Abstract
Malignant mesothelioma (MM) is a very aggressive asbestos-related cancer, for which no therapy proves to be effective. We have recently shown that the oncolytic adenovirus dl922-947 had antitumor effects in MM cell lines and murine xenografts. Previous studies demonstrated that dl922-947-induced host cell cycle checkpoint deregulation and consequent DNA lesions associated with the virus efficacy. However, the cellular DNA damage response (DDR) can counteract this virus action. Therefore, we assessed whether AZD1775, an inhibitor of the G2/M DNA damage checkpoint kinase WEE1, could enhance MM cell sensitivity to dl922-947. Through cell viability assays, we found that AZD1775 synergized with dl922-947 selectively in MM cell lines and increased dl922-947-induced cell death, which showed hallmarks of apoptosis (annexinV-positivity, caspase-dependency, BCL-XL decrease, chromatin condensation). Predictably, dl922-947 and/or AZD1775 activated the DDR, as indicated by increased levels of three main DDR players: phosphorylated histone H2AX (γ-H2AX), phospho-replication protein A (RPA)32, phospho-checkpoint kinase 1 (CHK1). Dl922-947 also increased inactive Tyr-15-phosphorylated cyclin-dependent kinase 1 (CDK1), a key WEE1 substrate, which is indicative of G2/M checkpoint activation. This increase in phospho-CDK1 was effectively suppressed by AZD1775, thus suggesting that this compound could, indeed, abrogate the dl922-947-induced DNA damage checkpoint in MM cells. Overall, our data suggest that the dl922-947-AZD1775 combination could be a feasible strategy against MM.
Keywords: malignant mesothelioma, oncolytic adenovirus, dl922-947, WEE1, AZD1775, MK-1775, adavosertib, DNA damage response, G2/M checkpoint, apoptosis
1. Introduction
Malignant mesothelioma (MM) is a very aggressive asbestos-related cancer that arises from the mesothelium lining the body cavities. The most common MM type develops in the pleura, the serous membrane covering the lungs and the chest cavity. Despite the ban on asbestos use in many countries, MM burden is still substantial (over 30,000 MM cases and over 25,000 deaths worldwide in 2018) [1] and is predicted to further rise owing to the long-latency time between exposure and diagnosis [2]. Moreover, asbestos is still used in the developing world and the employment of other asbestos-like fibers that are known to cause MM, such as erionite, is not strictly regulated [3]. The prognosis for patients with MM of the pleura is very poor, with a median survival of approximately 1 year from diagnosis [4]. No current therapeutic strategy is curative: surgery is often challenging and associated with morbidity and mortality [4,5,6] and the only approved first-line chemotherapeutic treatment, consisting of a combination of cisplatin and pemetrexed, has shown limited effects [4,7]. Therefore, there is a great need to identify new targets for the development of effective therapeutic strategies.
Considering that virotherapy-based approaches have recently found a successful application in the clinical setting for different cancer types [8,9,10] and given that MM is a good candidate for this strategy because the pleural location provides direct access for the intra-tumoral injection of the virus [11], we have recently assessed the effects of a selectively replicating oncolytic virus (OV) in MM cell lines and murine xenografts [12]. We used, in particular, the adenovirus dl922-947, the efficacy of which has previously been shown by our group and others in cells from different tumors [13,14,15,16,17]. This OV carries a 24-bp deletion in the E1A-Conserved Region 2, which renders viral replication dependent on the inactivation of the retinoblastoma (RB1) pathway [18]. Since disruption of the oncosuppressive RB1 pathway is an almost universal hallmark of human cancers, including MM [19,20], dl922-947 can kill tumor cells, while sparing normal cells in which the RB1 pathway is functional. We showed that dl922-947 had antitumor effects in both MM cell lines and xenografts [12]. In particular, dl922-947 affected cell cycle progression, triggered immunogenic cell death, and reduced the production of pro-angiogenic factors, consistent with the ability of OVs to induce an antitumor immune response [21,22] and a re-shaping of the tumor microenvironment [23,24].
Beyond the above-mentioned mechanisms of dl922-947 action, the deregulation of multiple cell cycle checkpoints, which accelerates the host cell progression through the cycle, plays an important role for the activity of this OV [25]. Abrogation of these checkpoints results in genomic DNA over-replication and, consequently, in the accumulation of DNA lesions [26,27], which have been found to associate with higher sensitivity to dl922-947 [27]. However, the virus-induced DNA damage activates the host cell DNA damage response (DDR) signaling, which can counteract the virus action [27,28]. Consistently, we and others showed that inhibitors of crucial factors of the DNA damage signaling and repair, such as ataxia telangiectasia mutated (ATM), checkpoint kinase 1 (CHK1), and poly(ADP-ribose) polymerase (PARP), enhanced the effects of dl922-947 [26,27,28].
Among the drugs targeting the DDR pathway, AZD1775 (MK-1775, adavosertib), an inhibitor of the tyrosine kinase WEE1, has shown efficacy in sensitizing many cancer types to DNA damaging agents in both preclinical studies and phase I/II clinical trials [29,30,31,32,33,34]. WEE1 is a crucial activator of the G2/M checkpoint, which stalls the cell cycle in response to DNA damage, by phosphorylating and inhibiting cyclin-dependent kinase 1/2 (CDK1/CDK2). WEE1 inhibition leads to G2/M checkpoint override, unscheduled mitotic entry, increased replication stress, subsequent nucleotide starvation, and loss of genomic integrity [30]. G2/M checkpoint abrogation through WEE1 inhibition was originally conceived as a strategy to selectively sensitize cancer cells to DNA damaging agents, given that most human cancers rely on the G2/M checkpoint to detect and repair damaged DNA [35]. Indeed, the G1/S checkpoint is defective in almost all cancers because of the loss of the p53 tumor suppressor. Therefore, tumor cells treated with a WEE1 inhibitor are forced to enter aberrant and lethal mitosis in the presence of DNA damage; conversely, non-neoplastic cells, which retain G1/S checkpoint activity, are unaffected by this treatment. Based on this rationale, many studies focused on the effects of WEE1 inhibition in combination with DNA damaging agents in tumors bearing TP53 mutations. However, other mechanisms, such as DDR aberrations, nucleotide starvation, replicative stress, and, as more recently found, loss of the ATRX chromatin remodeler gene [36] and low phosphatase and tensin homolog (PTEN) expression [37], contribute to sensitize cancer cells to WEE1 inhibition, which, thus, proved monotherapy activity even in TP53-wild-type cancer cells [29,30,38]. Moreover, WEE1 inhibition showed efficacy also in combination with inhibitors of other DDR factors, such as PARP [39,40,41,42], CHK1 [29,43,44,45,46,47], and ataxia telangiectasia and Rad3 related (ATR) kinase [48,49,50], and also when combined with different anticancer targeted agents [29,51,52,53,54,55,56,57,58,59,60,61] and immunotherapeutic approaches [29,62,63,64].
We have previously demonstrated that WEE1 inhibition sensitizes MM cells to the DNA-damaging agent cisplatin by forcing them to enter mitosis despite damaged DNA [65], as further confirmed also by others in a more recent study [66]. We have also previously observed that dl922-947 induces DNA over-replication in MM cells [12], which could be indicative of possible DNA damage generation. In the present study, we found that dl922-947 induces, indeed, a DDR in MM cells and that WEE1 inhibition through AZD1775 synergizes with dl922-947 by abrogating the DNA damage checkpoint and increasing cell death. Thus, our data suggest that the combination of these agents could be a feasible strategy against MM.
2. Results
2.1. AZD1775 Synergizes with dl922-947 in MM Cell Lines
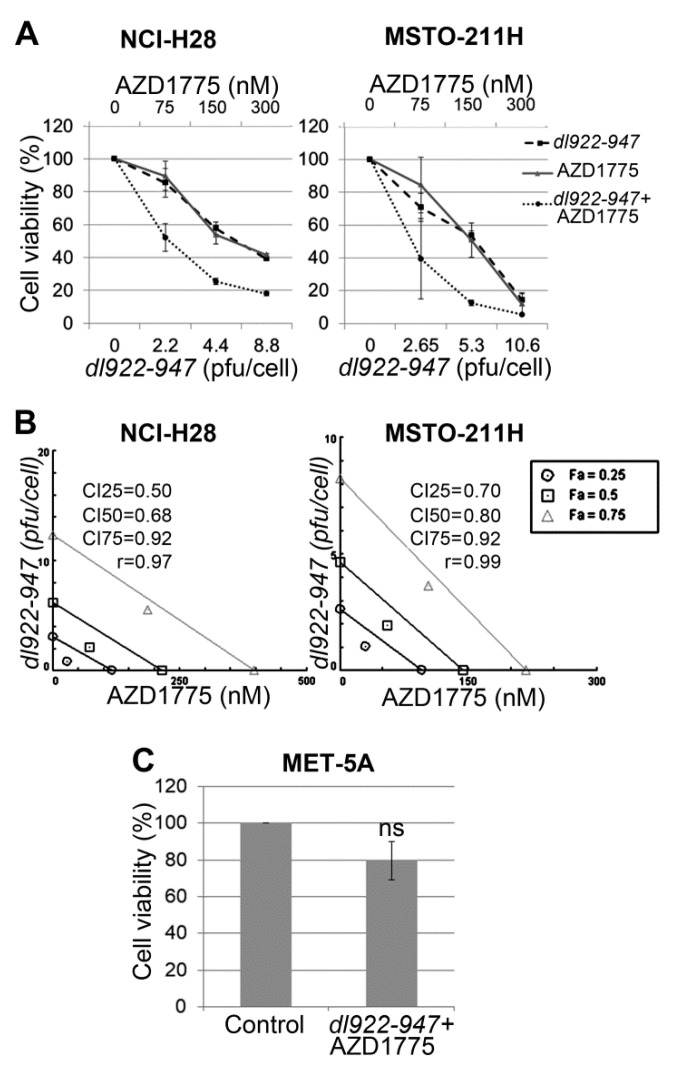
To evaluate whether WEE1 inhibition by AZD1775 enhances dl922-947 efficacy in MM cells, we challenged NCI-H28 and MSTO-211H cell lines for 5 days with the two agents, both alone and in combination at different concentrations in a constant ratio. In particular, the agents were added in 2-fold serial dilutions above and below their 5-day half maximal inhibitory concentration (IC50) values, which were 4.4 and 5.3 pfu/cell of dl922-947 in NCI-H28 and MSTO-211H, respectively (as we previously reported [12]), and 150 nM of AZD1775 in both cell lines. Cell viability data were obtained through sulforhodamine B (SRB) assay (Figure 1A) and evaluated by isobologram analysis, which showed synergism between AZD1775 and dl922-947 in both cell lines (Figure 1B).
Figure 1.
Synergistic effect of dl922-947-AZD1775 combination on malignant mesothelioma (MM) cell lines. (A) Dose–response curves for dl922-947 alone, AZD1775 alone, and dl922-947-AZD1775 combination in NCI-H28 and MSTO-211H cell lines 5 days after treatment. Results represent the means with standard deviation of 2 independent experiments, each conducted in triplicate, and are expressed as percentages of cell viability calculated with respect to control cells treated with DMSO alone. (B) Isobologram analysis to evaluate synergism between dl922-947 and AZD1775. Isobolograms are derived from the mean values of the dose–response experiments reported in (A), through the CompuSyn software 1.0 (ComboSyn, Inc., Paramus, NJ, USA), at effect levels (Fa, fraction affected) of 25, 50, and 75%. Data points on the line indicate additivity; points below the line indicate synergy; points above the line indicate antagonism. The combination indexes (CIs) at 25, 50, and 75% of cell killing (CI25, CI50, CI75, respectively) and r values are also reported. Combination index (CI) values < 1 indicate synergism. (C) Histogram representing MET-5A cell viability analyzed 5 days after dl922-947-AZD1775 co-treatment. Cell viability was calculated as a percentage with respect to control cells treated with DMSO alone. Results represent the means ± standard deviation of 3 experiments, each conducted in triplicate. The absorbance values of treated and control cells were subjected to paired Student’s t-test and showed no significant difference (ns, not significant).
To rule out possible cytotoxic effects of the dl922-947-AZD1775 combination on non-neoplastic cells, we treated MET-5A cell line, derived from normal mesothelium, with this drug combination (at approximately the IC50 value identified in tumor cells). We observed only a slight, not significant toxicity after 5 days of treatment (Figure 1C).
2.2. AZD1775 Increases the dl922-947-Induced Cell Death in MM Cells
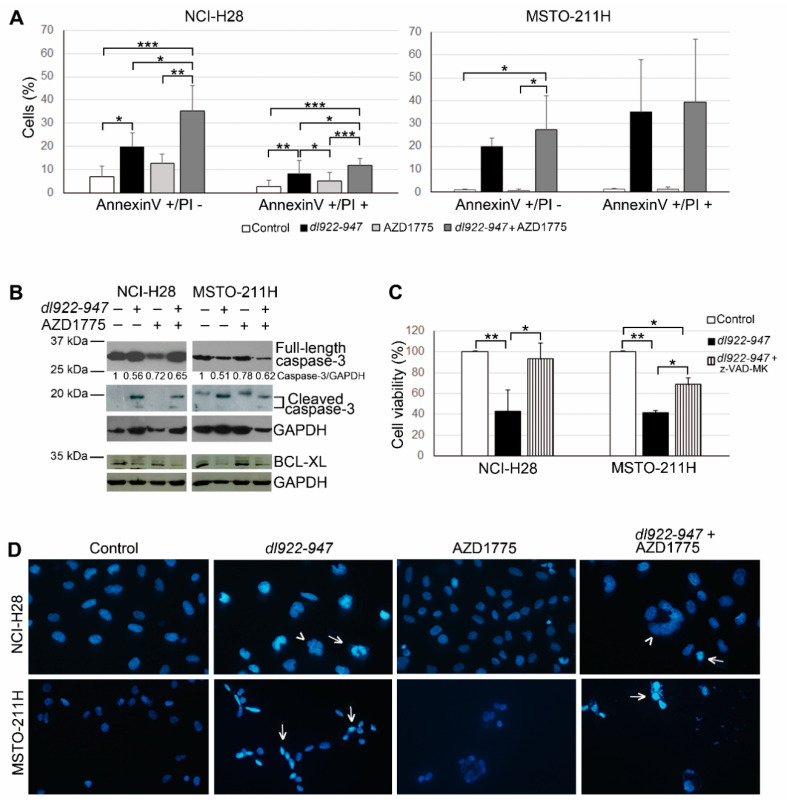
To assess cell death induction by dl922-947 and/or AZD1775 in NCI-H28 and MSTO-211H cell lines, we analyzed, through FACS, double staining with annexinV–FITC, which detects an early apoptosis marker, and propidium iodide (PI), which indicates membrane permeabilization in necrotic/late apoptotic cells. Ninety-six hours after treatment with the two agents at their IC50 values, we observed an increase in the percentage of both annexinV-positive–PI-negative cells (indicative of early apoptosis) and annexinV-positive–PI-positive cells (indicative of late apoptosis/necrosis), which was higher after dl922-947 and AZD1775 co-treatment than after dl922-947 infection alone (Figure 2A). Conversely, treatment with AZD1775 alone did not induce a significant increase in annexinV positivity.
Figure 2.
Cell death induction in malignant mesothelioma (MM) cells treated with dl922-947 and/or AZD1775. (A) Histograms report the means with standard deviations of at least three independent experiments representing the percentage of positive cells stained with annexinV–FITC and propidium iodide (PI) 96 h after treatment with dl922-947 and/or AZD1775 or DMSO, as a control. Statistically significant differences were evaluated by one-way repeated measures ANOVA with Tukey post-test and indicated as follows: * p < 0.05, significant; ** p < 0.01, very significant; and *** p < 0.001, extremely significant. (B) Caspase-3 and BCL-XL protein levels were analyzed by Western blotting in NCI-H28 and MSTO-211H cells treated as reported above. The antibody against caspase-3 detects both the full-length protein and the active cleaved form, which are shown separately at different exposure times (the full-length protein bands are shown at a shorter exposure time because they become overexposed at the time necessary for cleaved caspase-3 bands to appear). GAPDH was used as a loading control. Full-length caspase-3 band densities were quantified by densitometric analysis and normalized with the GAPDH band densities. Data are presented as relative values with respect to control values, set at 1. (C) Histograms reporting NCI-H28 and MSTO-211H cell viability 96 h after treatment with dl922-947 alone and in combination with Z-VAD-FMK. Results represent the means with standard deviations of at least 2 independent experiments, each conducted in triplicate, and are expressed as percentages of cell viability calculated with respect to untreated control cells. Statistically significant differences were evaluated by one-way repeated measures ANOVA with Tukey post-test and indicated as follows: * p < 0.05, significant; ** p < 0.01, very significant. (D) Representative fluorescence micrographs of DAPI-stained NCI-H28 and MSTO-211H cells treated with dl922-947 and/or AZD1775 as reported above. Arrows indicate some of the nuclei with clumped or condensed chromatin; arrowheads indicate some multilobed nuclei or multinucleation.
We also evaluated the activation of the apoptosis marker caspase-3 in NCI-H28 and MSTO-211H cells treated as above, by Western blotting analysis; we observed that dl922-947, both alone and in combination with AZD1775, induced an increase in the active cleaved caspase-3 levels and a concurrent slight decrease in the full-length protein (Figure 2B). To confirm the role of caspase activation in dl922-947-induced cell death, we co-treated NCI-H28 and MSTO-211H cells with dl922-947 and the pan-caspase inhibitor Z-VAD-FMK. We found that these cells had a significantly higher cell viability than cells treated with dl922-947 alone (Figure 2C).
Considering that, among BCL-2 family members, the anti-apoptotic protein BCL-XL was previously found to be particularly important for survival of MM cells, including NCI-H28 and MSTO-211H [67], we analyzed by Western blotting its expression in these cell lines treated with dl922-947 and/or AZD1775, as described above. We observed decreased BCL-XL levels in both cell lines upon treatment with dl922-947 and dl922-947-AZD1775 combination (Figure 2B).
Moreover, DAPI staining showed that NCI-H28 and MSTO-211H cells, treated with dl922-947 and its combination with AZD1775, had clumped or condensed chromatin, which is compatible with the apoptotic cell death. These cells also had other alterations in nuclear morphology (multilobed nuclei, multinucleation), which could be in line with aberrant mitoses (Figure 2D). Conversely, cells treated with AZD1775 alone had uncondensed and homogeneously distributed chromatin, similar to untreated control cells. This is consistent with the lack of a significant increase in apoptotic markers that we observed in AZD1775-treated cells.
2.3. AZD1775 Inactivates the DNA Damage Checkpoint Induced by dl922-947 in MM Cells
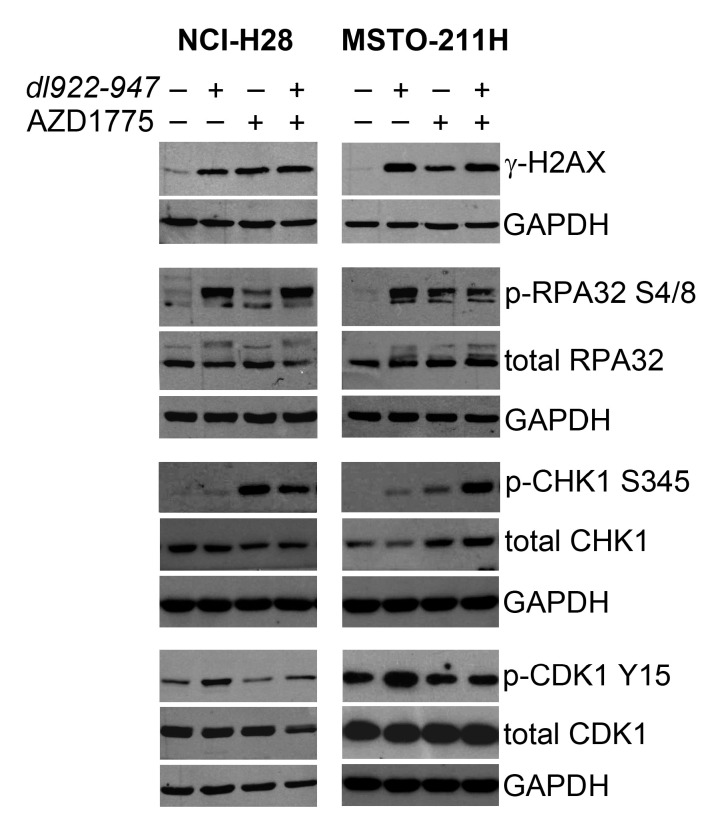
To study the molecular mechanism whereby WEE1 inhibition by AZD1775 sensitizes MM cells to dl922-947, we analyzed the effects of these agents on the WEE1 direct substrate, CDK1, and also on crucial factors of the DDR signaling. In particular, we analyzed phosphorylated histone H2AX (γ-H2AX), which is a well-known marker of double-strand breaks, and replication protein A (RPA) and CHK1, which have previously been implicated in the DDR pathway induced by dl922-947 [27,28] or AZD1775 [44,68,69,70,71,72,73] in other cancer cell types.
RPA is a heterotrimeric protein complex, consisting of RPA70, RPA32, and RPA14 subunits, which acts as a sensor of DNA damage and replication stress, by associating with single-stranded DNA [74]. DNA damaging agents induce RPA32 N-terminus phosphorylation, which is involved in the checkpoint response mediated by the ATR kinase, leading to CHK1 phosphorylation and activation [74]. CHK1 transduces the damage signals to a variety of effectors, resulting in cell cycle checkpoint activation, cell cycle arrest, DNA repair, or cell death [75].
Through Western blotting, we observed that dl922-947 and AZD1775, both alone and in combination at their respective IC50 values, induced the expression of γ-H2AX, phospho-RPA32 Ser 4/Ser 8, and phospho-CHK1 Ser 345 in both MM cell lines, which is indicative of DDR activation (Figure 3). Consistently, we observed that dl922-947 increased the levels of the inactive Tyr-15-phosphorylated form of CDK1, denoting G2/M checkpoint activation (Figure 3). As expected, this increase in phospho-CDK1 was suppressed by AZD1775 (Figure 3), showing that this inhibitor can effectively prevent the WEE1-mediated phosphorylation and inactivation of CDK1, which could thereby abrogate the dl922-947-induced DNA damage checkpoint in MM cell lines.
Figure 3.
Effect of dl922-947 and AZD1775 on the activation of cyclin-dependent kinase 1 (CDK1) and DNA damage signaling factors in malignant mesothelioma (MM) cell lines. NCI-H28 and MSTO-211H cell lines were treated with dl922-947 and AZD1775, both alone and in combination, and analyzed through Western blotting for the following factors: phosphorylated histone H2AX (γ-H2AX); phospho-replication protein A (RPA)32 Ser 4/Ser 8 and total RPA32; phospho-checkpoint kinase 1 (CHK1) Ser 345 and total CHK1; phospho-CDK1 Tyr 15 and total CDK1. DMSO was added to untreated control cells. An anti-GAPDH antibody was used for loading control.
3. Discussion
MM is a very aggressive asbestos-associated cancer for which at present there is no curative modality. Although significant efforts have been made to reduce occupational exposure to asbestos, MM incidence is expected to rise because of the long-latency time between exposure and diagnosis [2]. Moreover, asbestos and other mineral fibers that are known to cause MM are still employed in some countries [3]. Therefore, there is an urgent need to identify new therapeutic avenues.
Considering that MM is a good candidate for innovative virotherapy-based approaches because the pleural location provides direct access for the intra-tumoral injection of the virus [11], we have recently analyzed the effects of the oncolytic adenovirus dl922-947 in MM cells. The replication of this OV, bearing a deletion in the RB1 binding site of the E1A region, is dependent on RB1 inactivation [18], which occurs very frequently in MM. Indeed, although RB1 mutations are extremely rare in this cancer type (COSMIC, the Catalogue of Somatic Mutations in Cancer, http://cancer.sanger.ac.uk), the homozygous deletion of the cyclin-dependent kinase inhibitor 2A (CDKN2A) locus, which results in RB1 functional inactivation, is one of the most common mutations in MM cells [19,20], including the NCI-H28 and MSTO-211H cell lines under study (COSMIC). We have found that dl922-947 has antitumor effects in both MM cell lines and xenografts by affecting cell cycle progression, triggering immunogenic cell death, and reducing the production of pro-angiogenic factors [12].
In particular, we have observed that dl922-947 induces DNA over-replication in MM cells, which can generate DNA damage. Since the ability of dl922-947 to induce DNA lesion accumulation associates with its efficacy [27] and given that DDR inhibitors can favor this mechanism of action [26,27,28], in the present study, we analyzed whether the abrogation of the G2/M DNA damage checkpoint through the WEE1 inhibitor AZD1775 enhanced MM cell sensitivity to dl922-947. Through cell viability assays and isobologram analysis, we found that AZD1775 synergized with dl922-947 in MM cell lines.
We previously reported that both dl922-947 [12] and AZD1775 [65] sensitized MM cells to cisplatin, the drug currently used in MM chemotherapy. The comparison of the present data with our previous findings shows that, at the same treatment time and doses, dl922-947 affects cell viability similarly or even more efficaciously when combined with AZD1775 than when combined with cisplatin in MSTO-211H and NCI-H28 cells, respectively. Therefore, the dl922-947-AZD1775 co-treatment seems worthy of further investigations; in particular, its efficacy should be evaluated in animal models, also in comparison with that of other mono- and combination therapies.
We also found that dl922-947-AZD1775 combination did not significantly affect the viability of the normal mesothelial cell line MET-5A, thus again encouraging further testing of this strategy.
To analyze cell death induction, we performed the annexinV assay, which detects a well-known apoptosis marker, namely the exposure of phosphatidylserine to the cell surface. We observed an increase in the percentage of annexinV-positive cells, which was higher after the combination treatment with dl922-947 and AZD1775 than after the treatment with dl922-947 alone. Moreover, upon treatment with dl922-947, both alone and in combination with AZD1775, we also observed the activation of the apoptotic marker caspase-3. We confirmed the caspase-dependency of the dl922-947-induced cell death by using the pan-caspase inhibitor Z-VAD-FMK, which significantly increased cell viability. We also observed a decreased expression of the anti-apoptotic protein BCL-XL in NCI-H28 and MSTO-211 cells following treatment with dl922-947 and dl922-947-AZD1775 combination. This is in line with previous data indicating a crucial role of BCL-XL in regulating cell death of these cell lines [67]. Furthermore, we observed a nuclear morphology compatible with the apoptotic cell death upon treatment with dl922-947 and its combination with AZD1775. Conversely, in previous studies on ovarian cancer cells, classical hallmarks of apoptosis were not detected after treatment with dl922-947 or other oncolytic adenoviral mutants, which have been suggested to induce a different type of programmed cell death [76,77]. However, it seems plausible that dl922-947 could trigger different cell death processes in diverse cancer cell types, although further studies are necessary to understand the exact mode of cell death induced by this OV in MM. In particular, the characterization of the whole process requires a more thorough definition of the factors involved and the extension of the analysis to a wider set of MM cell lines, taking into account their possible dependency on different mechanisms to control cell death.
We observed that AZD1775 and dl922-947, both alone and in combination, activated the DDR pathway, as indicated by the increase in expression of γ-H2AX, phospho-RPA32 Ser 4/Ser 8, and phospho-CHK1 Ser 345, and in line with what was previously found in other cancer cell types [27,28,44,68,69,70,71,72,73]. Moreover, we observed that dl922-947 increased the levels of the inactive Tyr-15-phosphorylated form of CDK1, which is a surrogate marker of G2/M checkpoint activation. This increase in phospho-CDK1 was suppressed by AZD1775. Thus, this molecule seems indeed to abrogate the dl922-947-induced DNA damage checkpoint in MM cell lines by effectively preventing the WEE1-mediated phosphorylation and inactivation of CDK1.
G2/M checkpoint abrogation through WEE1 inhibition in combination with DNA-damaging agents has initially been suggested to be mainly effective on cancer cells bearing mutations in the key G1/S checkpoint regulator TP53 since these cells rely on the G2/M checkpoint to detect and repair damaged DNA [35]. However, this approach proved to be successful also in preclinical studies on TP53 wild-type MM cells most likely because of the occurrence in these cells of G1/S checkpoint inactivation through mechanisms other than direct TP53 mutation [65,66]. In particular, the homozygous deletion of the CDKN2A locus, which, as stated above, is very common in MM cells, results in the functional inactivation of both the key tumor suppressors controlling the G1/S checkpoint, p53 and RB1 [20]. Consistent with these observations, in this study, we found that the dl922-947-AZD1775 combination was effective in NCI-H28 and MSTO-211H cell lines, which, while expressing wild-type TP53 [78], both carry a homozygous deletion of the tumor suppressor locus CDKN2A (COSMIC, http://cancer.sanger.ac.uk). Thus, disruption of crucial tumor-suppressive pathways, which largely underlies MM development [7], seems to offer therapeutic opportunities for this cancer. Indeed, two main anticancer strategies can be used to target tumor suppressors: the first aims to exploit their loss, whereas the second aims to reactivate their function [79,80,81]. Accordingly, in our previous preclinical studies on MM, we have shown the potential anticancer efficacy of strategies based on the reactivation of the oncosuppressive functions of p53 [78], RBL2/p130, which is another crucial member of the RB family [82], and p27, which is a cell cycle inhibitor co-regulated with RBL2/p130 [82,83]; conversely, in the present study, we used agents that exploit tumor suppressor inactivation for their selective anticancer action: the oncolytic adenovirus dl922-947, which depends on RB1 inactivation for its replication, and AZD1775, which is considered to be mainly effective in cancer cells defective in G1/S checkpoint regulators.
Similar combinations between dl922-947 and other DDR inhibitors were analyzed in previous studies on different cancer types by both our group and others [26,27,28]. Here, we combined dl922-947 with AZD1775 based on our previous observation of the efficacy of WEE1 inhibition in sensitizing MM cells to the DNA-damaging agent cisplatin [65]. Moreover, in a recent kinome-wide CRISPR/Cas9 knockout screening, which has identified several kinases whose deficiency improves chemotherapy efficacy in MM, WEE1 knockout has proved to induce the most significant effect [66].
Although different DDR inhibitors have partially overlapping effects, they have also unique and complementary modes of action, which are responsible for their synergy [68,70,72,84,85,86]. Thus, rational combinations of these inhibitors could be useful to overcome or prevent resistance [30]. Recent studies have shown that triplet regimens consisting of chemotherapy plus WEE1 and CHK1 inhibitors could be particularly efficacious [72,87]. Possible triple treatments combining the oncolytic adenovirus dl922-947 with DDR inhibitors deserve future investigation.
In conclusion, we found that WEE1 inhibition through AZD1775 sensitizes MM cells to dl922-947 by abrogating the DNA damage checkpoint induced by the virus. Although the dl922-947-AZD1775 combination has yet to be assessed in animal models, this selective anticancer strategy, which depends on oncosuppressive pathway disruption, could be an optimal approach against a tumor, such as MM, that mainly develops through the loss of tumor suppressor functions.
4. Materials and Methods
4.1. Cell Cultures and Adenovirus Preparation
NCI-H28 and MSTO-211H mesothelioma cell lines and MET-5A mesothelial cells were purchased from American Type Culture Collection (ATCC; Manassas, VA USA). NCI-H28 and MSTO-211H cells were grown in RPMI-1640 supplemented with 10% fetal bovine serum (FBS), 1% penicillin-streptomycin, and 1% glutamine. MET-5A cells were grown in Medium 199 with 10% FBS, 0.5% penicillin-streptomycin, 1% glutamine, and 3.3 nM epidermal growth factor, 400 nM hydrocortisone, and 870 nM insulin. All cell culture reagents were obtained from Sigma-Aldrich (St Louis, MO, USA), and cells were maintained in a humidified incubator set at 37 °C and 5% CO2. Cells were routinely tested with the PlasmoTest™ Mycoplasma Detection kit (Invivogen, San Diego, CA, USA) for the presence of mycoplasma, which was eradicated with Plasmocin™ Mycoplasma Elimination Reagent (Invivogen), when necessary. Dl922-947 was expanded in the human embryonic kidney cell line HEK-293 (ATCC), purified, stored, and quantified in 8.3 × 108 pfu/mL viral stocks, as previously described [12].
4.2. Drug Combination, Sulforhodamine B (SRB) Assay, and Synergism Analysis
MM cells were seeded in 96-well plates 24 h before treatment with dl922-947 and AZD1775 (MK1775, purchased from Axon Medchem), both alone and in combination at various concentrations in a constant ratio. Five days after treatment, cells were fixed with 50% (v/v) trichloroacetic acid and stained with 0.4% (w/v) SRB in 1% (v/v) acetic acid, following the manufacturer’s instructions. Synergism, additivity, or antagonism were determined through isobologram analysis using the CompuSyn software 1.0 (ComboSyn, Inc., Paramus, NJ, USA). Combination index (CI) values were also calculated by the CompuSyn software, which uses the Chou–Talalay method. CI < 1 indicates synergism, CI = 1 additivity, and CI > 1 antagonism. The r value represents the linear correlation coefficient of the median effect plot, which indicates the conformity of the data to the mass action law.
4.3. Apoptosis Analysis
Apoptosis was assessed through flow cytometric analysis (BD FACSCalibur, Becton Dickinson BD Biosciences, Franklin Lakes, NJ, USA) of MM cells treated for 96 h with dl922-947 and/or AZD1775 at their IC50 values and stained with annexinV–FITC and propidium iodide (AnnexinV-FITC Kit, Biolegend, San Diego, CA, USA) according to the manufacturer’s instructions.
To evaluate whether the dl922-947-induced cell death was caspase-dependent, the cells were treated with dl922-947 alone and in combination with the pan-caspase inhibitor Z-VAD-FMK (R&D Systems, Minneapolis, MN, USA) at the concentration of 100 µM. Ninety-six hours after treatment, cell viability was evaluated by MTS assay (CellTiter 96® AQueous One Solution Cell Proliferation Assay, Promega, Madison, WI, USA), following the manufacturer’s instructions.
4.4. DAPI Staining
NCI-H28 and MSTO-211H cells were grown on coverslips and treated with dl922-947 and AZD1775, both alone and in combination. Ninety-six hours after treatment, the cells were fixed in 3% paraformaldehyde for 10 min and permeabilized with 0.5% triton-X 100 for 10 min. Samples were then blocked in 1% BSA for 10 min. The coverslips were mounted using the ProLong Gold Antifade Reagent with DAPI (Life Technologies, Carlsbad, CA, USA). Images were obtained using the Nikon Eclipse E600 microscope (Nikon, Minato, Tokyo, Japan).
4.5. Western Blotting Analysis
For total protein extraction, cells were lysed on ice for 30 min in a buffer consisting of 50 mM Tris-HCl pH 7.5, 1 mM EDTA pH 8.0, 150 mM NaCl, 1% NP-40, supplemented with protease and phosphatase inhibitor cocktails (Roche, Basilea, Switzerland). The protein samples were resolved by SDS-PAGE and blotted onto nitrocellulose membranes, which were then incubated with antibodies against: ɣ-H2AX (Cat. #05-636) from Merk Millipore, Burlington, MA, USA; phospho-RPA32 Ser 4/Ser 8 (Cat. #A300–245A) and RPA32 (Cat. #A300–244A) from Bethyl Laboratories, Montgomery, TX, USA; phospho-CHK1 Ser 345 (Cat. #2348), CHK1 (Cat. #2360), phospho-CDK1 Tyr15 (Cat. #4539), CDK1 (Cat. #9116S), and caspase-3 (Cat. #9662S) from Cell Signaling Technologies, Danvers, MA, USA; BCL-XS/L (Cat. #sc-1041) and GAPDH (Cat. #sc-25778) from Santa Cruz Biotechnology, Santa Cruz, CA, USA. After incubation with horseradish peroxidase-conjugated secondary antibodies, signals were detected through ECL (Amersham Biosciences, GE Healthcare, Little Chalfont, UK). The chemiluminescent images were analyzed by ImageQuant LAS 500 (GE Healthcare, Little Chalfont, UK).
4.6. Statistical Analysis
Results were expressed as means ± standard deviation and derived from at least two independent experiments. Statistical analyses were performed through one-way repeated measures ANOVA with Tukey post-test (to compare multiple matched groups) and through paired Student’s t test (to compare two matched groups) using the GraphPad Software 5.01 (GraphPad Software, San Diego, CA, USA). p values < 0.05 were considered as significant.
Acknowledgments
We are grateful to Aurora Costa and to Pasquale Barba for technical help and to Alessandra Trocino, Librarian at IRCCS “G. Pascale” of Naples, Italy, for bibliographic assistance.
Abbreviations
| ATM | Ataxia telangiectasia mutated |
| ATR | Ataxia telangiectasia and Rad3 related |
| CDK | Cyclin-dependent kinase |
| CDKN2A | Cyclin-dependent kinase inhibitor 2A |
| CHK1 | Checkpoint kinase 1 |
| CI | Combination index |
| COSMIC | Catalogue of Somatic Mutations in Cancer |
| DDR | DNA damage response |
| Fa | Fraction affected |
| FBS | Fetal bovine serum |
| IC50 | Half maximal inhibitory concentration |
| MM | Malignant mesothelioma |
| OV | Oncolytic virus |
| PARP | Poly(ADP-ribose) polymerase |
| PI | Propidium iodide |
| RB | Retinoblastoma |
| RPA | Replication protein A |
| SRB | Sulforhodamine B |
| γ-H2AX | Phosphorylated histone H2AX |
Author Contributions
Conceptualization, F.P. and G.P.; methodology, C.A.I., I.M.F., S.D.S., A.M.M., M.B.; software, C.A.I., P.I., I.M.F.; formal analysis, P.I.; investigation, C.A.I., I.M.F., S.D.S.; writing—original draft preparation, P.I.; writing—review and editing, P.I., F.P., G.P.; supervision, A.G.; funding acquisition, F.P., G.P., A.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by: the Italian Ministry of Health progetto di Ricerca Corrente (M4/7) “Identificazione di nuovi approcci per la diagnosi e terapia del mesothelioma pleurico”; Mesothelioma Applied Research Foundation, GRANT ID 483418; the Sbarro Health Research Organization (www.shro.org); the Commonwealth of Pennsylvania; the Programme STAR, financially supported by UniNA and Compagnia San Paolo. S.D.S. was supported by a FIRC-AIRC fellowship.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Baumann F., Carbone M. Environmental risk of mesothelioma in the United States: An emerging concern-epidemiological issues. J. Toxicol. Environ. Health B Crit. Rev. 2016;19:231–249. doi: 10.1080/10937404.2016.1195322. [DOI] [PubMed] [Google Scholar]
- 3.Carbone M., Yang H. Mesothelioma: Recent highlights. Ann. Transl. Med. 2017;5:238. doi: 10.21037/atm.2017.04.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Lancet Respiratory Medicine Pleural mesothelioma: Tackling a deadly cancer. Lancet Respir. Med. 2019;7:99. doi: 10.1016/S2213-2600(19)30004-9. [DOI] [PubMed] [Google Scholar]
- 5.Scherpereel A., Opitz I., Berghmans T., Psallidas I., Glatzer M., Rigau D., Astoul P., Bölükbas S., Boyd J., Coolen J., et al. ERS/ESTS/EACTS/ESTRO guidelines for the management of malignant pleural mesothelioma. Eur. Respir. J. 2020;55 doi: 10.1183/13993003.00953-2019. [DOI] [PubMed] [Google Scholar]
- 6.Robinson B.W., Musk A.W., Lake R.A. Malignant mesothelioma. Lancet. 2005;366:397–408. doi: 10.1016/S0140-6736(05)67025-0. [DOI] [PubMed] [Google Scholar]
- 7.Hinz T.K., Heasley L.E. Translating mesothelioma molecular genomics and dependencies into precision oncology-based therapies. Semin. Cancer Biol. 2020;61:11–22. doi: 10.1016/j.semcancer.2019.09.014. [DOI] [PubMed] [Google Scholar]
- 8.Russell S.J., Peng K.W., Bell J.C. Oncolytic virotherapy. Nat. Biotechnol. 2012;30:658–670. doi: 10.1038/nbt.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marelli G., Howells A., Lemoine N.R., Wang Y. Oncolytic Viral Therapy and the Immune System: A Double-Edged Sword against Cancer. Front. Immunol. 2018;9:866. doi: 10.3389/fimmu.2018.00866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malfitano A.M., Di Somma S., Iannuzzi C.A., Pentimalli F., Portella G. Virotherapy: From single agents to combinatorial treatments. Biochem. Pharmacol. 2020;177:113986. doi: 10.1016/j.bcp.2020.113986. [DOI] [PubMed] [Google Scholar]
- 11.Pease D.F., Kratzke R.A. Oncolytic Viral Therapy for Mesothelioma. Front. Oncol. 2017;7:179. doi: 10.3389/fonc.2017.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Somma S., Iannuzzi C.A., Passaro C., Forte I.M., Iannone R., Gigantino V., Indovina P., Botti G., Giordano A., Formisano P., et al. The Oncolytic Virus dl922-947 Triggers Immunogenic Cell Death in Mesothelioma and Reduces Xenograft Growth. Front. Oncol. 2019;9:564. doi: 10.3389/fonc.2019.00564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhattacharyya M., Francis J., Eddouadi A., Lemoine N.R., Hallden G. An oncolytic adenovirus defective in pRb-binding (dl922-947) can efficiently eliminate pancreatic cancer cells and tumors in vivo in combination with 5-FU or gemcitabine. Cancer Gene Ther. 2011;18:734–743. doi: 10.1038/cgt.2011.45. [DOI] [PubMed] [Google Scholar]
- 14.Lockley M., Fernandez M., Wang Y., Li N.F., Conroy S., Lemoine N., McNeish I. Activity of the adenoviral E1A deletion mutant dl922-947 in ovarian cancer: Comparison with E1A wild-type viruses, bioluminescence monitoring, and intraperitoneal delivery in icodextrin. Cancer Res. 2006;66:989–998. doi: 10.1158/0008-5472.CAN-05-2691. [DOI] [PubMed] [Google Scholar]
- 15.Botta G., Perruolo G., Libertini S., Cassese A., Abagnale A., Beguinot F., Formisano P., Portella G. PED/PEA-15 modulates coxsackievirus-adenovirus receptor expression and adenoviral infectivity via ERK-mediated signals in glioma cells. Hum. Gene Ther. 2010;21:1067–1076. doi: 10.1089/hum.2009.181. [DOI] [PubMed] [Google Scholar]
- 16.Libertini S., Iacuzzo I., Perruolo G., Scala S., Ierano C., Franco R., Hallden G., Portella G. Bevacizumab increases viral distribution in human anaplastic thyroid carcinoma xenografts and enhances the effects of E1A-defective adenovirus dl922-947. Clin. Cancer Res. 2008;14:6505–6514. doi: 10.1158/1078-0432.CCR-08-0200. [DOI] [PubMed] [Google Scholar]
- 17.Malfitano A.M., Somma S.D., Prevete N.G., Portella G. Virotherapy as a Potential Therapeutic Approach for the Treatment of Aggressive Thyroid Cancer. Cancers. 2019;11:1532. doi: 10.3390/cancers11101532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heise C., Hermiston T., Johnson L., Brooks G., Sampson-Johannes A., Williams A., Hawkins L., Kirn D. An adenovirus E1A mutant that demonstrates potent and selective systemic anti-tumoral efficacy. Nat. Med. 2000;6:1134–1139. doi: 10.1038/80474. [DOI] [PubMed] [Google Scholar]
- 19.Forte I.M., Giordano A., Pentimalli F. Molecular markers of mesothelioma aiding in diagnostic challenges: The combined use of p16 and BAP1. In: Giordano A., Franco R., editors. Malignant Pleural Mesothelioma: A Guide for Clinicians. Academic Press; Cambridge, MA, USA: 2019. pp. 109–115. [Google Scholar]
- 20.Prins J.B., Williamson K.A., Kamp M.M., Van Hezik E.J., Van der Kwast T.H., Hagemeijer A., Versnel M.A. The gene for the cyclin-dependent-kinase-4 inhibitor, CDKN2A, is preferentially deleted in malignant mesothelioma. Int. J. Cancer. 1998;7:649–653. doi: 10.1002/(SICI)1097-0215(19980209)75:4<649::AID-IJC25>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 21.Howells A., Marelli G., Lemoine N.R., Wang Y. Oncolytic Viruses-Interaction of Virus and Tumor Cells in the Battle to Eliminate Cancer. Front. Oncol. 2017;7:195. doi: 10.3389/fonc.2017.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bartlett D.L., Liu Z., Sathaiah M., Ravindranathan R., Guo Z., He Y., Guo Z.S. Oncolytic viruses as therapeutic cancer vaccines. Mol. Cancer. 2013;12:103. doi: 10.1186/1476-4598-12-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berkey S.E., Thorne S.H., Bartlett D.L. Oncolytic Virotherapy and the Tumor Microenvironment. Adv. Exp. Med. Biol. 2017;1036:157–172. doi: 10.1007/978-3-319-67577-0_11. [DOI] [PubMed] [Google Scholar]
- 24.Passaro C., Borriello F., Vastolo V., Di Somma S., Scamardella E., Gigantino V., Franco R., Marone G., Portella G. The oncolytic virus dl922-947 reduces IL-8/CXCL8 and MCP-1/CCL2 expression and impairs angiogenesis and macrophage infiltration in anaplastic thyroid carcinoma. Oncotarget. 2016;7:1500–1515. doi: 10.18632/oncotarget.6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Connell C.M., Wheatley S.P., McNeish I.A. Nuclear survivin abrogates multiple cell cycle checkpoints and enhances viral oncolysis. Cancer Res. 2008;68:7923–7931. doi: 10.1158/0008-5472.CAN-08-0817. [DOI] [PubMed] [Google Scholar]
- 26.Passaro C., Volpe M., Botta G., Scamardella E., Perruolo G., Gillespie D., Libertini S., Portella G. PARP inhibitor olaparib increases the oncolytic activity of dl922-947 in in vitro and in vivo model of anaplastic thyroid carcinoma. Mol. Oncol. 2015;9:78–92. doi: 10.1016/j.molonc.2014.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Connell C.M., Shibata A., Tookman L.A., Archibald K.M., Flak M.B., Pirlo K.J., Lockley M., Wheatley S.P., McNeish I.A. Genomic DNA damage and ATR-Chk1 signaling determine oncolytic adenoviral efficacy in human ovarian cancer cells. J. Clin. Investig. 2011;121:1283–1297. doi: 10.1172/JCI43976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Passaro C., Abagnale A., Libertini S., Volpe M., Botta G., Cella L., Pacelli R., Hallden G., Gillespie D., Portella G. Ionizing radiation enhances dl922-947-mediated cell death of anaplastic thyroid carcinoma cells. Endocr. Relat. Cancer. 2013;20:633–647. doi: 10.1530/ERC-13-0001. [DOI] [PubMed] [Google Scholar]
- 29.Fu S., Wang Y., Keyomarsi K., Meric-Bernstam F., Meric-Bernstein F. Strategic development of AZD1775, a Wee1 kinase inhibitor, for cancer therapy. Expert Opin. Investig. Drug. 2018;27:741–751. doi: 10.1080/13543784.2018.1511700. [DOI] [PubMed] [Google Scholar]
- 30.Pilié P.G., Tang C., Mills G.B., Yap T.A. State-of-the-art strategies for targeting the DNA damage response in cancer. Nat. Rev. Clin. Oncol. 2019;16:81–104. doi: 10.1038/s41571-018-0114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cuneo K.C., Morgan M.A., Sahai V., Schipper M.J., Parsels L.A., Parsels J.D., Devasia T., Al-Hawaray M., Cho C.S., Nathan H., et al. Dose Escalation Trial of the Wee1 Inhibitor Adavosertib (AZD1775) in Combination With Gemcitabine and Radiation for Patients With Locally Advanced Pancreatic Cancer. J. Clin. Oncol. 2019;37:2643–2650. doi: 10.1200/JCO.19.00730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cole K.A., Pal S., Kudgus R.A., Ijaz H., Liu X., Minard C.G., Pawel B.R., Maris J.M., Haas-Kogan D.A., Voss S.D., et al. Phase I Clinical Trial of the Wee1 Inhibitor Adavosertib (AZD1775) with Irinotecan in Children with Relapsed Solid Tumors: A COG Phase I Consortium Report (ADVL1312) Clin. Cancer Res. 2020;26:1213–1219. doi: 10.1158/1078-0432.CCR-19-3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kato H., de Souza P., Kim S.W., Lickliter J.D., Naito Y., Park K., Kumar S., Mugundu G.M., Bang Y.J. Safety, Pharmacokinetics, and Clinical Activity of Adavosertib in Combination with Chemotherapy in Asian Patients with Advanced Solid Tumors: Phase Ib Study. Target. Oncol. 2020;15:75–84. doi: 10.1007/s11523-020-00701-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oza A.M., Estevez-Diz M.D.P., Grischke E.M., Hall M., Marmé F., Provencher D.M., Uyar D.S., Weberpals J.I., Wenham R.M., Laing N., et al. A biomarker-enriched, randomized Phase II trial of adavosertib (AZD1775) plus paclitaxel and carboplatin for women with platinum-sensitive TP53-mutant ovarian cancer. Clin. Cancer Res. 2020;26:4767–4776. doi: 10.1158/1078-0432.CCR-20-0219. [DOI] [PubMed] [Google Scholar]
- 35.Indovina P., Giordano A. Targeting the checkpoint kinase WEE1: Selective sensitization of cancer cells to DNA-damaging drugs. Cancer Biol. Ther. 2010;9:523–525. doi: 10.4161/cbt.9.7.11276. [DOI] [PubMed] [Google Scholar]
- 36.Liang J., Zhao H., Diplas B.H., Liu S., Liu J., Wang D., Lu Y., Zhu Q., Wu J., Wang W., et al. Genome-Wide CRISPR-Cas9 Screen Reveals Selective Vulnerability of ATRX-Mutant Cancers to WEE1 Inhibition. Cancer Res. 2020;80:510–523. doi: 10.1158/0008-5472.CAN-18-3374. [DOI] [PubMed] [Google Scholar]
- 37.Brunner A., Suryo Rahmanto A., Johansson H., Franco M., Viiliäinen J., Gazi M., Frings O., Fredlund E., Spruck C., Lehtiö J., et al. PTEN and DNA-PK determine sensitivity and recovery in response to WEE1 inhibition in human breast cancer. Elife. 2020;9:e57894. doi: 10.7554/eLife.57894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Young L.A., O’Connor L.O., de Renty C., Veldman-Jones M.H., Dorval T., Wilson Z., Jones D.R., Lawson D., Odedra R., Maya-Mendoza A., et al. Differential Activity of ATR and WEE1 Inhibitors in a Highly Sensitive Subpopulation of DLBCL Linked to Replication Stress. Cancer Res. 2019;79:3762–3775. doi: 10.1158/0008-5472.CAN-18-2480. [DOI] [PubMed] [Google Scholar]
- 39.Lallo A., Frese K.K., Morrow C.J., Sloane R., Gulati S., Schenk M.W., Trapani F., Simms N., Galvin M., Brown S., et al. The Combination of the PARP Inhibitor Olaparib and the WEE1 Inhibitor AZD1775 as a New Therapeutic Option for Small Cell Lung Cancer. Clin. Cancer Res. 2018;24:5153–5164. doi: 10.1158/1078-0432.CCR-17-2805. [DOI] [PubMed] [Google Scholar]
- 40.Lin X., Chen D., Zhang C., Zhang X., Li Z., Dong B., Gao J., Shen L. Augmented antitumor activity by olaparib plus AZD1775 in gastric cancer through disrupting DNA damage repair pathways and DNA damage checkpoint. J. Exp. Clin. Cancer Res. 2018;37:129. doi: 10.1186/s13046-018-0790-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haynes B., Murai J., Lee J.M. Restored replication fork stabilization, a mechanism of PARP inhibitor resistance, can be overcome by cell cycle checkpoint inhibition. Cancer Treat. Rev. 2018;71:1–7. doi: 10.1016/j.ctrv.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fang Y., McGrail D.J., Sun C., Labrie M., Chen X., Zhang D., Ju Z., Vellano C.P., Lu Y., Li Y., et al. Sequential Therapy with PARP and WEE1 Inhibitors Minimizes Toxicity while Maintaining Efficacy. Cancer Cell. 2019;35:851–867. doi: 10.1016/j.ccell.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Russell M.R., Levin K., Rader J., Belcastro L., Li Y., Martinez D., Pawel B., Shumway S.D., Maris J.M., Cole K.A. Combination therapy targeting the Chk1 and Wee1 kinases shows therapeutic efficacy in neuroblastoma. Cancer Res. 2013;73:776–784. doi: 10.1158/0008-5472.CAN-12-2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qi W., Xie C., Li C., Caldwell J.T., Edwards H., Taub J.W., Wang Y., Lin H., Ge Y. CHK1 plays a critical role in the anti-leukemic activity of the wee1 inhibitor MK-1775 in acute myeloid leukemia cells. J. Hematol. Oncol. 2014;7:53. doi: 10.1186/s13045-014-0053-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chaudhuri L., Vincelette N.D., Koh B.D., Naylor R.M., Flatten K.S., Peterson K.L., McNally A., Gojo I., Karp J.E., Mesa R.A., et al. CHK1 and WEE1 inhibition combine synergistically to enhance therapeutic efficacy in acute myeloid leukemia ex vivo. Haematologica. 2014;99:688–696. doi: 10.3324/haematol.2013.093187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ghelli Luserna Di Rorà A., Bocconcelli M., Ferrari A., Terragna C., Bruno S., Imbrogno E., Beeharry N., Robustelli V., Ghetti M., Napolitano R., et al. Synergism Through WEE1 and CHK1 Inhibition in Acute Lymphoblastic Leukemia. Cancers. 2019;11:1654. doi: 10.3390/cancers11111654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deneka A.Y., Einarson M.B., Bennett J., Nikonova A.S., Elmekawy M., Zhou Y., Lee J.W., Burtness B.A., Golemis E.A. Synthetic Lethal Targeting of Mitotic Checkpoints in HPV-Negative Head and Neck Cancer. Cancers. 2020;12:306. doi: 10.3390/cancers12020306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jin J., Fang H., Yang F., Ji W., Guan N., Sun Z., Shi Y., Zhou G., Guan X. Combined Inhibition of ATR and WEE1 as a Novel Therapeutic Strategy in Triple-Negative Breast Cancer. Neoplasia. 2018;20:478–488. doi: 10.1016/j.neo.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bukhari A.B., Lewis C.W., Pearce J.J., Luong D., Chan G.K., Gamper A.M. Inhibiting Wee1 and ATR kinases produces tumor-selective synthetic lethality and suppresses metastasis. J. Clin. Investig. 2019;129:1329–1344. doi: 10.1172/JCI122622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qi W., Xu X., Wang M., Li X., Wang C., Sun L., Zhao D., Sun L. Inhibition of Wee1 sensitizes AML cells to ATR inhibitor VE-822-induced DNA damage and apoptosis. Biochem. Pharmacol. 2019;164:273–282. doi: 10.1016/j.bcp.2019.04.022. [DOI] [PubMed] [Google Scholar]
- 51.Cozzi M., Giorgi F., Marcelli E., Pentimalli F., Forte I.M., Schenone S., D’Urso V., De Falco G., Botta M., Giordano A., et al. Antitumor activity of new pyrazolo[3,4-d]pyrimidine SRC kinase inhibitors in Burkitt lymphoma cell lines and its enhancement by WEE1 inhibition. Cell Cycle. 2012;11:1029–1039. doi: 10.4161/cc.11.5.19519. [DOI] [PubMed] [Google Scholar]
- 52.Ghelli Luserna Di Rorà A., Beeharry N., Imbrogno E., Ferrari A., Robustelli V., Righi S., Sabattini E., Verga Falzacappa M.V., Ronchini C., Testoni N., et al. Targeting WEE1 to enhance conventional therapies for acute lymphoblastic leukemia. J. Hematol. Oncol. 2018;11:99. doi: 10.1186/s13045-018-0641-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Caiola E., Frapolli R., Tomanelli M., Valerio R., Iezzi A., Garassino M.C., Broggini M., Marabese M. Wee1 inhibitor MK1775 sensitizes KRAS mutated NSCLC cells to sorafenib. Sci. Rep. 2018;8:948. doi: 10.1038/s41598-017-18900-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee J.W., Parameswaran J., Sandoval-Schaefer T., Eoh K.J., Yang D.H., Zhu F., Mehra R., Sharma R., Gaffney S.G., Perry E.B., et al. Combined Aurora Kinase A (AURKA) and WEE1 Inhibition Demonstrates Synergistic Antitumor Effect in Squamous Cell Carcinoma of the Head and Neck. Clin. Cancer Res. 2019;25:3430–3442. doi: 10.1158/1078-0432.CCR-18-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.De Jong M.R.W., Langendonk M., Reitsma B., Herbers P., Nijland M., Huls G., van den Berg A., Ammatuna E., Visser L., van Meerten T. WEE1 Inhibition Enhances Anti-Apoptotic Dependency as a Result of Premature Mitotic Entry and DNA Damage. Cancers. 2019;11:1743. doi: 10.3390/cancers11111743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Corella A.N., Cabiliza Ordonio M.V.A., Coleman I., Lucas J.M., Kaipainen A., Nguyen H.M., Sondheim D., Brown L.G., True L.D., Lee J.K., et al. Identification of Therapeutic Vulnerabilities in Small-cell Neuroendocrine Prostate Cancer. Clin. Cancer Res. 2020;26:1667–1677. doi: 10.1158/1078-0432.CCR-19-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu W., Zeng X., Yin Y., Li C., Yang W., Wan W., Shi L., Wang G., Tao K., Zhang P. Targeting the WEE1 kinase strengthens the antitumor activity of imatinib via promoting KIT autophagic degradation in gastrointestinal stromal tumors. Gastric Cancer. 2020;23:39–51. doi: 10.1007/s10120-019-00977-1. [DOI] [PubMed] [Google Scholar]
- 58.Takashima Y., Kikuchi E., Kikuchi J., Suzuki M., Kikuchi H., Maeda M., Shoji T., Furuta M., Kinoshita I., Dosaka-Akita H., et al. Bromodomain and extraterminal domain inhibition synergizes with WEE1-inhibitor AZD1775 effect by impairing nonhomologous end joining and enhancing DNA damage in nonsmall cell lung cancer. Int. J. Cancer. 2020;146:1114–1124. doi: 10.1002/ijc.32515. [DOI] [PubMed] [Google Scholar]
- 59.Hu J., Wang T., Xu J., Wu S., Wang L., Su H., Jiang J., Yue M., Wang J., Wang D., et al. WEE1 inhibition induces glutamine addiction in T-cell acute lymphoblastic leukemia. Haematologica. 2020 doi: 10.3324/haematol.2019.231126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xing L., Lin L., Yu T., Li Y., Cho S.F., Liu J., Wen K., Hsieh P.A., Kinneer K., Munshi N., et al. A novel BCMA PBD-ADC with ATM/ATR/WEE1 inhibitors or bortezomib induce synergistic lethality in multiple myeloma. Leukemia. 2020;34:2150–2162. doi: 10.1038/s41375-020-0745-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liang L., He Y., Wang H., Zhou H., Xiao L., Ye M., Kuang Y., Luo S., Zuo Y., Feng P., et al. The Wee1 kinase inhibitor MK1775 suppresses cell growth, attenuates stemness and synergises with bortezomib in multiple myeloma. Br. J. Haematol. 2020;191:62–67. doi: 10.1111/bjh.16614. [DOI] [PubMed] [Google Scholar]
- 62.Sun L., Moore E., Berman R., Clavijo P.E., Saleh A., Chen Z., Van Waes C., Davies J., Friedman J., Allen C.T. WEE1 kinase inhibition reverses G2/M cell cycle checkpoint activation to sensitize cancer cells to immunotherapy. Oncoimmunology. 2018;7:e1488359. doi: 10.1080/2162402X.2018.1488359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Patel P., Sun L., Robbins Y., Clavijo P.E., Friedman J., Silvin C., Van Waes C., Cook J., Mitchell J., Allen C. Enhancing direct cytotoxicity and response to immune checkpoint blockade following ionizing radiation with Wee1 kinase inhibition. Oncoimmunology. 2019;8:e1638207. doi: 10.1080/2162402X.2019.1638207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hai J., Zhang H., Zhou J., Wu Z., Chen T., Papadopoulos E., Dowling C.M., Pyon V., Pan Y., Liu J.B., et al. Generation of Genetically Engineered Mouse Lung Organoid Models for Squamous Cell Lung Cancers Allows for the Study of Combinatorial Immunotherapy. Clin. Cancer Res. 2020;26:3431–3442. doi: 10.1158/1078-0432.CCR-19-1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Indovina P., Marcelli E., Di Marzo D., Casini N., Forte I.M., Giorgi F., Alfano L., Pentimalli F., Giordano A. Abrogating G(2)/M checkpoint through WEE1 inhibition in combination with chemotherapy as a promising therapeutic approach for mesothelioma. Cancer Biol. Ther. 2014;15:380–388. doi: 10.4161/cbt.27623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu D., Liang S.Q., Yang H., Bruggmann R., Berezowska S., Yang Z., Marti T.M., Hall S.R.R., Gao Y., Kocher G.J., et al. CRISPR Screening Identifies WEE1 as a Combination Target for Standard Chemotherapy in Malignant Pleural Mesothelioma. Mol. Cancer Ther. 2020;19:661–672. doi: 10.1158/1535-7163.MCT-19-0724. [DOI] [PubMed] [Google Scholar]
- 67.Varin E., Denoyelle C., Brotin E., Meryet-Figuière M., Giffard F., Abeilard E., Goux D., Gauduchon P., Icard P., Poulain L. Downregulation of Bcl-xL and Mcl-1 is sufficient to induce cell death in mesothelioma cells highly refractory to conventional chemotherapy. Carcinogenesis. 2010;31:984–993. doi: 10.1093/carcin/bgq026. [DOI] [PubMed] [Google Scholar]
- 68.Guertin A.D., Martin M.M., Roberts B., Hurd M., Qu X., Miselis N.R., Liu Y., Li J., Feldman I., Benita Y., et al. Unique functions of CHK1 and WEE1 underlie synergistic anti-tumor activity upon pharmacologic inhibition. Cancer Cell Int. 2012;12:45. doi: 10.1186/1475-2867-12-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang G., Niu X., Zhang W., Caldwell J.T., Edwards H., Chen W., Taub J.W., Zhao L., Ge Y. Synergistic antitumor interactions between MK-1775 and panobinostat in preclinical models of pancreatic cancer. Cancer Lett. 2015;356:656–668. doi: 10.1016/j.canlet.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sakurikar N., Thompson R., Montano R., Eastman A. A subset of cancer cell lines is acutely sensitive to the Chk1 inhibitor MK-8776 as monotherapy due to CDK2 activation in S phase. Oncotarget. 2016;7:1380–1394. doi: 10.18632/oncotarget.6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Busch C.J., Kröger M.S., Jensen J., Kriegs M., Gatzemeier F., Petersen C., Münscher A., Rothkamm K., Rieckmann T. G2-checkpoint targeting and radiosensitization of HPV/p16-positive HNSCC cells through the inhibition of Chk1 and Wee1. Radiother. Oncol. 2017;122:260–266. doi: 10.1016/j.radonc.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 72.Koh S.B., Wallez Y., Dunlop C.R., Bernaldo de Quirós Fernández S., Bapiro T.E., Richards F.M., Jodrell D.I. Mechanistic Distinctions between CHK1 and WEE1 Inhibition Guide the Scheduling of Triple Therapy with Gemcitabine. Cancer Res. 2018;78:3054–3066. doi: 10.1158/0008-5472.CAN-17-3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hauge S., Macurek L., Syljuåsen R.G. p21 limits S phase DNA damage caused by the Wee1 inhibitor MK1775. Cell Cycle. 2019;18:834–847. doi: 10.1080/15384101.2019.1593649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maréchal A., Zou L. RPA-coated single-stranded DNA as a platform for post-translational modifications in the DNA damage response. Cell Res. 2015;25:9–23. doi: 10.1038/cr.2014.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Patil M., Pabla N., Dong Z. Checkpoint kinase 1 in DNA damage response and cell cycle regulation. Cell. Mol. Life Sci. 2013;70:4009–4021. doi: 10.1007/s00018-013-1307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Baird S.K., Aerts J.L., Eddaoudi A., Lockley M., Lemoine N.R., McNeish I.A. Oncolytic adenoviral mutants induce a novel mode of programmed cell death in ovarian cancer. Oncogene. 2008;27:3081–3090. doi: 10.1038/sj.onc.1210977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weigert M., Binks A., Dowson S., Leung E.Y.L., Athineos D., Yu X., Mullin M., Walton J.B., Orange C., Ennis D., et al. RIPK3 promotes adenovirus type 5 activity. Cell Death Dis. 2017;8:3206. doi: 10.1038/s41419-017-0110-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Di Marzo D., Forte I.M., Indovina P., Di Gennaro E., Rizzo V., Giorgi F., Mattioli E., Iannuzzi C.A., Budillon A., Giordano A., et al. Pharmacological targeting of p53 through RITA is an effective antitumoral strategy for malignant pleural mesothelioma. Cell Cycle. 2014;13:652–665. doi: 10.4161/cc.27546. [DOI] [PubMed] [Google Scholar]
- 79.Knudsen E.S., Wang J.Y. Targeting the RB-pathway in cancer therapy. Clin. Cancer Res. 2010;16:1094–1099. doi: 10.1158/1078-0432.CCR-09-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Indovina P., Pentimalli F., Casini N., Vocca I., Giordano A. RB1 dual role in proliferation and apoptosis: Cell fate control and implications for cancer therapy. Oncotarget. 2015;6:17873–17890. doi: 10.18632/oncotarget.4286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Indovina P., Pentimalli F., Conti D., Giordano A. Translating RB1 predictive value in clinical cancer therapy: Are we there yet? Biochem. Pharmacol. 2019;166:323–334. doi: 10.1016/j.bcp.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 82.Pentimalli F., Forte I.M., Esposito L., Indovina P., Iannuzzi C.A., Alfano L., Costa C., Barone D., Rocco G., Giordano A. RBL2/p130 is a direct AKT target and is required to induce apoptosis upon AKT inhibition in lung cancer and mesothelioma cell lines. Oncogene. 2018;37:3657–3671. doi: 10.1038/s41388-018-0214-3. [DOI] [PubMed] [Google Scholar]
- 83.Indovina P., Giorgi F., Rizzo V., Khadang B., Schenone S., Di Marzo D., Forte I.M., Tomei V., Mattioli E., D’Urso V., et al. New pyrazolo[3,4-d]pyrimidine SRC inhibitors induce apoptosis in mesothelioma cell lines through p27 nuclear stabilization. Oncogene. 2012;31:929–938. doi: 10.1038/onc.2011.286. [DOI] [PubMed] [Google Scholar]
- 84.Bridges K.A., Chen X., Liu H., Rock C., Buchholz T.A., Shumway S.D., Skinner H.D., Meyn R.E. MK-8776, a novel chk1 kinase inhibitor, radiosensitizes p53-defective human tumor cells. Oncotarget. 2016;7:71660–71672. doi: 10.18632/oncotarget.12311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moiseeva T.N., Qian C., Sugitani N., Osmanbeyoglu H.U., Bakkenist C.J. WEE1 kinase inhibitor AZD1775 induces CDK1 kinase-dependent origin firing in unperturbed G1- and S-phase cells. Proc. Natl. Acad. Sci. USA. 2019;116:23891–23893. doi: 10.1073/pnas.1915108116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nojima H., Homma H., Onozato Y., Kaida A., Harada H., Miura M. Differential properties of mitosis-associated events following CHK1 and WEE1 inhibitor treatments in human tongue carcinoma cells. Exp. Cell Res. 2020;386:111720. doi: 10.1016/j.yexcr.2019.111720. [DOI] [PubMed] [Google Scholar]
- 87.Chung S., Vail P., Witkiewicz A.K., Knudsen E.S. Coordinately Targeting Cell-Cycle Checkpoint Functions in Integrated Models of Pancreatic Cancer. Clin. Cancer Res. 2019;25:2290–2304. doi: 10.1158/1078-0432.CCR-18-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]