Abstract
Background
The optimal treatment sequence for patients with advanced rectal cancer and synchronous resectable liver metastases is controversial. We examined the outcomes associated with an individualized selection of classic, reversed, or combined approaches.
Methods
Between 1999–2014, 268 patients with rectal cancer and synchronous liver-only metastases underwent curative-intent multimodality therapy. Demographics, tumor and treatment details were reviewed. Survival outcomes were examined across treatment sequences and time periods (1999–2003, 2004–2008, and 2009–2014).
Results
150 (56.0%) patients underwent primary tumor resection first (“classic” approach); 44 (16.4%) patients, simultaneous resection of the primary and liver metastases (“combined”), and 74 (27.6%) patients, liver resection first (“reversed”). Patients who underwent the reversed approach had more liver metastases (3 [2–5]) at presentation (vs. 1 [1–2.5] in combined or 1 [1–3] in classic; p<0.001). Over time (from 1999–2003 to 2009–2014), both patients undergoing curative-intent treatment (62 to 122 patients) and the relative proportion undergoing reversed approach (6.4 to 37.7%) significantly increased. Despite higher disease burden, the 5-year overall survival (OS) was higher for patients treated in 2009–2014 vs. 1999–2003 (76% vs. 45%, p<0.002). 210 patients (78%) were rendered free of disease. 58 were not, due to disease progression or treatment complications, and their 5-year-OS was poor at 6%.
Conclusions
Individualized selection of treatment sequence based on the liver metastases and primary tumor disease burden allowed most patients to complete resection of all gross disease, and is associated with a 5-year OS approaching that for stage III rectal cancer in the most recent era.
INTRODUCTION
Between 15–25% of all colorectal cancer patients present with synchronous liver metastases.1 Such a presentation is thought to be associated with less favorable cancer biology and survival when compared with presenting with metachronous liver metastases.1,2 While the benefit of a coordinated multidisciplinary approach to these patients is well recognized, the optimal treatment sequencing to achieve best oncologic outcome remains an area of significant controversy.3 Among CRC patients with synchronous liver metastases, the subgroup with a primary cancer in the rectum is especially challenging: compared to colon cancer, most patients with stage IV rectal cancer will have a locally advanced primary cancer that is at risk for local complications may require pelvic radiation in addition to resection, and carries higher risks of anastomotic complications and of potentially delays in systemic therapy.4–6
Each patient with rectal cancer and synchronous liver metastases requires a personalized assessment of disease burden and treatment planning. Three common treatment approaches have been reported. The “classic” treatment sequence consists of resection of the rectal cancer, followed by resection of the liver metastases. A “combined” treatment sequence where pelvic surgery and hepatic resection are performed simultaneously can be safely pursued in select patients. The “reversed” treatment sequence approach as originally described by Mentha involved upfront systemic chemotherapy and resection of the liver metastases prior to resection of the primary tumor.7 Since 2000, chemotherapy regimens for metastatic CRC have significantly advanced, with high response rates (>50%) and long median survival (30 months) being observed today.8 Indeed, the progress in systemic therapy had increased the ability to convert patients to resectable metastatic disease, and had been concurrent with the development of the “reversed” approach to maximize the chances of resecting all gross disease in patients with very advanced liver metastatic disease.7–9
Determining the optimal treatment sequence for patients with specifically rectal cancer and synchronous liver metastases remains a clinical challenge. Over a 15-year period, we assess the adoption of “reversed” surgical approach and its impact, and we evaluate the evolution of treatment selection to achieve optimal outcomes.
METHODS
Patient cohort
After approval by the Institutional Review Board, a prospectively collected institutional database of patients who undergo surgical resection for CRC liver metastases was queried. We identified adult patients (>18 years old) who were diagnosed with synchronous liver metastases from rectal or rectosigmoid adenocarcinoma between 1999 and 2014. Synchronous liver metastases were defined as those identified within 90 days of the diagnosis of the primary tumor. Rectal or rectosigmoid adenocarcinomas that arose within 20 cm of the anal verge were included. Patients who were considered eligible to undergo curative-intent surgical treatment at their initial evaluation, with goal of resecting all gross disease from all sites, were included (N=300). No patient had extrahepatic disease. Patients who underwent 2-stage hepatectomy were excluded (N=32), leaving 268 patients in our study cohort. The institutional database and medical records, including records from outside institutions, were reviewed for demographics, tumor pathology, operative procedures, follow-up, patterns of recurrence, and survival.
At our institution, decisions regarding the treatment sequence for patients with rectal adenocarcinoma and liver metastases are made after multidisciplinary assessment. All liver resections were performed with the curative intent of resecting all gross disease. In this study, we defined three treatment sequences: classic (resection of rectal primary tumor first), combined (resection of rectal primary tumor and liver metastases in the same operation), and reversed (resection of liver metastases first).10 The number and size of the metastases identified on pretreatment imaging and prior to liver resection were recorded. Liver resection was categorized as either major hepatectomy (including ≥3 contiguous liver segments) or minor hepatectomy (including ≤2 contiguous liver segments). Resection margins were classified as R0 (microscopically negative) or R1 (microscopically positive), versus R2 (grossly positive).
Actuarial overall survival (OS) and disease-free survival (DFS) rates were calculated from the date of final resection, which was the date on which the patient was rendered free of gross disease for the patients who completed their entire intended treatment sequence.
Statistical analysis
Continuous variables were expressed with median and interquartile range; categorical variables were expressed as number and percentage. The entire study period was divided into 3 periods of equal length: 1999–2003, 2004–2008, and 2009–2014. Patients were compared by surgical treatment and by treatment period. Comparisons were analyzed with the chi-square test, the Mann-Whitney U test, and 1-way ANOVA, as appropriate. OS and DFS rates were calculated using the Kaplan-Meier method and compared using the log-rank test. P-values less than 0.05 was considered statistically significant.
A Cox proportional hazards model was constructed to identify independent variables for OS. The assumption of proportionality was tested by analysis of the Schoenfeld residuals; variance inflation factor calculations were used to evaluate for multicollinearity. Statistical analysis for the Cox regression model was performed using R (R; R Foundation for Statistical Computing, Vienna, Austria). All other statistical analyses were performed using SPSS version 17.2.
RESULTS
Pre-treatment characteristics of patients selected for different treatment sequences
Overall, 150 patients (56.0%) underwent classic, 44 patients (16.4%) underwent combined, and 74 patients (27.6%) underwent reversed treatment sequencing (Table 1). Patients were similar among the groups except those who were selected for the reversed sequence were more likely to have a primary tumor lower in the rectum (87.8% vs. 64.7% for classic and 79.5% for combined sequences; p<0.001). In addition, they had significantly more liver metastases at diagnosis (median 3 lesions, vs. 1 for classic and 1 for combined sequences; p<0.001), higher incidence of patients with bilobar liver metastases (56.8% vs. 38.7% for classic and 34.1% for combined sequences; p=0.016), and larger liver metastases at diagnosis (median 3.4 cm vs. 2.7 cm for classic and 2.2 cm for combined sequences; p<0.001; Table 1). Despite a higher proportion of symptoms among patients who underwent the “classic” treatment sequence (30.3%, vs. 18.2% for combined and 18.9% for reversed), the difference did not reach statistical significance (Table 1).
Table 1.
Clinicopathologic Characteristics of Patients with Rectal Cancer and Synchronous Liver Metastases by Selected Treatment Sequence
| Characteristic | All (n=268) | Classic (n=150) | Combined (n=44) | Reverse (n=74) | p Value |
|---|---|---|---|---|---|
| PRETREATMENT | |||||
| Age at diagnosis, median (IQR), years | 54.1 (45.1, 61.0) | 55.3 (46.3, 62.4) | 53.6 (44.5, 60.9) | 51.4 (43.3, 59.3) | 0.054 |
| Race/ethnicity | 0.10 | ||||
| White | 210 (79.5) | 117 (78.0) | 31 (72.1) | 62 (87.3) | |
| Black | 12 (4.5) | 5 (3.3) | 5 (11.6) | 2 (2.8) | |
| Hispanic | 25 (9.5) | 16 (10.7) | 5 (11.6) | 4 (5.6) | |
| Asian | 17 (6.4) | 12 (8.0) | 2 (4.7) | 3 (4.2) | |
| Other | 4 | 0 | 1 | 3 | |
| Female | 92 (34.3) | 45 (30.0) | 15 (34.1) | 32 (43.2) | 0.15 |
| Primary tumor location | <0.001 | ||||
| Rectosigmoid | 71 (26.5) | 53 (35.3) | 9 (20.5) | 9 (12.2) | |
| Rectum | 197 (73.5) | 97 (64.7) | 35 (79.5) | 65 (87.8) | |
| KRAS status | 0.21 | ||||
| Not tested | 169 | 101 | 27 | 41 | |
| Wild-type | 58 (58.6) | 33 (67.4) | 8 (47.1) | 17 (51.5) | |
| Mutant | 41 (41.4) | 16 (32.6) | 9 (52.9) | 16 (48.5) | |
| Number of liver lesions on CT at diagnosis, median (IQR) | 2 (1,3) | 1 (1,3) | 1 (1,2.25) | 3 (2, 5) | <0.001 |
| Distribution of liver lesions | |||||
| Unilobar | 153 (57.1) | 92 (61.3) | 29 (65.9) | 32 (43.2) | 0.016 |
| Bilobar | 115 (42.9) | 58 (38.7) | 15 (34.1) | 42 (56.8) | |
| Size of largest liver lesion on CT at diagnosis, median (IQR), cm | 2.8 (1.8, 4.8) | 2.7 (1.7, 4.5) | 2.2 (1.3, 2.9) | 3.4 (2.3, 5.7) | <0.001 |
| Rectal cancer symptom reported | |||||
| Absent | 67 (25.0) | 45 (30.3) | 8 (18.2) | 14 (18.9) | 0.10 |
| Present | 201 (75.0) | 105 (70.0) | 36 (81.8) | 60 (81.1) | |
| Clinical N staging | |||||
| Negative | 18 (12.2) | 7 (9.6) | 5 (15.2) | 6 (14.3) | 0.63 |
| Positive | 130 (87.7) | 66 (90.4) | 28 (84.8) | 36 (85.7) | |
| Unknown | 120 | 77 | 11 | 32 | |
| OPERATIVE AND POST-OPERATIVE | |||||
| Major hepatectomy | 149 (55.6) | 94 (62.7) | 5 (11.4) | 50 (67.6) | <0.001 |
| Portal vein embolization | 28 (10.5) | 15 (10.0) | 3 (6.8) | 10 (13.5) | 0.49 |
| Number of metastases resected by pathology report, median (IQR) | 1 (1,3) | 1 (1,3) | 1 (1,1) | 2 (1,3) | 0.0001 |
| Size of largest metastasis by pathology report, median (IQR), cm | 2 (1.2, 3.5) | 2.2 (1.3, 3.6) | 1.2 (0.5, 2) | 1.8 (1.1, 4) | 0.0018 |
| Liver metastases, pathologic response (% viable), median (IQR) | 60 (40, 90) | 50 (30, 85) | 60 (40, 94.5) | 75 (50, 95) | 0.039 |
| Condition of liver parenchyma at liver surgery | 0.37 | ||||
| Fibrosis | 1 (0.4) | 0 | 1 (2.3) | 0 | |
| Normal | 131 (48.9) | 73 (48.7) | 24 (54.5) | 34 (45.9) | |
| Sinusoidal obstruction syndrome | 20 (7.5) | 10 (6.7) | 4 (9.1) | 6 (8.1) | |
| Steatosis | 63 (23.5) | 41 (27.3) | 6 (13.6) | 16 (21.62) | |
| Steatohepatitis | 53 (19.8) | 26 (17.3) | 9 (20.5) | 18 (24.3) | |
CT, computed tomography; IQR, interquartile range.
Surgical details by treatment sequence
A significantly higher proportion of patients in the reversed group underwent major hepatectomy (67.6%) when compared to those in the classic (62.7%) or combined (11.4%) groups (p<0.001; Table 1). Portal vein embolization (PVE) hepatectomy was performed in 13.5% of patients with reversed approach. The number of liver metastases resected based on pathology reports was higher in the reversed group than in the other 2 groups (p=0.0001; Table 1). The size of largest liver metastasis resected based pathology reports was smaller in the combined group than in the other 2 groups (p=0.0018; Table 1). Degree of pathologic response was significantly greater in the reversed group than in the classic group (p=0.039). There was no difference among the three groups with respect to condition of the liver parenchyma (p=0.37; Table 1).
Of the 268 patients in the study, 152 (56.7%) underwent pelvic irradiation prior to resection of the rectal primary tumor, with 141 (92.8%) patients receiving long-course (50.4 Gy in 30 fractions) and 11 (7.2%) patients receiving short-course radiation therapy (25 Gy in 5 fractions). The procedures for resection of the rectal primary tumor included: low anterior resection (n=171), ultra-low anterior resection or coloanal anastomosis (n=27), and abdominal perineal resection (n=27). Two other patients had clinical complete response after chemoradiation and did not undergo any resection.
Evolution in the selection of treatment sequencing over time
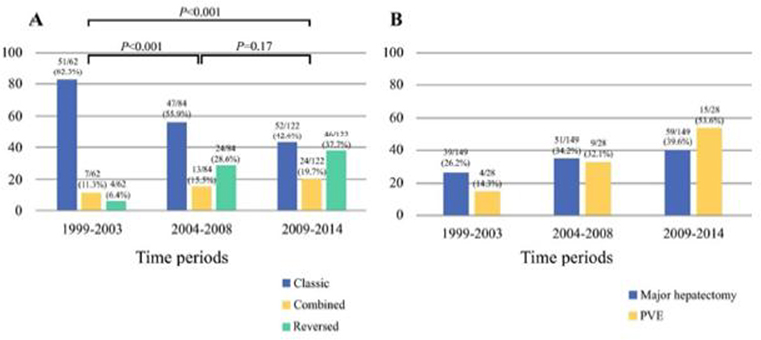
The total number of patients with rectal cancer and liver metastases undergoing resection considered eligible for complete resection of all disease with curative intent significantly increased over time (Table 2). The selection of the reversed treatment sequence also increased over time. There was a corresponding decrease over time in the selection of patients via the classic approach (Figure 1a). The patients deemed to have disease amendable to complete resection appeared to have more significant disease burden over time, with more number of metastases (Table 2). Reflective of the increased burden of liver disease, the more recent time periods accounted for increasing proportions of all patients undergoing major hepatectomy and PVE (Table 2; Figure 1b). For example, 39.6% of all major hepatectomies, and 53.6% of the PVEs were performed during 2009–2014 (p=0.01; Figure 1b).
Table 2.
Surgical Treatment for Patients by Treatment Period
| All (N=268) | 1999–2003 (N=62) | 2004–2008 (N=84) | 2009–2014 (N=122) | p | |
|---|---|---|---|---|---|
| Treatment Feature | |||||
| No. of liver metastases on CT at diagnosis, median (IQR) | 2 (1,3) | 1 (1,2) | 2 (1, 4) | 2 (1,3) | 0.028 |
| No. of liver metastases resected according to pathology report, median (IQR) | 1 (1,3) | 1 (1,3) | 2 (1,4) | 1 (1, 2) | 0.064 |
| Size of largest liver metastasis on CT at diagnosis, median (IQR), cm | 2.8 (1.8, 4.8) | 4.0 (2.3, 6.6) | 2.3 (1.6, 3.5) | 2.7 (1.8, 6.4) | 0.003 |
| Size of largest liver metastasis according to pathology report, median (IQR), cm | 2 (1.2, 3.5) | 2.8 (1.5, 5) | 1.8 (1.2, 3.5) | 1.7 (1.0, 2.7) | 0.006 |
| Major hepatectomy | 149 | 39 (26.2) | 51 (34.2) | 59 (39.6) | 0.089 |
| PVE | 28 | 4 (14.3) | 9 (32.1) | 15 (53.6) | 0.44 |
IQR, interquartile range; PVE, portal vein embolization
Figure 1. Individual selection of treatment sequences over time.
The armamendarium of treatment sequence options has expanded over time to increasingly include the utilization of the “reversed” treatment sequence approach (a). This was associated with an increase in major hepatectomy and resection of significant liver disease burden, including 2-stage hepatectomy with portal vein embolization (b).
Survival outcomes associated with individualized treatment sequence selection
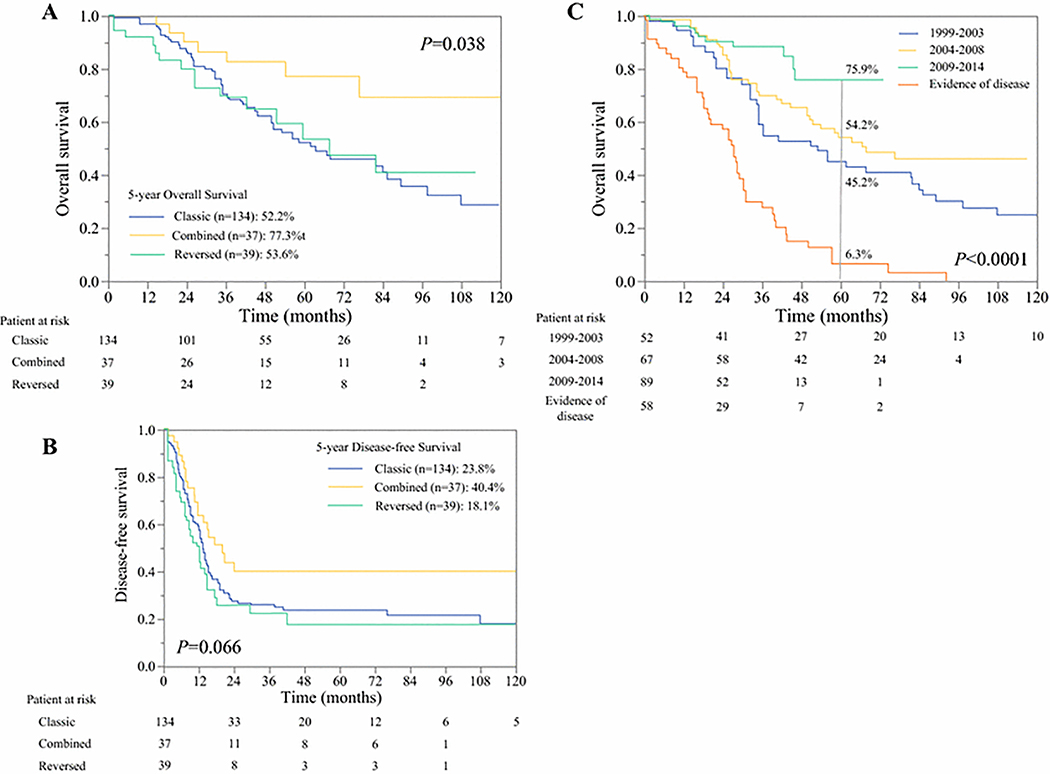
After a median follow-up of 44.1 months, 210 patients (78%) had completed all intended surgical treatment. The OS and DFS of these patients according to treatment sequence are shown in Figure 2a,b. Five-year survival rates were highest in patients selected to undergo the combined approach (OS: 77%, p=0.038; DFS: 40%, p=0.06). Five-year survival rates did not differ significantly between the selected groups that completed the classic and the reversed approaches (OS: 52% and 54%, respectively; DFS: 24% and 18%, respectively).
Figure 2.
(a) Overall survival among patients rendered disease-free according to their selected treatment sequence of “Classic”, “Reversed”, or “Combined” approaches. Good risk patients undergoing a “Combined” approach had the best survival of 75%. “Reversed” group achieved comparable outcomes to “Classic” despite a significantly higher metastatic tumor burden. (Combined = thick solid line; Reversed = thin dotted line; Classic = thick dotted line)
(b) Disease-free survival among patients rendered disease-free according to their selected treatment sequence. (Combined = thick solid line; Reversed = thin dotted line; Classic = thick dotted line)
(c) Overall survival by treatment period. Outcomes of 210 patients rendered disease free in different treatment period (1999–2003 = thick dotted line; 2003–2008=solid line; 2009–2014= thin dotted line), with reference to the outcome of 58 patients who were not rendered disease free.
Higher number of liver metastasis prior to resection (Hazard ratio [HR]: 1.1; 95% confidence interval [CI], 1.03–1.27; p=0.01), and larger the size of the maximal liver metastasis (HR: 1.1; 95% CI, 1.03–1.27; p=0.02) were independent variable influencing OS. No association was observed for the type of liver resection (major versus minor; p=0.65) or for the selected treatment sequence (classic, combined, versus reversed; p=0.81) among patients who completed resection of all gross disease.
Indeed, among the 210 patients, OS improved significantly over time (p=0.007; Figure 2b). The 5-year OS rate was 45% for patients treated during 1999–2003 and 76% for those treated during 2009–2014.
A total of 58 patients (22%) did not complete resection of all gross disease. The 5-year OS rate of these patients was only 6.3% (Figure 2b). The most common reasons for lack of completion of all planned surgery and failure to render No-Evidence-of-Disease status was disease progression (n=39, 45.3%; n=29: liver progression; n=10: multiple distant sites). Other reasons were postoperative complications precluding completion of the entire treatment sequence (n=5, 20%), complete response at primary site (n=2, 4%), and other reason (n=11, 31%). Progression of the rectal primary tumor was never a cause for lack of surgical completion.
DISCUSSION
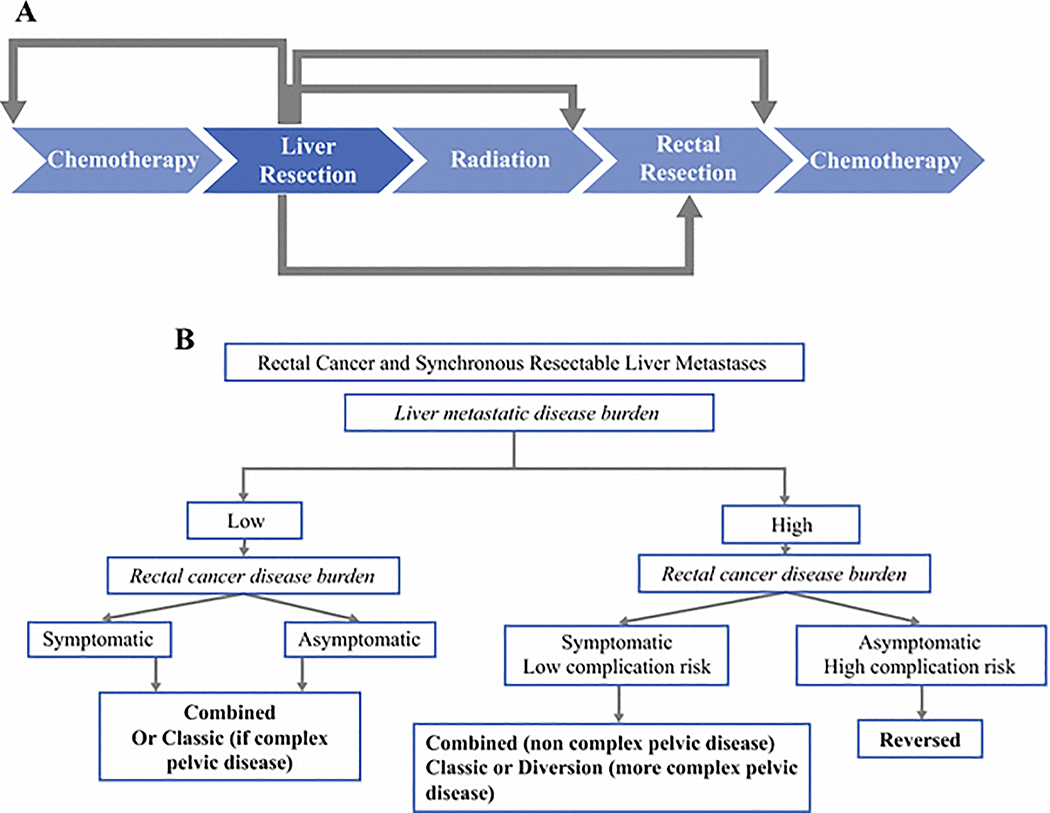
Over a 15-year period, we identified an increasing number of patients with rectal cancer and synchronous liver metastases whose disease had been considered amendable to surgical resection with curative intent, despite a trend toward a greater metastatic burden in the liver. With an expanded armamentarium of treatment sequencing options (increasingly to include the “reversed” sequence in addition to “classic” and “combined”) we were able to optimize oncologic outcomes over time through an individualized approach where treatment sequencing was tailored to the specific patient and his/her metastatic and primary disease burden (Figure 3a). In the most recent years, patients have enjoyed an estimated 5-year survival rate that approaches that of patients with stage III rectal cancer.11
Figure 3.
(a) Possible permutations of treatment interventions for patients with resectable synchronous liver metastasis from rectal cancer.
(b) Individualized treatment algorithm for patients with resectable synchronous liver metastasis from rectal cancer depending on liver disease burden and symptomatology form primary.
After a median follow-up of 44.1 months, our observed 5-year OS rate of 45% (1999–2003) rose to 76% (2009–2014) and compares favorably to outcomes previously reported in the literature for rectal cancer patients with synchronous liver metastases. Among 53 patients treated between 2004–2012, Gall et al reported 5-year OS of 39% after a median follow-up of 39 months.12 Similarly, Boostrom et al reported a 5-year OS of 32% among 45 patients treated between 1991–2005.13 At our institution, we have systematically adopted an individualized approach to treatment sequence selection (Figure 3b). With accumulated experience in patients who present with significantly advanced metastatic burden and locally advanced rectal cancer that may require pelvic-directed neoadjuvant chemoradiation and/or two-stage procedures, “reversed” sequence was found to be useful in rendering these patients free of all visible disease. For patients whose metastatic liver disease demonstrates major response (type I) to systemic chemotherapy,14 addressing metastatic liver disease first allows timely capture of a window for aggressive surgical resection.15–18 After the metastatic disease is under control, full attention can be devoted to the primary rectal cancer in a unhurried fashion. Pelvic radiotherapy, when needed, could be administered; temporary diversion and two-stage procedures could be planned; and potential morbidities such as anastomotic leak that may threaten completion of the entire treatment sequence could be minimized.19–21 However, when patients present with pelvic symptoms attributable to rectal primary, we would favor the “combined” or “classic” treatment sequence, depending on the magnitude of the anticipated morbidity from the planned primary rectal and liver resections.
Our individualized selection of treatment approach was associated with a 78% rate of completing resection of all gross disease. The importance of adopting the optimal treatment sequence that will maximize the chances of rendering patients free of all disease was underscored by a poor 5-year OS rate of only 6% for patients who failed to be rendered free of disease. Among the patients who did not complete surgery, failure was most commonly (45%) due to progression of the liver metastases or systemic disease outside the liver. Thus, disease biology and identifying patients who are optimal candidates for aggressive surgical resection is critical.10 Secondly, another 20% of the patients failed complete resection of all gross disease because of operative complications associated with one component along the treatment sequence. Further, we have shown previously that even when patients undergoing resection for colorectal liver metastasis, a dose-response association exists between complications and oncologic outcome.22 Therefore, minimizing operative morbidity is another important goal for careful treatment planning. For example, patients with very limited liver disease burden were stratified to the combined approach. Indeed, this group enjoyed the most favorable 5-year OS among all groups, while also benefiting from having been spared the morbidity and added cost of staged operations.13,23,24 On the other hand, the “reversed” approach allowed completion of complex liver resections, without risking morbidity from rectal surgery. Finally, primary progression to unresectabilty was not found in our cohort to be a reason for failure to resect all gross disease, reflecting that utilizing the classic approach when rectal cancers present with obstruction or are borderline unresectable, is appropriate.
While, the present study is, to our knowledge, the largest single-institutional series of patients with synchronous liver metastases and rectal cancer treated by a variety of but individually selected treatment sequences, it has several limitations. This is a retrospective study and the reported outcomes must be interpreted as a reflection of surgical decision-making and careful selection. They should not be interpreted to reflect superiority of one treatment sequence over another among an unselected patient population. Further, our findings reflect the tertiary referral nature of our real-life clinical practice. Some of the patients treated with the classic approach had their primary tumors addressed prior to referral to our institution for resection of the liver disease, and in many cases, it was not possible to retrospectively determine whether the patients indeed had impending complications from the rectal cancer at initial presentation.
In conclusion, we identified evolution in the individualized selection of curative-intent treatment sequencing among patients with liver metastatic disease and rectal cancer, with increasing incorporation of the “reversed” approach into the armamentarium of treatment sequence options over time. Despite significant metastatic disease burden and locally advanced primary rectal cancer, the selection of treatment sequence can be tailored to the individual’s respective disease burdens and to the goal of minimizing operative morbidities. This approach was associated with a high rate of successfully rendering patients free of gross disease, and with excellent 5-year oncologic outcomes.
Synopsis.
This cohort study highlights the outcomes associated with individualized treatment sequence selection based on metastatic and primary disease burden in 268 patients with rectal cancer and synchronous liver metastases. Clinical characteristics, surgical details, and survival outcomes over time were analyzed.
Acknowledgments
Funding: None. The authors have no financial or proprietary interest in the subject matter or materials discussed in the article.
REFERENCES
- 1.Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg August 2006;244(2):254–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conrad C, You N, Vauthey JN. In patients with colorectal liver metastases, can we still rely on number to define treatment and outcome? Oncology (Williston Park). November 2013;27(11):1078, 1083–1074, 1086. [PubMed] [Google Scholar]
- 3.Butte JM, Gonen M, Ding P, et al. Patterns of failure in patients with early onset (synchronous) resectable liver metastases from rectal cancer. Cancer. November 1 2012;118(21):5414–5423. [DOI] [PubMed] [Google Scholar]
- 4.Merkow RP, Bentrem DJ, Cohen ME, et al. Effect of cancer surgery complexity on short-term outcomes, risk predictions, and hospital comparisons. J Am Coll Surg. October 2013;217(4):685–693. [DOI] [PubMed] [Google Scholar]
- 5.Farid SG, Aldouri A, Morris-Stiff G, et al. Correlation between postoperative infective complications and long-term outcomes after hepatic resection for colorectal liver metastasis. Ann Surg January 2010;251(1):91–100. [DOI] [PubMed] [Google Scholar]
- 6.Adam R, de Gramont A, Figueras J, et al. Managing synchronous liver metastases from colorectal cancer: a multidisciplinary international consensus. Cancer Treat Rev November 2015;41(9):729–741. [DOI] [PubMed] [Google Scholar]
- 7.Mentha G, Majno PE, Andres A, Rubbia-Brandt L, Morel P, Roth AD. Neoadjuvant chemotherapy and resection of advanced synchronous liver metastases before treatment of the colorectal primary. Br J Surg July 2006;93(7):872–878. [DOI] [PubMed] [Google Scholar]
- 8.Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol July 5 2016. [DOI] [PubMed] [Google Scholar]
- 9.Mentha G, Majno P, Terraz S, et al. Treatment strategies for the management of advanced colorectal liver metastases detected synchronously with the primary tumour. Eur J Surg Oncol December 2007;33 Suppl 2:S76–83. [DOI] [PubMed] [Google Scholar]
- 10.You YN, Eng C, Aloia T. Multidisciplinary management of stage IV colon cancer. Seminars in Colon and Rectal Surgery. 2016;27(4):213–218. [Google Scholar]
- 11.Rodel C, Graeven U, Fietkau R, et al. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol August 2015;16(8):979–989. [DOI] [PubMed] [Google Scholar]
- 12.Gall TM, Basyouny M, Frampton AE, et al. Neoadjuvant chemotherapy and primary-first approach for rectal cancer with synchronous liver metastases. Colorectal Dis June 2014;16(6):O197–205. [DOI] [PubMed] [Google Scholar]
- 13.Boostrom SY, Vassiliki LT, Nagorney DM, et al. Synchronous rectal and hepatic resection of rectal metastatic disease. J Gastrointest Surg. September 2011;15(9):1583–1588. [DOI] [PubMed] [Google Scholar]
- 14.Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. March 22 2008;371(9617):1007–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chun YS, Vauthey JN, Boonsirikamchai P, et al. Association of computed tomography morphologic criteria with pathologic response and survival in patients treated with bevacizumab for colorectal liver metastases. JAMA December 2 2009;302(21):2338–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shindoh J, Chun YS, Loyer EM, Vauthey JN. Non-size-based response criteria to preoperative chemotherapy in patients with colorectal liver metastases: the morphologic response criteria. Curr Colorectal Cancer Rep. June 1 2013;9(2):198–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shindoh J, Loyer EM, Kopetz S, et al. Optimal morphologic response to preoperative chemotherapy: an alternate outcome end point before resection of hepatic colorectal metastases. J Clin Oncol. December 20 2012;30(36):4566–4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ayez N, Burger JW, van der Pool AE, et al. Long-term results of the “liver first” approach in patients with locally advanced rectal cancer and synchronous liver metastases. Dis Colon Rectum. March 2013;56(3):281–287. [DOI] [PubMed] [Google Scholar]
- 19.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. October 21 2004;351(17):1731–1740. [DOI] [PubMed] [Google Scholar]
- 20.Tzeng CW, Aloia TA, Vauthey JN, et al. Morbidity of staged proctectomy after hepatectomy for colorectal cancer: a matched case-control analysis. Ann Surg Oncol. February 2013;20(2):482–490. [DOI] [PubMed] [Google Scholar]
- 21.Smith JD, Butte JM, Weiser MR, et al. Anastomotic leak following low anterior resection in stage IV rectal cancer is associated with poor survival. Ann Surg Oncol. August 2013;20(8):2641–2646. [DOI] [PubMed] [Google Scholar]
- 22.Yamashita S, Sheth RA, Niekamp AS, et al. Comprehensive Complication Index Predicts Cancer-specific Survival After Resection of Colorectal Metastases Independent of RAS Mutational Status. Ann Surg October 04 2016. [DOI] [PubMed] [Google Scholar]
- 23.Silberhumer GR, Paty PB, Temple LK, et al. Simultaneous resection for rectal cancer with synchronous liver metastasis is a safe procedure. Am J Surg. June 2015;209(6):935–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abbott DE, Cantor SB, Hu CY, et al. Optimizing clinical and economic outcomes of surgical therapy for patients with colorectal cancer and synchronous liver metastases. J Am Coll Surg August 2012;215(2):262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]