Abstract
Background
Over the last few years, epidemiological studies have shown that infection with Helicobacter pylori has a major effect on micronutrient deficiency as well as on adverse pregnancy outcomes. Importantly, there are gaps in understanding the linkage of H. pylori infection with micronutrients deficiency in pregnant women.
Objective
We conducted a systematic review and meta-analysis to estimate the association between H. pylori infection and micronutrient deficiencies in pregnant women.
Methods
A systematic literature search was conducted for relevant articles using PubMed, Web of Science, and Scopus database from inception to March 2020. The OR with 95% CIs was determined by meta-analysis of data extracted from the selected studies.
Results
From 2384 primary articles, 6 studies were selected for systematic reviews and 4 studies distinctively (with 1274 participants: 553 cases and 721 controls) were selected for meta-analysis. The meta-analysed fixed effect model estimated the odds of having H. pylori infection was not significantly higher among pregnant women with micronutrient deficiencies than those without deficiencies (OR=1.12, 95% CI 0.88 to 1.42, p=0.37). In the subgroup analysis, no correlation was found between H. pylori infection and vitamin B12 (OR=0.74, 95% CI 0.45 to 1.21, p=0.22), folate (OR=1.07, 95% CI 0.73 to 1.58, p=0.73), and ferritin (OR=0.81, 95% CI 0.51 to 1.31, p=0.4). However, a positive correlation was found between iron-deficiency anaemia (IDA) and H. pylori infection (OR=16.23, 95% CI 4.19 to 62.93, p<0.0001) during pregnancy.
Conclusion
H. pylori infection is associated with increased risk of IDA but not with deficiency of other micronutrients in pregnancy.
PROSPERO registration number
CRD42019135683.
Keywords: vitamins, micronutrients, Helicobacter pylori, meta-analysis, malabsorption
Introduction
The balanced store of nutrient during pregnancy plays a crucial role in preserving the maternal well-being, as well as for the optimal growth and development of the fetus. Macronutrients (carbohydrate, protein and fat) serve as building blocks and provide energy whereas micronutrients, including vitamins and minerals, are indispensable for metabolism, immune function, and brain development.1 2 In 2015, WHO reported that globally, 32 million women became anaemic during pregnancy which was 15%–20% of total pregnant women,3 noticeably around 19 million were reported to be vitamin A deficient, and millions suffer from insufficient iron, folate, zinc or iodine stores. Moreover, about 20 million babies with low birth weight (LBW) are born weighing less than 2500 g at birth.4 An estimated 15 million babies are born premature, and many more are born with other complications which increase their risk of morbidity and mortality during childhood.4 It has been shown that poor micronutrient status is highly correlated with adverse pregnancy outcomes and subsequent childhood health.3 Several micronutrient supplementations programmes have been implemented to improve the micronutrient status of mother. A review by Alison et al3 reported that multiple micronutrient supplementations reduced the risk of poor pregnancy outcomes, while other studies found that maternal health was unchanged after supplementation of folic acid, iron, vitamin B12 and other micronutrients.5–7 In addition to poor health conditions, inadequate dietary intake, low socioeconomic condition and lack of sanitary knowledge, the prevalence of acute and chronic diseases caused by H. pylori is also responsible for the nutrient deficiency during pregnancy.8
H. pylori, a curved gram-negative ciliated bacillus, is responsible for stomach infection in 44.3% of the global population.9 H. pylori infection is highly prevalent in pregnant women, as pregnancy itself is a risk factor for the infection.10 Hormonal changes and immunological alterations that ensure maternal tolerance towards the fetus can trigger dormant H. pylori into infectious during pregnancy.11 This pathogen further alters gastric physiology with a chronic inflammatory response of the gastric mucosa. It also produces phospholipase A2 and C, and glycosulfatase enzymes that play vital roles in damaging the gastric mucosal layer.12 This eventually gives rise to higher gastric pH,13 which can affect nutrient and drug absorption.14 15 Moreover, H. pylori is associated with severe nausea and vomiting or hyperemesis gravidarum in pregnancy period and this could result in malnutrition as well, especially the loss of water-soluble vitamins.16 Prior research exhibited that H. pylori infection has a role in the pathogenesis of various pregnancy related disorders including iron-deficiency anaemia (IDA), fetal malformations and fetal growth restriction.17 18 IDA may coexist with vitamin B12 and folate deficiency which are associated with poor birth outcomes such as premature birth, intrauterine growth retardation, infertility or recurrent spontaneous abortion.19 Besides, it is probable that dietary vitamin B12 deficiency may be exacerbated due to malabsorption caused by H. pylori infection.15
Although there are some ambiguous findings, research has not been carried out to address systematically the association between H. pylori infection and micronutrients deficiency in pregnant women. Previous systematic reviews have highlighted the association between H. pylori infection and negative pregnancy outcomes20 and the association between H. pylori infection and micronutrient deficiencies in the general population.21 Therefore, our objective was to conduct a systematic review and meta-analysis to investigate whether H. pylori infection is linked with reduced concentration of micronutrients among pregnant women, who are highly vulnerable to infection and malnutrition.
Materials and methods
Registration of review protocol
The study was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses and Meta-analysis of Observational Studies in Epidemiology guidelines for systematic review and meta-analysis. We registered the protocol prospectively in PROSPERO.
Literature search
Search strategies were prepared and designated according to the PICOS principle method:
P (population): pregnant OR mother OR pregnancy OR child bearing age OR women.
I (intervention/exposure): H. pylori OR Helicobacter pylori.
C (comparison): without H. pylori infection.
O (outcome): nutrition OR malnutrition OR micronutrients OR nutrients OR undernutrition OR nutritional deficiency OR vitamin OR mineral OR trace element OR iron OR folate OR homocysteine OR zinc OR copper OR Iodine OR manganese OR calcium OR magnesium.
S (study design): no restriction.
Articles were searched using PubMed, Web of Science, and Scopus database from inception to March 2020 without restriction to geography. The Boolean operation (ie, AND, OR) was employed to compile the keywords that were searched in all fields format in the free text writing boxes. Manual searches of the relevant article references were also performed. The search strategies of three databases are given in online supplemental materials.
bmjgast-2020-000490supp001.pdf (108.5KB, pdf)
Selection criteria
Eligible studies were selected based on the following criteria:
Full text English-language original articles including randomised control trial (RCT), cross-sectional study, case-control study, or cohort-study designs.
Articles, reported any micronutrient deficiency (at least one) in pregnant women with H. pylori infection.
Studies that reported the assessment levels of micronutrients in H. pylori positives and H. pylori negative patients.
The exclusion criteria were as follows:
Review articles, case reports, editorials, guidelines, letter to editors and conference articles.
Articles written in languages other than English.
Studies in non-human, non-pregnant and animal studies.
Studies lacking the standard tests for detection of H. pylori infection or those with unusual diagnostic methodology.
Articles with incomplete or obscure methodology or results.
Data extraction
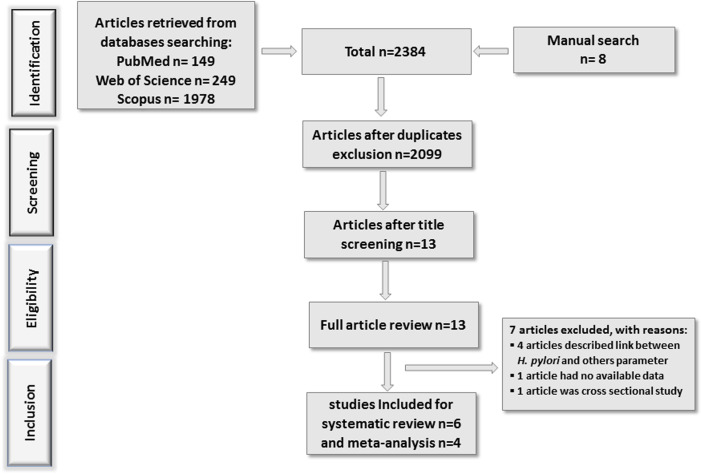
Two authors independently screened and evaluated the article title and abstract of the included studies to categorise the relevant research for full-text assessment. Discrepancies were settled by consensus through discussion with the third author (TJS). Using the search strategy, a total of 2384 unique records were initially found. Through cross-referencing and other resources, 8 additional studies were identified. We excluded the irrelevant and duplicated articles from the pooled search results considering the exclusion and inclusion criteria. After that, authors MNAA and ZNJ screened the title and abstract of 2099 articles. MNAA and ZNJ claimed for 15 and 18 articles for full review, respectively. Among those, 11 articles were common. After discussion and meeting with the author TJS, we were on agreement to select 13 articles for full review. On careful reading and scrutinising, we had no dispute to select 6 articles from those 13 articles. The list of excluded articles is given in online supplemental materials. Finally, we selected four articles for quantitative analysis and additionally two articles (total six) for systematic reviews. The following data were retrieved to present the description of the study:
Study information
First author, publication year, country, type of study design, total sample size, number of cases, number of controls and H. pylori detection method.
Baseline characteristics
Age, gestational age, location and body mass index (BMI).
Outcomes
Vitamin and trace elements depletion such as vitamin B12, folate, iron, copper, zinc and prevalence of IDA was considered for the effect measure. The most recent and comprehensive report was taken into evaluation, when data were reported from overlapping study samples.
Study quality evaluation
The qualities of the included studies were assessed by using relevant version of the Critical Appraisal Skills Programme (CASP) checklists tools.22 CASP checklist consists of 3 sections with 11 questions in total. Each section explores the trustworthiness, results and relevance of the studies; section A is for validation of the result, section B is for description of the result, and section C is for implementation of the result. Quality of the all included studies was assessed by two authors (MNAA, ZNJ) independently. In case of any divergences, open discussion with the third author (TJS) was executed and the issues were resolved. All the questions were designed for three answers (Yes, Not and Can’t tell). If the answer was clearly described, we marked it “Yes” and for the vice versa we marked it “No”. If the answer was ambiguous then we marked it “Can’t tell”. We excluded two questions (questions 7 and 8), not relevant to our studies. Similar with CASP checklist assessment, we appraised the risk of bias (ROB) of case-control studies through the Newcastle-Ottawa (NOS) Scale.23 We used a scoring method to assess each of the study. The scoring method mainly consists of three aspects: selection of participant (four points), comparability (two points) and outcome (three points).24 From a total of 9 points, studies with 7–9 were deemed low ROB, whereas studies with 5–6 and <5 points were considered moderate ROB and high ROB, respectively.25 26
Statistical analysis
The risk of poor micronutrient status outcome among H. pylori infected pregnant women was estimated by the OR with its 95% CIs as the effect size. P<0.05 was considered statistically significant. Interstudy heterogeneity was tested by Cochrane Q (X2) test with limiting p<0.10 level as a significance and estimated by the I2 statistic with values of 25%, 50%, and 75% indicating low, moderate, and high degrees of heterogeneity, respectively.27 Funnel plot was constructed to assess publication bias. Along with meta-analysis, subgroup analyses for each of the nutrients (vitamin B12, folate, ferritin and iron) were performed and represented by the forest plot. All the statistical analyses were carried out by using Review Manager Software (RevMan V.5.3).
Results
Description of the included studies
We recorded 2384 articles according to the previously described search strategy and retrieved article from PubMed (n=149), Web of Sciences (n=249), Scopus (n=1978) and other sources (n=8). In the initial screening, 272 studies were excluded as they were duplicated. Later, 2099 records were excluded due to irrelevant title or abstract, animal trial, or no relevance, leaving 13 studies. After complete reviewing, seven articles were eliminated with reasons: four articles were disqualified because they focused on the association between H. pylori seropositivity and gestational weight gain,28 socioeconomic condition,29 LBW30 and diabetic pregnant women.31 Three articles32–34 indicated, in result sections that H. pylori were the responsible factor for lower iron status in pregnant women, but quantitative data could not be retrieved. Only one study in Nigeria35 reported that there was no significant association between H. pylori infection and reduced level of trace element (Cu, Zn, Fe) in pregnant women. However, their published results were presented by mean value; therefore, eligible quantitative data were not found. Another article by Mubarak et al36 concluded that there was no association between H. pylori infection and anaemia, iron deficiency, and thrombocytopenia during pregnancy. However, the study was observational in nature, that is, cross sectional. The overall study search and selection was illustrated in figure 1.
Figure 1.
Flow diagram of study inclusion for systematic review and meta-analysis.
Most of the studies had specific exclusion criteria, such as hyperthyroid, gastrointestinal problems, diabetes mellitus, multiple gestation, and prepartum haemorrhage other than H. pylori infection. Serum antibody (IgG, IgA or IgM) analysis was performed by using the ELISA method to detect H. pylori infection in most of the studies. All four included articles for meta-analysis were case-control study.37–40 The demographic characteristics, study results and summary are described in tables 1 and 2, respectively. In the forest plot (figure 2), individuals with micronutrient deficiency were defined as an experimental group, while individuals with adequate status were classified as control group. Events were determined by number of individual infected with H. pylori.
Table 1.
Demographic characteristics of the included study participants
| Study (year) | Study design | Location | Gestation (week) (Mean±SD) |
Patients (n) | Age (mean±SD) | BMI (mean±SD) | ||||
| HP (+ve) | HP (−ve) | HP(+ve) | HP (−ve) | HP (+ve) | HP (−ve) | HP (+ve) | HP (−ve) | |||
| Ali et al (2018)40 | Case control study | Iraq | 34.8±5.5 | 33.6±5.9 | 50 | 50 | 28.1±6.2 | 29.1±5.6 | NM | NM |
| Felkner et al (2007)38 | Case control study | USA | NM | NM | 95 | 147 | 22.9±4.2 | 23.5±4.7 | 24±2.3 | 24±3.8 |
| Golalipour et al (2012)37 | Case control study | Iran | 40±3.3 | 41±3.3 | 35 | 53 | 25.3±5.4 | 25.4±5.1 | NM | NM |
| Mulayim et al (2008)39 | Case control study | Turkey | 30±2.4 | 30±2.7 | 72 | 45 | 28.5±3.47 | 30±3.1 | 27±2.1 | 24±3.6 |
| Mubarak et al (2014)36 | Cross-sectional study | Sudan | 25.1±2.6 | 25.1±2.6 | 125 | 54 | 26±6.8 | 26±6.8 | 28.8±6.32 | 28.8±6.32 |
| Ugwuja et al (2010)35 | Mixed method | Nigeria | 21.77±3.14 | 21.77±3.14 | 84 | 265 | 28.19±4.89 | 26.7±4.65 | 29.0±3.89 | 26.86±4.1 |
BMI, body mass index; HP(-ve), Helicobacter pylori negative; HP(+ve), Helicobacter pylori positive; NM, not mentioned.
Table 2.
Study results evaluating markers of H. pylori infection in patients with micronutrients deficiency
| Study (Year) | H. pylori diagnosis | Description of the Indicators |
Outcomes | Limitations/strength | |
| Antibody/targeted molecule | Method | ||||
| Ali et al (2018)40 | Anti-Helicobacter pylori IgA | ELISA | Iron | Significant positive correlation between iron deficiency and H. pylori-positive cases. | Smaller sample size, limited access data, study purpose was highly focused. |
| Felkner et al (2007)38 | Anti-Helicobacter pylori IgG | ELISA | Ferritin, folate, vitamin B12 | No association was found between micronutrients and H. pylori-positive cases. | Larger sample size, well-validated study, sample was collected in just after post partum. |
| Golalipour et al (2012)37 |
Anti-Helicobacter pylori IgG | ELISA | Ferritin, folate, vitamin B12 | No association was found between micronutrients and H. pylori-positive cases. | Smaller sample size, sample was collected in just after post partum. |
| Mulayim et al (2008)39 |
Urease | 14C-urea breath test | Iron | Significant positive correlation between iron deficiency and H. pylori-positive cases. | Data were not normally distributed when calculated for iron deficiency. |
| Mubarak et al (2014)36 |
Anti-Helicobacter pylori IgA | ELISA | Ferritin | There is a no link between iron deficiency, anaemia, and thrombocytopenia with H. pylori-positive cases. | Larger sample size, well-validated study, limited biasness |
| Ugwuja et al (2010)35 |
Anti-Helicobacter pylori IgG | ELISA | Copper, iron and zinc | Trace elements (Cu, Fe, and Zn) are not significantly associated with H. pylori positive cases | Larger sample size, limited access data, highly biased. |
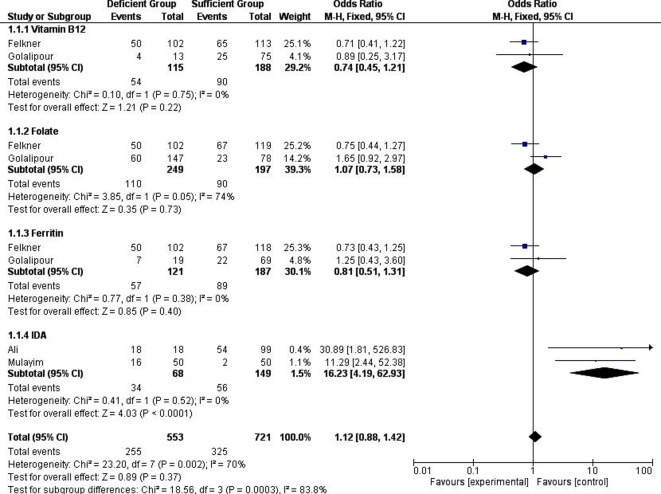
Figure 2.
Forest plot of the association between H. pylori infection and micronutrient deficiency.
Overall meta-analysis
Overall, the four eligible studies represented data from four different countries including USA, Iran, Iraq, and Turkey. A total of 553 cases and 721 controls were recruited in the meta-analysis to find out association between micronutrients deficiency and H. pylori infection among the pregnant women. As shown in figure 2, the overall meta-analysis in the fixed effect model suggested that the experimental (micronutrient deficient) group was not associated with H. pylori infection (OR=1.12, 95% CI 0.88 to 1.42, p=0.37) in comparison with control group (micronutrient sufficient). However, a moderate heterogeneity among the studies was observed (I2=70%, p<0.002).
Subgroup meta-analysis
Association of vitamin B12 with H. pylori infection
We compared the blood vitamin B12 level in case control studies in which the patients were infected with H. pylori. A forest plot (figure 2) of 2 studies comprising 188 controls and 115 experimental participants (cases) were included for statistical analysis. There was no significant role of H. pylori infection in either group (OR=0.74, 95% CI 0.45 to 1.21, p=0.22). No evidence of statistical heterogeneity (I2=0%, p=0.75) was observed in the meta-analysis.
Association of folate with H. pylori infection
A subgroup analysis of folate was carried out. There was no significant effect of H. pylori infection on blood folate status in pregnant women (OR=1.04, 95% CI 0.73 to 1.58, p=0.73). From 3 studies, 333 controls and 270 cases were included for statistical pooling in the forest plot. Moderate heterogeneity was found in this subgroup with significant p value (I2=74%, p=0.05).
Association of iron status with H. pylori infection
In usual conditions, iron status can be measured by assessing serum levels of ferritin.41 Figure 2 showed that H. pylori infection was not associated to reduce the ferritin status (OR=0.81, 95% CI 0.51 to 1.31, p=0.4). From 3 studies, 171 cases and 316 control participants were pooled in the forest plot. However, no heterogeneity (I2=0%, p=0.38) was found in this subgroup.
Two studies reported a positive correlation between IDA status with H. pylori infection (OR=16.23, 95% CI 4.19 to 62.93, p<0.0001). Although the sample size was small (68 cases and 149 control), both studies described highly significant correlation of increasing the IDA condition when participants were infected with H. pylori. Minimal heterogeneity (I2=0%, p=0.52) was found in this subgroup.
From the forest plot (figure 2), it could be assumed that without IDA subgroup, the sign of the diamond might be directed to left side. When this subgroup was omitted, the overall result was not changed (OR=0.89, 95% CI 0.69 to 1.15, p=0.39), while the total heterogeneity decreased (I2=0%, p=0.25). The subgroup meta-analysis data were summarised in table 3.
Table 3.
Subgroup metanalysis of indicator of micronutrients
| Subgroup | Included studies (n) | Participants (n) | OR (95% CI) | P value | Heterogenicity (χ2) | |
| Case | Control | |||||
| Vitamin B12 | 2 | 115 | 188 | 0.74 (0.45 to 1.21) | 0.22 | 0.1 |
| Folate | 2 | 249 | 197 | 1.07 (0.73 to 1.58) | 0.73 | 3.85 |
| Ferritin | 2 | 121 | 187 | 0.81 (0.51 to 1.31) | 0.40 | 0.77 |
| IDA | 2 | 68 | 149 | 16.23 (4.19 to 62.93) | <0.001 | 0.41 |
Publication bias
We created a funnel plot and Egger’s test (figure 3) to measure the publication bias objectively for outcomes from four studies, although it is less powerful for the less than 10 studies.42 No potential publication bias was found though some asymmetry was found in the funnel plot.
Figure 3.
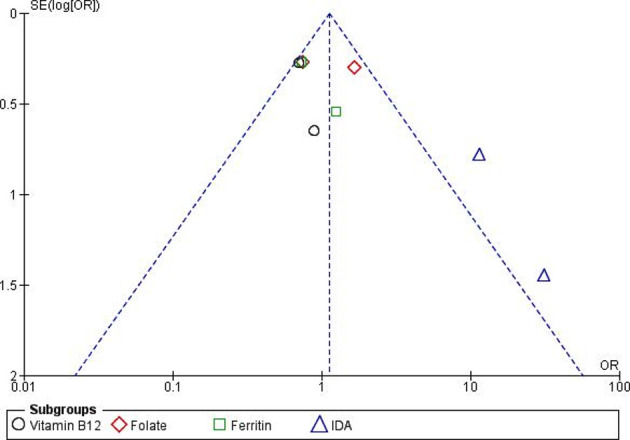
Funnel plot with estimated 95% CIs for meta-analysis of the effect of H. pylori on micronutrient status. IDA, iron-deficiency anaemia.
Quality assessment
The CASP checklist was used to evaluate the quality of the selected articles which include 10 questions to highlight the study rigour, biasness, research methods, risk factor, relevance and research integrity. We deduced a high percentage of interjudges’ agreement (75.00 %), while minimal were indecision (15.00%) and disagreement (10.00%). CASP scale score results showed that all of our enlisted studies had reasonable quality. The results of the quality assessment were summarised in table 4. Three studies (Golalipour et al,37 Felkner et al38 and Mubarak et al36) had higher score (90%) and others three (Ali et al,40 Mulayim et al39 and Ugwuja et al35) had 70%, 60% and 50% score that are considered as medium quality. The results of ROB assessment by using NOS was illustrated in supplementary materials. Two studies achieved low ROB (Ali et al40 and Mulayim et al39) and other two studies achieved medium ROB (Golalipour et al37 and Felkner et al.38).
Table 4.
The Critical Appraisal Skills Programme (CASP) checklists results for assessing the methodological quality of the included studies
| Questions | Ali et al 201740 | Felkner et al 200738 | Golalipour et al 201237 | Mulayim et al 200839 | Mubarak et al 201436 | Ugwuja et al 201035 | |
| 1 | Did the study address a clearly focused issue? | Yes | Yes | Yes | Yes | Yes | Yes |
| 2 | Did the authors use an appropriate method to answer their question? | Yes | Yes | Yes | No | Yes | No |
| 3 | Were the cases recruited in an acceptable way? | Yes | Yes | Yes | Yes | Yes | Can’t Tell |
| 4 | Were the controls selected in an acceptable way? | Yes | Yes | Yes | Yes | Yes | Yes |
| 5 | Was the exposure accurately measured to minimise bias? | Can’t Tell | Yes | Yes | Yes | Yes | Can’t Tell |
| 6 | Aside from the experimental intervention, were the groups treated equally? | Yes | Can’t Tell | Can’t Tell | Can’t Tell | Can’t Tell | Can’t Tell |
| 7 | Have the authors taken account of the potential confounding factors in the design and/or in their analysis? | No | Yes | Yes | No | Yes | No |
| 8 | Do you believe the results? | Yes | Yes | Yes | Yes | Yes | Yes |
| 09 | Can the results be applied to the local population? | Can’t Tell | Yes | Yes | No | Yes | Yes |
| 10 | Do the results of this study fit with other available evidence? | Yes | Yes | Yes | Yes | Yes | Yes |
| Individual study CASP score | 7/10=70% | 9/10=90% | 9/10=90% | 6/10=60% | 9/10=90% | 5/10=50% | |
Overall CASP score: Yes, 45/60=75.00%; Can’t Tell, 9/60=15.00%; No, 6/60=10%.
Discussion
The present meta‐analysis shows that H. pylori infection in pregnancy has no major effect on the risk of micronutrients deficiency. Subgroup analysis also suggests that the infection is highly associated with increased risk of IDA during pregnancy but not with vitamin B12, folate, and ferritin. To date, this systematic review and meta-analysis have been most comprehensive and updated on the likely link between H. pylori and micronutrient deficiency in pregnant women.
In our meta-analysis, we included case-control and cross-sectional studies alone. Given the slew of potential confounding factors, RCTs might be more valuable in such case. However, due to divergence from the inclusion criteria, we could not include RCT, quasi-RCT, and other mixed-method studies. The stringent regulations for study inclusions which comprise the PICOS principle, study design, subject type, data availability, study quality, and adequate data to measure the effect size, restricted the scope for meta-analysis to include only four case control studies. In addition, two studies were discussed systematically in the result section. Although numerous literatures were screened, assessed, and selected methodologically, we found moderate heterogeneity in the overall meta-analysis (I2=70%) with significant P value (p=0.002). This heterogeneity may be of clinical, methodological or statistical origin.43 Characteristics of the study participants and severity of H. pylori infection may also cause high clinical heterogeneity since demographic traits cover a wide ranges data including age, BMI and gestational age. We assume that methodological heterogeneity does not affect overall heterogeneity. On the other hand, statistical heterogeneity may contribute to high heterogeneity since we have subgroups and some are inconsistent.
The association between H. pylori infection and vitamin B12 status in pregnancy is a little investigated field of research. Earlier study reported the presence H. pylori-related gastritis in 57% of patients with macrocytic anaemia caused by B12 deficiency.44 Dietary vitamin B12 is tightly bound to haptocorrin and optimal stomach pH is essential to dissociate the vitamin from the protein enabling absorption.45 Several studies revealed that H. pylori infection has dual effects on stomach pH; acute infection induces hypochlorhydria (higher pH) while chronic infections can exert either hyperchlorhydria or hypochlorhydria (lower pH), depending on the site of the infection.46 47 However, our included studies are incapacious to define individual patient’s H. pylori infection statuses. Two studies corroborate our findings of no significant association between H. pylori infection and vitamin B12 deficiency,48 49 although these were among elderly populations. Another water-soluble B vitamin is folate, and its absorption is negatively influenced by higher pH triggered by H. pylori infection. A sample of either serum or red blood cell (RBC) is commonly used in the quantification of folic acid. But assessment of serum folate is favoured by many clinicians compared with RBC folate.50 Most importantly, RBC folate is superior to serum folate in pregnancy as it provides better folate status.51 In our meta-analysis, half of the studies estimated folate from serum sample and remaining half estimated from RBC sample. This can probably explain our results which do not support the link between H. pylori positivity and reduced folate. Similar to our study, Rasool et al52 found no impact of H. pylori on human serum vitamin B12, folate and homocysteine level.
Serum ferritin levels closely correlate with total body iron stores and measurement of the serum ferritin level is the most accurate test to diagnose IDA.53 In this study, we found that H. pylori infection was not associated with ferritin concentrations. This lack of association may be confounded by the extent that H. pylori infection resides in the stomach. One study found that H. pylori positive patients with peptic ulcer disease had a negative correlation between H. pylori and serum ferritin.54 In patients with normal gastrointestinal tract, no difference in ferritin levels were observed due to presence or absence of H. pylori infections.55 Interestingly, we observed a strong positive correlation between H. pylori infection and IDA (OR=16.23, 95% CI 4.19 to 62.93, p<0.0001). Several cogent biological mechanisms exist through which H. pylori elicits iron depletion. First, H. pylori is responsible for haemorrhagic gastritis, gastric adenocarcinoma, and peptic ulcer disease, all of which increase iron loss.56 Second, corporal gastritis attributable to H. pylori may curtail gastric acid secretion via gland atrophy, which would lead to decrease iron absorption.57 Third, the CagA protein of H. pylori is involved in interstitial holotransferrin direct iron acquisition.58 Iron uptake via the CagA protein further promotes H. pylori growth and intestinal colonisation.18 Our results are supported by a meta-analysis of a case-control studies that confirmed an increased risk of IDA with H. pylori infection.59
There may have unspecified factors that affected our study findings. Misclassification of H. pylori infection is one of them, since H. pylori specific immunological parameters IgA and IgG are considered as diagnostic markers that differ in various studies. The half-life of the IgA and IgG immunoglobulins is greater than 21 days. Participants may have been free of H. pylori when specimens were collected, yet the antibody may have been in circulation due to prior infection.60 The reverse was also possible, that is, subjects were infected during periconception but were no longer producing IgG or IgA antibodies at the time the blood specimens were drawn. In addition, cagA is a virulence factor for pathogenicity of H. pylori and it causes more severe gastric diseases with higher gastric pH.61–63 The presence of cagA toxin in H. pylori could results serious micronutrients deficiency. On the other hand, the absence of cagA toxin might be responsible for adequate micronutrients level although patients were infected with H. pylori. However, our included studies did not detect this virulence factor. Further, in the studies where we extracted data, there were no indications of antibiotic or drug use. This additional information would have been valuable to include in our analysis because the uses of antibiotics can reduce nutritional absorbance.64 65 Additional reasons for diminished micronutrient absorbance were other infections,66 such as Diphyllobothrium pacificum67 or Giardia,68 which might cause iron and vitamin B12 deficiency. Any other infection was not clearly described in the studies we meta-analysed.
Conclusion
H. pylori infection and micronutrient deficiencies are both endemic and widespread in economically disadvantaged populations especially in pregnant women. This meta-analysis shows that H. pylori infection is associated with increased risk of IDA but not with vitamin B12, folate, and ferritin during pregnancy. Therefore, it is important to screen H. pylori infection, especially for effective treatment of IDA in pregnant women. Our result is inconclusive due to limitation of the study number, insufficient data, weakness of the studies and current epidemiological evidences. Additional well-designed longitudinal studies are warranted to clarify the role of H. pylori infection that may elicit micronutrient deficiency during gestation. We recommend several important factors that must be considered for future study design; these factors are the incidence of H. pylori infection, the magnitude of infected area, duration of infection, dietary information, drug uses and other subclinical infections prior to and during the pregnancy.
Acknowledgments
The authors are grateful to all of the participants for their valuable participation in this study. They gratefully acknowledge to all their donors for their support and commitment to icddr, b’s research efforts. Current donors include the Government of the People’s Republic of Bangladesh; Global Affairs Canada (GAC), Canada; Swedish International Development Cooperation Agency (SIDA); and the Department for International Development (UKAid). They extend their gratitude to Anne Williams, PhD, MPH for editing and linguistic revision of the manuscript.
Footnotes
Contributors: All authors contributed to the protocol for this work. MNAA performed study design, data collection, data analysis, data interpretation and drafting of the manuscript. ZNJ did data collection, data interpretation and critical review of the paper. MAIB and ZI contributed the critical revision of the manuscript. TJS performed study conception, data interpretation, critical revision of the manuscript and supervision of the study.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information. We do not have any other available data. However, additional data are available upon reasonable request from the reviewer.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Georgieff MK. Nutrition and the developing brain: nutrient priorities and measurement. Am J Clin Nutr 2007;85:614s–20. 10.1093/ajcn/85.2.614S [DOI] [PubMed] [Google Scholar]
- 2.Williamson CS. Nutrition in pregnancy. Nutr Bull 2006;31:28–59. 10.1111/j.1467-3010.2006.00541.x [DOI] [Google Scholar]
- 3.Gernand AD, Schulze KJ, Stewart CP, et al. Micronutrient deficiencies in pregnancy worldwide: health effects and prevention. Nat Rev Endocrinol 2016;12:274–89. 10.1038/nrendo.2016.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bale JR, Stoll BJ, Lucas AO. The problem of low birth weight. in improving birth outcomes: meeting the challenge in the developing world. National Academies Press (US), 2003. [PubMed] [Google Scholar]
- 5.Schulze KJ, Mehra S, Shaikh S, et al. Antenatal multiple micronutrient supplementation compared to Iron-Folic acid affects micronutrient status but does not eliminate deficiencies in a randomized controlled trial among pregnant women of rural Bangladesh. J Nutr 2019;149:1260–70. 10.1093/jn/nxz046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christian P, Shrestha J, LeClerq SC, et al. Supplementation with micronutrients in addition to iron and folic acid does not further improve the hematologic status of pregnant women in rural Nepal. J Nutr 2003;133:3492–8. 10.1093/jn/133.11.3492 [DOI] [PubMed] [Google Scholar]
- 7.Vijayaraghavan K, Brahmam GN, Nair KM, et al. Evaluation of national nutritional anemia prophylaxis programme. Indian J Pediatr 1990;57:183–90. 10.1007/BF02722084 [DOI] [PubMed] [Google Scholar]
- 8.Vandenplas Y. Helicobacter pylori infection. World J Gastroenterol 2000;6:20–31. 10.3748/wjg.v6.i1.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zamani M, Ebrahimtabar F, Zamani V, et al. Systematic review with meta-analysis: the worldwide prevalence of Helicobacter pylori infection. Aliment Pharmacol Ther 2018;47:868–76. 10.1111/apt.14561 [DOI] [PubMed] [Google Scholar]
- 10.Poveda GF, Carrillo KS, Monje ME, et al. Helicobacter pylori infection and gastrointestinal symptoms on Chilean pregnant women. Rev Assoc Med Bras 2014;60:306–10. 10.1590/1806-9282.60.04.008 [DOI] [PubMed] [Google Scholar]
- 11.Lanciers S, Despinasse B, Mehta DI, et al. Increased susceptibility to Helicobacter pylori infection in pregnancy. Infect Dis Obstet Gynecol 1999;7:195–8. 10.1155/S1064744999000332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dzierzanowska-Fangrat K, Dzierzanowska D. Helicobacter pylori: microbiology and interactions with gastrointestinal microflora. J Physiol Pharmacol 2006;57(Suppl 3):5–14. [PubMed] [Google Scholar]
- 13.Feldman M, Cryer B, Lee E. Effects of Helicobacter pylori gastritis on gastric secretion in healthy human beings. Am J Physiol 1998;274:G1011–7. 10.1152/ajpgi.1998.274.6.G1011 [DOI] [PubMed] [Google Scholar]
- 14.Capurso G, Marignani M, Delle Fave G, et al. Iron-deficiency anemia in premenopausal women: why not consider atrophic body gastritis and Helicobacter pylori role? Am J Gastroenterol 1999;94:3084–5. 10.1111/j.1572-0241.1999.03084.x [DOI] [PubMed] [Google Scholar]
- 15.Franceschi F, Annalisa T, Teresa DR, et al. Role of Helicobacter pylori infection on nutrition and metabolism. World J Gastroenterol 2014;20:12809–17. 10.3748/wjg.v20.i36.12809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ng QX, Venkatanarayanan N, De Deyn MLZQ, et al. A meta-analysis of the association between Helicobacter pylori (H. pylori) infection and hyperemesis gravidarum. Helicobacter 2018;23. 10.1111/hel.12455. [Epub ahead of print: 26 Nov 2017]. [DOI] [PubMed] [Google Scholar]
- 17.Cardaropoli S, Rolfo A, Todros T. Helicobacter pylori and pregnancy-related disorders. World J Gastroenterol 2014;20:654. 10.3748/wjg.v20.i3.654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muhsen K, Cohen D. Helicobacter pylori infection and iron stores: a systematic review and meta-analysis. Helicobacter 2008;13:323–40. 10.1111/j.1523-5378.2008.00617.x [DOI] [PubMed] [Google Scholar]
- 19.de Benoist B. Conclusions of a WHO technical consultation on folate and vitamin B12 deficiencies. Food Nutr Bull 2008;29:S238–44. 10.1177/15648265080292S129 [DOI] [PubMed] [Google Scholar]
- 20.Zhan Y, Si M, Li M, et al. The risk of Helicobacter pylori infection for adverse pregnancy outcomes: A systematic review and meta‐analysis. Helicobacter 2019;24:e12562. 10.1111/hel.12562 [DOI] [PubMed] [Google Scholar]
- 21.Lahner E, Persechino S, Annibale B. Micronutrients (Other than iron) and Helicobacter pylori infection: a systematic review. Helicobacter 2012;17:1–15. 10.1111/j.1523-5378.2011.00892.x [DOI] [PubMed] [Google Scholar]
- 22.Critical Appraisal Skills Programme , 2018. Available: https://casp-uk.net/wp-content/uploads/2018/03/CASP-Case-Control-Study-Checklist-2018_fillable_form.pdf
- 23.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 24.Newcastle - Ottawa quality assessment scale. Case–control studies. Available: https://webcache.googleusercontent.com/search?q=cache:KSLOW_qrSycJ:https://www.ncbi.nlm.nih.gov/books/NBK99082/bin/appb-fm3.pdf+&cd=2&hl=en&ct=clnk&gl=bd
- 25.Machado V, Escalda C, Proença L, et al. Is there a bidirectional association between polycystic ovarian syndrome and periodontitis? A systematic review and meta-analysis. J Clin Med 2020;9:1961 10.3390/jcm9061961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gong H-B, Gao S-T, Pu X-M, et al. Association of rs610604 in TNFAIP3 and rs17728338 in TNIP1 gene polymorphisms with psoriasis susceptibility: a meta-analysis of case-control studies. BMC Med Genet 2020;21:103. 10.1186/s12881-020-01041-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wanyama R, Obai G, Odongo P, et al. Effect of maternal Helicobacter Pylori infection on gestational weight gain in an urban community of Uganda. Pan Afr Med J 2017;28:145. 10.11604/pamj.2017.28.145.9989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baingana RK, Kiboko Enyaru J, Davidsson L. Helicobacter pylori infection in pregnant women in four districts of Uganda: role of geographic location, education and water sources. BMC Public Health 2014;14:915. 10.1186/1471-2458-14-915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haggaz AD, Radi EA, Adam I. Anaemia and low birthweight in Western Sudan. Trans R Soc Trop Med Hyg 2010;104:234–6. 10.1016/j.trstmh.2009.07.013 [DOI] [PubMed] [Google Scholar]
- 31.Alshareef SA, Rayis DA, Adam I, et al. Helicobacter pylori infection, gestational diabetes mellitus and insulin resistance among pregnant Sudanese women. BMC Res Notes 2018;11:517. 10.1186/s13104-018-3642-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malik R, Guleria K, Kaur I, et al. Effect of Helicobacter pylori eradication therapy in iron deficiency anaemia of pregnancy - a pilot study. Indian J Med Res 2011;134:224–31. [PMC free article] [PubMed] [Google Scholar]
- 33.Nashaat EH, Mansour GM. Helicobacter pylori and anemia with pregnancy. Arch Gynecol Obstet 2014;289:1197–202. 10.1007/s00404-013-3138-8 [DOI] [PubMed] [Google Scholar]
- 34.Weyermann M, Rothenbacher D, Gayer L, et al. Role of Helicobacter pylori infection in iron deficiency during pregnancy. Am J Obstet Gynecol 2005;192:548–53. 10.1016/j.ajog.2004.08.028 [DOI] [PubMed] [Google Scholar]
- 35.Ugwuja EI, Akubugwo EI. Impact of maternal Helicobacter pylori infection on trace elements (copper, iron and zinc) and pregnancy outcomes. Online J Health Allied Sci 2010;8. [Google Scholar]
- 36.Mubarak N, Gasim GI, Khalafalla KE, et al. Helicobacter pylori, anemia, iron deficiency and thrombocytopenia among pregnant women at Khartoum, Sudan. Trans R Soc Trop Med Hyg 2014;108:380–4. 10.1093/trstmh/tru044 [DOI] [PubMed] [Google Scholar]
- 37.Golalipour MJ, Sedehi M, Qorbani M. Does maternal Helicobacter pylori infection increase the risk of occurrence of neural tube defects in newborns in Northern Iran? Neurosciences 2012;17:219–25. [PubMed] [Google Scholar]
- 38.Felkner M, Suarez L, Liszka B, et al. Neural tube defects, micronutrient deficiencies, and Helicobacter pylori : A new hypothesis. Birth Defects Res A Clin Mol Teratol 2007;79:617–21. 10.1002/bdra.20382 [DOI] [PubMed] [Google Scholar]
- 39.Mulayim B, Celik NY, Yanik FF. Helicobacter pylori infection detected by 14C-urea breath test is associated with iron deficiency anemia in pregnant women. J Obstet Gynaecol Res 2008;34:980–5. 10.1111/j.1447-0756.2008.00822.x [DOI] [PubMed] [Google Scholar]
- 40.Ali MAH, et al. The impact of Helicobacter pylori infection on iron deficiency anemia in pregnancy. Iraqi J Hematol 2017;6:60. [Google Scholar]
- 41.Daru J, Colman K, Stanworth SJ, et al. Serum ferritin as an indicator of iron status: what do we need to know? Am J Clin Nutr 2017;106:1634S–9. 10.3945/ajcn.117.155960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ioannidis JPA, Trikalinos TA. The appropriateness of asymmetry tests for publication bias in meta-analyses: a large survey. CMAJ 2007;176:1091–6. 10.1503/cmaj.060410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Melsen WG, Bootsma MCJ, Rovers MM, et al. The effects of clinical and statistical heterogeneity on the predictive values of results from meta-analyses. Clin Microbiol Infect 2014;20:123–9. 10.1111/1469-0691.12494 [DOI] [PubMed] [Google Scholar]
- 44.Annibale B, Capurso G, Delle Fave G. Consequences of Helicobacter pylori infection on the absorption of micronutrients. Dig Liver Dis 2002;34(Suppl 2):S72–7. 10.1016/S1590-8658(02)80170-0 [DOI] [PubMed] [Google Scholar]
- 45.Shum HY, O'Neill BJ, Streeter AM. Effect of pH changes on the binding of vitamin B12 by intrinsic factor. J Clin Pathol 1971;24:239–43. 10.1136/jcp.24.3.239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smolka AJ, Schubert ML. Helicobacter pylori-induced changes in gastric acid secretion and upper gastrointestinal disease. Curr Top Microbiol Immunol 2017;400:227–52. 10.1007/978-3-319-50520-6_10 [DOI] [PubMed] [Google Scholar]
- 47.Bücker R, Azevedo-Vethacke M, Groll C, et al. Helicobacter pylori colonization critically depends on postprandial gastric conditions. Sci Rep 2012;2:994. 10.1038/srep00994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pilott A, Fabrello R, Franceschi M, et al. Helicobacter pylori infection in asymptomatic elderly subjects living at home or in a nursing home: effects on gastric function and nutritional status. Age Ageing 1996;25:245–9. 10.1093/ageing/25.3.245 [DOI] [PubMed] [Google Scholar]
- 49.van Asselt DZ, de Groot LC, van Staveren WA, et al. Role of cobalamin intake and atrophic gastritis in mild cobalamin deficiency in older Dutch subjects. Am J Clin Nutr 1998;68:328–34. 10.1093/ajcn/68.2.328 [DOI] [PubMed] [Google Scholar]
- 50.Farrell C-JL, Kirsch SH, Herrmann M. Red cell or serum folate: what to do in clinical practice? Clin Chem Lab Med 2013;51:555–69. 10.1515/cclm-2012-0639 [DOI] [PubMed] [Google Scholar]
- 51.Pillay TS, Oosthuizen NM. Why are we still measuring red cell folate instead of just serum folate? J Clin Pathol 2014;67:289. 10.1136/jclinpath-2013-202086 [DOI] [PubMed] [Google Scholar]
- 52.Rasool S, Abid S, Iqbal MP, et al. Relationship between vitamin B12, folate and homocysteine levels and H. pylori infection in patients with functional dyspepsia: a cross-section study. BMC Res Notes 2012;5:206. 10.1186/1756-0500-5-206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bouri S, Martin J. Investigation of iron deficiency anaemia. Clin Med 2018;18:242–4. 10.7861/clinmedicine.18-3-242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Monzón H, Forné M, Esteve M, et al. Helicobacter pylori infection as a cause of iron deficiency anaemia of unknown origin. World J Gastroenterol 2013;19:4166–71. 10.3748/wjg.v19.i26.4166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saler T, Keşkek Şakir Özgür, Kırk S, et al. H. pylori may not be associated with iron deficiency anemia in patients with normal gastrointestinal tract endoscopy results. Adv Hematol 2014;2014:375915. 10.1155/2014/375915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tan H-J, Goh K-L. Extragastrointestinal manifestations of Helicobacter pylori infection: facts or myth? A critical review. J Dig Dis 2012;13:342–9. 10.1111/j.1751-2980.2012.00599.x [DOI] [PubMed] [Google Scholar]
- 57.Capurso G, Lahner E, Marcheggiano A, et al. Involvement of the corporal mucosa and related changes in gastric acid secretion characterize patients with iron deficiency anaemia associated with Helicobacter pylori infection. Aliment Pharmacol Ther 2001;15:1753–61. 10.1046/j.1365-2036.2001.01101.x [DOI] [PubMed] [Google Scholar]
- 58.Boyanova L. Role of Helicobacter pylori virulence factors for iron acquisition from gastric epithelial cells of the host and impact on bacterial colonization. Future Microbiol 2011;6:843–6. 10.2217/fmb.11.75 [DOI] [PubMed] [Google Scholar]
- 59.Qu X-H, Huang X-L, Xiong P, et al. Does Helicobacter pylori infection play a role in iron deficiency anemia? A meta-analysis. World J Gastroenterol 2010;16:886–96. 10.3748/wjg.v16.i7.886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bonilla FA. Pharmacokinetics of immunoglobulin administered via intravenous or subcutaneous routes. Immunol Allergy Clin North Am 2008;28:803–19. 10.1016/j.iac.2008.06.006 [DOI] [PubMed] [Google Scholar]
- 61.Hamlet A, Thoreson AC, Nilsson O, et al. Duodenal Helicobacter pylori infection differs in cagA genotype between asymptomatic subjects and patients with duodenal ulcers. Gastroenterology 1999;116:259–68. 10.1016/S0016-5085(99)70121-6 [DOI] [PubMed] [Google Scholar]
- 62.Chang W-L, Yeh Y-C, Sheu B-S. The impacts of H. pylori virulence factors on the development of gastroduodenal diseases. J Biomed Sci 2018;25:68. 10.1186/s12929-018-0466-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roesler BM, Rabelo-Gonçalves EMA, Zeitune JMR. Virulence factors of Helicobacter pylori: a review. Clin Med Insights Gastroenterol 2014;7:9–17. 10.4137/CGast.S13760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bushra R, Aslam N, Khan AY. Food-Drug interactions. Oman Med J 2011;26:77–83. 10.5001/omj.2011.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Péter S, Navis G, de Borst MH, et al. Public health relevance of drug-nutrition interactions. Eur J Nutr 2017;56:23–36. 10.1007/s00394-017-1510-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kraehenbuhl JP, Pringault E, Neutra MR. Review article: intestinal epithelia and barrier functions. Aliment Pharmacol Ther 1997;11(Suppl 3):3–9. 10.1111/j.1365-2036.1997.tb00803.x [DOI] [PubMed] [Google Scholar]
- 67.Jimenez JA, Rodriguez S, Gamboa R, et al. Diphyllobothrium pacificum infection is seldom associated with megaloblastic anemia. Am J Trop Med Hyg 2012;87:897–901. 10.4269/ajtmh.2012.12-0067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cordingley FT, Crawford GP. Giardia infection causes vitamin B12 deficiency. Aust N Z J Med 1986;16:78–9. 10.1111/j.1445-5994.1986.tb01127.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgast-2020-000490supp001.pdf (108.5KB, pdf)