Abstract
Zoonotic animal tuberculosis (TB) is a One Health paradigm infectious disease, caused by Mycobacterium tuberculosis complex bacteria, that affects different host species with varying levels of management. In most developed countries, official surveillance and control strategies support the longitudinal reporting of herd and/or animal prevalence. However, for under resourced countries without surveillance plans, this information may be obtained from cross-sectional studies only. The objective of this meta-analysis was to perform a worldwide estimate of the overall prevalence of animal TB in different livestock species whose importance in production systems varies according to the region of the world. The ISI's Web of Science and Google Scholar were searched combining keywords and related database-specific subject terms to identify relevant cohort or cross-sectional work published in this topic. A total of 443 articles were retrieved, screened, and a final set of 182 references included. Potential sources of variation were investigated using subgroup analyses and meta-regression. Prevalence estimates in five mammalian host groups were stratified according to host species, host characteristics, anatomical localization of lesions, sample size, geographical location, and diagnostic tests. The multivariable meta-regression analysis accounted for a range between 0% (farmed wild boar) and 68.71% (camelids) of the overall observed heterogeneity, indicating that the pondered predictors partially explain the observed variability. Differences in the overall prevalence of TB across hosts were small, with most groups showing values around 10%, except farmed wild boar (41%). The sample size emerged as an important moderator, with small size studies leading to the overestimation of prevalence. TB prevalence rates were very heterogeneous across continents and depended on the host, with lower values (below 10%) in Africa and Asia, while North America (33.6%, cattle), Europe (51%, goats), and South America (85.7%, pigs) exhibited higher rates, possibly related to greater densities of specific host groups managed on more intensive production systems. Stratification by diagnostic tests evidenced heterogeneous prevalence rates depending on the host group, possibly reflecting differences in test performance across different hosts. Results from this study highlight different TB burden scenarios, pinpointing host groups and diagnostics that should be prioritized in surveillance systems in different regions, thus providing policy-relevant information to catalyse TB control in settings with lower installed capacity and better resource allocation at the human-animal-environment interface.
Keywords: Animal tuberculosis, Livestock, One health, Meta-analysis, Animal-human-environment interface
1. Introduction
Animal tuberculosis (TB) is a zoonotic chronic disease that impacts the productivity of livestock systems, limits animal movement and trade, and threatens animal and public health. Among the ecotypes grouped within the Mycobacterium tuberculosis complex (MTC), M. bovis and M. caprae are recognized as the most relevant TB-causing agents in livestock [1,2]. The disease is characterized by the formation of granulomatous lesions, localized in lymph nodes, and/or several internal organs, mainly affecting the respiratory, digestive and excretory systems. The anatomic localization of lesions is commonly associated with infection transmission routes [[3], [4], [5]].
This disease affects several hosts, namely humans, farmed animals and free-ranging wildlife hosts across all continents, with the exception of Antarctica [[7], [8], [9], [10]]. Many other species besides cattle are managed in more or less intensive production systems and these are seldom under the focus of official surveillance and control plans. So, TB data in livestock species regarded as non-prototypical hosts, such as goats, sheep, or pigs, or animals regarded as wildlife but which are highly managed and maintained in confined or highly humanized environments for economical purposes, such as tourist activities (e.g. dromedaries), transportation of humans and goods (e.g. llamas), game farms, zoological parks, and others, is heterogeneously available and reported on a regional, national or global level.
Animal TB surveillance systems implemented in developed countries have supported disease eradication in the cattle population or contributed to lower individual and herd prevalence. However, the inherent high costs and technical demands for the implementation and maintenance at different levels are obstacles to the systematic application of eradication programs in developing countries [11]. Generally, these programs are based on test and slaughter schemes, with the detection of reactors and abattoir surveillance [12]. Therefore, the detection of infected animals is crucial for the effectiveness of control actions. Testing of live animals is performed by the intradermal tuberculin skin test, in single or comparative versions, and the interferon-gamma (IFN-γ) assay that is most commonly used as a complementary blood test [12,13]. The performance of both tests can be influenced by host intrinsic factors. Standard application conditions and result interpretation are normally described for cattle, requiring optimization when transposed to other species [14,15]. The post mortem evaluation by gross pathology, histopathology and bacteriological techniques are the reference diagnostic methods for animal TB [13].
Animal TB is included in the list of mandatory notifiable diseases of the World Organization of Animal Health (OIE). While the application of systematic surveillance and control programs enables notification and reporting of official prevalence values [12,17,18], countries without an official surveillance strategy have non-systematic, irregular, and geographically heterogeneous disease information (if any) provided by a few cross-sectional studies with ad-hoc diagnostic strategies [19].
To suppress knowledge limitations on the prevalence of animal TB in cattle and other affected host species worldwide and to generate a robust evidence basis for decision-making on community control and resource allocation, a systematic literature search and meta-analysis was performed in this work. The objective was to estimate, on a worldwide scale, the overall prevalence of animal TB in different livestock host species whose importance in production systems varies according to the region of the world. For this purpose, relevant information from cohort or cross-sectional works published in this topic for the last decades were dissected by means of a meta-analysis approach. The potential sources of prevalence variation were investigated using subgroup analyses and meta-regression. Prevalence figures were stratified according to host species, host characteristics, anatomical localization of lesions, sample size, geographical location, and diagnostic tests. Systematization of this knowledge may inform policy decisions and hierarchize field priorities to control this long-lasting disease at the animal-human-environment interface.
2. Methodology
2.1. Search strategy
A meta-analysis was conducted following the procedures provided by the PRISMA guidelines (Supplementary Fig. 1) [20]. Search for peer-reviewed literature was conducted through the ISI's Web of Science online interface (http://www.isiknowledge.com) and Google Scholar, and was last updated on 15th February 2020.
A complex search strategy was implemented using the interception of terms in three groups (#1 AND #2 AND #3), being group #1 “tuberculosis”; group #2 “epidemiology” OR “transmission”; and group #3 “Livestock” OR “Farmed” OR “Cattle” OR “Sheep” OR “Ovine” OR “Goat” OR “Pig” OR “Equine” OR “Camel” OR “Dromedary” OR “Alpaca” OR “Llama”. Related database-specific subject terms to identify relevant cohort or cross-sectional studies including species maintained in confined environments with human intervention, such as farmed game species, zoo animals, and animals used for tourist activities, were also used in the search scheme under the expression “farmed”.
No time or geographical location restrictions were placed on these searches, only those published in English and Portuguese were retrieved, and the reports performed by national or international authorities concerning official data submitted by each country were not considered.
2.2. Data extraction and selection criteria
All articles retrieved from the global search (n = 1443) were screened, based on title and abstract, and the manuscripts that did not contain information concerning animal TB epidemiology in livestock were eliminated (Supplementary Fig. 1). A group with a total of 443 articles remained based on inclusion criteria, and a systematic bibliometric review considering temporal and spatial disease evolution, host, infectious agent, and progress in research fields in animal TB has been published elsewhere [21]. After a thorough screening, a group of 182 articles followed to meta-analysis (Supplementary Table 1). The inclusion criteria were related with the explicit or implicit indication of animal TB prevalence figures associated with a given host, obtained by any diagnostic test, and with a sample size equal or superior to 30 individuals. Data was extracted considering author, publication year, study design (geographic location by continent, mammalian host), sample size, and diagnostic test used. Diagnostic tests included in the study were the delayed hypersensitivity test (namely, the tuberculin test), blood-based laboratory tests (namely, the IFN-γ assay and the Enzyme-Linked Immunosorbent Assay [ELISA]), histopathological examination (considering both macroscopic and microscopic lesions), culture, nucleic acid-based methods, and biochemical-based methods (biochemical characterization of isolates). The selected works were published between 1993 and 2020 (27 years) and encompassed eight host groups, namely cattle (number of articles = 151), goat (n = 12), pig (n = 12), buffalo (n = 11), camelids (n = 9; camel [n = 5], dromedary [n = 3], alpaca [n = 1]), sheep (n = 7), farmed cervids (n = 5; red deer [n = 3], fallow deer [n = 1], elk [n = 1]) and farmed wild boar (n = 4).
The discriminated animal TB prevalence values considered were grouped as follows: 1) at the whole host population level, summing the values obtained by different diagnostic tests; 2) clustered by sex, age class (cubs, sub-adults and adults), body condition, and/or breed; or 3) anatomical location of lesions according to infected tract (respiratory tract, digestive tract, or generalized lesions present in at least two different tracts) (Supplementary Table 2).
2.3. Statistical analyses
The “meta” and “metafor” libraries in R statistical package were used to estimate models for different hosts [[22], [23], [24]]. All analyses were performed using RStudio [25].
The estimated prevalence values from each study were logit transformed and the pooled prevalence was estimated using a random-effects model. Two model statistics were obtained, the Cochran's Q statistic [26] to test for heterogeneity and Higgin's statistic (I2 > 50% represents at least moderate heterogeneity) [27] to quantify the proportion of true variation due to heterogeneity between studies.
A univariable meta-regression model, with random-effects, was applied to select a group of moderator factors for multivariate analyses and to determine which percentage of heterogeneity is accounted for by each moderator. The accounted moderators were: geographic location by continent, publication period, sample size, diagnostic test, age class, sex, breed, body condition and anatomical location of lesions. Depending on the mammalian host group, some moderators were not included in the model due to the lack of available information. To examine the effects of geographic location, the study areas of articles were grouped by continents and potential sources of variation were investigated using subgroup analyses; and to evaluate the effect of time, three time periods were considered: 1990–1999, 2000–2010, and 2011–2020 (Supplementary Table 2). Multivariate meta-regression was performed to evaluate the percentage of heterogeneity accounted for by the full set of moderators. Analysis of data normality with the Q-Q plot was performed after meta-regression.
The potential evidence of publication bias was assessed by visual inspection of a funnel plot asymmetry, and Begg's rank correlation [28] and the Egger's weighted regression [29] methods were used to assess the significance level of the underlying bias (p-value <0.05).
The moderator significance within the full model was obtained with an analysis of variance (ANOVA), and variables with p-value <0.25, as previously set in other similar reviews and meta-analyses [19,30,31], were retained for inclusion in the final model.
3. Results and discussion
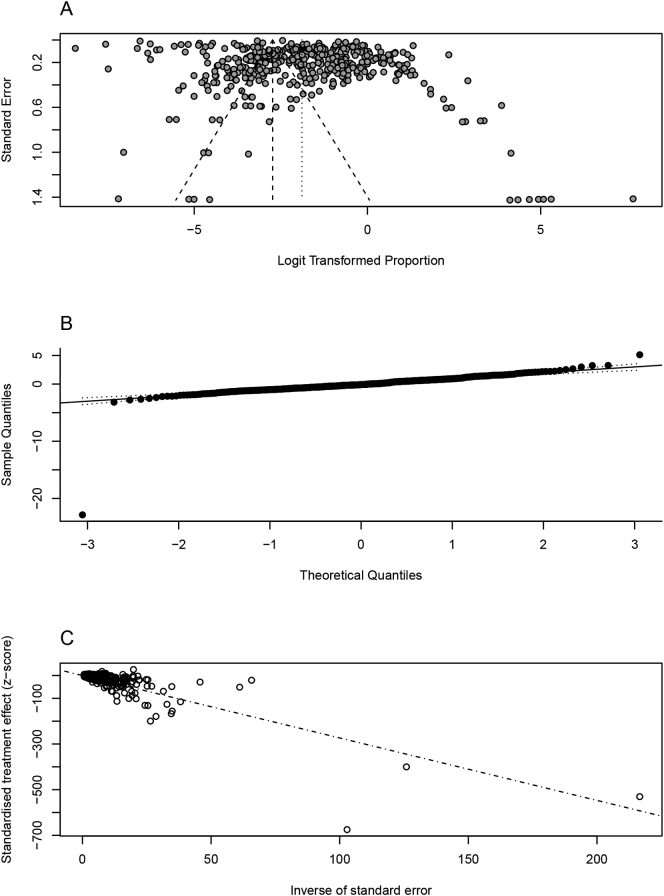
A total of 443 articles covering the last four decades was screened and 182 were included in this meta-analysis (Supplementary Table 1). All models were assessed for publication bias regarding sample size, occurrence and quantification of true heterogeneity, and data normality after logit transformation. Publication bias was not calculated in both farmed cervids and wild boar, due to the low amount of available data (n < 10 articles). From the remaining host groups, publication bias was only found in sheep data (p-value <0.05) and cattle data (p-value <0.001), towards lower and higher sample size studies, respectively (Fig. 1A; Supplementary Figs. 2–8). All models registered the existence of heterogeneity (Q value with p-value <0.001), with the proportion of true variation due to heterogeneity between studies (I2) ranging from 85.4% (farmed cervids) to 99.9% (cattle). Thus, all models show higher proportions of heterogeneity due to true differences in effect between studies and not by sampling errors. Data did not present a cleat fit to normality in the Q-Q plot for pig, goat, and sheep data, but presented a good fit to the Q-Q plot for the remaining host groups (Fig. 1B; Supplementary Figs. 2–8).
Fig. 1.
Animal tuberculosis prevalence in cattle: parameters of asymmetry and heterogeneity. A – Funnel plot of logit transformed prevalence and standard error; B - Q-Q norm plot for normality assessment; C –Galbraith plot: assessment of heterogeneity in the retrieved studies focusing the epidemiology of animal tuberculosis in cattle (scatter plot of standardized effect estimates against inverse standard error).
3.1. Bovinae
Using a random-effects model, the pooled prevalence of worldwide animal TB in cattle was estimated as 13.12% (95% Cl: 11.24% - 15.26%) (Supplementary Fig. 9). Galbraith plot enabled graphical assessment of heterogeneity across studies in cattle as most data fits outside the confidence bounds, indicating a higher contribution to heterogeneity (Fig. 1C).
Univariable meta-regression analysis of the nine moderators pondered as indicated in methods was performed. The proportion of each predictor variable's effect on heterogeneity (R2) ranged from 0% to 29.81% (Table 1; Supplementary Table 3). Further, the highest values of R2 were observed for sample size (29.81%), diagnostic test (6.69%), geographic location at the continental scale (4.71%), and anatomical localization of lesions (infected tract; 4.62%); the lowest values were observed for breed (1.67%), sex (1.46%), publication period (1.26%), and age class (0.84%) variables, while body condition exhibited no effect on heterogeneity (R2 = 0%) (Table 1; Supplementary Table 3).
Table 1.
Heterogeneity accounted by each moderator and overall heterogeneity calculated by host or host groups.
| Continent | Publication period | Diagnostic test | Sample size | Breed | Infected tract | Age class | Sex | Body condition | Overall | |
|---|---|---|---|---|---|---|---|---|---|---|
| Cattle | 4.71* | 1.26 | 6.69* | 29.81* | 1.67 | 4.62* | 0.84 | 1.46* | 0.00 | 34.65 |
| Buffalo | 37.17 | 0.00 | 4.63 | 61.32 | NA | NA | NA | NA | NA | 34.92 |
| Farmed cervids | 0.00* | 0.00 | 0.00* | 15.67* | NA | NA | NA | NA | NA | 18.97 |
| Camelids | 0.00 | 0.00 | 13.69* | 61.59* | NA | 0.00 | 0.00 | 0.00 | 0.00 | 68.71 |
| Goat | 23.99* | 6.52* | 0.00 | 47.5* | 12.07 | 17.48* | 0.00* | 0.00* | NA | 48.86 |
| Sheep | 4.31 | 21.18 | 0.00 | 42.57* | NA | NA | NA | NA | NA | 20.36 |
| Pig | 36.42* | 14.24* | 4.25 | 50.09* | NA | 0.00* | NA | NA | NA | 53.53 |
| Farmed wild boar | 0.00 | 0.00 | 0.00 | 0.00 | NA | NA | NA | NA | NA | 0.00 |
⁎ - ANOVA p-values <0.25; NA – non-applicable.
All nine moderators were also included in multivariable meta-regression analysis, accounting for 34.65% of the observed heterogeneity, meaning that these explain only a third of the variability observed. Analysis of variance (ANOVA) test indicated that five of the nine moderators were significant (p-value <0.25) (Table 1; Supplementary Table 3): geographic location by continent, diagnostic test, sample size, anatomical localization of lesions and gender.
The estimated stratified disease prevalence using significant moderators was then calculated. Assessing geographical location by continent, North America (33.6%) showed a significantly higher animal TB prevalence than Africa (10.3%) and Asia (13.8%) (Table 2). Cattle are widespread across continents, with the highest densities being registered in Asia (particularly, India), East Africa (particularly, Ethiopia), Northern Europe, North America (particularly, Mexico) and South America [32,33]. The differences in the disease prevalence estimates between North America (developed countries) and Asia and Africa (higher proportion of developing countries) might be influenced by the differential survey efforts and the lack of animal tuberculosis control programs in developing countries. Regarding diagnostic tests, the prevalence of animal TB estimated through biochemical- (39.1%), IFN-γ - (34.4%), and culture-based methods (26.6%) registered significantly higher values than histopathology (11.2%), tuberculin- (9.2%) or ELISA-based methods (6.8%) (Table 2). Moreover, studies reporting TB prevalence based on ELISA showed significantly lower rates compared to studies using any other diagnostic methods, except for tuberculin-based. These results can be explained by the different number of studies reporting prevalence rates using each test and because the sensitivity and specificity of each test are highly variable, with tuberculin-based tests usually reporting high sensitivity and moderate specificity, IFN-γ assays reporting moderate sensitivity and high specificity, and ELISA-based tests reporting low sensitivity and higher specificity [34,35]. Furthermore, studies with less than 100 sampled individuals (39.5%) showed significantly higher prevalence rates, followed by studies that sample between 100 and 200 individuals (25.3%), with all the remaining sample size categories showing lower prevalence results (Table 2). The bias in disease prevalence estimates can arise from different sample size studies, with lower sampling studies showing a bias towards higher disease prevalence. Respiratory tract lesions (47.8%) tend to be present at a higher prevalence, followed by digestive tract lesions (32.5%), and finally by generalized lesions (16.7%) (Table 2). These results were expected since aerial transmission is the most frequent infection route in cattle, leading to lesions in the respiratory tract and associated lymph nodes, followed by oral transmission through the ingestion of bacilli, leading to lesions in the digestive tract and associated lymph nodes; generalized, systemic infection is associated with terminal disease, a rare situation due to the periodical testing of cattle in vivo that diminishes disseminated TB cases [6,36]. The prevalence rates tend to be higher in female (8.7%) than male cattle (4.8%), which might be related with differences in the production systems (Table 2). Females are more associated with milk farms and males with beef farms. Some works associate milk farms with more intensive regimes and longer animal lifetime history, factors that could contribute to higher TB prevalence.
Table 2.
Discriminated prevalence of animal TB by host and moderator. Values in brackets represent CI 95%.
| Moderators | Cattle | Farmed cervids | Camelids | Goats | Sheep | Pigs | |
|---|---|---|---|---|---|---|---|
| Continent | Africa | 10.3% [8.8–25.9%] | NA | NS | 1.1% [0.3–3.6%] | NS | 0.9% [0.1–9.6%] |
| Asia | 13.8% [8.5–11.9%] | NA | NS | 1.9% [1.5–2.5%] | NS | 1.3% [0.8–2.0%] | |
| Europe | 17.8% [11.8–25.9%] | 10.3% [5.4–18.9%] | NS | 51.0% [16.5–84.6%] | NS | 24.1% [12.4–41.6%] | |
| North America | 33.6% [25.2–43.2%] | 7.9% [4.4–14.0%] | NS | NA | NS | NA | |
| South America | 20.5% [6.6–48.4%] | NA | NS | NA | NS | 85.7% [73.8–92.7%] | |
| Publication period | 1990–1999 | NS | NS | NS | 99.6% [93.8–100.0%] | NS | NA |
| 2000–2010 | NS | NS | NS | 7.0% [0.7–46.6%] | NS | 1.1% [0.1–19.1%] | |
| 2011–2020 | NS | NS | NS | 5.3% [2.1–12.7%] | NS | 20.9% [10.2–38.3%] | |
| Diagnostic test | Tuberculin | 9.2% [7.6–11.0%] | NS | 3.4% [1.8–6.6%] | NS | NS | NS |
| IFN-γ | 34.4% [18.0–55.4%] | NS | NA | NS | NS | NS | |
| ELISA | 6.8% [5.4–8.4%] | NS | NA | NS | NS | NS | |
| Histopathology | 11.2% [8.6–14.6%] | 9.3% [6.2–13.9%] | 16.1% [9.9–25.0%] | NS | NS | NS | |
| Culture | 26.6% [21.0–33.0%] | 7.4% [2.8–18.1%] | 46.6% [22.7–72.2%] | NS | NS | NS | |
| Nucleic acid | 24.7% [14.2–39.4%] | NS | 6.5% [1.6–22.4%] | NS | NS | NS | |
| Biochemical | 39.1% [29.9–49.1%] | NS | NA | NS | NS | NS | |
| Sample size (Cattle) | <100 | 39.5% [34.0–45.2%] | NA | NA | NA | NA | NA |
| 100–250 | 25.3% [20.7–30.5%] | NA | NA | NA | NA | NA | |
| 250–500 | 13.6% [10.4–17.5%] | NA | NA | NA | NA | NA | |
| 500–1000 | 6.1% [4.7–7.8%] | NA | NA | NA | NA | NA | |
| 1000–1500 | 7.8% [5.7–10.6%] | NA | NA | NA | NA | NA | |
| >1500 | 3.9% [2.7–5.7%] | NA | NA | NA | NA | NA | |
| Sample size (Others) | <100 | NA | 14.2% [4.3–38.0%] | 26.7% [18.1–37.6%] | 41.6% [21.3–65.2%] | 50.0% [18.5–81.5%] | 50.3% [27.6–72.8%] |
| 100–250 | NA | NA | 14.3% [9.2–21.5%] | 64.6% [5.3–98.3%] | 29.3% [21.9–37.9%] | 58.6% [3.6–98.1%] | |
| 250–1000 | NA | 8.0% [5.3–11.9%] | 9.3% [6.7–12.8%] | 1.5% [0.8–2.9%] | 20.0% [7.4–43.7%] | 25.1% [3.2–77.2%] | |
| >1000 | NA | NA | 0.1% [0.07–0.2%] | 0.1% [0.0–0.6%] | 0.1% [0.0–2.9%] | 0.7% [0.2–2.6%] | |
| Infected tract | Respiratory | 47.8% [33.5–62.4%] | NA | NS | 98.3% [90.6–99.7%] | NA | 12.5% [5.3–26.7%] |
| Digestive | 32.5% [16.8–53.4%] | NA | NS | 3.0% [1.0–8.9%] | NA | 40.0% [26.2–55.7%] | |
| Generalized | 16.7% [6.0–38.7%] | NA | NS | NA | NA | 47.5% [32.7–62.7%] | |
| Age class | Cub | NS | NA | NS | 1.7% [1.0–3.1%] | NA | NA |
| Sub-adult | NS | NA | NS | 2.6% [1.5–4.3%] | NA | NA | |
| Adult | NS | NA | NS | NA | NA | NA | |
| Sex | Female | 8.7% [4.4–16.4%] | NA | NS | 8.0% [4.9–12.9%] | NA | NA |
| Male | 4.8% [2.0–10.7%] | NA | NS | 1.4% [0.2–9.1%] | NA | NA | |
NS - non-significant; NA - non-applicable; hosts without statistically significant moderators not included.
The pooled prevalence in buffalos was 9.88% (95% Cl: 7.13% - 13.54%) (Supplementary Fig. 10). Individual R2 ranged from 0% to 61.32%, with the highest values of R2 being observed for sample size (61.32%) and geographic location by continent (37.17%), followed by diagnostic test (4.63%), while the publication period exhibited no effect on heterogeneity (R2 = 0%) (Table 1; Supplementary Table 3). Similarly, to the cattle model, multivariate regression analysis showed 34.92% of overall heterogeneity. ANOVA test indicated that none of the four moderators were significant (Table 1; Supplementary Table 3). The lack of statistically significant moderators can result from the low number of articles and data from this species validated to incorporate this meta-analysis (n = 13 articles).
3.2. Farmed cervids
Concerning farmed cervids, the pooled animal TB prevalence was estimated as 9.13% (95% Cl: 6.19% - 13.27%) (Supplementary Fig. 11), with an individual R2 ranging from 0% to 15.67%. Three moderators exhibited no effect on heterogeneity (R2 = 0%), with the exception of sample size (15.67%) (Table 1; Supplementary Table 3). The multivariate regression analysis indicated an overall heterogeneity of 18.97% and the ANOVA test indicated three out of four moderators as significant (geographical location by continent, diagnostic test, and sample size; p-value <0.25) (Table 1; Supplementary Table 3). However, no specific parameter showed significantly different effect on prevalence values, with Europe (10.3%) showing a tendency for higher prevalence than North America (7.9%) and histopathology-based diagnostics (9.3%) tending to report higher prevalence values than culture-based methods (7.4%) (Table 2). The differences across continents can be the result of host-specific susceptibilities to animal TB, since in Europe red deer is the most studied deer species, contrarily to North America that mainly focuses on elk. Histopathology-based methods have lower specificity than culture-based ones, due to several other infectious agents that can lead to granuloma-like lesions, such as M. avium subsp. paratuberculosis and Corynebacterium spp. [37].
3.3. Camelids
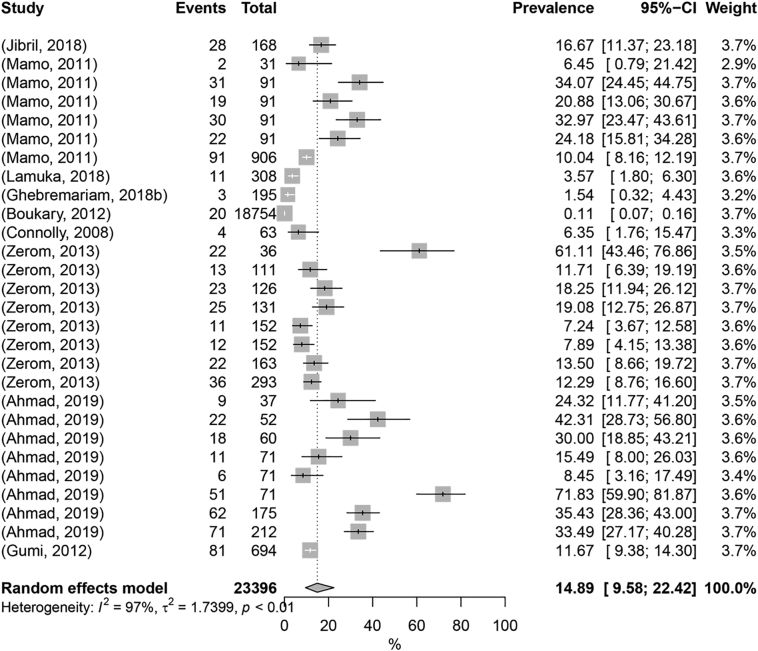
The pooled prevalence of animal TB in camelids worldwide was 14.89% (95% Cl: 9.58% - 22.42%) (Fig. 2). The univariable meta-regression analysis integrating different variables yielded the highest values of R2 for sample size (61.59%) and diagnostic test (13.69%), while all other six moderators did not exert an effect on heterogeneity (R2 = 0) (Table 1; Supplementary Table 3). A total of 68.71% overall observed heterogeneity was reported by the multivariable meta-regression analysis, meaning that the moderators included in the model explain more than two-thirds of the observed variability. ANOVA test indicated that two (sample size and diagnostic test) out of eight moderators were significant (p-value <0.25) when all variables were considered (Table 1; Supplementary Table 3).
Fig. 2.
Forest plot visualization of animal tuberculosis prevalence in Camelidae. “Total” refers to the sample size in each publication; “Events” refers to the number of TB-positive animals; “Prevalence” refers to TB prevalence in each publication.
The estimated disease prevalence rate, according to sample size and diagnostic test, was thereafter compared. Culture-based (46.6%) yielded significantly higher prevalence values than tuberculin-based (3.4%) and nucleic acid-based methods (6.5%), which could be related with herd culling after a positive in vivo test (that avoids doing tuberculin-testing for all the animals in the herd) and subsequent laboratorial confirmation for the subset of slaughtered suspect animals within the group (Table 2). Nevertheless, tuberculin and nucleic acid-based methods are used in higher sampling studies due to their high-throughput nature, representing a more reliable prevalence estimation strategy.
Studies with more than 1000 sampled individuals (0.11%) showed significantly lower prevalence values than all the remaining sample size categories (<1000 sampled individuals), which then again reinforces the notion that different sample-size studies may introduce bias, with studies with higher sampling number showing more reliable prevalence rates (Table 2).
3.4. Small ruminants
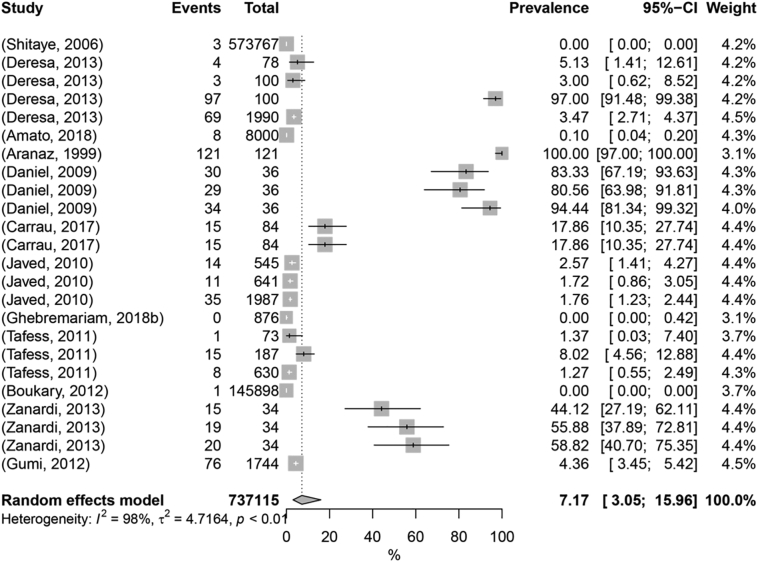
The category small ruminants includes works on two hosts: goats and sheep. First, focusing on goat works, the pooled prevalence for animal TB was estimated as 7.17% (95% Cl: 3.05% -15.96%) (Fig. 3, with Q value of 1276.95 (p-value <0.0001) and I2 of 98.2%, revealing high levels of heterogeneity. A total of eight moderators were used in the univariable meta-regression and R2 values obtained ranged between 0% to 47.50%, in decreasing order: sample size (47.50%), geographical location per continent (23.99%), anatomical localization of lesions (17.48%), breed (12.07%), and publication period (6.52%); diagnostic test, sex and age class exhibited no effect on heterogeneity (R2 = 0) (Table 1; Supplementary Table 3). The multivariable meta-regression analysis accounted for 48.86% of the overall observed heterogeneity, indicating that significant predictors explained almost half of the observed variability. Continent, publication period, sample size, and infected tract were considered significant (p-value <0.25) by ANOVA (Table 1; Supplementary Table 3).
Fig. 3.
Forest plot visualization of animal tuberculosis prevalence in goat. “Total” refers to the sample size in each publication; “Events” refers to the number of TB-positive animals; “Prevalence” refers to TB prevalence in each publication.
When focusing on geographic location, Europe has an estimated prevalence of animal TB of 51%, a marked higher value than Africa or Asia, with estimated values close to 1% (1.0% and 1.9%, respectively) (Table 2). This discrepancy could be related with differences in production systems within the different continents and with differences in the resources allocated to monitor TB in the livestock population. On the one hand, these results show that the epidemiological scenario in Europe is well-characterized and that the TB burden is significant, while on the other they highlight that the scarcity of data in underdeveloped countries may favour the artificial notion that TB prevalence is low. Goat breeds raised in Europe could be more susceptible to MTC agents and, in fact, M. caprae, the goat-adapted ecotype, is almost exclusively reported in Europe and mainly from goats [[38], [39], [40]]. Similar to models performed for other hosts, larger sample sizes (>250) were associated with lower prevalence numbers, corroborating that a higher sampling effort is more accurate to estimate prevalence values; but may also be influenced by production systems and management conditions, with intensive or extensive regimes impacting differently on disease prevalence (Table 2). The articles published between 1990 and 1999 reported higher prevalence rates than the studies from the most recent periods (Table 2). This might be related to the number of works included in each time interval and the diagnostic test privileged over time, which could impact the sensitivity and specificity of methodologies underlying the reports of prevalence figures. Moreover, the prevalence values obtained for 2000–2010 (7.02%) and 2011–2020 (5.34%) are similar to the pooled prevalence estimate for goats (Table 2). Respiratory lesions were more prevalent (98%) than lesions in the digestive tract (3%), suggesting that MTC transmission in goats might mainly occur through aerosols (Table 2).
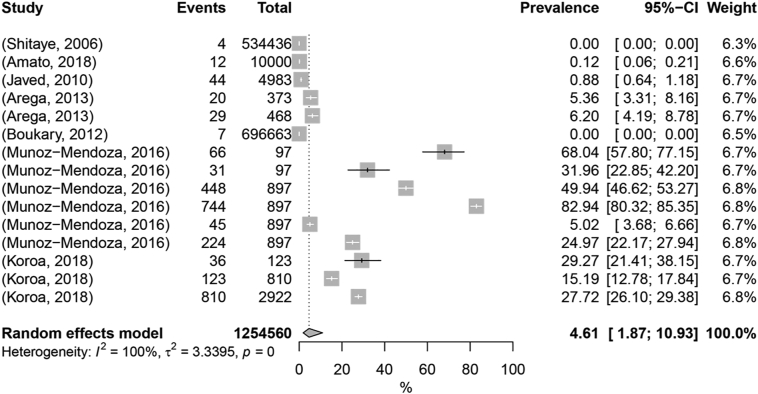
Considering sheep, the pooled prevalence was estimated as 4.61% (95% Cl: 1.87% - 10.93%) (Fig. 4). Geographic location by continent, publication period, diagnostic test, and sample size were the moderators included in the univariable meta-regression, with R2 values ranging between 0 and 42.57% (Table 1; Supplementary Table 3). The highest value was sample size (42.57%), followed by publication period (21.18%), geographic location (4.31%), and finally diagnostic test (0%). The multivariable meta-regression accounted for 20.36% of heterogeneity, and according to the ANOVA test, only sample size was a significant predictor (p-value <0.25) (Table 1; Supplementary Table 3). Similar to the goats' dataset, in the sheep-related works the larger sample sizes (>250) were associated with lower prevalence numbers (Table 2).
Fig. 4.
Forest plot visualization of animal tuberculosis prevalence in sheep. “Total” refers to the sample size in each publication; “Events” refers to the number of TB-positive animals; “Prevalence” refers to TB prevalence in each publication.
3.5. Suidae
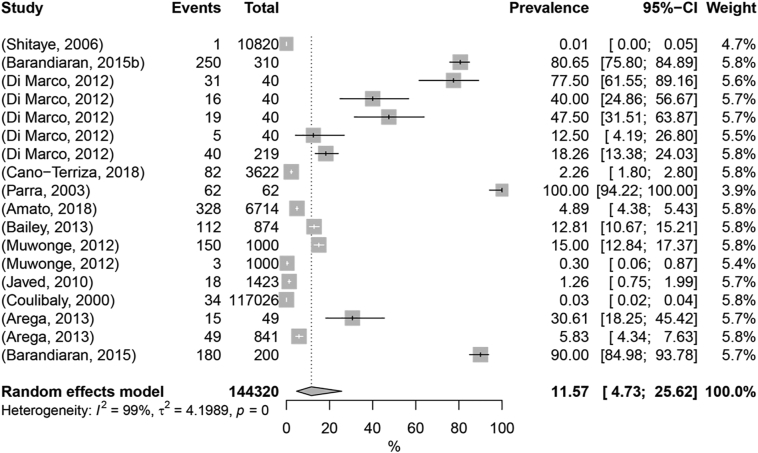
The pooled prevalence of animal TB in pigs was estimated as 11.57% (95% CI: 4.73% - 25.62%) (Fig. 5). Five moderators were considered in the univariate meta-regression: geographic location by continent, publication period, diagnostic test, sample size, and anatomical location of lesions. R2 varied between 0% and 50.09%, with the higher values registered for sample size (50.09%) and geographic location (36.42%); and the lower values for publication period (14.24%), diagnostic test (4.25%), and anatomical localization of lesions (0.00%). (Table 1; Supplementary Table 3). Multi-regression including all the cited moderators accounted for 53.53% of heterogeneity. The ANOVA tests indicated that geographical location by continent, publication period, and sample size were significant predictors (p-value <0.25) (Table 1; Supplementary Table 3). Those moderators were found to have different effects on the prevalence of TB in pigs. According to the model, the prevalence was significantly different in Africa (0.91%), South America (85.7%), and Europe (24.1%) (Table 2). When comparing Africa and Europe, the prevalence estimates may be influenced by the fact that the population density of pigs in Europe is much higher than in Africa [32], therefore Europe could be investing more resources in monitoring TB or in other trade-relevant disease in this species that enables TB suspicion and confirmation. However, South America, which shows on most of the sub-continent values of pig population density similar to those found in Africa [32], has an estimate which clearly weights data from two studies [41,42], both from Argentina. A single occurrence in Asia was retrieved in which the prevalence is 1.26%. This lack of data for Asia suggests knowledge gaps in a continent with countries with high population densities of pigs such as China [32]. The prevalence could also be stratified by the publication periods: from 2000 to 2010, the estimated prevalence was 1%, while between 2011 and 2020 the estimated prevalence was 20.9% (Table 2). Those differences in estimations may be biased by the low number of occurrences found in the first time interval. When comparing estimated prevalence rates across sample sizes, there is a wider range of values, beginning at 0.67% in the larger samples (>1000) and established at 50.2% in the smaller samples (Table 2). As so, only the ranges at the higher and lower extremes of the spectrum are found to have significantly different prevalence estimates, and similarly to other hosts, larger samples appear to represent smaller prevalence.
Fig. 5.
Forest plot visualization of animal tuberculosis prevalence in pig. “Total” refers to the sample size in each publication; “Events” refers to the number of TB-positive animals; “Prevalence” refers to TB prevalence in each publication.
Concerning farmed wild boar, a smaller set of data was retrieved, once most reports refer to free-ranging populations and not so frequently to farmed animals. As so, the dataset was comprised of seven occurrences. The pooled prevalence of animal TB in farmed wild boar was estimated as 40.51% (95% CI: 29.1%, 53.04%) (Supplementary Fig. 12). Univariate meta-regression was conducted to evaluate the amount of heterogeneity accounted for each moderator under study, namely publication period, diagnostic test, and sample size. R2 was found to be 0% for every moderator evaluated, meaning that none of the variables under study was responsible for the heterogeneity comprised in the model (Table 1; Supplementary Table 3). We thus conclude that the retrieved dataset, including occurrences for Europe only, is not robust enough to indicate the sources of heterogeneity nor to provide an estimated prevalence.
3.6. Overview
From all the eight mammalian host groups under study, farmed wild boar was the only one with insufficient data to attain any conclusion.
The majority of models did not show sample size bias across studies, except for cattle (higher sample size) and sheep (lower sample size) models. The overall heterogeneity found in the meta-analysis models ranged from 85.4% (farmed cervids) to 99.9% (cattle). However, the multivariable regression analysis calculated an overall heterogeneity, provided by the conjugation of the analysed moderators, that ranged between 0% (farmed wild boar) and 68.71% (camelids). Those results indicate that all moderators included in the regression models explain only part of the observed variability. The remaining variability can result from several other variables not considered in the models, but acknowledged to date to impact TB prevalence, such as management measures (e.g. biosecurity procedures, herd features, population density, and mixed host farming), animal movements, livestock-wildlife interactions, livestock-human interactions, among others, that were not represented or reported with adequate frequency in this dataset and thus were not adequate for robust and rigorous meta-analyses.
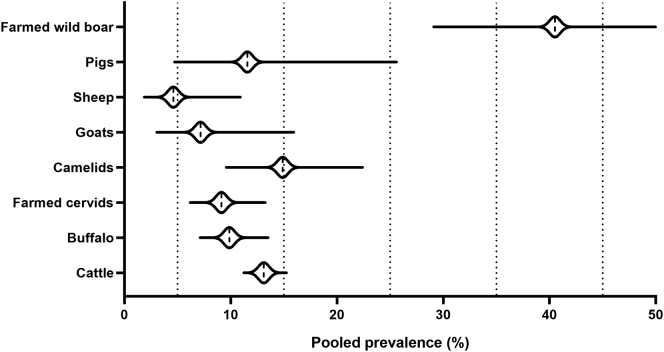
The differences between the overall prevalence of animal TB across each host group are low, with all host groups showing prevalence values around 10%, except farmed wild boar (41%) (Fig. 6). The remaining host groups showed disease prevalence rates between 5% (sheep) and 15% (camelids). The higher disease prevalence rate in farmed wild boar may result from the low number of articles included in the model, together with the low sample size and restricted geographical location of those studies.
Fig. 6.
Comparison of animal tuberculosis prevalence across hosts.
Of the different moderators included in the several models, geographical location by continent (four out of eight models) and sample size (six out of eight) were the most statistically relevant ones. The sample size proved to be important as a possible bias factor in small sample size studies, overestimating TB prevalence. Regarding TB prevalence per continent, it was markedly heterogeneous depending on the host group, with Africa and Asia being the continents where disease prevalence was generally low (between 1% and 10%) independently of the host. In contrast, North America (33.6%, associated to cattle), Europe (51%, associated to goats), and South America (85.7%, associated to pigs) showed higher disease prevalence. In all these continents, the specific host groups occur in high densities in livestock production systems [32,33]. The differences in disease prevalence estimates might be influenced by the differential survey efforts across countries, with lack of reported data particularly from low−/middle-income countries, but also due to the lack of control programs for specific hosts, even in high-income countries, which altogether obscure the animal TB scenario drawn here. In agreement, in a recent systematic review, Asia was identified as a region where research efforts in this field should be reinforced [21]. The stratified figures calculated in this work regarding the prevalence of animal tuberculosis in the world prefigure a situation that requires further confirmation and more insightful studies to clarify uncertainties pointed out above.
Besides those moderators, the anatomical location of lesions (namely, the infected tract) and the diagnostic test used were relatively important predictors for the analysed models. The respiratory tract is the predominant local with visible lesions, followed by the digestive tract, and finally by generalized infection signs, in both cattle and goats. Concerning disease prevalence estimates by diagnostic tests, the outputs were very heterogeneous depending on the host: in cattle, higher prevalence was attained when biochemical identification (39.1%) or interferon-gamma (34.4%) are used and lower prevalence when histopathology (11.2%) or tuberculin-based (9.2%) approaches are performed; in contrast, camelids showed a higher disease prevalence when culture-based methods (46.6%) are used and lower prevalence when tuberculin-based methods (3.4%) are implemented. These differences may result from the high range of sensitivities and specificities of the different diagnostic tests that give highly heterogeneous responses depending on the host.
4. Conclusion
In this meta-analysis, the worldwide overall animal tuberculosis prevalence of the different host groups was estimated, showing that the host groups considered hold high importance in the maintenance of animal TB. The encountered geographical differences are mainly associated with higher animal densities in higher disease prevalence locations. Additionally, a higher diversity of diagnostic tests leads to a higher diversity of estimated prevalence values due to differential sensitivity and specificity between tests and hosts, which can influence disease perception on a specific geographical and temporal scenario.
This work highlights the scarcity of studies focusing on the quantification of management measures, animal movements, livestock-wildlife interactions, and livestock-human interactions, which led to the lack of heterogeneity explained by the overall moderators used in the meta-regression. The lack of studies in these fields is a worldwide lacuna that calls for guided financial and human resources allocation in order to gain a more holistic discernment of animal tuberculosis epidemiology predictors in all possible livestock reservoirs.
This study highlights the requirement of integrated approaches to animal TB and the need to consider socio-economic and environmental contexts, informing decision-makers from different geographical regions on the required concrete measures to be applied in the surveillance of specific mammalian hosts, production systems, also highlighting which combination of diagnostic tests suits best the surveillance of each disease panorama. In particular, surveillance of cattle in North America could benefit from the increased use of INF-γ diagnostics, followed by post-mortem examination of animal respiratory and digestive tracts at slaughterhouses, together with a strict restriction on livestock-wildlife contacts, especially due to the role of white-tailed deer as reservoir. Moreover, in Europe, goats should become a focus of concern and augmented surveillance, with particular emphasis to infections caused by M. caprae. The inclusion of this host species in national control and eradication programs of animal TB in countries where it is managed under high densities and under the extensive regimen, e.g. Spain, Portugal and Austria, is thus to be commended based on our computed data. Results from this meta-analysis also point out to the reinforcement of abattoir surveillance of goat at the respiratory tract level. Furthermore, in South America, the need to increase surveillance in pigs, with the incorporation of in vivo diagnostic assays in the national control and eradication programs of animal TB, also stands out.
Author contributions
MVC conceived the study and methodological approaches. BR, ACP, and ACR analysed the data under the regular supervision of MVC and wrote the first draft of the manuscript. MVC critically revised and redirected all drafts. All authors gave intellectual input to the conceptualization of the final manuscript.
Funding
This work was funded by Programa Operacional de Competitividade e Internacionalização (POCI) (FEDER component), Programa Operacional Regional de Lisboa, and Fundação para a Ciência e a Tecnologia (FCT), Portugal, in the scope of project ‘Colossus: Control Of tubercuLOsiS at the wildlife/livestock interface uSing innovative natUre-based Solutions’ (ref. POCI-01-0145- FEDER- 029783) and strategic funding to cE3c and BioISI Research Units (UID/BIA/00329/2020 and UID/Multi/04046/2020). ACR and ACP were supported by FCT through doctoral grants (PD/BD/128031/2016 and SFRH/BD/136557/2018).
Declaration of Competing Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be perceived as a potential conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.onehlt.2020.100169.
Appendix A. Supplementary data
Supplementary material
References
- 1.Brites D. A new phylogenetic framework for the animal-adapted Mycobacterium tuberculosis complex. Front. Microbiol. 2018;9:2820. doi: 10.3389/fmicb.2018.02820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gagneux S. Ecology and evolution of Mycobacterium tuberculosis. Nat. Rev. Microbiol. 2018;16(4):202–213. doi: 10.1038/nrmicro.2018.8. [DOI] [PubMed] [Google Scholar]
- 3.Palmer M.V. Mycobacterium bovis: a model pathogen at the Interface of livestock, wildlife, and humans. Vet. Med. Int. 2012;2012:236205. doi: 10.1155/2012/236205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phillips C.J. The transmission of Mycobacterium bovis infection to cattle. Res. Vet. Sci. 2003;74(1):1–15. doi: 10.1016/S0034-5288(02)00145-5. [DOI] [PubMed] [Google Scholar]
- 5.Pollock J.M., Neill S.D. Mycobacterium bovis infection and tuberculosis in cattle. Vet. J. 2002;163(2):115–127. doi: 10.1053/tvjl.2001.0655. [DOI] [PubMed] [Google Scholar]
- 6.Liebana E. Pathology of naturally occurring bovine tuberculosis in England and Wales. Vet. J. 2008;176(3):354–360. doi: 10.1016/j.tvjl.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Broughan J.M. Mycobacterium bovis infections in domesticated non-bovine mammalian species. Part 1: review of epidemiology and laboratory submissions in Great Britain 2004-2010. Vet. J. 2013;198(2):339–345. doi: 10.1016/j.tvjl.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Gortázar C. The status of tuberculosis in European wild mammals. Mammal Rev. 2012;42(3):193–206. doi: 10.1111/j.1365-2907.2011.00191.x. [DOI] [Google Scholar]
- 9.Mohamed A. Bovine tuberculosis at the human–livestock–wildlife interface and its control through one health approach in the Ethiopian Somali pastoralists: a review. One Health. 2020;9:100113. doi: 10.1016/j.onehlt.2019.100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pesciaroli M. Tuberculosis in domestic animal species. Res. Vet. Sci. 2014;97:S78–S85. doi: 10.1016/j.rvsc.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 11.ECDC, E.A The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2015. EFSA J. 2016;14(12):4634. doi: 10.2903/j.efsa.2016.4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rivière J. Bovine tuberculosis surveillance in cattle and free-ranging wildlife in EU member states in 2013: a survey-based review. Vet. Microbiol. 2014;173(3–4):323–331. doi: 10.1016/j.vetmic.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 13.OIE . Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. Vol. 1. OIE; 2018. Bovine Tuberculosis; pp. 1058–1074. [Google Scholar]
- 14.Broughan J.M. Mycobacterium bovis infections in domesticated non-bovine mammalian species. Part 2: a review of diagnostic methods. Vet. J. 2013;198(2):346–351. doi: 10.1016/j.tvjl.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Pereira A.C. Animal tuberculosis: impact of disease heterogeneity in transmission, diagnosis and control. Transbound. Emerg. Dis. 2020;67(5):1828–1846. doi: 10.1111/tbed.13539. [DOI] [PubMed] [Google Scholar]
- 17.De Kantor I.N. Mycobacterium bovis infection in humans and animals with an emphasis on countries in Central and South America. In: Thoen C.O., editor. In Zoonotic Tuberculosis: Mycobacterium bovis and Other Pathogenic Mycobacteria. 3rd. John Wiley & Sons, Inc.; 2014. pp. 35–49. [Google Scholar]
- 18.Naugle A.L. Bovine tuberculosis eradication in the United States. A century of progress. In: Thoen C.O., editor. In Zoonotic Tuberculosis: Mycobacterium bovis and Other Pathogenic Mycobacteria. 3rd. John Wiley & Sons, Inc.; 2014. pp. 235–251. [Google Scholar]
- 19.Srinivasan S. Prevalence of bovine tuberculosis in India: a systematic review and meta-analysis. Transbound. Emerg. Dis. 2018;65(6):1627–1640. doi: 10.1111/tbed.12915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moher D. Preferred reporting items for systematic reviews and Meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reis A.C. Transbound. Emerg. Dis.; 2020. Global Trends of Epidemiological Research in Livestock Tuberculosis for the Last Four Decades. [DOI] [PubMed] [Google Scholar]
- 22.Balduzzi S., Rücker G., Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid. Based Mental Health. 2019;22(4):153–160. doi: 10.1136/ebmental-2019-300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwarzer G., Antes G., Schumacher M. A test for publication bias in meta-analysis with sparse binary data. Stat. Med. 2007;26(4):721–733. doi: 10.1002/sim.2588. [DOI] [PubMed] [Google Scholar]
- 24.Viechtbauer W. 36(3) J. Stat. Soft.; 2010. Conducting Meta-Analyses in R with the metafor Package. [DOI] [Google Scholar]
- 25.RStudio Team . 2015. RStudio: Integrated Development for R. [Google Scholar]
- 26.Cochran W.G. The combination of estimates from different experiments. Biometrics. 1954;10(1):101–129. doi: 10.2307/3001666. [DOI] [Google Scholar]
- 27.Higgins J.P. Measuring inconsistency in meta-analyses. Bmj. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Begg C.B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 29.Egger M. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dohoo I.R., Martin S.W., Stryhn H. Sampling. In: McPike S.M., editor. Veterinary Epidemiologic Research. VER, Inc; 2009. pp. 27–52. [Google Scholar]
- 31.Sibhat B. Bovine tuberculosis in Ethiopia: a systematic review and meta-analysis. Prev. Vet. Med. 2017;147:149–157. doi: 10.1016/j.prevetmed.2017.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gilbert M. Global distribution data for cattle, buffaloes, horses, sheep, goats, pigs, chickens and ducks in 2010. Sci. Data. 2018;5(1):180227. doi: 10.1038/sdata.2018.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robinson T.P. Mapping the global distribution of livestock. PLoS One. 2014;9(5) doi: 10.1371/journal.pone.0096084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alvarez J. Evaluation of the sensitivity and specificity of bovine tuberculosis diagnostic tests in naturally infected cattle herds using a Bayesian approach. Vet. Microbiol. 2012;155(1):38–43. doi: 10.1016/j.vetmic.2011.07.034. [DOI] [PubMed] [Google Scholar]
- 35.Singhla T. Determination of the sensitivity and specificity of bovine tuberculosis screening tests in dairy herds in Thailand using a Bayesian approach. BMC Vet. Res. 2019;15(1):149. doi: 10.1186/s12917-019-1905-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Domingo M., Vidal E., Marco A. Pathology of bovine tuberculosis. Res. Vet. Sci. 2014;97(Suppl):S20–S29. doi: 10.1016/j.rvsc.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 37.Sleeman J.M. Johne’s disease in a free-ranging white-tailed deer from Virginia and subsequent surveillance for Mycobacterium avium subspecies paratuberculosis. J. Wildl. Dis. 2009;45(1):201–206. doi: 10.7589/0090-3558-45.1.201. [DOI] [PubMed] [Google Scholar]
- 38.Reis A.C. Polyclonal infection as a new scenario in Mycobacterium caprae epidemiology. Vet. Microbiol. 2020;240 doi: 10.1016/j.vetmic.2019.108533. [DOI] [PubMed] [Google Scholar]
- 39.Rodríguez S. Mycobacterium caprae infection in livestock and wildlife, Spain. Emerg. Infect. Dis. J. 2011;17(3):532–535. doi: 10.3201/eid1703.100618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vidal E. Transmission of tuberculosis caused by Mycobacterium caprae between dairy sheep and goats. Small Rumin. Res. 2018;158:22–25. doi: 10.1016/j.smallrumres.2017.11.010. [DOI] [Google Scholar]
- 41.Barandiaran S. Tuberculosis in swine co-infected with Mycobacterium avium subsp. hominissuis and Mycobacterium bovis in a cluster from Argentina. Epidemiol. Infect. 2015;143(5):966–974. doi: 10.1017/S095026881400332X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barandiaran S. Bovine tuberculosis in domestic pigs: genotyping and distribution of isolates in Argentina. Res. Vet. Sci. 2015;103:44–50. doi: 10.1016/j.rvsc.2015.09.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material