Abstract

Electronic nicotine delivery systems (ENDS), by virtue of their highly engineered construction (plastics, glass, e-liquids), may contain a number of emerging chemicals of concern (ECCs), including phthalates, phenolic compounds, and flame retardants. Current knowledge regarding the safety of ENDS may underestimate the health risks from ECCs. In this study, we examined the types and levels of those three groups of chemicals in the components and parts of ENDS devices, including refill liquids, tanks/cartridges, atomizers, drip tips/mouthpieces, and sealing materials. Our results suggest that phthalates were the most prevalent chemicals in all tested samples, followed by parabens and organophosphate flame retardants (OPFRs). Particularly, all measured chemicals had significantly higher detection rates in cartridges/tanks, drip tips/mouthpieces, and sealing materials in contrast to e-liquids and coil samples. Among all those three types of ENDS components, phthalates generally had the highest concentrations (0.279–3790 ng/unit) in the drip tip/mouthpiece samples, followed by the sealing materials (0.380–508.8 ng/unit) and the empty tank/cartridge samples (up to 761.7 ng/unit). For parabens, highest concentrations were observed in drip tip/mouthpiece samples (1.152–130.1 ng/unit), followed by sealing materials (0.220–30.08 ng/unit) and the tank/cartridge samples (1.794–34.24 ng/unit). For OPFRs, tris(1,3-dichloro-2-propyl) phosphate had the highest concentrations (39.40–774.1 ng/unit) in all component samples. High concentrations (20.25–260.4 ng/unit) were also observed for several OPFRs in sealing materials and drip tip/mouthpiece samples. These findings will contribute to addressing the information gaps pertinent to the presence of ECCs in ENDS and will warrant further studies for understanding the potential negative health effects and to what extent those chemicals may cause potential negative health effects when using the ENDS. The findings will also contribute to developing evidence-based standards for the regulatory control of the types and levels of ECCs in ENDS products.
1. Introduction
Electronic nicotine delivery systems (ENDS) have been promoted and are often perceived by consumers as “safer” products than combustible tobacco products (e.g., cigarettes) in part because of lower toxic levels observed in ENDS aerosols compared with those identified in cigarette smoke.1 ENDS are increasingly used in the United States (US)2 and worldwide,3 and they have especially become very popular with young people over the past few years.4,5
Current debate regarding the safety of ENDS has focused on the toxicants known to be present in cigarettes6 that are absent or substantially reduced in ENDS (e.g., nitrosamines, polyaromatic hydrocarbons (PAHs), volatile organic compounds (VOCs), nitrogen oxides (NOxs), CO).6 This may underestimate long-term novel health risks posed by several other types of emerging chemicals of health concern (ECCs), such as phthalate plasticizers, phenolic compounds, and flame retardants. The concern that ENDS may contain a number of ECCs arises from the engineered construction of ENDS devices. It specifically builds on the following observations: (1) ENDS are highly engineered and contain plastic parts (e.g., cartridge, mouthpieces, and sealing materials); (2) there is potential contamination of e-liquids by ECCs along the manufacturing and distribution process from production to sale; and (3) there is contamination of nonplastics materials used in ENDS, i.e., glass and metal parts. Contamination could also occur during manufacturing and packaging processes. Those ECCs are not typical in combustible cigarettes but are widely used as plasticizers, flame retardants, lubricants, preservatives, antifoaming stabilizers, and surfactants in plastics, electronics, medical devices, packaging materials, and other consumer goods.7−11 Because many of these substances are not chemically bonded to the materials in which they are present, they can leach or outgas with time and use, and consequently, this can lead to human exposure.7,11−13
Identification of ECCs in consumable goods has prompted concerns about subsequent human exposure, and a number of studies have documented that ECC exposures are associated with adverse health outcomes, including carcinogenic activity, neurotoxicity, endocrine disruption, and reproductive and developmental abnormalities.14−24 To date, information regarding the presence of ECCs in ENDS is limited to only a few studies.25−29 Chung et al.27 detected moderate to elevated levels of polybrominated diphenyl ethers (PBDEs), a class of flame retardants, in 5 out of the 13 ENDS aerosol samples. For instance, the level of BDE-47 in one brand was >180 ng/mL. The authors also made the argument that the potential source may be the PBDEs leaching from ENDS atomizers and the associated protective casing.
The Chung findings are lent further relevance by our recent study in which we found ENDS users had the highest detection rates for the metabolites of organophosphate flame retardants (OPFRs) in urine samples from the National Health and Nutrition Examination Survey (NHANES) during 2013–2014.26 Among the measured metabolites, significantly higher urinary metabolite levels of tris(2-chloroethyl) phosphate (TCEP) were observed in ENDS users than in nonusers (p = 0.0124). TCEP is regarded as toxic and carcinogenic.20,23 Limited data related to ENDSs in the NHANES precluded a full evaluation of other ECC biomarkers in ENDS users. Considering similar exposure pathways, co-occurrence of ECCs in ENDSs is possible and forms the main hypothesis to be examined in this research.
In this study, we examined phthalate plasticizers, phenolic compounds, and organophosphate flame retardants in the components and parts of ENDS devices, including refill liquids, tanks/cartridges, atomizers, drip tips/mouthpieces, and sealing materials. While further research is still needed to assess the potential adverse health effects that might be associated with the use of ENDS that contain ECCs in their components, the analytical results obtained in the present study will contribute to addressing the information gaps pertinent to the presence of ECCs in ENDS and to the development of evidence-based standards to regulate the types and levels of ECCs in ENDS.
2. Experimental Procedures
2.1. Reagents and Standards
Native reference standards, including dibutyl phthalate (DBP), diethyl phthalate (DEP), di-2-ethylhexyl phthalate (DEHP), benzylbutyl phthalate (BBP), diisononyl phthalate (DiNP), di-n-octyl phthalate (DNOP), diisodecyl phthalate (DiDP), bis(2-butoxyethyl) phthalate (DBEP), dihexyl phthalate (DHP), di(2-ethylhexyl) terephthalate (DEHTP), dimethyl phthalate (DMP), di(isononyl) cyclohexane-1,2-dicarboxylate (DINCH), tris(1,3-dichloro-2-propyl)phosphate (TDCPP), tris(2-chloroethyl)phosphate (TCEP), tris(1-chloro-2-propyl)phosphate (TCPP), bis(1,3-dichloro-2-propyl)phosphate (BDCPP), tricresyl phosphate (TMPP), diphenyl phosphate (DPhP), triphenyl phosphate (TPhP), tributyl phosphate (TnBP), tri-isobutyl phosphate (TiBP), triethyl phosphate (TEP), tripropyl phosphate (TPP), tris(2-ethylhexyl)phosphate (TEHP), tris(2-butoxyethyl)phosphate (TBOEP), tris(2,3-dibromopropyl)phosphate (TDBPP), 2-ethylhexyl diphenyl phosphate (EHDPP), resorcinol bis(diphenyl phosphate) (RDP), tris(2,3-dibromopropyl)phosphate (TDBPP), trabromobisphenol A (TBBPA), and isotope labeled standards, including DHP-d4, DBP-d4, DEP-d4, DMP-d4, and DCHP-d4, were purchased from AccuStandard (New Haven, CT, USA). Native standards, including, methyl-, ethyl-, propyl-, and butyl-parabens, and isotopically labeled compounds, including TDCPP-d15, TPrP-d12, BBP-d4, TBP-d27, TEP-d51, TCPP-d18, TDBPP-d15, TPhP-d15, TCEP-d12, TBOEP-d27, butyl-d9, methyl-d4, and ethyl-d5 parabens, were purchased from Toronto Research Chemicals (North York, ON, Canada). All chemicals were used without further purification. Liquid chromatography–mass spectrometry (LC–MS) grade water, acetonitrile, methanol, formic acid, ammonium format, and USP-grade PG/VG were purchased from Fisher Scientific (Fairlawn, NJ, USA). Artificial saliva was purchased from Pickering Laboratories, Inc. (Mountain View, CA). TruView LC–MS Certified Glass inject vials were purchased from Waters (Milford, MA, USA). SPE (C18, 100 mg) columns were bought from Biotage (Charlotte, NC, USA). The Kinetex EVO C18 column (100 mm × 2.1 mm, particle size 2.6 μm) was purchased from Phenomenex (Torrance, CA, USA).
2.2. ENDS Samples
There are many types of ENDS products, and the product designs and materials used largely vary across brands. It is expected that types and levels of ECCs in ENDS products will largely vary across brands as well. We selected the brands of ENDS available in the US market based on the survey data from the Population Assessment of Tobacco and Health (PATH) Study,2,30 with combination of the latest available report on sales of ENDS products in the US,31 and purchased them either in local retail stores or online commercial sources during the period from October 2018 to January 2020. The brands that were reviewed and examined included VUSE, JUUL, NJOY, MarkTen, Halo Cigs, Mister-E-Liquid, Green Smoke, JoyeTech, Vaporfi, South Beach, White Cloud, Vapor4Life, KangerTech, Innokin, Aspire, ELeaf, ePuffer, Envii, VaporFi, Veppo, RI e-Cig & Vapes, and Element Vape. For each product, we only analyzed one manufacturer lot. Therefore, we did not examine lot-to-lot variability in this study. Samples were logged into a custom database categorized, kept in their original packages, and stored in Ziploc bags in a refrigerator (4 °C) until analysis. While not all types of brands were examined in this study, we believe that the results obtained by analyzing those most common brands available in US market will be meaningful for understanding the presence (types and levels) of ECCs in ENDS.
2.3. Standards and Sample Preparation
2.3.1. Standard Solution Preparation
The protocols for the preparation of the standard working solutions followed the International Conference on Harmonization guidelines32 and common procedures described elsewhere.25,33−35 Briefly, calibrators, quality control (QC) samples, and internal standard solution were prepared from serial dilutions of primary stock solutions with 60% methanol in water, and these solutions were stored in Teflon-capped amber glass vials at −20 °C. Twelve-point calibrators were prepared for each analyte encompassing concentrations ranging from 0.001 to 500 ng/mL. Three levels of QC samples, with their concentrations ranging from 0.15 to 500 ng/mL, were used to evaluate the accuracy of the results. A mixed spiking solution of 50 ng/mL for all internal standards was prepared and used for all analysis. Samples with concentrations exceeding the upper calibration standard were diluted, reprepared, and reanalyzed.
2.4. Sample Preparation
2.4.1. E-Liquid Sample Preparation
Detailed sample preparation protocol for measuring ECCs in e-liquids was reported in a previous study by Wei et al.25 Briefly, 50 μL of internal standard solution (50 ng/mL for each isotope labeled standard) was first added into each LC injection vial. Then, 50 μL of each sample (e.g., e-liquids, QCs, calibrators, and laboratory control blanks) and 400 μL of methanol and water (v/v 60:40) were transferred into the same vial. After gently mixing, 10 μL of each sample was injected into the LC system.
2.4.2. Sample Preparation for Refillable Empty Cartridge/Tank, Coils, and Sealing Components
PG was used as the matrix to extract potential ECCs on ENDS parts, including refillable empty cartridges/tanks, coils, and sealing materials that have direct contact with e-liquids. Using PG as an extraction solvent provides comparable, although not fully identical, characteristics with e-liquids, in terms of solvent strength and extraction capability. We added and recorded the maximum volume of PG into each empty cartridge/tank sample along with 50 μL of internal standard solution. Coils and sealing materials were separately extracted with 5 mL of PG and 50 μL of internal standard solution. There is no standard testing method for quantifying ECCs presented on ENDS parts. Extraction was performed at room temperature for a duration of 30 min. This duration is similar to the time used for the quantification of bisphenol A in plastic bottles in the ASTM standard method (D 7574-09).36 Based on the actual sample volume, an appropriate volume of HPLC grade water was added to dilute the sample, yielding a maximum percentage of <15% for total organic constituents (mainly PG) in the solution. This is important to obtain a sufficient recovery on subsequent SPE extraction.25 Samples were gently vortex-mixed and loaded onto SPE cartridges, equilibrated with 1.0 mL of methanol, 1.0 mL of acetonitrile, and 1.0 mL of water, and the solutions were pushed through the SPE under approximately 1.0 psi positive pressure. Samples were subsequently washed with 1.0 mL of water and 1.0 mL of methanol and water (v/v 15:85). After being dried for 15 min with nitrogen (25 psi), the samples were eluted with 1.0 mL of methanol, collected in 1.5 mL LC injection vials, and evaporated under a gentle nitrogen stream to dryness. The residuals were reconstituted in 100 μL of methanol and water (v/v 50:50) prior to analysis.
2.4.3. Sample Preparation for Drip Tips/Mouthpieces
Artificial saliva was used as the matrix to extract ECCs on ENDS drip tips/mouthpieces. We chose to use artificial saliva mainly because of the comparable characteristics with those of human saliva. It is also important to note that background levels of ECCs in artificial salvia can be well-controlled. To prepare the sample, each mouthpiece was put into 15 mL of artificial saliva and extracted for 30 min at a temperature of 37 °C, which is comparable to human body temperature. Extracted mouthpiece samples went through the same, complete SPE cleanup procedures for extracted ENDS component samples, except that artificial saliva extraction samples were not diluted with water prior to SPE cleanup.
2.5. Instrumentation Analysis
All pretreated samples were chromatographically resolved using a Kinetex EVO C18 column on a Shimadzu UPLC system (Columbia, MD, USA) and were analyzed by a Sciex triple quadrupole 6500+ mass spectrometer with a TurboIonSpray source (Foster City, CA, USA) under the conditions described in a previous study by Wei et al.25 Analyst software (version 1.7.0) was used for chromatographic data acquisition, and MultiQuant (version 3.0.3) was employed to process the analytic data and to quantitate the sample concentrations. Calibration curves were constructed using peak area ratios of analytes to corresponding internal standards for each batch via linear least-squares regression with a 1/x weighting factor.
2.6. Quality Control and Quality Assurance Measures
The following quality control (QC) and quality assurance (QA) measures were used during the entire study period to ensure the reliability of the analytical data: (a) Calibration standard solutions were stored ≤−20 °C, and the calibration curves were evaluated every three months using standard solutions prepared from secondary production lots; (b) calibrators, QC samples, and laboratory control blanks were prepared and analyzed following the same preparation and analysis procedures used for the unknown samples in each analytical batch. Blank controls are especially vital when analyzing ECCs which could present in many common laboratory consumables and instruments. In addition to including blank control samples in each batch, efforts to minimize the contamination during analysis included using prescreened high purity solvents and using pretested glassware; (c) QC samples were prepared in PG or artificial saliva to correct for potential matrix effects, and sample concentrations were calculated using 12-point curves. (d) Instruments were evaluated to maintain high sensitivity prior to each batch analysis; (e) the following parameters were characterized to ensure the data quality, including retention time, ion ratio (qualitative/quantitative peak area), and acceptable thresholds (e.g., blank contamination, extreme concentration, calibration linearity, QC concentration range, and carry over).
3. Results
3.1. Detection Rates and Characteristics
3.1.1. E-Liquid Samples
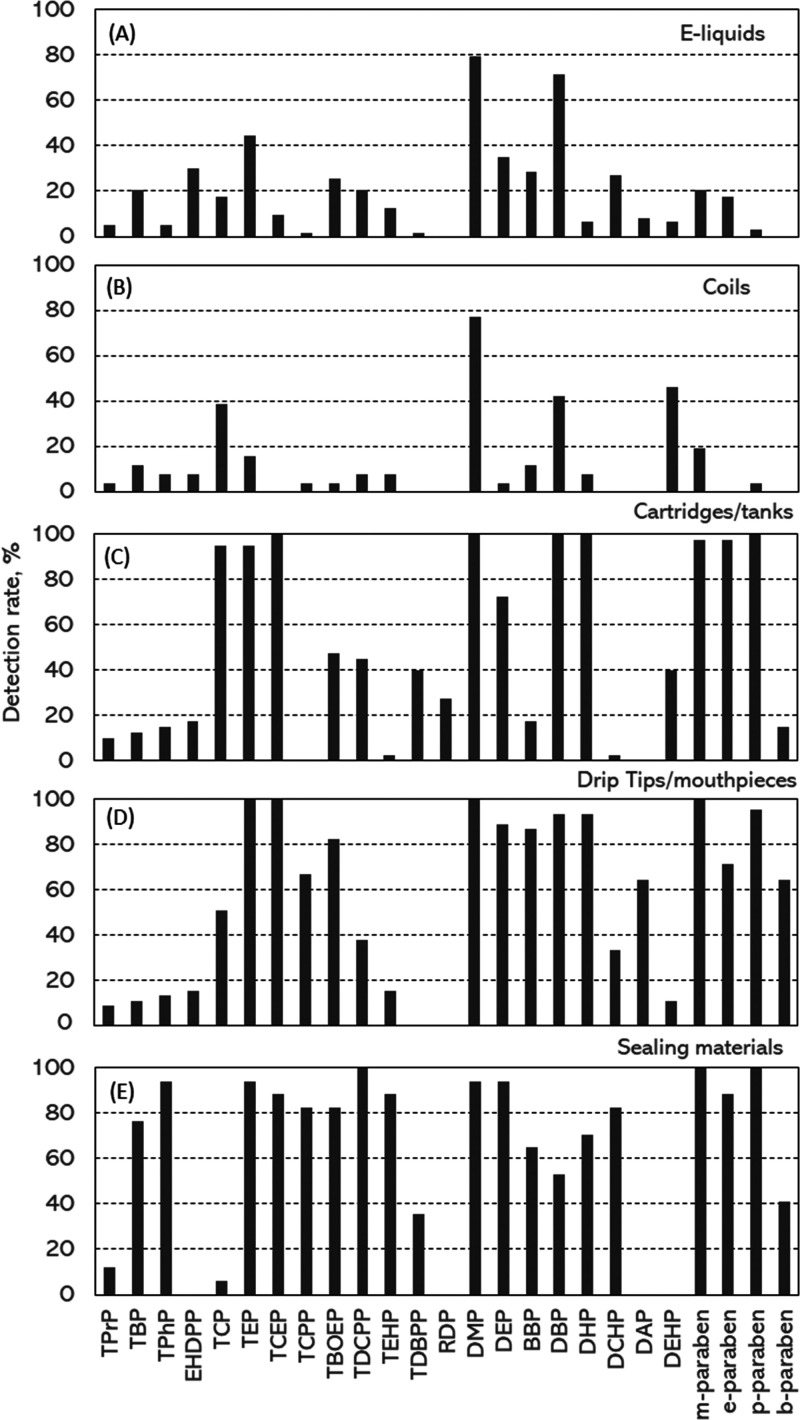
For e-liquid samples (detailed sample information provided in Table 1, n = 54), phthalates, including DMP, DBP, and DEP, were the most prevalent chemicals whose concentrations were at or higher than their limits of detection (LODs) (hereafter called detection rate) (Figure 1A). Other phthalates such as BBP, DCHP, DAP, and DEHP were detected in 6.3–28.6% of the samples. Among OPFRs measured in this study, TEP had the highest detection rate (44.4%) in e-liquids, followed by EHDPP (30.2%) and TBOEP (25.4%). Detection rates for other OPFRs were <21%. For parabens, methyl paraben was detected in 20.6% of e-liquid samples, followed by ethyl paraben (17.5%) and propyl paraben (3.2%). Butyl paraben was not detected in any samples.
Table 1. Description of the E-Liquid Samplesa.
| brand name | flavor | PG/VG | country of origin |
|---|---|---|---|
| ElementVape — A | American Red Premium American Tobacco | PG | USA with foreign ingredients |
| ElementVape — B | Vortex Vanilla Graham Custard | PG | USA with foreign ingredients |
| ElementVape — C | Kiwi Melon | 30/70 | MV Enterprises LLC, DBA OG Eliquids, USA |
| ElementVape — D | Blue Raspberry | 20/80 | JRU Inc., USA |
| ePuffer — A | Appletini | 70/30 | USA |
| ePuffer — B | Grape | 70/30 | USA |
| ePuffer — C | Menthol | 70/30 | USA |
| ePuffer — D | New London | 70/30 | USA |
| JoyTech — A | Tropical Blend | 40/60 | MyVapors Liquids, USA |
| JoyTech — B | Menthol | 40/60 | MyVapors Liquids, USA |
| JoyTech — C | Tobacco | 40/60 | MyVapors Liquids, USA |
| JoyTech — D | Orange Cream | 40/60 | MyVapors Liquids, USA |
| JUUL — A | Classic Tobacco | PG/VG | JUUL, USA |
| JUUL — B | Fruit | PG/VG | JUUL, USA |
| JUUL — C | Mint | PG/VG | JUUL, USA |
| JUUL — D | Mango | PG/VG | JUUL, USA |
| HaloCigs — A | Cherry | PG | Nicopure Laboratories LLC, USA |
| HaloCigs — B | Berry Blend | PG | Nicopure Laboratories LLC, USA |
| HaloCigs — C | Mint menthol | PG | Nicopure Laboratories LLC, USA |
| HaloCigs — D | Smooth Tobacco | PG | Nicopure Laboratories LLC, USA |
| Low Vis Liquids — A | Overcast | 20/80 | USA |
| Low Vis Liquids — B | Stratus | 20/80 | USA |
| MigCigs — A | It is Nuts | 50/50 | MigVapor, USA |
| MigCigs — B | Tobacco with a kick | 50/51 | MigVapor, USA |
| MigCigs — C | Caribbean Freeze | 50/50 | MigVapor, USA |
| Mister-E-Liquid — A | Psychobilly | 30/70 | Mister-E-Liquid LLC, USA |
| Mister-E-Liquid — B | Mister-E’s Menthol | 67/33 | Mister-E-Liquid LLC, USA |
| Mister-E-Liquid — C | Neptune | 67/33 | Mister-E-Liquid LLC, USA |
| Mister-E-Liquid — D | Blue Voodoo | 67/33 | Mister-E-Liquid LLC, USA |
| Mister-E-Liquid — E | Dime Piece | 67/33 | Mister-E-Liquid LLC, USA |
| Mister-E-Liquid — F | Gray Matter | 67/33 | Mister-E-Liquid LLC, USA |
| RI e-Cig & Vapes — A | Tobacco | 50/50 | USA |
| RI e-Cig & Vapes — B | Grape Frost | 50/50 | USA |
| RI e-Cig & Vapes — C | Menthol | 50/50 | USA |
| RI e-Cig & Vapes — D | The Berg | 30/70 | InneVape Laboratories USA, USA |
| South Beach Smoke — A | unflavored | 70/30 | USA |
| South Beach Smoke — B | unflavored | 70/31 | USA |
| South Beach Smoke — C | unflavored | 70/32 | USA |
| VaporFi — A | flavoring ingredients listed, not specified | 50/50 | VaporFi, USA |
| VaporFi — B | flavoring ingredients listed, not specified | 50/50 | VaporFi, USA |
| VaporFi — C | flavoring ingredients listed, not specified | 50/50 | VaporFi, USA |
| Vapor4Life — A | Oasis | PG/VG, ratio not specified | USA |
| Vapor4Life — B | Refreshmint | PG/VG, ratio not specified | USA |
| Vapor4Life — C | Grape | PG/VG, ratio not specified | USA |
| Veppo — A | Tobacco | PG | China |
| Veppo — B | Menthol | PG | China |
| Veppo — C | My Burro | PG | China |
| White Cloud — A | Regular | PG/VG, ratio not specified | China |
| White Cloud — B | What a Melon | PG/VG, ratio not specified | White Cloud Electronic Cigarettes, USA |
| White Cloud — C | Cin | PG/VG, ratio not specified | China |
| White Cloud — D | Menthol | PG/VG, ratio not specified | China |
| The House of Vapor — A | Menthol Sensation | PG/VG, ratio not specified | The House of Vapor LLC, USA |
| The House of Vapor — B | Tobacco | PG/VG, ratio not specified | The House of Vapor LLC, USA |
| The House of Vapor — C | Ghostberry | PG/VG, ratio not specified | The House of Vapor LLC, USA |
Descriptions include brand name, flavor types, PG/VG ratio, and country of origin. Samples were purchased during the period from October 2018 to January 2020.
Figure 1.
Detection rates of ECCs in e-liquids, cartridges/tanks, coils drip tips/mouthpieces, and sealing materials.
3.1.2. Coil Samples
For coil extraction samples (n = 20), phthalates were the most frequently detected chemicals. DMP had the highest detection rate of 76.9%, followed by DEHP (46.2), DBP (42.3%), DHP (7.7%), and DEP (3.8%) (Figure 1B). Neither DCHP nor DAP was detected in any samples. Among OPFRs, TCP had the highest detection rate of 38.5%, followed by TBP which was detected in 11.5% of the samples. Detection rates for other OPFRs were ≤11.5%. For parabens, only methyl- and propyl-parabens were detected in <20% samples.
3.1.3. Cartridges/Tanks
For cartridges/tanks (n = 40), phthalates including DMP, DBP, and DHP were detected in all extraction samples, followed by DEP and DEHP which were detected in 72.5 and 40% of samples (Figure 1C). Methyl-, ethyl-, and propyl-parabens were detected in ≥97.5% samples. Butyl-paraben was only detected in 15% of extraction samples. Among OPFRs, TCEP had the highest detection rate of 100%, followed by TCP (95%) and TEP (95%). Detection rates for other OPFRs were in the range 2.5–40%.
3.1.4. Drip Tip and Mouthpiece Samples
For drip tips/mouthpiece samples (Table 2, n = 41), parabens had the highest average detection rate among those three groups of chemicals, ranging from 64.4 to 100% (Figure 1D). DMP, DBP, DHP, BBP, and DEP were detected in above 86% of all extraction samples, and other phthalate analytes were detected in 11.1–64.4% of samples. Among OPFRs, TEP and TCEP were detected in all samples, followed by TBOEP, TCPP, TCP, and TDCPP which had the detection rates of 82.2, 66.7, 51.1, and 37.8%, respectively. Detection rates for EHDPP, TPhP, TBP, and TPrP were in a range 8.9–15.6%. Neither TDBPP nor RDP was detected in any samples.
Table 2. Description of ENDS Productsa.
| component
tested |
|||||||
|---|---|---|---|---|---|---|---|
| product description | purchase source | manufacturer | manufacturer location | drip tip | sealing material | coil | cartridge/tank |
| Ultimo atomizer | JoyeTech.com | Joyetech Electronics Co., Ltd. | China | √ | √ | √ | √ |
| ProCore X atomizer | JoyeTech.com | Joyetech Electronics Co., Ltd. | China | √ | √ | √ | √ |
| ProCore Aries atomizer | JoyeTech.com | Joyetech Electronics Co., Ltd. | China | √ | √ | √ | √ |
| Topbox Mini Platinum | Kangeronline.com | KangerTech Technology Co., Ltd. | China | √ | √ | ||
| Subtank Mini-C | Kangeronline.com | KangerTech Technology Co., Ltd. | China | √ | √ | √ | |
| Toptank Mini/Topfill | Kangeronline.com | KangerTech Technology Co., Ltd. | China | √ | √ | ||
| CLOCC | Kangeronline.com | KangerTech Technology Co., Ltd. | China | √ | |||
| Clear Cartomizer | Kangeronline.com | KangerTech Technology Co., Ltd. | China | √ | |||
| CC Clear Cartomizer | Kangeronline.com | KangerTech Technology Co., Ltd. | China | √ | |||
| Dual Coil Unit | Kangeronline.com | KangerTech Technology Co., Ltd. | China | √ | |||
| Seal ring | Kangeronline.com | KangerTech Technology Co., Ltd. | China | √ | |||
| Mini Protank 3/EVOD Glassomizer | Kangeronline.com | KangerTech Technology Co., Ltd. | China | √ | √ | ||
| Refillable Mini Tank | Whitecloudcigs.com | White Cloud Electronic Cigarettes | China | √ | √ | ||
| Nord Pod | HaloCigs.com | Shenzhen IVPS Technology Co., Ltd. | China | √ | √ | ||
| Reactor Subohm Tank | HaloCigs.com | Nicopure Laboratories | USA | √ | √ | ||
| Journey to Infinity/K Lite Tank, Aspire | HaloCigs.com | Shenzhen Eigate Technology Co., Ltd. | China | √ | √ | ||
| Suorin Air Cartridge | HaloCigs.com | Shenzhen Bluemark Technology Co., Ltd. | China | √ | |||
| Nautilus XS Tank Kit | Aspirecig.com | Shenzhen Eigate Technology Co., Ltd. | China | √ | √ | √ | √ |
| Nautilus XS (Mesh Coil) | Aspirecig.com | Shenzhen Eigate Technology Co., Ltd. | China | √ | |||
| K1 BVC Glassomizer | Aspirecig.com | Shenzhen Eigate Technology Co., Ltd. | China | √ | |||
| Breeze 2 Pod | Aspirecig.com | Shenzhen Eigate Technology Co., Ltd. | China | √ | |||
| Lemo Drop atomizer | eleafus.com | iSmoka | China | √ | √ | ||
| iJust 2 atomizer | eleafus.com | Eleaf Electronics Co., Ltd. | China | √ | √ | √ | |
| Lyche atomizer | eleafus.com | Eleaf Electronics Co., Ltd. | China | √ | √ | √ | √ |
| Melo 4 atomizer | eleafus.com | Eleaf Electronics Co., Ltd. | China | √ | √ | √ | √ |
| Ello Duro atomizer | eleafus.com | Eleaf Electronics Co., Ltd. | China | √ | √ | √ | √ |
| GS Drive atomizer | eleafus.com | Eleaf Electronics Co., Ltd. | China | √ | √ | ||
| Rotor atomizer | eleafus.com | Eleaf Electronics Co., Ltd. | China | √ | √ | √ | √ |
| Nord Pod | Vaprzon.com | Shenzhen IVPS Technology Co., Ltd. | China | √ | |||
| ROLO Badge Pod | Vaprzon.com | Shenzhen IVPS Technology Co., Ltd. | China | √ | |||
| SMOK, Fit Kit | Vaprzon.com | Shenzhen IVPS Technology Co., Ltd. | China | √ | |||
| Cobble Replacement Pod | Vaprzon.com | Shenzhen Eigate Technology Co., Ltd. | China | √ | |||
| AVP (Mesh Coil)/Journey to Infinity/AVP Pod | Vaprzon.com | Shenzhen Eigate Technology Co., Ltd. | China | √ | |||
| Suorin Vagon Cartridge | Vaprzon.com | Shenzhen Bluemark Technology Co., Ltd. | China | √ | |||
| Suorin Air | Vaprzon.com | Shenzhen Bluemark Technology Co., Ltd. | China | √ | |||
| Suorin ishare | Vaprzon.com | Shenzhen Bluemark Technology Co., Ltd. | China | √ | |||
| Zero Pod | Vaprzon.com | Renova Vaporesso | China | √ | |||
| Teros | Vaprzon.com | Joyetech Electronics Co., Ltd. | China | √ | |||
| eGo AIO | VaporFi.com | Joyetech | China | √ | √ | √ | √ |
| Wotofo Profile unity RTA, nexMESH | VaporFi.com | Shenzhen Wotofo Technology Co., Ltd. | China | √ | √ | ||
| Go Max, Multi-Use Disposable Tank | VaporFi.com | Shenzhen Innokin Technology Co., Ltd. | √ | ||||
| vSIX Subohm Tank | VaporFi.com | VaporFi | China | √ | √ | ||
| Crown IV Tank Checkmate | VaporFi.com | Shenzhen UWELL Technology Co., Ltd. | China | √ | √ | √ | |
| Herakles III 24 | VaporFi.com | Cigreat | √ | √ | √ | ||
| FreeMax, Fireluke M | VaporFi.com | Shenzhen Freemax Technology Co., Ltd. | China | √ | √ | √ | |
| Zlide | VaporFi.com | Shenzhen Innokin Technology Co., Ltd. | China | √ | √ | √ | |
| Scion II | VaporFi.com | Shenzhen Innokin Technology Co., Ltd. | China | √ | |||
| Hakutaku/Sxmini | VaporFi.com | YiHitech | China | √ | √ | √ | |
| Drip Tips | The House of Vapor | China | √ | ||||
| Super Tank Mini Resin Tip | The House of Vapor | China | √ | ||||
| Resin Tip | The House of Vapor | √ | |||||
| Drip Tip Blue | The House of Vapor | √ | |||||
| Profile Drip Tips Black | The House of Vapor | √ | |||||
| Drip Tip | The House of Vapor | √ | |||||
| Regis Mini Kit | The House of Vapor | Geekvape Technology Co., Ltd. | √ | √ | |||
| Kroma-A Zenith Kit | The House of Vapor | Shenzhen Innokin Technology Co., Ltd. | China | √ | |||
| Smoov | The House of Vapor | The House of Vapor | USA | √ | √ | √ | |
| Amulet Pod | The House of Vapor | Shenzhen UWELL Technology Co., Ltd. | China | √ | |||
| Nord Pod | The House of Vapor | Shenzhen IVPS Technology Co., Ltd. | China | √ | √ | ||
| EQ Pod | The House of Vapor | Shenzhen Innokin Technology Co., Ltd. | China | √ | |||
| Arco HorizonTech Tank | The House of Vapor | Shenzhen Horizon Technology Co., Ltd. | China | √ | √ | ||
Descriptions include product name, purchase source, manufacturer, country of production, and specific testing components. Samples were purchased during the period from October 2018 to January 2020.
3.1.5. Sealing Materials
For sealing samples (Table 2, n = 17), the trends for the detection rates for those three types of chemicals were similar to those for the drip tip/mouthpiece samples (Figure 1E). Parabens (methyl-, propyl-, and ethyl-parabens) had the highest average detection rate of 82.4%, followed by OPFRs (58.4%) and phthalates (57.4%).
3.2. Concentrations and Comparisons
For e-liquid samples (detailed sample information provided in Table 1), as the detection rates for the majority of the analytes were below 40%, except for TEP, DMP, and DBP, only blank-corrected 75% percentiles and maximum concentration values are presented (Table 3). Despite the general low detection rates, we observed that the concentrations of some chemicals in e-liquids can be remarkably high. For instance, the highest concentrations for TBOEP, TEHP, TDCPP, DMP, DCHP, DEHP, ethyl-, and propyl-parabens were all above 140 ng/mL. DBP, which was detected in 71.4% of e-liquids, had a median concentration of 149 ng/mL and a maximum concentration of 3539 ng/mL in those tested samples.
Table 3. Blank-Corrected Percentiles for Phthalate and Paraben Concentrations in E-Liquid Samples (ng/mL).
| detection rate | percentile |
||||
|---|---|---|---|---|---|
| % | 25% | 50% | 75% | maximum | |
| TPrP | 4.8 | 0.153 | 0.271 | ||
| TBP | 20.6 | 7.995 | 44.03 | ||
| TPhP | 4.8 | 4.119 | 4.619 | ||
| EHDPP | 30.2 | 0.736 | 6.218 | ||
| TCP | 17.5 | 0.476 | 7.703 | ||
| TEP | 44.4 | 5.413 | 32.65 | ||
| TCEP | 9.5 | 0.938 | 10.25 | ||
| TCPP | 1.6 | 54.61 | 54.61 | ||
| TBOEP | 25.4 | 44.28 | 214.1 | ||
| TDCPP | 20.6 | 75.81 | 202.6 | ||
| TEHP | 12.7 | 151.2 | 241.0 | ||
| TDBPP | 1.6 | 17.37 | 17.37 | ||
| RDP | 0.0 | ||||
| DMP | 79.4 | 0.195 | 2.493 | 2.493 | 228.4 |
| DEP | 34.9 | 53.46 | 1825 | ||
| BBP | 28.6 | 2.979 | 52.92 | ||
| DBP | 71.4 | 11.03 | 149.1 | 149.1 | 3539 |
| DHP | 6.3 | 1.181 | 3.010 | ||
| DCHP | 27.0 | 67.39 | 155.9 | ||
| DAP | 7.9 | 0.270 | 0.304 | ||
| DEHP | 6.3 | 85.30 | 135.7 | ||
| m-Paraben | 20.6 | 3.598 | 1040 | ||
| e-Paraben | 17.5 | 10.26 | 149.0 | ||
| p-Paraben | 3.2 | 109.9 | 146.1 | ||
| b-Paraben | 0.0 | ||||
Because detection rates in those ENDS component samples, including cartridges/tanks, drip tips/mouthpieces, and the sealing materials, except for coil samples, were generally higher in contrast to those of e-liquid samples, blank-corrected 25%, 50%, and 75% percentiles and the maximum values with detection rates of >50% are presented in Tables 4 and 5. Among those three types of ENDS components, phthalates generally had the highest concentrations (0.279–3790 ng/unit) in the drip tip/mouthpiece samples (Table 4), followed by the sealing materials (0.380–508.8 ng/unit) and the empty tank/cartridge samples (<LOD–761.7 ng/unit). Similarly, for parabens, highest concentrations were observed in drip tip/mouthpiece samples (1.152–130.1 ng/unit), followed by sealing materials (0.220–30.08 ng/unit) and the tank/cartridge samples (1.794–34.24 ng/unit) (Table 4). Among those OPFRs measured in this study, TDCPP had the highest concentrations (39.40–774.1 ng/unit) in all ENDS component samples (Table 5). High concentrations (20.25–260.4 ng/unit) were also observed for TCEP, TBOEP, TEP, TBP, TPhP, and TEHP in sealing materials and drip tip/mouthpiece samples. Concentrations for TPrP, EHDPP, TCP, TDBPP, and RDP were generally <10 ng/unit in those four types of ENDS component samples.
Table 4. Blank-Corrected Percentiles for Phthalate and Paraben Concentrations in ENDS Extraction Samples (ng/Unit).
| phthalate |
paraben |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DMP | DEP | BBP | DBP | DHP | DCHP | DAP | DEHP | methyl | ethyl | propyl | butyl | ||
| empty tank/cartridge | 25% | 2.015 | 1.394 | 82.43 | 0.082 | 1.196 | 0.055 | 0.480 | |||||
| 50% | 4.815 | 5.418 | 144.8 | 0.119 | 3.840 | 0.092 | 1.273 | ||||||
| 75% | 22.76 | 16.45 | 1.476 | 261.5 | 0.167 | 0.317 | 6.793 | 0.338 | 3.270 | 0.281 | |||
| max | 333.5 | 82.79 | 5.962 | 761.7 | 0.504 | 39.26 | 0.756 | 34.24 | 1.843 | 14.63 | 1.794 | ||
| coil | 25% | 0.139 | 4.310 | ||||||||||
| 50% | 0.229 | 10.41 | 0.274 | ||||||||||
| 75% | 0.674 | 2.760 | 24.30 | 0.484 | 0.326 | 0.378 | |||||||
| max | 5.492 | 1.531 | 2.840 | 510.7 | 0.560 | 0.470 | 0.904 | 0.467 | |||||
| drip tip/mouthpiece | 25% | 2.696 | 2.068 | 0.212 | 20.85 | 0.095 | 0.034 | 26.06 | 0.094 | 0.352 | 0.030 | ||
| 50% | 9.874 | 5.208 | 0.340 | 42.97 | 0.143 | 0.058 | 28.14 | 0.230 | 0.765 | 0.055 | |||
| 75% | 21.58 | 11.75 | 0.600 | 62.31 | 0.253 | 18.38 | 0.110 | 2.447 | 32.75 | 0.398 | 3.427 | 0.083 | |
| max | 3790 | 75.64 | 3.344 | 275.4 | 2.583 | 32.64 | 0.279 | 2.759 | 130.1 | 1.152 | 78.32 | 2.851 | |
| sealing material | 25% | 5.858 | 1.799 | 0.167 | 6.646 | 0.141 | 3.701 | 6.549 | 0.130 | 1.800 | |||
| 50% | 10.21 | 5.685 | 0.218 | 9.229 | 0.185 | 7.491 | 12.42 | 0.313 | 2.706 | ||||
| 75% | 40.77 | 19.87 | 0.558 | 16.32 | 0.282 | 10.12 | 15.88 | 0.422 | 5.386 | 0.128 | |||
| max | 508.8 | 58.80 | 0.940 | 17.85 | 0.380 | 17.99 | 30.08 | 1.468 | 15.39 | 0.220 | |||
Table 5. Blank-Corrected Percentiles for OPFR Concentrations in ENDS Extraction Samples (ng/Unit).
| percentile | TPrP | TBP | TPhP | EHDPP | TCP | TEP | TCEP | TCPP | TBOEP | TDCPP | TEHP | TDBPP | RDP | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| coil | 25% | |||||||||||||
| 50% | 1.718 | |||||||||||||
| 75% | 0.573 | 2.039 | 1.170 | 0.046 | 0.202 | 597.1 | 88.67 | |||||||
| maximum | 0.004 | 0.769 | 2.359 | 1.250 | 0.143 | 0.513 | 35.50 | 24.69 | 774.1 | 110.8 | ||||
| empty tank/cartridge | 25% | 0.043 | 0.410 | 0.050 | ||||||||||
| 50% | 0.083 | 0.563 | 0.116 | |||||||||||
| 75% | 0.044 | 2.520 | 0.500 | 0.449 | 0.176 | 1.374 | 0.213 | 8.865 | 26.08 | 1.938 | 0.135 | 0.355 | ||
| maximum | 0.107 | 22.55 | 1.973 | 2.633 | 1.717 | 7.827 | 2.547 | 25.80 | 39.39 | 1.938 | 0.802 | 3.214 | ||
| sealing material | 25% | 0.104 | 0.215 | 0.256 | 3.582 | 5.047 | 50.21 | 8.816 | ||||||
| 50% | 0.124 | 0.562 | 5.036 | 0.345 | 8.177 | 13.07 | 106.8 | 14.10 | ||||||
| 75% | 0.008 | 0.274 | 0.790 | 13.52 | 0.435 | 10.04 | 21.14 | 181.2 | 19.58 | 0.592 | ||||
| maximum | 0.008 | 2.684 | 1.180 | 0.055 | 25.11 | 64.42 | 12.80 | 38.66 | 226.3 | 38.87 | 0.870 | |||
| drip tip/mouthpiece | 25% | 0.009 | 0.480 | 0.254 | 1.413 | 5.394 | ||||||||
| 50% | 0.032 | 1.271 | 0.412 | 6.963 | 14.41 | |||||||||
| 75% | 1.070 | 1.256 | 0.163 | 0.101 | 2.604 | 0.643 | 10.50 | 32.78 | 63.38 | 12.65 | ||||
| maximum | 0.021 | 14.01 | 20.25 | 0.180 | 0.623 | 33.38 | 115.6 | 21.39 | 260.4 | 287.1 | 25.92 |
4. Discussion
Considering the highly engineered characteristics of ENDS which contain plastic, glass, and metal parts, as well as e-liquids which are packaged in similar materials, on the basis of our preliminary study, we performed this study and tested the hypothesis that ECCs, e.g., phthalate plasticizers, phenolic compounds, and flame retardants, that have been widely identified in consumable goods, are present in ENDSs. There is still limited research on types and levels of ECCs in ENDS, although a number of studies have documented other harmful or potentially harmful constituents (HPHCs),6 e.g., metals, PAHs, VOCs, and TSNAs, in ENDS/e-liquids/aerosols. This study fills this information gap for the first time by examining the types and levels of ECCs in ENDS.
The samples under each brand/category were purchased first on the basis of popularity, and those with the highest user self-reported prevalence were considered and selected. As the main goal of the present study is to examine the presence (types and levels) of ECCs in ENDS, we did not evaluate whether specific ingredients (e.g., flavors and nicotine) in e-liquids are potential sources of ECCs, mainly because the types and levels of the ingredients utilized by different manufactures are diversified and, to some extent, complicated. Given available resources, we were unable to purchase and assay sufficient samples, especially those flavored products, to categorize and evaluate the influences of different flavors on the types and levels of ECCs. Eliminating the flavored products and only measuring nonflavored ones would not reflect the main portion of ENDS use.
In addition to aforementioned limitations, in this study, we did not evaluate whether the presence of those ECCs, regardless of their levels in ENDS products, will eventually lead to adverse health consequences in ENDS users. However, previous epidemiological and toxicological studies have shown that many of those measured ECCs are potentially endocrine disrupting chemicals (EDCs) which can mimic or interfere with the hormone functions even at low levels, consequently resulting in many adverse health consequences, including carcinogenic activity, neurotoxicity, endocrine disruption, and reproductive and developmental abnormalities.14−24 Previously, we observed that there are significant associations between exposure to ECCs and the sex hormone levels in the general US population.24 For instance, we observed that the adjusted geometric means of serum sex-hormone-binding globulin increased by more than 36% in female children and female adolescents from the first to fourth quartiles of the urinary levels of diphenyl phosphate (DPhP), a metabolite of TPhP, bis(1,3-dichloro-2-propyl) phosphate (BDCPP), a metabolite of TDCPP, and dibutyl phosphate (DBuP), a metabolite of TBP. We also found that the adjusted GMs of serum estradiol (EST) decreased by more than 64% in female children and adolescents from the first to fourth quartiles of the urinary DBuP levels. As such, the findings obtained in this research warrant further toxicological and epidemiological studies for understanding the negative health effects and to what extent those chemicals may cause negative health effects when using the ENDS.
In June 2009, the FDA acquired the authority to regulate the manufacture, marketing, and distribution of cigarettes and smokeless and roll-your-own tobacco products to protect public health. In May 2016, the FDA extended its jurisdiction to all tobacco products, including ENDS, which gives the agency authority over the manufacturing, marketing, and distribution of e-cigarettes.37 There is significant concern about the adverse health outcomes that are found to be associated with ECCs exposure.14−22 The results of this study, specific to the types and levels of ECCs in different ENDS components, and of our previous work on biomarkers26 indicate that ENDS can be a source of exposure to ECCs. Although specific guidelines are not in place for regulatory control of the types and levels of ECCs in ENDS products, authorities have begun to regulate certain ECCs in consumer and industrial goods. For instance, in 2012, the FDA’s Center for Drug Evaluation and Research (CDER) released guidelines on the use of dibutyl phthalate (DBP) and di(2-ethylhexyl) phthalate (DEHP) in CDER-regulated drug and biologic products.9 This and future research can contribute to the development of the evidence-based standards for the regulatory control of the types and levels of ECCs in ENDS. Given emerging data, ENDS manufacturers should evaluate their manufacturing and packaging practices and minimize or eliminate ECCs from their products.
The research in this study was performed in Dr. Wei’s laboratory at Roswell Park Comprehensive Cancer Center and was supported by the National Institute of Environmental Health Sciences (NIEHS) and the US FDA Center for Tobacco Products (CTP) (NIH Grant No. R21 ES030028). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
The authors declare the following competing financial interest(s): M.L.G. received a research grant from Pfizer and served as a member of the scientific advisory board to Johnson&Johnson.
References
- Goniewicz M. L.; Knysak J.; Gawron M.; Kosmider L.; Sobczak A.; Kurek J.; Prokopowicz A.; Jablonska-Czapla M.; Rosik-Dulewska C.; Havel C. (2014) Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tobacco control 23, 133. 10.1136/tobaccocontrol-2012-050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman B. N.; Rostron B.; Johnson S. E.; Ambrose B. K.; Pearson J.; Stanton C. A.; Wang B.; Delnevo C.; Bansal-Travers M.; Kimmel H. L. (2017) Electronic cigarette use among US adults in the Population Assessment of Tobacco and Health (PATH) Study, 2013–2014. Tobacco control 26, e117. 10.1136/tobaccocontrol-2016-053462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutra L. M.; Glantz S. A. (2014) Electronic cigarettes and conventional cigarette use among US adolescents: a cross-sectional study. JAMA pediatrics 168 (7), 610–617. 10.1001/jamapediatrics.2013.5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen K. A.; Ambrose B. K.; Gentzke A. S.; Apelberg B. J.; Jamal A.; King B. A. (2018) Notes from the field: Use of electronic cigarettes and any tobacco product among middle and high school students—United States, 2011–2018. Morbidity and Mortality Weekly Report 67 (45), 1276. 10.15585/mmwr.mm6745a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miech R.; Johnston L.; O’Malley P. M.; Bachman J. G.; Patrick M. E. (2019) Trends in Adolescent Vaping, 2017–2019. N. Engl. J. Med. 381, 1490. 10.1056/NEJMc1910739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US-FDA Harmful and Potentially Harmful Constituents in Tobacco Products and Tobacco Smoke: Established List, U.S. Food & Drug Administration https://www.fda.gov/TobaccoProducts/Labeling/RulesRegulationsGuidance/ucm297786.htm (accessed 2019-08-07).
- Ospina M.; Jayatilaka N. K.; Wong L.-Y.; Restrepo P.; Calafat A. M. (2018) Exposure to organophosphate flame retardant chemicals in the US general population: Data from the 2013–2014 National Health and Nutrition Examination Survey. Environ. Int. 110, 32–41. 10.1016/j.envint.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim U.-J.; Oh J. K.; Kannan K. (2017) Occurrence, Removal, and Environmental Emission of Organophosphate Flame Retardants/Plasticizers in a Wastewater Treatment Plant in New York State. Environ. Sci. Technol. 51 (14), 7872–7880. 10.1021/acs.est.7b02035. [DOI] [PubMed] [Google Scholar]
- US-FDA Limiting the use of certain phthalates as excipients in CDER-regulated products https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm330792.htm (accessed 2018-01-09).
- Hauser R.; Duty S.; Godfrey-Bailey L.; Calafat A. M. (2004) Medications as a source of human exposure to phthalates. Environ. Health Perspect. 112 (6), 751. 10.1289/ehp.6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch H. M.; Calafat A. M. (2009) Human body burdens of chemicals used in plastic manufacture. Philos. Trans. R. Soc., B 364 (1526), 2063–2078. 10.1098/rstb.2008.0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat A. M.; Ye X.; Wong L.-Y.; Reidy J. A.; Needham L. L. (2008) Exposure of the US population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ. Health Perspect. 116 (1), 39–44. 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva M. J.; Barr D. B.; Reidy J. A.; Malek N. A.; Hodge C. C.; Caudill S. P.; Brock J. W.; Needham L. L.; Calafat A. M. (2004) Urinary levels of seven phthalate metabolites in the US population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environ. Health Perspect. 112 (3), 331. 10.1289/ehp.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornehag C.-G.; Sundell J.; Weschler C. J.; Sigsgaard T.; Lundgren B.; Hasselgren M.; Hägerhed-Engman L. (2004) The association between asthma and allergic symptoms in children and phthalates in house dust: a nested case-control study. Environ. Health Perspect. 112 (14), 1393. 10.1289/ehp.7187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya S. V.; Kulkarni H. (2012) Association of urinary bisphenol A concentration with allergic asthma: results from the National Health and Nutrition Examination Survey 2005–2006. J. Asthma 49 (8), 800–806. 10.3109/02770903.2012.721041. [DOI] [PubMed] [Google Scholar]
- Araki A.; Saito I.; Kanazawa A.; Morimoto K.; Nakayama K.; Shibata E.; Tanaka M.; Takigawa T.; Yoshimura T.; Chikara H.; et al. (2014) Phosphorus flame retardants in indoor dust and their relation to asthma and allergies of inhabitants. Indoor air 24 (1), 3–15. 10.1111/ina.12054. [DOI] [PubMed] [Google Scholar]
- Meeker J. D.; Stapleton H. M. (2010) House dust concentrations of organophosphate flame retardants in relation to hormone levels and semen quality parameters. Environ. Health Perspect. 118 (3), 318. 10.1289/ehp.0901332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippat C.; Mortamais M.; Chevrier C.; Petit C.; Calafat A. M.; Ye X.; Silva M. J.; Brambilla C.; Pin I.; Charles M.-A.; et al. (2012) Exposure to phthalates and phenols during pregnancy and offspring size at birth. Environ. Health Perspect. 120 (3), 464. 10.1289/ehp.1103634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loganathan S. N.; Kannan K. (2011) Occurrence of bisphenol A in indoor dust from two locations in the eastern United States and implications for human exposures. Arch. Environ. Contam. Toxicol. 61 (1), 68–73. 10.1007/s00244-010-9634-y. [DOI] [PubMed] [Google Scholar]
- US-DHHS TOXICOLOGY AND CARCINOGENESIS: a STUDIES OF TRIS(2-CHLOROETHYL)PHOSPHATE https://ntp.niehs.nih.gov/ntp/htdocs/lt_rpts/tr391.pdf (accessed 2018-01-11).
- (2016) OEHHA, Current Proposition 65 List. California Office of Environmental Health Hazard Assessment. [Google Scholar]
- Ehrlich S.; Williams P. L.; Missmer S. A.; Flaws J. A.; Ye X.; Calafat A. M.; Petrozza J. C.; Wright D.; Hauser R. (2012) Urinary bisphenol A concentrations and early reproductive health outcomes among women undergoing IVF. Hum. Reprod. 27 (12), 3583–3592. 10.1093/humrep/des328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (1998) World Health Organization, Environmental Health Criteria 209, Flame Retardants: 420 Tris(chloropropyl) Phosphate and Tris(2-chloroethyl) Phosphate, World Health Organization, Geneva, Switzerland. [Google Scholar]
- Wei B.; O’Connor R.; Goniewicz M.; Hyland A. (2020) Association between Urinary Metabolite Levels of Organophosphorus Flame Retardants and Serum Sex Hormone Levels Measured in a Reference Sample of the US General Population. Exposure and Health 1–12. 10.1007/s12403-020-00353-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei B.; Goniewicz M.; O’Connor R. J. (2019) Concurrent Quantification of Emerging Chemicals of Health Concern in e-Cigarette Liquids by High-Performance Liquid Chromatography-Tandem Mass Spectrometry. ACS Omega 4 (13), 15364–15372. 10.1021/acsomega.9b01255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei B.; Goniewicz M. L.; O’Connor R. J.; Travers M. J.; Hyland A. J. (2018) Urinary Metabolite Levels of Flame Retardants in Electronic Cigarette Users: A Study Using the Data from NHANES 2013–2014. Int. J. Environ. Res. Public Health 15 (2), 201. 10.3390/ijerph15020201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S.-S.; Zheng J.-S.; Kwong A. C.; Lai V. W. (2018) Harmful flame retardant found in electronic cigarette aerosol. J. Cleaner Prod. 171, 10. 10.1016/j.jclepro.2017.09.286. [DOI] [Google Scholar]
- Oh J.-A.; Shin H.-S. (2015) Identification and quantification of several contaminated compounds in replacement liquids of electronic cigarettes by gas chromatography-mass spectrometry. J. Chromatogr. Sci. 53 (6), 841–848. 10.1093/chromsci/bmu146. [DOI] [PubMed] [Google Scholar]
- Moldoveanu S. C.; Yerabolu R. (2018) Critical evaluation of several techniques for the analysis of phthalates and terephthalates: Application to liquids used in electronic cigarettes. J. Chromatogr A 1540, 77–86. 10.1016/j.chroma.2018.02.001. [DOI] [PubMed] [Google Scholar]
- US-FDA(b) FDA and NIH Study: Population Assessment of Tobacco and Health https://www.fda.gov/TobaccoProducts/NewsEvents/ucm337005.htm (accessed 2018-01-10).
- Nielsen United States: Wells Fargo: Nielsen - Tobacco ‘All Channel’ Data https://www.tma.org/article/2017/united-states-wells-fargo-nielsen-tobacco-all-channel-data-through-128 (accessed 2018-01-16).
- ICH ICH HARMONISED TRIPARTITE GUIDELINE VALIDATION OF ANALYTICAL PROCEDURES: TEXT AND METHODOLOGY, Q2(R1) https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1__Guideline.pdf (accessed 2018-01-18).
- Wei B.; Feng J.; Rehmani I. J.; Miller S.; McGuffey J. E.; Blount B. C.; Wang L. (2014) A high-throughput robotic sample preparation system and HPLC-MS/MS for measuring urinary anatabine, anabasine, nicotine and major nicotine metabolites. Clin. Chim. Acta 436, 290–297. 10.1016/j.cca.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei B.; Wang L.; Blount B. C. (2015) Analysis of cannabinoids and their metabolites in human urine. Anal. Chem. 87 (20), 10183–10187. 10.1021/acs.analchem.5b02603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei B.; McGuffey J. E.; Blount B. C.; Wang L. (2016) Sensitive Quantification of Cannabinoids in Milk by Alkaline Saponification-Solid Phase Extraction Combined with Isotope Dilution UPLC-MS/MS. ACS Omega 1 (6), 1307–1313. 10.1021/acsomega.6b00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASTM (2009) ASTM Method: D7574–09, Standard Test Method for Determination of Bisphenol A in Environmental Waters by Liquid Chromatography/Tandem Mass Spectrometry, ASTM International, West Conshohocken, PA: www.astm.org. [Google Scholar]
- US-FDA(d) FDA’s New Regulations for E-Cigarettes, Cigars, and All Other Tobacco Products https://www.fda.gov/TobaccoProducts/Labeling/RulesRegulationsGuidance/ucm394909.htm#rule (accessed 2018-01-31).