Abstract
Background
Globally, there is a growing use of Information and Communication Technology (ICT), including mobile phones, tablets and computers, which are being integrated into people’s daily activities. An ICT-based intervention called F@ce was developed in order to provide a structure for the process in stroke rehabilitation and facilitate change by integrating a global problem-solving strategy using SMS alerts. The aim of the study was to evaluate the feasibility of i) F@ce within in-patient and primary care rehabilitation after stroke, ii) the study design and outcome measures used, and iii) the fidelity, adherence and acceptability of the intervention.
Methods
Three teams comprising occupational therapists and physiotherapists who work in neurological rehabilitation participated in a preparatory workshop on F@ce and then enrolled 10 persons with stroke to participate in the intervention. Goals were set using the Canadian Occupational Performance Measure (COPM) and the participants with stroke rated their performance and satisfaction with the activities associated with the three goals every day for 8 weeks. Data were collected at inclusion, at four and 8 weeks, using the COPM, Stroke Impact Scale, Frenchay Activities Index, Life Satisfaction Checklist, Self-Efficacy Scale, Hospital Anxiety and Depression Scale, Fatigue Severity Scale, follow-up survey, daily ratings on the web platform and logbooks.
Results
All of the participants showed increased scores in the primary outcome (COPM) and a clinically meaningful improvement of ≥2 points was found in four participants regarding performance and in six participants regarding satisfaction. Overall fidelity to the components of F@ce was good. The response rates to the F@ce web platform were 44–100% (mean 78%). All of the participants stated that F@ce had supported their rehabilitation.
Conclusion
The results indicate that the most beneficial part of F@ce was the person-centred, goal-setting process and SMS alerts. All participants were satisfied with F@ce and highlighted the benefits of receiving daily alerts about their goals. This encouraged them to be more active. The only downside mentioned was that they felt under an obligation to practice, although this was described as “a positive obligation”.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12883-020-01968-x.
Keywords: ADL, Disability, eHealth, Mobile phone, Occupational therapy, Participation in everyday life, Physiotherapy, Tablet, Tele rehabilitation, Telehealth
Background
Digitalisation in society, as well as in health care and rehabilitation, has increased rapidly in recent years [1, 2]. In line with this development, the Swedish government has created a vision of becoming a global leader in digital health solutions by 2025 [2]. Digitalisation can be a valuable tool for increased participation in society for people with disabilities as after stroke [3, 4]. There are a range of concepts and definitions that address different aspects of digitalisation in health care such as e-health, tele rehabilitation and health informatics. In this study, the term Information and Communication Technology (ICT) is used, including all technologies that are used interactively for communication and transfer of information, such as mobile phones, tablets and computers, as well as the applications and software of such devices [5].
The ability to manage activities in daily living (ADL) and participate in everyday life, including work, leisure and social activities, is often restricted after a stroke [6–9]. Thus, the everyday life after stroke has been described as chaotic and receiving rehabilitation to manage ADL is often a priority [8, 10]. The development of more user-friendly ICT solutions has created opportunities to provide ICT-supported rehabilitation services that could reduce some of the unmet needs of rehabilitation that are reported by people who have had stroke [9]. Although the evidence concerning the effectiveness of ICT is inconclusive [3, 4], a recent review has shown that interventions using ICT have beneficial effects on motor, higher cortical and mood disorders [4]. It has also been shown that ICT used as an alternative to face-to-face interventions could improve participation in daily life after stroke [3].
ICT could be utilised in rehabilitation after stroke to monitor rehabilitation progress and interact at a distance [3, 4]. The use of a mobile phone or computer has been shown to promote participation in everyday life and create a sense of security [11, 12]. Furthermore, the use of ICT-based interventions could reduce the number of home visits, thereby saving time and travel costs, particularly in rural areas [12, 13]. ICT solutions have also shown to enable person-centred care [14, 15] and facilitate communication and feedback from healthcare professionals [3, 16]. A concern among people with stroke is their potentially limited ability to manage different ICT devices. Earlier research has found that people could encounter a range of difficulties [11, 17, 18] but that people with acquired brain injury such as stroke could benefit from using ICT in their daily lives [11, 19]. Moreover, ICT could be successfully introduced and used within rehabilitation after acquired brain injury, regardless of age or previous use [20]. However, support is often needed, particularly when using a new device or when something unexpected happens [11].
A client-centred ADL intervention (CADL) was developed with the aim of enabling agency in activities and participation in everyday life among persons with stroke [21, 22]. The CADL was based on phenomenology with the lived experiences of the person as a point of departure for the intervention [23]. The client-centred approach included building a therapeutic relationship and ensuring that the person was actively involved in the goal setting and planning of the rehabilitation [24–26]. The CADL was delivered by occupational therapists and evaluated in a randomized controlled trial (RCT) [21, 22] along with qualitative studies [27–29]. The results of the RCT [21, 22] were inconclusive but the qualitative studies emphasized that sharing [28] and transparency [29] between therapists and the patients were benefits of using a client-centred approach. It was also shown that the CADL appeared to enhance the involvement of patients in goal setting and individualisation of the rehabilitation. In the present study, the CADL was further developed by following the Medical Research Council (MRC) guidelines for the development of complex interventions [30].
The results of the CADL study is a part of the evidence base in the development of the new intervention called F@ce that is presented in this study. One conclusion from the CADL evaluations was that all members of a stroke rehabilitation team should use the intervention. This is also recommended in the Swedish national guidelines for stroke care [31] and in this new intervention F@ce, the multidisciplinary teams were included. In line with the new multidisciplinary approach, the term client-centred was replaced with person-centred. The terms client-centred and person-centred are based on the same underlying theories as described by Rogers [32]. The person-centred approach views the person as having the potential to change and the therapist as being a facilitator in this process [32, 33].
The potential benefits and obstacles for using ICT within a person-centred rehabilitation intervention for people after stroke remain largely unexplored. Although healthcare professionals and persons with stroke have reported high levels of acceptance and satisfaction when using ICT interventions in stroke care, few studies have explored the outcome of such interventions [3, 34]. Thus, to meet the vision of the Swedish government [2], further research on the development and use of ICT within rehabilitation is needed.
Our assumption was that ICT could be used as a tool for reinforcing person-centred rehabilitation through increased sharing [28] and transparency [29]. According to the MRC guidelines, an important stage in the development of new interventions is conducting a feasibility study before testing on a larger scale [30]. Thus, this study had the following aim: to evaluate the feasibility of i) F@ce within in-patient and primary care rehabilitation after stroke, ii) the study design and outcome measures used, and iii) the fidelity, adherence and acceptability of the intervention.
Method
Study design
A single group design was used to evaluate the feasibility of F@ce, a person-centred, team-based intervention for rehabilitation after stroke supported by ICT. The CONSORT 2010 statement for randomised pilot and feasibility trials [35] was used as a structure (available as Supplementary Material).
Recruitment of participants with stroke
The six team members were responsible for identifying and recruiting 4–5 participants with stroke from each unit in September 2017. The inclusion criteria for the person with stroke were 1) referred to one of the participating units, 2) able to participate in an eight-week intervention, and 3) able to communicate in Swedish. Since a stroke often leads to long-term consequences on the person’s ability to return to daily life [36, 37], the team members suggested that it would be beneficial to evaluate F@ce also after the initial rehabilitation period i.e. to recruit participants regardless of time since stroke.
Professionals at the in-patient unit only recruited participants with stroke who, following discharge, could continue the F@ce intervention at one of the two participating primary care rehabilitation units. One of the participating team members notified persons with stroke who met the inclusion criteria about the study and those persons who agreed to participate signed a written consent form. The recruitment process was documented in logbooks kept by the team members and in field notes taken by the second author. In this feasibility study, a prospective sample size was not calculated.
Setting and recruitment of staff
Convenience sampling was used for recruitment of professionals to carry out F@ce. In total, six professionals, members of rehabilitation teams at one urban hospital based inpatient rehabilitation unit and two corresponding primary care rehabilitation units were recruited. Other professionals were also part of the teams, including speech and language therapists, medical social workers and dieticians. However, these professionals often worked in several different teams and were only involved as consultants when needed. The teams at the in-patient units also included physicians and nurses who were responsible for medical care.
The F@ce
The training of team members
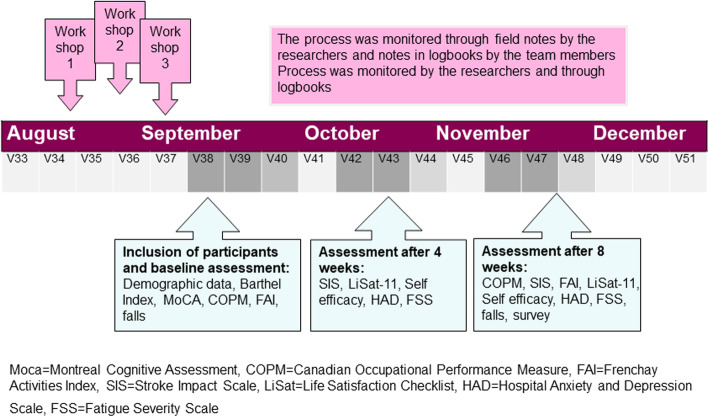
The team members participated in three workshops, 2 h each week for 3 weeks. The timeline is presented in Fig. 1. The aim of the workshops was to deepen the knowledge of person-centredness and participation and become familiar with the components of the F@ce intervention. The workshops contained short presentations on the theories, concepts and research underlying F@ce, as well as practical exercises of the goal setting and ICT used in the intervention.
Fig. 1.
Timeline of the study. The process of the study including the data collection timeline and the instrument used
The intervention
The F@ce intervention was an eight-week intervention that aimed to increase perceived participation in everyday life and self-efficacy, and to reduce the impact of stroke. The F@ce intervention was based on three basic principles from the CADL: applying a person-centred approach, enabling sharing throughout the rehabilitation process and using a transparent goal-setting process (see Supplementary material, Fig. 3).
The first basic principle of the F@ce intervention was the person-centred approach. The team members focused on building a relationship with the person with stroke [33, 38] by listening to the person’s narrative [33] and unique life experiences [23, 39] in order to understand their previous habits, roles and how they performed daily activities [25].
The second basic principle of the F@ce intervention was sharing throughout the rehabilitation process [28, 34]. For example, the team members and the person with stroke watched a video recording of an activity performed by the person with stroke in order to gain a shared overview of the person’s abilities.
The third basic principle of the F@ce intervention was using a transparent goal-setting process [38]. The Canadian Occupational Performance Measure (COPM) [40] was used to set goals and evaluate after 8 weeks when the intervention had ended. Three goals, based on activities that the person with stroke needed and/or wanted to do, as well as strategies for supporting the performance of the activities, were formulated in collaboration with the team.
ICT used within the project
Website
The research team developed a website including contact information of the participating researchers and reference literature for the intervention. The aims and content of each workshop were also available on the website to enable the team members to prepare and reflect before and after each workshop.
Stroke rehabilitation platform
A web-based platform, password protected, for the F@ce intervention was developed with three interfaces: 1) an administrator view used by the researchers in order to monitor the F@ce intervention process, 2) a team view in which team members registered the results of the COPM and monitored their patients’ daily ratings, and 3) a view for the participants with stroke in which daily alerts regarding their goals and strategies were set by the researcher and sent automatically as an SMS from a server at Stockholm University to the participant’s mobile phone or tablet each morning. This was followed by a short survey each afternoon that asked the following question: “How well did you manage to work on your goals today?” on a scale from 1 (did not perform any activity) - 5 (performed the activity very well). A low rating (1, 2) was marked in red in the system, a medium rating (3) was marked in yellow and a high rating (4, 5) was marked in green. The goals were transparent since they were visible and available to the participants on their mobile phones.
Database
An online, password protected database was developed for data collection used by the researcher. The results were then stored on a secure server.
Data collection
The timeline for the data collection is presented in Fig. 1. Demographic data on age, gender, months post stroke, employment before stroke and living situation were collected from the persons with stroke by the second author within the first week of inclusion. The persons with stroke were also asked to choose from three response categories in relation to their current use of ICT: Basic (use their mobile phones to make calls and receive text messages only), Moderate (use a smartphone and/or a tablet for searching on the internet) and Advanced (use a smartphone and/or a tablet and/or a computer for advanced activities).
Stroke severity was assessed using the Barthel Index [41]and scores were categorized as severe (< 15), moderate (15–49) or mild (50–100). Cognitive function was assessed using the Montreal Cognitive Assessment (MoCA) [42] with a score range of 0–30 and a score of < 26 indicating cognitive impairment. In total these baseline assessments took about 20–40 min to perform.
Primary outcome measures
The COPM [40] was used at inclusion and after the intervention to assess performance and satisfaction with self-care, productivity and leisure from the perspective of persons with stroke. The participants were first asked to rate their performance in the three chosen activities on a scale ranging from 1 (extremely poor/could not manage) to 10 (extremely well) and, secondly, to rate their satisfaction with their performance using the same scale. Weighted scores of performance and satisfaction of the chosen activities were summarized separately to create two total scores. A change of two points was seen as a clinically meaningful change [40].
The Stroke Impact Scale (SIS) 3.0 [43] was used at 4 weeks after inclusion to assess the perceived impact of stroke over the last 1–4 weeks. The SIS 3.0 has eight domains: strength, memory and thinking, emotions, communication, ADL/IADL, mobility, hand function, and participation. The scores range from 0 to 100 and the higher the score the less the impact of stroke. In addition, perceived recovery after stroke is rated on a visual analogue scale ranging from 0 (no recovery) to 100 (full recovery). An increased score of ≥15 points has been defined as constituting a clinically meaningful change [44].
The Frenchay Activities Index (FAI) [45] was used at inclusion and after the intervention to assess frequency of participation in everyday social and domestic activities over the last 3–6 months, thereby serving as a pre-stroke measure at inclusion for those participants with recent stroke. The scores range from 0 (inactive) to 45 (very active). For participants with recent stroke, a return to a pre-stroke score, as well as participants who had their stroke more than 6 months before inclusion, any improvement in score was considered a positive outcome.
Secondary outcome measures
The Life Satisfaction Checklist (LiSat-11) [46] was used at 4 weeks after inclusion and after the intervention to assess the participants’ satisfaction with life in general. Scores were dichotomised into not satisfied (alternatives 1 to 4) and satisfied (alternatives 5 and 6) [47].
The Self-Efficacy Scale [48] was used at 4 weeks after inclusion and after the intervention to assess the participants’ confidence in their ability to perform 18 predetermined activities. The scores range from 1 (not confident at all) to 10 (completely confident) for each activity. A score of > 5 is considered to represent confidence in the ability to perform activities in daily life [48].
The Hospital Anxiety and Depression Scale (HAD) [49] was used at 4 weeks after inclusion and after the intervention to assess anxiety and depression. The HAD has two subscales, each ranging from 0 to 21. Scores were categorised as no anxiety and depression (0–7), mild (8–10) or moderate to severe anxiety and depression (10–21) [49].
The Fatigue Severity Scale (FSS) [50] was used at 4 weeks after inclusion and after the intervention to assess fatigue. The final score is the mean of the nine items graded between 1 (strongly disagree) and 7 (strongly agree). Scores were categorized as no fatigue (1–3) or fatigue (4–7) [51].
Feasibility outcome measures
Fidelity
The team members were provided with a logbook template to take notes regarding their encounters with the participants in order to study the extent to which the delivery of the F@ce intervention followed the originally developed plan. The teams’ logbooks were collected at the end of the intervention. During the project, from the first workshop to final data collection (September 2017–February 2018), the second author was personally available to the teams each week (in conjunction with data collection) and via phone or email. Lunch meetings were also held with each of the teams around once a month in order to support the teams in their recruitment of participants and in conducting the intervention.
The second author also made field notes after each contact with the teams regarding their fidelity to F@ce.
Adherence
Data on goals and daily scorings of the participants with stroke were collected from the F@ce web platform in order to study how the participants adhered to the F@ce intervention. The maximum number of scorings for the participants was one scoring for each of the three goals a day, 7 days a week during the eight-week intervention.
Acceptability
Participants with stroke were asked to complete a survey containing open-ended questions about their experiences of F@ce concerning its potential benefits in rehabilitation and/or in everyday life, negative and positive aspects of using F@ce, as well as any technical difficulties/issues. The survey was distributed during the follow-up after the intervention.
Safety
Since falls are common after stroke the number of falls over the last 3 months were noted for each participant at inclusion and at follow-up after the intervention to monitor the safety of the intervention.
Data analysis
Descriptive statistics were used to analyse and present the results regarding recruitment, the outcome measure used, adherence, acceptability, and safety. The team members’ fidelity to the F@ce intervention was analysed by comparing the descriptions of how the teams performed the intervention, as described in the teams’ logbook notes and the second author’s field notes, with the components of F@ce.
Results
Participants and recruitment
Two and a half weeks after the final workshop, the first eligible participant with stroke was identified and the recruitment started. Figure 1 shows the timeline of the study. From September–December 2017 a total of 10 participants were included to participate in the eight-week intervention, of which four participants started at an in-patent unit. Figure 2 illustrates the flowchart of the recruitment of participants with stroke. Out of 33 assessed for eligibility 13 were included i.e. the recruitment rate was 39%. Reasons for not meeting the inclusion criteria were: did not start or continue rehabilitation in any of the participating primary care units, severe fatigue, severe aphasia, foot fracture, and depression. Three participants withdrew participation after inclusion i.e. the dropout rate was 23%.
Fig. 2.
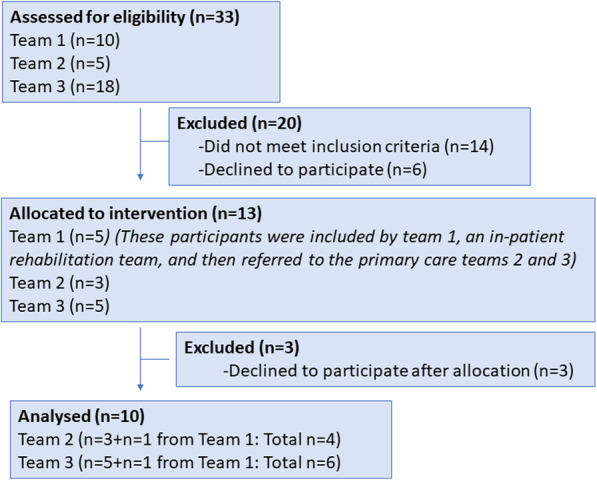
Flowchart of the recruitment of persons with stroke. The inclusion process of the participants in the study including assessed for eligibility, allocated to the intervention and the numbers of excluded
Mean age of the ten participants was 65 years (SD 12), five were men, four lived alone, and five had worked pre-stroke. All participants had suffered a mild stroke. Five had a recent stroke i.e. had suffered a stroke at ≤1 month before inclusion and five had suffered a chronic stroke i.e. from 10 to 32 months before inclusion. Three participants had a score on MoCA< 26 indicating cognitive impairment. However, one participant was unable to answer tall of the questions in MOCA due to aphasia and thus only scored 9. The participants’ current use of ICT was basic in one participant, moderate in three, and advanced in six participants.
Primary outcome measures
COPM
All of the participants showed increased scores and a clinically meaningful improvement of ≥2 points was detected in the performance of four of the participants and in the satisfaction of six of the participants. Clinically meaningful improvements were found in both participants with recent stroke as well as participants with chronic stroke. (See Table 1).
Table 1.
Participant goals and difference in the Canadian Occupational Performance Measure between inclusion and after the eight- week intervention
| Pat | Goals | Goal 2 | Goal 3 | Difference in performance (mean) | Difference in satisfaction (mean) |
|---|---|---|---|---|---|
| 1 | Sing | Play the guitar | Give a lecture | 1.7 | 6.7a |
| 2 | Write name | Brush teeth | Play the piano | 1.2 | 0.7 |
| 3 | Using the stairs | Walk outside | Water plants in garden | 0.0 | 0.7 |
| 4 | Ride the metro/ bus | Use a knife and fork | Tie shoelaces | 7.4a | 6.3a |
| 5 | Visit the gym | Ride the metro/bus | Return to work | 5.4a | 5.6a |
| 6 | Flip through pages in book | Reach for a glass | Open a mobile phone | 0.7 | 0.3 |
| 7 | Walk up and down a staircase | Visit and manage to use the toilet independently | Put on a t-shirt | 2.3a | 4.0a |
| 8 | Walk up and down a staircase | Reach for a glass | Make a sandwich | 2.6a | 2.6a |
| 9 | Walk using walking sticks | Take the initiative to carry out activities | Improve balance | 1.9 | 4.3a |
| 10 | Improve handwriting | Use knife and fork | Pick up object with foot | 0.3 | 0.3 |
aA clinically meaningful change in score of > 2
SIS 3.0
Clinically meaningful positive changes were shown in the domains of strength (n = 3), memory (n = 3), emotions (n = 3), participation (n = 3), recovery (n = 3), communication (n = 2), ADL/IADL (n = 2) and hand function (n = 1). Clinically meaningful negative changes were shown in the domains of hand function (n = 3), communication (n = 2), ADL/IADL (n = 2) and participation (n = 2) as well as mobility (n = 1), emotions (n = 1) and recovery (n = 1) (see Table 2).
Table 2.
Participant outcomes according to the Stroke Impact Scale 3.0
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Strength at 4 weeks | 100 | 56 | 81 | 63 | 100 | 38 | 13 | 38 | 75 | 63 |
| at follow up (0–100) | 100 | 100a | 94 | 69 | 100 | 56a | 38a | 31 | 88 | 50 |
| Memory at 4 weeks | 89 | 64 | 96 | 100 | 50 | 86 | 82 | 96 | 50 | 100 |
| at follow up (0–100) | 93 | 93a | 100a | 93 | 68a | 86 | 68 | 96 | 43 | 100 |
| Emotions at 4 weeks | 58 | 67 | 100 | 94 | 58 | 78 | 58 | 89 | 58 | 64 |
| at follow up (0–100) | 92a | 92a | 56 | 86 | 70 | 69 | 44 | 94 | 47 | 88a |
| Communication at 4 weeks | 93 | 64 | 100 | 100 | 79 | 93 | 89 | 57 | 71 | 100 |
| at follow up (0–100) | 93 | 93a | 100 | 100 | 61 | 93 | 64 | 86a | 68 | 100 |
| ADL/IADL at 4 weeks | 100 | 65 | 93 | 88 | 73 | 48 | 48 | 65 | 73 | 68 |
| at follow up (0–100) | 98 | 98a | 65 | 96 | 96a | 56 | 31 | 77 | 73 | 73 |
| Mobility at 4 weeks | 78 | 83 | 83 | 94 | 94 | 78 | 72 | 64 | 50 | 53 |
| at follow up (0–100) | 86 | 86 | 78 | 94 | 94 | 78 | 67 | 75 | 61 | 36 |
| Hand function at 4 weeks | 100 | 40 | 90 | 94 | 94 | 0 | 0 | 20 | 70 | 25 |
| at follow up (0–100) | 100 | 100a | 85 | 94 | 94 | 0 | 0 | 5 | 10 | 10 |
| Participation at 4 weeks | 81 | 31 | 81 | 94 | 28 | 56 | 28 | 59 | 31 | 72 |
| at follow up (0–100) | 72 | 72a | 63 | 88 | 53a | 53 | 16 | 78a | 41 | 56 |
| Recovery at 4 weeks | 78 | 30 | 70 | 70 | 50 | 60 | 35 | 40 | 75 | 60 |
| at follow up (0–100) | 70 | 45a | 95a | 75 | 80a | 60 | 30 | 50 | 50 | 50 |
ADL Activities of Daily Living, IADL Instrumental activities of Daily Living
aA clinically significant improvement of > 15 points (or reaching the maximum score of 100)
FAI
Only one of the participants with recent stroke had returned to a pre-stroke level of participation. Four of the five participants with chronic stroke had improved their scores and one participant had a lower score compared to their score at inclusion (see Table 3).
Table 3.
Participant outcomes according to FAI, HAD, LiSat-11, Self-efficacy scale, FSS
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Months post stroke | < 1 | < 1 | 1 | < 1 | < 1 | 10 | 19 | 22 | 24 | 32 |
| FAI at inclusion | 38a | 33a | 24a | 34a | 33a | 17 | 5 | 12 | 18 | 13 |
| at follow-up (0–45) | 36 | 14 | 9 | 34 | 30 | 11 | 9 | 30 | 23 | 19 |
| HAD-A at inclusion | 3 | 8 | 1 | 11 | 2 | 13 | 3 | 1 | 10 | 1 |
| at follow up (0–21) | 2 | 6 | 4 | 3 | 4 | 2 | 10 | 1 | 9 | 0 |
| HAD-D at inclusion | 1 | 5 | 1 | 1 | 8 | 11 | 2 | 0 | 11 | 1 |
| at follow-up (0–21) | 2 | 4 | 3 | 0 | 10 | 6 | 16 | 3 | 9 | 0 |
| LiSat-11 at 4 weeks | 6 | 4 | 5 | 4 | 5 | 6 | 2 | 3 | 3 | 4 |
| at follow up (1–6) | 5 | 5 | 4 | 5 | 4 | 5 | 1 | 4 | 5 | 4 |
| Self-efficacy scale at 4 weeks | 7.7 | 6.2 | 8.1 | 9.8 | 8.4 | 7.1 | 5.0 | 7.4 | 6.3 | 7.8 |
| at follow-up (1–10) | 8.8 | 7.4 | 8.0 | 9.7 | 8.9 | 6.6 | 5.3 | 8.8 | 7.7 | 7.9 |
| FSS at 4 weeks | 5.1 | 6.7 | 2.6 | 1.9 | 6.3 | 4.6 | 5.8 | 4.3 | 6.8 | 1.3 |
| at follow-up (1–7) | 4.8 | 6.4 | 1.0 | 2.9 | 5.7 | 6.0 | 7.0 | 4.0 | 6.4 | 2.2 |
FAI Frenchay Activities Index, HAD-A Hospital Anxiety and Depression Scale – subscale anxiety, HAD-D Hospital Anxiety and Depression Scale – subscale depression, LiSat-11 Life Satisfaction Checklist, FSS Fatigue severity scale
aPre-stroke measure
Secondary outcome measures
LiSat-11
Among participants with recent stroke, two reported a positive change from not satisfied to satisfied and two reported a negative change. Among participants with chronic stroke, only one reported a positive change.
Self-efficacy scale
All participants were confident in their ability to perform ADL at both 4 weeks and at follow-up (see Table 3).
HAD
Levels of depression and anxiety did not change in most patients. However, one participant with recent stroke had a positive change in score from moderate/severe anxiety to no anxiety. One participant with chronic stroke had a positive change from moderate/severe anxiety and depression to no anxiety and depression. Another participant with chronic stroke had a negative change from no anxiety and depression to mild anxiety and moderate/severe depression.
FSS
No changes regarding fatigue were shown.
Feasibility of F@ce in terms of fidelity, adherence, acceptability and potential harm
Fidelity
According to the teams’ logbooks, overall fidelity to the components of F@ce was good i.e. the components of the interventions were followed by the participating team members and persons with stroke. In accordance with the first component of F@ce, the teams initially met the participants individually (i.e. face-to-face meetings) in their rooms at the in-patient unit, or in their homes, in the case of those participants who lived at home. Two team members were usually present. Team 3, which included participants with ongoing rehabilitation, made an additional home visit at inclusion in F@ce to start the intervention and set new goals. In many cases, participants stated that initial technical issues (lack of experience, lack of internet connection, etc.) took time and energy to solve and were frustrating for the teams and the participants with stroke.
The assessments were conducted according to the F@ce intervention using the mobile phone of the person with stroke to record a video of an activity as the basis for sharing and discussing the performance together with the participant. All participants’ progress was followed up by occupational therapists using the COPM after the end of the eight-week intervention. This was described as an extra effort for the therapists. Participants with recent stroke who had been admitted to the in-patient unit found it difficult to set realistic goals once they had returned home. For these participants, the goals or strategies were adjusted. The participants’ goals are presented in Table 1.
During the eight-week intervention the participants had individual or group sessions with the team (once/twice a week). Issues with goals, strategies or scoring were followed up at these meetings by the team instead of logging into the stroke rehabilitation platform in order to monitor the participants’ scorings. The follow-up meetings were described by the team members in the logbooks using unstructured field notes.
Adherence
According to data from the stroke rehabilitation platform, the participants responded to 44–100% of the text messages they received (mean 78%). According to the follow-up survey, five participants with stroke reported no technical difficulties, two reported some technical difficulties, two reported many technical difficulties and one reported that the technic did not work at all.
According to the researcher’s (second author) field notes, the teams contacted the researcher as soon as technical issues occurred in order to discuss potential solutions. One participant was unable to use their mobile phone (or tablet) to rate the goals and was therefore asked to write their ratings on paper after a discussion with the team members. Another participant who lived in a rural area had difficulties with the internet connection and therefore wrote their ratings on paper for 79% of the time. Additionally, two participants experienced initial problems using the stroke rehabilitation platform before they learnt how to register their ratings; they would write their ratings on paper when necessary.
Acceptability
According to the follow-up survey, all of the participants (100%) stated that using F@ce had been positive and described the overall experience as interesting (n = 6), enjoyable (n = 2), supportive (n = 1) or a reminder (n = 1). All of the participants stated that F@ce had supported their rehabilitation to a very high extent (n = 5), a high extent (n = 2) or to some extent (n = 3). The participants also stated that F@ce had supported their everyday lives to a very high extent (n = 2), a high extent (n = 3), to some extent (n = 4) or not at all (n = 1).
Five participants stated that visualizing their goals, being reminded and aware of their abilities and how to perform their activities were perceived as a benefit of F@ce. Three participants stated that exercising more and “having to perform their activities was positive”. One of the participants stated that everything associated with F@ce was positive and another participant stated that it gave them an opportunity to utilise once memory function and the mobile phone.
Regarding the negative aspects of F@ce, two of the participants wished that they had been able to adjust their goals along the way. One participant referred to the technical issues and three participants stated that being “forced” to do the activities and feeling guilty when they did not do the activities were negative aspects. Some of participants would recommend F@ce to someone else without a doubt (n = 5) or would be likely (n = 5) to recommend F@ce to someone else.
Among the participants with recent stroke, one reported falling once before leaving the hospital and another reported falling four times after returning home. Among the participants with chronic stroke, one reported falling two times before the intervention and three times during the intervention. Another participant with chronic stroke reported falling two times before the intervention and not falling at all during the intervention. No additional harm or safety issues were reported in the logbooks or researcher’s field notes.
Discussion
The results of this feasibility study indicate that the F@ce intervention was feasible to use within both in-patient and primary care rehabilitation after stroke. The outcome measures that were used were feasible and took approximately 20–40 min to complete. Even though this study was not designed to evaluate the effects of F@ce as such, clinically significant improvements in the COPM and in the SIS were found in several of the participants after only 4 weeks, which is seen as promising. Overall, the participating teams and the persons with stroke were satisfied with F@ce, and adherence and acceptability were high. The fidelity of the teams to the intervention requires some improvement, for example, more time for workshop planning and preparation and better procedures for team members for following-up the intervention.
One of the main findings was that persons with stroke appreciated and adhered to F@ce to a large extent. In particular, they stated that the goals they had formulated, and the daily alerts were beneficial for their recovery. The results of the COPM also showed that the participants’ perceived performance and satisfaction increased. Thus, also using the COPM [40] as part of the intervention appeared to be appropriate and the therapists reported no difficulties in using the measurement. A culturally and contextually adapted version of the F@ce intervention showed that the COPM was usable and also showed significant results when evaluated in Uganda [12]. In this study, the COPM was performed by occupational therapists only, possibly because they were more used to using the instrument and because it was originally developed by occupational therapists. Other team members may therefore not have been accustomed to using the instrument. However, the COPM has previously been used in a team-based intervention and has been shown to improve person-centredness and participation in goal setting [52]. It could be that more introduction and guidance is required in using the instrument since it is considered appropriate for physiotherapists to use the instrument [53]. Following a stroke and/or other brain injuries, self-awareness could be an issue that could make goal setting difficult [47]. Nevertheless, it has been shown that the COPM can be used to set goals despite self-awareness issues, although support from significant others or a therapist may be necessary [54].
The F@ce intervention was developed to meet the current and future needs for rehabilitation of stroke patients. In this century, progress in the field of medical research has been greater than ever before in areas such as the development of new treatments and providing high-quality care [55]. Swedish health care is ranked amongst the best in the world in treating cancer, acute illness and vaccinations [51]. However, when it comes to caring for people with long-term illnesses, safe patient care and patient satisfaction, Swedish health care is ranked amongst the bottom third of over 40 countries worldwide [51]. The Swedish Health and Social Care Inspectorate (IVO) has reported that there are flaws in person-centredness and in the coordination of care in the Swedish healthcare system [56]. The IVO further reports the need to develop digital tools that are simple and usable for communication and follow-up, as well as to create a model for inter-professional teamwork [56]. The development of F@ce has taken such needs into account when creating a model for team-based rehabilitation with the support of ICT in order to enhance communication and enable follow-up from a distance. According to the teams’ logbooks, the overall fidelity to the components of F@ce was good. Also, even though the team had no daily contact and communication with the participants, they appeared to be motivated by the daily alerts and the rating system. Some of the participants reported that the goals needed to be adjusted more frequently, which could indicate that the professionals required further support in how to monitor and follow up the patients’ ratings.
Nevertheless, the results indicate that the most beneficial aspects of F@ce was the person-centred goal-setting process and SMS alerts. The findings show that the participants set their goals based on activities they needed and/or wanted to perform in their everyday lives. This is in line with the results of the F@ce study in Uganda in which all participants were positive about the reminder system and felt that it helped them regain their abilities and that the follow-up system was beneficial to their rehabilitation process [16]. According to the national stroke guidelines [31] and rehabilitation research, setting goals is an essential part of the rehabilitation process [57, 58]. This study shows that team members are important for providing support and guiding goal setting, but also for adjusting the goals during the rehabilitation process.
The time spent on preparing and training the teams to use F@ce was restricted to a two-hour workshop once a week for 3 weeks. This was less time for preparation compared to other studies performed within the research group. For example, in the CADL study, there were five full days of preparation over one month [28] and in Uganda the participating therapists took part in a series of workshops over eight half days [12]. In the evaluations of the implementation of the CADL intervention, the collaborative relationship between the occupational therapists and the researchers was described as a relationship that enabled the fusion of scientific knowledge and practice [27]. Thus, it is important for researchers to spend sufficient time building a relationship with professionals and sharing knowledge and experiences using a “healthcare professional-centredness” perspective. Successful implementation could also depend on personal factors such as if the professionals are motivated and have sufficient knowledge of the underlying theory and the implementation process [59]. Organisational factors such as having the necessary resources and support from management, as was the case in this study, have also been shown to be important [59]. Thus, flexible and supportive collaboration throughout the implementation process is important, especially when something unexpected occurs. During the implementation of F@ce, the second researcher was present at the units each week while collecting data and was therefore able to maintain a relationship with the teams and provide support. However, in future testing and implementation of F@ce, it would be beneficial to prolong the workshops and have an even closer collaboration between professionals and researchers in order for the teams to have time to implement new knowledge in relation to the intervention.
The stroke rehabilitation web platform was developed to provide an opportunity for the teams to collaborate with the participants by sharing their daily ratings, enabling follow-up when necessary. However, this platform was not used to the extent expected. Team members stated that they lacked the time or that using the web platform had not yet been incorporated into their routines. Instead, they usually followed-up the participants’ ratings and progress in face-to-face meetings. Some of the participants with stroke stated that they wanted better follow-ups and adjustments of their goals. These results are in line with previous research that highlights the challenges of working with person-centredness within a team, including communication and collaboration with a person and within a team as key elements of goal setting and rehabilitation planning [60].
A limitation of this study could be that several outcome measures, for example, SIS, were used at four and 8 weeks after inclusion, i.e. only 4 weeks apart. It would have been preferable if the inclusion and follow-up could have been further apart to identify plausible changes over time. Furthermore, the F@ce intervention needs to be evaluated through qualitative interviews with users, team members, patients, and their significant others in order to evaluate their experience of participating in the intervention. Health economic evaluations should be performed to analyse the cost of the intervention in terms of purchasing hardware and software. Another limitation is the lack of control group and the small sample size. A larger sample size could have provided greater precision of scores for the outcome measures. However there is no definitive sample size recommended for feasibility studies, rather a range from 10 to 50 participants or more [61]. A large scale RCT study needs to be performed to evaluate the effects of F@ce. The recruitment rate of 39% indicates a need for clearer inclusion criteria and a close communication with the recruiting team member during the recruitment process. However, only three participants dropped out after inclusion which is a promising result of the intervention. It should be noted that all participants had had a mild stroke. Nevertheless, a strength of the study is that F@ce was evaluated and found to be beneficial for participants with a recent stroke as well as for participants who had a stroke several years ago.
Lastly, in this study, ICT was used as a tool throughout the rehabilitation process to enable sharing and transparency between the rehabilitation team and participants with stroke by providing them with alerts and feedback. This is in line with the results of a recent scoping review which shows that ICT can be used as an alternative to face-to-face interventions in order to improve participation in daily life after stroke [3]. Nevertheless, it is important to consider which individuals to target so that nobody is excluded from rehabilitation when using ICT. For example, older people might prefer face-to-face interactions or phone calls in their contact with healthcare professionals and for such people non-digital alternatives must be available [62]. Thus, it is important to provide support for people who are inexperienced in the use of ICT or who have cognitive or physical impairments that might hinder their use of ICT. However, since the use of ICT support in health care and rehabilitation will probably be necessary in the future, the development and evaluation of the F@ce intervention have contributed to such a digital development process.
Conclusion
The F@ce interventions appeared to remind and inspire the participants to perform activities and to improve their participation in daily activities after stroke. However, the teams must identify routines for follow-up in order to ensure that they provide appropriate support.
Using the COPM seems to be suitable for evaluation of this type of interventions. Additionally, the results of this study found that several of the patients improved their self-perceived performance and satisfaction of the activities according to the COPM over 8 weeks.
Supplementary information
Additional file 1 Supplementary Figure 3. Components of the F@ce intervention modelling from CADL,1,2,3. Two general strategies are combined and should be used by the teams (i.e. during the entire intervention process) in order to enable change: 1) using the client’s lived experience as a point of departure and 2) enabling significant experience to be gained from performing valued daily activities.
Acknowledgements
The authors would like to thank the rehabilitation teams, persons with stroke and their significant others for participating in the study.
Abbreviations
- ADL
Activities in Daily Living
- CADL
Client-centred ADL intervention
- COPM
Occupational Performance Measure
- ICT
Information and Communication Technology
- FAI
Frenchay Activities Index
- FSS
Fatigue Severity Scale
- HAD
Hospital Anxiety and Depression Scale
- LiSat-11
Life Satisfaction Checklist
- MoCA
Montreal Cognitive Assessment
- MRC
Medical Research Council
- RCT
Randomized Controlled Trial
- SIS
Stroke Impact Scale
Authors’ contributions
SG, KT, and CY were responsible for the study design. MG was responsible for data collection and together with SG and CY performed the analyses and interpreted the results. MA contributed with medical competence. UF was responsible for the ICT development together with SG, MG and CY. All authors contributed to, read, and approved the final manuscript.
Funding
Financial support was provided by the Doctoral School in Health Care Sciences (FiV) at Karolinska Institutet, the Swedish Research Council (VR), the Swedish Research Council for Health, Working Life and Welfare (FORTE) and the Swedish Stroke Association. The funders had no role in study design, data collection and analysis, or in preparation of the manuscript. Open Access funding provided by Karolinska Institute.
Availability of data and materials
The datasets supporting the conclusions of this article are available at the Division of Occupational therapy, Department of Neurobiology Care Sciences and Society, Karolinska Institutet, Stockholm, Sweden. E-mail: susanne.guidetti@ki.se.
Ethics approval and consent to participate
Ethical approval was obtained from the Regional Ethics Committee in Stockholm (2017/1414–32). Before data collection in the present study, the participants were given both oral and written information regarding the aim of the study, purpose of the interview, research methods as well as methods for ensuring confidentiality, and informed written consent was obtained from all participants.
Consent for publication
Written consent for publication was obtained from all participants.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Susanne Guidetti, Email: susanne.guidetti@ki.se.
Martha Gustavsson, Email: martha.gustavsson@ki.se.
Kerstin Tham, Email: kerstin.tham@mau.se.
Magnus Andersson, Email: magnus.la.andersson@karolinska.se.
Uno Fors, Email: uno@dsv.su.se.
Charlotte Ytterberg, Email: charlotte.ytterberg@ki.se.
References
- 1.World Health Organisation . Global diffusion of eHealth: making universal health coverage achievable. Report of the third global survey on eHealth. Geneva: World Health Organization; 2016. [Google Scholar]
- 2.Swedish gouvernment and Swedish Assosiation of Local Authorities and Regions . Vision for e-health 2025. Stockholm: Swedish gouvernment and Swedish Assosiation of Local Authorities and Regions; 2017. [Google Scholar]
- 3.Zonneveld M, Patomella AH, Asaba E, Guidetti S. The use of information and communication technology in healthcare to improve participation in everyday life: a scoping review. Disabil Rehabil. 2019:1–8. [DOI] [PubMed]
- 4.Sarfo FS, Ulasavets U, Opare-Sem OK, Ovbiagele B. Tele-rehabilitation after stroke: an updated systematic review of the literature. J Stroke Cerebrovasc Dis. 2018;27:2306–2318. doi: 10.1016/j.jstrokecerebrovasdis.2018.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chandler D, Munday R. A dictionary of media and communication. 1. Oxford: Oxford University Press; 2011. [Google Scholar]
- 6.Ekstam L, Uppgard B, von Koch L, Tham K. Functioning in everyday life after stroke: a longitudinal study of elderly people receiving rehabilitation at home. Scand J Caring Sci. 2007;21:434–446. doi: 10.1111/j.1471-6712.2006.00488.x. [DOI] [PubMed] [Google Scholar]
- 7.Fallahpour M, Tham K, Joghataei MT, Jonsson H. Perceived participation and autonomy: aspects of functioning and contextual factors predicting participation after stroke. J Rehabil Med. 2011;43:388–397. doi: 10.2340/16501977-0789. [DOI] [PubMed] [Google Scholar]
- 8.Fallahpour M, Jonsson H, Joghataei MT, Nasrabadi AN, Tham K. “I am not living my life”: lived experience of participation in everyday occupations after stroke in Tehran. J Rehabil Med. 2013;45:528–534. doi: 10.2340/16501977-1143. [DOI] [PubMed] [Google Scholar]
- 9.Kamwesiga JT, von Kock LK, Eriksson GM, Guidetti SGE. The impact of stroke on people living in Central Uganda: a descriptive study. Afr J Disabil. 2018;7:438. doi: 10.4102/ajod.v7i0.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guidetti S, Asaba E, Tham K. The lived experience of recapturing self-care. Am J Occup Ther. 2007;61:303–310. doi: 10.5014/ajot.61.3.303. [DOI] [PubMed] [Google Scholar]
- 11.Gustavsson M, Ytterberg C, Nabsen Marwaa M, Tham K, Guidetti S. Experiences of using information and communication technology within the first year after stroke - a grounded theory study. Disabil Rehabil. 2018;40:561–568. doi: 10.1080/09638288.2016.1264012. [DOI] [PubMed] [Google Scholar]
- 12.Kamwesiga JT, Eriksson GM, Tham K, et al. A feasibility study of a mobile phone supported family-centred ADL intervention, F@ce, after stroke in Uganda. Glob Health. 2018;14:82. doi: 10.1186/s12992-018-0400-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krpic A, Savanovic A, Cikajlo I. Telerehabilitation: remote multimedia-supported assistance and mobile monitoring of balance training outcomes can facilitate the clinical staff's effort. Int J Rehabil Res. 2013;36:162–171. doi: 10.1097/MRR.0b013e32835dd63b. [DOI] [PubMed] [Google Scholar]
- 14.Wildevuur SE, Simonse LW. Information and communication technology-enabled person-centered care for the “big five” chronic conditions: scoping review. J Med Internet Res. 2015;17:e77. doi: 10.2196/jmir.3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernocchi P, Vanoglio F, Baratti D, et al. Home-based telesurveillance and rehabilitation after stroke: a real-life study. Top Stroke Rehabil. 2016;23:106–115. doi: 10.1080/10749357.2015.1120453. [DOI] [PubMed] [Google Scholar]
- 16.Fors U, Kamwesiga JT, Eriksson GM, von Koch L, Guidetti S. User evaluation of a novel SMS-based reminder system for supporting post-stroke rehabilitation. BMC Med Inf Decis Making. 2019;19:122. doi: 10.1186/s12911-019-0847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gustavsson M, Ytterberg C, Guidetti S. Exploring future possibilities of using information and communication technology in multidisciplinary rehabilitation after stroke – a grounded theory study. Scand J Occup Ther. 2020;27(3):223–30. doi: 10.1080/11038128.2019.1666918. [DOI] [PubMed] [Google Scholar]
- 18.Engström A-LL, Lexell J, Lund ML. Difficulties in using everyday technology after acquired brain injury: a qualitative analysis. Scand J Occup Ther. 2010;17:233–243. doi: 10.3109/11038120903191806. [DOI] [PubMed] [Google Scholar]
- 19.Larsson Lund M, Lvgren-Engstrm A-L, Lexell J. Using everyday technology to compensate for difficulties in task performance in daily life: experiences in persons with acquired brain injury and their significant others. Disabil Rehabil: Assist Technol. 2011;6:402–411. doi: 10.3109/17483107.2011.574309. [DOI] [PubMed] [Google Scholar]
- 20.White J, Janssen H, Jordan L, Pollack M. Tablet technology during stroke recovery: a survivor's perspective. Disabil Rehabil. 2015;37:1186–1192. doi: 10.3109/09638288.2014.958620. [DOI] [PubMed] [Google Scholar]
- 21.Bertilsson A-S, Ranner M, Von Koch L, et al. A client-centred ADL intervention: three-month follow-up of a randomized controlled trial. Scand J Occup Ther. 2014;21:377–391. doi: 10.3109/11038128.2014.880126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guidetti S, Ranner M, Tham K, Andersson M, Ytterberg C, Von Koch L. A client-centred activities of daily living intervention for persons with stroke: one-year follow-up of a randomized controlled trial. J Rehabil Med. 2015;47:605. doi: 10.2340/16501977-1981. [DOI] [PubMed] [Google Scholar]
- 23.Merleau-Ponty M. Phenomenology of perception. London: Routledge; 1989. [Google Scholar]
- 24.Townsend EA, Polatajko HJ. Enabling occupation II : advancing an occupational therapy vision for health, well-being & justice through occupation. Ottawa: CAOT Publications ACE; 2007. [Google Scholar]
- 25.Taylor RR. Kielhofner's model of human occupation: theory and application. Philadelphia: Wolters Kluwer; 2017. [Google Scholar]
- 26.Law M, Baptiste S, Mills J. Client-centred practice: what does it mean and does it make a difference? Can J Occup Ther. 1995;62:250–257. doi: 10.1177/000841749506200504. [DOI] [PubMed] [Google Scholar]
- 27.Eriksson C, Eriksson G, Johansson U, Guidetti S. Occupational therapists’ perceptions of implementing a client-centered intervention in close collaboration with researchers: A mixed methods study. Scand J Occup Ther. 2020;27(2):142–53. doi: 10.1080/11038128.2019.1573917. [DOI] [PubMed] [Google Scholar]
- 28.Ranner M, Guidetti S, Von Koch L, Tham K. Experiences of participating in a client-centred ADL intervention after stroke. Disabil Rehabil. 2019;41(25):3025–33. doi: 10.1080/09638288.2018.1483434. [DOI] [PubMed] [Google Scholar]
- 29.Ranner M, Von Koch L, Guidetti S, Tham K. Client-centred ADL intervention after stroke: occupational therapists’ experiences. Scand J Occup Ther. 2016;23:81–90. doi: 10.3109/11038128.2015.1115549. [DOI] [PubMed] [Google Scholar]
- 30.Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. Int J Nurs Stud. 2013;50:587. doi: 10.1016/j.ijnurstu.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 31.National Board of Health and Wellfare . Swedish national guidelines for stroke care 2017. Stockholm: National Board of helath and wellfare; 2017. [Google Scholar]
- 32.Rogers CR. Client Centred therapy (new Ed) [Elektronisk resurs] New York: Constable & Robinson; 2012. [Google Scholar]
- 33.Ekman I, Swedberg K, Taft C, et al. Person- centered care — ready for prime time. Eur J Cardiovasc Nurs. 2011;10:248–251. doi: 10.1016/j.ejcnurse.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 34.Dumitrascu OM, Demaerschalk BM. Telestroke. Curr Cardiol Rep. 2017;19:85. doi: 10.1007/s11886-017-0895-1. [DOI] [PubMed] [Google Scholar]
- 35.Eldridge SM, Chan CL, Campbell MJ, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. Pilot Feasibility Stud. 2016;2:64. doi: 10.1186/s40814-016-0105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hartman-Maeir A, Soroker N, Ring H, Avni N, Katz N. Activities, participation and satisfaction one-year post stroke. Disabil Rehabil. 2007;29:559–566. doi: 10.1080/09638280600924996. [DOI] [PubMed] [Google Scholar]
- 37.National Board of Health and Welfare. Life situation two years after stroke – a follow up of stroke victims and their relatives. National Board of Health and Welfare; 2004. Published www.socialstyrelsen.se.
- 38.Guidetti S, Tham K. Therapeutic strategies used by occupational therapists in self-care training: a qualitative study. Occup Ther Int. 2002;9:257–276. doi: 10.1002/oti.168. [DOI] [PubMed] [Google Scholar]
- 39.Guidetti S, Asaba E, Tham K. Meaning of context in recapturing self-care after stroke or spinal cord injury.(report) Am J Occup Ther. 2009;63:323. doi: 10.5014/ajot.63.3.323. [DOI] [PubMed] [Google Scholar]
- 40.Law M, Baptiste S, Carswell A, McColl MA, Polatajko H, Pollock N. Canadian occupational performance measure. 5. Ottawa: CAOT Publications ACE; 2014. [DOI] [PubMed] [Google Scholar]
- 41.Mahoney FI, Barthel DW. Functional evaluation: the Barthel index. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 42.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 43.Duncan PW, Wallace D, Lai SM, Johnson D, Embretson S, Laster LJ. The stroke impact scale version 2.0. Evaluation of reliability, validity, and sensitivity to change. Stroke. 1999;30:2131–2140. doi: 10.1161/01.STR.30.10.2131. [DOI] [PubMed] [Google Scholar]
- 44.Duncan PW, Bode RK, Min Lai S, Perera S. Rasch analysis of a new stroke- specific outcome scale: the stroke impact scale. Arch Phys Med Rehabil. 2003;84:950–963. doi: 10.1016/S0003-9993(03)00035-2. [DOI] [PubMed] [Google Scholar]
- 45.Schuling J, de Haan R, Limburg M, Groenier KH. The Frenchay activities index. Assessment of functional status in stroke patients. Stroke. 1993;24:1173. doi: 10.1161/01.STR.24.8.1173. [DOI] [PubMed] [Google Scholar]
- 46.Melin R, Fugl-Meyer KS, Fugl-Meyer AR. Life satisfaction in 18- to 64-year-old swedes: in relation to education, employment situation, health and physical activity. J Rehabil Med. 2003;35:84–90. doi: 10.1080/16501970306119. [DOI] [PubMed] [Google Scholar]
- 47.Toglia J, Kirk U. Understanding awareness deficits following brain injury. NeuroRehabilitation. 2000;15:57–70. doi: 10.3233/NRE-2000-15104. [DOI] [PubMed] [Google Scholar]
- 48.Bandura A. Guide for constructing self-efficacy scales. In: Pajares F, Urdan T, editors. Self-efficacy beliefs of adolescents, vol. 5. Greenwich: Information Age Publishing; 2006. p. 307–37.
- 49.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 50.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46:1121–1123. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 51.Organisation for economic co-operation and development . Statistics from OECD countries. 2015. [Google Scholar]
- 52.Wressle E, Lindstrand J, Neher M, Marcusson J, Henriksson C. The Canadian occupational performance measure as an outcome measure and team tool in a day treatment programme. Disabil Rehabil. 2003;25:497–506. doi: 10.1080/0963828031000090560. [DOI] [PubMed] [Google Scholar]
- 53.Yang SY, Lin CY, Lee YC, Chang JH. The Canadian occupational performance measure for patients with stroke: a systematic review. J Phys Ther Sci. 2017;29:548–555. doi: 10.1589/jpts.29.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Doig E, Kuipers P, Prescott S, Cornwell P, Fleming J. Development of self-awareness after severe traumatic brain injury through participation in occupation-based rehabilitation: mixed-methods analysis of a case series. Am J Occupat Ther. 2014;68:578–588. doi: 10.5014/ajot.2014.010785. [DOI] [PubMed] [Google Scholar]
- 55.World Health Organisation. European 2018 health report, more than numbers -evidence for all. 2018. http://www.euro.who.int/en/publications/abstracts/european-health-report-2018.-more-than-numbers-evidence-for-all-20182018.
- 56.Health and Social Care Inspectorate (IVO). What has IVO seen 2018? (translated title). 2018. https://www.ivo.se/globalassets/dokument/publicerat/rapporter/rapporter-2019/vad-har-ivo-sett-2018-digital.pdf2018.
- 57.Doig E, Fleming J, Kuipers P. Achieving optimal functional outcomes in community-based rehabilitation following acquired brain injury: a qualitative investigation of therapists’ perspectives. Br J Occup Ther. 2008;71:360–370. doi: 10.1177/030802260807100902. [DOI] [Google Scholar]
- 58.Wressle E, Eeg-Olofsson AM, Marcusson J, Henriksson C. Improved client participation in the rehabilitation process using a client-centred goal formulation structure. J Rehabil Med. 2002;34:5–11. doi: 10.1080/165019702317242640. [DOI] [PubMed] [Google Scholar]
- 59.Fixsen DL. Implementation research [Elektronisk resurs] : a synthesis of the literature. National Implementation Research Network: Tampa; 2005. [Google Scholar]
- 60.Scobbie L, Dixon D, Wyke S. Goal setting and action planning in the rehabilitation setting: development of a theoretically informed practice framework. Clin Rehabil. 2011;25:468–482. doi: 10.1177/0269215510389198. [DOI] [PubMed] [Google Scholar]
- 61.Hertzog MA. Considerations in determining sample size for pilot studies. Res Nurs Health. 2008;31:180–191. doi: 10.1002/nur.20247. [DOI] [PubMed] [Google Scholar]
- 62.Jakobsson E, Nygard L, Kottorp A, Malinowsky C. Experiences from using eHealth in contact with health care among older adults with cognitive impairment. Scand J Caring Sci. 2019;33:380–9. doi: 10.1111/scs.12634. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1 Supplementary Figure 3. Components of the F@ce intervention modelling from CADL,1,2,3. Two general strategies are combined and should be used by the teams (i.e. during the entire intervention process) in order to enable change: 1) using the client’s lived experience as a point of departure and 2) enabling significant experience to be gained from performing valued daily activities.
Data Availability Statement
The datasets supporting the conclusions of this article are available at the Division of Occupational therapy, Department of Neurobiology Care Sciences and Society, Karolinska Institutet, Stockholm, Sweden. E-mail: susanne.guidetti@ki.se.