Abstract
Recent advances in cancer immunotherapy have clearly shown that checkpoint-based immunotherapy is effective in a small subgroup of cancer patients. However, no effective predictive biomarker has been identified so far. The major histocompatibility complex, better known in humans as human leukocyte antigen (HLA), is a very polymorphic gene complex consisting of more than 200 genes. It has a crucial role in activating an appropriate host immune response against pathogens and tumor cells by discriminating self and non-self peptides. Several lines of evidence have shown that down-regulation of expression of HLA class I antigen derived peptide complexes by cancer cells is a mechanism of tumor immune escape and is often associated to poor prognosis in cancer patients. In addition, it has also been shown that HLA class I and II antigen expression, as well as defects in the antigen processing machinery complex, may predict tumor responses in cancer immunotherapy. Nevertheless, the role of HLA in predicting tumor responses to checkpoint-based immunotherapy is still debated. In this review, firstly, we will describe the structure and function of the HLA system. Secondly, we will summarize the HLA defects and their clinical significance in cancer patients. Thirdly, we will review the potential role of the HLA as a predictive biomarker for checkpoint-based immunotherapy in cancer patients. Lastly, we will discuss the potential strategies that may restore HLA function to implement novel therapeutic strategies in cancer patients.
Keywords: major histocompatibility complex (MHC), human leukocyte antigen (HLA), antigen processing machinery (APM) molecules, carcinogenesis, tumor predisposition, biomarker, cancer immunotherapy
1. Human Leucocyte Antigen and Antigen Presentation Machinery Molecules: An Overview
1.1. HLA Class I: Structure and Function
The major histocompatibility complex (MHC), better known in humans as human leukocyte antigen (HLA), is a very polymorphic gene complex encoding for cell surface molecules specialized to present and recognize self and non-self peptides [1,2,3,4,5,6,7,8]. HLA complex contains more than 200 identified loci located close together on a 3 Mbp stretch within the short arm of chromosome 6 [6,8,9]. Population surveys have identified several thousands of allelic variants of HLA molecules which mainly affect the nature and composition of their peptide-binding groove, regulating the peptide repertoire presented on the cell membrane [6,8,9,10]. These allelic variants can be associated with an increased risk of various diseases including cancer [11].
HLA is categorized into three groups on the basis of function and structure: class I, II and III [12,13]. HLA class I molecules are expressed on the surface of nucleated cells, except for germ line and some neuronal cells [14]. HLA class I molecules display on cell membrane peptide fragments derived from endogenously degraded self or non-self proteins to T-cell receptor (TCR) of CD8+ cytotoxic T lymphocytes (CTLs) [7,15,16,17,18]. Peptides derived from unmutated (self) proteins are normally ignored by CTLs, whereas those derived from mutated (self) or pathogen (non-self) proteins are recognized and trigger an adaptive immune response [15,16,17]. Particularly, tumor cells are characterized by mutated genes and aberrantly expressed cellular proteins from which derived tumor specific antigens (TSAs) and tumor-associated antigens (TAAs). Through the presentation of TAAs/TSAs, tumor cells become susceptible to CTL-mediated lysis. Using this immune surveillance system, CTLs eradicate intracellular pathogens and exert potent antitumor activity, eliminating the transformed or infected cells through the adaptive immune response [19]. In addition, HLA class I molecules can also present peptides generated from exogenous proteins, a process known as cross-presentation [14,20]. This process is necessary to recognize and destroy tumor cells as well as viruses that do not readily infect antigen-presenting cells, stimulating naïve T cells into activated CTLs [14,20,21]. Specifically, cross-presentation involves dendritic cells (DCs) which present TAAs/TSAs or pathogen-derived peptides in their HLA class I complex to naïve T cells [22,23,24]. Extracellular peptide loading on HLA class I complex differs from the canonical way followed by intracellular peptides and it will be discussed below in this review. However, recently, several lines of evidence demonstrated that macrophages are also able to implement a cross-presentation process, subverting the original belief that it is an exclusive characteristic of DCs [22].
Besides mediating an adaptive immune response, HLA class I molecules also play a key role in the innate immune response since they serve as ligands of inhibitory killer cell immunoglobulin-like receptors (KIRs) of Natural Killer (NK) cells [25]. Because the majority of healthy nucleated cells express HLA class I molecules, inhibitory KIRs ensure that NK cells do not attack normal cells which express HLA class I molecules but eliminate infected and tumor cells which may have reduced expression of HLA class I molecules [25,26]. Structurally, HLA class I molecules are heterodimers that consist of two polypeptide chains, alpha (α) heavy chain and β2-microglobulin (β2-m) light chain [27]. The β2-m subunit is not polymorphic and is encoded on human chromosome 15. In contrast, the α chain is polymorphic and is encoded by HLA class I genes, further categorized in HLA-A, -B, and –C, according to the locus of their encoding gene [28,29,30]. The α heavy chain has three extracellular domains (α 1-3, with α1 being at the N-terminus), a transmembrane region and a C-terminal cytoplasmic tail [30,31,32]. The only invariant region is the Ig-like α3 domain, essential for non-covalent association with the β2-m light chain [30,31,32]. The α3-CD8 interaction holds the HLA class I molecules in place, while the TCR binds to α1-α2 and checks the coupled peptide for antigenicity [30,32]. The α1 and α2 domains fold to make up a groove for peptides to bind. Bound peptides are predominantly 8-10 amino acid in length, but longer peptides have also been reported [30,32,33].
Besides HLA-A, B, and C, some other HLA class I molecules are also encoded by non-classical HLA loci. Those include HLA-E, which primarily presents various peptides that are derived from the leader sequence of some HLA class I molecules. It blocks conventional NKs expressing the inhibitory heterodimeric NKG2A/CD94 receptor. Lastly, HLA-F mainly resides intracellularly and rarely reaches the cell surface; HLA-G, plays a role in protecting the fetus from the maternal immune responses [34,35,36,37,38,39].
1.2. HLA Class I Antigen Processing Machinery Complex and Antigen Presentation
The generation and expression of HLA class I antigen-derived peptide complexes is a multistep process and requires an integral and functional HLA class I antigen processing machinery (APM) [25]. This is constituted by several distinct components, such as the proteasome complex, the ubiquitination system, the transporters associated with antigen processing (TAP)1 and TAP2, the endoplasmic chaperone molecules (calnexin, calreticulin, ERp57, and tapasin), and the Golgi apparatus [25,40,41,42]. The generation and expression of HLA class I antigen derived peptide complexes and their presentation to naïve CD8+ T cells require four main tasks: (i) peptide generation and trimming; (ii) peptide transport; (iii) assembly of the HLA class I loading complex; and (iv) antigen presentation [25,30,43,44,45,46,47,48]. Firstly, proteins are targeted for degradation by the covalent attachment of multiple copies of the 76-residue protein ubiquitin to free amino groups of Lys [25,49]. Subsequently, they are transferred to the proteasome, where the catalytic core, called the 20S proteasome, contains α and β subunits. The catalytic core interacts with regulatory particles and creates a physical barrier to regulate access to the gate. The latter has protease catalytic activity [25,50,51,52]. Three of the 20S proteasome’s β subunits δ(β1), Z(β5), and MB1(β2) may be replaced by the functionally different counterparts low molecular proteins (LMP) as LMP2 (also called β1i), LMP7 (β5i), and LMP10 (β2i), respectively [25,53,54,55]. Proteasome incorporating LMP2, LMP7 and LMP10 is called immunoproteasomes because it develops under conditions of intensified immune response [25]. The immunoproteasome formation is induced during inflammation by stimulation with type I (α and β) or type II (γ) interferons (IFNs) [25,56,57]. Moreover, the immunoproteasome is involved in other activities such as generation of cytokines as well as regulation of T cell differentiation, survival and function during thymocyte development [25,58,59]. Peptides generated in the proteasome are then actively transported from the cytosol into the endoplasmic reticulum (ER) lumen by TAP [25]. TAP is a heterodimeric complex composed of two half-transporters, TAP1 and TAP2, members of the adenosine triphosphate (ATP)-binding cassette transporter family. This complex forms a transmembrane pore in the ER membrane whose opening and closing depend on ATP binding and hydrolysis, respectively (ATP switch model) [25,60,61,62,63]. TAP transports most efficiently peptides of a well-defined length (8–12 residues), while longer peptides can be further trimmed in the ER lumen or, alternatively, can be transported back to the cytosol where they are trimmed by cytosolic peptidases and recycle back to the ER [25,64,65,66,67,68,69,70]. Peptides transported into the ER by TAP are loaded onto nascent HLA class I molecules with the assistance of four chaperone proteins: calnexin, the thiol oxidoreductase ERp57, calreticulin, and tapasin [25,71,72,73,74,75,76,77,78,79]. Specifically, the HLA class I α heavy chain interacts with calnexin, which facilitates its complete folding and, by acting in concert with ERp57, ensures the correct oxidation [25,80,81]. At this point, the conformation of the α heavy chain is recognizable by β2-m [25,82]. Their binding triggers the release of calnexin [25,82,83]. The resulting conformational changes give the α heavy chain/β2-m heterodimer an “open” form that interacts with calreticulin [25,74]. High affinity peptide binding requires the additional participation of tapasin, which links the complex to nascent HLA class I molecules [25,75,79]. After peptide loading, HLA class I derived peptide complex dissociates from TAP as well as from ER-resident chaperones and clusters at export sites on the ER membrane, where it is selectively recruited into cargo vesicles for transport to the Golgi apparatus and then to the cell membrane [25]. On the membrane, the HLA class I derived peptide complex is extracellularly exposed to be recognized by the TCR of naïve T cells, potentially triggering an adaptive immune response when non self or mutated self antigen derived peptides are expressed [19,25].
During cross-presentation extracellular antigens need to enter into canonical HLA class I route and they can exploit various ways. (i) Extracellular peptides can be directly transferred from infected or tumor cells to the cytosol of DCs through the Gap junctions. (ii) ER components can fuse with endosomal/phagosomal pathway and the exogenous peptides are exported from phagosome into cytosol through the ER-associated protein degradation system. (iii) Recycling HLA class I molecules are loaded with extracellular peptides into recycling endosome. (iv) Exosomes secreted by infected or tumor cells can directly bind to DCs [84,85].
1.3. HLA Class II: Structure and Functions
In contrast to HLA class I molecules, HLA class II molecules are usually present only on professional antigen-presenting cells (APCs) (B cells, macrophages, DCs, Langerhans cells), thymic epithelium and activated (but not resting) T cells [86,87,88]. In all other nucleated cells, HLA class II antigen expression can be induced by IFN-γ [88,89,90]. HLA class II molecules promote the switch of naïve T cells into activated T cells by presenting exogenously derived antigen peptides to CD4+ T cells [86,87,88,89,90]. Moreover, HLA class II molecules regulate the functions of B cells, macrophages and T cells [87,88,91]. They are encoded by genes in the HLA-DP, -DQ and -DR loci of the chromosome 6 cluster [6]. HLA class II molecules consist of 2 highly polymorphic polypeptides, the α and β chains [87,92,93,94]. Only the β2 domain of the β chain is a non-polymorphic region. It constitutes the binding site for the CD4+ T cell co-receptor [87,92,93,94,95,96]. HLA class II molecules have a peptide-binding domain, an Ig-like domain and a transmembrane region with a cytoplasmic tail and are responsible for binding peptides (15-24 amino acids) derived from extracellular sources [87,92,93,94,95,96]. Therefore, HLA class II binds peptides longer than HLA class I and accommodates peptide side chains within its binding pocket. These two features increase HLA class II peptide diversity [97,98].
1.4. HLA Class II Antigen Presentation System: How it Works
Compared to HLA class I, also the HLA class II antigen presentation process is characterized by different tasks. This involves several molecules and protein complexes [99,100]. Firstly, α and β chains are assembled in the ER with the invariant chain (li, CD74), forming the (α/β-li)3 complexes [99,100,101]. Invariant chain li occupies the peptide binding groove of HLA class II, preventing peptide loading within ER [98,102]. Li targets HLA class II containing vesicles to acidic endosomes. Then, into these acidic endosomes, called MHC class II compartments (MIICs), the li chain undergoes selective proteolytic digestion, forming the class II-associated I chain peptide (CLIP). This peptide occupies the groove of HLA class II dimers [100,103]. Subsequently, CLIP is exchanged by tightly bound peptides derived from proteins degraded into the endosomal pathway [99,100]. HLA-DM molecules are crucial to facilitate this exchange by promoting CLIP removal and stabilizing the peptide free status of HLA class II molecules. Moreover, HLA-DM also catalyzes the release of weakly bound peptide, ensuring that only strong bound peptide HLA class II complexes reach the cell surface [98]. Finally, the HLA class II derived peptides complexes are exposed on APCs [100,103,104].
1.5. HLA Class I and II Transcription Regulation
The transcription of genes encoding for the components of HLA class I and II complex is tightly regulated according with the crucial role of these molecules to obtain an effective adaptive immune response. HLA class I genes, except for HLA-G, contain several conserved cis-acting regulatory elements. Specifically, three different elements are important for both constitutive and inducible expression. The first element, called enhancer A, contains a binding-site for the nuclear factor kB (NF-kB). The second one corresponds to an IFN-sensitive response element (ISRE) and allows the binding of IFN Regulatory Factors 1 (IRF1). Lastly, the third one is an SXY module comprising four different boxes: W/S, X1, X2 and Y. Equally, the promoter of β2-m, but not those of other genes involved in antigen processing and presentation such as TAP or LMP, contains all three cis-acting regulatory elements in its proximal region [105]. Conversely, HLA class II gene proximal promoters contain only the SXY module which is bound in its X1 box by the regulatory factor X (RFX) complex, which comprises RFX5, RFX-associated ankyrin-containing protein (RFXANK) and RFX-associated protein (RFXAP). The cAMP-responsive element binding protein 1 (CREB1) and the activating transcription factor 1 (ATF1) bind the X2 box; the nuclear transcription factor Y (NFY) complex interacts with the Y box. Instead, the elements interacting with W/S box remains poorly defined [106,107]. The interactors with SXY module of genes belonging to both HLA class I and II are crucial elements in the transcriptional control. Indeed, since the identification in 1993 of the class II trans-activator CIITA [108] and, more recently of the NOD-like receptor 5 (NLRC5) [109], also called class I trans-activator CITA, it has been clearly highlighted that both these NLR proteins miss of a DNA-binding domain. Therefore, both NLRC5/CITA and CIITA need to cooperate with the multiprotein complex that is assembled on the SXY module to exert their transactivation activity (forming CITA- and CIITA enhanceosomes) [110,111]. Different studies exploiting CIITA-deficient mice [112] and several molecular analyses performed in patients affected by bare lymphocyte disease (BLS) with HLA class II deficiency confirmed CIITA as the master regulator of HLA class II expression [92,113,114]. Differently NLRC5/CITA is defined as a key regulator of HLA class I, especially in selected immune cell subsets. Indeed, the generation of NLRC5/CITA knockout mice in three independent studies has allowed to show a retention of HLA class I expression in professional APCs also in the absence of the trans-activator [115,116,117], suggesting the presence of a compensatory mechanism. These results agreed with previous findings regarding the ability of CIITA to contribute to HLA class I expression control [118]. Moreover, NLRC5/CITA regulates the expression of other genes involved in HLA class I presentation and processing, such as β2-m, LMP2, and TAP1 [109]. Interestingly, the up-regulation of both NLRC5/CITA and CIITA is critical for the efficient induction of HLA class I and II, respectively, by IFN-γ stimulation. The induction of NLRC5/CITA by IFN-γ precedes HLA class I gene expression as well as CIITA transcript levels are induced earlier than HLA class II genes upon IFN-γ stimulation [119].
1.6. HLA Class III: A Poorly Characterized Class
The structure and function of HLA class III molecules are poorly defined. They are not involved in antigen binding but in inflammatory processes. Their gene cluster is present between those of class I and class II molecules and encodes important molecules involved in inflammatory processes including complement components C2 and C4, factor B, tumor necrosis factor (TNF)-α, lymphotoxin, and heat shock proteins [12,120,121,122].
1.7. Carcinoma Cells as Non-Professional APC: A Novel Role for HLA Class II Complex
As mentioned above, HLA class II is usually express only on APCs’ surface, playing a crucial role in CD4+ T cell activation [86,87,88]. However, several lines of evidence showed that many type of cancer cells can also express MHC class II complex regardless tissue origin [123,124,125,126,127,128,129,130]. So far, the role of tumor specific MHC class II (tsMHC-II) expression remains unclear. Conflicting evidences are reported about how tsMHC-II regulates cancer progression as well as immune checkpoint inhibitor (ICI)-based immunotherapy response [98]. The expression of tsMHC-II has been related to longer progression free survival (PFS) and overall survival (OS) in melanoma and Hodgkin lymphoma patients treated with programmed death cell 1 (PD-1)/programmed death-ligand 1 (PD-L1) monoclonal antibodies (mAbs) [123,125,126]. In contrast, a similar association was not found in melanoma patients treated with a cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) mAb [126]. Two independent studies on breast cancer specimens, evaluating tsMHC-II expression by both immunohistochemistry (IHC) and RNA sequencing demonstrated that tsMHC-II expression positivity correlated with longer disease free survival (DFS) and PFS [124,131]. These results were observed also in advanced-stage serous ovarian cancer [132]. However, further clinical trials are needed to define the role of tsMHC-II expression as a potential biomarker for ICI-based immunotherapy in cancer patients.
2. Defects and Clinical Significance of HLA in Human Cancer
2.1. HLA Class I Molecule Defects
Aberrations in expression of HLA class I derived peptide complex have frequently been observed in several types of cancer both in vivo and in vitro. Their frequency ranges from 0–90%. Depending on tumor types, these defects have been associated with aggressive histopathological features as well as poor survival [133,134,135,136,137,138,139,140,141,142,143,144,145,146,147]. Most of the defects are caused by genetic or epigenetic mutations as well as by transcriptional or post-translational modifications [145,148,149]. These types of alterations can induce a total loss or down-regulation of HLA class I derived peptide complex as well as selective loss of HLA class I haplotypes or alleles (Figure 1) [150,151,152].
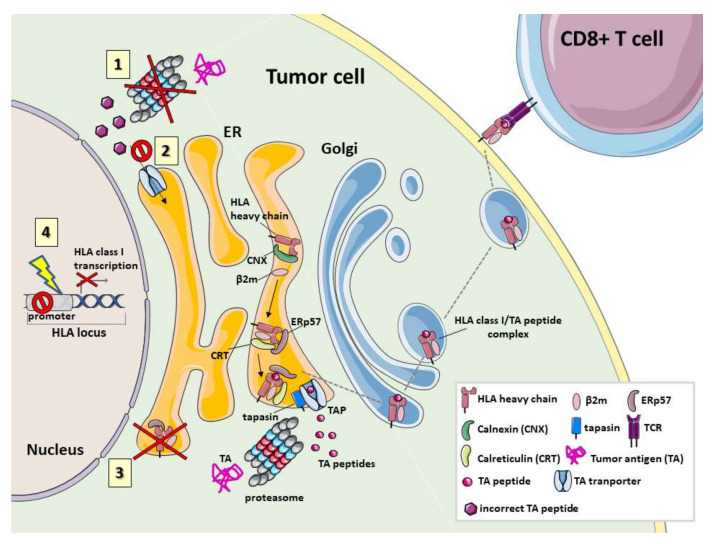
Figure 1.
Defects in tumor antigen processing, translocation and loading on HLA class I. Normally tumor antigens (TAs) are degraded by proteasome/immunoproteasome into TA peptides and translocated into the endoplasmic reticulum (ER) through ATP-dependent activation of TAP transporters. Then, different chaperones: tapasin, calnexin (CNX), calreticulin (CRT) and ERp57 form a multimeric complex that provides for the correct assembly of HLA class I and for peptide loading. The lack of TA presentation during tumor development can be determined by different defective mechanisms depicted in the cartoon. 1. Mutations in genes coding for proteasome subunits or deregulation of their expression implicate an incorrect TA degradation and the production of modified TA peptides. 2. Mutations in TAP genes, associated with down-regulation of their expression or to their dysfunction, reduce the translocation of TA peptides into the ER. 3. Defects in the expression of chaperones reduce the stable assembly of the “peptide-free” HLA class I molecule and of the HLA class I molecule-TA peptide complexes inhibiting a correct and efficient TA peptide presentation. 4. Defects in HLA class I gene expression involve the total loss of these genes or mechanisms that control their transcription resulting in HLA class I molecule down-regulation.
A complete loss of HLA class I derived peptide complex requires two genetic events: a mutation in one copy of the wild-type β2-m and loss of the other non-mutated copy. This phenomenon leads to the loss of heterozygosity (LOH), a genetic abnormality frequently found in malignant cells [153,154,155]. The mutations in β2-m can range from large deletions to single nucleotide mutations. Both types of alterations in most cases inhibit the translation of β2-m mRNA or abolish the disulfide linkage required for the native structure of β2-m, preventing its binding to HLA class I heavy chains [138,139,153]. Although mutations in β2-m can be randomly distributed, a mutation hot spot located in the CT repeat region of exon 1 has been identified in more than 75% of tumor cells, reflecting an increased genetic instability of this region during malignant transformation. As a result tumor cells present total HLA class I molecule loss since the HLA class I heavy chain-β2-m-peptide complex is not formed and not transported to the cell membrane [156,157,158,159]. In contrast, selective HLA class I allospecificity loss requires only one genetic event. This involves mutations of HLA class I allele(s) which inhibit HLA class I molecule transcription or translation. The other allele remains intact and no LOH is required. Loss of one HLA class I haplotype, e.g., HLA-A24, -B56, -Cw7, appears to be frequently caused by loss of segments of the short arm of chromosome 6, where HLA class I genes reside. LOH at chromosome 6 represents a frequent mechanism that contributes to selective HLA haplotype loss in tumors [160,161].
In addition, multiple types of alterations can induce down-regulation of HLA class I molecules [150,151,152]. Specifically, transcriptional activity of HLA class I heavy chain genes can be suppressed by: (i) the presence of silencer localized at the distal promoter region of HLA class I heavy chain gene [162,163]; (ii) epigenetic mechanisms which alter chromatin structure of the HLA class I heavy chain gene promoters; and (iii) DNA hypermethylation [162,164,165,166]. It is well known that the constitutive patterns of DNA methylation in solid and hematopoietic human malignancies are characterized by global hypomethylation with concomitant localized hypermethylation of DNA [148,167,168]. Furthermore, an impaired function of one of the APM components can also reduce the expression of HLA class I derived peptide complex [145]. Lastly, down-regulation of HLA class I antigen complex can be caused by alterations in the transcription factors forming the enhanceosome which bind SXY module on HLA class I heavy chain promoters [148,169,170,171,172]. Specifically, the expression and function of the NLRC5/CITA trans-activator can be affected by promoter methylation, copy number loss and somatic mutations [173,174]. About 60% of somatic mutations result in the inactivation of NLRC5/CITA [174].
2.2. Proteasome Defects
Alterations of proteasome subunits have been identified by utilizing mAbs that allow semi-quantitative analyses of the constitutive subunits δ, Z and MB1, as well as of the immunoproteasome subunits LMP2, LMP7 and LMP10 [25]. Down-regulation of one of these proteins caused by mutations at coding microsatellites or single nucleotide polymorphisms have been described in several types of tumors including colorectal, bladder, and ovarian carcinomas, as well as in acute myeloid leukemia and melanoma [147,175,176,177,178,179]. As previously described, the proteasome plays a key role in immune regulation. Consequently, inhibition or loss of function in one of the proteasomal components inhibits antigen processing and presentation and modifies the characteristics of processed peptides, decreasing the efficiency of epitope generation and altering tumor cell recognition by naïve T cells [25,169,171].
2.3. Defects in TAP1, TAP2 and Other Chaperones
Among the APM components, TAP genes have been most extensively investigated. At genetic level, mutations in TAP genes, resulting in total protein loss or expression of a non-functional protein, have been described in breast, lung, gastric, colorectal, and cervical carcinomas. Their frequency ranges from 10-84% in the cases analyzed [100,180,181,182,183,184]. TAP abnormalities reduce the translocation of peptides into the ER, resulting in a decreased formation of stable HLA class I derived peptide complexes or expression of “peptide-free” HLA class I molecules [171,172]. Interestingly, TAP-deficient individuals do not succumb to viral infections, suggesting that CD8+ T-cell immunity is sufficiently supported by an increased number of alternative TAP-independent processing pathways [171,172]. Identification of these alternative loading mechanisms into peptide-receptive HLA class I molecules still needs further investigation. It is reported that peptides can walk on multiple different paths before ending up in the grooves of HLA class I molecules [185]. Lastly, a substantial down-regulation in chaperone expression have been also associated to several types of malignancies due to defects in proper loading and assembly of HLA class I molecules, altering their maturation and stability [25,88].
2.4. HLA Class II Defects
Contrasting results have been described about the clinical significance of alterations in HLA class II molecule expression in cancer. Defects in HLA class II pathway, as well as induction of HLA class II molecule expression by non-immune cells have been involved in carcinogenesis [100]. In addition, HLA class II expression by cancer cells has been associated with poor prognosis and disease progression in melanoma and osteosarcomas [186,187,188]. However, an improved overall survival has been also associated to HLA class II expression by cancer cells in several types of cancer including melanoma, laryngeal, breast, cervical and colorectal cancer [100,188,189,190,191,192,193,194,195,196,197]. In various type of cancer (plasmacytoma, small cell lung cancer, and hepatocarcinoma) defects in HLA class II molecules have been associated to CIITA defects. The latter results in a reversible detrimental HLA class II expression that can be restored by CIITA transfection [100,198,199,200]. Moreover, other HLA class II presentation antigen pathway defects have been also described in Hodgkin’s disease cancer cells [100,201].
3. Role of HLA as A Predictive Biomarker for ICI-Based Immunotherapy
3.1. Impact of HLA Class I and II on ICI-Based Immunotherapy In Vivo
As we have described above, HLA class I antigen derived peptide complex is crucial for tumor antigen presentation to naïve T cells. Binding of HLA class I antigen derived peptide complex to the TCR of naïve T cells allows T cell activation and consequently the recognition and the lysis of altered tumor cells [7,15,16,17,18]. However, binding of HLA class I derived peptide complex to TCR is not sufficient to activate naïve T cells. Naïve T cell activation requires the interaction between the CD28 family receptors on T cell with their co-stimulatory ligands belonging to B7 family molecules on APCs. Therefore, T cell activation is tightly controlled by co-stimulating or co-inhibiting signaling which are triggered by the interaction between immune checkpoint molecules such as PD-1 and CTLA-4 and their ligands PD-L1 and CD80/CD86 [202,203,204,205], expressed on naïve T cells and APCs, respectively. Actually, the interaction between PD-1 and PD-L1 is crucial in the activation phase of T cells as well as in their effector phase, due to PD-L1 expression also on tumor cells. Several lines of evidence, both in vitro and in vivo, have shown that blockade of the co-inhibitory signaling, including PD-1/PD-L1 axis, by mAbs promotes a host immune response against cancer cells by releasing T cells activation [206]. This novel therapeutic approach, called ICI-based immunotherapy, is revolutionizing the treatment of solid tumors [207]. Several clinical trials in various types of malignancies, such as melanoma, head and neck, triple negative breast, lung, kidney and bladder cancer, have demonstrated that administration of mAbs, which inhibit the interaction of immunoregulatory checkpoint molecules, such as CTLA-4 and PD-1, with their ligands CD80, CD86, and PD-L1, can have a major and lasting effect on their clinical course, significantly improving clinical outcomes as compared with standard chemotherapy [208]. However, this type of therapy is effective only in a subgroup of cancer patients, regardless of the tumor type. Therefore, there is an urgent need to identify the mechanisms of resistance as well as predictive biomarkers which may help to select patients who may benefits from this type of therapy [209,210,211]. Several molecules have been investigated as potential predictive biomarkers of ICI-based immunotherapy [212,213,214,215]. Among the postulated escape mechanisms utilized by tumor cells to avoid recognition and destruction by the host’s immune system, are defects in the ability of tumor cells to process and present tumor antigens to naïve T cells [216]. This phenomenon is mediated by defects in the expression of HLA class I antigen-tumor antigen derived peptide complexes. Therefore, there has been an interest in investigating whether decreased or complete loss of HLA class I and II molecules as well as defects in the APM molecules might predict the efficacy of ICI-based immunotherapy by impairing naïve T cells activation induced by anti-checkpoint molecules. Several lines of evidence in vivo indicate that HLA class I or II modulation play a major role in the efficacy of ICI-based immunotherapy and various humanized mouse models that reliably reflect the complexity of the human heterogeneous tumour and its TME, have been developed in order to evaluate the potential role of HLA class I and II in predicting the efficacy of ICI-based immunotherapy as well as immune adverse effects for different types of cancer [217]. In the study of Lechner MG et al., six murine solid tumor models (CT26, 4T1, MAD109, RENCA, LLC, and B16) were used to demonstrate that MHC class I expression on tumor cells is an excellent surrogate marker of the overall tumor immunogenicity level as well as a predictor of response to immunotherapy. Specifically, tumor growth rate correlated indirectly with MHC class I expression and overall immunogenicity of the tumor model, with fastest growth in B16, LLC, and MAD109 and slowest growth in CT26, RENCA, and 4T1 [218]. Ashizawa et al. reported that HLA class I and class II KO NOG mice (NOG dKO) transplanted with human PBMCs and tumor cell lines showed high anticancer effects following a PD-1 antibody treatment [219]. Gettinger et al. functionally demonstrated that loss of HLA class I expression by CRISPR-mediated knock-out of β2-m in an immunocompetent cancer mouse model (A/J mice transplanted with murine lung cancer cell line UN-SCC680AJ) confers resistance to PD-1 blockade and tumour progression [220]. β2-m gene deactivation in a mouse oncogenic TC-1 cell line derived from primary lung epithelial cells has also been shown to lead to negative surface MHC-I expression along with reduced proliferation and tumor rejection. Despite stimulation with IFN-γ, tumour cells were only weakly responsive to combined immunotherapy [221]. In addition to HLA class I expression, it is important to acknowledge that HLA haplotypes have also been shown to correlate with immunotherapy response in vivo. Rangan L et al. described a tumor cell line generated from a naturally occurring tumor in HLA-A*0201/DRB1*0101 (A2/DR1) mouse named SARC-L1 with a very low expression of HLA-A*0201 molecules, absence of HLA-DRB1*0101 and weak but constitutive expression of PD-L1. Histological and genes signature analysis supported the sarcoma origin of this cell line. According to the high frequency of these HLA alleles in the world population, this mouse model gained considerable interest in the field of tumor immunology and it has been used as preclinical tool for the evaluation of antitumor immunotherapies. Interestingly, both HLA-A*0201 and PD-L1 expressions increased on SARC-L1 after IFN-γ exposure in vitro defining this tumor very sensitive to several drugs commonly used to treat sarcoma and susceptible to anti-PD-L1 mAb therapy in vivo [222]. As we have described above, tumor cells might also express tsHLA-II molecules [123,124,125,126,127,128,129,130]. In the majority of studies, tsHLA-II molecule expression by cancer cells is associated with better prognosis, improved response to ICI in humans and increased tumor rejection in mouse models of breast cancer, sarcoma, lung cancer and colon cancer [98]. However, contrasting reports have shown that tsHLA-II or CIITA has no effect or, in some cases, accelerates tumor growth. In a mouse model of lung cancer Mortara L et al. demonstrated that single cell clones derived from a CIITA transduced population grew more aggressively in mice when cell surface MHC-II was highly expressed [223]. These contrasting results are likely to reflect different variables present in the different mouse model and cancer cells utilized, including (i) the ability of TAAs to be presented differentially on MHC class I or II in each model system, (ii) the number of mutations and therefore number of candidate neo-antigens expressed, (iii) the number of tumor cells injected, (iv) the injection site of cancer cells in the mouse, and (v) the mouse strains used that are characterized by different immunological status. An intriguing but underexplored hypothesis is that induction of HLA class I-II molecules may lead to up-regulate immunoinhibitory molecules on tumor-infiltrating lymphocytes (TILs), such as lymphocyte activation gene 3 (LAG-3) that binds HLA class II and negatively regulates cellular proliferation, activation and homeostasis of T cells, in a similar fashion to CTLA-4 and PD-1. This phenomenon has been reported to play a role in regulatory T cell (Treg) suppressive function creating a tolerizing microenvironment for tumor growth. ICIs directed to LAG-3 have been shown to have synergy with PD-1 inhibition in mouse models, suggesting that co-signaling blockade could restore a favorable immune microenvironment that can respond to antigenic stimulation [224].
3.2. HLA Class I and II as Predictive Biomarker for ICI-Based Immunotherapy: Clinical Evidences
Actually, few clinical studies have been investigating the potential role of HLA class I and II antigens in predicting the efficacy of ICI-based immunotherapy and no large clinical cohort analysis of patient population has been performed. Rodig et al. retrospectively evaluated whether HLA proteins confer differential sensitivity to CTLA-4 and PD-1 blockade in pre-treated metastatic melanoma patients. Tumor biopsies were obtained from patients enrolled in two different trials: CheckMate 064 and CheckMate 069. In these trials, patients were treated with the anti-CTLA-4 monoclonal antibody ipilimumab followed by the anti-PD-1 mAb nivolumab, nivolumab followed by ipilimumab, ipilimumab alone, or concurrent nivolumab plus ipilimumab. In this study, Rodig et al. demonstrated that (i) reduced tumor HLA class I molecule expression (≤ 30%) correlated with lack of response to ipilimumab; (ii) HLA class II molecule expression (>1%) correlated with tumor response to nivolumab; and (iii) among nivolumab plus ipilimumab treated patients, reduced HLA class I molecule expression was not associated with progressive disease as well as a decreased tumor response and overall survival. As a result, HLA class I molecule expression appeared a reliable predictive biomarker of tumor response to anti-CTLA-4 mAb ipilimumab but not to anti-PD-1 mAb nivolumab. In contrast, HLA class II molecule expression might represent a useful predictive biomarker for nivolumab but not ipilimumab therapy [126]. In addition, Chowell et al. retrospectively performed high-resolution HLA class I genotyping of 1535 advanced cancer patients treated with ICI-based immunotherapy. Patients affected by non-small cell lung cancer or melanoma were treated with anti-CTLA-4, anti-PD-1/PD-L1 or combinations of both. Results from this study demonstrated that patients carrying maximal heterozygosity at HLA class I loci (HLA-A, HLA-B, or HLA-C) have an improved overall survival as compared to patients who were homozygous for at least one HLA locus. Moreover, patients carrying HLA-B44 supertype had extended survival, while those carrying HLA-B62 supertype (including HLA-B*15:01) or somatic loss of heterozygosity at HLA class I had poor survival outcomes [225].
4. Restoring HLA Class I Expression as A Novel Therapeutic Strategy for Cancer Immunotherapy
Identification of the molecular aberrations responsible for altered tumor expression of HLA class I derived peptide complexes is crucial for the success of cancer T cell-based immunotherapy as well as for the rational design of novel immunotherapeutic strategies which restore an integral expression of HLA class I derived peptide complexes. Most of the defects of HLA class I derived peptide complex in human cancers are distinguished in “hard” or “soft” lesions [152]. “Hard” lesions are caused by structural gene alterations that induce loss of expression of HLA class I derived peptide complexes. They are reported in about 30–40% of human cancers [226]. LOH and β2-m gene mutations at chromosomes 6 and 15, respectively, represent the major cause of “hard” defects. In addition, a homologous point mutation in codon 67 of the β2-m gene also results in the total loss of HLA class I molecule expression [227,228]. These types of alterations cannot be repaired by any signaling pathway inhibitors as well as chemotherapeutic or immunotherapeutic agents. Del Campo et al. showed that infection of cells carrying β2-m mutations with an adenoviral vector expressing the human β2-m gene caused a total restoration of HLA class I molecule expression [229]. Thus, based on the type of mutated genes, transfection with a wild type gene, such as HLA class I heavy chain or β2-m genes, can potentially restore the expression of HLA class I derived peptide complex [152].
On the other hand, “soft” defects are caused by transcriptional or post-transcriptional modifications of one of HLA class I APM component genes. Activation of pro-tumorigenic pathways or epigenetic modifications which induce a reduced expression of HLA class I APM components are the major causes of “soft” defects [230]. Activation of pro-tumorigenic pathways includes inhibition of Jak/STAT pathway or activation of mitogen-activated protein kinase (MAPK) pathway. Epigenetic modifications include those that cause impairment in gene regulation such as hypermethylation of the HLA-A, B, and C heavy chains, β2m and APM component encoding gene promoter regions, or unbalanced histone acetylation [152,227,228,231]. Lastly, post-transcriptional alterations are induced by aberrant function of micro-RNAs (miRNAs). All these types of alterations can be restored by signaling pathway inhibitors, chemotherapeutic or immunotherapeutic agents (Figure 2). In Table 1, we have summarized the information available in the literature on the main molecules able to restore HLA class I expression in cases associated with “soft” defects.
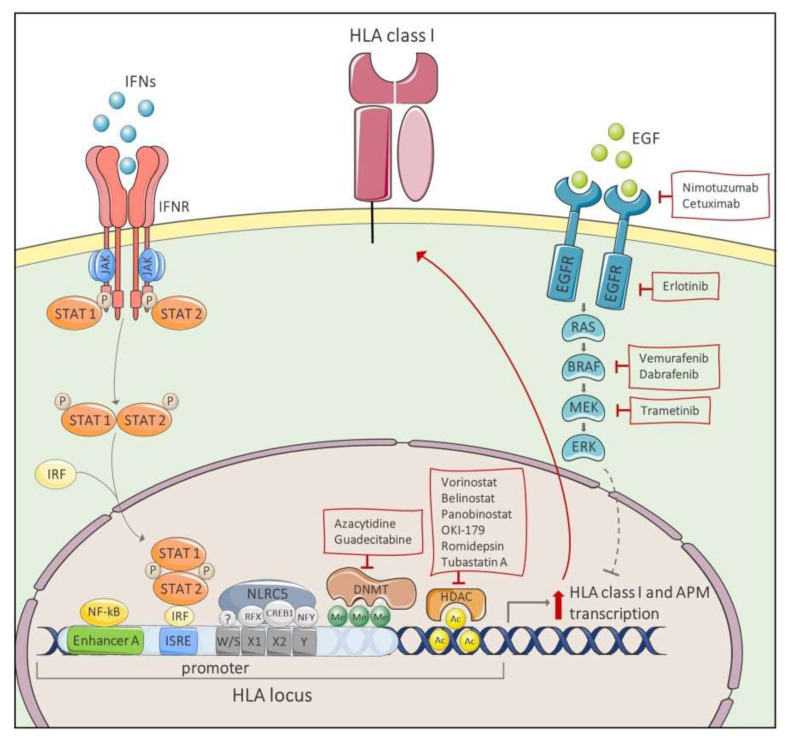
Figure 2.
Mechanisms for restoring HLA class I expression. IFN binding to IFNR triggers Jak/STAT transduction pathway. STAT1/STAT2/IRF complex translocates to the nucleus where it binds to ISRE motifs located in HLA promoter region, inducing HLA gene transcription. EGFR and MAPK down-stream pathways suppress HLA class I surface expression. EGFR inhibitors, such as nimotuzumab, cetuximab and erlotinib, BRAF inhibitors, vemurafenib and dabrafenib, and MEK inhibitor trametinib can increase expression of both HLA class I antigens and APM components. DNMT inhibitors (azacytidine and guadecitabine) and HDAC inhibitors (vorinostat, belinostat, panobinostat, OKI-179, romidepsin and tubastatin A) avoid hypermethylation of HLA promoter region and histones hypoacetylation that cause HLA genes silencing. Abbreviations: HLA, human leukocyte antigen; IFNs, interferons; IFNR, interferon receptor; IRF, IFN regulatory factor; ISRE, IFN-sensitive response element; EGF, epidermal growth factor; EGFR, epidermal growth factor receptor; DNMT, DNA methyltransferase; HDAC, histone deacetylase; APM, antigen-processing machinery.
Table 1.
Drugs for restoring MHC class I expression [228,230,232,233,234,235,236,237,238,239,240,241,242,243,244,245,246,247,248,249,250,251,252,253].
| Name | Target | Combination Therapy | Cancer Type | References |
|---|---|---|---|---|
| Kinase Inhibitors | ||||
| Nimotuzumab Cetuximab Erlotinib |
EGFR | IFN-γ | epidermoid carcinoma lung cancer head and neck carcinoma lung adenocarcinoma |
Garrido G. et al. 2017 [236] Srivastava et al. 2015 [234] Im et al. 2016 [235] |
| Trametinib | MEK1/2 | IFN-γ | mesothelioma melanoma lung adenocarcinoma colon adenocarcinoma thyroid carcinoma breast cancer head and neck carcinoma |
Brea et al. 2016 [232] Loi et al. 2016 [233] Kang et al. 2019 [237] |
| Vemurafenib | BRAFV600E | IFN-γ IFNα-2b |
Melanoma | Sapkota et al. 2013 [238] Sabbatino et al. 2016 [239] |
| Dabrafenib | Trametinib | Hu-Lieskovan et al. 2015 [240] | ||
| Epigenetic Agents | ||||
| Vorinostat | HDAC class I, II, and IV | Mithramycin A | Merkel cell carcinoma | Ritter et al. 2017 [241] |
| B-cell lymphoma cutaneous T-cell lymphoma acute myeloid leukemia glioma |
Chacon et al. 2016 [247] Banik et al. 2019 [228] Sun et al. 2019 [242] Yang et al. 2019 [243] Wang et al. 2020 [253] |
|||
| Belinostat | peripheral T-cell lymphoma B-cell lymphoma |
Banik et al. 2019 [228] Wang et al. 2020 [253] |
||
| Panobinostat | multiple myeloma B-cell lymphoma |
Banik et al. 2019 [228] Wang et al. 2020 [253] |
||
| OKI-179 | diffuse large B-cell lymphoma | Wang et al. 2019 [230] | ||
| Romidepsin | HDAC 1-2 | B-cell lymphoma peripheral T cell lymphoma |
Banik et al. 2019 [228] Wang et al. 2020 [253] |
|
| Tubastatin A | HDAC 6 | Melanoma | Woan et al. 2015 [244] | |
| Azacytidine | DNMT | lung carcinoma melanoma |
Fonsatti et al. 2007 [246] Chacon et al. 2016 [247] |
|
| Guadecitabine | breast cancer | Luo et al. 2018 [248] | ||
| Tazemetostat | EZH2 | diffuse large B-cell lymphoma | Ennishi et al. 2019 [245] | |
| Chemotherapeutics | ||||
| Cisplatin | DNA | Vinorelbine or5-fluorouracil | lung cancer head and neck carcinoma |
De Biasi et al. 2014 [249] |
| Epothilone B Taxol Vinblastine |
Microtubules | ovarian cancer | Pellicciotta et al. 2011 [251] | |
| Doxorubicin | Topoisomerase | nasopharyngeal carcinoma | Faè et al. 2016 [250] | |
| Topotecan Etoposide | breast cancer | Wan et al. 2012 [252] | ||
The administration of IFNs might effectively counteract HLA class I down-regulation in cancer cells by boosting the presentation of tumor specific-associated antigens [254,255,256,257]. A phase 0 clinical trial showed that systemic administration of IFN-γ increases not only HLA class I expression on tumor cells, but also T-cell infiltration in cold tumors [258] IFN-mediated up-regulation of HLA class I derived peptide complexes occurs via activation of the Jak/STAT pathway which induces the binding of IRFs to ISRE motifs located in HLA class I promoter region [107,254]. In addition, IFN-γ-mediated HLA class I derived peptide complex up-regulation is also associated to an increased histone demethylation and acetylation of APM genes in the MHC locus, particularly of histone H3 in TAP1 promoter locus [259]. Down-regulation of Jak/STAT signal transduction pathway and therefore of HLA class I derived peptide complex is strictly linked to protein kinase activation. Activation of either epithelial growth factor receptor (EGFR) or Ras/MAPK downstream pathway directly suppresses expression of HLA class I derived peptide complex and IFN-induced antigen presentation [232,233]. In this case the combination of IFNs and tyrosine kinase inhibitors (TKIs) can represent a potential therapeutic strategy for recovering HLA class I derived peptide complex expression in cancer cells. EGFR inhibitors such as the monoclonal antibodies nimotuzumab and cetuximab and the tyrosine kinase inhibitor erlotinib have been shown to increase membrane expression of HLA class I APM components in cells with EGFR activation [234,235,236]. Im and collaborators showed that the sensitivity of lung cancer cells to erlotinib positively correlates with the increase of expression of HLA class I derived peptide complex following IFN-γ treatment [235]. Moreover, the MAPK/ERK kinase (MEK) inhibitor trametinib, in combination with IFN-γ, has been shown to enhance tumor immunogenicity by modulating HLA class I derived peptide complex in different types of malignancies [232], including triple-negative breast cancer [233] and head and neck squamous cell carcinoma [237]. In melanoma patients harboring the BRAFV600E mutations, BRAF inhibitor vemurafenib strengthens the induction of HLA class I antigen expression by both IFN-γ and IFN-α2b [238,239]. Furthermore, this effect was enhanced when BRAF inhibition was combined with the MEK inhibitor trametinib [240].
However, it is important to mention that several tumors develop IFN-γ signaling insensitivity. The lack of response to IFN-γ stimulation results from cellular defects on IFNγR1receptor or downstream components of the signaling pathway such as Janus kinases (JAK) 1 and 2 [260]. In addition, the absence of STAT1 expression and/or tyrosine-phosphorylation and epigenetic regulation of IRF1 can contribute to the lack of HLA class I expression, restoring in vitro following IFN-γ administration [261,262]. Lastly, given the addiction of IFN-γ-induced HLA class I and II up-regulation by NLRC5/CITA and CIITA, respectively, genetic or functional defects on these two trans-activators abrogate the ability of IFN-γ to boost antigen processing and presentation [106,260].
Inhibition of histone deacetylases (HDACs), enzymes that remove acetyl group from lysines on histones, also promotes the increase of expression of HLA class I APM components. HDAC inhibitors (HDACi) vorinostat, belinostat, and panobinostat, targeting HDAC class I, II, and IV, and romidepsin, a specific HDAC 1 and 2 inhibitor, are currently approved for hematological malignancies [228,230]. Vorinostat, also known as SAHA, in combination with mithramycin A, a Sp1 inhibitor, reverses the histone hypoacetylation which causes APM gene silencing in Merkel cell carcinoma [241]. In addition, vorinostat promotes tumor cell recognition by CTLs in glioma cells [242,243]. In diffuse large B-cell lymphoma, the HDAC class I inhibitor OKI-179 reverts down-regulation of HLA class I derived peptide complex, a typical feature of this hematological cancer [230]. Likewise, the selective inhibition of HDAC6 with tubastatin A improves the immunogenicity of melanoma cells by increasing HLA class I expression [244]. Mutations in EZH2 gene, encoding for a histone-lysine N-methyltransferase, are strictly connected to loss of expression of HLA class I derived peptide complex in large B-cell lymphoma. Treatment with EZH2 inhibitor tazemetostat (EPZ-6438) restores the expression of HLA class I derived peptide complex in EZH2-mutant cell lines [245]. In cases of DNA hypermethylation, therapy with DNA methyltransferase inhibitors (DNMTi) such as azacytidine and decitabine restores the expression of HLA class I derived peptide complex [263]. Azacytidine increased the transcription of APM genes and HLA class I molecule expression in lung carcinoma and melanoma cells [246,247]. Guadecitabine, a novel DNMTi, significantly up-regulated both basal and IFN-γ-dependent expression of HLA class I derived peptide complex in breast cancer cells [248]. In the case of HLA class I APM component down-regulation mediated by aberrant miRNA function, it has been shown that suppression of miR-9 and miR-19 expression restores the expression of HLA class I derived peptide complex [264,265]. Lastly, several chemotherapeutic agents can promote the expression of HLA class I derived peptide complex. In many types of cancer cell lines cisplatin alone or in combination with vinorelbine or 5-fluorouracil [249], doxorubicin [250], the microtubule-destabilizers epothilone B, taxol and vinblastine [251] increase the expression of HLA class I APM components. Moreover, some other chemotherapeutic agents, such as the topoisomerase-I inhibitors topotecan and etoposide, indirectly induce the up-regulation of HLA class I derived peptide complex by stimulating IFN-β autocrine/paracrine signaling of tumor cells [252].
5. Conclusions
In the last decades, important progress has been made pertaining to the knowledge of the structure and the function of HLA class I and II antigen presentation pathways. An improved knowledge of molecular mechanisms underlying HLA class I APM defects will be crucial to better understand the mechanisms determining carcinogenesis, tumor progression and immune escape. Moreover, recent knowledge of the pathways leading to restored HLA expression could be utilized to conceive new cancer therapeutic strategies. The identification of molecular defects resulting in acquired insensitivity to the stimulation with specific adjuvants, such as IFNs, could allow to a better selection of cancer patients who can take advantage from this approach for HLA expression restoring. Because of their crucial role in tumor cell recognition, additional studies are urgently needed to validate the role of expression of HLA class I and II antigens, as well as of APM components as novel potential predictive biomarkers in cancer immunotherapy. Moreover, where HLA class I and II gene expression is not recoverable, the development of different immunotherapeutic approaches able to target native cell surface antigens, and therefore independent from HLA expression, such as chimer antigen receptor (CAR)-T cell or bi-specific T-cell engager antibodies (BiTES) should be taken in consideration.
Abbreviations
| ACT | adoptive cell-transfer |
| APC | antigen presenting cell |
| APM | antigen processing machinery |
| ATF1 | activating transcriptor factor 1 |
| ATP | adenosine triphosphate |
| BiTES | bi-specific T-cell engager antibodies |
| BLS | bare lymphocyte disease |
| CAR | chimeric antigen receptor |
| CIITA | class II trans-activator |
| CIITA | class I trans activator |
| CLIP | class II-associated I chain peptide |
| CNX | calnexin |
| CREB1 | cAMP-responsive element binding protein 1 |
| CRT | calreticulin |
| CTL | cytotoxic T lymphocyte |
| CTLA-4 | cytotoxic T-lymphocyte antigen 4 |
| DC | dendritic cell |
| DFS | disease free survival |
| DNMTi | DNA methyltransferase inhibitor |
| EGFR | epithelial growth factor receptor |
| ER | endoplasmic reticulum |
| HDAC | histone deacetylase |
| HLA | human leukocyte antigen |
| ICI | immune checkpoint inhibitor |
| IFN | interferon |
| IRF | IFN Regulatory Factor |
| ISRE | IFN-sensitive response element |
| KIR | killer cell immunoglobulin-like receptor |
| JAK | janus kinase |
| LAG-3 | lymphocyte activation gene 3 |
| LMP | low molecular weight protein |
| LOH | loss of heterozygosity |
| mAb | monoclonal antibody |
| MAPK | mitogen-activated protein kinase |
| MEK | MAPK/ERK kinase |
| MHC | major histocompatibility complex |
| MIIC | MHC class II compartment |
| NF-kB | nuclear factor kB |
| NFY | nuclear transcriptor factor Y |
| NK | natural killer |
| NLRC5 | NOD-like receptor 5 |
| OS | overall survival |
| PD-1 | programmed cell death 1 |
| PD-L1 | programmed cell death ligand 1 |
| PFS | progression free survival |
| RFX | regulatory factor X |
| RFXANK | RFX-associated ankyrin-containing protein |
| RFXAP | RFX associated protein |
| TAP | transporters associated with antigen processing |
| TCR | T-cell receptor |
| TKI | tyrosine kinase inhibitor |
| TNF-α | tumor necrosis factor alpha |
| TA | tumor antigen |
| TAA | tumor associated antigen |
| TAM | Type II tumour-associated macrophages |
| TIL | tumor-infiltrating lymphocyte |
| TME | tumor microenvironment |
| Treg | regulatory T cell |
| TSA | tumor specific antigen |
| tsMHC-II | tumor specific MHC class II |
| β2-m | β2-microglobulin |
Author Contributions
Conception and design: F.S. and J.D.C. Writing, review, and/or revision of the manuscript: F.S., L.L., I.S., G.P., G.C., and J.D.C. Study supervision: F.S., V.C., C.S., and S.P. Other (discussed results and implications of findings): F.S., S.P., V.C., F.A.S., and C.S. All authors have read and agreed to the published version of the manuscript.
Funding
The work was supported by Ministero dell’Università e della Ricerca (Progetti di Rilevante Interesse Nazionale (PRIN), 2017, CODICE 2017PHRC8X_003) (to SP) and by “Fondazione con il Sud” (Brains2South 2015-PDR-0224) (to J.D.C.).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Zinkernagel R.M., Doherty P.C. Restriction of in vitro T cell-mediated cytotoxicity in lymphocytic choriomeningitis within a syngeneic or semiallogeneic system. Nature. 1974;248:701–702. doi: 10.1038/248701a0. [DOI] [PubMed] [Google Scholar]
- 2.Townsend A.R., Skehel J.J. Influenza A specific cytotoxic T-cell clones that do not recognize viral glycoproteins. Nature. 1982;300:655–657. doi: 10.1038/300655a0. [DOI] [PubMed] [Google Scholar]
- 3.Townsend A.R., Rothbard J., Gotch F.M., Bahadur G., Wraith D., McMichael A.J. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell. 1986;44:959–968. doi: 10.1016/0092-8674(86)90019-X. [DOI] [PubMed] [Google Scholar]
- 4.Bjorkman P.J., Saper M.A., Samraoui B., Bennett W.S., Strominger J.L., Wiley D.C. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987;329:506–512. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- 5.Rötzschke O., Falk K., Deres K., Schild H., Norda M., Metzger J., Jung G., Rammensee H.G. Isolation and analysis of naturally processed viral peptides as recognized by cytotoxic T cells. Nature. 1990;348:252–254. doi: 10.1038/348252a0. [DOI] [PubMed] [Google Scholar]
- 6.Trowsdale J. Genomic structure and function in the MHC. Trends Genet. 1993;9:117–122. doi: 10.1016/0168-9525(93)90205-V. [DOI] [PubMed] [Google Scholar]
- 7.Flutter B., Gao B. MHC class I antigen presentation--recently trimmed and well presented. Cell. Mol. Immunol. 2004;1:22–30. [PubMed] [Google Scholar]
- 8.Norman P.J., Norberg S.J., Guethlein L.A., Nemat-Gorgani N., Royce T., Wroblewski E.E., Dunn T., Mann T., Alicata C., Hollenbach J.A., et al. Sequences of 95 human MHC haplotypes reveal extreme coding variation in genes other than highly polymorphic HLA class I and II. Genome Res. 2017;27:813–823. doi: 10.1101/gr.213538.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horton R., Wilming L., Rand V., Lovering R.C., Bruford E.A., Khodiyar V.K., Lush M.J., Povey S., Talbot C.C., Wright M.W., et al. Gene map of the extended human MHC. Nat. Rev. Genet. 2004;5:889–899. doi: 10.1038/nrg1489. [DOI] [PubMed] [Google Scholar]
- 10.Vandiedonck C., Knight J.C. The human Major Histocompatibility Complex as a paradigm in genomics research. Brief. Funct. Genom. Proteom. 2009;8:379–394. doi: 10.1093/bfgp/elp010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trowsdale J., Knight J.C. Major histocompatibility complex genomics and human disease. Annu. Rev. Genom. Hum. Genet. 2013;14:301–323. doi: 10.1146/annurev-genom-091212-153455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Complete sequence and gene map of a human major histocompatibility complex. The MHC sequencing consortium. Nature. 1999;401:921–923. doi: 10.1038/44853. [DOI] [PubMed] [Google Scholar]
- 13.Milner C.M., Campbell R.D. Genetic organization of the human MHC class III region. Front. Biosci. 2001;6:D914–D926. doi: 10.2741/A653. [DOI] [PubMed] [Google Scholar]
- 14.Neefjes J., Jongsma M.L.M., Paul P., Bakke O. Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nat. Rev. Immunol. 2011;11:823–836. doi: 10.1038/nri3084. [DOI] [PubMed] [Google Scholar]
- 15.Ajitkumar P., Geier S.S., Kesari K.V., Borriello F., Nakagawa M., Bluestone J.A., Saper M.A., Wiley D.C., Nathenson S.G. Evidence that multiple residues on both the alpha-helices of the class I MHC molecule are simultaneously recognized by the T cell receptor. Cell. 1988;54:47–56. doi: 10.1016/0092-8674(88)90178-X. [DOI] [PubMed] [Google Scholar]
- 16.Garcia K.C., Degano M., Stanfield R.L., Brunmark A., Jackson M.R., Peterson P.A., Teyton L., Wilson I.A. An alphabeta T cell receptor structure at 2.5 A and its orientation in the TCR-MHC complex. Science. 1996;274:209–219. doi: 10.1126/science.274.5285.209. [DOI] [PubMed] [Google Scholar]
- 17.Garboczi D.N., Ghosh P., Utz U., Fan Q.R., Biddison W.E., Wiley D.C. Structure of the complex between human T-cell receptor, viral peptide and HLA-A2. Nature. 1996;384:134–141. doi: 10.1038/384134a0. [DOI] [PubMed] [Google Scholar]
- 18.Blum J.S., Wearsch P.A., Cresswell P. Pathways of antigen processing. Annu. Rev. Immunol. 2013;31:443–473. doi: 10.1146/annurev-immunol-032712-095910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farhood B., Najafi M., Mortezaee K. CD8+ cytotoxic T lymphocytes in cancer immunotherapy: A review. J. Cell. Physiol. 2019;234:8509–8521. doi: 10.1002/jcp.27782. [DOI] [PubMed] [Google Scholar]
- 20.Cruz F.M., Colbert J.D., Merino E., Kriegsman B.A., Rock K.L. The Biology and Underlying Mechanisms of Cross-Presentation of Exogenous Antigens on MHC-I Molecules. Annu. Rev. Immunol. 2017;35:149–176. doi: 10.1146/annurev-immunol-041015-055254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rock K.L., Shen L. Cross-presentation: Underlying mechanisms and role in immune surveillance. Immunol. Rev. 2005;207:166–183. doi: 10.1111/j.0105-2896.2005.00301.x. [DOI] [PubMed] [Google Scholar]
- 22.Muntjewerff E.M., Meesters L.D., Van den Bogaart G. Antigen Cross-Presentation by Macrophages. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.01276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bevan M.J. Minor H antigens introduced on H-2 different stimulating cells cross-react at the cytotoxic T cell level during in vivo priming. J. Immunol. 1976;117:2233–2238. [PubMed] [Google Scholar]
- 24.Embgenbroich M., Burgdorf S. Current Concepts of Antigen Cross-Presentation. Front. Immunol. 2018;9 doi: 10.3389/fimmu.2018.01643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leone P., Shin E.-C., Perosa F., Vacca A., Dammacco F., Racanelli V. MHC class I antigen processing and presenting machinery: Organization, function, and defects in tumor cells. J. Natl. Cancer Inst. 2013;105:1172–1187. doi: 10.1093/jnci/djt184. [DOI] [PubMed] [Google Scholar]
- 26.Thielens A., Vivier E., Romagné F. NK cell MHC class I specific receptors (KIR): From biology to clinical intervention. Curr. Opin. Immunol. 2012;24:239–245. doi: 10.1016/j.coi.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 27.Wieczorek M., Abualrous E.T., Sticht J., Álvaro-Benito M., Stolzenberg S., Noé F., Freund C. Major Histocompatibility Complex (MHC) Class I and MHC Class II Proteins: Conformational Plasticity in Antigen Presentation. Front. Immunol. 2017;8:292. doi: 10.3389/fimmu.2017.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cunningham B.A. Structure and significance of beta2-microglobulin. Fed. Proc. 1976;35:1171–1176. [PubMed] [Google Scholar]
- 29.Cunningham B.A., Berggård I. Structure, evolution and significance of beta2-microglobulin. Transplant. Rev. 1974;21:3–14. doi: 10.1111/j.1600-065X.1974.tb01543.x. [DOI] [PubMed] [Google Scholar]
- 30.Madden D.R. The three-dimensional structure of peptide-MHC complexes. Annu. Rev. Immunol. 1995;13:587–622. doi: 10.1146/annurev.iy.13.040195.003103. [DOI] [PubMed] [Google Scholar]
- 31.Wilson I.A., Fremont D.H. Structural analysis of MHC class I molecules with bound peptide antigens. Semin. Immunol. 1993;5:75–80. doi: 10.1006/smim.1993.1011. [DOI] [PubMed] [Google Scholar]
- 32.Persson K., Schneider G. Three-dimensional structures of MHC class I-peptide complexes: Implications for peptide recognition. Arch. Immunol. Ther. Exp. 2000;48:135–142. [PubMed] [Google Scholar]
- 33.Mage M.G., Dolan M.A., Wang R., Boyd L.F., Revilleza M.J., Robinson H., Natarajan K., Myers N.B., Hansen T.H., Margulies D.H. The peptide-receptive transition state of MHC class I molecules: Insight from structure and molecular dynamics. J. Immunol. 2012;189:1391–1399. doi: 10.4049/jimmunol.1200831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Braud V.M., Allan D.S., O’Callaghan C.A., Söderström K., D’Andrea A., Ogg G.S., Lazetic S., Young N.T., Bell J.I., Phillips J.H., et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature. 1998;391:795–799. doi: 10.1038/35869. [DOI] [PubMed] [Google Scholar]
- 35.Carosella E.D., HoWangYin K.-Y., Favier B., LeMaoult J. HLA-G-dependent suppressor cells: Diverse by nature, function, and significance. Hum. Immunol. 2008;69:700–707. doi: 10.1016/j.humimm.2008.08.280. [DOI] [PubMed] [Google Scholar]
- 36.Lepin E.J., Bastin J.M., Allan D.S., Roncador G., Braud V.M., Mason D.Y., Van der Merwe P.A., McMichael A.J., Bell J.I., Powis S.H., et al. Functional characterization of HLA-F and binding of HLA-F tetramers to ILT2 and ILT4 receptors. Eur. J. Immunol. 2000;30:3552–3561. doi: 10.1002/1521-4141(200012)30:12<3552::AID-IMMU3552>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 37.Persson G., Jørgensen N., Nilsson L.L., Andersen L.H.J., Hviid T.V.F. A role for both HLA-F and HLA-G in reproduction and during pregnancy? Hum. Immunol. 2020;81:127–133. doi: 10.1016/j.humimm.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 38.Hackmon R., Pinnaduwage L., Zhang J., Lye S.J., Geraghty D.E., Dunk C.E. Definitive class I human leukocyte antigen expression in gestational placentation: HLA-F, HLA-E, HLA-C, and HLA-G in extravillous trophoblast invasion on placentation, pregnancy, and parturition. Am. J. Reprod. Immunol. 2017;77 doi: 10.1111/aji.12643. [DOI] [PubMed] [Google Scholar]
- 39.Joosten S.A., Sullivan L.C., Ottenhoff T.H.M. Characteristics of HLA-E Restricted T-Cell Responses and Their Role in Infectious Diseases. J. Immunol. Res. 2016;2016:2695396. doi: 10.1155/2016/2695396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raghavan M., Del Cid N., Rizvi S.M., Peters L.R. MHC class I assembly: Out and about. Trends Immunol. 2008;29:436–443. doi: 10.1016/j.it.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paulsson K.M. Evolutionary and functional perspectives of the major histocompatibility complex class I antigen-processing machinery. Cell. Mol. Life Sci. 2004;61:2446–2460. doi: 10.1007/s00018-004-4113-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wearsch P.A., Cresswell P. The quality control of MHC class I peptide loading. Curr. Opin. Cell Biol. 2008;20:624–631. doi: 10.1016/j.ceb.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Antoniou A.N., Powis S.J., Elliott T. Assembly and export of MHC class I peptide ligands. Curr. Opin. Immunol. 2003;15:75–81. doi: 10.1016/S0952-7915(02)00010-9. [DOI] [PubMed] [Google Scholar]
- 44.Jensen P.E. Recent advances in antigen processing and presentation. Nat. Immunol. 2007;8:1041–1048. doi: 10.1038/ni1516. [DOI] [PubMed] [Google Scholar]
- 45.Kloetzel P.M. Antigen processing by the proteasome. Nat. Rev. Mol. Cell Biol. 2001;2:179–187. doi: 10.1038/35056572. [DOI] [PubMed] [Google Scholar]
- 46.Sant A., Yewdell J. Antigen processing and recognition. Curr. Opin. Immunol. 2003;15:66–68. doi: 10.1016/S0952-7915(02)00020-1. [DOI] [PubMed] [Google Scholar]
- 47.Schölz C., Tampé R. The peptide-loading complex--antigen translocation and MHC class I loading. Biol. Chem. 2009;390:783–794. doi: 10.1515/BC.2009.069. [DOI] [PubMed] [Google Scholar]
- 48.Kotsias F., Cebrian I., Alloatti A. Antigen processing and presentation. Int. Rev. Cell Mol. Biol. 2019;348:69–121. doi: 10.1016/bs.ircmb.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 49.Koegl M., Hoppe T., Schlenker S., Ulrich H.D., Mayer T.U., Jentsch S. A novel ubiquitination factor, E4, is involved in multiubiquitin chain assembly. Cell. 1999;96:635–644. doi: 10.1016/S0092-8674(00)80574-7. [DOI] [PubMed] [Google Scholar]
- 50.Goldberg A.L. Functions of the proteasome: The lysis at the end of the tunnel. Science. 1995;268:522–523. doi: 10.1126/science.7725095. [DOI] [PubMed] [Google Scholar]
- 51.Kloetzel P.-M. The proteasome and MHC class I antigen processing. Biochim. Biophys. Acta. 2004;1695:225–233. doi: 10.1016/j.bbamcr.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 52.Rechsteiner M., Hoffman L., Dubiel W. The multicatalytic and 26 S proteases. J. Biol. Chem. 1993;268:6065–6068. [PubMed] [Google Scholar]
- 53.Griffin T.A., Nandi D., Cruz M., Fehling H.J., Kaer L.V., Monaco J.J., Colbert R.A. Immunoproteasome assembly: Cooperative incorporation of interferon gamma (IFN-gamma)-inducible subunits. J. Exp. Med. 1998;187:97–104. doi: 10.1084/jem.187.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Groettrup M., Standera S., Stohwasser R., Kloetzel P.M. The subunits MECL-1 and LMP2 are mutually required for incorporation into the 20S proteasome. Proc. Natl. Acad. Sci. USA. 1997;94:8970–8975. doi: 10.1073/pnas.94.17.8970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nandi D., Woodward E., Ginsburg D.B., Monaco J.J. Intermediates in the formation of mouse 20S proteasomes: Implications for the assembly of precursor beta subunits. EMBO J. 1997;16:5363–5375. doi: 10.1093/emboj/16.17.5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aki M., Shimbara N., Takashina M., Akiyama K., Kagawa S., Tamura T., Tanahashi N., Yoshimura T., Tanaka K., Ichihara A. Interferon-gamma induces different subunit organizations and functional diversity of proteasomes. J. Biochem. 1994;115:257–269. doi: 10.1093/oxfordjournals.jbchem.a124327. [DOI] [PubMed] [Google Scholar]
- 57.Shin E.-C., Seifert U., Kato T., Rice C.M., Feinstone S.M., Kloetzel P.-M., Rehermann B. Virus-induced type I IFN stimulates generation of immunoproteasomes at the site of infection. J. Clin. Investig. 2006;116:3006–3014. doi: 10.1172/JCI29832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen W., Norbury C.C., Cho Y., Yewdell J.W., Bennink J.R. Immunoproteasomes shape immunodominance hierarchies of antiviral CD8(+) T cells at the levels of T cell repertoire and presentation of viral antigens. J. Exp. Med. 2001;193:1319–1326. doi: 10.1084/jem.193.11.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Melnikova V.I., Sharova N.P., Maslova E.V., Voronova S.N., Zakharova L.A. Ontogenesis of rat immune system: Proteasome expression in different cell populations of the developing thymus. Cell. Immunol. 2010;266:83–89. doi: 10.1016/j.cellimm.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 60.Schmitt L., Tampé R. Structure and mechanism of ABC transporters. Curr. Opin. Struct. Biol. 2002;12:754–760. doi: 10.1016/S0959-440X(02)00399-8. [DOI] [PubMed] [Google Scholar]
- 61.Arora S., Lapinski P.E., Raghavan M. Use of chimeric proteins to investigate the role of transporter associated with antigen processing (TAP) structural domains in peptide binding and translocation. Proc. Natl. Acad. Sci. USA. 2001;98:7241–7246. doi: 10.1073/pnas.131132198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Higgins C.F., Linton K.J. The ATP switch model for ABC transporters. Nat. Struct. Mol. Biol. 2004;11:918–926. doi: 10.1038/nsmb836. [DOI] [PubMed] [Google Scholar]
- 63.Parcej D., Tampé R. ABC proteins in antigen translocation and viral inhibition. Nat. Chem. Biol. 2010;6:572–580. doi: 10.1038/nchembio.410. [DOI] [PubMed] [Google Scholar]
- 64.Herget M., Baldauf C., Schölz C., Parcej D., Wiesmüller K.-H., Tampé R., Abele R., Bordignon E. Conformation of peptides bound to the transporter associated with antigen processing (TAP) Proc. Natl. Acad. Sci. USA. 2011;108:1349–1354. doi: 10.1073/pnas.1012355108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Koopmann J.O., Post M., Neefjes J.J., Hämmerling G.J., Momburg F. Translocation of long peptides by transporters associated with antigen processing (TAP) Eur. J. Immunol. 1996;26:1720–1728. doi: 10.1002/eji.1830260809. [DOI] [PubMed] [Google Scholar]
- 66.Momburg F., Roelse J., Howard J.C., Butcher G.W., Hämmerling G.J., Neefjes J.J. Selectivity of MHC-encoded peptide transporters from human, mouse and rat. Nature. 1994;367:648–651. doi: 10.1038/367648a0. [DOI] [PubMed] [Google Scholar]
- 67.Momburg F., Roelse J., Hämmerling G.J., Neefjes J.J. Peptide size selection by the major histocompatibility complex-encoded peptide transporter. J. Exp. Med. 1994;179:1613–1623. doi: 10.1084/jem.179.5.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Momburg F., Hämmerling G.J. Generation and TAP-mediated transport of peptides for major histocompatibility complex class I molecules. Adv. Immunol. 1998;68:191–256. doi: 10.1016/s0065-2776(08)60560-x. [DOI] [PubMed] [Google Scholar]
- 69.Schumacher T.N., Kantesaria D.V., Heemels M.T., Ashton-Rickardt P.G., Shepherd J.C., Fruh K., Yang Y., Peterson P.A., Tonegawa S., Ploegh H.L. Peptide length and sequence specificity of the mouse TAP1/TAP2 translocator. J. Exp. Med. 1994;179:533–540. doi: 10.1084/jem.179.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Roelse J., Grommé M., Momburg F., Hämmerling G., Neefjes J. Trimming of TAP-translocated peptides in the endoplasmic reticulum and in the cytosol during recycling. J. Exp. Med. 1994;180:1591–1597. doi: 10.1084/jem.180.5.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Diedrich G., Bangia N., Pan M., Cresswell P. A role for calnexin in the assembly of the MHC class I loading complex in the endoplasmic reticulum. J. Immunol. 2001;166:1703–1709. doi: 10.4049/jimmunol.166.3.1703. [DOI] [PubMed] [Google Scholar]
- 72.Hughes E.A., Cresswell P. The thiol oxidoreductase ERp57 is a component of the MHC class I peptide-loading complex. Curr. Biol. 1998;8:709–712. doi: 10.1016/S0960-9822(98)70278-7. [DOI] [PubMed] [Google Scholar]
- 73.Morrice N.A., Powis S.J. A role for the thiol-dependent reductase ERp57 in the assembly of MHC class I molecules. Curr. Biol. 1998;8:713–716. doi: 10.1016/S0960-9822(98)70279-9. [DOI] [PubMed] [Google Scholar]
- 74.Sadasivan B., Lehner P.J., Ortmann B., Spies T., Cresswell P. Roles for calreticulin and a novel glycoprotein, tapasin, in the interaction of MHC class I molecules with TAP. Immunity. 1996;5:103–114. doi: 10.1016/S1074-7613(00)80487-2. [DOI] [PubMed] [Google Scholar]
- 75.Momburg F., Tan P. Tapasin-the keystone of the loading complex optimizing peptide binding by MHC class I molecules in the endoplasmic reticulum. Mol. Immunol. 2002;39:217–233. doi: 10.1016/S0161-5890(02)00103-7. [DOI] [PubMed] [Google Scholar]
- 76.Ortmann B., Copeman J., Lehner P.J., Sadasivan B., Herberg J.A., Grandea A.G., Riddell S.R., Tampé R., Spies T., Trowsdale J., et al. A critical role for tapasin in the assembly and function of multimeric MHC class I-TAP complexes. Science. 1997;277:1306–1309. doi: 10.1126/science.277.5330.1306. [DOI] [PubMed] [Google Scholar]
- 77.Dick T.P., Bangia N., Peaper D.R., Cresswell P. Disulfide bond isomerization and the assembly of MHC class I-peptide complexes. Immunity. 2002;16:87–98. doi: 10.1016/S1074-7613(02)00263-7. [DOI] [PubMed] [Google Scholar]
- 78.Ortmann B., Androlewicz M.J., Cresswell P. MHC class I/beta 2-microglobulin complexes associate with TAP transporters before peptide binding. Nature. 1994;368:864–867. doi: 10.1038/368864a0. [DOI] [PubMed] [Google Scholar]
- 79.Wright C.A., Kozik P., Zacharias M., Springer S. Tapasin and other chaperones: Models of the MHC class I loading complex. Biol. Chem. 2004;385:763–778. doi: 10.1515/BC.2004.100. [DOI] [PubMed] [Google Scholar]
- 80.Ellgaard L., Helenius A. ER quality control: Towards an understanding at the molecular level. Curr. Opin. Cell Biol. 2001;13:431–437. doi: 10.1016/S0955-0674(00)00233-7. [DOI] [PubMed] [Google Scholar]
- 81.Oliver J.D., Roderick H.L., Llewellyn D.H., High S. ERp57 functions as a subunit of specific complexes formed with the ER lectins calreticulin and calnexin. Mol. Biol. Cell. 1999;10:2573–2582. doi: 10.1091/mbc.10.8.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Degen E., Cohen-Doyle M.F., Williams D.B. Efficient dissociation of the p88 chaperone from major histocompatibility complex class I molecules requires both beta 2-microglobulin and peptide. J. Exp. Med. 1992;175:1653–1661. doi: 10.1084/jem.175.6.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nössner E., Parham P. Species-specific differences in chaperone interaction of human and mouse major histocompatibility complex class I molecules. J. Exp. Med. 1995;181:327–337. doi: 10.1084/jem.181.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Groothuis T.A.M., Neefjes J. The many roads to cross-presentation. J. Exp. Med. 2005;202:1313–1318. doi: 10.1084/jem.20051379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Neefjes J., Sadaka C. Into the Intracellular Logistics of Cross-Presentation. Front. Immunol. 2012;3 doi: 10.3389/fimmu.2012.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lechler R.I. Structure-function relationships of MHC class II molecules. Immunol. Suppl. 1988;1:25–26. [PubMed] [Google Scholar]
- 87.Stern L.J., Potolicchio I., Santambrogio L. MHC class II compartment subtypes: Structure and function. Curr. Opin. Immunol. 2006;18:64–69. doi: 10.1016/j.coi.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 88.Rock K.L., Reits E., Neefjes J. Present Yourself! By MHC Class I and MHC Class II Molecules. Trends Immunol. 2016;37:724–737. doi: 10.1016/j.it.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chang C.H., Flavell R.A. Class II transactivator regulates the expression of multiple genes involved in antigen presentation. J. Exp. Med. 1995;181:765–767. doi: 10.1084/jem.181.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Collins T., Korman A.J., Wake C.T., Boss J.M., Kappes D.J., Fiers W., Ault K.A., Gimbrone M.A., Strominger J.L., Pober J.S. Immune interferon activates multiple class II major histocompatibility complex genes and the associated invariant chain gene in human endothelial cells and dermal fibroblasts. Proc. Natl. Acad. Sci. USA. 1984;81:4917–4921. doi: 10.1073/pnas.81.15.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Holling T.M., Schooten E., Van Den Elsen P.J. Function and regulation of MHC class II molecules in T-lymphocytes: Of mice and men. Hum. Immunol. 2004;65:282–290. doi: 10.1016/j.humimm.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 92.Reith W., LeibundGut-Landmann S., Waldburger J.-M. Regulation of MHC class II gene expression by the class II transactivator. Nat. Rev. Immunol. 2005;5:793–806. doi: 10.1038/nri1708. [DOI] [PubMed] [Google Scholar]
- 93.Ting J.P.-Y., Trowsdale J. Genetic control of MHC class II expression. Cell. 2002;109(Suppl. S21–S33) doi: 10.1016/S0092-8674(02)00696-7. [DOI] [PubMed] [Google Scholar]
- 94.Boss J.M., Jensen P.E. Transcriptional regulation of the MHC class II antigen presentation pathway. Curr. Opin. Immunol. 2003;15:105–111. doi: 10.1016/S0952-7915(02)00015-8. [DOI] [PubMed] [Google Scholar]
- 95.Neumann J., Koch N. Assembly of major histocompatibility complex class II subunits with invariant chain. FEBS Lett. 2005;579:6055–6059. doi: 10.1016/j.febslet.2005.09.070. [DOI] [PubMed] [Google Scholar]
- 96.Harton J., Jin L., Hahn A., Drake J. Immunological Functions of the Membrane Proximal Region of MHC Class II Molecules. F1000Res. 2016;5 doi: 10.12688/f1000research.7610.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Arnold P.Y., La Gruta N.L., Miller T., Vignali K.M., Adams P.S., Woodland D.L., Vignali D.A.A. The majority of immunogenic epitopes generate CD4+ T cells that are dependent on MHC class II-bound peptide-flanking residues. J. Immunol. 2002;169:739–749. doi: 10.4049/jimmunol.169.2.739. [DOI] [PubMed] [Google Scholar]
- 98.Axelrod M.L., Cook R.S., Johnson D.B., Balko J.M. Biological Consequences of MHC-II Expression by Tumor Cells in Cancer. Clin. Cancer Res. 2019;25:2392–2402. doi: 10.1158/1078-0432.CCR-18-3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pieters J. MHC class II restricted antigen presentation. Curr. Opin. Immunol. 1997;9:89–96. doi: 10.1016/S0952-7915(97)80164-1. [DOI] [PubMed] [Google Scholar]
- 100.Seliger B., Maeurer M.J., Ferrone S. Antigen-processing machinery breakdown and tumor growth. Immunol. Today. 2000;21:455–464. doi: 10.1016/S0167-5699(00)01692-3. [DOI] [PubMed] [Google Scholar]
- 101.Cresswell P. Invariant chain structure and MHC class II function. Cell. 1996;84:505–507. doi: 10.1016/S0092-8674(00)81025-9. [DOI] [PubMed] [Google Scholar]
- 102.Busch R., Cloutier I., Sékaly R.P., Hämmerling G.J. Invariant chain protects class II histocompatibility antigens from binding intact polypeptides in the endoplasmic reticulum. EMBO J. 1996;15:418–428. doi: 10.1002/j.1460-2075.1996.tb00372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Denzin L.K., Cresswell P. HLA-DM induces CLIP dissociation from MHC class II alpha beta dimers and facilitates peptide loading. Cell. 1995;82:155–165. doi: 10.1016/0092-8674(95)90061-6. [DOI] [PubMed] [Google Scholar]
- 104.Kropshofer H., Arndt S.O., Moldenhauer G., Hämmerling G.J., Vogt A.B. HLA-DM acts as a molecular chaperone and rescues empty HLA-DR molecules at lysosomal pH. Immunity. 1997;6:293–302. doi: 10.1016/S1074-7613(00)80332-5. [DOI] [PubMed] [Google Scholar]
- 105.Van Den Elsen P.J. Expression Regulation of Major Histocompatibility Complex Class I and Class II Encoding Genes. Front. Immunol. 2011;2 doi: 10.3389/fimmu.2011.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kobayashi K.S., Van den Elsen P.J. NLRC5: A key regulator of MHC class I-dependent immune responses. Nat. Rev. Immunol. 2012;12:813–820. doi: 10.1038/nri3339. [DOI] [PubMed] [Google Scholar]
- 107.Jongsma M.L.M., Guarda G., Spaapen R.M. The regulatory network behind MHC class I expression. Mol. Immunol. 2019;113:16–21. doi: 10.1016/j.molimm.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 108.Steimle V., Otten L.A., Zufferey M., Mach B. Complementation cloning of an MHC class II transactivator mutated in hereditary MHC class II deficiency (or bare lymphocyte syndrome) Cell. 1993;75:135–146. doi: 10.1016/S0092-8674(05)80090-X. [DOI] [PubMed] [Google Scholar]
- 109.Meissner T.B., Liu Y.-J., Lee K.-H., Li A., Biswas A., Van Eggermond M.C.J.A., Van den Elsen P.J., Kobayashi K.S. NLRC5 cooperates with the RFX transcription factor complex to induce MHC class I gene expression. J. Immunol. 2012;188:4951–4958. doi: 10.4049/jimmunol.1103160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Drozina G., Kohoutek J., Jabrane-Ferrat N., Peterlin B.M. Expression of MHC II genes. Curr. Top. Microbiol. Immunol. 2005;290:147–170. doi: 10.1007/3-540-26363-2_7. [DOI] [PubMed] [Google Scholar]
- 111.Gobin S.J., Van Zutphen M., Westerheide S.D., Boss J.M., Van den Elsen P.J. The MHC-specific enhanceosome and its role in MHC class I and beta(2)-microglobulin gene transactivation. J. Immunol. 2001;167:5175–5184. doi: 10.4049/jimmunol.167.9.5175. [DOI] [PubMed] [Google Scholar]
- 112.Harton J.A., Ting J.P. Class II transactivator: Mastering the art of major histocompatibility complex expression. Mol. Cell. Biol. 2000;20:6185–6194. doi: 10.1128/MCB.20.17.6185-6194.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Reith W., Mach B. The bare lymphocyte syndrome and the regulation of MHC expression. Annu. Rev. Immunol. 2001;19:331–373. doi: 10.1146/annurev.immunol.19.1.331. [DOI] [PubMed] [Google Scholar]
- 114.Krawczyk M., Reith W. Regulation of MHC class II expression, a unique regulatory system identified by the study of a primary immunodeficiency disease. Tissue Antigens. 2006;67:183–197. doi: 10.1111/j.1399-0039.2006.00557.x. [DOI] [PubMed] [Google Scholar]
- 115.Robbins G.R., Truax A.D., Davis B.K., Zhang L., Brickey W.J., Ting J.P.-Y. Regulation of Class I Major Histocompatibility Complex (MHC) by Nucleotide-binding Domain, Leucine-rich Repeat-containing (NLR) Proteins. J. Biol. Chem. 2012;287:24294–24303. doi: 10.1074/jbc.M112.364604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Biswas A., Meissner T.B., Kawai T., Kobayashi K.S. Cutting edge: Impaired MHC class I expression in mice deficient for Nlrc5/class I transactivator. J. Immunol. 2012;189:516–520. doi: 10.4049/jimmunol.1200064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ludigs K., Jandus C., Utzschneider D.T., Staehli F., Bessoles S., Dang A.T., Rota G., Castro W., Zehn D., Vivier E., et al. NLRC5 shields T lymphocytes from NK-cell-mediated elimination under inflammatory conditions. Nat. Commun. 2016;7:10554. doi: 10.1038/ncomms10554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Martin B.K., Chin K.C., Olsen J.C., Skinner C.A., Dey A., Ozato K., Ting J.P. Induction of MHC class I expression by the MHC class II transactivator CIITA. Immunity. 1997;6:591–600. doi: 10.1016/S1074-7613(00)80347-7. [DOI] [PubMed] [Google Scholar]
- 119.Meissner T.B., Li A., Kobayashi K.S. NLRC5: A newly discovered MHC class I transactivator (CITA) Microbes Infect. 2012;14:477–484. doi: 10.1016/j.micinf.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gruen J.R., Weissman S.M. Human MHC class III and IV genes and disease associations. Front. Biosci. 2001;6:D960–D972. doi: 10.2741/A658. [DOI] [PubMed] [Google Scholar]
- 121.Deakin J.E., Papenfuss A.T., Belov K., Cross J.G.R., Coggill P., Palmer S., Sims S., Speed T.P., Beck S., Graves J.A.M. Evolution and comparative analysis of the MHC Class III inflammatory region. BMC Genom. 2006;7:281. doi: 10.1186/1471-2164-7-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Xie T., Rowen L., Aguado B., Ahearn M.E., Madan A., Qin S., Campbell R.D., Hood L. Analysis of the gene-dense major histocompatibility complex class III region and its comparison to mouse. Genome Res. 2003;13:2621–2636. doi: 10.1101/gr.1736803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Johnson D.B., Estrada M.V., Salgado R., Sanchez V., Doxie D.B., Opalenik S.R., Vilgelm A.E., Feld E., Johnson A.S., Greenplate A.R., et al. Melanoma-specific MHC-II expression represents a tumour-autonomous phenotype and predicts response to anti-PD-1/PD-L1 therapy. Nat. Commun. 2016;7:10582. doi: 10.1038/ncomms10582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Park I.A., Hwang S.-H., Song I.H., Heo S.-H., Kim Y.-A., Bang W.S., Park H.S., Lee M., Gong G., Lee H.J. Expression of the MHC class II in triple-negative breast cancer is associated with tumor-infiltrating lymphocytes and interferon signaling. PLoS ONE. 2017;12:e0182786. doi: 10.1371/journal.pone.0182786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Roemer M.G.M., Redd R.A., Cader F.Z., Pak C.J., Abdelrahman S., Ouyang J., Sasse S., Younes A., Fanale M., Santoro A., et al. Major Histocompatibility Complex Class II and Programmed Death Ligand 1 Expression Predict Outcome After Programmed Death 1 Blockade in Classic Hodgkin Lymphoma. J. Clin. Oncol. 2018;36:942–950. doi: 10.1200/JCO.2017.77.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rodig S.J., Gusenleitner D., Jackson D.G., Gjini E., Giobbie-Hurder A., Jin C., Chang H., Lovitch S.B., Horak C., Weber J.S., et al. MHC proteins confer differential sensitivity to CTLA-4 and PD-1 blockade in untreated metastatic melanoma. Sci. Transl. Med. 2018;10 doi: 10.1126/scitranslmed.aar3342. [DOI] [PubMed] [Google Scholar]
- 127.Seliger B., Kloor M., Ferrone S. HLA class II antigen-processing pathway in tumors: Molecular defects and clinical relevance. Oncoimmunology. 2017;6 doi: 10.1080/2162402X.2016.1171447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Oldford S.A., Robb J.D., Watson P.H., Drover S. HLA-DRB alleles are differentially expressed by tumor cells in breast carcinoma. Int. J. Cancer. 2004;112:399–406. doi: 10.1002/ijc.20441. [DOI] [PubMed] [Google Scholar]
- 129.Bustin S.A., Li S.R., Phillips S., Dorudi S. Expression of HLA class II in colorectal cancer: Evidence for enhanced immunogenicity of microsatellite-instability-positive tumours. Tumour Biol. 2001;22:294–298. doi: 10.1159/000050630. [DOI] [PubMed] [Google Scholar]
- 130.Younger A.R., Amria S., Jeffrey W.A., Mahdy A.E.M., Goldstein O.G., Norris J.S., Haque A. HLA class II antigen presentation by prostate cancer cells. Prostate Cancer Prostatic Dis. 2008;11:334–341. doi: 10.1038/sj.pcan.4501021. [DOI] [PubMed] [Google Scholar]
- 131.Forero A., Li Y., Chen D., Grizzle W.E., Updike K.L., Merz N.D., Downs-Kelly E., Burwell T.C., Vaklavas C., Buchsbaum D.J., et al. Expression of the MHC Class II Pathway in Triple-Negative Breast Cancer Tumor Cells Is Associated with a Good Prognosis and Infiltrating Lymphocytes. Cancer Immunol. Res. 2016;4:390–399. doi: 10.1158/2326-6066.CIR-15-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Callahan M.J., Nagymanyoki Z., Bonome T., Johnson M.E., Litkouhi B., Sullivan E.H., Hirsch M.S., Matulonis U.A., Liu J., Birrer M.J., et al. Increased HLA-DMB expression in the tumor epithelium is associated with increased CTL infiltration and improved prognosis in advanced-stage serous ovarian cancer. Clin. Cancer Res. 2008;14:7667–7673. doi: 10.1158/1078-0432.CCR-08-0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dierssen J.W.F., De Miranda N.F.C.C., Ferrone S., Van Puijenbroek M., Cornelisse C.J., Fleuren G.J., Van Wezel T., Morreau H. HNPCC versus sporadic microsatellite-unstable colon cancers follow different routes toward loss of HLA class I expression. BMC Cancer. 2007;7:33. doi: 10.1186/1471-2407-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.De Miranda N.F.C.C., Nielsen M., Pereira D., Van Puijenbroek M., Vasen H.F., Hes F.J., Van Wezel T., Morreau H. MUTYH-associated polyposis carcinomas frequently lose HLA class I expression—A common event amongst DNA-repair-deficient colorectal cancers. J. Pathol. 2009;219:69–76. doi: 10.1002/path.2569. [DOI] [PubMed] [Google Scholar]
- 135.Bicknell D.C., Kaklamanis L., Hampson R., Bodmer W.F., Karran P. Selection for beta 2-microglobulin mutation in mismatch repair-defective colorectal carcinomas. Curr. Biol. 1996;6:1695–1697. doi: 10.1016/S0960-9822(02)70795-1. [DOI] [PubMed] [Google Scholar]
- 136.Cabrera T., Collado A., Fernandez M.A., Ferron A., Sancho J., Ruiz-Cabello F., Garrido F. High frequency of altered HLA class I phenotypes in invasive colorectal carcinomas. Tissue Antigens. 1998;52:114–123. doi: 10.1111/j.1399-0039.1998.tb02274.x. [DOI] [PubMed] [Google Scholar]
- 137.Cabrera T., Maleno I., Collado A., Lopez Nevot M.A., Tait B.D., Garrido F. Analysis of HLA class I alterations in tumors: Choosing a strategy based on known patterns of underlying molecular mechanisms. Tissue Antigens. 2007;69(Suppl. S1):264–268. doi: 10.1111/j.1399-0039.2006.00777.x. [DOI] [PubMed] [Google Scholar]
- 138.Benitez R., Godelaine D., Lopez-Nevot M.A., Brasseur F., Jiménez P., Marchand M., Oliva M.R., Van Baren N., Cabrera T., Andry G., et al. Mutations of the beta2-microglobulin gene result in a lack of HLA class I molecules on melanoma cells of two patients immunized with MAGE peptides. Tissue Antigens. 1998;52:520–529. doi: 10.1111/j.1399-0039.1998.tb03082.x. [DOI] [PubMed] [Google Scholar]
- 139.Hicklin D.J., Wang Z., Arienti F., Rivoltini L., Parmiani G., Ferrone S. beta2-Microglobulin mutations, HLA class I antigen loss, and tumor progression in melanoma. J. Clin. Investig. 1998;101:2720–2729. doi: 10.1172/JCI498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tang Q., Zhang J., Qi B., Shen C., Xie W. Downregulation of HLA class I molecules in primary oral squamous cell carcinomas and cell lines. Arch. Med. Res. 2009;40:256–263. doi: 10.1016/j.arcmed.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 141.Maleno I., López-Nevot M.A., Cabrera T., Salinero J., Garrido F. Multiple mechanisms generate HLA class I altered phenotypes in laryngeal carcinomas: High frequency of HLA haplotype loss associated with loss of heterozygosity in chromosome region 6p21. Cancer Immunol. Immunother. 2002;51:389–396. doi: 10.1007/s00262-002-0296-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cabrera T., Angustias Fernandez M., Sierra A., Garrido A., Herruzo A., Escobedo A., Fabra A., Garrido F. High frequency of altered HLA class I phenotypes in invasive breast carcinomas. Hum. Immunol. 1996;50:127–134. doi: 10.1016/0198-8859(96)00145-0. [DOI] [PubMed] [Google Scholar]
- 143.Qifeng S., Bo C., Xingtao J., Chuanliang P., Xiaogang Z. Methylation of the promoter of human leukocyte antigen class I in human esophageal squamous cell carcinoma and its histopathological characteristics. J. Thorac. Cardiovasc. Surg. 2011;141:808–814. doi: 10.1016/j.jtcvs.2010.04.031. [DOI] [PubMed] [Google Scholar]
- 144.Facoetti A., Nano R., Zelini P., Morbini P., Benericetti E., Ceroni M., Campoli M., Ferrone S. Human leukocyte antigen and antigen processing machinery component defects in astrocytic tumors. Clin. Cancer Res. 2005;11:8304–8311. doi: 10.1158/1078-0432.CCR-04-2588. [DOI] [PubMed] [Google Scholar]
- 145.Cai L., Michelakos T., Yamada T., Fan S., Wang X., Schwab J.H., Ferrone C.R., Ferrone S. Defective HLA class I antigen processing machinery in cancer. Cancer Immunol. Immunother. 2018;67:999–1009. doi: 10.1007/s00262-018-2131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Garrido F., Algarra I. MHC antigens and tumor escape from immune surveillance. Adv. Cancer Res. 2001;83:117–158. doi: 10.1016/s0065-230x(01)83005-0. [DOI] [PubMed] [Google Scholar]
- 147.Kageshita T., Hirai S., Ono T., Hicklin D.J., Ferrone S. Down-regulation of HLA class I antigen-processing molecules in malignant melanoma: Association with disease progression. Am. J. Pathol. 1999;154:745–754. doi: 10.1016/S0002-9440(10)65321-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Campoli M., Ferrone S. HLA antigen changes in malignant cells: Epigenetic mechanisms and biologic significance. Oncogene. 2008;27:5869–5885. doi: 10.1038/onc.2008.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chang C.-C., Pirozzi G., Wen S.-H., Chung I.-H., Chiu B.-L., Errico S., Luongo M., Lombardi M.L., Ferrone S. Multiple structural and epigenetic defects in the human leukocyte antigen class I antigen presentation pathway in a recurrent metastatic melanoma following immunotherapy. J. Biol. Chem. 2015;290:26562–26575. doi: 10.1074/jbc.M115.676130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Garrido F., Cabrera T., Concha A., Glew S., Ruiz-Cabello F., Stern P.L. Natural history of HLA expression during tumour development. Immunol. Today. 1993;14:491–499. doi: 10.1016/0167-5699(93)90264-L. [DOI] [PubMed] [Google Scholar]
- 151.Ljunggren H.G., Kärre K. In search of the “missing self”: MHC molecules and NK cell recognition. Immunol. Today. 1990;11:237–244. doi: 10.1016/0167-5699(90)90097-S. [DOI] [PubMed] [Google Scholar]
- 152.Garrido F., Cabrera T., Aptsiauri N. “Hard” and “soft” lesions underlying the HLA class I alterations in cancer cells: Implications for immunotherapy. Int. J. Cancer. 2010;127:249–256. doi: 10.1002/ijc.25270. [DOI] [PubMed] [Google Scholar]
- 153.Paschen A., Arens N., Sucker A., Greulich-Bode K.M., Fonsatti E., Gloghini A., Striegel S., Schwinn N., Carbone A., Hildenbrand R., et al. The coincidence of chromosome 15 aberrations and beta2-microglobulin gene mutations is causative for the total loss of human leukocyte antigen class I expression in melanoma. Clin. Cancer Res. 2006;12:3297–3305. doi: 10.1158/1078-0432.CCR-05-2174. [DOI] [PubMed] [Google Scholar]
- 154.Del Campo A.B., Aptsiauri N., Méndez R., Zinchenko S., Vales A., Paschen A., Ward S., Ruiz-Cabello F., González-Aseguinolaza G., Garrido F. Efficient recovery of HLA class I expression in human tumor cells after beta2-microglobulin gene transfer using adenoviral vector: Implications for cancer immunotherapy. Scand. J. Immunol. 2009;70:125–135. doi: 10.1111/j.1365-3083.2009.02276.x. [DOI] [PubMed] [Google Scholar]
- 155.Maleno I., Aptsiauri N., Cabrera T., Gallego A., Paschen A., López-Nevot M.A., Garrido F. Frequent loss of heterozygosity in the β2-microglobulin region of chromosome 15 in primary human tumors. Immunogenetics. 2011;63:65–71. doi: 10.1007/s00251-010-0494-4. [DOI] [PubMed] [Google Scholar]
- 156.Pérez B., Benitez R., Fernández M.A., Oliva M.R., Soto J.L., Serrano S., López Nevot M.A., Garrido F. A new beta 2 microglobulin mutation found in a melanoma tumor cell line. Tissue Antigens. 1999;53:569–572. doi: 10.1034/j.1399-0039.1999.530607.x. [DOI] [PubMed] [Google Scholar]
- 157.Chang C.-C., Campoli M., Restifo N.P., Wang X., Ferrone S. Immune selection of hot-spot beta 2-microglobulin gene mutations, HLA-A2 allospecificity loss, and antigen-processing machinery component down-regulation in melanoma cells derived from recurrent metastases following immunotherapy. J. Immunol. 2005;174:1462–1471. doi: 10.4049/jimmunol.174.3.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bernal M., Ruiz-Cabello F., Concha A., Paschen A., Garrido F. Implication of the β2-microglobulin gene in the generation of tumor escape phenotypes. Cancer Immunol. Immunother. 2012;61:1359–1371. doi: 10.1007/s00262-012-1321-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Garrido F., Romero I., Aptsiauri N., Garcia-Lora A.M. Generation of MHC class I diversity in primary tumors and selection of the malignant phenotype. Int. J. Cancer. 2016;138:271–280. doi: 10.1002/ijc.29375. [DOI] [PubMed] [Google Scholar]
- 160.Browning M., Petronzelli F., Bicknell D., Krausa P., Rowan A., Tonks S., Murray N., Bodmer J., Bodmer W. Mechanisms of loss of HLA class I expression on colorectal tumor cells. Tissue Antigens. 1996;47:364–371. doi: 10.1111/j.1399-0039.1996.tb02571.x. [DOI] [PubMed] [Google Scholar]
- 161.Sette A., Chesnut R., Fikes J. HLA expression in cancer: Implications for T cell-based immunotherapy. Immunogenetics. 2001;53:255–263. doi: 10.1007/s002510100334. [DOI] [PubMed] [Google Scholar]
- 162.Khleif S., editor. Tumor Immunology and Cancer Vaccines. Springer; New York, NY, USA: 2005. Cancer Treatment and Research. [Google Scholar]
- 163.Murphy C., Nikodem D., Howcroft K., Weissman J.D., Singer D.S. Active repression of major histocompatibility complex class I genes in a human neuroblastoma cell line. J. Biol. Chem. 1996;271:30992–30999. doi: 10.1074/jbc.271.48.30992. [DOI] [PubMed] [Google Scholar]
- 164.Nie Y., Yang G., Song Y., Zhao X., So C., Liao J., Wang L.D., Yang C.S. DNA hypermethylation is a mechanism for loss of expression of the HLA class I genes in human esophageal squamous cell carcinomas. Carcinogenesis. 2001;22:1615–1623. doi: 10.1093/carcin/22.10.1615. [DOI] [PubMed] [Google Scholar]
- 165.Coral S., Sigalotti L., Gasparollo A., Cattarossi I., Visintin A., Cattelan A., Altomonte M., Maio M. Prolonged upregulation of the expression of HLA class I antigens and costimulatory molecules on melanoma cells treated with 5-aza-2’-deoxycytidine (5-AZA-CdR) J. Immunother. 1999;22:16–24. doi: 10.1097/00002371-199901000-00003. [DOI] [PubMed] [Google Scholar]
- 166.Coral S., Sigalotti L., Altomonte M., Engelsberg A., Colizzi F., Cattarossi I., Maraskovsky E., Jager E., Seliger B., Maio M. 5-aza-2’-deoxycytidine-induced expression of functional cancer testis antigens in human renal cell carcinoma: Immunotherapeutic implications. Clin. Cancer Res. 2002;8:2690–2695. [PubMed] [Google Scholar]
- 167.Esteller M. Epigenetics provides a new generation of oncogenes and tumour-suppressor genes. Br. J. Cancer. 2006;94:179–183. doi: 10.1038/sj.bjc.6602918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lettini A.A., Guidoboni M., Fonsatti E., Anzalone L., Cortini E., Maio M. Epigenetic remodelling of DNA in cancer. Histol. Histopathol. 2007;22:1413–1424. doi: 10.14670/HH-22.1413. [DOI] [PubMed] [Google Scholar]
- 169.Ferris R.L., Hunt J.L., Ferrone S. Human leukocyte antigen (HLA) class I defects in head and neck cancer: Molecular mechanisms and clinical significance. Immunol. Res. 2005;33:113–133. doi: 10.1385/IR:33:2:113. [DOI] [PubMed] [Google Scholar]
- 170.Ferrone S., Marincola F.M. Loss of HLA class I antigens by melanoma cells: Molecular mechanisms, functional significance and clinical relevance. Immunol. Today. 1995;16:487–494. doi: 10.1016/0167-5699(95)80033-6. [DOI] [PubMed] [Google Scholar]
- 171.Concha-Benavente F., Srivastava R., Ferrone S., Ferris R.L. Immunological and clinical significance of HLA class I antigen processing machinery component defects in malignant cells. Oral Oncol. 2016;58:52–58. doi: 10.1016/j.oraloncology.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Seliger B., Ferrone S. HLA Class I Antigen Processing Machinery Defects in Cancer Cells-Frequency, Functional Significance, and Clinical Relevance with Special Emphasis on Their Role in T Cell-Based Immunotherapy of Malignant Disease. Methods Mol. Biol. 2020;2055:325–350. doi: 10.1007/978-1-4939-9773-2_15. [DOI] [PubMed] [Google Scholar]
- 173.Yoshihama S., Roszik J., Downs I., Meissner T.B., Vijayan S., Chapuy B., Sidiq T., Shipp M.A., Lizee G.A., Kobayashi K.S. NLRC5/MHC class I transactivator is a target for immune evasion in cancer. Proc. Natl. Acad. Sci. USA. 2016;113:5999–6004. doi: 10.1073/pnas.1602069113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yoshihama S., Vijayan S., Sidiq T., Kobayashi K.S. NLRC5/CITA: A key player in cancer immune surveillance. Trends Cancer. 2017;3:28–38. doi: 10.1016/j.trecan.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kloor M., Becker C., Benner A., Woerner S.M., Gebert J., Ferrone S., Von Knebel Doeberitz M. Immunoselective pressure and human leukocyte antigen class I antigen machinery defects in microsatellite unstable colorectal cancers. Cancer Res. 2005;65:6418–6424. doi: 10.1158/0008-5472.CAN-05-0044. [DOI] [PubMed] [Google Scholar]
- 176.Cathro H.P., Smolkin M.E., Theodorescu D., Jo V.Y., Ferrone S., Frierson H.F. Relationship between HLA class I antigen processing machinery component expression and the clinicopathologic characteristics of bladder carcinomas. Cancer Immunol. Immunother. 2010;59:465–472. doi: 10.1007/s00262-009-0765-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Leffers N., Gooden M.J.M., Mokhova A.A., Kast W.M., Boezen H.M., Ten Hoor K.A., Hollema H., Daemen T., Van der Zee A.G.J., Nijman H.W. Down-regulation of proteasomal subunit MB1 is an independent predictor of improved survival in ovarian cancer. Gynecol. Oncol. 2009;113:256–263. doi: 10.1016/j.ygyno.2008.12.030. [DOI] [PubMed] [Google Scholar]
- 178.Hoves S., Aigner M., Pfeiffer C., Laumer M., Obermann E.C., Mackensen A. In situ analysis of the antigen-processing machinery in acute myeloid leukaemic blasts by tissue microarray. Leukemia. 2009;23:877–885. doi: 10.1038/leu.2008.391. [DOI] [PubMed] [Google Scholar]
- 179.Kamarashev J., Ferrone S., Seifert B., Böni R., Nestle F.O., Burg G., Dummer R. TAP1 down-regulation in primary melanoma lesions: An independent marker of poor prognosis. Int. J. Cancer. 2001;95:23–28. doi: 10.1002/1097-0215(20010120)95:1<23::AID-IJC1004>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 180.Hicklin D.J., Marincola F.M., Ferrone S. HLA class I antigen downregulation in human cancers: T-cell immunotherapy revives an old story. Mol. Med. Today. 1999;5:178–186. doi: 10.1016/S1357-4310(99)01451-3. [DOI] [PubMed] [Google Scholar]
- 181.Vitale M., Rezzani R., Rodella L., Zauli G., Grigolato P., Cadei M., Hicklin D.J., Ferrone S. HLA class I antigen and transporter associated with antigen processing (TAP1 and TAP2) down-regulation in high-grade primary breast carcinoma lesions. Cancer Res. 1998;58:737–742. [PubMed] [Google Scholar]
- 182.Atkins D., Breuckmann A., Schmahl G.E., Binner P., Ferrone S., Krummenauer F., Störkel S., Seliger B. MHC class I antigen processing pathway defects, ras mutations and disease stage in colorectal carcinoma. Int. J. Cancer. 2004;109:265–273. doi: 10.1002/ijc.11681. [DOI] [PubMed] [Google Scholar]
- 183.Hirata T., Yamamoto H., Taniguchi H., Horiuchi S., Oki M., Adachi Y., Imai K., Shinomura Y. Characterization of the immune escape phenotype of human gastric cancers with and without high-frequency microsatellite instability. J. Pathol. 2007;211:516–523. doi: 10.1002/path.2142. [DOI] [PubMed] [Google Scholar]
- 184.Chen H.L., Gabrilovich D., Tampé R., Girgis K.R., Nadaf S., Carbone D.P. A functionally defective allele of TAP1 results in loss of MHC class I antigen presentation in a human lung cancer. Nat. Genet. 1996;13:210–213. doi: 10.1038/ng0696-210. [DOI] [PubMed] [Google Scholar]
- 185.Oliveira C.C., Van Hall T. Alternative Antigen Processing for MHC Class I: Multiple Roads Lead to Rome. Front. Immunol. 2015;6:298. doi: 10.3389/fimmu.2015.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Van Duinen S.G., Ruiter D.J., Broecker E.B., Van der Velde E.A., Sorg C., Welvaart K., Ferrone S. Level of HLA antigens in locoregional metastases and clinical course of the disease in patients with melanoma. Cancer Res. 1988;48:1019–1025. [PubMed] [Google Scholar]
- 187.Zaloudik J., Moore M., Ghosh A.K., Mechl Z., Rejthar A. DNA content and MHC class II antigen expression in malignant melanoma: Clinical course. J. Clin. Pathol. 1988;41:1078–1084. doi: 10.1136/jcp.41.10.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Trieb K., Lechleitner T., Lang S., Windhager R., Kotz R., Dirnhofer S. Evaluation of HLA-DR expression and T-lymphocyte infiltration in osteosarcoma. Pathol. Res. Pract. 1998;194:679–684. doi: 10.1016/S0344-0338(98)80126-X. [DOI] [PubMed] [Google Scholar]
- 189.Moretti S., Pinzi C., Berti E., Spallanzani A., Chiarugi A., Boddi V., Reali U.M., Giannotti B. In situ expression of transforming growth factor beta is associated with melanoma progression and correlates with Ki67, HLA-DR and beta 3 integrin expression. Melanoma Res. 1997;7:313–321. doi: 10.1097/00008390-199708000-00006. [DOI] [PubMed] [Google Scholar]
- 190.Diederichsen A.C., Stenholm A.C., Kronborg O., Fenger C., Jensenius J.C., Zeuthen J., Christensen P.B., Kristensen T., Ostenhom A.C. Flow cytometric investigation of immune-response-related surface molecules on human colorectal cancers. Int. J. Cancer. 1998;79:283–287. doi: 10.1002/(SICI)1097-0215(19980619)79:3<283::AID-IJC13>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 191.Hilders C.G., Houbiers J.G., Van Ravenswaay Claasen H.H., Veldhuizen R.W., Fleuren G.J. Association between HLA-expression and infiltration of immune cells in cervical carcinoma. Lab. Investig. 1993;69:651–659. [PubMed] [Google Scholar]
- 192.Coleman N., Stanley M.A. Analysis of HLA-DR expression on keratinocytes in cervical neoplasia. Int. J. Cancer. 1994;56:314–319. doi: 10.1002/ijc.2910560303. [DOI] [PubMed] [Google Scholar]
- 193.Cromme F.V., Meijer C.J., Snijders P.J., Uyterlinde A., Kenemans P., Helmerhorst T., Stern P.L., Van den Brule A.J., Walboomers J.M. Analysis of MHC class I and II expression in relation to presence of HPV genotypes in premalignant and malignant cervical lesions. Br. J. Cancer. 1993;67:1372–1380. doi: 10.1038/bjc.1993.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Jackson P.A., Green M.A., Marks C.G., King R.J., Hubbard R., Cook M.G. Lymphocyte subset infiltration patterns and HLA antigen status in colorectal carcinomas and adenomas. Gut. 1996;38:85–89. doi: 10.1136/gut.38.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Matsushita K., Takenouchi T., Kobayashi S., Hayashi H., Okuyama K., Ochiai T., Mikata A., Isono K. HLA-DR antigen expression in colorectal carcinomas: Influence of expression by IFN-gamma in situ and its association with tumour progression. Br. J. Cancer. 1996;73:644–648. doi: 10.1038/bjc.1996.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Norheim Andersen S., Breivik J., Løvig T., Meling G.I., Gaudernack G., Clausen O.P., Schjölberg A., Fausa O., Langmark F., Lund E., et al. K-ras mutations and HLA-DR expression in large bowel adenomas. Br. J. Cancer. 1996;74:99–108. doi: 10.1038/bjc.1996.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Anichini A., Mortarini R., Nonaka D., Molla A., Vegetti C., Montaldi E., Wang X., Ferrone S. Association of Antigen-Processing Machinery and HLA Antigen Phenotype of Melanoma Cells with Survival in American Joint Committee on Cancer Stage III and IV Melanoma Patients. Cancer Res. 2006;66:6405–6411. doi: 10.1158/0008-5472.CAN-06-0854. [DOI] [PubMed] [Google Scholar]
- 198.Sartoris S., Valle M.T., Barbaro A.L., Tosi G., Cestari T., D’Agostino A., Megiovanni A.M., Manca F., Accolla R.S. HLA class II expression in uninducible hepatocarcinoma cells after transfection of AIR-1 gene product CIITA: Acquisition of antigen processing and presentation capacity. J. Immunol. 1998;161:814–820. [PubMed] [Google Scholar]
- 199.Yazawa T., Kamma H., Fujiwara M., Matsui M., Horiguchi H., Satoh H., Fujimoto M., Yokoyama K., Ogata T. Lack of class II transactivator causes severe deficiency of HLA-DR expression in small cell lung cancer. J. Pathol. 1999;187:191–199. doi: 10.1002/(SICI)1096-9896(199901)187:2<191::AID-PATH206>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 200.Mach B., Steimle V., Martinez-Soria E., Reith W. Regulation of MHC class II genes: Lessons from a disease. Annu. Rev. Immunol. 1996;14:301–331. doi: 10.1146/annurev.immunol.14.1.301. [DOI] [PubMed] [Google Scholar]
- 201.Bosshart H., Jarrett R.F. Deficient major histocompatibility complex class II antigen presentation in a subset of Hodgkin’s disease tumor cells. Blood. 1998;92:2252–2259. doi: 10.1182/blood.V92.7.2252. [DOI] [PubMed] [Google Scholar]
- 202.Fife B.T., Bluestone J.A. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol. Rev. 2008;224:166–182. doi: 10.1111/j.1600-065X.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 203.Mueller D.L., Jenkins M.K., Schwartz R.H. Clonal expansion versus functional clonal inactivation: A costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu. Rev. Immunol. 1989;7:445–480. doi: 10.1146/annurev.iy.07.040189.002305. [DOI] [PubMed] [Google Scholar]
- 204.Schneider H., Downey J., Smith A., Zinselmeyer B.H., Rush C., Brewer J.M., Wei B., Hogg N., Garside P., Rudd C.E. Reversal of the TCR stop signal by CTLA-4. Science. 2006;313:1972–1975. doi: 10.1126/science.1131078. [DOI] [PubMed] [Google Scholar]
- 205.Nishimura H., Honjo T. PD-1: An inhibitory immunoreceptor involved in peripheral tolerance. Trends Immunol. 2001;22:265–268. doi: 10.1016/S1471-4906(01)01888-9. [DOI] [PubMed] [Google Scholar]
- 206.Ribas A. Releasing the Brakes on Cancer Immunotherapy. N. Engl. J. Med. 2015;373:1490–1492. doi: 10.1056/NEJMp1510079. [DOI] [PubMed] [Google Scholar]
- 207.Webb E.S., Liu P., Baleeiro R., Lemoine N.R., Yuan M., Wang Y.-H. Immune checkpoint inhibitors in cancer therapy. J. Biomed. Res. 2018;32:317–326. doi: 10.7555/JBR.31.20160168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Keung E.Z., Wargo J.A. The Current Landscape of Immune Checkpoint Inhibition for Solid Malignancies. Surg. Oncol. Clin. N. Am. 2019;28:369–386. doi: 10.1016/j.soc.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 209.Pitt J.M., Vétizou M., Daillère R., Roberti M.P., Yamazaki T., Routy B., Lepage P., Boneca I.G., Chamaillard M., Kroemer G., et al. Resistance Mechanisms to Immune-Checkpoint Blockade in Cancer: Tumor-Intrinsic and -Extrinsic Factors. Immunity. 2016;44:1255–1269. doi: 10.1016/j.immuni.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 210.Restifo N.P., Smyth M.J., Snyder A. Acquired resistance to immunotherapy and future challenges. Nat. Rev. Cancer. 2016;16:121–126. doi: 10.1038/nrc.2016.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Jenkins R.W., Barbie D.A., Flaherty K.T. Mechanisms of resistance to immune checkpoint inhibitors. Br. J. Cancer. 2018;118:9–16. doi: 10.1038/bjc.2017.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Meng X., Huang Z., Teng F., Xing L., Yu J. Predictive biomarkers in PD-1/PD-L1 checkpoint blockade immunotherapy. Cancer Treat. Rev. 2015;41:868–876. doi: 10.1016/j.ctrv.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 213.Maleki Vareki S., Garrigós C., Duran I. Biomarkers of response to PD-1/PD-L1 inhibition. Crit. Rev. Oncol. Hematol. 2017;116:116–124. doi: 10.1016/j.critrevonc.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 214.Manson G., Norwood J., Marabelle A., Kohrt H., Houot R. Biomarkers associated with checkpoint inhibitors. Ann. Oncol. 2016;27:1199–1206. doi: 10.1093/annonc/mdw181. [DOI] [PubMed] [Google Scholar]
- 215.Gibney G.T., Weiner L.M., Atkins M.B. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 2016;17:e542–e551. doi: 10.1016/S1470-2045(16)30406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Zaretsky J.M., Garcia-Diaz A., Shin D.S., Escuin-Ordinas H., Hugo W., Hu-Lieskovan S., Torrejon D.Y., Abril-Rodriguez G., Sandoval S., Barthly L., et al. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N. Engl. J. Med. 2016;375:819–829. doi: 10.1056/NEJMoa1604958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kametani Y., Ohno Y., Ohshima S., Tsuda B., Yasuda A., Seki T., Ito R., Tokuda Y. Humanized Mice as an Effective Evaluation System for Peptide Vaccines and Immune Checkpoint Inhibitors. Int. J. Mol. Sci. 2019;20:6337. doi: 10.3390/ijms20246337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Lechner M.G., Karimi S.S., Barry-Holson K., Angell T.E., Murphy K.A., Church C.H., Ohlfest J.R., Hu P., Epstein A.L. Immunogenicity of murine solid tumor models as a defining feature of in vivo behavior and response to immunotherapy. J. Immunother. 2013;36:477–489. doi: 10.1097/01.cji.0000436722.46675.4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ashizawa T., Iizuka A., Nonomura C., Kondou R., Maeda C., Miyata H., Sugino T., Mitsuya K., Hayashi N., Nakasu Y., et al. Antitumor Effect of Programmed Death-1 (PD-1) Blockade in Humanized the NOG-MHC Double Knockout Mouse. Clin. Cancer Res. 2017;23:149–158. doi: 10.1158/1078-0432.CCR-16-0122. [DOI] [PubMed] [Google Scholar]
- 220.Gettinger S., Choi J., Hastings K., Truini A., Datar I., Sowell R., Wurtz A., Dong W., Cai G., Melnick M.A., et al. Impaired HLA Class I Antigen Processing and Presentation as a Mechanism of Acquired Resistance to Immune Checkpoint Inhibitors in Lung Cancer. Cancer Discov. 2017;7:1420–1435. doi: 10.1158/2159-8290.CD-17-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Lhotakova K., Grzelak A., Polakova I., Vackova J., Smahel M. Establishment and characterization of a mouse tumor cell line with irreversible downregulation of MHC class I molecules. Oncol. Rep. 2019;42:2826–2835. doi: 10.3892/or.2019.7356. [DOI] [PubMed] [Google Scholar]
- 222.Rangan L., Galaine J., Boidot R., Hamieh M., Dosset M., Francoual J., Beziaud L., Pallandre J.-R., Lauret Marie Joseph E., Asgarova A., et al. Identification of a novel PD-L1 positive solid tumor transplantable in HLA-A*0201/DRB1*0101 transgenic mice. Oncotarget. 2017;8:48959–48971. doi: 10.18632/oncotarget.16900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Mortara L., Castellani P., Meazza R., Tosi G., De Lerma Barbaro A., Procopio F.A., Comes A., Zardi L., Ferrini S., Accolla R.S. CIITA-induced MHC class II expression in mammary adenocarcinoma leads to a Th1 polarization of the tumor microenvironment, tumor rejection, and specific antitumor memory. Clin. Cancer Res. 2006;12:3435–3443. doi: 10.1158/1078-0432.CCR-06-0165. [DOI] [PubMed] [Google Scholar]
- 224.Murciano-Goroff Y.R., Warner A.B., Wolchok J.D. The future of cancer immunotherapy: Microenvironment-targeting combinations. Cell Res. 2020;30:507–519. doi: 10.1038/s41422-020-0337-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Chowell D., Morris L.G.T., Grigg C.M., Weber J.K., Samstein R.M., Makarov V., Kuo F., Kendall S.M., Requena D., Riaz N., et al. Patient HLA class I genotype influences cancer response to checkpoint blockade immunotherapy. Science. 2018;359:582–587. doi: 10.1126/science.aao4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Garrido F., Aptsiauri N., Doorduijn E.M., Garcia Lora A.M., Van Hall T. The urgent need to recover MHC class I in cancers for effective immunotherapy. Curr. Opin. Immunol. 2016;39:44–51. doi: 10.1016/j.coi.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Garrido F., Aptsiauri N. Cancer immune escape: MHC expression in primary tumours versus metastases. Immunology. 2019;158:255–266. doi: 10.1111/imm.13114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Banik D., Moufarrij S., Villagra A. Immunoepigenetics Combination Therapies: An Overview of the Role of HDACs in Cancer Immunotherapy. Int. J. Mol. Sci. 2019;20:2241. doi: 10.3390/ijms20092241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Del Campo A.B., Carretero J., Muñoz J.A., Zinchenko S., Ruiz-Cabello F., González-Aseguinolaza G., Garrido F., Aptsiauri N. Adenovirus expressing β2-microglobulin recovers HLA class I expression and antitumor immunity by increasing T-cell recognition. Cancer Gene Ther. 2014;21:317–332. doi: 10.1038/cgt.2014.32. [DOI] [PubMed] [Google Scholar]
- 230.Wang X., Waschke B.C., Woolaver R.A., Chen Z., Zhang G., Piscopio A.D., Liu X., Wang J.H. Histone Deacetylase Inhibition Sensitizes PD1 Blockade-Resistant B-cell Lymphomas. Cancer Immunol. Res. 2019;7:1318–1331. doi: 10.1158/2326-6066.CIR-18-0875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Ramsuran V., Kulkarni S., O’huigin C., Yuki Y., Augusto D.G., Gao X., Carrington M. Epigenetic regulation of differential HLA-A allelic expression levels. Hum. Mol. Genet. 2015;24:4268–4275. doi: 10.1093/hmg/ddv158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Brea E.J., Oh C.Y., Manchado E., Budhu S., Gejman R.S., Mo G., Mondello P., Han J.E., Jarvis C.A., Ulmert D., et al. Kinase Regulation of Human MHC Class I Molecule Expression on Cancer Cells. Cancer Immunol. Res. 2016;4:936–947. doi: 10.1158/2326-6066.CIR-16-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Loi S., Dushyanthen S., Beavis P.A., Salgado R., Denkert C., Savas P., Combs S., Rimm D.L., Giltnane J.M., Estrada M.V., et al. RAS/MAPK Activation Is Associated with Reduced Tumor-Infiltrating Lymphocytes in Triple-Negative Breast Cancer: Therapeutic Cooperation Between MEK and PD-1/PD-L1 Immune Checkpoint Inhibitors. Clin. Cancer Res. 2016;22:1499–1509. doi: 10.1158/1078-0432.CCR-15-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Srivastava P.K. Neoepitopes of Cancers: Looking Back, Looking Ahead. Cancer Immunol. Res. 2015;3:969–977. doi: 10.1158/2326-6066.CIR-15-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Im J.S., Herrmann A.C., Bernatchez C., Haymaker C., Molldrem J.J., Hong W.K., Perez-Soler R. Immune-Modulation by Epidermal Growth Factor Receptor Inhibitors: Implication on Anti-Tumor Immunity in Lung Cancer. PLoS ONE. 2016;11:e0160004. doi: 10.1371/journal.pone.0160004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Garrido G., Rabasa A., Garrido C., Chao L., Garrido F., García-Lora Á.M., Sánchez-Ramírez B. Upregulation of HLA Class I Expression on Tumor Cells by the Anti-EGFR Antibody Nimotuzumab. Front. Pharmacol. 2017;8:595. doi: 10.3389/fphar.2017.00595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Kang S.-H., Keam B., Ahn Y.-O., Park H.-R., Kim M., Kim T.M., Kim D.-W., Heo D.S. Inhibition of MEK with trametinib enhances the efficacy of anti-PD-L1 inhibitor by regulating anti-tumor immunity in head and neck squamous cell carcinoma. Oncoimmunology. 2019;8:e1515057. doi: 10.1080/2162402X.2018.1515057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Sapkota B., Hill C.E., Pollack B.P. Vemurafenib enhances MHC induction in BRAFV600E homozygous melanoma cells. Oncoimmunology. 2013;2:e22890. doi: 10.4161/onci.22890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Sabbatino F., Wang Y., Scognamiglio G., Favoino E., Feldman S.A., Villani V., Flaherty K.T., Nota S., Giannarelli D., Simeone E., et al. Antitumor Activity of BRAF Inhibitor and IFNα Combination in BRAF-Mutant Melanoma. J. Natl. Cancer Inst. 2016;108 doi: 10.1093/jnci/djv435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Hu-Lieskovan S., Mok S., Homet Moreno B., Tsoi J., Robert L., Goedert L., Pinheiro E.M., Koya R.C., Graeber T.G., Comin-Anduix B., et al. Improved antitumor activity of immunotherapy with BRAF and MEK inhibitors in BRAF(V600E) melanoma. Sci. Transl. Med. 2015;7:279ra41. doi: 10.1126/scitranslmed.aaa4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Ritter C., Fan K., Paschen A., Reker Hardrup S., Ferrone S., Nghiem P., Ugurel S., Schrama D., Becker J.C. Epigenetic priming restores the HLA class-I antigen processing machinery expression in Merkel cell carcinoma. Sci. Rep. 2017;7:2290. doi: 10.1038/s41598-017-02608-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Sun T., Li Y., Yang W., Wu H., Li X., Huang Y., Zhou Y., Du Z. Histone deacetylase inhibition up-regulates MHC class I to facilitate cytotoxic T lymphocyte-mediated tumor cell killing in glioma cells. J. Cancer. 2019;10:5638–5645. doi: 10.7150/jca.34471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Yang W., Li Y., Gao R., Xiu Z., Sun T. MHC class I dysfunction of glioma stem cells escapes from CTL-mediated immune response via activation of Wnt/β-catenin signaling pathway. Oncogene. 2020;39:1098–1111. doi: 10.1038/s41388-019-1045-6. [DOI] [PubMed] [Google Scholar]
- 244.Woan K.V., Lienlaf M., Perez-Villaroel P., Lee C., Cheng F., Knox T., Woods D.M., Barrios K., Powers J., Sahakian E., et al. Targeting histone deacetylase 6 mediates a dual anti-melanoma effect: Enhanced antitumor immunity and impaired cell proliferation. Mol. Oncol. 2015;9:1447–1457. doi: 10.1016/j.molonc.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Ennishi D., Takata K., Béguelin W., Duns G., Mottok A., Farinha P., Bashashati A., Saberi S., Boyle M., Meissner B., et al. Molecular and Genetic Characterization of MHC Deficiency Identifies EZH2 as Therapeutic Target for Enhancing Immune Recognition. Cancer Discov. 2019;9:546–563. doi: 10.1158/2159-8290.CD-18-1090. [DOI] [PubMed] [Google Scholar]
- 246.Fonsatti E., Nicolay H.J.M., Sigalotti L., Calabrò L., Pezzani L., Colizzi F., Altomonte M., Guidoboni M., Marincola F.M., Maio M. Functional up-regulation of human leukocyte antigen class I antigens expression by 5-aza-2’-deoxycytidine in cutaneous melanoma: Immunotherapeutic implications. Clin. Cancer Res. 2007;13:3333–3338. doi: 10.1158/1078-0432.CCR-06-3091. [DOI] [PubMed] [Google Scholar]
- 247.Chacon J.A., Schutsky K., Powell D.J. The Impact of Chemotherapy, Radiation and Epigenetic Modifiers in Cancer Cell Expression of Immune Inhibitory and Stimulatory Molecules and Anti-Tumor Efficacy. Vaccines. 2016;4:43. doi: 10.3390/vaccines4040043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Luo N., Nixon M.J., Gonzalez-Ericsson P.I., Sanchez V., Opalenik S.R., Li H., Zahnow C.A., Nickels M.L., Liu F., Tantawy M.N., et al. DNA methyltransferase inhibition upregulates MHC-I to potentiate cytotoxic T lymphocyte responses in breast cancer. Nat. Commun. 2018;9:248. doi: 10.1038/s41467-017-02630-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.De Biasi A.R., Villena-Vargas J., Adusumilli P.S. Cisplatin-induced antitumor immunomodulation: A review of preclinical and clinical evidence. Clin. Cancer Res. 2014;20:5384–5391. doi: 10.1158/1078-0432.CCR-14-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Faè D.A., Martorelli D., Mastorci K., Muraro E., Dal Col J., Franchin G., Barzan L., Comaro E., Vaccher E., Rosato A., et al. Broadening Specificity and Enhancing Cytotoxicity of Adoptive T Cells for Nasopharyngeal Carcinoma Immunotherapy. Cancer Immunol. Res. 2016;4:431–440. doi: 10.1158/2326-6066.CIR-15-0108. [DOI] [PubMed] [Google Scholar]
- 251.Pellicciotta I., Yang C.-P.H., Goldberg G.L., Shahabi S. Epothilone B enhances Class I HLA and HLA-A2 surface molecule expression in ovarian cancer cells. Gynecol. Oncol. 2011;122:625–631. doi: 10.1016/j.ygyno.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 252.Wan S., Pestka S., Jubin R.G., Lyu Y.L., Tsai Y.-C., Liu L.F. Chemotherapeutics and radiation stimulate MHC class I expression through elevated interferon-beta signaling in breast cancer cells. PLoS ONE. 2012;7:e32542. doi: 10.1371/journal.pone.0032542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Wang X., Waschke B.C., Woolaver R.A., Chen S.M.Y., Chen Z., Wang J.H. HDAC inhibitors overcome immunotherapy resistance in B-cell lymphoma. Protein Cell. 2020 doi: 10.1007/s13238-020-00694-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Zhou F. Molecular mechanisms of IFN-gamma to up-regulate MHC class I antigen processing and presentation. Int. Rev. Immunol. 2009;28:239–260. doi: 10.1080/08830180902978120. [DOI] [PubMed] [Google Scholar]
- 255.De Charette M., Marabelle A., Houot R. Turning tumour cells into antigen presenting cells: The next step to improve cancer immunotherapy? Eur. J. Cancer. 2016;68:134–147. doi: 10.1016/j.ejca.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 256.Medrano R.F.V., Hunger A., Mendonça S.A., Barbuto J.A.M., Strauss B.E. Immunomodulatory and antitumor effects of type I interferons and their application in cancer therapy. Oncotarget. 2017;8:71249–71284. doi: 10.18632/oncotarget.19531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Selinger E., Reiniš M. Epigenetic View on Interferon γ Signalling in Tumour Cells. Folia Biol. (Praha) 2018;64:125–136. doi: 10.14712/fb2018064040125. [DOI] [PubMed] [Google Scholar]
- 258.Zhang S., Kohli K., Black R.G., Yao L., Spadinger S.M., He Q., Pillarisetty V.G., Cranmer L.D., Van Tine B.A., Yee C., et al. Systemic Interferon-γ Increases MHC Class I Expression and T-cell Infiltration in Cold Tumors: Results of a Phase 0 Clinical Trial. Cancer Immunol. Res. 2019;7:1237–1243. doi: 10.1158/2326-6066.CIR-18-0940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Vlková V., Štěpánek I., Hrušková V., Šenigl F., Mayerová V., Šrámek M., Šímová J., Bieblová J., Indrová M., Hejhal T., et al. Epigenetic regulations in the IFNγ signalling pathway: IFNγ-mediated MHC class I upregulation on tumour cells is associated with DNA demethylation of antigen-presenting machinery genes. Oncotarget. 2014;5:6923–6935. doi: 10.18632/oncotarget.2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Castro F., Cardoso A.P., Gonçalves R.M., Serre K., Oliveira M.J. Interferon-Gamma at the Crossroads of Tumor Immune Surveillance or Evasion. Front. Immunol. 2018;9 doi: 10.3389/fimmu.2018.00847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Rodríguez T., Méndez R., Del Campo A., Jiménez P., Aptsiauri N., Garrido F., Ruiz-Cabello F. Distinct mechanisms of loss of IFN-gamma mediated HLA class I inducibility in two melanoma cell lines. BMC Cancer. 2007;7:34. doi: 10.1186/1471-2407-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Sucker A., Zhao F., Pieper N., Heeke C., Maltaner R., Stadtler N., Real B., Bielefeld N., Howe S., Weide B., et al. Acquired IFNγ resistance impairs anti-tumor immunity and gives rise to T-cell-resistant melanoma lesions. Nat. Commun. 2017;8:15440. doi: 10.1038/ncomms15440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Aspeslagh S., Morel D., Soria J.-C., Postel-Vinay S. Epigenetic modifiers as new immunomodulatory therapies in solid tumours. Ann. Oncol. 2018;29:812–824. doi: 10.1093/annonc/mdy050. [DOI] [PubMed] [Google Scholar]
- 264.Gao F., Zhao Z.-L., Zhao W.-T., Fan Q.-R., Wang S.-C., Li J., Zhang Y.-Q., Shi J.-W., Lin X.-L., Yang S., et al. miR-9 modulates the expression of interferon-regulated genes and MHC class I molecules in human nasopharyngeal carcinoma cells. Biochem. Biophys. Res. Commun. 2013;431:610–616. doi: 10.1016/j.bbrc.2012.12.097. [DOI] [PubMed] [Google Scholar]
- 265.Li J., Lin T.-Y., Chen L., Liu Y., Dian M.-J., Hao W.-C., Lin X.-L., Li X.-Y., Li Y.-L., Lian M., et al. miR-19 regulates the expression of interferon-induced genes and MHC class I genes in human cancer cells. Int. J. Med. Sci. 2020;17:953–964. doi: 10.7150/ijms.44377. [DOI] [PMC free article] [PubMed] [Google Scholar]