Abstract
This study reports the results of a molecular screening for Wolbachia (Wb) infection in Aedes albopictus (Skuse) populations recently established in the Yucatan Peninsula, Mexico. To do so, collections of free-flying adults with BG traps and emerged adults from eggs after ovitrap field collections were performed in three suburban localities of the city of Merida, Yucatan. Overall, local populations of Ae. albopictus present a natural Wb infection rate of ~40% (18 of 45). Wb infection was detected in both field-collected adults (76.5%, 13 of 17) and eggs reared (17.8%, 5 of 28) and in 37.9% (11/29) of females and 43.7% (7/16) of male Ae. albopictus mosquitoes. An initial screening for Wolbachia strain typing showed that native Ae. albopictus were naturally coinfected with both wAlbA and wAlbB strains. The knowledge of the prevalence and diversity of Wolbachia strains in local populations of Aedes mosquitoes is part of the baseline information required for current and future Wolbachia-based vector control approaches to be conducted in Mexico.
Keywords: Wolbachia, Aedes albopictus, Aedes aegypti, wAlbA, wAlbB
Aedes (Stegomyia) albopictus (Skuse), commonly known as the ‘Asian tiger mosquito’, is considered a secondary but competent vector of arboviruses of great importance in public health including dengue (DENV), chikungunya (CHIKV; Bonizzoni et al. 2013), and Zika (ZIKV; McKenzie et al. 2019). Although it is native to Asia, it has invaded and colonized many countries in the Americas, Europe, and Africa (Battaglia et al. 2016, Kraemer et al. 2019). In Mexico, Ae. albopictus has been recorded in 16 states including Mexico City, Guanajuato, Jalisco, Coahuila, Chiapas, Hidalgo, Morelos, Nuevo León, Oaxaca, San Luis Potosí, Sinaloa, Tabasco, Tamaulipas, Veracruz, Yucatán, and Quintana Roo (Villegas-Trejo et al. 2010, Salomón-Grajales et al. 2012, Ortega-Morales et al. 2018, Contreras-Perera et al. 2019, Dávalos-Becerril et al. 2019, González-Acosta et al. 2020), but its geographical distribution is expected to increase because the macro- and micro ecological conditions suitable for its establishment, particularly throughout South Mexico (Pech-May et al. 2016, Yañez-Arenas et al. 2018).
Wolbachia (Rickettsiaceae) (hereafter called as Wb) are ubiquitous obligate bacterial endosymbionts that naturally infect ~20% of all insect species (Hilgenboecker et al. 2008). Wb is maternally transmitted in insects, and it is known to exert a profound impact on host biology, mainly at the reproductive level. The most common reproductive effect is called cytoplasmic incompatibility (CI). Wb-induced CI modulates the production of viable eggs once an uninfected female mates a Wb-infected male, whereas Wb-infected females can successfully breed with either infected or uninfected males (Werren et al. 2008).
Wb naturally infects many mosquito species including Ae. albopictus but not the primary dengue vector Aedes aegypti (Ross et al. 2020). Wb introduced into Ae. aegypti via transinfection can cause early embryonic arrest (CI) and egg hatch failure (Mains et al. 2019). Interestingly, Wb can also cause pathogen interference in Ae. aegypti as Wb-infected mosquitoes are less susceptible to be infected or coinfected with important arboviruses such as DENV, CHIKV, ZIKV, Mayaro virus, and yellow fever virus (Moreira et al. 2009, Walker et al. 2011, Aliota et al. 2016, Pereira et al. 2018). This has led to inundative field-releases of Wb-infected mosquitoes as a potential control strategy for Ae. aegypti, either by population suppression (Crawford et al. 2020) or by population replacement (World Mosquito Program 2017, Nazni et al. 2019, Ryan et al. 2019).
In Mexico, both Wolbachia-based approaches, one using Wb strain wMel (Wolbachia pipientis from Drosophila melanogaster; Dutra et al. 2015, Nguyen et al. 2015) for population replacement in Baja California, and another using wAlbB (Wolbachia pipientis from Ae. albopictus; Xi et al. 2005) for population suppression in Yucatan, are under initial phases of implementation and evaluation for the control of Ae. aegypti. Data on natural infection frequency are critical to evaluate the potential of using Wolbachia as a vehicle to modify insect vector populations (Turelli and Hoffmann 1999, Kitrayapong et al. 2002). However, the prevalence and characteristics of Wolbachia in natural mosquito populations are yet poorly known in Mexico.
Aedes albopictus was recently reported in the periphery of Merida, the capital of the state of Yucatan, and the city with the largest population and major epidemiological importance in the Peninsula of Yucatan for the transmission of DENV, CHIKV, and ZIKV (Contreras-Perera et al. 2019). Aedes albopictus is very soon expected to invade and coexist with populations of Ae. aegypti in Merida. Thus, this study showed the molecular screening for Wolbachia infection of field-collected adult Ae. albopictus mosquitoes in the suburban areas of Merida, Yucatan.
Materials and Methods
Study Area
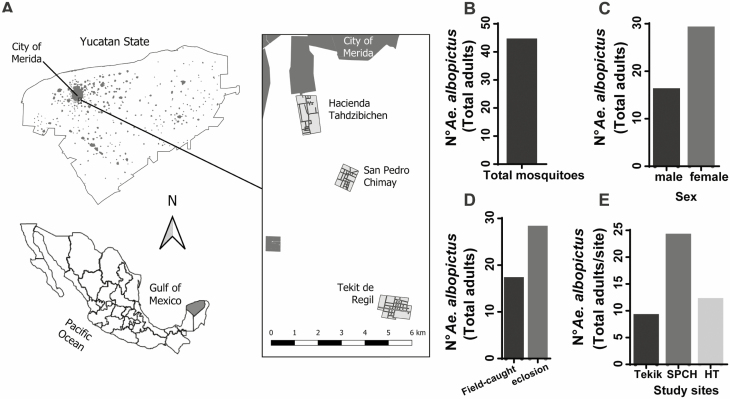
Field collections of local Aedes populations (adults and eggs) were performed every week from April to December of 2019 at San Pedro Chimay (20°51′55″N 89°34′46″O), Hacienda Tahdzibichen (20°53′06″N 89°35′52″O), and Tekik de Regil (hereafter Tekik; 20°48′59″N 89°33′39″O), all suburban areas in the periphery of the city of Merida in the Peninsula of Yucatan (southeast Mexico; Fig. 1A). Sociodemographic features of these localities include an average of 1,200 inhabitants per locality with an average of 6 households and 31 inhabitants per hectare (INEGI 2016). They share similar urban and ecological landscapes such as type of housing and share large vegetated backyards with vegetation (coverage > 60%). The average altitude of the localities is 9 m above sea level. The climate is mainly warm with an annual average temperature of 26°–27°C (36°C max–18°C min), relative humidity of 70–75%, and two distinct climate phases during the year: a rainy season, from May/June to October with a rainfall of 882.5 mm, and a dry season, from November to April with rainfall of 167.9 mm (INEGI 2017).
Fig. 1.
Study area: distribution and collection sites of Aedes albopictus in suburban areas of Merida, Yucatan. (A) Location of localities used for field-collection of Aedes albopictus adults and eggs. (B) Total of individual adult mosquitoes included in this study and their distribution by (C) sex, (D) collection method, and (E) locality.
Mosquito Collection and Rearing
Aedes adults were collected using outdoor BG-sentinel traps (20–30 traps per locality) for 24 h/periods, one per week), as part of the routine surveillance for control of Ae. aegypti in the suburban communities of Merida performed by the Collaborative Unit for Entomological Bioassays (UCBE) and the Universidad Autonoma de Yucatan (UADY). According to the CDC and other studies, BG-sentinel traps are currently the most used and the gold-standard adult traps for the sampling, monitoring, and surveillance of outdoor Aedes and Culex species in field trials (Li et al. 2016, CDC 2018). Collected specimens were transported to UCBE-UADY for their identification using standard taxonomic keys (Rueda 2004). As part of the vector control program protocol in Mexico, corroboration and validation of larvae and adult specimens of mosquitoes is supported by the National Reference Center at the Instituto de Diagnóstico y Referencia Epidemiológicos (InDRE) of the Ministry of Health in Mexico. Voucher specimens are deposited at the Colección Entomológica Regional (CER)—Universidad Autónoma de Yucatán (UADY). All Ae. albopictus were separated from Ae. aegypti, which is also present in the localities. Aedes eggs were collected using ovitraps (approximately one ovitrap per Ha) in each locality and served weekly. Paper strips with eggs were sent to the LCB-UADY for mosquito rearing following standard operating procedures of the LCB-UADY. Briefly, eggs were incubated for embryo development (48 h) at room temperature. Larvae were reared and fed 6% larvae feeding solution (Food product for tilapia [Biofinguerlin]: yeast powder [Pronat Ultra], 9:1, respectively) in plastic containers with water. Pupae obtained were maintained within BugDorm-1 Insect Rearing Cages. Adults emerged were maintained for 24 h under standard insectary conditions at 80 ± 5% humidity, 26 ± 1°C, and 12/12 light/dark cycle. Then, specimens were cold immobilized for taxonomic classification and stored at −20°C until further analyses.
DNA Extraction and PCR Screening for Wolbachia Infection
Total genomic DNA from individual adult mosquitoes was extracted using a Blood and Tissue DNEasy Kit (Qiagen, Hilden, Germany) following the manufacturer’s instructions with some in-house modifications. Briefly, individual adult mosquitoes were firstly disinfected inside sterile Eppendorf tubes (70% ethanol) at room temperature (2 h) and later mechanically homogenized using a sterile pestle and electric homogenizer after adding the lysis buffer from the DNA extraction kit (Qiagen). After elution, DNA was quantified using a nanodrop (Thermo Scientific) and stored at −20°C until further analyses. To detect Wolbachia infection in Aedes mosquitoes, an initial set of primers was used to specifically amplify the 16S rRNA from Wolbachia as previously described: 16-2F (5′-AGCTTCGAGTGAAACCAATTC-3′) and 16-2R (5′-GAAGATAATGACGGTACTCAC-3′; Simoes et al. 2011). An end point PCR protocol was performed using a Mastercycler EP Gradient-Thermal-Cycler (Eppendorf) and Taq DNA polymerase recombinant kit including PCR buffer (10×), MgCl2 (50 mM), dNTPs mix (10 mM), forward and reverse primers (10 µM), Taq DNA polymerase (5 U/µl), and extracted DNA template (100–200 ng per reaction). Amplification parameters were established as follows: initial denaturation at 95°C for 5 min; 35 cycles of denaturation at 95°C for 1 min, Tm annealing at 55°C for 1 min, and extension at 72°C for 1 min; final extension at 72°C for 3 min. Genomic DNA extracted from a local strain of Ae. aegypti infected with Wolbachia strain B (hereafter referred to wMIDB, F9, kindly donated by Dr. Xi, Michigan State University) as previously described (Xi et al. 2005) was used as PCR-positive control. On the other hand, DNA extracted from Wolbachia-free (Wb) Ae. aegypti strain collected from Yucatan (hereafter referred to wtMID, F4) was used as the negative control. Amplified DNA was visualized using agarose gel (1.5%) stained with Saber safe (Thermo Scientific) and UV excitation. The amplified 16S rRNA gene was approximately 1,000 bp.
Molecular Typing of Wolbachia Infection in Ae. albopictus
A set of samples that were positive using the 16-2 primers described above were additionally processed for typification of the Wolbachia strain in infected Ae. albopictus mosquitoes using two sets of primers previously described to specifically amplify the Wolbachia surface protein (wsp) gene from strains A and B (Zhou et al. 1998, Ahmad et al. 2017). Briefly, a PCR mixture was prepared to contain extracted DNA template (200 ng per reaction), PCR buffer (10×), MgCl2 (50 mM), dNTPs mix (10 mM), Taq DNA polymerase (5 U/µl), RNAse/DNAse-free water, and forward and reverse primers (10 µM) to amplify DNA genome from Wolbachia strain A and B described as follows: primers 328F (5′-CCAGCAGATACTATTGCG-3′) and 691R (5′-AAAAATTAAACGCTACTCCA-3′) for wAlbA strain, and primers 183F (5′-AAGGAACCGAAGTTCATG-3′) and 691R (describe above) for wAlbB. Amplification parameters were established as described above for 16-2 primers. DNA extracted from Ae. aegypti mosquitoes either Wb free (wtMID) or Wb strain B infected (wMIDB) was used as negative and positive controls for the PCR assay, respectively, following published protocols (Xi et al. 2005). These two sets of primers amplify a DNA fragment ranging from 400 to 600 bp depending on the individual Wb strain.
Results and Discussion
In total, 45 Ae. albopictus adult mosquitoes (64% female–36% male), among of which was 17 captured with BG-sentinel traps and 28 reared from field-collected eggs using ovitraps, were examined for Wb infection by PCR from the three localities (Fig. 1A–D). The trapping Ae. albopictus mosquitoes in both adult and egg stages provide further evidence of the early invasion of this mosquito species in the suburban areas of the municipality of Merida (Salomón-Grajales et al. 2012, Ortega-Morales et al. 2018, Contreras-Perera et al. 2019).
In this study, using a universal set of primer nucleotides to amplify a sequence of the Wb 16S rDNA gene (Table 1), we initially assessed the circulation of Wb in native Ae. albopictus mosquitoes from all the localities, either from field-caught and free-flying adults or those reared from eggs (Fig. 2). This initial PCR assay resulted in the amplification of approximately 1,000 base pairs long DNA fragment, identified as positive for Wb infection (Fig. 2A), as it has been previously reported (Simoes et al. 2011, Carvajal et al. 2019).
Table 1.
Primer nucleotide sequences used for amplification and typification of Wolbachia genome using DNA extracted from adult Aedes albopictus mosquitoes
| Primer name | Nucleotide sequence (5′–3′) | PCR product size (bp) | Wolbachia strain | Reference(s) |
|---|---|---|---|---|
| 16-2F | AGCTTCGAGTGAAACCAATTC | ~1,000 | Multistrain | Simoes et al. (2011) |
| 16-2R | GAAGATAATGACGGTACTCAC | |||
| 328F | CCAGCAGATACTATTGCG | ~300–400 | A | Zhou et al. (1998); Ahmad et al. (2017) |
| 691R | AAAAATTAAACG CTACTCCA | |||
| 183F | AAGGAACCGAAGTTCATG | ~500–600 | B | Zhou et al. (1998); Ahmad et al. (2017) |
| 691R | AAAAATTAAACG CTACTCCA |
Fig. 2.
Detection of Wolbachia infection in field-caught adult Aedes albopictus mosquitoes of the suburban areas of Merida, Yucatan. (A) PCR amplification of Wb DNA genome from total genomic DNA extracted from individual Ae. albopictus mosquitoes. A representative image showing a group of positive and negative samples (lanes 2–8). An amplicon of ~1,000 bp was considered positive for Wb infection (lanes: 4–9); positive control: genomic DNA from Aedes aegypti infected with Wb strain B as previously described (wMIDB; Xi et al. 2005; lane 9, female); negative control: genomic DNA from wild-type Ae. aegypti (Wb free) from Yucatan (wtMID) [lanes 10 (male)–11 (female)]; negative controls of PCR reaction mix without added genomic DNA (lanes 12–13). (B) Percentage of total Wb positive mosquitoes detected by PCR. (C) Sex distribution of Wb infection in Ae. albopictus. (D) Percentage of Wb infection detected in Ae. aegypti mosquitoes either adult field-caught or adult-reared under laboratory conditions from field-collected eggs. (E) Percentage of Wb infection and location of Wb-infected Aedes albopictus collected from the distinct localities in the suburban areas of Merida.
Overall Wb infection rate in Ae. albopictus from all the localities was of 40% (18/45; 37.9% of females, 43.7% of males; Table 2, Fig. 2A and B). Average Wb infection rate by locality was 13.3% and varied between localities (Table 2; Fig. 2E), with Tekik showing the highest percentage of Wb infection (>60%) of all mosquitoes tested in that site (33.3% males, 33.3% females; Fig. 2). The overall Wb infection rate (40%) found in this study lies between several field surveys around the world using field-collected specimens, which have found variable frequency rates of Wb infection ranging from 11 to 100% (Kitrayapong et al. 2002, de Albuquerque et al. 2011, Zhang et al. 2014, Noor Afizah et al. 2015, Ahmad et al. 2017, Guo et al. 2018, Martin et al. 2019, Hu et al. 2020).
Table 2.
Wolbachia infection of individual adult Aedes albopictus mosquitoes collected in distint suburban areas of Merida, Yucatan
| Study sites | Sample typea,b | Sex (no. of individuals) | Wolbachia infection (no. of PCR + individuals) | Wolbachia infection rate (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F | M | Total | F | M | Total | Fc | Md | Total& | ||
| Tekik | Free-flying adults from BG traps | 3 | 1 | 9 | 3 | 1 | 6 | 33.3 | 33.3 | 13.3 |
| Adults emerged from eggs | 0 | 5 | 0 | 2 | ||||||
| SPCH | Free-flying adults from BG traps | 6 | 1 | 24 | 4 | 1 | 8 | 16.6 | 16.6 | 17.8 |
| Adults emerged from eggs | 9 | 8 | 0 | 3 | ||||||
| HT | Free-flying adults from BG traps | 6 | 0 | 12 | 4 | 0 | 4 | 33.3 | 0.0 | 8.9 |
| Adults emerged from eggs | 5 | 1 | 0 | 0 | ||||||
| Total | 29 | 16 | 45 | 11 | 7 | 18 | 24.4 | 15.6 | 40 | |
F, female; M, male; Tekik, Tekik de Regil; SPCH, San Pedro Chimay; HT, Hacienda Tahdzibichen.
aField-collected free-flying adults (collected at BG traps).
bField-collected adults emerged from eggs (collected in the field with ovitraps).
cEstimated from Wolbachia positive females or males and the total of mosquitoes captured per locality.
dEstimated from total of Wolbachia positive (female/male) mosquitoes and the total of mosquitoes tested.
A 76.5% (13/17) of all adult mosquitoes directly captured in the field were positive for Wb infection. However, only few adults of Ae. albopictus reared from field-collected eggs (17.8%, 5/28) showed the presence of Wb genome. Wb are intracellular bacterium that can be vertically transmitted from infected females to their offspring (transovarial transmission) and considered its primary mode of dissemination within host species (Werren et al. 2008). Particularly in eggs, the density of Wb infection can be affected under certain environmental circumstances such as high temperature or prolonged light exposure, which reduced the ability of Wb to invade and persist in the mosquito populations (Ross et al. 2019). Nevertheless, our results showed that vertical transmission of Wb occurs in wild populations of Ae. albopictus in the suburban areas of Merida.
Although the natural infection of wild Ae. albopictus with Wb was expected, so far only one single report exists describing the circulation of Wb in mosquitoes of Mexico. Roblero-Andrade et al. (2019) reported Wb infections from free-flying Ae. albopictus populations collected from cemeteries in Chiapas, Southeast of Mexico. In total, 42% (135/343) of females collected were positive for Wb infection; however, only one male mosquito was tested in the study and this was Wb negative. Additionally, large variability in the infection rates (7.7%–100%) was also observed and no identification of the infecting Wb strain was performed (Roblero-Andrade et al. 2019). Our study in Yucatan we found similar rates of overall Wb infection in total adults Ae. albopictus (40%) and total females (37.9%) screened; and we provide information on the Wb infection rate of male Ae. albopictus mosquitoes for the first time in Mexico.
Naturally occurring populations of Ae albopictus can be single-infected or coinfected with two types of Wb strains known as wAlbA (supergroup A) and wAlbB (supergroup B; Werren et al. 1995, Zhou et al. 1998). Here, a set of five mosquito samples were further analyzed by PCR to amplify a segment of the wsp gene to determine which strain of Wb was circulating in these populations of Ae. albopictus of Yucatan. We identified strains A and B as the infecting Wb endosymbiont of Ae. albopictus (Fig. 3A and B). All samples analyzed showed an amplification product with a molecular size lower than 400 bp corresponding to Wb strain A (Fig. 3A, lanes 2–6), and four of these samples (Fig. 3B, lanes 2–5) showed an amplification fragment for Wb strain B (approx. 500 bp). These results indicate that coinfection with Wb strains A and B occurs in Ae. albopictus of Yucatan.
Fig. 3.
Molecular characterization of Wolbachia strains infecting adult Aedes albopictus mosquitoes of the suburban areas of Merida, Yucatan. PCR amplification of Wb DNA genome from total genomic DNA extracted from individual Ae. albopictus mosquitoes using two set of primers specific for Wb strain A (A) and B (B). A representative image showing a group of five positive samples (lanes 2–7). An amplicon of 400 and 500 bp was considered positive for Wb infection with strain A and B, respectively; positive control: genomic DNA from Aedes aegypti infected with Wb strain B (wMIDB; Xi et al. 2005; lane 8); negative control: genomic DNA from wild-type Ae. aegypti (Wb free) from Yucatan (wtMID; lane 9). DNA marker of 100 bp (lanes 1 and 10).
As expected, the DNA used as positive control obtained from Ae. aegypti previously infected with Wb strain B (wMIDB) showed an amplification fragment for wAlbB, but not wAlbA (Fig. 3A and B, lane 8). No amplicon was obtained when genomic DNA obtained from the native Ae. aegypti strain (wtMID) was used (Fig. 2A, lanes 10–11). The lack of any amplification product confirmed that the native populations of Ae. aegypti mosquitoes in the localities included in the study are not hosting Wb strains.
Here, this study provides evidence of the circulation of Wb strains A and B in native populations of Ae. albopictus of Mexico. Further analyses using a bigger set of regionally/nationally field-collected mosquitoes, and the surveillance of Wb prevalence, as well as advanced molecular tools such as the multilocus sequence typing system or deep sequencing analyses of distinct Wb genes will help to better characterize the Wb strains that circulate in the populations of Aedes mosquitoes from Yucatan and Mexico. A better understanding of the distribution and diversity of Wolbachia in Mexico is valuable, as the infection of native populations of Ae. albopictus with Wolbachia can limit some arboviral infections, and it is also useful for the establishment of biological banks of locally occurring Wb strains that can be evaluated for the control of Aedes-transmitted diseases. Knowing the natural occurrence, infection frequency, and types of native Wb strains infecting both female and male Ae. albopictus—and other mosquito species—will provide a foundation for the design, implementation, and evaluation of Wolbachia-based interventions to control Aedes mosquitoes and ultimately, dengue, chikungunya, and Zika in Mexico.
Acknowledgments
We acknowledge the field-surveillance team at UCBE for species determination and mosquito sampling. We also like to thank Dr. Zhiyong Xi at Michigan State University and Sun Yat-sen University for kindly donating Aedes aegypti mosquitoes artificially infected with the Wolbachia strain B, used as a positive control in the molecular characterization and validation of Wolbachia infection in Aedes albopictus of Yucatan by PCR. Abdiel Martin-Park is supported by the Catedras-CONACYT program. Research funding was provided by Fondo Mixto CONACyT (Mexico)–Gobierno del Estado de Yucatan (Project YUC-2017-03-01-556) and USAID (Project AID-OAA-F-16-00082).
Contributor Information
UCBE-LCB Team:
Jorge Palacio-Vargas, Javier Pérez-Ojeda, Juan Navarrete-Carballo, Wilbert Bibiano Marin, and Anuar Medina Barreiro
Author Contributions
Collection, taxonomic classification, maintenance, and rearing of mosquitoes: A.M.P., A.C.M., Y.C.P., S.P.C., and UCBE-LCB team. Conceived and designed the experiments: H.P.G., A.M.P., Y.C.P., S.P., and P.M.S. Performed the experiments: H.P.G., S.P.C., A.M.P., Y.C.P. Analyzed the data: H.P.G., A.M.P., Y.C.P., S.P.C., A.C.M., and the UCBE-LCB team. Contributed reagents/materials/analysis tools: P.M.S., G.T.A., and G.V.P.. Wrote the paper: H.P.G., A.M.P., Y.C.P., A.C.M., and P.M.S. The following names represent the active members of the UCBE-LCB team at UADY that significantly contributed to the development of this study: Jorge Palacio-Vargas (also at the Secretary of Health, Yucatan), Javier Pérez-Ojeda, Juan Navarrete-Carballo, Wilbert Bibiano Marin, and Anuar Medina Barreiro.
Conflict of Interest
The authors declare no conflicts of interest.
References Cited
- Ahmad N A, Vythilingam I, Lim Y A L, Zabari N Z A M, and Lee H L. . 2017. Detection of Wolbachia in Aedes Albopictus and their effects on chikungunya virus. Am. J. Trop. Med. Hyg. 96: 148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliota M T, Peinado S A, Velez I D, and Osorio J E. . 2016. The wMel strain of Wolbachia reduces transmission of Zika virus by Aedes aegypti. Sci. Rep. 6: 28792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia V, Gabrieli P, Brandini S, Capodiferro M R, Javier P A, Chen X G, Achilli A, Semino O, Gomulski L M, Malacrida A R, . et al. 2016. The worldwide spread of the tiger mosquito as revealed by mitogenome haplogroup diversity. Front. Genet. 7: 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonizzoni M, Gasperi G, Chen X, and James A A. . 2013. The invasive mosquito species Aedes albopictus: current knowledge and future perspectives. Trends Parasitol. 29: 460–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvajal T M, Hashimoto K, Harnandika R K, Amalin D M, and Watanabe K. . 2019. Detection of Wolbachia in field-collected Aedes aegypti mosquitoes in metropolitan Manila, Philippines. Parasit. Vectors 12: 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. 2018. Surveillance and Control of Aedes aegypti and Aedes albopictus in the United States, pp. 1–16. In CDC (ed.). CDC website. [Google Scholar]
- Contreras-Perera Y J, Briceño-Mendez M, Flores-Suárez A E, Manrique-Saide P, Palacio-Vargas J A, Huerta-Jimenez H, and Martin-Park A. . 2019. New record of Aedes albopictus in a suburban area of Merida, Yucatan, Mexico. J. Am. Mosq. Control Assoc. 35: 210–213. [DOI] [PubMed] [Google Scholar]
- Crawford J E, Clarke D W, Criswell V, Desnoyer M, Cornel D, Deegan B, Gong K, Hopkins K C, Howell P, Hyde J S, . et al. 2020. Efficient production of male Wolbachia-infected Aedes aegypti mosquitoes enables large-scale suppression of wild populations. Nat. Biotechnol. 38: 482–492. [DOI] [PubMed] [Google Scholar]
- Dávalos-Becerril E, Correa-Morales F, González-Acosta C, Santos-Luna R, Peralta-Rodríguez J, Pérez-Rentería C, Ordoñez-Álvarez J, Huerta H, Carmona-Perez M, Díaz-Quiñonez J A, . et al. 2019. Urban and semi-urban mosquitoes of Mexico City: a risk for endemic mosquito-borne disease transmission. PLoS One 14: e0212987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutra H L, Dos Santos L M, Caragata E P, Silva J B, Villela D A, Maciel-de-Freitas R, and Moreira L A. . 2015. From lab to field: the influence of urban landscapes on the invasive potential of Wolbachia in Brazilian Aedes aegypti mosquitoes. PLoS Negl. Trop. Dis. 9: e0003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Albuquerque A L, Magalhães T, and Ayres C F. . 2011. High prevalence and lack of diversity of Wolbachia pipientis in Aedes albopictus populations from Northeast Brazil. Mem. Inst. Oswaldo Cruz 106: 773–776. [DOI] [PubMed] [Google Scholar]
- González-Acosta M, Marín F, Puliafito B, Bonifaci N, Fernández A, Navarro M, Salvador H, Balaguer F, Iglesias S, Velasco A, . et al. 2020. High-sensitivity microsatellite instability assessment for the detection of mismatch repair defects in normal tissue of biallelic germline mismatch repair mutation carriers. J. Med. Genet. 57: 269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Song Z, Luo L, Wang Q, Zhou G, Yang D, Zhong D, and Zheng X. . 2018. Molecular evidence for new sympatric cryptic species of Aedes albopictus (Diptera: Culicidae) in China: a new threat from Aedes albopictus subgroup? Parasit. Vectors 11: 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgenboecker K, Hammerstein P, Schlattmann P, Telschow A, and Werren J H. . 2008. How many species are infected with Wolbachia? A statistical analysis of current data. FEMS Microbiol. Lett. 281: 215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Xi Z, Liu X, Wang J, Guo Y, Ren D, Wu H, Wang X, Chen B, and Liu Q. . 2020. Identification and molecular characterization of Wolbachia strains in natural populations of Aedes albopictus in China. Parasit. Vectors 13: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INEGI 2016. Panorama sociodemográfico de Yucatán 2015. Panorama sociodemográfico 2015. Instituto Nacional de Estadistica y Geografia. INEGI, c2016, Mexico. 237p. [Google Scholar]
- INEGI 2017. Anuario estadístico y geográfico de Yucatán 2017. Anuario estadístico y geográfico. Instituto Nacional de Estadistica y Geografia. INEGI, c2017, Mexico. 708p. [Google Scholar]
- Kitrayapong P, Baimai V, and O’Neill S L. . 2002. Field prevalence of Wolbachia in the mosquito vector Aedes albopictus. Am. J. Trop. Med. Hyg. 66: 108–111. [DOI] [PubMed] [Google Scholar]
- Kraemer M U G, Reiner R C Jr., Brady O J, Messina J P, Gilbert M, Pigott D M, Yi D, Johnson K, Earl L, Marczak L B, . et al. 2019. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat. Microbiol. 4: 854–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Su X, Zhou G, Zhang H, Puthiyakunnon S, Shuai S, Cai S, Gu J, Zhou X, Yan G, . et al. 2016. Comparative evaluation of the efficiency of the BG-Sentinel trap, CDC light trap and mosquito-oviposition trap for the surveillance of vector mosquitoes. Parasit. Vectors 9: 446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mains J W, Kelly P H, Dobson K L, Petrie W D, and Dobson S L. . 2019. Localized control of Aedes aegypti (Diptera: Culicidae) in Miami, FL, via inundative releases of Wolbachia-infected male mosquitoes. J. Med. Entomol. 56: 1296–1303. [DOI] [PubMed] [Google Scholar]
- Martin E, Borucki M K, Thissen J, Garcia-Luna S, Hwang M, Wise de Valdez M, Jaing C J, Hamer G L, and Frank M. . 2019. Mosquito-borne viruses and insect-specific viruses revealed in field-collected mosquitoes by a monitoring tool adapted from a microbial detection array. Appl. Environ. Microbiol. 85: e01202-01219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie B A, Wilson A E, and Zohdy S. . 2019. Aedes albopictus is a competent vector of Zika virus: a meta-analysis. PLoS One 14: e0216794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira L A, Iturbe-Ormaetxe I, Jeffery J A, Lu G, Pyke A T, Hedges L M, Rocha B C, Hall-Mendelin S, Day A, Riegler M, . et al. 2009. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell 139: 1268–1278. [DOI] [PubMed] [Google Scholar]
- Nazni W A, Hoffmann A A, NoorAfizah A, Cheong Y L, Mancini M V, Golding N, Kamarul G M R, Arif M A K, Thohir H, NurSyamimi H, . et al. 2019. Establishment of Wolbachia strain walbb in malaysian populations of Aedes aegypti for dengue control. Curr. Biol. 29: 4241–4248.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T H, Nguyen H L, Nguyen T Y, Vu S N, Tran N D, Le T N, Vien Q M, Bui T C, Le H T, Kutcher S, . et al. 2015. Field evaluation of the establishment potential of wMelPop Wolbachia in Australia and Vietnam for dengue control. Parasit. Vectors 8: 563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noor Afizah A, Roziah A, Nazni W A, and Lee H L. . 2015. Detection of Wolbachia from field collected Aedes albopictus Skuse in Malaysia. Indian J. Med. Res. 142: 205–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Morales A I, Bond G, Méndez-López R, Garza-Hernández J A, Hernández-Triana L M, and Casas-Martínez M. . 2018. First record of invasive mosquito Aedes albopictus in Tabasco and Yucatan, Mexico. J. Am. Mosq. Control Assoc. 34: 120–123. [DOI] [PubMed] [Google Scholar]
- Pech-May A, Moo-Llanes D A, Puerto-Avila M B, Casas M, Danis-Lozano R, Ponce G, Tun-Ku E, Pinto-Castillo J F, Villegas A, Ibáñez-Piñon C R, . et al. 2016. Population genetics and ecological niche of invasive Aedes albopictus in Mexico. Acta Trop. 157: 30–41. [DOI] [PubMed] [Google Scholar]
- Pereira T N, Rocha M N, Sucupira P H F, Carvalho F D, and Moreira L A. . 2018. Wolbachia significantly impacts the vector competence of Aedes aegypti for Mayaro virus. Sci. Rep. 8: 6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roblero-Andrade A E, Rosales-Ramírez G, Torres-Monzón J A, López-Ordóñez T, Avendaño-Rabiell A, and Casas-Martínez M. . 2019. Distribución de la infección por Wolbachia sp. en mosquitos de cementerios del sur de Chiapas, México. Entomol. Mex. 6: 484–489. [Google Scholar]
- Ross P A, Ritchie S A, Axford J K, and Hoffmann A A. . 2019. Loss of cytoplasmic incompatibility in Wolbachia-infected Aedes aegypti under field conditions. PLoS Negl. Trop. Dis. 13: e0007357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross P A, Callahan A G, Yang Q, Jasper M, Arif M A K, Afizah A N, Nazni W A, and Hoffmann A A. . 2020. An elusive endosymbiont: does Wolbachia occur naturally in Aedes aegypti? Ecol. Evol. 10: 1581–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda L. 2004. Pictorial keys for the identification of mosquitoes (Diptera: Culicidae) associated with Dengue Virus Transmission. Zootaxa 589: 60. [Google Scholar]
- Ryan P A, Turley A P, Wilson G, Hurst T P, Retzki K, Brown-Kenyon J, Hodgson L, Kenny N, Cook H, Montgomery B L, . et al. 2019. Establishment of wMel Wolbachia in Aedes aegypti mosquitoes and reduction of local dengue transmission in Cairns and surrounding locations in northern Queensland, Australia. Gates Open Res. 3: 1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomón-Grajales J, Lugo-Moguel G V, Tinal-Gordillo V R, de La Cruz-Velázquez J, Beaty B J, Eisen L, Lozano-Fuentes S, Moore C G, and García-Rejón J E. . 2012. Aedes albopictus mosquitoes, Yucatan Peninsula, Mexico. Emerg. Infect. Dis. 18: 525–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoes P M, Mialdea G, Reiss D, Sagot M F, and Charlat S. . 2011. Wolbachia detection: an assessment of standard PCR protocols. Mol. Ecol. Resour. 11: 567–572. [DOI] [PubMed] [Google Scholar]
- Turelli M, and Hoffmann A A. . 1999. Microbe-induced cytoplasmic incompatibility as a mechanism for introducing transgenes into arthropod populations. Insect Mol. Biol. 8: 243–255. [DOI] [PubMed] [Google Scholar]
- Villegas-Trejo A, Manrique-Saide P, Che-Mendoza A, Cruz-Canto W, Fernandez M G, González-Acosta C, Dzul-Manzanilla F, Huerta H, and Arredondo-Jiménez J I. . 2010. First report of Aedes albopictus and other mosquito species in Morelos, Mexico. J. Am. Mosq. Control Assoc. 26: 321–323. [DOI] [PubMed] [Google Scholar]
- Walker T, Johnson P H, Moreira L A, Iturbe-Ormaetxe I, Frentiu F D, McMeniman C J, Leong Y S, Dong Y, Axford J, Kriesner P, . et al. 2011. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature 476: 450–453. [DOI] [PubMed] [Google Scholar]
- Werren J H, Zhang W, and Guo L R. . 1995. Evolution and phylogeny of Wolbachia: reproductive parasites of arthropods. Proc. Biol. Sci. 261: 55–63. [DOI] [PubMed] [Google Scholar]
- Werren J H, Baldo L, and Clark M E. . 2008. Wolbachia: master manipulators of invertebrate biology. Nat. Rev. Microbiol. 6: 741–751. [DOI] [PubMed] [Google Scholar]
- World Mosquito Program 2017. World mosquito program—eliminate dengue. https://www.worldmosquitoprogram.org/en/global-progress/mexico [Google Scholar]
- Xi Z, Khoo C C, and Dobson S L. . 2005. Wolbachia establishment and invasion in an Aedes aegypti laboratory population. Science 310: 326–328. [DOI] [PubMed] [Google Scholar]
- Yañez-Arenas C, Rioja-Nieto R, Martín G A, Dzul-Manzanilla F, Chiappa-Carrara X, Buenfil-Ávila A, Manrique-Saide P, Correa-Morales F, Díaz-Quiñónez J A, Pérez-Rentería C, . et al. 2018. Characterizing environmental suitability of Aedes albopictus (Diptera: Culicidae) in Mexico based on regional and global niche models. J. Med. Entomol. 55: 69–77. [DOI] [PubMed] [Google Scholar]
- Zhang D, Zhan X, Wu X, Yang X, Liang G, Zheng Z, Li Z, Wu Y, and Zheng X. . 2014. A field survey for Wolbachia and phage WO infections of Aedes albopictus in Guangzhou City, China. Parasitol. Res. 113: 399–404. [DOI] [PubMed] [Google Scholar]
- Zhou W, Rousset F, and O’Neil S. . 1998. Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. Proc. Biol. Sci. 265: 509–515. [DOI] [PMC free article] [PubMed] [Google Scholar]