Abstract
Mucormycosis is an invasive mould that can cause aggressive infection, particularly in immunocompromised patients. Though oesophageal mucormycosis is relatively rare, it remains an elusive and devastating manifestation of this disease. The management is also challenging, due to surgical morbidity and contraindications such as thrombocytopenia in immunocompromised hosts. In this report, we present the case of a 60-year-old Lebanese man with newly diagnosed acute myeloid leukaemia who developed oesophageal mucormycosis after induction chemotherapy with idarubicin/cytarabine (7+3). The diagnosis was made when the patient developed febrile neutropenia and odynophagia. CT scan of the chest revealed a thickened oesophagus. Oesophagogastroduodenoscopy with biopsy, histopathology and PCR were performed, resulting in the diagnosis of Rhizopus microsporus. The patient was successfully treated with liposomal amphotericin B and salvage posaconazole therapy without surgical intervention. We reviewed the clinical characteristics of the six published oesophageal mucormycosis reports from the literature.
Keywords: infectious diseases, infections, infection (gastroenterology), haematology (incl blood transfusion)
Background
Mucormycosis is a devastating invasive fungal disease that portends poor outcomes even with maximal medical and surgical management. Though gastrointestinal mucormycosis represents an infrequent manifestation of the disease, comprising only 8% of cases, the diagnosis is often both elusive and devastating. Delayed diagnoses may partially account for the high mortality (54%) observed with this entity.1 Even among gastrointestinal sites of infection, oesophageal involvement is the least common with scant literature to guide management and treatment.2 Oesophageal mucormycosis management is particularly challenging given the difficulty in obtaining surgical source control. We present a case of gastro-oesophageal mucormycosis successfully treated with liposomal amphotericin B (LAMB) by salvage posaconazole without surgical intervention and review the available literature for cases of gastrointestinal mucormycosis to better characterise the clinical presentation, management and outcomes of this disease.
Case presentation
A 60-year-old Lebanese man presented with fever, night sweat and migratory polyarthralgia of his large and small joints. A complete blood count in the emergency room showed 61% blasts; subsequently, a diagnosis of FMS-like tyrosine kinase 3 (FLT3) negative acute myeloid leukaemia was made. He was admitted to the hospital and started on idarubicin/cytarabine (7+3) along with acyclovir, voriconazole and moxifloxacin for neutropenia prophylaxis.3
His medical history was notable for coronary artery disease, hypertension and hyperlipidaemia. He had a 30 pack-year smoking history but was not currently smoking. He did not smoke marijuana or take herbal supplements. The patient did reveal that he rolled grape leaves with his family prior to admission. He worked in information technology and his recent travel was notable for travel to the Caribbean just prior to his diagnosis. His only allergy was a cough to lisinopril.
Seven days after induction, he became febrile to 102.7°F, with an absolute neutrophil count (ANC) of 0. Neutropenia prophylaxis was continued through this time and moxifloxacin was broadened to vancomycin and piperacillin/tazobactam. Vancomycin and piperacillin/tazobactam were discontinued after 2 and 7 days, respectively, after documenting negative blood cultures and resolution of fever. He also developed some abdominal discomfort and odynophagia without dysphagia that was attributed to mucositis.
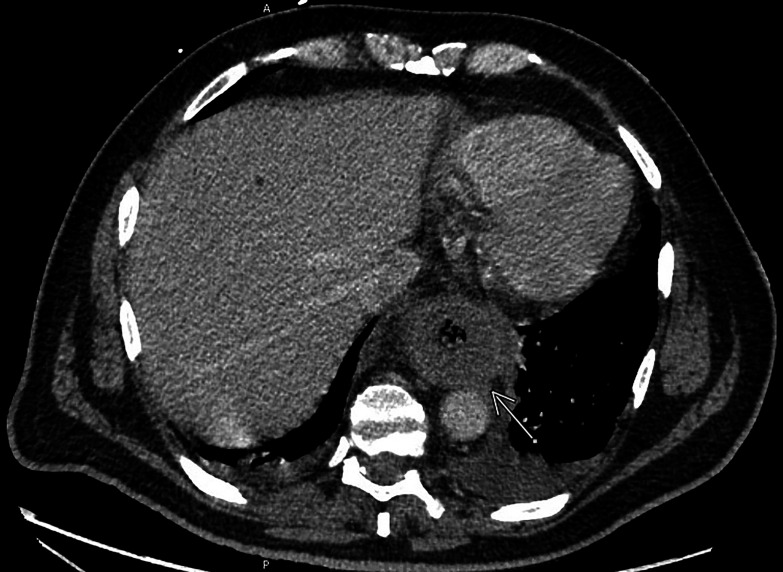
On day 17, after induction, he became febrile to 101.4 with an ANC of 0 and was re-started on vancomycin and piperacillin/tazobactam. A chest radiograph showed a new left basilar infiltrate. The following day, he became confused and lethargic. Physical examination was negative for oral eschars or sinus tenderness. CT of the chest, abdomen and pelvis revealed significant oesophageal mural thickening and dilation with intraluminal debris and surrounding infiltrative changes (figure 1). Oral voriconazole prophylaxis was changed to intravenous anidulafungin due to dysphagia.
Figure 1.
CT chest reveals significant oesophageal mural thickening and dilatation with intraluminal debris (indicated by the arrow), most pronounced distally to the level of the gastro-oesophageal junction.
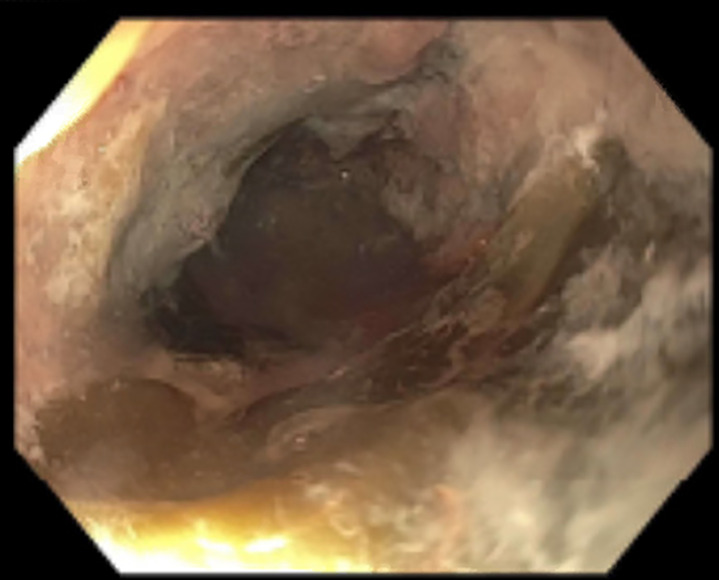
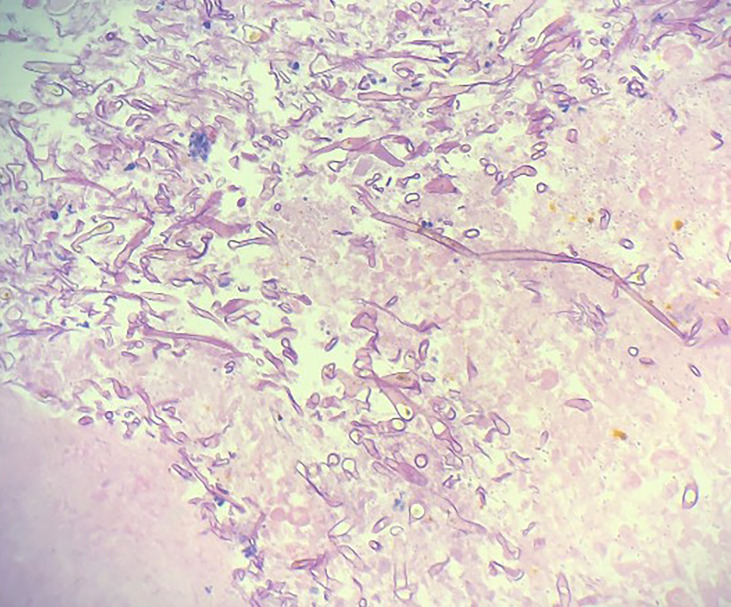
An oesophagogastroduodenoscopy (OGD) performed the following day showed severe circumferential oesophagitis with adherent food residue (figure 2). The oesophagus was almost entirely ulcerated but there was no active bleeding at the base. Five non-bleeding cratered gastric ulcers with a clean base were encountered in the gastric fundus and cardia. Biopsies were obtained for histopathology but none were sent for fungal culture due to low suspicion for a fungal infection at the time of OGD. Histopathological examination of biopsy specimens demonstrated aseptate, wide hyphae branching at 90º, consistent with a Mucorales infection (figure 3). Stains for cytomegalovirus, herpes simplex virus and adenovirus were negative. Broad-range 18S PCR sequencing was performed on tissue at a reference laboratory, resulting as positive for Rhizopus microsporus. The patient had resolution of neutropenia at this time, so acyclovir, vancomycin, piperacillin/tazobactam and anidulafungin were discontinued. Appropriate antifungal therapy was initiated.
Figure 2.
Oesophagogastroduodenoscopy revealed severe circumferential oesophagitis with significant ulceration.
Figure 3.
Histopathological examination of biopsy specimens demonstrated aseptate, wide hyphae branching at 90°, consistent with a Mucorales infection.
Treatment
The patient was started on LAMB and anidulafungin was stopped. As his symptomatology rapidly improved, a repeat OGD was not immediately performed and surgical management was not pursued. Three weeks into his treatment, he developed an oesophageal stricture with recurrent odynophagia and regurgitation of food. Repeat OGD showed a large oesophageal ulcer and severe oesophageal stenosis with circumferential necrosis that required oesophageal dilation, but repeat pathology and cultures were negative. Since no clinical isolate was obtained, he completed 7.5 weeks of LAMB and was then maintained on empiric posaconazole based on lower minimum inhibitory concentration 50(MIC50) for R. microsporus in the literature compared with isavuconazole (MIC50 0.5 vs 1).4
Outcome and follow-up
He continued to require dilation for oesophageal strictures, and eventually, lumen-opposing metal stents were placed 5 months after this admission, and a percutaneous endoscopic gastrostomy (PEG) tube was placed a month later.
Discussion
Oesophageal mucormycosis is a poorly characterised, yet often catastrophic, form of invasive Mucorales infection. On reviewing the literature for published cases, we found six reports of oesophageal mucormycosis5–10 (table 1). Of seven total patients (including the present case), six were immunocompromised (four with haematologic malignancies and two with end-stage renal disease). Of seven patients, one was documented to have type 2 diabetes mellitus and six of the seven were men.
Table 1.
Clinical presentation, diagnostics, treatment and outcomes of confirmed cases of oesophageal mucormycosis5–10
| Study | Age (years) | Sex | Predisposing condition | Additional sites of involvement | Presentation | Antifungal prophylaxis | Imaging | Diagnosis | Medical treatment | Surgical treatment | Outcome |
| Lee et al9 | 51 | M | AML | Bronchus | Fever and cough with neutropenia | Caspofungin | CT scan revealed extensive necrotising right-sided pneumonia and was suspicious for broncho-oesophageal fistula | Flexible bronchoscopy Fungal culture revealed Mucor sp OGD revealed large linear defect of the oesophageal wall that perhaps connected with the bronchus |
LAMB | – | Died on the day of diagnosis due to haemoptysis |
| Mezhir et al5 | 50 | M | HCV, ESRD and polysubstance use | Stomach and liver | Abdominal pain and haematemesis | None | Abdominal X-ray revealed pneumoperitoneum CT scan revealed cystic lesion in the right lobe of liver |
OGD revealed numerous necrotic lower oesophageal and gastric ulcers Fungal culture revealed Mucor sp | Initial: LAMB (5 mg/kg/day)+oral AMB (50 mg/day) Successful treatment: LAMB +posaconazole (200 mg every 6 hours) Maintenance: oral posaconaole (400 mg every 12 hours) for 1 year |
– | Resolution on serial CT scan at 1, 2, 7 and 12 months |
| Boatright et al6 | 63 | M | TIIDM and APML | None | Dysphagia | None | None | OGD of distal oesophagus revealed circumferential wall thickening and black discoloration Fungal culture revealed Mucor sp | LAMB, echinocandins, fluconazole and broad-spectrum antibiotics | – | Died 24 days after diagnosis (cause of death not documented) |
| Gani et al8 | 79 | M | ESRD s/p deceased donor kidney transplant on immunosuppression, TIIDM | Stomach | Dysphagia and odynophagia | None | None | OGD revealed oesophagitis Fungal culture revealed Rhizopus sp |
Isavuconazole: initial 372 mg every 8 hours then indefinite 372 mg once a day | – | Repeat OGD post-operative day 20 revealed partial resolution Remained asymptomatic at 6-month follow-up |
| Kraft10 | 55 | M | Myeloid leukaemia | None | Fever, dysphagia and neutropenia | Posaconazole | CT scan revealed thickened oesophageal wall with signs of mediastinitis | Oesophagectomy Fungal culture revealed Rhizopus sp | LAMB | Oesophagectomy with salivary fistula | Died 5 days after surgery due to multiorgan dysfunction |
| Raviraj et al7 | 19 | W | Haemolytic uremic syndrome | Stomach | Fever, haematemesis and melena | None | CT scan revealed leakage from large gastric ulcer | OGD revealed large bleeding ulcer over the oesophagus and stomach Fungal culture revealed Mucor sp |
LAMB +posaconazole for 8 weeks and 2 weeks, respectively | Partial gastrectomy | Resolution on OGD at 3-month follow-up |
| Present case | 60 | M | AML | Stomach | Fever, odynophagia and neutropenia | Voriconazole | CT scan revealed oesophageal wall thickening consistent with severe oesophagitis | OGD revealed severe circumferential oesophagitis PCR testing revealed Rhizopus microsporus |
LAMB for 55 days followed by maintenance posaconazole | Resolution on repeat OGD at 27 days after diagnosis |
AML, acute myeloid leukaemia; APML, acute promyelocytic leukaemia; ESRD, end-stage renal disease; HCV, hepatitis C; LAMB, liposomal amphotericin B; M, man; OGD, oesophagogastroduodenoscopy; TIIDM, type 2 diabetes mellitus; W, woman.
The most common presenting symptoms were dysphagia/odynophagia (four out of seven cases) and fever (four out of seven cases) followed by haematemesis (two out of seven cases). The most common additional site of involvement was the stomach (four out of seven cases). Three out of seven patients were on antifungal prophylaxis (caspofungin, posaconazole and voriconazole). Two out of seven cases showed oesophageal wall thickening on CT scan. OGD was performed in all the seven cases and revealed significant changes ranging from oesophagitis and ulceration to necrosis. In six out of seven cases, the diagnosis was confirmed with fungal culture whereas the present case was confirmed with PCR. A Mucorales mold s. was isolated in four out of seven and Rhizopus spp in three out of seven cases.
Mortality occurred in three out of seven cases and was attributed to haemoptysis in one and multiorgan system failure in one, while there was no documentation in one. Two of seven patients underwent surgical intervention (one died and one survived). Among the patients who survived (four out seven patients), three received LAMB and one received isavuconazole. Complications in the non-surgical group included fistula formation (one out of seven patients) and oesophageal strictures requiring stenting and PEG tube placement (one out of seven patients).
Taken together, findings from previously reported cases and the current case suggest that though oesophageal mucormycosis is an uncommon and protean syndrome, certain parameters may increase the index of suspicion for this disease. Immunocompromised individuals remain the predominant host population, especially during times of intense immunosuppression as with induction chemotherapy or in the early post-transplant period. The two most common presenting symptoms are fever and dysphagia. However, the common occurrence of chemotherapy-related mucositis in patients undergoing induction chemotherapy (four out of seven patients in this review) as well as other infectious causes such as herpetic and candidal mucositis that lead to dysphagia make this symptom less discriminatory.11 Odynophagia may be an important symptom warranting additional investigation for an infectious oesophagitis. This review also suggests that CT scan can be a useful tool for the diagnosis of oesophageal mucormycosis, as CT scan revealed oesophageal wall thickening in two of seven cases, a relatively uncommon and perhaps more specific finding for oesophagitis that may lead to further evaluation with OGD. OGD and biopsy was performed in all seven reported cases. There was initial concern with proceeding with an OGD in this case as the patient was profoundly neutropenic but evidence from the literature has not shown OGD-related adverse effects in this setting.12 Therefore, it is reasonable to proceed with OGD for definitive diagnosis when there is clinical suspicion for an invasive mould infection.
In the described case, the diagnosis was made by histopathology, which demonstrated wide, aseptate ribbon-like hyphae consistent with Mucorales. This was then confirmed by broad-range PCR as Rhizopus sp. A study of 28 patients with suspected invasive fungal infection aimed to compare direct microscopy with culture and PCR for the diagnosis of Mucorales infection in blood and tissue specimens.13 Seven patients were confirmed positive by direct smears, of which six were PCR positive and five were fungal culture positive. Although small, this study suggests a potential role for PCR testing for the diagnosis of Mucorales infections. Furthermore, in a study of 29 confirmed cases of Mucorales, PCR testing was especially helpful for culture-negative patients.14 In this study, 12 patients were determined to be culture positive, of which 10 were PCR positive while 15 patients were culture negative, of which 12 were PCR positive. Though PCR is an important adjunctive diagnostic method, fungal culture remains the mainstay of diagnosis as it provides information on susceptibility. Indeed Mucorales species have variable susceptibility15 to the advanced oral azoles posaconazole and isavuconazole, which are important step-down options after initial therapy with LAMB.
Despite the limited number of cases documented in the literature, there are several trends worth noting. Invasive mucormycosis is classically treated with a combination medical therapy and surgery.16 However, oesophageal surgery is complicated and may be high-risk due to existing chemotherapy-induced cytopenias. This literature review suggests that although mortality remains high overall, oesophageal infection may be treated with medical therapy alone. The first-line antifungal therapy in these patients is LAMB. There is insufficient evidence regarding combination therapy of LAMB with triazoles.16 Of the four survivors in this literature review, two received combination therapy with LAMB and posaconazole. There is also some evidence that posaconazole can be successfully used as salvage therapy.17 Two of the four survivors were maintained on salvage posaconazole and have had favourable outcomes despite some ongoing morbidity.
Our patient’s preparation of grape leaves (which involves rolling of leaves) for a traditional Middle Eastern dish likely predisposed him to the disease through inhaling and swallowing of fungal spores. Though epidemiological risk factors were not reported in published cases, eliciting high-risk exposures such as sustained exposure to raw vegetation during profound neutropenia may represent another clue when suspecting oesophageal mucormycosis.
Learning points.
Diagnosis of oesophageal mucormycosis requires high clinical suspicion in high-risk patient populations but fever, dysphagia and odynophagia in the setting of intense immunosuppression are suggestive.
CT scan showing oesophageal thickening should be further investigated with an oesophagogastroduodenoscopy to obtain biopsies for histopathology, culture and potentially PCR.
Management remains challenging; this review suggests that locally invasive infection can be successfully treated with medical therapy alone.
Footnotes
Contributors: Conception, planning and design, acquisition of data or analysis and interpretation of data: MR, LP and MMA. Drafting the article or revising it critically for important intellectual content: MR, LP and MMA. Final approval of the version published: MR, LP and MMA. Agreement to be accountable for the article and to ensure that all questions regarding the accuracy or integrity of the article are investigated and resolved: MR, LP and MMA.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer-reviewed.
References
- 1.Jeong W, Keighley C, Wolfe R, et al. The epidemiology and clinical manifestations of mucormycosis: a systematic review and meta-analysis of case reports. Clin Microbiol Infect 2019;25:26–34. 10.1016/j.cmi.2018.07.011 [DOI] [PubMed] [Google Scholar]
- 2.Dioverti MV, Cawcutt KA, Abidi M, et al. Gastrointestinal mucormycosis in immunocompromised hosts. Mycoses 2015;58:714–8. 10.1111/myc.12419 [DOI] [PubMed] [Google Scholar]
- 3.Baden LR, Swaminathan S, Angarone M, et al. Prevention and treatment of cancer-related infections, version 2.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2016;14:882–913. 10.6004/jnccn.2016.0093 [DOI] [PubMed] [Google Scholar]
- 4.Arendrup MC, Jensen RH, Meletiadis J. In vitro activity of isavuconazole and comparators against clinical isolates of the mucorales order. Antimicrob Agents Chemother 2015;59:7735–42. 10.1128/AAC.01919-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mezhir JJ, Mullane KM, Zarling J, et al. Successful nonoperative management of gastrointestinal mucormycosis: novel therapy for invasive disease. Surg Infect 2009;10:447–51. 10.1089/sur.2008.049 [DOI] [PubMed] [Google Scholar]
- 6.Boatright B, Tang S-jiang, Whatley ZJ, et al. Esophageal mucormycosis. Video J Encyclopedia GI Endoscop 2014;1:658–60. 10.1016/j.vjgien.2013.05.002 [DOI] [Google Scholar]
- 7.Raviraj KS, Miglani P, Garg A, et al. Gastric mucormycosis with hemolytic uremic syndrome. J Assoc Physicians India 2015;63:75–6. [PubMed] [Google Scholar]
- 8.Gani I, Doroodchi A, Falkenstrom K, et al. Gastric mucormycosis in a renal transplant patient treated with isavuconazole monotherapy. Case Rep Transplant 2019;2019:9839780. 10.1155/2019/9839780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee J-H, Hyun J-S, Kang D-Y, et al. Rare complication of bronchoesophageal fistula due to pulmonary mucormycosis after induction chemotherapy for acute myeloid leukemia: a case report. J Med Case Rep 2016;10:195. 10.1186/s13256-016-0991-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kraft F. A case report: mucormycosis in a neutropenic patient. Oncol Res Treat 2014;37. [Google Scholar]
- 11.Dysphagia Section, Oral Care Study Group, Multinational Association of Supportive Care in Cancer (MASCC)/International Society of Oral Oncology (ISOO), Raber-Durlacher JE, Brennan MT, et al. Swallowing dysfunction in cancer patients. Support Care Cancer 2012;20:433–43. 10.1007/s00520-011-1342-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abu-Sbeih H, Ali FS, Coronel E, et al. Safety of endoscopy in cancer patients with thrombocytopenia and neutropenia. Gastrointest Endosc 2019;89:937–49. 10.1016/j.gie.2018.12.004 [DOI] [PubMed] [Google Scholar]
- 13.Badiee P, Arastefar A, Jafarian H. Comparison of histopathological analysis, culture and polymerase chain reaction assays to detect mucormycosis in biopsy and blood specimens. Iran J Microbiol 2013;5:406–10. [PMC free article] [PubMed] [Google Scholar]
- 14.Hammond SP, Bialek R, Milner DA, et al. Molecular methods to improve diagnosis and identification of mucormycosis. J Clin Microbiol 2011;49:2151–3. 10.1128/JCM.00256-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tissot F, Agrawal S, Pagano L, et al. ECIL-6 guidelines for the treatment of invasive candidiasis, aspergillosis and mucormycosis in leukemia and hematopoietic stem cell transplant patients. Haematologica 2017;102:433–44. 10.3324/haematol.2016.152900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sipsas NV, Gamaletsou MN, Anastasopoulou A, et al. Therapy of mucormycosis. J Fungi 2018;4:90 10.3390/jof4030090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Burik J-AH, Hare RS, Solomon HF, et al. Posaconazole is effective as salvage therapy in zygomycosis: a retrospective summary of 91 cases. Clin Infect Dis 2006;42:e61–5. 10.1086/500212 [DOI] [PubMed] [Google Scholar]