Abstract
Background and aims:
Calcific aortic valve disease is highly prevalent in patients with significant smoking history and is a marker of atherosclerosis. The aim of this study was to define the prognostic value of aortic valve calcification (AVC) derived from low dose, lung cancer screening computed tomography (LCSCT) for all-cause mortality in this higher risk population.
Methods
This is a single site, retrospective analysis of 1529 moderate-to-high atherosclerotic cardiovascular risk U.S. veterans (65 years [IQI: 61, 68] years; 96% male), who underwent clinically indicated LCSCT. CTs were scored for aortic valve calcification (AVC) and coronary artery calcification (CAC). The primary endpoint was all-cause mortality and secondary endpoints were nonfatal myocardial infarction (MI) and nonfatal cerebrovascular accident (CVA).
Results:
Over 4-year follow-up, 227 patients (15%) died, 112 patients (7%) had nonfatal MI, and 52 patients (3%) had nonfatal CVA. AVC was predictive of all-cause mortality (HR per 100: 1.041 [1.030–1.052], p < 0.001), and this association remained significant after multivariate adjustment for traditional atherosclerotic risk factors, including CAC (1.021 [1.007–1.036], p = 0.003). After excluding patients with severe aortic stenosis (AS) or severe AVC (≥1274 AU in women and ≥2065 AU in men), in a subset of 765 patients who had echocardiograms, this association remained significant after multivariate analysis (HR per 100: 1.052 [1.010–1.095], p = 0.014). Despite controlling for CAC in the models, AVC was still associated with MI (HR per 100: 1.021 [1.004–1.039], p = 0.017) and with CVA (HR per 100: 1.027 [1.002–1.051], p = 0.032).
Conclusions:
Scoring AVC derived from LCSCT is predictive of mortality, nonfatal MI, and nonfatal CVA in patients at known risk for cardiovascular disease, independent of coronary calcification or severe aortic valve stenosis.
Keywords: Calcific aortic valve disease, Aortic valve calcification, Computed tomography, Smoking, Atherosclerosis, Outcomes
1. Introduction
Calcific aortic valve disease (CAVD) is highly prevalent in the aging population and shares many common risk factors with coronary artery disease (CAD), including tobacco cigarette smoking [1,2]. Moreover, aortic valve sclerosis, a marker of atherosclerosis early in the CAVD process, has been associated with increased risk of cardiovascular events, supporting overlapping disease mechanisms [3]. However, there is also evidence of differing biology between CAD and CAVD; HMG-CoA reductase inhibitors (statins), which impact outcomes in CAD, do not appear to influence the natural progression of AS in patients with CAVD [4].
The presence of calcification on computed tomography (CT) imaging is a hallmark of both CAVD and CAD. Coronary artery calcification (CAC) is predictive of total atherosclerotic burden and risk of cardiovascular and all-cause mortality [5]. Aortic valve calcification (AVC) has been associated with physiologic measures of aortic valve stenosis (AS), and quantification of AVC in the range of severe AS has been predictive of worse prognosis in patients [6,7]. Nonzero AVC has been studied in relatively low-risk populations and was found to be predictive of worsening cardiovascular outcomes in a manner that appears partially dependent on CAC [8], and of all-cause mortality in a manner that appears incremental to CAC [9]. However, the independent prognostic value of AVC, as a continuous variable, for all-cause mortality independent of both CAC or severe AS in moderate-to-high atherosclerotic risk populations has not been evaluated.
Since smoking is a major risk factor for both CAVD and progressive CAD from atherosclerosis and current guidelines recommend routine lung cancer screening with low dose CT (LCSCT) in this population [10,11], we sought to determine the prognostic value of reporting AVC from LCSCT for all-cause mortality, independent of traditional cardiovascular risk factors including CAC, in this moderate-to-high risk cohort.
2. Materials and methods
This study was approved by the Providence Veteran Affairs Medical Center Institutional Review Board and complies with the Declaration of Helsinki, and all patient data were handled in compliance with the Health Insurance Portability and Accountability Act (HIPAA) regulations. All patient records were de-identified and analyzed anonymously. Through the Providence VA Medical Center and the office of the Associate Chief of Staff for Research at Providence VA Medical Center, all data, analytical methods, and study materials are available upon request to other researchers, who meet the Institutional Review Board criteria for access to VA confidential research data, for purposes of reproducing the results or replicating the study.
This was a single-center, retrospective analysis of 1654 U S. veterans who underwent U.S. Preventive Services Task Force guideline-recommended (i.e. 30-pack-year tobacco smoking history, 55–80 years of age, active or quit < 15 years previously) lung cancer screening CT between October 1, 2013, and July 31, 2014 [11]. One hundred twenty-five patients were excluded from the study due diagnosis of lung cancer or prior aortic valve replacement, resulting in 1529 patients for analyses.
CT examinations were not electrocardiographically (ECG)-gated and were performed with a 128-slice CT scanner (Siemens Healthcare, Erlangen, Germany); 128 × 0.6 mm collimation, 0.5-s rotation, pitch of 0.84, 380-mm field of view, 512×512 pixel matrix, tube voltage of 120 kV and tube current of 40 mA, image reconstruction thickness of 1.00–1.25 mm, and a minimum area required to identify calcium was 0.55 mm2. AVC and CAC scores were quantified using the Agatston method via a semiautomated imaging workstation with readers blinded to patient data [12,13]. The kappa for interobserver agreement was 0.91 (0.85–0.95) and 0.92 (0.88–0.96) for AVC and CAC, respectively.
The U.S. Department of Veterans Affairs electronic medical record (EMR) was searched for patient demographics and cardiovascular risk factors. CAD was defined as a history of myocardial infarction (MI), prior revascularization or abnormalities on cardiac testing (exercise treadmill testing, echocardiography, myocardial perfusion, cardiac computed tomography, or coronary angiography). MI was defined as EMR-documented evidence of elevated cardiac troponin values with at least one value above the 99th percentile upper reference limit plus clinical evidence of acute myocardial ischemia, consistent with universal definitions [14]. Cerebrovascular accident (CVA) included non-hemorrhagic, ischemic stroke and transient ischemic attack. The primary outcome was EMR-documented all-cause mortality. Secondary outcomes included nonfatal MI and nonfatal CVA. Adjudicators of clinical outcomes were blinded to AVC and CAC and vice versa.
Results are presented as mean (standard error) for continuous variables with normal distribution, median (interquartile range) for continuous variables without normal distribution, and number (percentage) for categorical data. The Shapiro-Wilk test for normality was used to test for normal distribution. Pearson product-moment correlation coefficient was carried out between AVC and CAC. A subset of 113 patients had both non-ECG-gated CTs and ECG-gated cardiac CTs. The Pearson product-moment correlation coefficient between AVC derived from non-ECG-gated and the AVC derived from ECG-gated studies was carried out, along with standard error of estimate, followed by a Bland-Altman plot for bias and agreement.
The relationship between AVC as a continuous variable and primary or secondary outcomes was determined using Cox regression analysis. The multivariate adjustments included age, body mass index (BMI), hypertension, diabetes mellitus (DM), prior CVA, prior CAD, chronic kidney disease (CKD) stage 3 or higher (GFR < 60 mL/min) and CAC, based on p values < 0.20 in the univariate analyses for the outcomes. Patients with nonzero AVC were divided categorically into quintiles by AVC, followed by Kaplan-Meier analysis and multivariate Cox regression analysis for outcomes using AVC = 0 AU as the reference group. Finally, we conducted a sensitivity analysis for the primary outcome on a subset of patients who had echocardiograms, excluding the 48 patients who met criteria for either severe AS by echocardiography or non-severe AS plus severe AVC (≥1274 AU in women and ≥2065 AU in men [6,7]) to exclude low flow, low gradient severe AS.
All statistical analyses were conducted with the use of Stata/SE (version 15.1; StataCorp LLC, College Station, TX) or Prism (version 7; GraphPad Software Inc, La Jolla, CA). A 2-sided p < 0.05 was considered statistically significant.
3. Results
We evaluated a total of 1529 patients who underwent clinically indicated low dose lung cancer screening CT at Providence VA Medical between October 1, 2013 and July 31, 2014 (Table 1). Indicative of the northeast U.S. veteran population, the median age of this mostly Caucasian (94%) male (96%) population was 65 years (interquartile interval [IQI]: 61, 68 years). The median atherosclerotic cardiovascular disease score was 19% (IQI: 13%, 28%). Sixty-two percent of patients had hypertension, 75% had dyslipidemia, 29% had diabetes, 15% had CKD, 6% had prior CVA or TIA, and 24% had CAD. The overall median CAC was 546 AU (IQI: 109, 1585). The overall median AVC was 66 AU (IQI: 0, 352). In the whole population, the Pearson correlation between AVC and CAC was weak with an R = 0.277 (CI: 0.230–0.322; p < 0.001). In the subset of patients who underwent both non-ECG-gated LCSCT and ECG-gated cardiac CT, Pearson correlation between AVC from non-ECG-gated (comparator) and ECG-gated (referent) was strong with an R = 0.9734 (CI: 0.9615–0.9816; SEE = 67.8). Bland-Altman analysis revealed a mean bias of 49 (−179, 277.6).
Table 1.
Baseline demographics and clinical characteristics of the overall population.
| Overall population (n = 1529) | |
|---|---|
| Age, years, median (IQI) | 65 (61, 68) |
| BMIa, median (IQI) | 28.6 (25.5, 32.5) |
| Male, n (%) | 1475 (96.4) |
| Caucasian, n (%) | 1441 (94.4) |
| DMa, n (%) | 440 (28.8) |
| Hypertension, n (%) | 949 (62.0) |
| Hyperlipidemia, n (%) | 1147 (75.0) |
| Total cholesterol, median (IQI) | 176 (153, 204) |
| HDLa cholesterol, median (IQI) | 43 (36, 52) |
| Statin use, n (%) | 914 (59.7) |
| Current smoker, n (%) | 819 (53.6) |
| Family history of early CADa, n (%) | 192 (12.5) |
| CADa, n (%) | 366 (23.9) |
| Prior MIa, n (%) | 158 (10.3) |
| Prior CABGa, n (%) | 67 (4.3) |
| Prior CVAa, n (%) | 98 (6.4) |
| CKDa, n (%) | 221 (14.5) |
| GFRa, median (IQI) | 79.9 (69.3, 92.4) |
| ASCVDa Risk Score, median (IQI) | 18.9 (12.6, 28.4) |
| CACSa, median (IQI) | 546 (109, 1585) |
| AVCa, median (IQI) | 66 (0, 352) |
| All-cause mortality, n (%) | 227 (14.8) |
| MIa, n (%) | 112 (7.3) |
| CVAa, n (%) | 52 (3.4) |
AVC = aortic valve calcium score; ASCVD = atherosclerotic cardiovascular disease; BMI = body mass index; CABG = coronary artery bypass graft surgery; CACS = coronary artery calcium score; CAD = coronary artery disease; CKD = chronic kidney disease; CVA = cerebrovascular accident; DM = diabetes mellitus; HDL = high-density lipoprotein; GFR = glomerular filtration rate; MI = myocardial infarction.
Death occurred in 227 patients (15%) during the 48-month follow-up. By Cox regression, AVC was associated with mortality (per 100; HR: 1.041; 95% CI: 1.030–1.052; p < 0.001), and this association remained significant after multivariate adjustment (per 100; HR: 1.021; 95% CI: 1.007–1.036; p = 0.003; Table 2). Sensitivity analysis, excluding 538 patients with AVC = 0 AU, revealed AVC was still associated with mortality (per 100; HR: 1.034; 95% CI: 1.022–1.046; p < 0.001), and this association remained significant after the multivariate adjustment (per 100; HR: 1.019; 95% CI: 1.004–1.034; p = 0.014). Categorical analysis demonstrated AVC to be associated with mortality with increasing quintiles (Fig. 1A andTable 3). After multivariate analysis, AVC continued to be associated with mortality at quintiles 4 and 5 (HR: 1.780; 95% CI: 1.116–2.838; p = 0.015 and HR: 2.139; 95% CI: 1.352–3.385; p = 0.001, respectively).
Table 2.
Relationship between aortic valve calcification and all-cause mortality, myocardial infarction and cerebrovascular accident.
| Event | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| All-cause mortality | Myocardial infarction | Cerebrovascular accident | |||||||
| Model | HR | (95% CI) | p value | HR | (95% CI) | p value | HR | (95% CI) | p value |
| Unadjusted | 1.041 | (1.030–1.052) | < 0.001 | 1.038 | (1.023–1.053) | < 0.001 | 1.039 | (1.019–1.060) | < 0.001 |
| Adjusted | 1.021 | (1.007–1.036) | 0.003 | 1.021 | (1.004–1.039) | 0.017 | 1.027 | (1.002–1.051) | 0.032 |
Fig. 1.
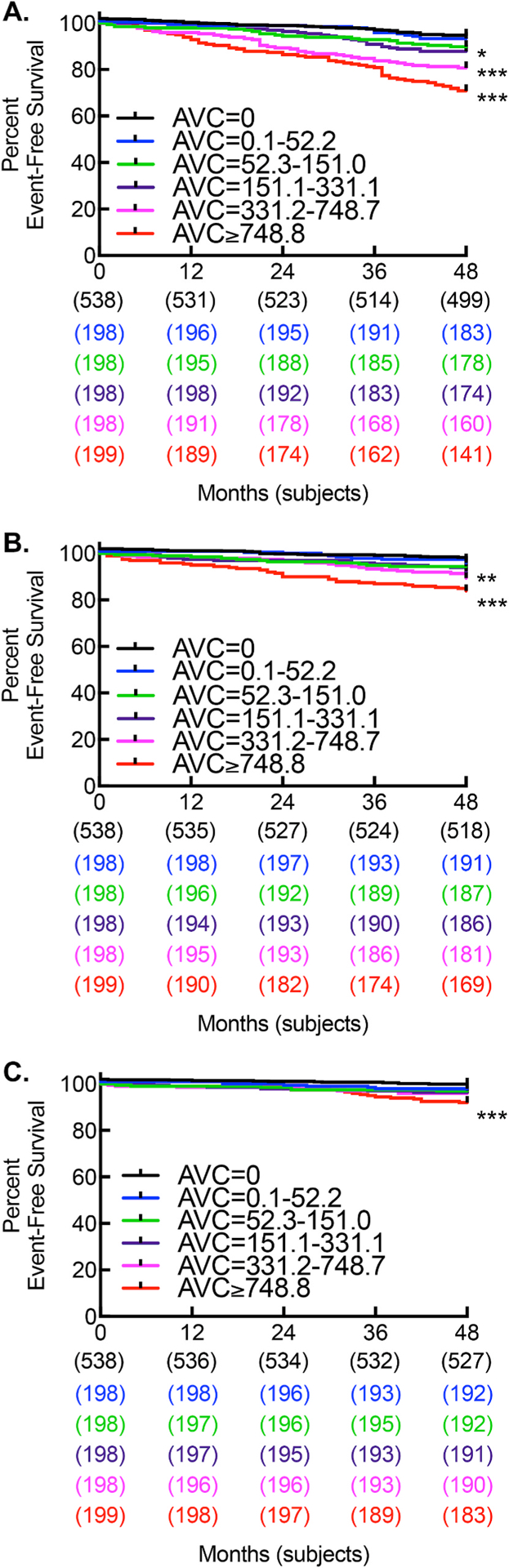
Increasing AVC is associated with increased mortality, myocardial infarction, and cerebrovascular events.
Kaplan-Meier curve for (A) survival (*p < 0.05; ***p < 0.001; log rank), (B) myocardial infarction (**p = 0.004; ***p < 0.001; log rank), and (C) cerebrovascular accident (***p < 0.001; log rank).
Table 3.
Adjusted relationship between aortic valve calcification by quintile and all-cause mortality, myocardial infarction and cerebrovascular accident.
| Event | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| All-cause mortality | Myocardial infarction | Cerebrovascular accident | |||||||
| Quintile (AVC range) | HR | (95% CI) | p value | HR | (95% CI) | p value | HR | (95% CI) | p value |
| 1 (0.1–52.2) | 1.001 | (0.552–1.817) | 0.997 | 1.018 | (0.490–2.117) | 0.961 | 1.472 | (0.543–3.991) | 0.447 |
| 2 (52.3–151.0) | 1.155 | (0.670–1.990) | 0.604 | 0.967 | (0.463–2.016) | 0.928 | 0.855 | (0.270–2.707) | 0.790 |
| 3 (151.1–331.1) | 1.221 | (0.727–2.054) | 0.450 | 1.090 | (0.563–2.111) | 0.797 | 1.370 | (0.519–3.614) | 0.525 |
| 4 (331.2–748.7) | 1.780 | (1.116–2.838) | 0.015 | 1.495 | (0.824–2.713) | 0.185 | 1.448 | (0.562–3.731) | 0.444 |
| 5 (> 748.8) | 2.139 | (1.352–3.385) | 0.001 | 1.875 | (1.048–3.355) | 0.034 | 2.528 | (1.070–5.977) | 0.035 |
A subset of 765 (50%) patients had undergone echocardiograms. The median left ventricular ejection fraction was 61% (IQI: 58, 63). Thirty-eight (5%) patients had a left ventricular ejection fraction less than 40%. We next performed a sensitivity analysis excluding 48 patients who met echocardiography criteria for severe AS or non-severe AS with severe AVC (low flow, low gradient). After exclusion, AVC was still associated with mortality (per 100; HR: 1.085; 95% CI: 1.047–1.125; p < 0.001) by Cox regression, and this association remained significant after multivariate adjustment (per 100; HR: 1.052; 95% CI: 1.010–1.095; p = 0.014).
In order to assess potential mechanisms contributing to increased all-cause mortality, we performed secondary time to event analysis on nonfatal MI and nonfatal CVA (Table 2). Nonfatal MI occurred in 112 patients (7%), and nonfatal CVA occurred in 52 patients (3%) during the 48-month follow-up. By Cox regression, AVC was associated with MI (per 100; HR: 1.038; 95% CI: 1.023–1.053; p < 0.001), and this association remained significant after multivariate adjustment (per 100; HR: 1.021; 95% CI: 1.004–1.039; p = 0.017). Categorical analysis demonstrated AVC to be associated with MI with increasing quintiles (Fig. 1B and Table 3). After multivariate analysis, AVC continued to be associated with MI at quintile 5 (HR: 1.875; 95% CI: 1.048–3.355; p = 0.034). By Cox regression, AVC was associated with CVA (per 100; HR: 1.039; 95% CI: 1.019–1.060; p < 0.001), and this association remained significant after multivariate adjustment (per 100; HR: 1.027; 95% CI: 1.002–1.051; p = 0.032). Categorical analysis demonstrated AVC to be associated with CVA with increasing quintiles (Fig. 1C and Table 3). After multivariate analysis, AVC was still associated with CVA in quintile 5 (HR: 2.528; 95% CI: 1.070–5.977; p = 0.035).
4. Discussion
CAVD may identify an inflammatory atherosclerotic process that impacts mortality irrespective of severe physiologic valve stenosis or even calcific coronary artery disease. Early echocardiography-based studies have supported an association between aortic sclerosis and atherosclerosis by demonstrating associations with all-cause mortality and increased cardiovascular events, though valve calcification was not formally quantified in those studies [3]. Later, ECG-gated, dedicated cardiac CT studies demonstrated threshold quantification of severe AVC to support a diagnosis of severe AS and to be predictive of mortality [6,7]. Additionally, nonzero AVC, as a binary, has been studied in relatively low-risk populations and was found to be predictive of worsening cardiovascular outcomes in a manner that appeared partially dependent on CAC [8], and of all-cause mortality in a manner that appeared incremental to CAC [9]. Here we found a weak association between AVC and CAC. We further demonstrated in an at-risk patient population with smoking history that AVC as a continuous variable is associated with all-cause mortality independent of both CAC and severe AS. To better understand whether an atherosclerotic process may be involved, we assessed the prognostic value of AVC for nonfatal MI and nonfatal CVA. After adjustment for cardiovascular risk and CAC, AVC was also predictive of both nonfatal MI and nonfatal CVA, suggestive of an independent value of AVC in prognosis of atherosclerotic risk.
This study was limited by selection biases inherent to the study design. This particular population of northeast U.S. veterans consisted mostly of older, white men with significant smoking histories. Events may be underestimated because of limitations inherent in extracting data or because care outside the Department of Veterans Affairs was not fully captured. To address these limitations, further studies are needed, including larger prospective multicenter studies in a more diverse patient population.
In conclusion, scoring AVC derived from LCSCT is helpful at predicting mortality, nonfatal MI and nonfatal CVA in patients at known risk for CAVD independent of the degree of coronary calcification or severe aortic valve stenosis. Analogous to what we and others have found in previous studies regarding use of non-ECG-gated CTs to score CAC [15], AVC derived from non-ECG-gated CTs shows strong agreement to AVC derived from ECG-gated CTs. Reporting AVC from LCSCT may help physicians identify patients who would benefit from more aggressive cardiovascular care. Additional prospective studies are required to further assess the mechanistic relationship between atherosclerotic events and mortality in patients with high AVC in order to determine whether this population would benefit from primary and secondary strategies involving aggressive lipid/inflammation-based cardiovascular treatment.
HIGHLIGHTS.
Aortic valve calcification (AVC) is predictive of mortality after adjustment for cardiovascular risk, aortic stenosis (AS), and coronary artery calcification (CAC), in patients with history of smoking.
AVC is also predictive of nonfatal myocardial infarction and nonfatal cerebrovascular accident in this population.
There is prognostic value in reporting AVC from low dose lung cancer screening computed tomography.
Acknowledgments
Research reported in this publication was supported by a Research Project Grant NIH NHLBI R01HL139795 (A.R.M.), Research Project Grant NIH NHLBI R01HL148727 (G.C.), and an Institutional Development Award (IDeA) from NIH NIGMS P20GM103652 (A.R.M.). This work was also supported by Career Development Award Number 7IK2BX002527 from the United States Department of Veterans Affairs Biomedical Laboratory Research and Development Program (A.R.M.) and by VA VHA CSR&D 1I01CX001892 (G.C.). The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government. This work was also supported in part by NIH NHLBI T35 HL094308 (A.C.) and NIH NHLBI R01 136431 and 147095 (E.A.).
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Lindman BR, Clavel MA, Mathieu P, Iung B, Lancellotti P, Otto CM, Pibarot P, Calcific aortic stenosis, Nat. Rev. Dis. Prim 2 (2016) 16006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB, American Heart Association Statistics C and Stroke Statistics S. Executive summary: heart disease and stroke statistics-2013 update: a report from the American Heart Association, Circulation 127 (2013) 143–152. [DOI] [PubMed] [Google Scholar]
- [3].Otto CM, Lind BK, Kitzman DW, Gersh BJ, Siscovick DS, Association of aorticvalve sclerosis with cardiovascular mortality and morbidity in the elderly, N. Engl. J. Med 341 (1999) 142–147. [DOI] [PubMed] [Google Scholar]
- [4].Teo KK, Corsi DJ, Tam JW, Dumesnil JG, Chan KL, Lipid lowering on progression of mild to moderate aortic stenosis: meta-analysis of the randomized placebo-controlled clinical trials on 2344 patients, Can. J. Cardiol 27 (2011) 800–808. [DOI] [PubMed] [Google Scholar]
- [5].Rennenberg RJ, Kessels AG, Schurgers LJ, van Engelshoven JM, de Leeuw PW, Kroon AA, Vascular calcifications as a marker of increased cardiovascular risk: a meta-analysis, Vasc. Health Risk Manag. 5 (2009) 185–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Clavel MA, Messika-Zeitoun D, Pibarot P, Aggarwal SR, Malouf J, Araoz PA, Michelena HI, Cueff C, Larose E, Capoulade R, Vahanian A, Enriquez-Sarano M, The complex nature of discordant severe calcified aortic valve disease grading: new insights from combined Doppler echocardiographic and computed tomographic study, J. Am. Coll. Cardiol 62 (2013) 2329–2338. [DOI] [PubMed] [Google Scholar]
- [7].Clavel MA, Pibarot P, Messika-Zeitoun D, Capoulade R, Malouf J, Aggarval S, Araoz PA, Michelena HI, Cueff C, Larose E, Miller JD, Vahanian A, Enriquez-Sarano M, Impact of aortic valve calcification, as measured by MDCT, on survival in patients with aortic stenosis: results of an international registry study, J. Am. Coll. Cardiol 64 (2014) 1202–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Owens DS, Budoff MJ, Katz R, Takasu J, Shavelle DM, Carr JJ, Heckbert SR, Otto CM, Probstfield JL, Kronmal RA, O’Brien KD, Aortic valve calcium independently predicts coronary and cardiovascular events in a primary prevention population, JACC Cardiovasc. Imag 5 (2012) 619–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Blaha MJ, Budoff MJ, Rivera JJ, Khan AN, Santos RD, Shaw LJ, Raggi P, Berman D, Rumberger JA, Blumenthal RS, Nasir K, Relation of aortic valve calcium detected by cardiac computed tomography to all-cause mortality, Am. J. Cardiol 106 (2010) 1787–1791. [DOI] [PubMed] [Google Scholar]
- [10].Owens DS, Katz R, Takasu J, Kronmal R, Budoff MJ, O’Brien KD, Incidence and progression of aortic valve calcium in the Multi-ethnic Study of Atherosclerosis (MESA), Am. J. Cardiol 105 (2010) 701–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Moyer VA, Force USPST, Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement, Ann. Intern. Med 160 (2014) 330–338. [DOI] [PubMed] [Google Scholar]
- [12].Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr., Detrano R, Quantification of coronary artery calcium using ultrafast computed tomography, J. Am. Coll. Cardiol 15 (1990) 827–832. [DOI] [PubMed] [Google Scholar]
- [13].Shavelle DM, Budoff MJ, Buljubasic N, Wu AH, Takasu J, Rosales J, Otto CM, Zhao XQ, O’Brien KD, Usefulness of aortic valve calcium scores by electron beam computed tomography as a marker for aortic stenosis, Am. J. Cardiol 92 (2003) 349–353. [DOI] [PubMed] [Google Scholar]
- [14].Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD and executive group on behalf of the joint European society of cardiology/American College of cardiology/American heart association/world heart federation Task Force for the universal definition of myocardial I. Fourth universal definition of myocardial infarction (2018), J. Am. Coll. Cardiol 72 (2018) 2231–2264. [DOI] [PubMed] [Google Scholar]
- [15].Christensen JL, Sharma E, Gorvitovskaia AY, Watts JP Jr., Assali M, Neverson J, Wu WC, Choudhary G, Morrison AR, Impact of slice thickness on the predictive value of lung cancer screening computed tomography in the evaluation of coronary artery calcification, J. Am. Heart Assoc 8 (2019) e010110. [DOI] [PMC free article] [PubMed] [Google Scholar]


