Abstract
Figulus binodulus Waterhouse is a small stag beetle distributed in East Asia. We determined the first mitochondrial genome of F. binodulus of which is 16,261-bp long including 13 protein-coding genes, two ribosomal RNA genes, 22 transfer RNAs, and a single large noncoding region of 1,717 bp. Gene order of F. binodulus is identical to the ancestral insect mitochondrial gene order as in most other stag beetle species. All of 22 tRNAs could be shaped into typical clover-leaf structure except trnSer1. Comparative analyses of 21 Lucanidae mitochondrial genomes was conducted in aspect of their length and AT-GC ratio. Nucleotide diversities analyses provide that cox1 and cox2 in Lucanidae are less diverse than those of Scarabaeoidea. Fifty simple sequence repeats (SSRs) were identified on F. binodulus mitochondrial genome. Comparative analysis of SSRs among five mitochondrial genomes displayed similar trend along with SSR types. Figulus binodulus was sister to all other available family Lucanidae species in the phylogenetic tree.
Keywords: Figulus binodulus, mitochondrial genome, Lucaninae, nucleotide diversity, simple sequence repeat
Figulus binodulus Waterhouse is a small stag beetle species of the tribe Figulini found in east Asian countries, such as Korea, Japan, China, Taiwan, and Vietnam (Hangay and De Keyzer 2017). They have a rather unusual life cycle for a stag beetle: While most stag beetles are herbivorous throughout their life time, the adult F. binodulus turn carnivorous, preying on beetle larvae or other insects living in the decaying wood (Mori and chiba 2009). Moreover, F. binodulus is also unique in that they live in small groups, where the adults pulverize the wood for the young ones, making it easier for the larvae to consume wood (Mori and Chiba 2009). As a result of this subsocial life style, they rarely leave the dead wood, with the only exceptions seen in the breeding seasons (Mori and Chiba 2009).
One of the critical issues in scientific researches is disproportionate research efforts. Only some groups of insects, such as Papilionidae, Lucanidae, and Cicindelinae, are well researched in that aspect (Stork 2018). Even in such well-examined taxa, the uneven study interest continues. For instance, while there are 20 complete mitochondrial genomes of Lucanidae available in the NCBI (As of February 2020), 15 of them originate from subfamily Lucaninae covering only three tribes, Dorcini, Lucanini, and Aegini (Table 2).
Table 2.
List of 21 available Lucanidae mitochondrial genomes including Figulus binodulus used in this study
| Sub-family | Species name | NCBI accession | Total length (bp) | Number of PGCs | Number of tRNAs | Number of rRNAs | GC ratio (%) | AT ratio (%) | GC Skew | AT Skew | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lucaninae | Figulus binodulus | MN180051 | 16,261 | 13 | 22 | 2 | 30.71 | 69.29 | −0.3206 | 0.1125 | This study |
| Dorcus hansi | NC_043928 | 18,130 | 13 | 22 | 2 | 29.62 | 70.38 | −0.3151 | 0.0552 | Unpublished | |
| Dorcus hopei | MF612067 | 16,026 | 13 | 22 | 2 | 31.95 | 68.05 | −0.3043 | 0.0915 | Chen et al., 2018b | |
| Dorcus seguyi | NC_038212 | 17,953 | 13 | 22 | 2 | 28.78 | 71.22 | −0.3066 | 0.0612 | Chen et al., 2018b | |
| Dorcus seguyi | MF612069 | 18,503 | 13 | 22 | 2 | 28.76 | 71.24 | −0.3031 | 0.0607 | Chen et al., 2018b | |
| Dorcus parallelipipedus | KT876887 | 17,561 | 13 | 22 | 2 | 30.88 | 69.12 | −0.2763 | 0.0662 | Linard et al., 2016 | |
| Lucanus cervus | MH595464 | 22,714 | 13 | 22 | 2 | 30.10 | 69.90 | −0.2536 | 0.0802 | Chen et al., 2019 | |
| Lucanus mazama | NC_013578 | 15,258 | 13 | 22 | 2 | 32.87 | 67.13 | −0.2723 | 0.0744 | Sheffield et al., 2009 | |
| Lucanus sp. | KT876903 | 20,631 | 13 | 22 | 2 | 30.16 | 69.84 | −0.2663 | 0.0683 | Linard et al., 2016 | |
| Neolucanus maximus | NC_039652 | 16,601 | 13 | 22 | 2 | 32.09 | 67.91 | −0.3321 | 0.1494 | Linard et al., 2016 | |
| Odontolabis cuvera fallaciosa | MF908524 | 19,614 | 13 | 22 | 2 | 29.50 | 70.50 | −0.2572 | 0.1335 | Wang et al., 2018 | |
| Prosopocoilus astacoides blanchardi | KF364622 | 21,628 | 13 | 22 | 2 | 32.99 | 67.01 | −0.2655 | 0.1069 | Kim et al., 2015 | |
| Prosopocoilus confucius | NC_036038 | 16,951 | 13 | 22 | 2 | 32.15 | 67.85 | −0.3616 | 0.0767 | Lin et al., 2017 | |
| Prosopocoilus gracilis | NC_027580 | 16,736 | 13 | 22 | 2 | 33.91 | 66.09 | −0.3318 | 0.1062 | Wu et al., 2016 | |
| Rhaetus westwoodii | MG159815 | 18,131 | 13 | 22 | 2 | 32.50 | 67.50 | −0.3222 | 0.0755 | Jing et al., 2018 | |
| Serrognathus platymelus | NC_044096 | 16,790 | 13 | 22 | 2 | 31.23 | 68.77 | −0.2837 | 0.0785 | Unpublished | |
| Syndesinae | Ceruchus minor | NC_042613 | 18,601 | 13 | 23 | 2 | 31.61 | 68.39 | −0.2799 | 0.1292 | Unpublished |
| Sinodendron rugosum | NC_042614 | 18,126 | 13 | 22 | 2 | 26.97 | 73.03 | −0.2979 | 0.0638 | Unpublished | |
| Sinodendron yunnanense | NC_036157 | 16,921 | 13 | 22 | 2 | 24.94 | 75.06 | −0.2706 | 0.0347 | Lin et al., 2017 | |
| Aesalinae | Aesalus sp. | MH120282 | 17,743 | 13 | 22 | 2 | 26.48 | 73.52 | −0.2129 | 0.0672 | Unpublished |
| Himaloaesalus gaoligongshanus | NC_042922 | 17,013 | 13 | 22 | 2 | 25.61 | 74.39 | −0.2564 | 0.1078 | Unpublished |
To extend our understanding of family Lucanidae especially in the aspect of mitochondrial genomes, we completed the first complete mitochondrial genome of F. binodulus in the tribe Figulini belongs to family Lucanidae. We compared the sequence with all 20 available mitochondrial genomes of Lucanidae in various aspects, including transfer RNA structure, mitochondrial genome configuration, nucleotide diversity throughout complete mitochondrial genomes, simple sequence repeats (SSRs), and phylogenetic analyses. Comparative data generated in this study would be useful for further understanding of phylogenetic relationship (Cameron et al. 2009, Li et al. 2019, Liu et al. 2018), for developing molecular markers to distinguish species or even populations within species based on nucleotide diversity and SSRs (Simon et al. 1994, Mousson et al. 2005), and for identifying cryptic species (Burger et al. 2014).
Materials and Methods
Sample Preparation and DNA Extraction of F. binodulus
Total DNA of F. binodulus was extracted from an adult individual collected in Gageodo, Jeollanam province (34°03′04.0″ N, 125°07′48.6″ E), Republic of Korea, using DNeasy Blood &Tissue Kit (QIAGEN, Hilden, Germany). DNA sample and specimen (95% ethanol) are deposited in the InfoBoss Cyber Herbarium (IN; J. Lee, INH-00021).
Genome Sequencing and de novo Assembly of Mitochondrial Genome of F. binodulus
Raw sequences were obtained from Illumina HiSeqX with constructing 350-bp insertion pair-end library at Macrogen Inc., Korea. Under the environment of Genome Information System (GeIS; http://geis.infoboss.co.kr/; Park et al., in preparation), raw sequence were filtered by Trimmomatic v0.33 (Bolger et al. 2014) and subjected to de novo assembly process done by Velvet v1.2.10 (Zerbino and Birney 2008) with k-mers ranging from 61 to 75 in order to obtain the complete mitochondrial genome sequences. Filling gap sequences as well as circular test were conducted with SOAPGapCloser v1.12 (Zhao et al. 2011). After that, all assembled bases were manually investigated using BWA v0.7.17 (Li et al. 2009) and SAMtools v1.9 (Li 2013) to correct misassembled sequences bases.
Mitochondrial Genome Annotation
Geneious R11 11.1.5 (Biomatters Ltd, Auckland, New Zealand) was used to annotate the mitochondrial genome based on sequence alignment with other Lucanid mitochondrial genomes. To confirm location and structure of transfer RNAs (tRNAs), the annotated GenBank format file of F. binodulus mitochondrial genome was subjected to the MITOS web server with genetic code ‘05-invertebrate’ (Bernt et al. 2013), and ARWEN server with default option (Laslett and Canbäck 2008). The prediction results were reviewed manually and drawn into final tRNA structure. The annotated GenBank format file of F. binodulus mitochondrial genome was used to draw the circular map using CGView with default options (Grant and Stothard 2008)
Identification of SSRs on F. binodulus Mitochondrial Genome
SSRs were identified on the chloroplast genome sequence using the pipeline of the SSR database (SSRDB; http://ssr.pe.kr/; Park et al., in preparation). Based on conventional definition of SSR on organelle genomes: monoSSR (unit sequence length is 1 bp) to hexaSSR (unit sequence length is 6 bp), that over 10-bp long. Since various criteria of SSRs was used for organelle genomes (Gandhi et al. 2010, Chen et al. 2015, Cheng et al. 2016, Shukla et al. 2018, Jeon and Kim, 2019, Li et al. 2019), we adopted the criteria used in organelle genomes of Dysphania ambrosioides (Kim et al. 2019) and Arabidopsis thaliana (Park et al. 2020b) monoSSR (unit sequence length is 1 bp) to hexaSSR (6 bp) are used as normal SSRs and heptaSSR (7 bp) to decaSSR (10 bp) were defined as extendedSSRs. Among normal SSRs, pentaSSRs, and hexaSSRs of which unit number was 2 were classified as potentialSSRs.
Nucleotide Diversity Analysis of F. binodulus Mitochondrial Genome
Nucleotide diversity of the 21 Lucanid mitochondrial genomes was calculated based on the method proposed by Nei and Li (Nei and Li 1979) using the perl script, one of analysis tools implemented in the GenomeArchive; http://www.genoomearchive.info/ (Park and Xi 2018, Park et al., in preparation). Window size and step size of sliding-window method were set as 500 and 200 bp, respectively. Genomic positions of each windows were compared with gene annotations of the mitochondrial genome.
All available 88 Scarabaeoid mitochondrial sequences including the 21 Lucanids mitochondrial genomes that contained all 13 protein-coding gene (PCGs) were retrieved from NCBI and sequences of each PCGs were extracted. Multiple sequence alignments for each PCG sequence for two datasets (21 Lucanids mitochondrial genomes and 88 Scarabaeiod mitochondrial genomes) were conducted with MAFFT v7.450 (Katoh and Standley 2013). Nucleotide diversity for each alignment was calculated based on Nei and Li (1979) using the perl script without sliding-window option.
Construction of Phylogenetic Trees
Thirteen PCGs and 2 ribosomal RNAs (rRNAs) were extracted from 21-stag beetle mitochondrial genomes and an outgroup cockchafer species (Rhopaea magnicornis; NC_027602). The 15 genes were first aligned individually using MAFFT v7.450 (Katoh and Standley 2013), then were concatenated to construct the phylogenetic trees. Maximum likelihood (number of bootstrap repeats is 1,000) and neighbor-joining (number of bootstrap repeats is 10,000) phylogenetic trees were constructed using MEGA X (Kumar et al. 2018). During the ML analysis, a heuristic search was used with nearest-neighbor interchange (NNI) branch swapping, the Tamura-Nei model, and uniform rates among sites. All other options were set to their default values. Bootstrap analyses with 1,000 pseudoreplicates were conducted with the same options. Bayesian inference (number of generations is 1,100,000) tree was constructed by Mr. Bayes v3.2.6 (Huelsenbeck and Ronquist 2001) under the environment of Geneious R11 1.1.5 (Biomatters Ltd, Auckland, New Zealand). The GTR model with gamma rates was used as a molecular model. A Markov-chain Monte Carlo (MCMC) algorithm was employed for 1,000,000 generations, sampling trees every 200 generations, with four chains running simultaneously. Trees from the first 100,000 generations were discarded as burn-in.
Results and Discussions
Complete Mitochondrial Genome of F. binodulus
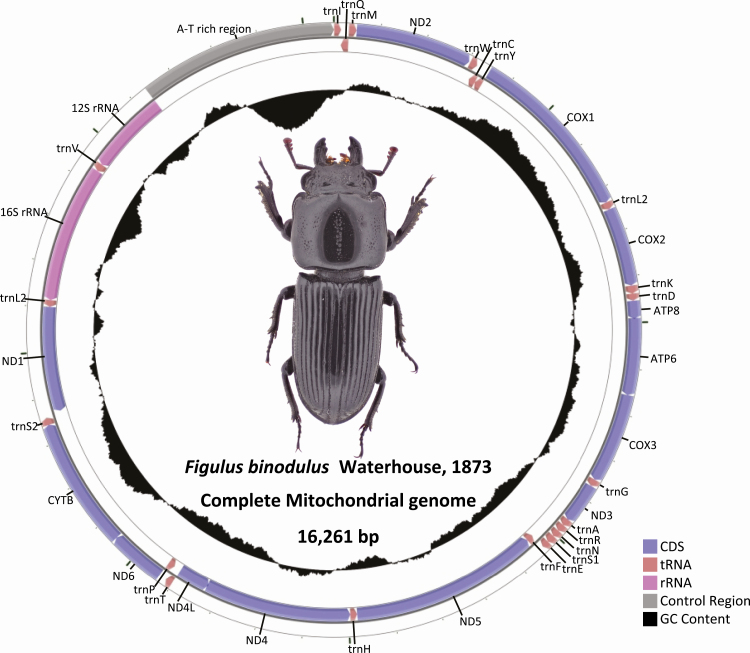
Figulus binodulus mitochondrial genome (GenBank MN180051) was 16,261-bp long and contained 13 PCGs, 2 rRNAs, and 22 tRNAs, which is the typical configuration of an insect mitochondrial genome (Figure 1; Table 1). Its GC ratio is 30.71% which was within the range of previously known species with the highest found in Prosopocoilus gracilis (33.91%) and the lowest in Sinodendron yunnanense (24.94%; Table 2). The order of the 37 genes found in F. binodulus mitochondrial genome was identical to the ancestral insect mitochondrial gene order (Cameron 2014). This order was conserved throughout all stag beetle lineages with only some minor modifications: 1) trnTyr duplication was found in Cherucus minor and 2) relocation of trnLeu2 was observed in Sinodendron species (Lin et al. 2017).
Fig. 1.
Complete mitochondrial genome sequence of Figulus binodulus. Black graph inside circular diagram presents GC ratio of mitochondrial genomes. Colorful bars on outer circular form indicates CDSs (blue), tRNAs (light brown), rRNAs (pink), and control region (gray).
Table 1.
Summary of Figulus binodulus mitochondrial genome
| Gene | Strand | Start (bp) | End (bp) | Size (bp) | Start codon | End codon | Anticodon | Intergenic length (bp) |
|---|---|---|---|---|---|---|---|---|
| trnIle | J | 2 | 63 | 62 | ACA | TA | GAU | N/A |
| trnGln | N | 61 | 129 | 69 | TAC | TAA | UUG | −3 |
| trnMet | J | 129 | 196 | 68 | AAA | TA | CAU | −1 |
| nad2 | J | 197 | 1,207 | 1011 | ATG | TAA | 0 | |
| trnTrp | J | 1,218 | 1,282 | 65 | AAG | TA | UCA | 10 |
| trnCys | N | 1,275 | 1,335 | 61 | AGC | T | GCA | −8 |
| trnTyr | N | 1,335 | 1,398 | 64 | GGT | A | GUA | −1 |
| cox1 | J | 1,400 | 2,930 | 1531 | AAC | T | 1 | |
| trnLeu | J | 2,931 | 2,995 | 65 | TCT | AA | UAA | 0 |
| cox2 | J | 2,996 | 3,680 | 685 | ATA | T | 0 | |
| trnLys | J | 3,681 | 3,751 | 71 | CAT | GA | CUU | 0 |
| trnAsp | J | 3,751 | 3,812 | 62 | AAA | TA | GUC | −1 |
| atp8 | J | 3,813 | 3,968 | 156 | ATT | TAA | 0 | |
| atp6 | J | 3,962 | 4,630 | 669 | ATG | TAA | −7 | |
| cox3 | J | 4,630 | 5,413 | 784 | ATG | T | −1 | |
| trnGly | J | 5,414 | 5,476 | 63 | ACT | GTA | UCC | 0 |
| nad3 | J | 5,477 | 5,830 | 354 | ATA | TAG | 0 | |
| trnAla | J | 5,829 | 5,892 | 64 | AGG | A | UGC | −2 |
| trnArg | J | 5,892 | 5,955 | 64 | AAA | A | UCG | −1 |
| trnAsn | J | 5,956 | 6,018 | 63 | TTA | AAA | GUU | 0 |
| trnSer | J | 6,019 | 6,085 | 67 | GGG | T | UCU | 0 |
| trnGlu | J | 6,086 | 6,147 | 62 | ATT | TA | UUC | 0 |
| trnPhe | N | 6,146 | 6,207 | 62 | ACT | TA | GAA | −2 |
| nad5 | N | 6,208 | 7,921 | 1714 | ATT | T | 0 | |
| trnHis | N | 7,922 | 7,984 | 63 | ACT | GTA | GUG | 0 |
| nad4 | N | 7,986 | 9,321 | 1336 | ATG | T | 1 | |
| nad4l | N | 9,315 | 9,602 | 288 | ATG | TAG | −7 | |
| trnThr | J | 9,605 | 9,666 | 62 | GTT | CT | UGU | 2 |
| trnPro | N | 9,667 | 9,728 | 62 | CAA | GA | UGG | 0 |
| nad6 | J | 9,733 | 10,224 | 492 | ATG | TAA | 4 | |
| cytb | J | 10,224 | 11,366 | 1143 | ATG | TAG | −1 | |
| trnSer | J | 11,365 | 11,429 | 65 | AGT | TT | UGA | −2 |
| nad1 | N | 11,455 | 12,405 | 951 | ATT | TAG | 25 | |
| trnLeu | N | 12,406 | 12,468 | 63 | ATT | ATA | UAG | 0 |
| 16S ribosomal RNA | N | 12,469 | 13,729 | 1261 | GTT | T | 0 | |
| trnVal | N | 13,730 | 13,799 | 70 | CAA | A | UAC | 0 |
| 12S ribosomal RNA | N | 13,800 | 14,544 | 745 | AAA | A | 0 |
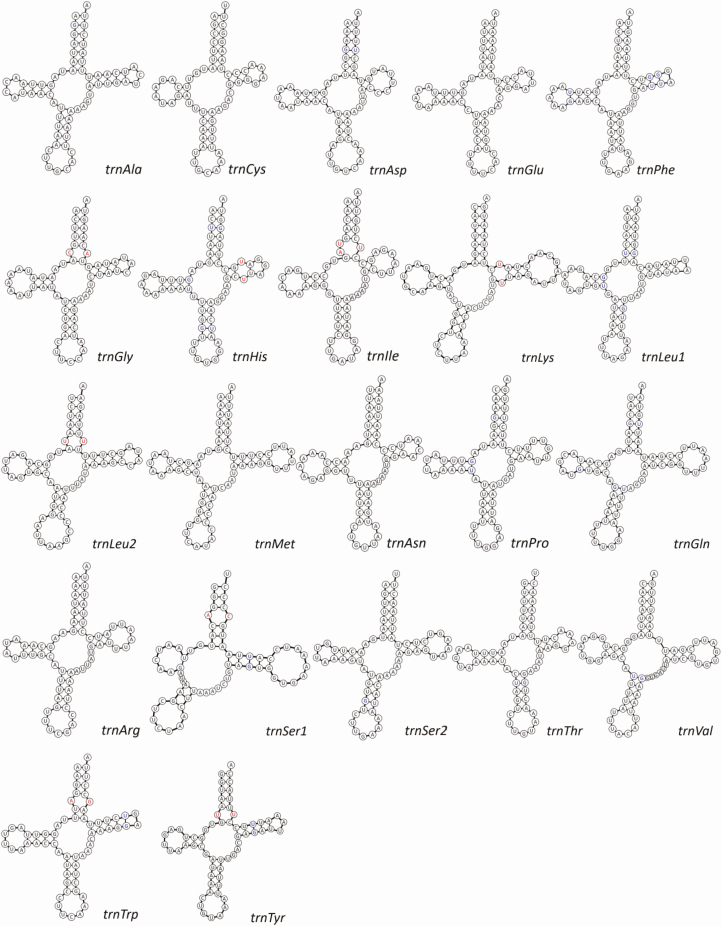
Total length of F. binodulus mitochondrial tRNAs ranged from 61 bp (trnCys) to 71 bp (trnLys; Table 1). The length of nucleotide-amino acid accepter arms (AA arm) was uniformly 7 bp in all 22 tRNAs, while the anticodon arms (AC arm) varied from 3 to 5 bp. Compared with the AA and AC arms, length of dihydrouridine arms (D arm), and TΨC arms (T arm) were much more variable, with the length varying from 1 to 5 bp (Yang et al. 2018). All tRNAs could be shaped into typical cloverleaf structure except trnSer1, which had a shortened D arm (Fig. 2). This, however, is a well-known phenomenon for metazoan mitochondrial genomes, repeatedly reported in many animal species (Wolstenholme 1992).
Fig. 2.
Twenty-two tRNA structure originated from Figulus binodulus mitochondrial genome. Structure of 22 tRNAs with base pair of tRNAs. Names of tRNAs and anticodon were displayed bottom right of each structure. Mismatches are indicated in red bases, and wobble-pairs (G-U) are indicated as blue.
Twenty-eight mismatched base pairs were identified from the predicted tRNA secondary structures. G-U wobble pairs accounted for most of the mismatches (19 out of 28, 67.86%), which is a common feature in tRNAs (Varani and McClain 2000). UA-U mismatch base pair was found in the AA arm of trnIle, which were supported by the both predictions of ARWEN and MITOS (Fig. 2). In addition, two G-A, two C-A, and four U-U mismatches were also identified (Fig. 2).
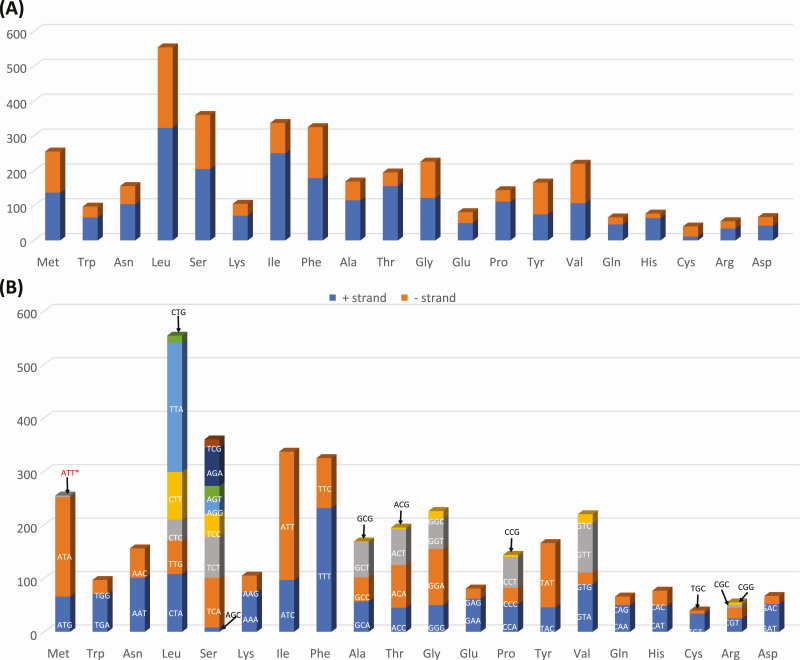
Leucine (Leu) was the most frequently used amino acid in F. binodulus mitochondrial PCGs (Fig. 3), congruent to previous insect mitochondrial researches (Negrisolo et al. 2011, Wei et al. 2014, Zhang et al. 2014, Xin et al. 2017, Chen et al. 2018a). Serine (Ser) was the second most frequent amino acid and was followed by Isoleucine (Ile) and Phenylalanine (Phe; Fig. 3A). Interestingly, the most frequent two amino acids (Leu and Ser) were the only amino acids that had two different mitochondrial tRNA genes. In addition, these four amino acids showed different ratio of plus and minus strands (Fig. 3A): for example, Ile had the lowest proportion of minus strand and Leu and Ser are the next (Fig. 3A).
Fig. 3.
Codon usage of Figulus binodulus mitochondrial genome. (A) X-axis presents amino acids and Y-axis presents number of amino acids along with direction of genes (+ strand is blue color and – strand is red color). (B) Frequency of codons along with amino acids. Codons are displayed on the bars or around bars with arrows. Red colored codons indicate exceptional cases caused by RNA editing event.
Each codon in one amino acid displayed different proportions, indicating codon bias of each amino acid (Fig. 3B). In total, 63 codons including the exceptional start codon, ATT, were found in F. binodulus mitochondrial PCGs. The ratio of each codon varied however, usually with A-T-based codons out numbering the G-C based codons (e.g., TTT vs TTG in Phe; Fig. 3B), which is a feature steadily found in insect mitochondrial genomes (Dai et al. 2015).
Twelve out of 13 PCGs started with a typical start codon, ATN: 7 with ATG (nad2, atp6, cox3, nad4, nad4l, nad6, and cytb), 3 with ATT (atp8, nad5, and nad1), and 2 with ATA (cox2 and nad3). Cox1, on the other hand, had AAC as a start cordon which is known to be an alternative start codon in Polyphaga beetles (Sheffield et al. 2008). Three stop codons types were identified: the typical TAA and TAG stop codons and an abnormal T stop codon. Four genes (nad2, atp8, atp6, and nad6) had TAA codons, and other four genes (nad3, nad4l, cytb, and nad1) had TAG codons. The rest of the five genes (cox1, cox2, cox3, nad5, and nad4) ended with an incomplete T codon which is thought to be completed into a TAA stop codon by a posttranscriptional polyadenylation (Boore 1999, Meng et al. 2016).
Comparisons of F. binodulus Mitochondrial Genomes with 21 Complete Lucanid Mitochondrial Genomes
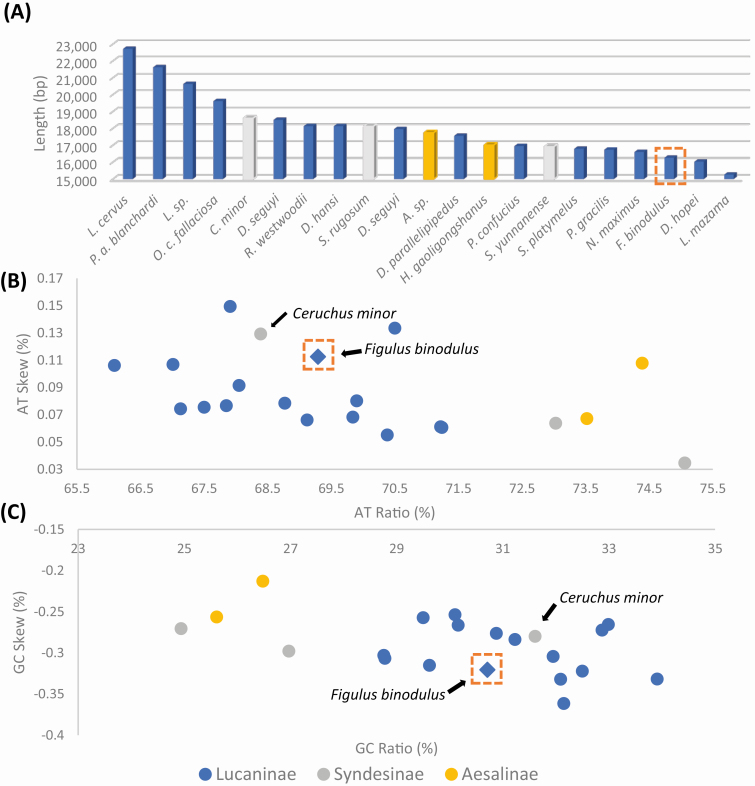
Including F. binodulus mitochondrial genome sequenced in this study, 21 complete mitochondrial genomes were available in family Lucanidae (Table 2). F. binodulus was third from the shortest, only longer than two species, Lucanus Mazama (NC_013578, 15,258 bp) and Dorcus hopei (MF612069, 16,026 bp; Fig. 4A). No correlation was found between the length of the mitochondrial genomes and the phylogenetic position of each species (see different bar colors in Fig. 4A) as the length of each sequence were mostly determined by species specific intergenic insertions (e.g., Prosopocoilus astacoides blanchardi: KF364622, 21,628 bp; Fig. 5B) or control region variations (e.g., Odontolabis curvera fallaciosa: MF908524, 19,614 bp).
Fig. 4.
Mitochondrial genome length, AT% vs AT-Skew, and GC% vs GC-Skew graphs in Lucanidae mitochondrial genomes. (A) X-axis indicates 21 Lucanidae mitochondrial genomes, and Y-axis means length of their complete mitochondrial genome. (B) X-axis present GC ratio and Y-axis show GC-skew. (C) X-axis present AT ratio and Y-axis show AT-skew. Orange dotted box indicates Figulus binodulus assembled in this study, and each color of symbol indicates subfamily
Fig. 5.
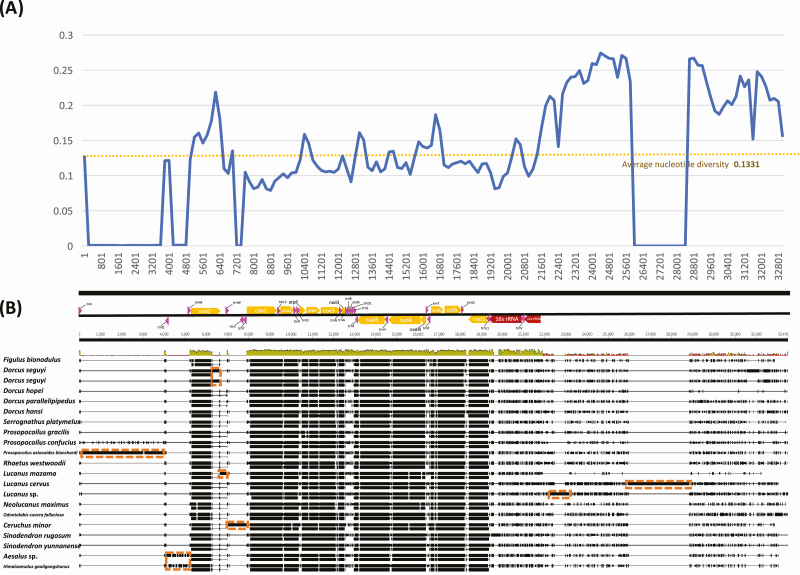
Nucleotide diversity and structural comparison of 21 Lucanidae mitochondrial genomes including Figulus binodulus. (A) X-axis indicates start position of sliding window (500 bp) used for calculating nucleotide diversity. Y-axis shows nucleotide diversity value. Blue line means nucleotide diversity of each window and yellow dotted line is average nucleotide diversity value. (B) Color arrow indicates PCGs (Orange), tRNAs (Pink), and rRNAs (Red). Orange dotted boxes indicate insertions.
Basal subfamilies, Syndesinae and Aesalinae, showed high AT ratio (from 73 to 76%; Table 2 and Fig. 4B) compared with that of Lucaninae except Ceruchus minor (Syndesinae; 68.39%); however, AT skew of the two subfamilies was similar to those of the Lucaninae mitochondrial genomes (Fig. 4B). This peculiarity of C. minor led to the dispersed positions of Syndesinae in both AT skew/AT ratio and GC skew/GC ratio graphs, where C. minor was clustered with the members of Lucaninae and Syndesinae (Fig. 4B and C). Excluding the exceptional C. minor, the three subfamilies could be roughly distinguished (Fig. 4B and C).
Nucleotide Diversity Analysis of F. binodulus Mitochondrial Genomes With 21 Complete Lucanid Mitochondrial Genomes
Based on multiple sequence alignments of available 21 stag beetle mitochondrial genomes (Table 2), nucleotide diversity was calculated. The total nucleotide diversity was 0.1331 (Fig. 5A), which was higher than those of family Ptinidae (0.08368; Park et al., under revision) and family Aphididae (0.0432; Park et al., in preparation). Cox1 presented the lowest nucleotide diversity among all PCGs (Fig. 5A). This phenomenon was also consistent with that of mitochondrial genomes of superfamily Scarabaeoidea including family Lucaenidae (Fig. 6). The nucleotide diversities of rRNA genes, region known to be well conserved in insect mitochondria (De Mandal et al. 2014) were very low (from 0.090 to 0.145; Fig. 5), were still higher than those of family Ptinidae (0.027 to 0.040; Park et al. under revision) and Aphididae (0.014 to 0.043; Park et al. in preparation). Nad2, nad6, atp8, and N-terminal of cytb showed nucleotide diversity ranges from 0.15 to 0.17 (Fig. 5A), higher than that of Ptinidae (from 0.05 to 0.13; Park et al., under revision). Nucleotide diversity of cox1 was flat from N-terminal to C-terminal (Fig. 5A), whereas atp6, nad5, and cytb showed sharp decrease of nucleotide diversity from N-terminal to C-terminal (Fig. 5A).
Fig. 6.
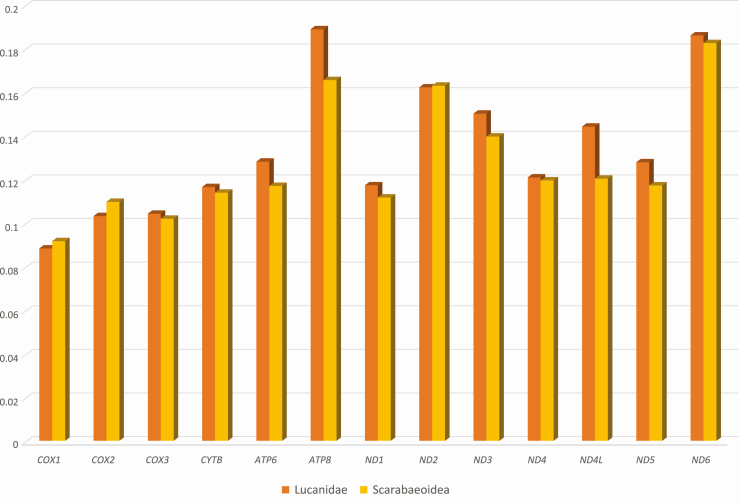
Nucleotide diversity of 13 PCGs in 21 Lucanidae complete mitochondrial genomes and 88 Scarabaeoidea mitochondrial genomes. X-axis indicates 13 PCGs and Y-axis indicates nucleotide diversity. Orange color indicates nucleotide diversities based on 21 Lucanidae mitochondrial genomes and Yellow color means nucleotide diversities from 88 Scarabaeoidea mitochondrial genomes.
Multiple insertions were found within the alignments (Fig. 5B), which resulted in drastic plummeting of nucleotide diversity (Fig. 5A). Most insertions were species specific (Fig. 5B); however, the insertion between trnGln and trnMet was shared in both species of subfamily Aesalinae (Fig. 5B). The AT-rich control region was the only region to have insertions while other insertions were also found in vicinity of the region (Fig. 5B). Even without the insertions, the control region showed extremely high nucleotide diversity (Fig. 5B).
All 13 PCGs displayed slightly different nucleotide diversities between Lucanidae and Scarabaeoidea groups (Fig. 6). While cox1, cox2, and nad2 were more conserved in Lucanid mitochondrial genomes than those of Scarabaeoidea, rest of the PCGs were more conserved in the superfamily level (Fig. 6). Atp8 and nad4l genes presented the largest differences between the two groups (Fig. 6), which were coincidentally the first and second shortest PCGs. Above half of the 88 available sequences (46 species) of Scarabaeoid mitochondrial genomes were from subfamily Scarabainae of family Scarabaidae, especially from genus Onthophagus (20 species). This unevenness in selected taxa may have reduced the diversities of the Scarabaeoidea group, thus the extreme conserveness of cox1 and cox2 of Lucanidae is a significant phenomenon. These diversity data of each gene will be useful in selecting proper molecular markers.
SSR Identified in F. binodulus Mitochondrial Genome
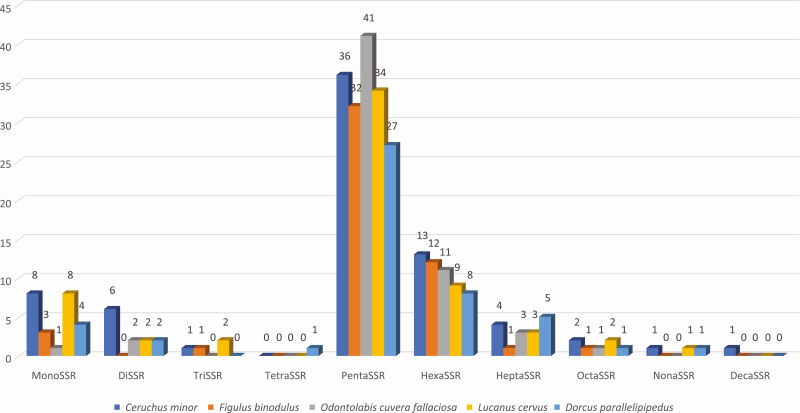
SSRs were rescued from F. binodulus mitochondrial genome sequences with the pipeline of the SSR database (see Materials and Methods). Among three types of SSRs, classified as SSRs, extended SSRs and potential SSRs, 4 SSRs (three monoSSRs, and one triSSRs), 2 extended SSRs (heptaSSR and octaSSR per each), and 44 potential SSRs were identified (Table 3). Along with unit sequence length, pentaSSRs occupied the most of all types of SSRs (32 SSRs; 64.00%; Fig. 7) and hexaSSRs covered the second largest proportion (12 SSRs; 32.00%; Fig. 7). Only one of each triSSR, heptaSSR, and octaSSR were identified (Fig. 7). Forty SSRs (80.00%) were in genic regions, whereas 10 SSRs (20.00%) were located in the control region.
Table 3.
List of 50 SSRs, extended SSRs, and potential SSRs from Figulus binodulus mitochondrial genomes
| No | Name | SSR type | Type | Coordination | Unit sequence | Repeat number | Position | Genes | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | cH0000001 | PotentialSSR | HexaSSR | 329 | 340 | AAAAAC | 2 | Genic | nad2 |
| 2 | cP0000001 | PotentialSSR | PentaSSR | 781 | 790 | ACTAT | 2 | Genic | nad2 |
| 3 | c70000001 | ExtenedSSR | HeptaSSR | 999 | 1012 | CCTTCTA | 2 | Genic | nad2 |
| 4 | cP0000002 | PotentialSSR | PentaSSR | 2623 | 2632 | AATTC | 2 | Genic | cox1 |
| 5 | cP0000003 | PotentialSSR | PentaSSR | 6208 | 6217 | ATAAA | 2 | Genic | nad5 |
| 6 | cP0000004 | PotentialSSR | PentaSSR | 6638 | 6647 | AAATA | 2 | Genic | nad5 |
| 7 | cP0000005 | PotentialSSR | PentaSSR | 6698 | 6707 | AAAAG | 2 | Genic | nad5 |
| 8 | cP0000006 | PotentialSSR | PentaSSR | 6775 | 6784 | AAATA | 2 | Genic | nad5 |
| 9 | cP0000007 | PotentialSSR | PentaSSR | 6920 | 6929 | CATTA | 2 | Genic | nad5 |
| 10 | cP0000008 | PotentialSSR | PentaSSR | 7575 | 7584 | CCATC | 2 | Genic | nad5 |
| 11 | cP0000009 | PotentialSSR | PentaSSR | 7598 | 7607 | AATTA | 2 | Genic | nad5 |
| 12 | cP0000010 | PotentialSSR | PentaSSR | 7994 | 8003 | AAACA | 2 | Genic | nad4 |
| 13 | cP0000011 | PotentialSSR | PentaSSR | 8562 | 8571 | CTAAT | 2 | Genic | nad4 |
| 14 | cP0000012 | PotentialSSR | PentaSSR | 8770 | 8779 | AAAAT | 2 | Genic | nad4 |
| 15 | cH0000002 | PotentialSSR | HexaSSR | 9197 | 9208 | CCAAAA | 2 | Genic | nad4 |
| 16 | cP0000013 | PotentialSSR | PentaSSR | 9304 | 9313 | AAATA | 2 | Genic | nad4 |
| 17 | cH0000003 | PotentialSSR | HexaSSR | 9462 | 9473 | ATACAA | 2 | Genic | nad4l |
| 18 | cH0000004 | PotentialSSR | HexaSSR | 9985 | 9996 | TTAACC | 2 | Genic | nad6 |
| 19 | cH0000005 | PotentialSSR | HexaSSR | 10289 | 10300 | ACCTTC | 2 | Genic | cytb |
| 20 | cP0000014 | PotentialSSR | PentaSSR | 10529 | 10538 | ATTAT | 2 | Genic | cytb |
| 21 | cP0000015 | PotentialSSR | PentaSSR | 11121 | 11130 | CTTAT | 2 | Genic | cytb |
| 22 | cP0000016 | PotentialSSR | PentaSSR | 11218 | 11227 | TTATT | 2 | Genic | cytb |
| 23 | cM0000001 | SSR | MonoSSR | 11471 | 11481 | A | 11 | Genic | nad1 |
| 24 | cH0000006 | PotentialSSR | HexaSSR | 11625 | 11636 | AAAAGA | 2 | Genic | nad1 |
| 25 | cH0000007 | PotentialSSR | HexaSSR | 11851 | 11862 | AAACAT | 2 | Genic | nad1 |
| 26 | cH0000008 | PotentialSSR | HexaSSR | 11934 | 11945 | TTAAAG | 2 | Genic | nad1 |
| 27 | cH0000009 | PotentialSSR | HexaSSR | 12006 | 12017 | AATTAG | 2 | Genic | nad1 |
| 28 | cP0000017 | PotentialSSR | PentaSSR | 12394 | 12403 | AAATA | 2 | Genic | nad1 |
| 29 | cP0000018 | PotentialSSR | PentaSSR | 12474 | 12483 | TTTTC | 2 | Genic | 16S rRNA |
| 30 | cP0000019 | PotentialSSR | PentaSSR | 12851 | 12860 | TTAAA | 2 | Genic | 16S rRNA |
| 31 | cP0000020 | PotentialSSR | PentaSSR | 12920 | 12929 | AAAAT | 2 | Genic | 16S rRNA |
| 32 | cP0000021 | PotentialSSR | PentaSSR | 13190 | 13199 | ATTAC | 2 | Genic | 16S rRNA |
| 33 | cP0000022 | PotentialSSR | PentaSSR | 13217 | 13226 | TTAAT | 2 | Genic | 16S rRNA |
| 34 | cH0000010 | PotentialSSR | HexaSSR | 13316 | 13327 | AAAAAT | 2 | Genic | 16S rRNA |
| 35 | cP0000023 | PotentialSSR | PentaSSR | 13421 | 13430 | AATTA | 2 | Genic | 16S rRNA |
| 36 | cP0000024 | PotentialSSR | PentaSSR | 13597 | 13606 | AAATA | 2 | Genic | 16S rRNA |
| 37 | cP0000025 | PotentialSSR | PentaSSR | 13947 | 13956 | AATTA | 2 | Genic | 12S rRNA |
| 38 | cP0000026 | PotentialSSR | PentaSSR | 14127 | 14136 | ACAGG | 2 | Genic | 12S rRNA |
| 39 | cP0000027 | PotentialSSR | PentaSSR | 14183 | 14192 | AACTA | 2 | Genic | 12S rRNA |
| 40 | cP0000028 | PotentialSSR | PentaSSR | 14280 | 14289 | AATAA | 2 | Genic | 12S rRNA |
| 41 | cP0000029 | PotentialSSR | PentaSSR | 14593 | 14602 | TAGTT | 2 | Intergenic | |
| 42 | cP0000030 | PotentialSSR | PentaSSR | 15021 | 15030 | CTAAA | 2 | Intergenic | |
| 43 | cP0000031 | PotentialSSR | PentaSSR | 15184 | 15193 | CTAAA | 2 | Intergenic | |
| 44 | cP0000032 | PotentialSSR | PentaSSR | 15466 | 15475 | TTTAT | 2 | Intergenic | |
| 45 | cM0000002 | SSR | MonoSSR | 15598 | 15612 | T | 15 | Intergenic | |
| 46 | cT0000001 | SSR | TriSSR | 15674 | 15691 | TAT | 6 | Intergenic | |
| 47 | cH0000011 | PotentialSSR | HexaSSR | 15856 | 15867 | TATTTA | 2 | Intergenic | |
| 48 | c80000001 | ExtenedSSR | OctaSSR | 15868 | 15883 | AATAAATG | 2 | Intergenic | |
| 49 | cH0000012 | PotentialSSR | HexaSSR | 16080 | 16091 | AAATTA | 2 | Intergenic | |
| 50 | cM0000003 | SSR | MonoSSR | 16168 | 16186 | A | 19 | Intergenic |
Fig. 7.
Distribution of number of SSRs along with unit length of SSRs identified on Figulus binodulus mitochondrial genome. X-axis presents SSR types and Y-axis indicates number of SSRs for each type. Numbers on bars means number of SSRs.
Four species of F. binodulus were selected to represent the neighboring lineages of F. binodulus: Ceruchus minor of tribe Ceruchini, and Odontolabis cuvera fallaciosa, Lucanus cervus, and Dorcus parallelipipedus of tribes Aegini, Lucanini, and Dorcini, respectively. SSRs were identified on the selected sequences using the same method (see Materials and Methods). Overall number of SSRs along with 10 types in the 5 species were similar to each other (Fig. 7): PentaSSRs and hexaSSRs explaining the first and second largest proportion of identified SSRs and extended SSRs, respectively. In detail, numbers of diSSRs, heptaSSRs, and octaSSRs in F. binodulus were the lowest among the five mitochondrial genomes (Fig. 7). Number of HexaSSRs showed interesting trend that C. minor was the largest and D. parallellpipedus is the smallest (Fig. 7), congruent to their phylogenetic relation (Fig. 8). In addition, mitochondrial genome of F. binodulus lacked diSSRs, whereas that of C. minor was the largest, which was same to that of L. cervus. It indicates that number of SSRs along with SSR types may not related to their phylogenetic position.
Fig. 8.
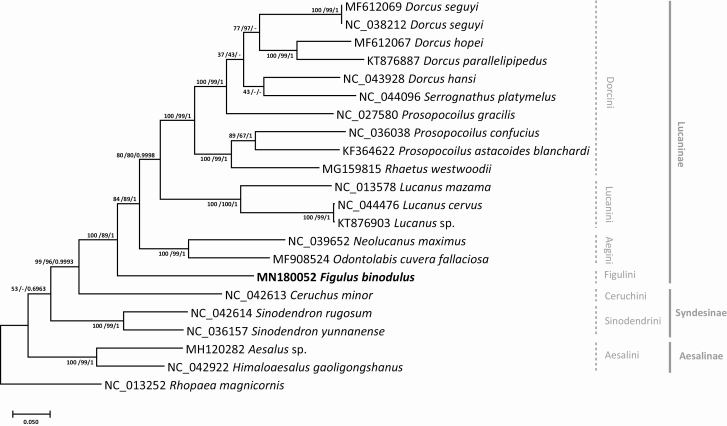
Phylogenetic trees of 21 complete mitochondrial genomes of Lucanidae. Neighbor joining (bootstrap repeat is 10,000) and maximum likelihood (bootstrap repeat is 1,000 phylogenetic trees of 21 Lucaninae, Aesalinae, and Syndesinae mitochondrial genomes with one Scarabaeidae complete mitochondrial genome as outgroup: Figulus binodulus (MN180052 in this study), Dorcus seguyi (MF612069), Dorcus seguyi (NC_038212), Dorcus hopei (MF612067), Dorcus parallelipipedus (KT876887), Dorcus hansi (NC_043928), Serrognathus platymelus (NC_044096), Prosopocoilus gracilis (NC_027580), Prosopocoilus Confucius (NC_036038), Prosopocoilus astacoides blanchardi (KF364622), Rhaetus westwoodii (MG159815), Lucanus Mazama (NC_013578), Lucanus cervus (NC_044476), Lucanus sp. (KT876903), Neolucanus maximus (NC_039652), Odontolabis cuvera fallaciosa (MF908524), Ceruchus minor (NC_042613), Sinodendron rugosum (NC_042614), Sinodendron yunnanense (NC_036157), Aesalus sp. (MH120282), Himaloaesalus gaoligongshanus (NC_042922), and Rhopaea magnicornis (NC_013252) as an out group. Phylogenetic tree was drawn based on maximum likelihood tree. The numbers above branches indicate bootstrap support values of maximum likelihood, neighbor joining, and Bayesian inference trees, respectively.
This trend of SSRs and extended SSRs was maintained outside of family Lucanidae. In S. paniceum (Coleoptera: Ptinidae), 21.11% of SSRs (Park et al., under revision) were located in the control region (Table 3), similar to the proportion found in F. binodulus control region of 10 out of 50 SSRs (20.00%), even though the total number of SSRs in F. binodulus was around half of that of S. paniceum (Park et al., under revision).
As SSRs are known to be prone to rapid evolutions (Stolle et al. 2013) as well as three mitochondrial genomes of S. paniceum, a cosmopolitan pest species, showed that 21.69% SSRs had variations among three individuals (Park et al., under revision), the SSRs identified from F. binodulus mitochondrial genomes could be used as molecular markers for distinguishing different populations or individuals enough.
Phylogenetic Interpretation of Lucanidae Mitochondrial Genomes
Thirty-seven genes on mitochondrial genomes covered >14 kb, which could be used for constructing high resolution phylogenetic trees (Simon et al. 1994, 2006; Boore and Brown 1998;Cameron 2014; Lavrov 2014; Smith and Keeling 2015; Yu and Liang 2018; Łukasik et al. 2019), displaying its usefulness to understand their taxonomic classification even though some exceptional cases were reported (Hwang et al. 2001, Park et al. 2020a).
We constructed phylogenetic trees based on 21 Lucanidae mitochondrial genomes covering three out of four subfamilies of Lucanidae except Lampriminae. Phylogenetic trees were constructed with three methods: maximum likelihood (ML), neighbor joining (NJ), and Bayesian inference (BI; see Materials and Methods). Inter-subfamily topology was congruent to the previously known phylogeny (Kim and Farrell 2015) in that Aesalinae is branched out first, then did tribe Sinodendrini and Ceruchini of Syndesinae, respectively (Fig. 8), showing a paraphyletic manner of subfamily Syndesinae. The support values of the phylogenetic trees, however, were low (Fig. 8); therefore, additional mitochondrial genomes from the minor subfamilies would be essential in future studies. Interestingly, the clade covering Ceruchini and all Lucaninae was well supported in all three trees (Fig. 8), which is congruent with that Cheruchus clustered with Lucaninae species not Sinodendron species in the AT ratio/AT skew and GC ratio/GC skew graphs (Fig. 4B and C).
Figulus binodulus was placed sister to all other Lucaninae mitochondrial genomes as previous studies on this subfamily were concentrated to several closely related species such as Dorcus spp. or Prosopocoilus spp. (Kim et al. 2015), not including a large proportion of the subfamily such as the Platycerini clade or the Gondwanan clade found in Kim and Farrell 2015. Lower classifications of the subfamily also turned out to be a mess: Prosopocoilus gracilis was shown to be more related to Dorcus spp. than other Prosopocoilus spp. (Fig. 8), which was a known phenomenon since the publication of the sequence (Wu et al. 2016). The recently revised genus Serrognathus was also not supported well as it did not form a separate clade (Fig. 8).
Acknowledgments
This study was carried out with the support of the two grants: InfoBoss Research Grant (IBG-0030) and ‘Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ013389052019)’, Rural Development Administration, Republic of Korea. Jongsun Park designed and managed this project, Jungmo Lee prepared the sample, Jungmo Lee, Jonghyun Park, Hong Xi, and Jongsun Park analyzed mitochondrial genomes Jungmo Lee, Jonghyun Park, Jongsun Park wrote the mansucript.
References Cited
- Bernt M, Donath A, Jühling F, Externbrink F, Florentz C, Fritzsch G, Pütz J, Middendorf M, and Stadler P F. . 2013. MITOS: improved de novo metazoan mitochondrial genome annotation. Mol. Phylogenet. Evol. 69: 313–319. [DOI] [PubMed] [Google Scholar]
- Bolger A M, Lohse M, and Usadel B. . 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 30: 2114–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boore J L. 1999. Animal mitochondrial genomes. Nucleic Acids Res. 27: 1767–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boore J L, and Brown W M. . 1998. Big trees from little genomes: mitochondrial gene order as a phylogenetic tool. Curr. Opin. Genet. Dev. 8: 668–674. [DOI] [PubMed] [Google Scholar]
- Burger T D, Shao R, and Barker S C. . 2014. Phylogenetic analysis of mitochondrial genome sequences indicates that the cattle tick, Rhipicephalus (Boophilus) microplus, contains a cryptic species. Mol. Phylogenet. Evol. 76: 241–253. [DOI] [PubMed] [Google Scholar]
- Cameron S L. 2014. Insect mitochondrial genomics: implications for evolution and phylogeny. Annu. Rev. Entomol. 59: 95–117. [DOI] [PubMed] [Google Scholar]
- Cameron S L, Sullivan J, Song H, Miller KB, and Whiting M F. . 2009. A mitochondrial genome phylogeny of the Neuropterida (lace-wings, alderflies and snakeflies) and their relationship to the other holometabolous insect orders. Zoologica Scripta. 38: 575–590. [Google Scholar]
- Chen J, Hao Z, Xu H, Yang L, Liu G, Sheng Y, Zheng C, Zheng W, Cheng T, and Shi J. . 2015. The complete chloroplast genome sequence of the relict woody plant Metasequoia glyptostroboides Hu et Cheng. Front. Plant Sci. 6: 447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Chen P Y, Xue X F, Hua H Q, Li Y X, Zhang F, and Wei S J. . 2018a. Extensive gene rearrangements in the mitochondrial genomes of two egg parasitoids, Trichogramma japonicum and Trichogramma ostriniae (Hymenoptera: Chalcidoidea: Trichogrammatidae). Sci. Rep. 8: 7034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Liu J, Cao Y, Zhou S, and Wan X. . 2018b. Two new complete mitochondrial genomes of Dorcus stag beetles (Coleoptera, Lucanidae). Genes Genomics. 40: 873–880. [DOI] [PubMed] [Google Scholar]
- Chen D, Liu J, Bartolozzi L, and Wan X. . 2019. The complete mitochondrial genome of stag beetle Lucanus cervus (Coleoptera: Lucanidae) and phylogenetic analysis. PeerJ. 7: e8274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Zhao Z, Li B, Qin C, Wu Z, Trejo-Saavedra D L, Luo X, Cui J, Rivera-Bustamante R F, Li S, . et al. 2016. A comprehensive characterization of simple sequence repeats in pepper genomes provides valuable resources for marker development in Capsicum. Sci. Rep. 6: 18919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai L, Qian C, Zhang C, Wang L, Wei G, Li J, Zhu B, and Liu C. . 2015. Characterization of the complete mitochondrial genome of Cerura menciana and comparison with other Lepidopteran insects. PLoS One. 10: e0132951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mandal S, Chhakchhuak L, Gurusubramanian G, and Kumar N S. . 2014. Mitochondrial markers for identification and phylogenetic studies in insects–A Review. DNA Barcodes. 2: 1–9. [Google Scholar]
- Gandhi S G, Awasthi P, and Bedi Y S. . 2010. Analysis of SSR dynamics in chloroplast genomes of Brassicaceae family. Bioinformation. 5: 16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant J R, and Stothard P. . 2008. The CGView Server: a comparative genomics tool for circular genomes. Nucleic Acids Res. 36: W181–W184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hangay G, and De Keyzer R. . 2017. A guide to stag beetles of Australia. CSIRO Publishing, Australia. [Google Scholar]
- Huelsenbeck J P, and Ronquist F. . 2001. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 17: 754–755. [DOI] [PubMed] [Google Scholar]
- Hwang U W, Friedrich M, Tautz D, Park C J, and Kim W. . 2001. Mitochondrial protein phylogeny joins myriapods with chelicerates. Nature. 413: 154–157. [DOI] [PubMed] [Google Scholar]
- Jeon J-H, and Kim S-C. . 2019. Comparative analysis of the complete chloroplast genome sequences of three closely related east-Asian wild roses (Rosa sect. Synstylae; Rosaceae). Genes. 10: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing L, Zhou S-J, Chen Y-J, and Wan X. . 2018. Mitogenome of the monotypic genus rhaetus (Coleoptera: Scarabaeidae: Lucanidae). J. Entomol. Sci. 53: 503–513. [Google Scholar]
- Katoh K, and Standley D M. . 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30: 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S I, and Farrell BD. . 2015. Phylogeny of world stag beetles (Coleoptera: Lucanidae) reveals a Gondwanan origin of Darwin’s stag beetle. Mol. Phylogenet. Evol. 86: 35–48. [DOI] [PubMed] [Google Scholar]
- Kim M J, Kim K G, Kim S R, and Kim I. . 2015. Complete mitochondrial genome of the two-spotted stag beetle, Metopodontus blanchardi (Coleoptera: Lucanidae). Mitochondrial DNA. 26: 307–309. [DOI] [PubMed] [Google Scholar]
- Kim Y, Park J, and Chung Y. . 2019. Comparative analysis of chloroplast genome of Dysphania ambrosioides (L.) mosyakin & clemants understanding phylogenetic relationship in genus dysphania R.Br. Korean J. Plant Resour. 32: 644–688. [Google Scholar]
- Kumar S, Stecher G, Li M, Knyaz C, and Tamura K. . 2018. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35: 1547–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laslett D, and Canbäck B. . 2008. ARWEN: a program to detect tRNA genes in metazoan mitochondrial nucleotide sequences. Bioinformatics. 24: 172–175. [DOI] [PubMed] [Google Scholar]
- Lavrov DV. 2014. Mitochondrial genomes in invertebrate animals. Molecular Life Sciences, Springer New York, pp. 1–8. [Google Scholar]
- Li H. 2013. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv preprint arXiv:1303.3997. [Google Scholar]
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, and Durbin R. . 2009. The sequence alignment/map format and SAMtools. Bioinformatics. 25: 2078–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Zhang C, Guo X, Liu Q, and Wang K. . 2019. Complete chloroplast genome of Camellia japonica genome structures, comparative and phylogenetic analysis. PLoS One. 14: e0216645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z-Q, Song F, Li T, Wu Y-Y, and Wan X. . 2017. New mitogenomes of two Chinese stag beetles (Coleoptera, Lucanidae) and their implications for systematics. J. Insect Sci. 17: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linard B, Arribas P, Andújar C, Crampton-Platt A, and Vogler A P. . 2016. Lessons from genome skimming of arthropod-preserving ethanol. Mol. Ecol. Resour. 16: 1365–1377. [DOI] [PubMed] [Google Scholar]
- Liu Y, Song F, Jiang P, Wilson J J, Cai W, and Li H. . 2018. Compositional heterogeneity in true bug mitochondrial phylogenomics. Mol. Phylogenet. Evol. 118: 135–144. [DOI] [PubMed] [Google Scholar]
- Łukasik P, Chong R A, Nazario K, Matsuura Y, Bublitz A C, Campbell M A, Meyer M C, Van Leuven J T, Pessacq P, Veloso C, . et al. 2019. One hundred mitochondrial genomes of cicadas. J. Hered. 110: 247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Z, Lei C, Chen X, and Jiang S. . 2016. Complete mitochondrial genome sequence of Heliconius melpomene rosina (Insecta: Lepidoptera: Nymphalidae). Mitochondrial DNA A. DNA Mapp. Seq. Anal. 27: 3911–3912. [DOI] [PubMed] [Google Scholar]
- Mori H, and Chiba S. . 2009. Sociality improves larval growth in the stag beetle Figulus binodulus (Coleoptera: Lucanidae). Eur. J. Entomol. 106: 379–383. [Google Scholar]
- Mousson L, Dauga C, Garrigues T, Schaffner F, Vazeille M, and Failloux A-B. . 2005. Phylogeography of Aedes (Stegomyia) aegypti (L.) and Aedes (Stegomyia) albopictus (Skuse)(Diptera: Culicidae) based on mitochondrial DNA variations. Genetics Res. 86: 1–11. [DOI] [PubMed] [Google Scholar]
- Negrisolo E, Babbucci M, and Patarnello T. . 2011. The mitochondrial genome of the ascalaphid owlfly Libelloides macaronius and comparative evolutionary mitochondriomics of neuropterid insects. BMC Genomics. 12: 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M, and Li W H. . 1979. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc. Natl. Acad. Sci. U. S. A. 76: 5269–5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J., and Xi H.. 2018. GenomeArchive: a standardized whole genome database. Plant and Animal Genome XXVI Conference (PAG 2018). doi: 10.13140/RG.2.2.27092.22408 [DOI] [Google Scholar]
- Park J, Xi H, and Park J. . 2020a. The complete mitochondrial genome of Ochetellus glaber (Mayr, 1862) (Hymenoptera: Formicidae). Mitochondrial DNA Part B. 5: 147–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J., Xi H., and Kim Y.. 2020b. The complete chloroplast genome of Arabidopsis thaliana isolated in Korea (Brassicaceae): an investigation of intraspecific variations of the chloroplast genome of Korean A. Thaliana. Int. J. Genom. 2020: 3236461. doi: 10.1155/2020/3236461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield N C, Song H, Cameron S L, and Whiting M F. . 2008. A comparative analysis of mitochondrial genomes in Coleoptera (Arthropoda: Insecta) and genome descriptions of six new beetles. Mol. Biol. Evol. 25: 2499–2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield N C, Song H, Cameron S L, and Whiting M F. . 2009. Nonstationary evolution and compositional heterogeneity in beetle mitochondrial phylogenomics. Syst. Biol. 58: 381–394. [DOI] [PubMed] [Google Scholar]
- Shukla N, Kuntal H, Shanker A, and Sharma SN. . 2018. Mining and analysis of simple sequence repeats in the chloroplast genomes of genus Vigna. Biotechnol. Res. Innovation. 2: 9–18. [Google Scholar]
- Simon C, Frati F, Beckenbach A, Crespi B, Liu H, and Flook P. . 1994. Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann. Entomol. Soc. Am. 87: 651–701. [Google Scholar]
- Simon C, Buckley T R, Frati F, Stewart J B, and Beckenbach A T. . 2006. Incorporating molecular evolution into phylogenetic analysis, and a new compilation of conserved polymerase chain reaction primers for animal mitochondrial DNA. Annu. Rev. Ecol. Evol. Syst. 37: 545–579. [Google Scholar]
- Smith D R, and Keeling P J. . 2015. Mitochondrial and plastid genome architecture: reoccurring themes, but significant differences at the extremes. Proc. Natl. Acad. Sci. U. S. A. 112: 10177–10184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolle E, Kidner J H, and Moritz R F. . 2013. Patterns of evolutionary conservation of microsatellites (SSRs) suggest a faster rate of genome evolution in Hymenoptera than in Diptera. Genome Biol. Evol. 5: 151–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stork N E. 2018. How many species of insects and other terrestrial arthropods are there on Earth? Annu. Rev. Entomol. 63: 31–45. [DOI] [PubMed] [Google Scholar]
- Varani G, and McClain W H. . 2000. The G x U wobble base pair. A fundamental building block of RNA structure crucial to RNA function in diverse biological systems. EMBO Rep. 1: 18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Liu J, Lin Z, and Wan X. . 2018. The complete mitochondrial genome of Odontolabis fallaciosa (Coleoptera: Lucanidae) with its phylogenetic implications. Zool. Syst. 43: 268–275. [Google Scholar]
- Wei L, He J, Jia X, Qi Q, Liang Z, Zheng H, Ping Y, Liu S, and Sun J. . 2014. Analysis of codon usage bias of mitochondrial genome in Bombyx mori and its relation to evolution. BMC Evol. Biol. 14: 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme D R. 1992. Animal mitochondrial DNA: structure and evolution. Int. Rev. Cytol. 141: 173–216. [DOI] [PubMed] [Google Scholar]
- Wu Y Y, Cao Y Y, Fang J, and Wan X. . 2016. The first complete mitochondrial genome of stag beetle from China, Prosopocoilus gracilis (Coleoptera, Lucanidae). Mitochondrial DNA A. DNA Mapp. Seq. Anal. 27: 2633–2634. [DOI] [PubMed] [Google Scholar]
- Xin Z -Z, Liu Y, Zhu X -Y, Wang Y, Zhang H-B, Zhang D-Z, Zhou C -L, Tang B -P, and Liu Q-N. . 2017. Mitochondrial genomes of two Bombycoidea insects and implications for their phylogeny. Sci. Rep. 7: 6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Zhang Y, Feng S, Liu L, and Li Z. . 2018. The first complete mitochondrial genome of the Japanese beetle Popillia japonica (Coleoptera: Scarabaeidae) and its phylogenetic implications for the superfamily Scarabaeoidea. Int. J. Biol. Macromol. 118: 1406–1413. [DOI] [PubMed] [Google Scholar]
- Yu F, and Liang A-P. . 2018. The Complete Mitochondrial Genome of Ugyops sp.(Hemiptera: Delphacidae). J. Insect Sci.. 18: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbino D R, and Birney E. . 2008. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 18: 821–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Nardi F, Hull-Sanders H, Wan X, and Liu Y. . 2014. The complete nucleotide sequence of the mitochondrial genome of Bactrocera minax (Diptera: Tephritidae). PLoS One. 9: e100558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q Y, Wang Y, Kong Y M, Luo D, Li X, and Hao P. . 2011. Optimizing de novo transcriptome assembly from short-read RNA-Seq data: a comparative study. BMC Bioinformatics. 12(Suppl 14): S2. [DOI] [PMC free article] [PubMed] [Google Scholar]