Abstract
Background
This study estimates the prevalence and identifies predictors of psychoactive medication use in adolescent survivors of childhood cancer (aged 12-18 years) and its associations with functional outcomes at young adulthood (aged 18-28 years).
Methods
This retrospective cohort study includes 5665 adolescent survivors of childhood cancer at no less than 5 years postdiagnosis (53.8% male, median age = 15 years, interquartile range [IQR] = 13-16 years) and 921 adolescent sibling controls. Parent-reported psychoactive medication use during adolescence was collected at baseline. After a median of 8 years, functional outcomes and social attainment were self-reported during adulthood (n = 3114, median age = 22 years, IQR = 20-24 years). Multivariable log-binomial models evaluated associations among risk factors, medication use, and adult outcomes.
Results
Higher prevalence of psychoactive medication use was reported in survivors compared with siblings (18.3% vs 6.6%; 2-sided P < .001), with trends for increasing antidepressant and stimulant use in recent treatment eras. After adjusting for cancer treatment and baseline cognitive problems, psychoactive medication use during adolescence was associated with impaired task efficiency (relative risk [RR] = 1.20, 95% confidence interval [CI] = 1.01 to 1.43) and memory (RR = 1.27, 95% CI = 1.05 to 1.52) during adulthood. Survivors who reported continued use of medications from adolescence to adulthood demonstrated poorer emotional regulation (RR = 1.68, 95% CI = 1.24 to 2.27) and organization (RR = 1.82, 95% CI = 1.28 to 2.59) compared with nonusers. Adolescent opioid use was associated with somatization symptoms (RR = 1.72, 95% CI = 1.09 to 2.73) during adulthood, after adjusting for cancer treatment and baseline behavioral problems. They were also more likely to not complete college (RR = 1.21, 95% CI = 1.04 to 1.41) or work full-time (RR = 1.60, 95% CI = 1.23 to 2.08) compared with nonusers.
Conclusion
Use of psychoactive medication is more prevalent among adolescent survivors compared with siblings and does not normalize adult outcomes, as evidenced by poorer functional outcomes during young adulthood.
Modern treatment approaches for childhood cancer have increased survival rates (1). However, this comes with a recognized risk for late effects from cancer and anticancer therapies (2,3). Long-term survivors are at risk for cancer- and treatment-related symptoms, such as sleep disturbances, fatigue, neurocognitive deficits, and chronic pain (4,5), which can lead to poorer health-related quality of life and reduced functional independence in long-term survivors.
In survivors, as in the general population, many of these distressing symptoms are treated pharmacologically. We previously reported that adult survivors of childhood cancer who were older than 18 years of age were up to 60% more likely to report the use of opioid analgesics, nonopioid analgesics, and anxiolytics, as compared with healthy sibling controls (6). The use of psychoactive medications was also concurrently associated with worse neurocognitive function in task efficiency and memory (7), as well as poorer social functioning and vitality (6).
Evidence on prevalence and predictors of psychoactive medication use in adolescent survivors of childhood cancer is lacking. In the United States, approximately 6% of adolescents aged 12 to 19 years are reported to have used psychotropic medications for the treatment of attention deficit hyperactivity disorder, insomnia, depression, and other mental health conditions between 2005 and 2010 (8). Adolescent survivors are at increased risk of developing emotional, behavioral, cognitive, and social problems (9–11), raising the question of whether there is more prevalent use of psychoactive medications among survivors. Further, little is known about the impact of psychoactive medication use in adolescence on long-term outcomes such as health status, educational attainment, and employment in later life. Given the unique neurodevelopmental processes taking place during adolescence, it is important to evaluate whether administration of psychoactive medications early in life can normalize mood and behavior problems in these survivors during adulthood.
Methods
Study Population
The Childhood Cancer Survivor Study (CCSS) is a multi-institutional retrospective cohort of 5-year survivors of childhood cancer (12,13). Eligibility criteria for the CCSS include 1) a diagnosis of cancer prior to 21 years of age between 1970 and 1999 and 2) survival to at least 5 years postdiagnosis. Multiple cancer diagnoses were included. Institutional review boards at the 31 participating institutions approved the CCSS study protocol, and participants provided informed consent. At cohort entry, survivors identified a living sibling nearest to them in age. A random sample of siblings were contacted to participate (12,13). With the exception of cancer-specific topics, information collected from the sibling cohort is identical to that obtained on the survivor population.
The study population for the current analyses included 5655 cancer survivors who were between 12 and 18 years old when their parent or guardian completed the baseline survey upon entry to the CCSS cohort (Supplementary Figure 1, available online). There were 921 adolescent siblings who served as a comparison group (see Table 1). The impact of psychoactive medication use on future functional outcomes was examined in 3114 of the survivors who were adults (18 to 28 years old) and provided functional outcomes data in a follow-up survey 8 years later (interquartile range [IQR]= 7-9 years). A subset of adult survivors (n = 585) who were older than 25 years of age during follow-up provided data on their social attainment outcomes (highest education attainment and employment status). Differences in characteristics and psychoactive medication use during adolescence were compared between survivors with adult follow-up vs survivors who did not complete follow-up (Supplementary Table 1, available online).
Table 1.
Characteristics of adolescent survivors of childhood cancer and sibling comparison populationa
| Clinical characteristics | Siblings | All survivorsb | Survivors with psychoactive medication | Survivors without psychoactive medication |
|---|---|---|---|---|
| (n = 921) |
(n = 5665) |
(n = 1037) |
(n = 4628) |
|
| No. (%) | No. (%) | No. (%) | No. (%) | |
| Year of completion of baseline survey | ||||
| 1992-1999 | 782 (84.9) | 4178 (66.2) | 740 (64.7) | 3438 (66.5) |
| 2000-2009 | 32 (3.5) | 421 (11.0) | 102 (13.8) | 319 (10.4) |
| 2010-2015 | 107 (11.6) | 1066 (22.8) | 195 (21.5) | 871 (23.1) |
| Sex | ||||
| Male | 488 (53.0) | 3047 (54.1) | 566 (55.3) | 2481 (53.8) |
| Female | 433 (47.0) | 2618 (45.9) | 471 (44.7) | 2147 (46.2) |
| Race | ||||
| White/non-Hispanic | 763 (82.8) | 4533 (79.9) | 868 (83.6) | 3665 (79.0) |
| Others | 118 (12.8) | 1017 (17.9) | 153 (14.8) | 864 (18.5) |
| Not specified | 40 (4.3) | 115 (2.3) | 16 (1.6) | 99 (2.4) |
| Age at assessment, yc | 15 (12-16) | 15 (13-16) | 15 (13-16) | 15 (13-16) |
| Health insurance | ||||
| Yes | 861 (93.5) | 4714 (84.1) | 793 (77.3) | 3921 (85.6) |
| No | 48 (5.2) | 316 (5.6) | 54 (5.7) | 262 (5.5) |
| Not specified | 12 (1.3) | 633 (10.3) | 188 (17.0) | 445 (8.8) |
| Household income | ||||
| < $60 000 | 508 (55.2) | 3311 (56.5) | 637 (58.8) | 2674 (56.0) |
| ≥$60 000 | 364 (39.5) | 1995 (37.1) | 340 (35.3) | 1655 (37.5) |
| Not specified | 49 (5.3) | 359 (6.4) | 60 (5.9) | 299 (6.5) |
| History of epilepsy | ||||
| No | 906 (98.5) | 5094 (90.6) | 700 (68.2) | 4394 (95.5) |
| Yes | 14 (1.5) | 551 (9.4) | 332 (31.8) | 219 (4.5) |
| History of migraine/headache | ||||
| No | 812 (88.3) | 4380 (77.9) | 648 (63.0) | 3732 (81.2) |
| Yes | 108 (11.7) | 1269 (22.1) | 385 (37.0) | 884 (18.8) |
| Cancer-related pain | ||||
| No | — | 4335 (86.1) | 617 (73.9) | 3718 (88.8) |
| Mild | — | 443 (8.8) | 111 (13.8) | 332 (7.9) |
| Moderate | — | 186 (3.7) | 71 (7.9) | 115 (2.5) |
| Severe | — | 72 (1.4) | 35 (4.3) | 37 (0.8) |
| Diagnosis | ||||
| Leukemia | — | 2276 (40.2) | 362 (41.0) | 1914 (47.5) |
| Central nervous system tumor | — | 906 (16.0) | 306 (26.7) | 600 (11.6) |
| Hodgkin disease/non-Hodgkin lymphoma | — | 290 (5.1) | 42 (3.7) | 248 (4.8) |
| Wilms tumor | — | 838 (14.8) | 109 (9.5) | 729 (14.1) |
| Neuroblastoma | — | 930 (16.4) | 145 (12.7) | 785 (15.2) |
| Soft tissue sarcoma/osteosarcoma | — | 425 (7.5) | 73 (6.4) | 352 (6.8) |
| Age at diagnosis, yc | — | 2.8 (1.5-4.3) | 2.8 (1.5-4.6) | 2.7 (1.5-4.2) |
| Chemotherapy | ||||
| Anthracycline | — | 1658 (43.6) | 266 (39.2) | 1392 (44.8) |
| Alkylating agent | — | 1961 (51.6) | 353 (52.1) | 1608 (51.7) |
| IV Methotrexate (cumulative),d mg/m2 | — | 7337 (81 188.2) | 3808 (32 343.5) | 8112 (88 353.1) |
| IT Methotrexate (cumulative),d mg/m2 | — | 98 (144.0) | 87 (138.6) | 100 (145.1) |
| Anti-tumor antibiotic | — | 2138 (51.1) | 351 (47.3) | 1787 (51.9) |
| Corticosteroids | — | 2043 (48.8) | 350 (47.2) | 1693 (49.1) |
| Enzymes | — | 1657 (39.6) | 263 (35.4) | 1394 (40.5) |
| Epipodophyllotoxins | — | 530 (13.9) | 97 (14.3) | 433 (13.9) |
| Heavy metals | — | 360 (8.6) | 95 (12.8) | 265 (7.7) |
| Plant alkaloids | — | 3007 (71.8) | 486 (65.5) | 2521 (73.2) |
| Radiation | ||||
| None | — | 3446 (66.9) | 558 (61.1) | 2889 (70.9) |
| Brain <20 Gy | — | 781 (15.2) | 129 (13.4) | 652 (14.8) |
| Brain 20-35 Gy | — | 330 (6.4) | 61 (6.8) | 269 (6.0) |
| Brain >35 Gy | — | 591 (11.5) | 196 (18.6) | 395 (8.3) |
| Body only (chest, abdomen, pelvis) | — | 1447 (23.6) | 287 (26.1) | 1160 (23.0) |
Summary statistics was calculated on total number of participants for whom data was available and weighted to reflect modified sampling of survivors in expansion cohort. IV = intravenous; IT = intrathecal.
Combination of both original and expansion cohorts.
Presented as median (interquartile range).
Presented as mean (SD). Intravenous and intrathecal methotrexate are known to be associated with dose-dependent neurotoxic late effects. Hence, subsequent analyses included methotrexate cumulative doses as a continuous variable.
Psychoactive Medications
Psychoactive medication use at baseline was the primary study outcome. Participants’ parents reported drugs prescribed by a physician and dispensed by a pharmacist that were taken consistently by the adolescent survivor for more than 1 month during the previous 2 years. Medications were classified using the American Hospital Formulary Service Drug Information database (14), consistent with previous publications (6,7). Eight therapeutic drug categories that include psychoactive properties were identified: antidepressants; anxiolytics, sedatives, or hypnotics; anticonvulsants; nonopioid analgesics; opioids; muscle relaxants; neuroleptics; and stimulants (Supplementary Box 1, available online).
Predictors and Covariates
Demographic and socioeconomic variables for adolescent survivors were provided by the survivor’s parent or guardian at the baseline survey and included sex, age at the time of survey completion, race and/or ethnicity, health insurance, and household income. Information on original cancer diagnosis and cancer treatment exposures were abstracted from medical records of treating institutions. The maximum radiation dose to 4 body regions (brain, chest, abdomen, and pelvis) was determined based on detailed review of radiation therapy records. The radiation dose was defined as the maximum prescribed dose within each region, which is taken as the total prescribed dose from all overlapping fields within the treated region. Neurologic variables included history of headache, presence and severity of bodily pain, and history of stroke or seizure.
Adolescent survivors’ behavioral and cognitive function at baseline were proxy rated using the Behavior Problem Index, a standardized questionnaire that has been normed on a large nationally representative sample (15). It consists of 5 symptom domains: depression and/or anxiety, headstrong behavior, attention deficit, peer conflict and/or social withdrawal, and antisocial behavior. Impairment within each domain was defined as a score equivalent to the top 10th percentile of the sibling group. Academic problems were defined as previous enrollment into a learning disabled or special education program.
Young Adulthood Outcomes
All functional outcomes at adulthood were self-reported by survivors at follow-up surveys. Neurocognitive outcomes were measured using the Childhood Cancer Survivor Study - Neurocognitive Questionnaire (CCSS-NCQ), a 25-item instrument developed and previously validated in the CCSS survivor and sibling population (16). It consists of 4 domains: task efficiency, emotional regulation, organization, and memory. A higher score is indicative of more problems. Neurocognitive impairment was defined as a score falling in the top 10th percentile of the sibling normative reference for each domain. Emotional distress was measured by the Brief-Symptom Inventory-18, which included subscales for anxiety, depression, and somatization (17). Sex-specific scores were calculated based on standardized normative values, and scores falling in the top 10th percentile were classified as representing a clinical level of acute distress on each subscale. Traumatic stress was assessed by the Posttraumatic Stress Diagnostic Scale, a 17-item, self-reported questionnaire to assess symptoms of posttraumatic stress disorder (18). Ratings on items are summed to create 3 subscales: reexperiencing, avoidance, and arousal. Consistent with previous CCSS studies (9,10), an overall positive endorsement of posttraumatic stress disorder was defined by the report of at least 1 reexperiencing symptom, at least 3 avoidance symptoms, and at least 2 arousal symptoms. Health-related quality of life was measured using the Medical Outcomes Survey 36-Item Short Form Health Survey, which includes 8 domains: general health, role physical, physical function, bodily pain, vitality, mental health, social function, and role emotional (19). The 36-Item Short Form provides age and sex-specific norms to generate T-scores. Impairment was defined as T-score falling less than 40.
Social attainment information on survivors’ highest education attainment (categorized as college graduate and above vs below) and employment status (categorized as full-time employment vs others) were obtained, and analyses were conducted on a subset of the cohort who were above the age of 25 years during follow-up.
Statistical Analysis
Descriptive analyses summarized the distribution of relevant outcome variables, predictors, and covariates according to reasonable groupings and consistent with previous CCSS manuscripts (6,7,12,20). Comparison of psychoactive medication use between survivors and siblings at baseline was conducted using multivariable log-binomial models (generalized linear models with Poisson error and log-link function) adjusting for potential confounders (sex, age, health insurance status, and household income). Relative risk (RR) estimates and 95% confidence intervals (CI) were reported and robust sandwich variance estimates accounted for intrafamily correlation between survivors and siblings. A similar model that incorporated calendar year was used to compare change in prevalence of psychoactive medication use over time between survivors and siblings by testing for interactions between time and survivor or sibling status.
Among survivors, multivariable log-binomial models identified clinical and cancer treatment factors associated with psychoactive medication during adolescence. Multivariable log-binomial models evaluated associations between psychoactive medication use and adolescent survivors’ concurrent behavioral and social functioning at baseline, adjusted for age at evaluation, sex, race, and cancer treatment variables. These variables were decided a priori because previous studies have demonstrated their associations with either psychoactive medication use or functioning outcomes in the general population or/and cancer survivors (6–8,11).
Multivariable log-binomial models were also used to evaluate the associations between psychoactive medication use during adolescence and later functional outcomes in adulthood, adjusted for variables shown to be associated with functional outcomes (4,5,7,11) (age at evaluation, sex, race, cancer treatment variables, behavior problems, and placement in special education during adolescence), as well as variables that were statistically significantly associated with psychoactive medication use during adolescence in the previous analysis. A similar multivariable log-binomial model was conducted for testing the associations between continued psychoactive medication use from adolescence to adulthood and subsequent functional outcomes in adulthood. All statistical estimates from the data, including percentages, were calculated with sampling weights to account for undersampling of survivors of acute lymphoblastic leukemia diagnosed from 1987 to 1999. All analyses were conducted in SAS (SAS 9.4, SAS Institute, Cary, NC). A P value of less than .05 was considered statistically significant, and all tests were 2-sided.
Results
Study Population Characteristics
Participants included 5665 adolescent survivors of childhood cancer (53.8% male; median [IQR] age = 2.7 [1.3-4.3] years at cancer diagnosis; median age = 15 [13-16] years at baseline) and 921 siblings (53.0% male; age = 15 [12-16] years at baseline) (Table 1). The majority of the survivors were diagnosed with leukemia (40.0%), central nervous system (CNS) malignancy (16.0%), and neuroblastoma (16.4%). One-quarter of the survivors were treated with cranial radiation (Table 1).
Prevalence of Psychoactive Medication Use
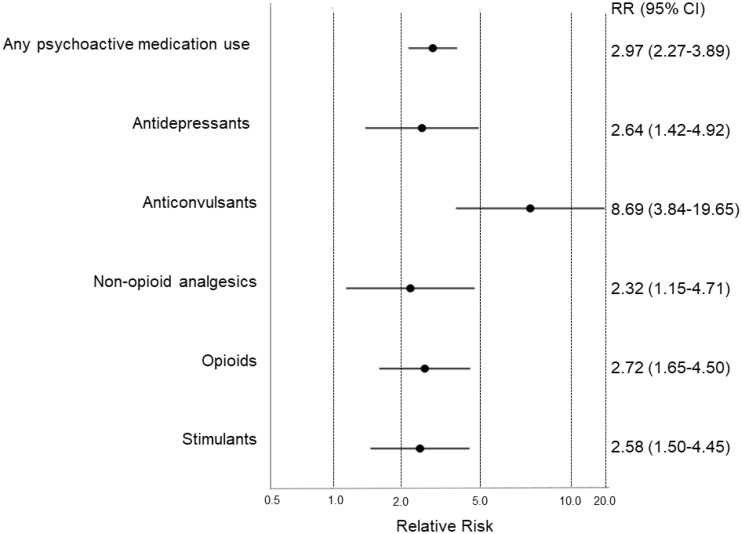
Compared to siblings, survivors were nearly three times more likely to use any psychoactive medication (18.3% vs 6.6%, RR = 2.97, 95% CI = 2.27 to 3.89; P < .001) (Figure 1; Supplementary Table 2, available online). Survivors had higher risk of using antidepressants (RR = 2.64, 95% CI = 1.42 to 4.92), stimulants (RR = 2.58, 95% CI = 1.50 to 4.45), and anticonvulsants (RR = 8.69, 95% CI = 3.84 to 19.65) than siblings (Figure 1). The use of both nonopioid (2.5% vs 0.9%; P = .002) and opioid (5.7% vs 1.7%; P < .001) analgesics were also higher in survivors, whereas neuroleptics and muscle relaxants were less prevalent in both groups (Supplementary Table 2, available online). Stratified analyses within diagnostic groups showed that, relative to siblings, the highest risk estimates for use of any psychoactive medications were observed in CNS tumor survivors, whereas higher use of antidepressants was observed in all survivors regardless of cancer diagnoses (Supplementary Figure 2, available online).
Figure 1.
Relative risks of psychoactive medicine use at baseline comparing adolescent survivors of childhood cancer and siblings. Each dot and whisker represents the relative risk (RR) estimate and 95% confidence interval (CI), respectively, on a logarithmic scale. Models are adjusted for age, sex, and race. Comparisons were not conducted for anxiolytics, sedatives, and hypnotics; muscle relaxants; and neuroleptics because of limited sample size within both the survivor and sibling groups. The exact proportion of survivors and siblings who reported psychoactive medication use and the relative risk (95% CI) estimates are presented in Supplementary Table 2 (available online).
The frequency of overall psychoactive medication use among the adolescent sample remained relatively stable from 1992 to 2015, with the exception of a slight increase from 1992-1999 to 2000-2009 eras (Supplementary Table 3, available online). Within survivors, from the era 1992-1999 to 2010-2015, the frequency of antidepressant use changed from 2.0% to 5.8%, and stimulant use changed from 2.7% to 5.6% (Supplementary Table 3, available online). However, the use of opioids dropped from 7.1% to 1.6% over this same time.
Factors Associated With Psychoactive Medication During Adolescence
Survivors of CNS malignancies were more likely than survivors with solid and soft tissue tumors to use opioids (RR = 1.44, 95% CI = 1.01 to 2.05) and anticonvulsants (RR = 9.11, 95% CI = 6.66 to 12.5) (Supplementary Table 4, available online). Moderate pain was strongly associated with use of antidepressants (RR = 2.55, 95% CI = 1.48 to 4.38), nonopioid (RR = 3.53, 95% CI = 2.08 to 5.99), and opioid (RR = 2.57, 95% CI = 1.59 to 4.16) analgesics compared with survivors without pain. Those who received more than 35 Gy of cranial radiation were more likely than nonirradiated survivors to use psychoactive medication (RR = 1.96, 95% CI = 1.64 to 2.33), especially anticonvulsants (RR = 5.36, 95% CI = 4.01 to 7.18) and opioids (RR = 1.64, 95% CI = 1.09 to 2.48).
Adolescent survivors who used psychoactive medication were more likely to have parent-reported cognitive and behavioral problems (Table 2). These adolescents were also more likely to have received special education services (RR = 1.71, 95% CI = 1.57 to 1.86).
Table 2.
Association of concurrent parent-reported cognitive and behavioral problems and special education service with psychoactive medication use in adolescent survivors of childhood cancer
| Behavioral problemsand special educationservice use | Any psychoactive medication |
Antidepressant |
Anxiolytics, sedatives, hypnotics |
Anticonvulsants |
Nonopioid analgesics |
Opioids |
Muscle relaxants |
Neuroleptics |
Stimulants |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RR (95% CI)b | RR (95% CI)b | RR (95% CI)b | RR (95% CI)b | RR (95% CI)b | RR (95% CI)b | RR (95% CI)b | RR (95% CI)b | RR (95% CI)b | ||||||||||
| Behavior problems indexa | ||||||||||||||||||
| Depression/Anxiety | 2.10c (1.82 to 2.42) |
3.04c (2.52- 3.67) |
1.67c (1.06 to 2.64) |
1.24 (0.94 to 1.64) |
1.85c (1.43 to 2.39) |
1.46c (1.15 to 1.86) |
1.25 (0.57 to 2.74) |
4.32c (3.45 to 5.42) |
2.36c (1.93 to 2.90) |
|||||||||
| Headstrong behavior | 2.09c (1.79 to 2.44) |
2.76c (2.22 to 3.44) |
1.51 (0.94 to 2.43) |
1.17 (0.88 to 1.56) |
1.95c (1.48 to 2.56) |
1.47c (1.13 to 1.91) |
1.12 (0.47 to 2.67) |
4.56c (3.66 to 5.67) |
2.68c (2.20 to 3.26) |
|||||||||
| Attention deficit | 3.03c (2.66 to 3.46) |
3.32c (2.77 to 3.97) |
1.76c (1.18 to 2.64) |
2.50c (2.08 to 3.00) |
1.44c (1.08 to 1.91) |
1.41c (1.12 to 1.79) |
1.48 (0.77 to 2.86) |
5.18c (4.36 to 6.14) |
4.30c (3.73 to 4.96) |
|||||||||
| Peer conflict/Social withdrawal | 1.59c (1.43 to 1.77) |
2.24c (1.97 to 2.55) |
1.21 (0.85 to 1.70) |
1.23c (1.03 to 1.47) |
1.23 (0.98 to 1.55) |
1.14 (0.94 to 1.38) |
1.18 (0.66 to 2.12) |
2.81c (2.31 to 3.43) |
1.90c (1.63 to 2.22) |
|||||||||
| Antisocial | 1.70c (1.44 to 2.00) |
2.24c (1.72 to 2.93) |
0.91 (0.48 to 1.72) |
1.05 (0.77 to 1.43) |
1.33 (0.94 to 1.88) |
1.29 (0.97 to 1.71) |
1.46 (0.67 to 3.16) |
3.65c (2.58 to 5.16) |
2.41c (1.94 to 3.01) |
|||||||||
| Placement in special education | ||||||||||||||||||
| Yes | 1.71c (1.57 to 1.86) |
1.84c (1.62 to 2.10) |
1.75c (1.40 to 2.18) |
1.94c (1.75 to 2.15) |
1.07 (0.86 to 1.32) |
1.12 (0.97 to 1.30) |
0.94 (0.54 to 1.65) |
1.98c (1.61 to 2.44) |
1.83c (1.61 to 2.07) |
|||||||||
Cognitive and behavioral impairments were defined as a score falling ≥90th percentile based on values obtained in the sibling cohort on the Brief Problem Index. CI = confidence interval; RR = relative risk.
Models are adjusted for age, sex, race, socioeconomic status, intravenous and intrathecal methotrexate doses, and cranial radiation dose.
Denotes statistical significance (P < .05).
Longitudinal Outcomes During Young Adulthood
From adolescence to young adulthood, 7.9% (n = 250) of the survivors were identified to be persistent users of psychoactive medications, whereas 14.8% (n = 479) were new-onset users, and 8.3% (n = 264) reported discontinuation of psychoactive medications (Supplementary Table 5, available online). At follow-up, approximately one-tenth of the survivors (10.7%) were found to be new-onset users of antidepressants.
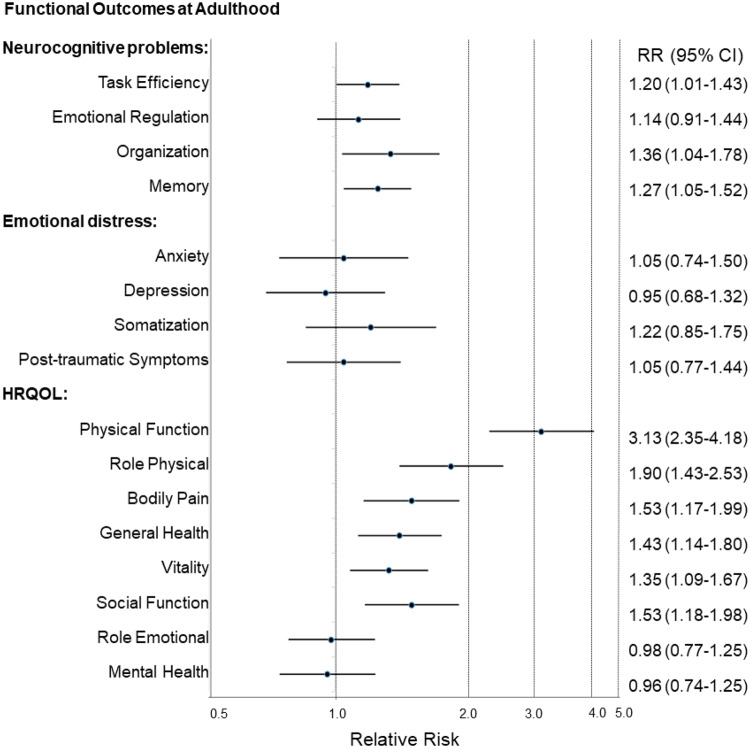
As compared with never-users, survivors who reported continued use of medications from adolescence to adulthood demonstrated higher risk of developing emotional regulation (RR = 1.68, 95% CI = 1.24 to 2.27) and organization (RR = 1.82, 95% CI = 1.28 to 2.59) problems (Table 3). Overall, survivors who used any psychoactive medication during adolescence were more likely to report neurocognitive problems, after adjusting for age, sex, race, cancer treatment exposures, and baseline cognitive problems (Figure 2). Adolescent stimulant use was associated with impairment on task efficiency (RR = 1.20, 95% CI = 1.01 to 1.43), memory (RR = 1.27, 95% CI = 1.05 to 1.52), emotional regulation (RR = 1.47, 95% CI = 1.04 to 2.08), and organization (RR = 1.80, 95% CI = 1.30 to 2.49) (Table 3).
Table 3.
Association between psychoactive medication use at adolescence and neurocognitive function at adulthood
| Use of psychoactive medications | Task efficiencya | Emotional regulationa | Organizationa | Memorya |
|---|---|---|---|---|
| (n = 2042) |
(n = 2043) |
(n = 2042) |
(n = 2045) |
|
| RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | |
| Pattern of psychoactive medicationuse from adolescence to adulthoodb | ||||
| Nonusers | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
| Persistent users | 1.51 (1.21 to 1.90)c | 1.68 (1.24 to 2.27)c | 1.82 (1.28 to 2.59)c | 1.52 (1.18 to 1.96)c |
| Former users | 1.29 (1.01 to 1.65)c | 1.12 (0.82 to 1.54) | 1.27 (0.89 to 1.83) | 1.35 (1.06 to 1.71)c |
| New-onset users | 1.69 (1.40 to 2.04)c | 1.96 (1.60 to 2.39)c | 1.63 (1.23 to 2.16)c | 1.56 (1.28 to 1.90)c |
| Any psychoactive medication use | 1.20 (1.01 to 1.43)c | 1.14 (0.91 to 1.44) | 1.36 (1.04 to 1.78)c | 1.27 (1.05 to 1.52)c |
| By categories | ||||
| Antidepressants | 0.99 (0.73 to 1.35) | 1.19 (0.82 to 1.73) | 1.16 (0.76 to 1.77) | 1.07 (0.78 to 1.46) |
| Anxiolytics, sedatives, hypnotics | 1.13 (0.61 to 2.07) | 0.75 (0.31 to 1.78) | 2.08 (0.94 to 4.57) | 1.62 (1.00 to 2.63)c |
| Anticonvulsants | 1.33 (1.07 to 1.65)c | 0.86 (0.57 to 1.31) | 1.37 (0.89 to 2.11) | 1.35 (1.04 to 1.74)c |
| Nonopioid analgesics | 1.00 (0.65 to 1.55) | 1.15 (0.73 to 1.82) | 1.22 (0.69 to 2.16) | 1.03 (0.65 to 1.63) |
| Opioids | 1.08 (0.84 to 1.40) | 1.12 (0.79 to 1.58) | 0.84 (0.47 to 1.50) | 1.05 (0.76 to 1.43) |
| Muscle relaxantsd | 2.28 (1.13 to 4.60)c | 1.22 (0.35 to 4.27) | 3.34 (1.29 to 8.64)c | 1.30 (0.48 to 3.54) |
| Neurolepticsd | 0.87 (0.42 to 1.76) | 1.32 (0.59 to 2.92) | 1.28 (0.52 to 3.13) | 1.25 (0.68 to 2.30) |
| Stimulants | 1.24 (0.95 to 1.61) | 1.47 (1.04 to 2.08)c | 1.80 (1.30 to 2.49)c | 1.19 (0.89 to 1.59) |
Neurocognitive impairment was defined as a score falling ≥90th percentile based on values obtained in the sibling cohort on the Childhood Cancer Survivor Study-Neurocognitive Questionnaire. Relative risks (RR) and 95% confidence intervals (CI) from multivariable models are adjusted for age, sex, and race; intravenous and intrathecal methotrexate doses and cranial radiation dose; attention problems and placement in special education at adolescence.
Nonusers were defined as survivors with no reported use of psychoactive medication at both adolescence and young adulthood. Persistent users were defined as survivors with reported use of psychoactive medication at both adolescence and young adulthood. Former users were defined as survivors with reported use of psychoactive medication at adolescence but did not report use at young adulthood. New-onset users were defined as survivors with no reported use of psychoactive medication at adolescence but reported use at young adulthood.
Denotes statistical significance (P < .05).
Caution needed in interpreting results for muscle relaxants and neuroleptics because of the small sample size.
Figure 2.
Association between any psychoactive medication use at adolescence and risk of functional impairment during adulthood. Each dot and whisker represents the relative risk (RR) estimate and 95% confidence interval (CI), respectively, on a logarithmic scale. Models are adjusted for age, sex, and race; intravenous and intrathecal methotrexate doses and cranial radiation dose; attention and placement in special education at adolescence. The exact relative risk (95% CI) estimates for each class of psychoactive medication use and functional outcomes (neurocognitive problems, emotional distress, and health-related quality of life) and model descriptions are presented in Table 3 and Supplementary Tables 6 and 7, respectively. HRQOL = Health-related quality of life.
Although a statistically significant association between overall psychoactive medication use at adolescence and long-term emotional distress was not identified (Figure 2), opioid use was associated with subsequent somatization symptoms (RR = 1.72, 95% CI = 1.09 to 2.73) during adulthood (Supplementary Table 6, available online).
After adjusting for age, sex, race, cancer treatment exposures, and baseline behavioral problems during adolescence, poorer health-related quality of life was identified in adult survivors who reported psychoactive medication use at adolescence (Figure 2). Survivors taking opioids were 2.5 times more likely to report reduced physical functioning (RR = 2.47, 95% CI = 1.70 to 3.59), as well as poorer vitality (RR = 1.70, 95% CI = 1.29 to 2.26) and more bodily pain (RR = 1.99, 95% CI = 1.44 to 2.74) (Supplementary Table 7, available online). Adolescent stimulant users were almost twice as likely to report impaired physical functioning (RR = 1.86, 95% CI = 1.18 to 2.96). Antidepressants were statistically significantly associated with poorer quality of life at adulthood, including physical role functioning, general health, social functioning, and emotional role functioning (Supplementary Table 7, available online).
Finally, social attainment outcomes were obtained from 585 survivors who were older than 25 years of age during follow-up (median age = 25 [25-26] years at follow-up). At follow-up, 39.3% of survivors had completed graduate education, and 67.2% were employed full-time (Supplementary Table 8, available online). After adjusting for age, sex, race, cancer treatment exposures, and placement in special education, adolescent survivors who used psychoactive medication were 20% more likely not to graduate college than those who did not use psychoactive medication (RR = 1.21, 95% CI = 1.04 to 1.41) and 60% (RR = 1.60, 95% CI = 1.23 to 2.08) more likely to not be working full-time. Those who used antidepressants, stimulants, opioids, and anxiolytics or sedatives were more likely to attain a lower education (Supplementary Table 9, available online). Those who used anticonvulsants and stimulants were twice as likely be working less than full-time during adulthood (Supplementary Table 9, available online).
Discussion
Overall, 18% of survivors reported using at least 1 psychoactive medication between 1992 and 2010, statistically significantly higher than the 6% of siblings and the 6% estimated in the general population (8). After adjusting for cancer treatment exposures and baseline cognitive problems, psychoactive medication use during adolescence was associated with poorer self-reported functional outcomes at adulthood, including neurocognitive problems and reduced health-related quality of life, but less so for emotional distress. Long-term educational and employment problems were also observed in adolescent users compared with nonusers of psychoactive medications. Our results support the urgent need to review current practices that rely on pharmacological interventions to manage distress and cancer-related symptoms in survivors.
Antidepressant use had a 3-fold increase among adolescent survivors surveyed from 1992 to 2010 (2.0% to 6.3%), whereas stimulant use had a 2-fold increase (2.7% to 6.8%). Similar patterns were detected in siblings, although the overall prevalence was half that seen in survivors during the same eras. Our findings may be reflective of the increasing trend of antidepressant and stimulant use within the general population (6,21). Of note, 10% of the survivors reported new-onset use of antidepressants when they reached young adulthood at follow-up assessment. This observation is consistent with reports of increasing antidepressant use over time within the general population in the United States and that antidepressants are one of the most commonly used prescription drugs in adults over the recent era (22).
Psychoactive medication use was concurrently associated with parent-reported pain and cognitive and behavior problems in adolescence. Most psychoactive medications investigated in this study are used primarily to treat the abovementioned problems in a clinical setting. Longitudinal follow-up results revealed that use of certain classes of psychoactive drugs, such as opioids and muscle relaxants, was associated with somatization and posttraumatic symptoms at adulthood, suggesting a potentially more eventful cancer or/and treatment experience in these survivors. Opioid and nonopioid analgesia users reported more bodily pain and poorer vitality later in life. This may reflect persistent pain and behavioral symptoms in the same group of survivors who were in need of prescription drugs during the early phase of cancer survivorship. Beyond the provision of pharmacological treatment, these at-risk survivors may benefit from additional interventions, such as social service and behavioral or cognitive rehabilitation, to address other predisposing factors.
Psychoactive medication use during adolescence was strongly associated with self-reported neurocognitive problems in multiple domains as adults, even after adjusting for neurotoxic cancer treatment variables and baseline cognitive problems. These findings are consistent with previous studies that adverse cognitive outcomes are observed in individuals treated with psychoactive agents in both cancer and noncancer populations (7,23). The association between psychoactive drugs and neurocognitive function is particularly evident for stimulants and anticonvulsants. During the neurodevelopment stage, critical processes such as synaptogenesis and axonal growth are sensitive to the effects of pharmacological interventions (24). Hence, treating children and adolescents with psychotropic agents such as serotonergic or dopaminergic drugs, opioids, benzodiazepines, and methylphenidate may have lasting influence on brain maturation. In the presence of multiple clinical and treatments factors that can lead to poorer neurocognitive outcomes in survivors of childhood cancer, it is clear from our results that the use of psychoactive medication does not normalize neurocognitive function over time.
Our previous work on adult survivors also reported association between psychoactive medication use and neurocognitive impairment (7). In combination with other reports that demonstrate psychoactive medication use may be associated with unfavorable functional outcomes (7,23), our results support the need for strategies to manage distress symptoms in survivors early, before they develop into clinical conditions that require pharmacological interventions. This is especially relevant in subgroups of adolescent survivors of leukemia, CNS tumors, or neuroblastoma and survivors treated with cranial radiation and intrathecal methotrexate, who were found to be at increased risk for adverse behavioral and social outcomes (11) .We have also previously established the impact of cancer treatment on emotional distress and neurocognitive function through the mediating effects of chronic conditions (25,26). Therefore, screening for late effects and mental health status in at‐risk subgroups may facilitate the early identification of distress symptoms and timely intervention before symptoms become chronic, functionally impairing, and requiring pharmacological interventions. The use of psychoactive medications to treat emotional distress symptoms is unfavorable in populations with multiple comorbidities, because of reasons such as medication burden, poor adherence, and risk of adverse events associated with polypharmacy (27). Within the general population, addictive properties of psychoactive drugs may lead to other psychoactive prescription drug use disorders such as substance abuse and risky health behaviors (28,29). These collective findings underscore the need for more effort in directing balanced and appropriate use of psychopharmacologic and nonpharmacological interventions to treat distress symptoms in survivors of childhood cancer.
Despite having a large, well-characterized sample with longitudinal follow-up and use of sibling controls, findings of this study should be considered in the context of several limitations. Our study relied exclusively on proxy-reported psychoactive medication use; external verification of reported medications with dispensing records was not conducted. A more elaborate and detailed description of medication utilization will be important for future studies to consider. Given the retrospective nature of the study design, temporal relationship between onset of medication use and functional outcomes cannot be established. Even though we adjusted for cancer treatment exposures and baseline cognitive or behavioral problems using multivariable models, adverse functional outcomes at adulthood may not be directly attributed to psychoactive medication use, because there may be other confounding psychological or behavioral issues that were not captured by the measures included in the study. The observed association must be interpreted with caution due to potential selection bias, because survivors who exhibited behavioral problems during adolescences and reported the use of psychoactive medications seemed less likely to provide functional performance evaluation during adulthood (Supplementary Table 1, available online). While the cancer treatment protocols represented in the CCSS cohort span from 1970 to 1999, these treatment exposures remain the backbone of contemporary therapies for childhood cancer (30,31), and hence, health outcomes of this cohort are still applicable to survivors treated in the newer era.
In conclusion, our findings examined utilization trends and identified clinical and cancer treatment predictors of psychoactive medication use among adolescent survivors of childhood cancer. This has the potential to inform screening and intervention practices that may likely benefit many childhood cancer survivors. This study also provides preliminary data to suggest an association between the use of specific psychoactive medications at adolescence and adverse functional outcomes during adulthood. Clinically, our study results underscore the need for future research on the psychopharmacologic treatment of survivors of childhood cancer and may potentially direct the development of guidelines for appropriate provision of psychoactive medications for at-risk survivors, as well as monitoring for adverse cognitive effects and functional impairments associated with their use.
Funding
This work was supported by the National Cancer Institute (CA55727, G.T. Armstrong, Principal Investigator). Support to St. Jude Children’s Research Hospital was also provided by the Cancer Center Support (CORE) grant (CA21765, C. Roberts, Principal Investigator) and the American Lebanese-Syrian Associated Charities (ALSAC).
Notes
Role of the funder: The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
Disclosures: All authors have no conflicts of interest to declare.
Role of the authors: All authors: Conception or design of the work. YTC, WL, KRK: Data collection. YTC, WL, DS, KRK: Data analysis and interpretation. All authors: Data interpretation. YTC, KRK: Drafting the article. All authors: Critical revision of the article. All authors: Final approval of the version to be published.
Data availability statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Supplementary Material
References
- 1. Armstrong GT, Chen Y, Yasui Y, et al. Reduction in late mortality among 5-year survivors of childhood cancer. N Engl J Med. 2016;374(9):833–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bhakta N, Liu Q, Ness KK, et al. The cumulative burden of surviving childhood cancer: an initial report from the St Jude lifetime cohort study (SJLIFE). Lancet. 2017;390(10112):2569–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Robison LL, Hudson MM. Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nat Rev Cancer. 2014;14(1):61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cheung YT, Brinkman TM, Mulrooney DA, et al. Impact of sleep, fatigue, and systemic inflammation on neurocognitive and behavioral outcomes in long-term survivors of childhood acute lymphoblastic leukemia. Cancer. 2017;123(17):3410–3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Krull KR, Cheung YT, Liu W, et al. Chemotherapy pharmacodynamics and neuroimaging and neurocognitive outcomes in long-term survivors of childhood acute lymphoblastic leukemia. J Clin Oncol. 2016;34(22):2644–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brinkman TM, Ullrich NJ, Zhang N, et al. Prevalence and predictors of prescription psychoactive medication use in adult survivors of childhood cancer: a report from the childhood cancer survivor study. J Cancer Surviv. 2013;7(1):104–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brinkman TM, Zhang N, Ullrich NJ, et al. Psychoactive medication use and neurocognitive function in adult survivors of childhood cancer: a report from the childhood cancer survivor study. Pediatr Blood Cancer. 2013;60(3):486–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jonas BS, Gu Q, Albertorio-Diaz JR. Psychotropic medication use among adolescents: United States, 2005-2010. NCHS Data Brief 135; 2013:1–8. [PubMed]
- 9. Gianinazzi ME, Rueegg CS, Wengenroth L, et al. Adolescent survivors of childhood cancer: are they vulnerable for psychological distress? Psychooncology. 2013;22(9):2051–2058. [DOI] [PubMed] [Google Scholar]
- 10. Krull KR, Huang S, Gurney JG, et al. Adolescent behavior and adult health status in childhood cancer survivors. J Cancer Surviv. 2010;4(3):210–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schultz KA, Ness KK, Whitton J, et al. Behavioral and social outcomes in adolescent survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2007;25(24):3649–3656. [DOI] [PubMed] [Google Scholar]
- 12. Leisenring WM, Mertens AC, Armstrong GT, et al. Pediatric cancer survivorship research: experience of the childhood cancer survivor study. J Clin Oncol. 2009;27(14):2319–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Robison LL, Armstrong GT, Boice JD, et al. The childhood cancer survivor study: a National Cancer Institute to Supported resource for outcome and intervention research. J Clin Oncol. 2009;27(14):2308–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American Society of Health-System Pharmacists. AHFS Drug Information; 2017. https://www.ahfsdruginformation.com/ahfs-pharmacologic-therapeutic-classification/. Accessed June 1, 2017.
- 15. Peterson JL, Zill N. Marital disruption, parent-child relationships, and behavior problems in children. J Marriage Fam. 1986;48(2):295–307.
- 16. Kenzik KM, Huang IC, Brinkman TM, et al. The childhood cancer survivor study-neurocognitive questionnaire (CCSS-NCQ) revised: item response analysis and concurrent validity. Neuropsychology. 2015;29(1):31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Derogatis LR. BSI 18, Brief Symptom Inventory 18: Administration, Scoring and Procedures Manual. Minneapolis, MN: NCS Pearson, Inc; 2001. [Google Scholar]
- 18. Foa EB. Posttraumatic Stress Diagnostic Scale. Minneapolis, MN: Pearson; 1995. [Google Scholar]
- 19. McHorney CA, Ware JE, Jr, Rogers W, et al. The validity and relative precision of MOS short- and long-form health status scales and Dartmouth COOP charts. Results from the medical outcomes study. Med Care. 1992;30(suppl 5):65. [DOI] [PubMed] [Google Scholar]
- 20. Brinkman TM, Zhang N, Recklitis CJ, et al. Suicide ideation and associated mortality in adult survivors of childhood cancer. Cancer. 2014;120(2):271–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Safer DJ. Recent trends in stimulant usage. J Atten Disord. 2016;20(6):471–477. [DOI] [PubMed] [Google Scholar]
- 22. U.S. Health. With special feature on racial and ethnic health disparities. 2016 ASI 4144-11; DHHS Publication No. 2016-1232, 2016; 2015.
- 23. Lakhan SE, Hagger-Johnson G. The impact of prescribed psychotropics on youth. Clin Pract Epidemiol Ment Health. 2007;3(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bottelier MA, Schouw MLJ, Klomp A, et al. The effects of psychotropic drugs on developing brain (ePOD) study: methods and design. BMC Psychiatry. 2014;14(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vuotto SC, Krull KR, Li C, et al. Impact of chronic disease on emotional distress in adult survivors of childhood cancer: a report from the childhood cancer survivor study. Cancer. 2017;123(3):521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cheung YT, Brinkman TM, Li C, et al. Chronic health conditions and neurocognitive function in aging survivors of childhood cancer: a report from the childhood cancer survivor study. J Natl Cancer Inst. 2018;110(4):411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Koopmans GT, Lamers LM. Chronic conditions, psychological distress and the use of psychoactive medications. A preliminary version of this article was presented as a paper at the 22nd European Conference on Psychosomatic Research, September 3-5, 1998; Manchester, UK; 2000. [DOI] [PubMed]
- 28. Hammond CJ, Mayes LC, Potenza MN. Neurobiology of adolescent substance use and addictive behaviors: treatment implications. Adolesc Med. 2014;25(1):15–32. [PMC free article] [PubMed] [Google Scholar]
- 29. TjäDerborn M. Psychoactive Prescription Drug Use Disorders, Misuse and Abuse. Linköping, Sweden: Department of Medical and Health Sciences, Linköping University; 2016. [Google Scholar]
- 30. Green DM, Kun LE, Matthay KK, et al. Relevance of historical therapeutic approaches to the contemporary treatment of pediatric solid tumors. Pediatr Blood Cancer. 2013;60(7):1083–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hudson MM, Neglia JP, Woods WG, et al. Lessons from the past: opportunities to improve childhood cancer survivor care through outcomes investigations of historical therapeutic approaches for pediatric hematological malignancies. Pediatr Blood Cancer. 2012;58(3):334–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.