Abstract
Dimethyldioxirane epoxidizes 4,5-benzoxepin to form the reactive 2,3-epoxyoxepin intermediate followed by very rapid ring-opening to an o-xylylene that immediately isomerizes to the stable product 1H-2-benzopyran-1-carboxaldehyde. The present study demonstrates that separate incubations of 4,5-benzoxepin with three cytochrome P450 isoforms (2E1, 1A2, and 3A4) as well as pooled human liver microsomes (pHLM) also produce 1H-2-benzopyran-1-carboxaldehyde as the major product, likely via the 2,3-epoxyoxepin. The reaction of 4,5-benzoxepin with cerium (IV) ammonium nitrate (CAN) yields a dimeric oxidized molecule that is also a lesser product of the P450 oxidation of 4,5-benzoxepin. The observation that P450 enzymes epoxidize 4,5-benzoxepin suggests that the 2,3-epoxidation of oxepin is a major pathway for the ring-opening metabolism of benzene to muconaldehyde.
Keywords: 2,3-epoxyoxepins; metabolic ring opening of benzene; cytochrome P450 isozymes
1. Introduction
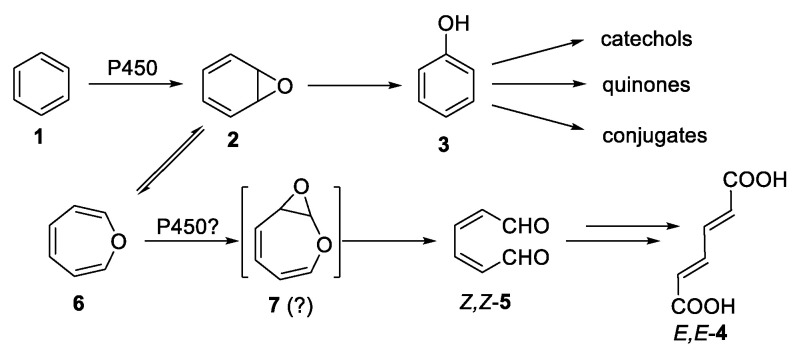
Benzene (1) is a very widespread environmental contaminant and human carcinogen with a complex metabolic fate (Scheme 1). The discovery [1] of the equilibrium between benzene oxide (2) and oxepin (6) laid the groundwork for the study by Davies and Whitham [2] in which they oxidized oxepins by employing meta-chloroperbenzoic acid (m-CPBA), a study relevant to the metabolism of benzene (1). The oxidation reactions produced acyclic dicarbonyl compounds (e.g., 5 through putative 2,3-epoxyoxepins (7, see Scheme 1). However, the reactive 2,3-epoxyoxepin intermediates could neither be isolated nor observed spectroscopically [2], due, in part, to the acidic conditions of their generation. The present study makes a comparison between the oxidative metabolism of benzene through benzene oxide/oxepin with that of a “non-natural metabolite” of naphthalene: 4,5-benzoxepin. It is worthwhile to very briefly compare these metabolic pathways.
Scheme 1.
Metabolic pathway of benzene to phenol and postulated pathway to E,E-muconic acid.
Benzene is a very widespread environmental contaminant and human carcinogen with a complex metabolic fate (Scheme 1) [3,4,5,6]. It is metabolized in the liver, primarily by cytochrome P450 2E1, mostly through epoxidation to benzene oxide (2) [7,8,9,10], followed by rearrangement to phenol (3) and subsequent further oxidation as well as bioconjugation reactions. A relatively small fraction (up to 10–20%) is metabolically ring opened by P450 to Z,Z-muconaldehyde (Z,Z-5) which isomerizes to E,E-5, which is oxidized by aldehyde dehydrogenase to E,E-muconic acid (E,E-4, detected in urine [11]) [12,13,14,15]. Muconaldehyde is a very potent inhibitor of the normal generation of blood cells [6]. It reacts with glutathione and can cross-link DNA and proteins and induces inhibition of gap junction intracellular communication [16,17,18].
Benzene is unique in its conversion to significant quantities of closed-ring (e.g., phenol, 3) and ring-opened (e.g., muconic acid, 4) metabolites via mammalian monooxygenases. Alkylated aromatics such as toluene, ethylbenzene, and xylenes are predominantly metabolically oxidized at the alkyl groups (e.g., toluene to benzyl alcohol [19]). Naphthalene is metabolized to 1-naphthol. Other, larger polycyclic aromatic hydrocarbons (PAH) also form ring-substituted rather than open-chain metabolites. While there is near-universal acceptance of the benzene oxide pathway to phenol, the pathway to muconaldehyde is less understood. Direct attack by hydroxyl (OH) on benzene [20] followed by a reaction of the initial radical with molecular oxygen appears to be relevant to atmospheric formation of muconaldehyde [21]. Of particular interest is the pathway suggested by Davies and Whitham [2] nearly four decades ago. They postulated the epoxidation of oxepin to “2,3-epoxyoxepin” (7) followed by rapid ring opening to Z,Z-muconaldehyde (Z,Z-5). Z,Z-5 isomerizes thermally as well as catalytically to E,Z-5 followed by catalyzed isomerization to E,E-5 (see Scheme 1) [22,23]. Using the acidic epoxidation reagents (e.g., m-CPBA) of the period, Davies and Whitham implicated the intermediacies of 2,3-epoxyoxepins without isolating or spectroscopically observing them [2].
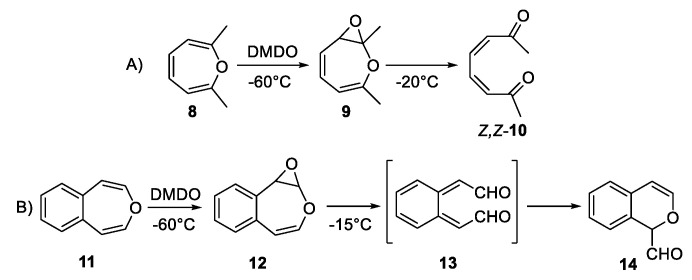
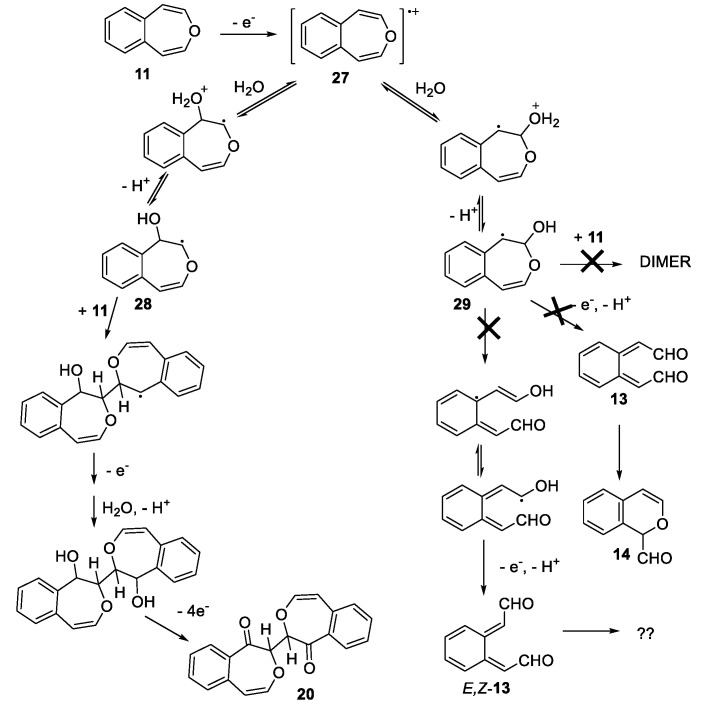
Ab initio calculations predicted a low barrier for the concerted ring opening of 7 to Z,Z-5 [24]. Interestingly, 3,6-bridged 2,3-epoxyoxepins were reported in 1982, synthesized using m-CPBA and exhibited melting points ca 100 °C [25]. These surprising stabilities are attributable to the influences of the bridges which both frustrate thermal rearrangements and somewhat stabilize the epoxides to acid [26]. The first report of a simple 2,3-epoxyoxepin involved reaction of 2,7-dimethyloxepin (8) with dimethyldioxirane (DMDO) [also methyl(trifluoromethyl)dioxirane] at −60 °C involved NMR observation of a trace amount of 2,3-epoxyoxepin 9 which isomerized to the diketone (Z,Z-10) at −20 °C (Scheme 2A) [27]. A similar study of the parent 2/6 equilibrium produced Z,Z-10 but no direct observation of 7 [28]. The reaction of DMDO with 4,5-benzoxepin (11) at −50 °C provided a near quantitative yield of 12 which rearranged rapidly at −15 °C to form 14 almost quantitatively, undoubtedly via the short-lived ortho-xylylene 13 (Scheme 2B) [29].
Scheme 2.
(A) Reaction of 2,7-dimethyloxepin with dimethyldioxirane (DMDO); (B) reaction of 4,5-benzoxepin with dimethyldioxirane.
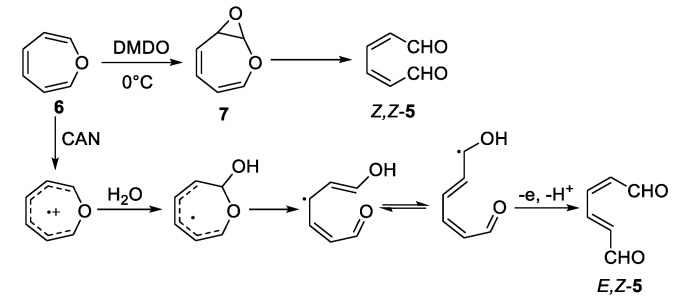
The growing acceptance that oxepin is an intermediate in the pathway to muconaldehyde has raised other subtleties. Low-temperature DMDO reactions with oxepin, 2-methyloxepin, and 2,7-dimethyloxepin form the Z,Z-isomers (e.g., Z,Z-5, Scheme 3) [28]. Viewing consecutive one-electron oxidations, as another reasonable pathway to muconaldehyde, the Golding group explored surrogate reactions [30]. The reaction of the above three oxepins with cerium(IV) ammonium nitrate (CAN) produced E,Z-isomers exclusively (e.g., E,Z-5 as well as E,Z-10, see Scheme 3), consistent with geometric isomerization of the ring-opened radical intermediate [30]. Without intercession of free radical intermediates, Z,Z-5 is converted to E,Z-5 at 55 °C in the dark in 16 h [22]. The cyclic 2H-pyran-2-carboxaldehyde is the higher-energy intermediate connecting these two stereoisomers. This thermal pathway does not isomerize E,Z-5 to E,E-5. E,Z-5 (as well as Z,Z-5) are readily converted to E,E-5 in the presence of a nucleophilic reagent (e.g., trimethylamine in acetonitrile) acting as a catalyst in reversible Michael additions [22]. In contrast, for the 4,5-benzo-substituted system (Scheme 2), 1H-2-benzopyran-1-carboxaldehyde (14), the cyclic molecule is more stable than the Z,Z-isomer (13) and effectively shuts down the pathway to the E,Z-isomer, and is also higher in energy.
Scheme 3.
Golding dimethyldioxirane (DMDO) and postulated cerium (IV) ammonium nitrate (CAN) oxidation mechanisms for oxepin [30].
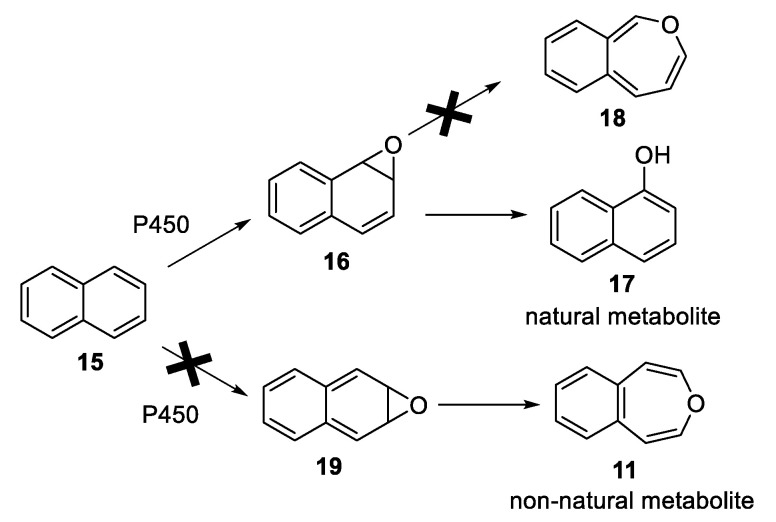
2,7-Dimethyloxepin (8, Scheme 2) is an interesting substrate for investigation of P450 metabolism. Although it is the only detectable tautomer, due to steric repulsion between the methyl substituents in the benzene oxide tautomer, the Curtin–Hammett Principle does not eliminate the undetectable tautomer from being the reactive substrate [31]. In principle, it could be derived by P450-catalyzed epoxidation of ortho-xylene. Since xylenes are oxidized at the methyl substituent, 8 should not form in significant quantity via mammalian P450 monooxygenases. One might think of it as a “non-natural metabolite further down a hypothetical pathway avoiding methyl oxidation”. 4,5-Benzoxepin (11) is even more interesting as a non-natural metabolite. Naturally occurring P450-catalyzed epoxidation at the 1,2-position of naphthalene (15) forms the 1,2-epoxide (16) which isomerizes to 1-naphthol (17) rather than to the less stable oxepin 18, which lacks aromatic stabilization [32,33]. Hypothetical epoxidation at the 2,3-position of naphthalene would eliminate aromaticity—the 2,3-epoxide (19) is far less stable than the 1,2-epoxide. It is never formed, and therefore its valence isomer 4,5-benzoxepin (11) is not produced naturally (see Scheme 4) [32,33]. Thus, 11 can also be considered as a “non-natural metabolite further down a hypothetical pathway avoiding natural 1,2-epoxidation of naphthalene”.
Scheme 4.
Comparison of Epoxidation Pathways for Naphthalene.
Although the benzene oxide isomer of 8 is undetectable by NMR, it is only a few kcal/mol higher in energy, so that rearrangement to dimethylphenol and attack by nucleophiles should be possible. In contrast, 19 is calculated to be over 30 kcal/mol less stable than 11 [33], eliminating reactions via the epoxide.
2. Results and Discussion
2.1. Oxidations of 4,5-Benzoxepin with DMDO and CAN
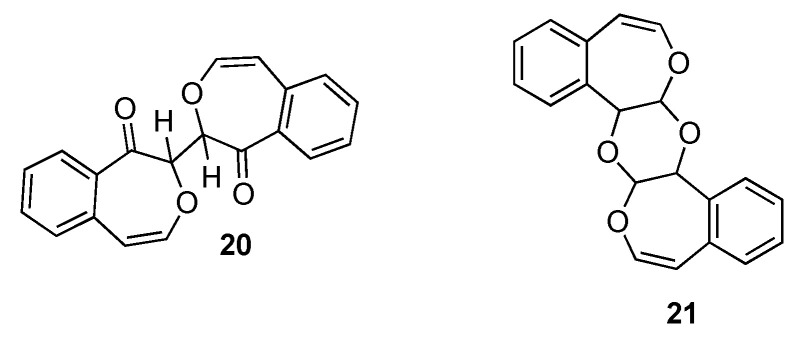
The present study compares the reaction products of DMDO and CAN oxidations of 2,7-dimethyloxepin (8) and 4,5-benzoxepin (11) with those derived from incubation with cytochrome P450 enzymes (synthetic procedures found in Appendix A and Supplementary Information). Whereas oxidation of 2,7-dimethyloxepin with DMDO produces Z,Z-10 and CAN oxidation yields E,Z-10 [29], the corresponding oxidations of 4,5-benzoxepin produced very different, non-isomeric products. A reaction with DMDO (1:1 molar ratio) produces 14 but reaction with CAN (2:1 molar ratio) produces two essentially dimeric molecules assigned structures 21 (C20H16O4) and 20 (C20H14O4) (Scheme 5). The two compounds appear to form via different pathways. As will be supported later in this paper, incubation of 11 with P450 isoforms yielded 20 as a lesser product with a minute quantity of 21.
Scheme 5.
Proposed products of the CAN reaction with 4,5-benzoxepin (11) assigned structure 21 (C20H16O4) and 20 (C20H14O4).
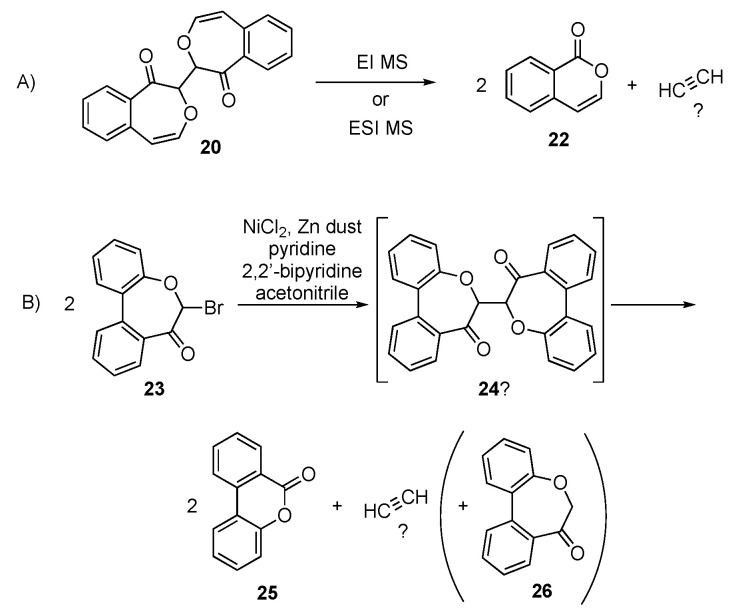
Spectroscopic data in support of the identifications of 20 and 21 are in the Experimental Section and further documented in the Supplementary Information. B3LYP/6-31G* calculations [34,35] predict that (R,R)- (or S,S)-20 is 1.4 kcal/mol lower in energy than meso-20. The anti- (Ci) diastereomer of 21 is calculated to be 5.7 kcal/mol lower in energy than the syn- (C2v) diastereomer. 13C NMR shows ten unique carbon atoms with shifts consistent with those expected for 20. 1H NMR shows seven distinct protons (4H: aromatic; 2H vinylic; 1H at 6.6 ppm roughly consistent with the downfield shift calculated for the dimer (meso or R,R/S,S)). Two-dimensional NMR (COSY, DEPT, NOESY, and HSQC) suggests the presence of a conjugated alkene (NOESY correlation) and confirms the presence of one proton (6.6 ppm) on the carbon alpha to the carbonyl (DEPT, HSQC correlations). IR spectroscopy indicates a strong absorbance at 1666 cm−1 and no absorbance due to hydroxyl groups. The UV absorbances (λmax = 273 nm and 338 nm) are also consistent with similar structural features. GC/EI-MS data as well as ESI/MS data were not definitive for a structure corresponding to 21 (expected m/z = 318, or 319 via ESI/MS). However, EI and ESI/MS are both consistent with isocoumarin which could arise from 20 through a loss of acetylene (or its equivalent, see Scheme 6A), presumably in a stepwise mechanism. Column chromatography did not provide samples of 20 or 21 suitable for elemental analysis or X-ray crystallographic study.
Scheme 6.
(A) Observed mass spectrometry fragmentation of 20 to 22; (B) Postulated pathway for observed chemical transformation of 23 to 26.
The EI mass spectrum observed corresponding to isocoumarin (22) includes an abundant m/z 90 (Parent - 2 CO) in agreement with a published mass spectrum [36]. Another published mass spectrum for isocoumarin [37] appears to erroneously report an abundant m/z 97 with no m/z 90 reported. Precise mass analysis corresponds to the formula C9H6O2 for the m/z 146 ion as expected for isocoumarin (see Supplementary Information). The ESI/MS data also correspond to isocoumarin. Further investigation of this system involved synthesis of the reported monomeric bromo derivative 23 [38]. Coupling using NiCl2 and Zn in the presence of pyridine and 2,2′-bipyridine to produce dimer 24 was attempted. It provided lactone 25 (Scheme 6B) in addition to reduced monomer 26, the latter likely due to residual moisture. Although the expulsion of acetylene appears to be unlikely, it is possible that the two carbons lost en route to 25 might arise in other forms under these reaction conditions. A single small-scale headspace analysis found no acetylene and only methane as the single gaseous component, which was very surprising and is worthy of future systematic investigation.
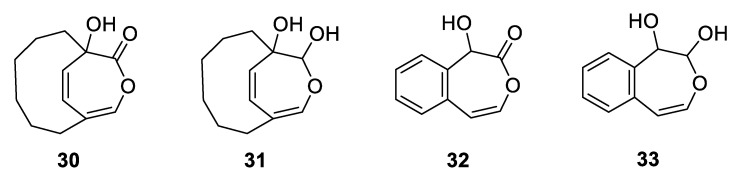
Scheme 7 describes a postulated reaction mechanism for the formation of the dimeric 20 from 4,5-benzoxepin under CAN oxidation. While the mechanism resembles that postulated by Golding et al. [30] in its initial steps, the ring opening of the postulated free radical 25 is unfavorable due to the need to lose aromaticity; hence, 14 (via 13) is not formed and isomerization to E,Z-13 should not occur. While the mechanism offered in Scheme 7 depicts dimerization through the reaction of the radical 24 with 11, one would expect analogous dimerization through 25 to be more sterically hindered. The central C–C linkage in dimeric molecule 20 might be anticipated to be somewhat weak due to the possibility of forming two capto-dative free radicals. However, a dimeric molecule capable of forming two PhCOC(OPh)(Ph) radicals showed no evidence of decomposition at 60 °C [39]. Indeed, a crystallographic study of bridged dimer containing this linkage provided a C–C bond length of 1.523 Ǻ [40]. Other stable examples are known [41,42]. The pathway to formation of 21 is suggested by the oxidation of the bridged oxepin to form 30, presumed to occur via glycol 31 [43]. The equivalent 32 and 33 are reasonable precursors to 21 (Scheme 8).
Scheme 7.
Postulated mechanism for formation of the dimeric molecule 20 from 4,5-benzoxepin in the presence of CAN.
Scheme 8.
Models (30 and 31) for and potential precursors (32 and 33) of 21.
2.2. Incubations of 4,5-Benzoxepin with P450 Isoforms
Incubations of 11 with P450 2E1, P450 1A2, P450 3A4, and pooled human liver microsomes (pHLM) were compared with the products of DMDO and CAN oxidations. The 2E1 isoform is known to be optimal for benzene; the 1A2 isoform is optimal for naphthalene; the 3A4 isoform has a larger active site and is relatively non-specific. The enzymes and pHLM were obtained commercially and the co-factor mixture is described in the Experimental Section. The primary analytical method for the enzyme incubation products was GC/MS since the scale is small.
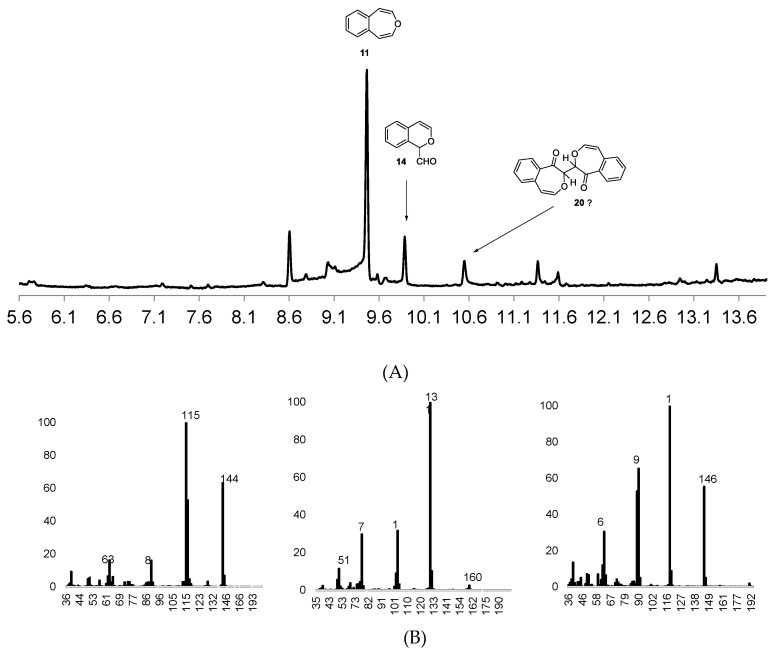
Figure 1A reproduces the GC/MS chromatogram derived from the incubation of 4,5-benzoxepin (11) with cytochrome P450 1A2. Highlighted in this chromatogram are unreacted 11, 1H-2-benzopyran-1-carboxaldehyde (14) and a smaller quantity of the dimer (20). Figure 1B reproduces the mass spectra of these three components. We have established that the epoxidation of 11 with DMDO cleanly produces 14 [29]. Trials with P450 2E1, P450 3A4 and pHLM all produced similar results. Incubations of 11 with 2E1 and 3A4 yielded a lower proportion of 21 relative to 14. The reaction of 11 with CAN produces not 14 but the oxidized dimeric molecule 21, as noted above. Control experiments without the P450 isoforms did not yield 14 or 20. Incubations with 2E1, 3A4, and pHLM each yield very similar results with 4,5-benzoxepin. They suggest that, for the cytochrome P450s tested, each epoxidize the oxepin structure as the major pathway. It may well be that consecutive single-electron oxidations constitute a relatively minor pathway.
Figure 1.
(A) Total Ion Chromatogram (TIC) for reaction of 4,5-benzoxepin 11 with P450 1A2; (B) mass spectra of compounds 11, 14, and 20, respectively.
2.3. Incubations of 2,7-Dimethyloxepin with P450 Isoforms
Incubations of 2,7-dimethyloxepin (8) with the same suite of enzymes as 11 under the same reaction conditions resulted in inconsistent observations of E,Z-10 in very trace amounts. Some of the trials provided dimethylphenols in appreciable yields and dimethyldihydroxybenzenes in low-to-moderate yields. Control experiments suggest that these products are the result of enzymatic reactions, not just thermal rearrangements. There has been no evidence of oxidation at the methyl groups of 8 to form carboxylic acid derivatives. The paper by Golding et al. [30] did not report P450 incubations. As noted earlier, the benzene oxide valence isomer of 2,7-dimethyloxepin is only a few kcal/mol higher in energy than the benzene oxide isomer. Benzene oxides exhibit very short half-lives in protic solvents consistent with the observation of dimethylphenol(s) and even dimethylcatechol(s). In contrast, the epoxide valence isomer (19) of 4,5-benzoxepin is energetically inaccessible, shutting down some pathways of competing reactions, thus permitting epoxidation of 11 and its rearrangement to 14. The GC/MS data for the incubations of 8 are included in the Supplementary Information.
3. Materials and Methods
Starting materials, reagents, and solvents were purchased from Aldrich, Alfa Aesar and Fisher Scientific and used without further purification unless otherwise indicated. Reaction products were purified by flash column chromatography using Silicycle Inc. silica gel (60 Å, 230–400 mesh). Thin-layer chromatography was performed on Agela Technologies TLC Silica plates (Agela Technologies, Tianjin, China) (silica gel 60 GF254) and visualization was accomplished with UV light (UVP, INC, San Gabriel, CA, USA). 2,7-dimethyloxepin (8) and 4,5-benzoxepin (11) were synthesized and purified according to the literature [44,45] using standard air-free techniques. Stock solutions of the oxepins 8 and 11 were prepared in acetonitrile and stored at −80°C for use in enzymatic studies. All intermediates and final products were characterized by NMR. 1H NMR and 13C NMR spectra were acquired with a Varian UnityINOVA 500 NMR (Varian, Palo Alto, CA, USA) or Varian Mercury 400 BB NMR (Varian, Palo Alto, CA, USA). Chemical shifts are reported in parts per million (ppm) relative to tetramethylsilane (TMS) unless otherwise noted and coupling constants (J values) are in Hertz (Hz). Infrared spectra were recorded on a Thermo Fischer Scientific Nicolet iS10 FTIR spectrometer (ThermoFischer Scientific, Waltham, MA, USA). UV–vis data were acquired on a Varian Cary 5E UV–vis-NIR spectrophotometer. NADPH and magnesium dichloride were purchased from Sigma Aldrich (St Louis, MO, USA). Potassium phosphate buffer (0.5 M), pooled human liver microsomes, and cytochrome P450 isoforms 1A2, 3A4, and 2E1 were purchased from Corning Life Sciences in Woburn, MA, USA. MilliQ water was used in all incubations, which were carried out in a 37 °C water bath. All organic extracts of incubation reactions in dichloromethane were characterized using a Shimazdu GCMS-QP2010 (Shimazdu, Kyoto, Japan) equipped with a 30.0 m SHRX1-5MS column (thickness: 0.25 um, diameter: 0.25 um) and 70 eV electron impact detector. The injector temperature (splitless injection) was held at 250 °C and the detector at 260 °C. The column temperature increased from 45 °C to 250 °C at 15 °C/min and was held at 250 °C for 0 min, after a solvent delay of 5.5 min. The flow rate was 1 mL/min and the total run time was 14.67 min. Standards of the reactants and expected metabolites (see Scheme S1) were prepared in dichloromethane, and retention times and mass spectra were recorded for comparison.
4. Conclusions
The incubation (P450 isoforms and pHLM) experiments employing 4,5-benzoxepin (11) clearly indicate epoxidation to form the reactive intermediate 2,3-epoxyoxepin as the major pathway. A lesser metabolic product is the dimeric molecule 20, a reaction product of the oxidation of 11 by CAN. There was no detectable quantity of 21, the other CAN product. These observations suggest that cytochrome P450 epoxidizes the oxepin as its major pathway but also catalyzes consecutive one-electron oxidations. Incubation of 2,7-dimethyloxepin with P450 isoforms and pHLM did not produce the anticipated ring-opened diketone. Instead, dimethylphenols and dimethyldihydroxybenzenes were found. Although these results are essentially consistent with the results of Stok et al. [44], who were the first to identify an oxepin as a minor product of incubation of t-butylbenzene with cytochrome P450, it must be noted that this was bacterial P450 rather than mammalian. In contrast to 4,5-benzoxepin, where the naphthalene oxide isomer is energetically inaccessible, the 1,2-dimethylbenzene oxide isomer is only a few kcal/mol higher in energy than 2,7-dimethyloxepin. While it is not self-evident how to differentiate the formation of muconaldehyde from benzene oxide versus oxepin, the work of Golding and colleagues (e.g., Scheme 3) on these tautomers and methyl- and dimethyl-substituted derivatives provides subtle stereochemical probes into these questions. The present work, relying on a “non-natural” metabolite of naphthalene suggests that, for this system, at least, the epoxidation of an oxepin to form a reactive 2,3-epoxyoxepin intermediate is an important, if not dominant, metabolic pathway.
Acknowledgments
We gratefully acknowledge the help of Professor Stacia Sower and her student Tim Marquis in providing the facilities and instruction for the incubation of substrates with enzymes and to Professor Sterling Tomellini for access to the gas chromatography/mass spectrometry apparatus. We are also grateful for the financial support of the University of New Hampshire Graduate School (Summer TA fellowship—HMG) and the Department of Chemistry at UNH (Daggett Award—RWF). We are thankful for access to instruments (NMR, FTIR, and UV–vis) within the University Instrumentation Center (UIC) at the University of New Hampshire in Durham, NH. We would like to thank Sebastian A. Pantovich for performing the headspace analysis. Finally, we acknowledge David Ashline for his help in obtaining the ESI-MS spectra and for providing his expertise.
Supplementary Materials
The following are available online: characterization data and spectra and analysis of incubation products.
Appendix A
Synthetic Procedures and Characterization
Preparation of 4,5-Benzoxepin (11) via Autooxidation
A 2% solution of 1,4-dihydro-1,4-epoxy-naphthalene (3.0 g) in absolute ethanol (150 mL) was prepared and divided into three quartz tubes [45]. The tubes were sealed and flushed with nitrogen. The reaction mixture was irradiated at 2537 Å for 24 h, then concentrated and purified by column chromatography (100% hexanes). Compound 11 eluted first and was collected in a 6% yield as a yellow solid. 1H NMR (400 MHz, CDCl3) δ = 5.06 (dd, 2H, J = 7.70, 3.99 Hz), 5.67 (dd, 2H, J = 7.38, 3.98 Hz), 6.61–6.66 (AA’XX’, 2H), 6.88-6.92 (AA’XX’, 2H). 13C NMR (100 MHz, CDCl3) δ = 146.2, 136.0, 129.2, 127.9, 113.4. EI-MS (DCM) = 144 (85), 115 (100), 89 (35). UV–vis (0.2 mg/mL in DCM) λmax = 310.2 nm.
Preparation of 2,7-Dimethyloxepin (8)
2,7-dimethyloxepin was prepared according to the literature procedure published by Paquette and Barrett [46].
Oxidation of 11 with DMDO. The NMR scale reaction was carried out according to the procedure by Nauduri and Greenberg [29].
Preparation of 20
Cerium ammonium nitrate (2 eq, 1.08 g, 1.9 mmol) was dissolved in acetone (15 mL) in a foil-covered 50 mL round bottom flask with stirring. The flask as then cooled to −78 °C, and 4,5-benzoxepin (1 eq, 136 mg, 0.94 mmol) dissolved in acetone (10 mL) was added dropwise. The reaction was monitored by TLC (3:1 hexanes:ether). After 10 min, the reaction was brought to room temperature and diluted with ether. The organic layer was washed with brine, dried over sodium sulfate, and concentrated under reduced pressure. The crude product was purified by column chromatography (60 mesh silica gel, 6:1 hexanes: EtOAc) and the second fraction was collected and characterized as follows. 1H NMR (400 MHz, CDCl3) δ = 5.79 (d, 1H, J = 8.17 Hz), 6.23 (d, 1H, J = 5.24 Hz), 6.36 (d, 1H, J = 8.17 Hz), 7.01 (d, 1H, J = 5.23 Hz), 7.30 (d, 1H, J = 7.65), 7.31 (t, 1H, J = 7.50 Hz), 7.40 (d, 1H, J = 7.21 Hz), 7.46 (t, 1H, J = 7.55 Hz). 13C NMR (125 MHz, CDCl3) δ = 79.2, 92.4, 107.5, 126.2, 127.0, 129.1, 129.7, 130.4, 133.8. 138.8. IR (ATR, neat) = 807 cm−1(C-H), 843 cm−1 (C-H), 1270 cm−1 (C-O), 1284 cm−1 (C-O), 1652 cm−1 (C=C-O). UV–vis (0.2 mg/mL in DCM) λmax = 233, 266, 288 nm.
Oxidation of 11 with CAN Preparation of 21
The reaction was carried out according to the procedure by Golding et al. [30]. Cerium ammonium nitrate (2 eq, 1.08 g, 1.9 mmol) was dissolved in acetone (15 mL) in a foil-covered 50 mL round bottom flask with stirring. The flask as then cooled to −78 °C, and 4,5-benzoxepin (1 eq, 136 mg, 0.94 mmol) dissolved in acetone (10 mL) was added dropwise. The reaction was monitored by TLC (3:1 hexanes:ether). After 1.5 h, the reaction was brought to room temperature and diluted with ether. The organic layer was washed with brine, dried over sodium sulfate, and concentrated under reduced pressure. The crude product was purified by column chromatography (60 mesh silica gel, 9:1 hexanes: EtOAc) and the first fraction was collected and characterized as follows. 1H NMR (400 MHz, CDCl3) δ = 5.94 (d, 1H, J = 7.53 Hz), 6.48 (d, 1H, 7.52 Hz), 6.66 (s, 1H), 7.35 (d, 1H, J = 7.79 Hz), 7.40 (td, 1H, J = 7.62, 1.18 Hz), 7.62 (td, 1H, J = 7.75, 1.47 Hz), 8.00 (dd, 1H, J = 7.88, 1.46 Hz). 13C NMR (125 MHz, CDCl3) δ = 98.1, 111.4, 127.4, 130.3, 130.8, 133.1, 134.2, 134.4, 140.4, 183.8. IR (ATR, neat) = 797 cm−1 ((Ar)-C-H), 1272 cm−1 (C-O), 1666 cm−1 (C=O) 2928 cm−1 (C-H). ESI-MS (dichloromethane) = 147 (100), 131 (50), 119 (47). EI-MS (DCM) = 146 (65), 118 (100), 90 (65). UV–vis (0.2 mg/mL in DCM) λmax = 273.0, 338.8 nm.
General Enzyme Incubation Procedure
The quenched reaction mixture was centrifuged (21,000× g) for 4 min to precipitate the pellet. The supernatant was transferred to a fresh tube and 150 uL of DCM was added. The tube was gently The substrates (300 uM) were incubated in 1.7 mL Eppindorf tubes. A 500 uL reaction mixture containing 100 mM buffer, 3.3 mM MgCl2, 1 mM NADPH in 0.5 M NaOH, and 300 uM substrate was preincubated for 5 min in a 37 °C water bath. The reactions were initiated by the addition of P450 isoforms (35 pmol/mL) or pHLM (0.5 mg/mL). After inverting the capped tubes twice, the reaction mixtures were incubated for 30 min to 1 h (depending on the enzyme and substrate). The reactions were terminated by the addition of 100 uL acetonitrile. shaken then centrifuged (21,000× g) for 3 min. The lower organic layer was removed with a micropipette and placed in a fresh tube. The aqueous layer was extracted twice more with DCM (150 uL each). The organic layer (2 uL) was injected into the GC-MS for analysis using the conditions above.
Author Contributions
Conceptualization, A.G., H.M.W.-G. and R.W.F.; methodology, A.G, H.M.W.-G., R.W.F. and N.A.C.; investigation, H.M.W.-G., R.W.F. and N.A.C.; writing—original draft preparation, A.G and H.M.W.-G.; writing—review and editing, A.G., H.M.W.-G., R.W.F. and N.A.C.; supervision, A.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References
- 1.Vogel E., Günther H. Benzene Oxide-Oxepin Valence Tautomerism. Angew. Chem. Int. Ed. Engl. 1967;6:385–401. doi: 10.1002/anie.196703851. [DOI] [Google Scholar]
- 2.Davies S.G., Whitham G.H. Benzene Oxide-Oxepin Oxidation to Muconaldehyde. J. Chem. Soc. Perkin Trans. 1977;1:1346–1347. doi: 10.1039/P19770001346. [DOI] [Google Scholar]
- 3.Witz G., Latriano L., Goldstein B.D. Metabolism and Toxicity of trans, trans-Muconaldehyde, an Open-Ring Microsomal Metabolite of Benzene. Environ. Health Persp. 1989;82:19–22. doi: 10.1289/ehp.898219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Snyder R., Witz G., Goldstein B.D. The Toxicology of Benzene. Environ. Health Persp. 1993;100:293–306. doi: 10.1289/ehp.93100293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Snyder R., Hedli C.C. An Overview of Benzene Metabolism. Environ. Health Persp. 1996;104:1165–1171. doi: 10.1289/ehp.961041165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Snyder R. Benzene’s Toxicity: A Consolidated Short Review of Human and Animal Studies by H.A. Khan. Hum. Exp. Toxicol. 2007;26:687–696. doi: 10.1177/0960327107083975. [DOI] [PubMed] [Google Scholar]
- 7.Lovern M.R., Turner M.J., Meyer M., Kedderis G.L., Bechtold W.E., Schlosser P.M. Identification of Benzene Oxide as a Product of Benzene Metabolism by Mouse, Rat, and Human Liver Microsomes. Carcinogenesis. 1997;18:1695–1700. doi: 10.1093/carcin/18.9.1695. [DOI] [PubMed] [Google Scholar]
- 8.Henderson A.P., Barnes M.L., Bleasdale C., Cameron R., Clegg W., Heath S.L., Lindstrom A.B., Rappaport S.M., Waidyanatha S., Watson W.P., et al. Reactions of Benzene Oxide with Thiols including Glutathione. Chem. Res. Toxicol. 2005;18:265–270. doi: 10.1021/tx049781y. [DOI] [PubMed] [Google Scholar]
- 9.Monks T.J., Butterworth M., Lau S.S. The Fate of Benzene Oxide. Chem. Biol. Interact. 2010;184:201–206. doi: 10.1016/j.cbi.2009.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zarth A.T., Murphy S.E., Hecht S.S. Benzene Oxide is a Substrate for Glutathione S-Transferases. Chem. Biol. Interact. 2015;242:390–395. doi: 10.1016/j.cbi.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weisel C.P. Benzene Exposure: An Overview of Monitoring Methods and Their Findings. Chem. Biol. Interact. 2010;184:58–66. doi: 10.1016/j.cbi.2009.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tomida I., Nakajima M.Z. The Chemistry of 3,5-Cyclohexadiene-1,2-diol VI. Metabolism of the Glycols and Muconic Dialdehyde. Physiol. Chem. 1960;318:171–178. doi: 10.1515/bchm2.1960.318.1.171. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein B.D., Witz G., Javid J., Amaruso M.A., Rossman T., Wolder B. Muconaldehyde, a Potential Toxic Metabolite of Benzene Metabolism. Adv. Exp. Med. Biol. 1982;136 Pt A:331–339. doi: 10.1007/978-1-4757-0674-1_20. [DOI] [PubMed] [Google Scholar]
- 14.Latriano L., Goldstein B.D., Witz G. Formation of Muconaldehyde, an Open-Ring Metabolite of Benzene, in Mouse Liver Microsomes: An Additional Pathway for Toxic Metabolism. Proc. Natl. Acad. Sci. USA. 1986;83:8356–8360. doi: 10.1073/pnas.83.21.8356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grotz V.L., Ji S.C., Kline S.A., Goldstein B.D., Witz G. Metabolism of Benzene and trans, trans-Muconaldehyde in the Isolated Perfused Rat Liver. Toxicol. Lett. 1994;70:281–290. doi: 10.1016/0378-4274(94)90122-8. [DOI] [PubMed] [Google Scholar]
- 16.Amin D.P., Witz G. DNA-Protein Crosslink and DNA Strand Break Formation in HL-60 Cells Treated with trans, trans-Muconaldehyde, Hydroquinone, and Their Mixture. Int. J. Toxicol. 2001;20:69–80. doi: 10.1080/10915810151115173. [DOI] [PubMed] [Google Scholar]
- 17.Rivedal E., Leithe E. The Benzene Metabolite trans, trans-Muconaldehyde Blocks Gap Junction Intercellular Communication by Cross-Linking Connexin 43. Toxicol. Appl. Pharmacol. 2008;232:463–468. doi: 10.1016/j.taap.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 18.Rivedal E., Witz G., Leithe E. Gap Unction Intercellular Communication and Benzene Toxicity. Chem. Biol. Interact. 2010;184:229–232. doi: 10.1016/j.cbi.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 19.Nakajima T., Wang R.-S., Elovaara E., Park S.S., Gelboin H.V., Hietanen E., Vainio H. Monoclonal Antibody-Directed Characterization of Cytochrome P450 Isozymes Responsible for Toluene Metabolism in Rat Liver. Biochem. Pharmacol. 1991;41:395–404. doi: 10.1016/0006-2952(91)90536-E. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Z.H., Goldstein B.D., Witz G. Iron-Stimulated Ring-Opening of Benzene in a Mouse Liver Microsomal System: Mechanistic Studies and Formation of a New Metabolite. Biochem. Pharmacol. 1995;50:1607–1617. doi: 10.1016/0006-2952(95)02043-8. [DOI] [PubMed] [Google Scholar]
- 21.Klotz B., Volkamer R., Hurley M.D., Anderson M.P.S., Nielsen O.J., Barnes I., Imamura T., Wirtz K., Becker K.H., Platt U., et al. OH-Initiated Oxidation of Benzene. Part II. Influence of Elevated NOx Concentrations. Phys. Chem. Chem. Phys. 2002;4:4399–4411. doi: 10.1039/b204398j. [DOI] [Google Scholar]
- 22.Golding B.T., Kennedy G., Watson W.P. Simple Synthesis of Isomers of Muconaldehyde and 2-Methylmuconaldehyde. Tetrahedron Lett. 1988;29:5991–5994. doi: 10.1016/S0040-4039(00)82248-0. [DOI] [Google Scholar]
- 23.Golding B.T., Bleasdale C., MacGregor J.O., Nieschalk J., Pearce K., Watson W.P. Chemistry of Muconaldehyde of Possible Relevance to the Toxicology of Benzene. Environ. Health Perspect. 1996;104:1201–1209. doi: 10.1289/ehp.961041201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenberg A., Bock C.W., George P., Glusker J.P. Energetics of the Metabolic Production of (E,E)-Muconaldehyde from Benzene via the Intermediates 2,3-Epoxyoxepin and (Z,Z)- and (E,Z)-Muconaldehyde: Ab Initio Molecular Orbital Calculations. Chem. Res. Toxicol. 1993;6:701–710. doi: 10.1021/tx00035a016. [DOI] [PubMed] [Google Scholar]
- 25.Rosner P., Wolff C., Tochtermann W. Syntheses of Medium and Large Rings. III. Reactions of β,β’ Hexano-Bridged Oxepins. Chem. Ber. 1982;115:1162–1169. [Google Scholar]
- 26.Morgan J.P., Greenberg A. Insights into the Formation and Isomerization of the Benzene Metabolite Muconaldehyde and Related Molecules: Comparison of Computational and Experimental Studies of Simple, Benzo-Annelated, and Bridged 2,3-Epoxyoxepins. J. Org. Chem. 2010;75:4761–4768. doi: 10.1021/jo100610g. [DOI] [PubMed] [Google Scholar]
- 27.Greenberg A., Ozari A., Carlin C.M. Reactions of 2,7-Dimethyloxepin with Dimethyldioxirane and Methyl(trifluoromethyl)dioxirane: Ring Opening and Probable Observation of the Intermediate “2,3-Epoxyoxepin”. Struct. Chem. 1998;9:223–236. doi: 10.1023/A:1022427232134. [DOI] [Google Scholar]
- 28.Bleasdale C., Cameron R., Edwards C., Golding B.T. Dimethyldioxirane Converts Benzene Oxide/Oxepin into (Z,Z)-Muconaldehyde and sym-Oxepin Oxide: Modeling the Metabolism of Benzene and its Photooxidation Degradation. Chem. Res. Toxicol. 1997;10:1314–1318. doi: 10.1021/tx970122d. [DOI] [PubMed] [Google Scholar]
- 29.Nauduri D., Greenberg A. Direct Observation by 1H-NMR of 4,5-Benzoxepin-2,3-oxide and its Surprisingly Rapid Ring-Opening Rearrangement to 1H-2-Benzopyran-1-carboxaldehyde. Tetrahedron Lett. 2004;45:4789–4793. doi: 10.1016/j.tetlet.2004.04.081. [DOI] [Google Scholar]
- 30.Golding B.T., Barnes M.L., Bleasdale C., Henderson A.P., Jiang D., Li X., Mutlu E., Petty H.J., Sadeghi M.M. Modeling the formation and reactions of benzene metabolites. Chem. Biol. Interact. 2010;184:196–200. doi: 10.1016/j.cbi.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 31.Morgan J.P., Greenberg A. Curtin-Hammett Principle: Application to Benzene Oxide-Oxepin Tautomers. Struct. Chem. 2013;24:1945–1956. doi: 10.1007/s11224-013-0274-5. [DOI] [Google Scholar]
- 32.Greenberg A., Bock C.W., George P., Glusker J.P. Mechanism of Metabolic Ring-Opening of Benzene and its Relation to Mammalian PAH Metabolism. Polycycl. Aromat. Compd. 1994;7:123–128. doi: 10.1080/10406639408014721. [DOI] [Google Scholar]
- 33.Boyd D.R., Sharma N.D. The Changing Face of Arene Oxide-Oxepine Chemistry. Chem. Soc. Rev. 1996;25:289–296. doi: 10.1039/CS9962500289. [DOI] [Google Scholar]
- 34.Frisch M.J., Trucks G.W., Schlegel H.B., Scuseria G.E., Robb M.A., Cheeseman J.R., Scalmani G., Barone V., Mennucci B., Petersson G.A., et al. Gaussian 09, Revision, D.01. Gaussian, Inc.; Wallingford, CT, USA: 2009. [Google Scholar]
- 35.Spartan 14, 1.1.0. Wavefunction, Inc.; Irvine, CA, USA: 2014. [Google Scholar]
- 36.Suzuki J., Watanabe T., Suzuki S. Formation of Mutagens by Photochemical Reaction of 2-Naphthol in Aqueous Nitrile Solution. Chem. Pharm. Bull. 1988;36:2204–2211. doi: 10.1248/cpb.36.2204. [DOI] [PubMed] [Google Scholar]
- 37.Hauser F.M., Baghdanov V.M. A New Procedure for Regiospecific Syntheses of Benzopyran-1-ones. J. Org. Chem. 1988;53:4676–4681. doi: 10.1021/jo00255a006. [DOI] [Google Scholar]
- 38.Thazana N., Worayuthakarn R., Kradanrat P., Hohn E., Young L., Ruchirawat S. Copper(I)-Mediated and Microwave-AssistedCaryl-Carboxylic Coupling: Synthesis of Benzopyranones and Isolamellarin Alkaloids. J. Org. Chem. 2007;72:9379–9382. doi: 10.1021/jo701599g. [DOI] [PubMed] [Google Scholar]
- 39.Cariou M., Carlier R., Simonet J. Enamines and Enediamines: Synthesis and Anodic Oxidation. Application to the Formation of Novel Heterocycles. Bull. Soc. Chim. France. 1986;67:81–792. [Google Scholar]
- 40.Fukuyama K., Fujita H., Tokushima S., Tsukihara T., Katsube Y., Motoyama T. Crystal and Molecular Structures of the Adduct Fluoreno[9,1-bc]pyrylium-3-olate with Methyl Cinnamate and the Dimer of Fluoreno[9,1-bc]-pyrylium-3-olate. Bull. Chem. Soc. Jpn. 1986;59:255–258. doi: 10.1246/bcsj.59.255. [DOI] [Google Scholar]
- 41.Cambie R.C., Joblin K.N., Preston A.F. Chemistry of the Podocarpaceae. XLIII. Utilization of the 8α,13- epoxylabd-14-ene and Related Compounds for the Preparation of Ambergris-Type Compounds. Aust. J. Chem. 1972;25:1767–1778. doi: 10.1071/CH9721767. [DOI] [Google Scholar]
- 42.Fuson R.C., McBurney C.H., Holland W.E. 1,2-Diacylethylene Glycols. J. Am. Chem. Soc. 1939;61:3246–3249. doi: 10.1021/ja01267a003. [DOI] [Google Scholar]
- 43.Tochtermann W., Rösner P. Eine Möglichkeit zur Ringerweiterung des Cyclooctins: Synthese und Reaktionen von 3,6-Hexano-oxepin-4,5-dicarbonsäure-diethylester. Tetrahedron Lett. 1980;21:4905–4908. doi: 10.1016/S0040-4039(00)71151-8. [DOI] [Google Scholar]
- 44.Stok J.E., Chow S., Krenske E.H., Soto C.F., Matyas C., Poirier R.A., Williams C.M., De Voss J.J. Direct Observation of an Oxepin from a Bacterial Cytochrome P450-Catalyzed Oxidation. Chem. Eur. J. 2016;22:4408–4412. doi: 10.1002/chem.201600246. [DOI] [PubMed] [Google Scholar]
- 45.Zeigler G.R. Mechanisms of Photochemical Reactions in Solution. LVII. Photorearrangement of 1,4-Epoxy-1,4-dihydronaphthalene to Benzo[f]oxepin. J. Am. Chem. Soc. 1969;91:446. doi: 10.1021/ja01030a040. [DOI] [Google Scholar]
- 46.Paquette L.A., Barrett J.H. 2,7-Dimethyloxepin. Org. Synth. 1969;49:62. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.