Abstract
Purpose
Tinnitus and equilibrium disorders such as dizziness and vertigo have been reported by patients with COVID-19; however, they have been rarely investigated. The aim of this study was to study the prevalence of subjective tinnitus and dizziness in a sample of COVID-19 patients using an online 10-item close-ended questionnaire.
Methods
A multicentric study that included 15 Italian hospitals in different regions was conducted using an online 10-item close-ended questionnaire developed to identify the presence of tinnitus and balance disorders in patients with COVID-19 between May 5 and June 10, 2020. The questionnaire was administered to 185 patients in a period of > 30 – < 60 days after diagnosis of COVID-19; responses were recorded in an online Excel spreadsheet. The questionnaire was composed of three sections: (1) demographic information; (2) presence and characteristics of tinnitus and dizziness after COVID-19 diagnosis; (3) possible association with migraine.
Results
Thirty-four patients (18.4%) reported equilibrium disorders after COVID-19 diagnosis. Of these, 32 patients reported dizziness (94.1%) and 2 (5.9%) reported acute vertigo attacks. Forty-three patients (23.2%) reported tinnitus; 14 (7.6%) reported both tinnitus and equilibrium disorders.
Conclusion
This study suggests that the presence of subjective otoneurological symptoms such as tinnitus and balance disorders can affect COVID-19 patients; further studies are necessary to investigate the prevalence and pathophysiological mechanisms underlying these subjective symptoms in COVID-19 patients.
Keywords: COVID-19, Tinnitus, Vestibular disorders, Dizziness, Screening
Introduction
Coronavirus disease 19 (COVID-19) is a pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1, 2]. Although the clinical features observed in COVID-19 patients mostly indicated the respiratory and circulatory systems as the primary targets of SARS-CoV-2, accumulating evidence report neurological manifestations that may affect up to 30% of COVID-19 patients [3, 4].
The neurotropic characteristics of SARS-CoV-2 are still being investigated; genome sequences were detected in the brain of affected patients, with evidence of some pathologic changes in the hypothalamus and cortex neurons [5, 6] and in the cerebrospinal fluids [7]. Several neurological manifestations such as impaired consciousness, headache, and dizziness have been reported in COVID-19 patients [8–10]; in addition, olfactory and taste alterations are common symptoms in patients affected by COVID-19 [11–15].
Even if there is growing evidence of neurological involvement of SARS-CoV-2, otoneurological subjective symptoms such as tinnitus and balance disorders have been only marginally investigated to date. The aim of this work was to investigate the prevalence of tinnitus and equilibrium disorders such as dizziness and vertigo in a sample of COVID-19 patients through an online questionnaire.
Materials and methods
A multicentric study that included 15 Italian hospitals in different regions investigated the presence of tinnitus and equilibrium disorders in COVID-19 patients; data were collected between May 5 and June 10, 2020 using an online 10-item close-ended questionnaire proposed by the authors (Table 1).
Table 1.
Online questionnaire
| Part 1: Equilibrium disorders (vertigo/dizziness) |
| Have you ever experienced vertigo/dizziness before COVID-19 diagnosis? (yes/no) |
| Have you started experiencing dizziness or vertigo after diagnosis of COVID-19? (yes/no) |
| If YES, please describe the characteristics of your symptoms (violent vertigo attacks/chronic dizziness/instability) |
| Indicate the severity of your vertigo/dizziness (0–10) |
| Part 2: Tinnitus |
| Have you ever experienced tinnitus before COVID-19 diagnosis? (yes/no) |
| Have you started experiencing tinnitus after diagnosis of COVID-19? (yes/no) |
| If yes, please specify the characteristics of your tinnitus (occasional/continuous floating/persistent/pulsatile/continuous) |
| Indicate the severity of your tinnitus (0–10) |
| Part 3: Migraine |
| Do you suffer from migraine? (yes/no) |
Participants
Patients with a positive nasopharyngeal swab for SARS-CoV-2 (first positive swab > 30 days– < 60 days before questionnaire administration) were included in the study, regardless of disease severity and necessity of oxygen support during treatment. Exclusion criteria were hospitalization in Intensive Care Unit due to COVID-19, subjective hearing loss in at least one ear, an anamnestic history of acoustic trauma or prolonged noise exposure, presence of known audiological pathologies before the diagnosis of COVID-19 or previous ear surgery, psychiatric, cardiovascular or circulatory comorbidities, or concurrent or previous medical treatment with chloroquine or hydroxychloroquine for their reported ototoxic effects [16–18].
A checkbox to provide informed consent to the use of provided data was included in the questionnaire. All participants gave their informed consent to be included in the study and to the use of anonymized data provided in the responses to the questionnaire. The study was consistent with the Helsinki Declaration for human rights.
Questionnaire details
The questionnaire was designed using the Google Forms software and was administered online to patients in several Italian regions > 30– < 60 days after diagnosis of COVID-19; responses were recorded in an online Excel spreadsheet. Access to the online response spreadsheet was limited to the principal investigator.
The questionnaire was composed of three parts, each one investigating a specific condition and its association with COVID-19: (1) presence and characteristics of equilibrium disorders; (2) presence and characteristics of tinnitus; (3) presence/absence of migraine. For each condition, severity was investigated using a Visual Analogue Scale (VAS) ranging from 0 (absent) to 10 (most severe); no decimals were included in the scale [19, 20].
Statistical analysis
Prevalence of symptoms was calculated using Statistical Packages for Social Sciences (SPSS), version 23, SPSS Inc., Chicago, IL, USA.
Results
One-hundred and eighty-five questionnaire responses from 185 patients with positive nasopharyngeal/oropharyngeal swabs for SARS-CoV-2 in a period of > 30– < 60 days from enrolment were received from May 5 to June 10, 2020. Eighty-six patients (46.5%) were females and 99 were males (53.5%). The mean age was 52.15 (SD ± 13; median 53) with a range of 19–81 years.
Equilibrium disorders
Thirty-four patients (18.4%), 20 females and 14 males, reported balance disorders after COVID-19 diagnosis. Of these, thirty-two patients reported dizziness (94.1%) and two (5.9%) reported acute vertigo attacks [Fig. 1]. VAS score mean for equilibrium disorders was 5.
Fig. 1.
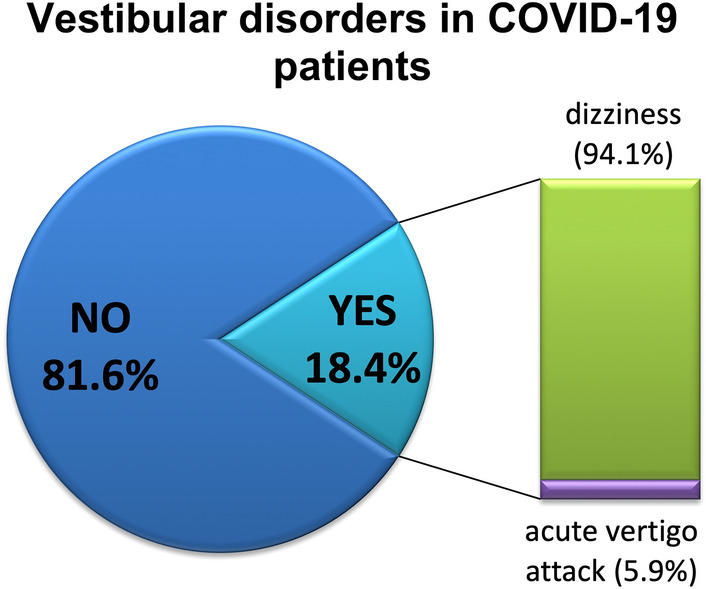
Equilibrium disorders in our sample after COVID-19 diagnosis
Tinnitus
Forty-three patients (23.2%), 15 females and 28 males, reported tinnitus after COVID-19 diagnosis. Tinnitus characteristics were as follows:
17/43 (39.5%) described their tinnitus as recurrent (comes and goes away during the day);
10/43 (23.3%) as occasional (episodic, sporadic);
7/43 (16.3%) as continuous floating (continuous with intensity changes throughout the day);
4/43 (9.3%) as persistent (always present, day and night);
3/43 (7.0%) as pulsatile (synchronous with heartbeat);
2/43 (4.6%) as continuous (always present with the same intensity, making it difficult to fall asleep) [Fig. 2]. VAS score mean for tinnitus was 5.
Fig. 2.
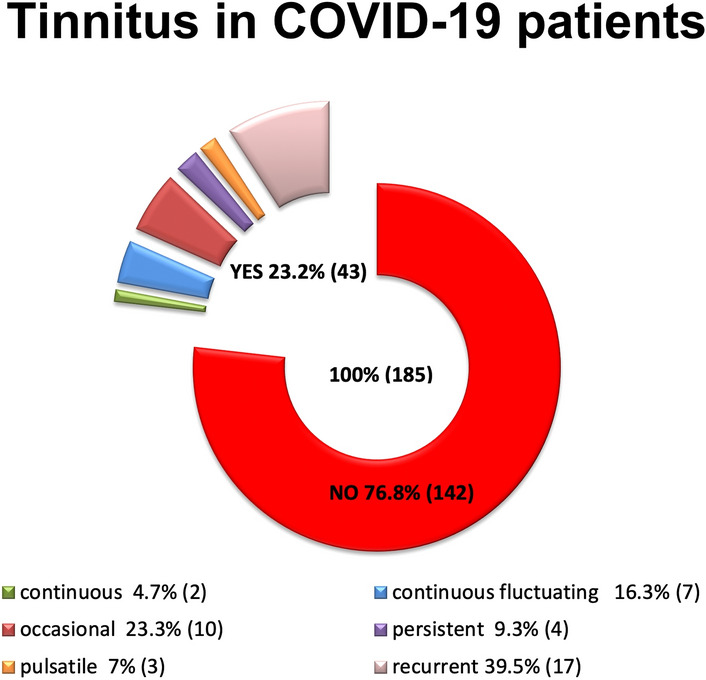
Tinnitus in our sample after COVID-19 diagnosis
Fourteen patients (7.6%) reported both tinnitus and balance disorders after COVID-19 diagnosis corresponding to 32.6% of the patients with tinnitus (14/43) and 41.2% of the patients with vestibular disorders (14/34).
Association between otoneurological disorders and migraine
Migraine was present in 57 patients (30.8%). Eleven (5.9%) patients with tinnitus diagnosis were also affected by migraine, corresponding to 25.6% of all patients with tinnitus following COVID-19 diagnosis (11/43).
Thirteen (7.0%) patients with equilibrium disorders were also affected by migraine, corresponding to 38.2% of all patients with dizziness following COVID-19 diagnosis (13/34).
Five (2.7%) patients with both tinnitus and balance disorders after COVID-19 diagnosis were also affected by migraine.
Discussion
Some viral infections are known to cause hearing loss, most of them typically damaging inner ear cells [21]; however, some viruses can also affect the auditory brainstem [22].
Coronavirus-related otoneurological symptoms, such as tinnitus and balance disorders, have been described so far [10, 23–29], as neurotrophic and neuroinvasive capabilities are typical of some coronaviruses [30]. Previous studies on other coronavirus infections showed a brain involvement, focusing attention on the possible neuro-auditory impairment following this infection [31, 32]. The effects of SARS-CoV-2 on the neuronal tissue could be due to a direct infection of the central nervous system or related to a vascular damage caused by vasculitis or vasculopathy, similarly to the mechanism described for varicella zoster virus (VZV) and human immunodeficiency virus (HIV) [31, 32]. The latter could be supported by the evidence that COVID-19 patients present direct signs of hypercoagulability [33].
Hearing alterations and balance disorders can be dependent on vascular damage because the inner ear structures are particularly susceptible to ischemia due to their characteristics of terminal vasculature and high-energy requirement. Both primary and secondary vasculitis are commonly associated to audiovestibular symptoms, and primary cardiovascular disease can presents episodes of dizziness [34, 35]. Several viral infections, such as hepatitis B and hepatitis C, can be associated with vasculitis and published evidence indicates that in COVID-19 vasculitis is one of the clinical manifestation [36]. Additionally, benign paroxysmal positional vertigo has been clinically reported in COVID-19 patients, although data have not been published yet. It could be hypothesized that prolonged hospitalization and bed rest may be responsible for otolith detachment.
Despite the growing amount of scientific literature on COVID-19, studies that correlate audiovestibular symptoms to SARS-CoV-2 infection are still limited and further investigation is necessary for a better estimate of their incidence [10, 23–29]. Furthermore, the incidence of audiovestibular symptoms among patients infected with other types of coronavirus (i.e., MERS and SARS) is debated [28].
The reported prevalence of audiovestibular symptoms in COVID-19 patients is very low. It is still unclear if this indicates that these symptoms are rare or if the attention of the researchers was more focused on potentially life-threatening symptoms. In the medical literature, tinnitus and balance disorder in COVID-19 patients have been reported in a few studies without identifying common clinical characteristics. Moreover, these reports do not describe in details the relationship with COVID-19 therapies, particularly with chloroquine or hydroxychloroquine, that are well-known causes of inner ear damage [16–18, 37]. Our results, although preliminary, suggest that subjective otoneurological symptoms such as tinnitus and balance disorders may be present in a significant percentage of COVID-19 patients, and should, therefore, be further investigated. It should be taken into account the role of the central nervous system in the onset of investigated symptoms, and especially for equilibrium disorders for which it is impossible to discriminate through our questionnaire the peripheral or central origin.
Limits of this study
This preliminary study presents several limitations that should be considered. The first is the absence of COVID-19 severity evaluation among patients included in the study. Therefore, the severity of the disease cannot be correlated to the presence of the investigated symptoms, as well as the effects of oxygen therapy on inner ear circulation that may be present in patients that underwent this type of support during treatment. The second is the absence of clinical evaluation of these patients (otoscopy, audiovestibular examination); however, the study investigates specifically the newly onset of these symptoms and exclude patients with pre-existing audiovestibular conditions. The third is that the study has been designed as an online reporting tool of subjective symptoms; such reporting may have been influenced by other factors that have not been investigated such as the psychological status of the patient.
Conclusion
Based on the preliminary results of this study, it could be hypothesized that subjective otoneurological symptoms such as tinnitus and balance disorders may be present in COVID-19 patients free from possible bias introduced by pharmacological treatments. Further studies on larger samples are necessary to investigate the pathophysiological mechanisms that underlie the presence of these symptoms in SARS-CoV-2 infection and the persistence of these symptoms over time and their possible transformation into chronic conditions.
Funding
The authors declare that they have no sources of funding.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animals rights
Not applicable.
Informed consent
A checkbox to provide informed consent to the use of provided data was included in the questionnaire. All participants gave their informed consent to the use of anonymized data provided in the responses to the questionnaire.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gentile I, Abenavoli L. COVID-19: perspectives on the potential novel global threat. Rev Recent Clin Trials. 2020 doi: 10.2174/1574887115999200228100745. [DOI] [PubMed] [Google Scholar]
- 2.Tian S, Hu N, Lou J, Chen K, Kang X, Xiang Z, Chen H, Wang D, Liu N, Liu D, Chen G, Zhang Y, Li D, Li J, Lian H, Niu S, Zhang L, Zhang J. Characteristics of COVID-19 infection in Beijing. J Infect. 2020;80(4):401–406. doi: 10.1016/j.jinf.2020.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmad I, Rathore FA. Neurological manifestations and complications of COVID-19: A literature review. J ClinNeurosci. 2020;77:8–12. doi: 10.1016/j.jocn.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, Miao X, Li Y, Hu B. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan. China: JAMA Neurol; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paniz-Mondolfi A, Bryce C, Grimes Z, Gordon RE, Reidy J, Lednicky J, Sordillo EM, Fowkes M. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) J Med Virol. 2020;92(7):699–702. doi: 10.1002/jmv.25915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gu J, Gong E, Zhang B, Zheng J, Gao Z, Zhong Y, Zou W, Zhan J, Wang S, Xie Z, Zhuang H, Wu B, Zhong H, Shao H, Fang W, Gao D, Pei F, Li X, He Z, Xu D, Shi X, Anderson VM, Leong AS. Multiple organ infection and the pathogenesis of SARS. J Exp Med. 2005;202(3):415–424. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang YH, Jiang D, Huang JT. SARS-CoV-2 detected in cerebrospinal fluid by PCR in a case of COVID-19 encephalitis. Brain BehavImmun. 2020;87:149. doi: 10.1016/j.bbi.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu Y, Xu X, Yang L, Liu C, Yang C. Nervous system damage after COVID-19 infection: presence or absence? Brain BehavImmun. 2020;87:55. doi: 10.1016/j.bbi.2020.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moriguchi T, Harii N, Goto J, Harada D, Sugawara H, Takamino J, Ueno M, Sakata H, Kondo K, Myose N, Nakao A, Takeda M, Haro H, Inoue O, Suzuki-Inoue K, Kubokawa K, Ogihara S, Sasaki T, Kinouchi H, Kojin H, Ito M, Onishi H, Shimizu T, Sasaki Y, Enomoto N, Ishihara H, Furuya S, Yamamoto T, Shimada S. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.OzcelikKorkmaz M, Egilmez OK, Ozcelik MA, Guven M. Otolaryngological manifestations of hospitalised patients with confirmed COVID-19 infection. Eur Arch Otorhinolaryngol. 2020 doi: 10.1007/s00405-020-06396-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ralli M, Stadio A, Greco A, Vincentiis M, Polimeni A. Defining the burden of olfactory dysfunction in COVID-19 patients. Eur Rev Med PharmacolSci. 2020;24(7):3440–3441. doi: 10.26355/eurrev_202004_20797. [DOI] [PubMed] [Google Scholar]
- 12.Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, Dequanter D, Blecic S, El Afia F, Distinguin L, Chekkoury-Idrissi Y, Hans S, Delgado IL, Calvo-Henriquez C, Lavigne P, Falanga C, Barillari MR, Cammaroto G, Khalife M, Leich P, Souchay C, Rossi C, Journe F, Hsieh J, Edjlali M, Carlier R, Ris L, Lovato A, De Filippis C, Coppee F, Fakhry N, Ayad T, Saussez S. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277(8):2251–2261. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giacomelli A, Pezzati L, Conti F, Bernacchia D, Siano M, Oreni L, Rusconi S, Gervasoni C, Ridolfo AL, Rizzardini G, Antinori S, Galli M. Self-reported olfactory and taste disorders in patients with severe acute respiratory coronavirus 2 infection: a cross-sectional study. Clin Infect Dis. 2020;71(15):889–890. doi: 10.1093/cid/ciaa330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spadera L, Viola P, Pisani D, Scarpa A, Malanga D, Sorrentino G, Madini E, Laria C, Aragona T, Leopardi G, Maggiore G, Ciriolo M, Boccuto L, Pizzolato R, Abenavoli L, Cassandro C, Ralli M, Cassandro E, Chiarella G. Sudden olfactory loss as an early marker of COVID-19: a nationwide Italian survey. Eur Arch Otorhinolaryngol. 2020 doi: 10.1007/s00405-020-06252-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiarella G, Pizzolato R, Malanga D, Pisani D, Abenavoli L, Viola P. Prevention of COVID-19 infection in the medical population: a possible help from anosmia? Rev Recent Clin Trials. 2020 doi: 10.2174/1574887115666200603152637. [DOI] [PubMed] [Google Scholar]
- 16.Altissimi G, Colizza A, Cianfrone G, Vincentiis M, Greco A, Taurone S, Musacchio A, Ciofalo A, Turchetta R, Angeletti D, Ralli M. Drugs inducing hearing loss, tinnitus, dizziness and vertigo: an updated guide. Eur Rev Med PharmacolSci. 2020;24(15):7946–7952. doi: 10.26355/eurrev_202008_22477. [DOI] [PubMed] [Google Scholar]
- 17.Prayuenyong P, Kasbekar AV, Baguley DM. Clinical Implications of Chloroquine and Hydroxychloroquine Ototoxicity for COVID-19 treatment: a mini-review. Front Public Health. 2020;8:252. doi: 10.3389/fpubh.2020.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ralli M, Lobarinas E, Fetoni AR, Stolzberg D, Paludetti G, Salvi R. Comparison of salicylate- and quinine-induced tinnitus in rats: development, time course, and evaluation of audiologic correlates. OtolNeurotol. 2010;31(5):823–831. doi: 10.1097/MAO.0b013e3181de4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raj-Koziak D, Gos E, Swierniak W, Rajchel JJ, Karpiesz L, Niedzialek I, Wlodarczyk E, Skarzynski H, Skarzynski PH. Visual analogue scales as a tool for initial assessment of tinnitus severity: psychometric evaluation in a clinical population. AudiolNeurootol. 2018;23(4):229–237. doi: 10.1159/000494021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dannenbaum E, Chilingaryan G, Fung J. Visual vertigo analogue scale: an assessment questionnaire for visual vertigo. J Vestib Res. 2011;21(3):153–159. doi: 10.3233/VES-2011-0412. [DOI] [PubMed] [Google Scholar]
- 21.Cohen BE, Durstenfeld A, Roehm PC. Viral causes of hearing loss: a review for hearing health professionals. Trends Hear. 2014 doi: 10.1177/2331216514541361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abramovich S, Prasher DK. Electrocochleography and brain-stem potentials in Ramsay Hunt syndrome. Arch Otolaryngol Head Neck Surg. 1986;112(9):925–928. doi: 10.1001/archotol.1986.03780090021002. [DOI] [PubMed] [Google Scholar]
- 23.Elibol E. Otolaryngological symptoms in COVID-19. Eur Arch Otorhinolaryngol. 2020 doi: 10.1007/s00405-020-06319-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malayala SV, Raza A. A Case of COVID-19-Induced Vestibular Neuritis. Cureus. 2020;12(6):e8918. doi: 10.7759/cureus.8918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munro KJ, Uus K, Almufarrij I, Chaudhuri N, Yioe V. Persistent self-reported changes in hearing and tinnitus in post-hospitalisation COVID-19 cases. Int J Audiol. 2020;1:1–2. doi: 10.1080/14992027.2020.1798519. [DOI] [PubMed] [Google Scholar]
- 26.Fiani B, Covarrubias C, Desai A, Sekhon M, Jarrah R. A Contemporary review of neurological sequelae of COVID-19. Front Neurol. 2020;11:640. doi: 10.3389/fneur.2020.00640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freni F, Meduri A, Gazia F, Nicastro V, Galletti C, Aragona P, Galletti C, Galletti B, Galletti F. Symptomatology in head and neck district in coronavirus disease (COVID-19): a possible neuroinvasive action of SARS-CoV-2. Am J Otolaryngol. 2020;41(5):102612. doi: 10.1016/j.amjoto.2020.102612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Almufarrij I, Uus K, Munro KJ. Does coronavirus affect the audio-vestibular system? A rapid systematic review. Int J Audiol. 2020;59(7):487–491. doi: 10.1080/14992027.2020.1776406. [DOI] [PubMed] [Google Scholar]
- 29.Karimi-Galougahi M, Naeini AS, Raad N, Mikaniki N, Ghorbani J. Vertigo and hearing loss during the COVID-19 pandemic - is there an association? ActaOtorhinolaryngolItal. 2020 doi: 10.14639/0392-100X-N0820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sriwijitalai W, Wiwanitkit V. Hearing loss and COVID-19: a note. Am J Otolaryngol. 2020;41(3):102473. doi: 10.1016/j.amjoto.2020.102473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chetty R, Batitang S, Nair R. Large artery vasculopathy in HIV-positive patients: another vasculitic enigma. Hum Pathol. 2000;31(3):374–379. doi: 10.1016/s0046-8177(00)80253-1. [DOI] [PubMed] [Google Scholar]
- 32.Gilden DH, Kleinschmidt-DeMasters BK, Wellish M, Hedley-Whyte ET, Rentier B, Mahalingam R. Varicella zoster virus, a cause of waxing and waning vasculitis: the New England Journal of Medicine case 5–1995 revisited. Neurology. 1996;47(6):1441–1446. doi: 10.1212/wnl.47.6.1441. [DOI] [PubMed] [Google Scholar]
- 33.Panigada M, Bottino N, Tagliabue P, Grasselli G, Novembrino C, Chantarangkul V, Pesenti A, Peyvandi F, Tripodi A. Hypercoagulability of COVID-19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. J ThrombHaemost. 2020;18(7):1738–1742. doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ralli M, Campo F, Angeletti D, Minni A, Artico M, Greco A, Polimeni A, de Vincentiis M. Pathophysiology and therapy of systemic vasculitides. EXCLI J. 2020;19:817–854. doi: 10.17179/excli2020-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ralli M, Di Stadio A, De Virgilio A, Croce A, de Vincentiis M. Autoimmunity and otolaryngology diseases. J Immunol Res. 2018;1:2747904. doi: 10.1155/2018/2747904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roncati L, Ligabue G, Fabbiani L, Malagoli C, Gallo G, Lusenti B, Nasillo V, Manenti A, Maiorana A. Type 3 hypersensitivity in COVID-19 vasculitis. ClinImmunol. 2020;217:108487. doi: 10.1016/j.clim.2020.108487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Luca P, Scarpa A, De Bonis E, Cavaliere M, Viola P, Gioacchini MF, Ralli M, Cassandro E, Cassandro C. Chloroquine and hydroxychloroquine ototoxicity; potential implications for SARS-CoV-2 treatment. A brief review of the literature. Am J Otol. 2020;8:102640. doi: 10.1016/j.amjoto.2020.102640. [DOI] [PMC free article] [PubMed] [Google Scholar]


