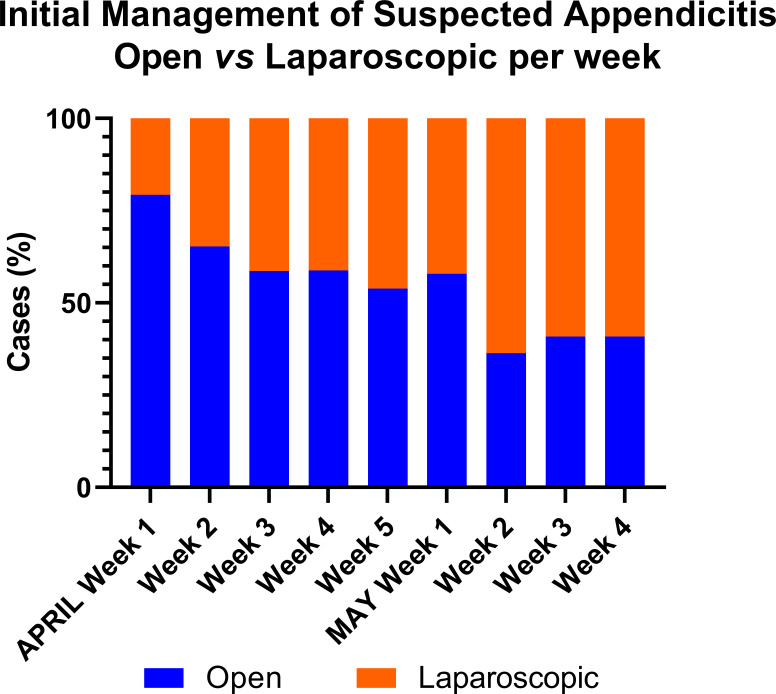
During the first week of April 2020, the proportion of cases initially treated non-operatively was 49% which reduced to 33% in the last week of May 2020 (figure 2)). Of those cases treated operatively, 79% were by open appendicectomy during the first week of April 2020 and this reduced to 41% in the last week of May 2020 (figure 3)
Figure 2.
Initial management strategy of appendicitis by week, operative versus non-operative. The orange bars represent operative treatment and the blue bars represent non-operative treatment demonstrating a trend towards operative treatment over time.
Figure 3.
Initial operative management strategy of appendicitis by week, open versus laparoscopic. The orange bars represent laparoscopic appendicectomy and the blue bars represent open appendicectomy demonstrating a trend towards laparoscopic appendicectomy over time.
Patient outcomes to 30 days
For 326 cases treated non-operatively, 81 (25%) children failed non-operative treatment during the initial admission. This failure rate in cases suspected to be simple appendicitis was 24% (62/259) and for those suspected to be complicated appendicitis was 30% (12/40). All these cases proceeded to appendicectomy which was approached via an open procedure in 35 (44%) cases and a laparoscopic procedure in 45 (56%) cases (data missing in 1 case). Where available (missing n=2), intra-operative findings in those who failed initial non-operative treatment were normal appendix in 4 (4.9%) children, simple appendicitis in 37 (47%) children, complicated appendicitis in 32 (41%) children and appendix mass in 6 (7.4%) children.
Overall, cases treated operatively had a longer length of initial inpatient stay compared with those treated initially non-operatively (3 (2–5) vs 2 (1–4) days, p<0.0001). Overall, the 30-day readmission rate was 12% (30/245) for non-operative treatment in those that were discharged home without an operation and 7.4% (38/512) in those treated initially with an operation. Reasons for readmission in cases treated operatively were abdominal collection/abscess (n=17), abdominal pain (n=11), fever (n=2), wound dehiscence/infection (n=6) and small bowel obstruction (n=1). Reasons for readmission in the group which underwent non-operative treatment without appendicectomy prior to discharge were abdominal collection/abscess (n=4), abdominal pain (n=20) and fever (n=4). Note that in some cases there were multiple reasons for readmission or the reason was not specified.
At 30 days there were no reported deaths. Children undergoing an open procedure had a similar rate of readmission (7 (n=19) vs 8% (n=19), p=0.87), wound infection (4 (n=10) vs 2% (n=4), p=0.18), bowel obstruction (1 (n=2) vs 1% (n=3), p=0.68), intra-abdominal collection (8 (n=21) vs 10% (n=25), p=0.44) and re-operation (3 (n=8) vs 5% (n=13), p=0.27) to those who had a laparoscopic procedure. An open procedure was associated with a shorter length of inpatient stay compared with a laparoscopic procedure (2 (2–4) vs 3 (2–6), p=0.005). These outcomes are further stratified by severity of appendicitis in table 4.
Table 4.
Comparative outcomes for cases treated initially operatively stratified by operative findings
| Simple or no appendicitis | Complicated appendicitis or appendix mass | |||||
| Open (n=141) | Laparoscopic (n=118) | P value | Open (n=120) | Laparoscopic (n=125) | P value | |
| Readmission (n, %) | 4 (2.8) | 4 (3.4) | 1 | 15 (13) | 15 (12) | 1 |
| Wound infection (n, %) | 0 (0) | 2 (1.7) | 0.21 | 10 (8.3) | 2 (1.6) | 0.02 |
| Bowel obstruction (n, %) | 0 (0) | 0 (0) | 1 | 2 (1.7) | 3 (2.4) | 1 |
| Intra-abdominal collection/abscess (n, %) | 4 (2.8) | 2 (1.7) | 0.69 | 17 (14) | 23 (18) | 0.39 |
| Reoperation /IR procedure (n, %) | 1 (0.7) | 0 (0) | 1 | 7 (5.8) | 13 (10) | 0.25 |
| Length of stay (days, median and IQR) | 2 (1-3) | 2 (1–3) | 0.33 | 4 (2–6) | 6 (4–8) | 0.001 |
IR, interventional radiology.
Discussion
This report confirms anecdotal suspicion that the management of children with appendicitis has been significantly changed during the coronavirus pandemic with a clear shift towards non-operative management and towards open appendicectomy in cases managed surgically. Although we do not present here any comparative data to a different time period, the use of non-operative treatment for children with acute appendicitis was extremely limited in a survey performed in 20183 and anecdotally we do not believe there has been a significant change in practice prior to the start of the pandemic. The fact that overall just under 40% of children with suspected appendicitis were treated initially non-operatively represents a huge change in practice. This change was seen from early in the pandemic as reported in our initial survey and was anticipated by the majority of survey respondents. A small number of recent reports suggest that such changes in the management of appendicitis are not unique to the UK but have been implemented in a number of countries.11–13
Interestingly, over the course of this data collection period the proportion of cases undergoing non-operative management decreased and the proportion of cases treated surgically having a laparoscopic procedure increased over time. We suspect this pattern is a reflection of initial guidance from professional bodies proposing non-surgical treatments be sought wherever possible and cautioning against the use of laparoscopy.5 Subsequent evolution of that guidance over time may have encouraged surgeons to resume their normal practice. We intend to monitor surgical practice longer term during the pandemic to identify any further changes in management.
The sudden widespread uptake of non-operative treatment of appendicitis seen to date during the pandemic presents an opportunity to evaluate non-operative treatment in a real-world setting across multiple centres in a way that until recently would not have been imagined possible. These early data suggest that non-operative treatment of acute appendicitis is effective and safe in a real-world setting. Further work is needed however, to determine whether the outcomes from non-operative management are acceptable to children with appendicitis, their parents and other stakeholders including surgeons. Cases treated non-operatively achieved a shorter length of stay than cases treated surgically, there were no deaths and the adverse event profile of each treatment approach was similar. These data suggest that surgeons selected less severe cases for non-operative treatment despite no formal guidelines existing for this purpose. Yet it remains likely that non-operative treatment has been used here outside the criteria used to date in formal research studies in which it has been evaluated.14–16 Of note, 12% of cases in which non-operative treatment was used as first-line therapy were suspected to be complicated appendicitis.
Both open and laparoscopic appendicectomy are recognised to be safe and effective treatments for children with appendicitis, although there has been increased uptake of laparoscopic appendicectomy in children in the UK in recent years.17 The reversal of this trend during the pandemic such that just 48% of all cases treated surgically were performed laparoscopically is likely in response to guidance from professional bodies that laparoscopy may increase the risk of SARS-CoV-2 transmission in positive cases.4 Anecdotally, we are aware that a move away from laparoscopy has been implemented by some individual surgeons and also by some institutions. The trend towards a higher proportion of cases being performed laparoscopically over time (figure 2) is consistent with updated guidance from professional bodies and a greater understanding about the epidemiology of COVID-19 in children.7
Recently the high negative appendicectomy rate observed in the UK (15.9% in children) has been highlighted and achieved significant public interest.18 19 It is therefore of particular note that the negative appendicectomy rate seen in this dataset is extremely low, and in fact one of the lowest rates reported in the UK to date.1 18 20 The caveat to this, however, is that we have reported intraoperative findings rather than histological findings and the rate based on histology may in fact be higher. Alongside this, radiological imaging (both US and CT) has been seen more frequently during the period of the pandemic than in a published national dataset (53% vs 41%).18 It is not clear whether increasing use of imaging has resulted in such a low negative appendicectomy rate but it is certainly a possibility. Other factors may also be contributory however, including increased use of non-operative treatment and potentially increased consultant involvement in decision making. Given cases have been selected for non-operative treatment by clinicians, it is possible that some children who would have otherwise undergone negative appendicectomy were selected for non-operative treatment instead. Although we do not have data on consultant involvement in decision making on a case-by-case basis within this dataset, just over 70% of consultants anticipated there would be greater consultant involvement in management of children with appendicitis in the survey undertaken at the beginning of the pandemic. This is certainly an area worthy of further investigation since a reduction in the negative appendicectomy rate may be seen as an unanticipated benefit that it would be beneficial to maintain in the longer term. The COVID-19 pandemic may inadvertently be presenting opportunities for quality improvement that should be realised beyond the period of the pandemic itself.
The strengths of this study are that data have been collected prospectively from multiple centres, including representation from all nations, across the UK and Ireland. We deliberately did not involve other international centres so as to achieve a region across which there is relative consistency in management of children with appendicitis. This early analysis has been performed following a change in surgical practice to inform ongoing management during the pandemic and in the event of a second wave. The findings are likely generalisable to other countries in whom management is similar to that in the UK and Ireland. As a pragmatic real-world study, it provides an overview of real-life outcomes outside the confines that would typically be achieved in a clinical trial. Conversely, some may view this pragmatism as a limitation since we have not used precise definitions for severity of appendicitis nor have we proposed criteria for different treatment strategies. This may explain a comparable failure rate of non-operative treatment in those with suspected simple and complicated appendicitis. An additional limitation of any study looking at non-operative treatment of appendicitis is that we cannot be sure whether those who underwent non-operative treatment definitely had appendicitis. While any such resulting ‘overtreatment’ may be viewed by some as regrettable, the risk/benefit profile of these two treatments is likely to be in favour of non-operative treatment over unnecessary operation for the majority of cases. However, improved positive identification of cases with appendicitis should remain the goal so that only children who truly have appendicitis receive treatment for it. A further limitation on terms of assessing the outcomes following non-operative treatment is that we have only reported outcomes to 30 days. We plan further analysis of a larger patient cohort to include long-term follow-up particularly of the group of children managed thus far without surgery. Finally, it is conceivable that the initial survey raised awareness of the use of alternative management strategies and may in itself have influenced practice, although 95% of respondents stated that an active decision was being made suggesting this is unlikely for the majority.
In conclusion, we present evidence that the COVID-19 pandemic has had a marked impact on the management of children with appendicitis with clear shifts towards increased use of non-operative treatment and open (as opposed to laparoscopic) appendicectomy. Despite the absence of formal guidelines, non-operative treatment appears safe and effective in children who have been selected for this treatment modality. Overall, these data should reassure surgeons about management strategy used during the pandemic in the face of restrictions to normal surgical services and may inform best practice in future times of limited surgical capacity.
Supplementary Material
Footnotes
Twitter: @gbethellUK, @doccmr, @nigel_j_hall
Collaborators: CASCADE study collaborators Anna-May Long, Florin Djendov, Victor Emordi, Cambridge University Hospitals; Mark Peter, Sarah Staight, Andrew Jackson, Huddersfield Royal Infirmary; Stewart Cleeve, Arun Kelay, Royal London Hospital; Michael Terry, Christina Major, Oscar Croysdale, Mike Nelson, St Mary’s Hospital; Eleri Cusick, Hannah Rhodes, Juliette King, Sophie Lewis, Bristol Children’s Hospital; Chris Driver, Gillian Winter, Royal Aberdeen Children's Hospital; Michael Wilson, Rachael Robertson, Duncan Rutherford, Kieran McGovern, Forth Valley Royal Hospital; Selena Curkovic, Raef Jackson, Bhushanrao Jadhav, University Hospital of Wales; Thomas Raymond, Vijay Gangalam, Deepak Selvakumar, Thomas Raymond, Royal Lancaster Infirmary; Khalid Elmalik, Reda Habak, Muslim Abdullah, Mohamed Ahmed Osama, Leicester Royal Infirmary; Milan Gopal, Laura Phillips, Khlud Asanai, Hany Gabra, Great North Children’s Hospital; Noman Zafar, Sophia Lewis, Florence Kashora, Dixa Thakrar, Northwick Park Hospital; Dean Rex, Annita Budzanowski, St George’s Hospital; Jennifer Binnington, Simon Timbrell, Megan Ridgeway, East Lancashire Hospital NHS Trust; Shirley Chan, Amani Asour, Adetayo Aderombi, Medway Maritime Hospital; Donald Menzies, Ali Murtada, Corina Dragu, Vincent Quan, Colchester Hospital; Alan Askari, Krashna Patel, Luton and Dunstable University Hospital; Merrill McHoney, Hetal Patel, Sesi Hotonu, Ashley Meikle, Royal Hospital for Children, Edinburgh; Andrew Beamish, Royal Gwent Hospital, Newport; Ajay Belgaumkar, Bankole Oyewole, Prabhat Narayan, Surrey and Sussex Healthcare NHS Trust; Marianne Hollyman, Angeliki Kosti, Thomas Badenoch, Musgrove Park Hospital; Frances Goulder, Katie Siggens, Kizzie Peters, Fiona Kirkham, Royal Hampshire County Hospital, Winchester; Paul Froggatt, Karen Lai, Cristina Navarro, Dorinda Chandrabose, Poole Hospital; Simon Toh, Portsmouth Hospital NHS Trust; Ingo Jester, Ben Martin, Birmingham Women’s and Children’s Hospital; Elizabeth Gemmill, King’s Mill Hospital; Richard Egan, Keira Lily, Morriston, Swansea; Mark Dilworth, Dimitrios Stamatiou, Birmingham Heartlands Hospital; Alasdair Macmillan, Joshua McIntyre, Danielle Clyde, Majid Rashid, Victoria Hospital Kirkcaldy; Gandrapu Srinivas, Torbay Hospital, Torquay Hospital; Katherine Buckley, Darren Smith, Wirral University Teaching Hospital NHS Trust; Henry Dowson, Gautam Singh, George Kerans, Ashwini Ghorpade, Muhammad Tobbal, Frimley Park Hospital; Seshu Kumar Bylapudi, Milton Keynes University Hospital; Louise Phillips, Kimberley Hallam, Marisa Clemente, Tanzeela Gala, Glan Clwyd Hospital; Karol Pal, Borders General Hospital, Melrose; George Ninkovic-Hall, Emila Paul, Leighton Hospital; Theo Pelly, Joe Vance-Daniel, Kingston Hospital; Venkatesh Kanakala, Edward J Nevins, James Dixon, Michael John, James Cook University hospital; Jude Prince, Dale Vimalachandran, Countess of Chester NHS Foundation Trust; Georgios Karagiannidis, Ipswich Hospital NHS Trust; Suzette Samlalsingh, Chrsitine Ozone, Amina Bouhelal, Queen’s Hospital, Barking, Havering and Redbridge University Hospitals NHS Trust; Siddhartha Handa, Furness General Hospital; Andrew Mitchell, Sathasivam Rajeev, Ellen Ross, Ali Wadah, Darlington Memorial Hospital; Tim Bradnock, John Hallett, Felicity Arthur, Royal Hospital for Children, Glasgow; Shirish Tewari, Vinay Shah, Vivek Gupta, Nick Reay-Jones, Lister Hospital; Salman Bodla, Nuha Yassin, New Cross Hospital Wolverhampton; Harriet Corbett, Sumita Chhabra, Alder Hey Children’s Hospital; Athanasios Tyraskis, Benjamin Allin, Angus Fitchie, Oxford University Hospital Trust; Michael Stanton, Dina Fouad, Southampton Children’s Hospital; Mark Vipond, Harry Dean, Matthew Boal, Oliver Brown, Gloucestershire Royal Hospital; Jonathan Goring, Mahmoud Marei, Christian Verhoef, Jonathan Ducey, Royal Manchester Children's Hospital; Clare Rees, Chipo Mushonga, Dan Frith, Imperial College Healthcare NHS Trust; Ashok Ram, Tristan Boam, Melissa Gabriel, Ferzine Mohamed, Norfolk and Norwich University Hospital; David Williams, Katie Cross, Nadine Dyar, Rick MacMahon, Mohammed Fakhrul-Aldeen, Iain Bain, David Bunting, North Devon District Hospital; Graham Branagan, Rachel Carten, Chee Wan Lai, Lydia Longstaff, Salisbury Hospital; Anindya Niyogi, Claudia Koh, Michael John, Christian Fox, Nottingham Children’s Hospital; Stavros Loukogeorgakis, Joe Curry, Kate Cross, Jayaram Sivaraj, Great Ormond Street Hospital; Sean Marven, Milda Jancauskaite, Helen Please, Wayne Fradley, Sheffield Children's Hospital; Fenella Welsh, Maki Jitsumara, Basingstoke Hospital; Sinead Hassett, Ancuta Muntean, Ionica Stoica, Sarah Yassin, Lukas O’Brien, Children’s Health Ireland, Crumlin; Suzanne Lawther, David Colvin, Ciaran Durand, Royal Belfast Hospital for Sick Children; Mohamed Eltom, Hull University Teaching Hospital; Iain Yardley, Kirsty Brennan, Clara Chong, Evelina Hospital; Hasan Mukhtar, Hany Khalil, Stephanie Clark, Whittington Health NHS trust; Ashish Desai, Ben Woodward, Kings College Hospital; Amulya Saxena, Joshua Cave, Chelsea and Westminster Hospital; Alistair Sharples, University Hospitals of North Midlands.
Contributors: Conception or design of the work: GSB, CMR, JS, NJH. Data collection: CASCADE study collaborators. Data analysis and interpretation: GSB, NJH. Drafting the article: GSB. Critical revision of the article: NJH. Final approval of the version to be published: GSB, CMR, JS, NJH.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: All authors have completed the Unified Competing Interest form (available on request from the corresponding author) and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous 3 years, no other relationships or activities that could appear to have influenced the submitted work.
Patient consent for publication: Not required.
Ethics approval: This study was registered at each site as a service evaluation, as defined by the health research authority guidance, as this was an observational study only collecting anonymised routine data with no change to clinical care pathways. The survey was approved by the research committee of the British Association of Paediatric Surgeons.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available on reasonable request. Relevant data available by reasonable request.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
CASCADE study Collaborators:
Anna-May Long, Florin Djendov, Victor Emordi, Mark Peter, Sarah Staight, Andrew Jackson, Stewart Cleeve, Arun Kelay, Michael Terry, Christina Major, Oscar Croysdale, Mike Nelson, Eleri Cusick, Hannah Rhodes, Juliette King, Sophie Lewis, Chris Driver, Gillian Winter, Michael Wilson, Selena Curkovic, Raef Jackson, Bhushanrao Jadhav, Thomas Raymond, Vijay Gangalam, Deepak Selvakumar, Thomas Raymond, Khalid Elmalik, Reda Habak, Muslim Abdullah, Mohamed Ahmed Osama, Milan Gopal, Laura Phillips, Khlud Asanai, Hany Gabra, Noman Zafar, Sophia Lewis, Florence Kashora, Dixa Thakrar, Dean Rex, Annita Budzanowski, Jennifer Binnington, Simon Timbrell, Megan Ridgeway, Shirley Chan, Amani Asour, Adetayo Aderombi, Donald Menzies, Ali Murtada, Corina Dragu, Vincent Quan, Alan Askari, Krashna Patel, Merrill McHoney, Hetal Patel, Sesi Hotonu, Ashley Meikle, Andrew Beamish, Ajay Belgaumkar, Bankole Oyewole, Prabhat Narayan, Marianne Hollyman, Angeliki Kosti, Thomas Badenoch, Frances Goulder, Katie Siggens, Kizzie Peters, Fiona Kirkham, Paul Froggatt, Karen Lai, Cristina Navarro, Dorinda Chandrabose, Simon Toh, Ingo Jester, Elizabeth Gemmill, Richard Egan, Keira Lily, Mark Dilworth, Dimitrios Stamatiou, Alasdair Macmillan, Joshua McIntyre, Danielle Clyde, Majid Rashid, Gandrapu Srinivas, Katherine Buckley, Darren Smith, Henry Dowson, Gautam Singh, Seshu Kumar Bylapudi, Louise Phillips, Kimberley Hallam, Marisa Clemente, Tanzeela Gala, Karol Pal, George Ninkovic-Hall, Emila Paul, Theo Pelly, Joe Vance-Daniel, Venkatesh Kanakala, Edward J Nevins, James Dixon, Michael John, Jude Prince, Dale Vimalachandran, Georgios Karagiannidis, Suzette Samlalsingh, Chrsitine Ozone, Amina Bouhelal, Siddhartha Handa, Andrew Mitchell, Sathasivam Rajeev, Ellen Ross, Ali Wadah, Tim Bradnock, John Hallett, Shirish Tewari, Vinay Shah, Vivek Gupta, Nick Reay-Jones, Salman Bodla, Nuha Yassin, Harriet Corbett, Sumita Chhabra, Athanasios Tyraskis, Benjamin Allin, Angus Fitchie, Michael Stanton, Dina Fouad, Mark Vipond, Harry Dean, Matthew Boal, Oliver Brown, Jonathan Goring, Mahmoud Marei, Christian Verhoef, Jonathan Ducey, Clare Rees, Chipo Mushonga, Dan Frith, Ashok Ram, Tristan Boam, Melissa Gabriel, Ferzine Mohamed, David Williams, Katie Cross, Nadine Dyar, Rick MacMahon, Mohammed Fakhrul-Aldeen, Iain Bain, David Bunting, Graham Branagan, Rachel Carten, Chee Wan Lai, Lydia Longstaff, Anindya Niyogi, Claudia Koh, Michael John, Christian Fox, Stavros Loukogeorgakis, Joe Curry, Kate Cross, Jayaram Sivaraj, Sean Marven, Milda Jancauskaite, Helen Please, Wayne Fradley, Fenella Welsh, Maki Jitsumara, Sinead Hassett, Ancuta Muntean, Ionica Stoica, Sarah Yassin, Suzanne Lawther, David Colvin, Ciaran Durand, Mohamed Eltom, Iain Yardley, Kirsty Brennan, Clara Chong, Hasan Mukhtar, Hany Khalil, Stephanie Clark, Ashish Desai, Amulya Saxena, Joshua Cave, Alistair Sharples, Lukas O’Brien, George Kerans, Ashwini Ghorpade, Felicity Arthur, Muhammad Tobbal, Rachael Robertson, Ben Martin, Ben Woodward, Kieran McGovern, and Duncan Rutherford
References
- 1. Giuliani S, Cecil EV, Apelt N, et al. . Pediatric emergency appendectomy and 30-day postoperative outcomes in district general hospitals and specialist pediatric surgical centers in England, April 2001 to March 2012: retrospective cohort study. Ann Surg 2016;263:184–90. 10.1097/SLA.0000000000001099 [DOI] [PubMed] [Google Scholar]
- 2. Di Saverio S, Podda M, De Simone B, et al. . Diagnosis and treatment of acute appendicitis: 2020 update of the WSES Jerusalem guidelines. World J Emerg Surg 2020;15:27. 10.1186/s13017-020-00306-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hall NJ. Conservative treatment of appendicitis in children a randomised controlled trial contract (feasibility study). health technol Assess 2020. [Google Scholar]
- 4. De Simone B, Chouillard E, Di Saverio S, et al. . Emergency surgery during the COVID-19 pandemic: what you need to know for practice. Ann R Coll Surg Engl 2020;102:323–32. 10.1308/rcsann.2020.0097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Joint guidance for surgeons: guidance for surgeons working during the COVID-19 pandemic from the surgical Royal Colleges of the United Kingdom and Ireland. Royal College of Surgeons of England. Available: https://www.rcseng.ac.uk/coronavirus/joint-guidance-for-surgeons-v1/2020
- 6. COVIDSurg Collaborative Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet 2020;396:27–38. 10.1016/S0140-6736(20)31182-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Munro AR D. The missing link? children and transmission of SARS-COV-2, 2020. Available: https://dontforgetthebubbles.com/the-missing-link-children-and-transmission-of-sars-cov-2/
- 8. Harris PA, Taylor R, Thielke R, et al. . Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sherratt FC, Allin BSR, Kirkham JJ, et al. . Core outcome set for uncomplicated acute appendicitis in children and young people. Br J Surg 2020;107:1013–22. 10.1002/bjs.11508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. von Elm E, Altman DG, Egger M, et al. . The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008;61:344–9. 10.1016/j.jclinepi.2007.11.008 [DOI] [PubMed] [Google Scholar]
- 11. Saleem A, Sajid MI, Arshad M. Acute appendicitis during SARS-CoV-2: a brief communication of patients and changes in clinical practice from a single Institute in Pakistan. J Pediatr Surg 2020. 10.1016/j.jpedsurg.2020.07.033. [Epub ahead of print: 05 Aug 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ogundele IO, Alakaloko FM, Nwokoro CC, et al. . Early impact of COVID-19 pandemic on paediatric surgical practice in Nigeria: a national survey of paediatric surgeons. BMJ Paediatr Open 2020;4:e000732. 10.1136/bmjpo-2020-000732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nasher O, Sutcliffe JR, Stewart RJ. Pediatric surgery during the COVID-19 pandemic: an international survey of current practice. Eur J Pediatr Surg 2020. 10.1055/s-0040-1714714. [Epub ahead of print: 26 Aug 2020]. [DOI] [PubMed] [Google Scholar]
- 14. Minneci PC, Mahida JB, Lodwick DL, et al. . Effectiveness of patient choice in Nonoperative vs surgical management of pediatric uncomplicated acute appendicitis. JAMA Surg 2015:1–8. [DOI] [PubMed] [Google Scholar]
- 15. Armstrong J, Merritt N, Jones S, et al. . Non-Operative management of early, acute appendicitis in children: is it safe and effective? J Pediatr Surg 2014;49:782–5. 10.1016/j.jpedsurg.2014.02.071 [DOI] [PubMed] [Google Scholar]
- 16. Gorter RR, van der Lee JH, Cense HA, et al. . Initial antibiotic treatment for acute simple appendicitis in children is safe: short-term results from a multicenter, prospective cohort study. Surgery 2015;157:916–23. 10.1016/j.surg.2015.01.008 [DOI] [PubMed] [Google Scholar]
- 17. Bethell G, Adams S, Johnson T, et al. . Laparoscopy uptake for paediatric appendicectomy – a comparison of adult general surgeons vs paediatric surgeons in England from 1997 to 2015. Nottingham, UK: British Association of Paediatric Surgeons Annual International Congress, 2019. [Google Scholar]
- 18. RIFT Study Group on behalf of the West Midlands Research Collaborative Appendicitis risk prediction models in children presenting with right iliac fossa pain (Rift study): a prospective, multicentre validation study. Lancet Child Adolesc Health 2020;4:271–80. 10.1016/S2352-4642(20)30006-7 [DOI] [PubMed] [Google Scholar]
- 19. Donnelly L. Women needlessly having their appendix out in almost one in three cases. The Daily Telegraph 2019. [Google Scholar]
- 20. Tiboni S, Bhangu A, Hall NJ, et al. . Outcome of appendicectomy in children performed in paediatric surgery units compared with general surgery units. Br J Surg 2014;101:707–14. 10.1002/bjs.9455 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjpo-2020-000831supp001.pdf (38.9KB, pdf)