Abstract
The present study dealt with the influence of temperature and feed on the nutritional value of Tenebrio molitor, especially on the content of crude protein, amino acids, fat, and fatty acid profile. Tenebrio molitor larvae were kept in 15, 20, and 25°C and fed with wheat bran, lentil flour, and mixture. The parameters were analyzed by international standard methods. Generally, with an increase of the lentils in the feed, the crude protein content increased. The changes in the temperature and the feed were most pronounced on the essential amino acids Val, Arg, and Leu. The highest average fat content was determined at 20°C in insects fed with wheat bran. The lowest fat content was determined at 15°C in bran-fed insects. The dependency of fat content on the temperature in feeding with lentil flour and a mixture of wheat bran and lentil flour was statistically insignificant (P > 0.05, Kruskal–Wallis, Mann–Whitney post hoc tests). The highest content of polyene fatty acids was achieved at a rearing temperature of 15°C and the bran diet. It was concluded that a higher proportion of protein diet could increase the content of crude protein in the insects. An increase in the temperature generally leads only to a slight increase in the content of nitrogenous substances. The influence of feed on this nutritional parameter is therefore much more significant than the effect of the rearing temperature. In general, it can be stated that the feed and the temperature also significantly affect the fat content.
Keywords: lentil flour, nutritional value, mealworm, temperature, wheat bran
Edible insects, especially those with a short life cycle and a high reproduction rate, are generally considered to be ecologically friendly food alternatives (Mlček et al. 2014). Because insects are poikilothermic (ectothermic) animals that do not require energy for thermoregulation, their feed conversion rate can be very high. Nutritional benefits include, for example, the higher content of polyunsaturated fatty acid (PUFA) together with the same protein content in comparison to common meat (van Huis 2016). Edible insects in general also fulfill the request of WHO for human nutrition, thankfully attributed to the suitable composition of essential amino acids (Rumpold and Schlüter 2013). However, nutritional properties depend on food and other living conditions. The nutritional value of insects is therefore not chemically constant.
The larvae of Tenebrio molitor (T. molitor) (Coleoptera: Tenebrionidae), known as mealworm, has long been used as food for insectivores worldwide (Józefiak et al. 2018, Sogari et al. 2019) and therefore its breeding is well-described. The advantage of breeding is unpretentiousness and short life cycle (Yi et al. 2013). In the adult stage, the mealworm is a black beetle growing to a size of 12–15 mm, which is harmful in flour warehouses, households, and small food establishments because of ingestion of various starch-containing substrates (Wang et al. 2012). When used as food, it is especially valued for proteins and amino acids, especially in areas with lack of conventional protein sources (Defoliart 1992, Ramos-Elorduy et al. 2011, van Broekhoven et al. 2015).
The T. molitor is recommended to be reared in the temperature range of 25–27.5°C evidenced in the literature (Grau et al. 2017). For the analysis in research, Ruschioni et al. (2020) kept the T. molitor in a similar temperature of 28 ± 1°C and at a RH of 60 ± 5%. The same temperature of 28°C in a room where the rearing chamber was located was used in research by Li et al. (2015). Studies of Kim et al. (2015) and Xu et al. (2012) stated the changes in rearing groups in dependency on the rearing temperature. van Broekhoven et al. (2015) described in their study the statistically significant difference of the nutritional values between the individual species with the same feed and rearing temperature. There was a difference in protein, fat content, and amino acid profile. Some other studies (Oonincx and van der Poel 2011, Oonincx et al. 2015, Dreassi et al. 2017) described the difference of nutritional values in the same species and same rearing temperature, but with different feed. Mancini et al. (2019) employed five different former foodstuff products as feeding substrates, and it was ascertained that T. molitor could be practically reared on these substrates to achieve higher nutritional values. Another feeding substrate has been studied by Ruschioni et al. (2020). They used 25% of olive pomace and 75% of wheat middlings as the best compromise between growth performance and nutritional properties. The larvae fed with this feed were shown to have the highest protein content, dry matter yield, and essential amino acids/nonessential amino acids ratio.
Though different studies suggest that there is a dependency of the nutritional values on the rearing temperature, still these studies do not state the exact dependency in full context.
It is presumed that with the right choice of temperature and feed (diet) it is possible to influence the nutritional value of insect. The present study deals with the influence of temperature and feed on the nutritional values and expands knowledge on this topic. The originality of this work lies in a broad description and characterization of four different basic nutritional values depending on the combination of selected breeding temperatures (15, 20, and 25°C) and two types of feed (wheat bran and lentil flour) in three composition ratios. The feed for the experimental groups was chosen purposefully, considering the availability on site, price, and especially the impact on the nutrition composition suitable for human (Bednářová et al. 2013).
Materials and Methods
Tenebrio molitor larvae were used for the analysis. Samples were bought from a company in Brno in the Czech Republic and rearing was done at the university in Brno for 4 wk in the plastic boxes with the size (600 × 400 × 125) mm3, at 55 ± 10% RH at three different temperatures of 15, 20, and 25°C. The insect was divided into three experimental groups. The first group was fed only by wheat bran, the second only by lentil flour, and the third group was fed by a mixture of 50% wheat bran and 50% lentil flour. For each experimental group, the feed was weighed, and the weight of the feed was the same for all groups. Nutrition values of the feed were reported in per 100 g.
The crude energy value of the wheat bran is as follows: 1,210 kJ/292 kcal, fat 5.3 g, of which saturated fatty acids were 0.88 g, carbohydrates 24.9 g, of which sugars were 2.2 g, fiber 40.2 g, protein 16.2 g, salt 0.1 g. The producer was Life s.r.o., Beroun, the Czech Republic.
The energy value of lentil flour is stated as follows: 1,250 kJ/298 kcal, protein 24.1 g, fats 2.0 g, of which saturated fatty acids were 0.5 g, carbohydrates 49.6 g, of which sugars were 2.2 g, fiber 11.4 g, salt 6.7 mg. The producer was Extrudo Bečice s.r.o., Bečice, the Czech Republic.
Before analysis, the samples were processed as follows: T. molitor larvae in ultimate and penultimate instar development (with a full body length just before pupation) were taken from the rearing. Next step was starving for 48 h, followed by killing with boiling water at 100°C and drying at 105°C. The samples (DM—dried material) prepared in this way were homogenized and stored in a cooling box at 4–7°C until analysis. Samples for excrement analysis were taken at 20°C, homogenized and stored at 4–7°C until analysis.
At the end of the experiment, the substrate with excrements was taken from the rearing boxes. These samples were homogenized and stored at 4–7°C until analysis.
The total content of protein was determined using the Kjeldahl method (ISO 1871:2009). For measurement, 1 g dried homogenized material from T. molitor larvae in ultimate and penultimate instar development, from feeds and excrements was used for each analysis. Samples were mineralized at 420°C for 105 min. Distillation was done using Kjeltec 2200 (FOSS, Hilleroed, Denmark) for 4 min. The amount of crude protein was calculated by multiplying the content of detected nitrogen by a coefficient of 6.25. The conversion factor was selected according to valid Regulation (EU) No. 1169/2011, Annex I. The crude protein content reported in [g kg−1] was measured four times.
A fat content determination was done using the Soxhlet method of extraction (Soxhlet 1871) using Gerhardt Soxtherm SOX414 (C. Gerhardt GmbH & Co. KG, Königswinter, Germany). Five grams of dried and homogenized samples (with the accuracy of 0.0001 g) was put into extraction shell and extracted with 150 ml of petroleum ether (chosen program: 70°C for 120 min). Extracted sample was then dried at 103°C and repeatedly weighted till a constant weight was obtained (the difference between two consecutive weighings less than 10 mg). The fat content reported in [g kg−1] was measured three times.
Fatty acid methyl esters (FAMEs) in analyzed samples were analyzed using gas chromatography with flame ionization detector (FID) on GC-2010 (Shimadzu, Kyoto, Japan) using highly polar chromatographic column HP-88 (100 m × 0.25 mm, 0.2 μm; Agilent Technologies, Santa Clara, CA), which is destined to identify cis/trans methyl esters of fatty acids. Chromatographic conditions were as follows: spray volume 1 μl, spray temperature 250°C, split ratio 1:100, carrier gas nitrogen, temperature program 80°C/5 min, 200°C/30 min, 250°C/15 min.
Quantitative evaluation of FAME content in the samples was done by using the method of inner normalization to content of inner standard—undecanoic acid methyl ester (Sigma-Aldrich, St. Louis, MO) using FAME Mixture C4-C24 (Supelco Inc. Bellefonte, PA) standard, which contained 37 selected fatty acids. The representation of individual fatty acids was recalculated to a percentage of the total methyl esters present. The fatty acid profile was measured four times. For measurement, the dried homogenized material from T. molitor larvae in ultimate and penultimate instar development, from feeds and excrements prepared by the above methodology was used for the analysis.
Acidic hydrolysis of samples was used as the first step for the determination of aspartic acid (Asp), threonine (Thr), serine (Ser), glutamic acid (Glu), proline (Pro), glycine (Gly), alanine (Ala), valine (Val), isoleucine (Ile), leucine (Leu), tyrosine (Tyr), phenylalanine (Phe), histidine (His), lysine (Lys), and arginine (Arg). The acidic hydrolysis of samples and determination of amino acids were performed in accordance with Mišurcová et al. (2014). Amino acids were determined and identified by using ion-exchange chromatography by the AAA400 analyzer (Ingos, Prague, the Czech Republic) with post-column ninhydrin derivation and spectrophotometric detection (wavelength 440 nm for proline, 570 nm for the other amino acids). Samples were measured four times. For measurement, 0.5 g dried homogenized material from T. molitor larvae in ultimate and penultimate instar development, from feeds and excrements was used for each analysis.
The results were expressed by the average (M) ± standard error (SE) in program Excel 2013 (Microsoft Corporation, Redmond, WA). Possibilities of program STATISTICA CZ version 12 (TIBCO Software Inc., Palo Alto, CA) were used for next data analysis. First, a Shapiro–Wilk normality test (α = 0.05) was performed on all measured data groups. Subsequently, the data groups were grouped according to observing a factor affecting properties at a constant value of another factor affecting the properties as well (e.g., a data group was created where the feed was changed but the temperature was constant at 15°C).
Homogeneity of variances in these groups was tested using the Levene’s test of homogeneity of variances (α = 0.05) and also the Brown–Forsythe test of homogeneity of variances (α = 0.05). If the preconditions were not met for any of the tests to verify the normality or homogeneity of the variances of the data sets (even for only one group of data), the calculation of the Kruskal–Wallis test (α = 0.05) was continued for the whole given measurement of a certain substance. Comparison of statistical differences between groups of data in individual groups was performed using Mann–Whitney post hoc test (α = 0.05). The results of the parametric ANOVA test (α = 0.05) and subsequent parametric post hoc tests performed on some data groups are not described in the present article, as only some groups and some comparisons met the tests of the ANOVA assumptions and the predictive level between samples were not comparable.
Results
Crude Protein Determination
For each experimental group, the crude protein content in DM was evaluated and the results are shown in Fig. 1. The crude protein content in general increases with growing temperature. In groups fed by wheat bran, the crude protein content was, on average, the lowest (apart from 25°C group) in comparison to the groups fed by different fodder, and statistically significant dependency on temperature was detected (H (2, N = 12) = 8.00; P = 0.02). The crude protein content rose by 8%. The influence of the growing temperature on crude protein content while using lentil flour and the mixture of wheat bran and lentil flour as feed was not evaluated as statistically significant (H (2, N = 12) = 4.31; P = 0.12; and H (2, N = 12) = 0.81; P = 0.67). In the group fed by the mixture of 50% lentil flour and 50% wheat bran, the values were approximately in the middle of the crude protein content range.
Fig. 1.
Dependency of the crude protein content in T. molitor on the temperature and feed. The error bars indicate standard errors. The ‘*’ on the column indicates a significant difference (P < 0.05, Kruskal–Wallis test, Mann–Whitney post hoc tests) for constant temperature. The ‘+’ on the column indicates a significant difference (P < 0.05, Kruskal–Wallis test, Mann–Whitney post hoc tests) for constant feed. The crude protein content was measured four times.
In general, with an increase in the lentils in the feed the content of the crude protein increases. In the groups kept at 15 and 20°C, a statistically significant difference was observed (H (2, N = 12) = 9.85; P = 0.01; and H (2, N = 12) = 9.84; P = 0.01).
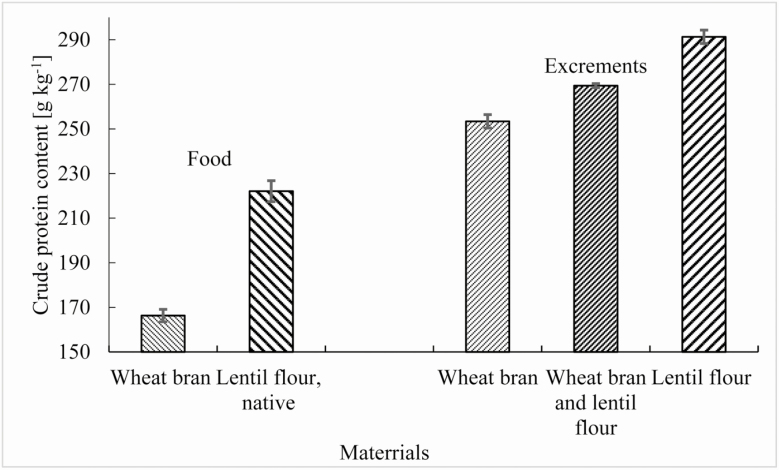
Besides the T. molitor larvae analysis, analyses of feed and excrements are shown in Fig. 2. As the exact conversion factor for feed and excrement was not known, a general conversion factor of 6.25 was used. Therefore, the observed values of the crude protein may not correspond to reality and cannot be compared with the values found in larvae. However, the values can be used to compare excrement with each other. There was a statistically significant difference (H (2, N = 12) = 9.85; P = 0.01) between a group with wheat bran feed and a group with lentil flour feed.
Fig. 2.
The crude protein content in dried material in feed and excrements (average ± standard error). The crude protein content was measured four times.
Determination of Amino Acid Content
The representation of each of the monitored amino acids in the analyzed T. molitor (in DM) is shown in Tables 1 and 2. A statistically significant difference (P < 0.05) between the content of individual amino acids in dependency on the feed at constant temperature is shown in Table 3. A statistically significant difference (P < 0.05) between the content of individual amino acids in dependency on the temperature using the same feed is shown in Table 4.
Table 1.
Representation of individual content of amino acids (average ± standard error) in dried material in T. molitor in the dependency on the feed and temperature: first part
| Sample type | Temp | Feed used | Amino acid [g kg−1] | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ALA | ARG | ASP | CYS | GLU | GLY | HIS | ILE | LEU | |||
| Feed | WB | 6.9 ± 0.2 | 12.2 ± 0.2 | 12.0 ± 0.3 | 3.7 ± 0.1 | 28.6 ± 0.8 | 7.9 ± 0.1 | 4.3 ± 0.1 | 5.0 ± 0.1 | 9.2 ± 0.2 | |
| Feed | LF | 7.5 ± 0.2 | 14.3 ± 0.4 | 23.6 ± 0.3 | 2.5 ± 0.1 | 31.2 ± 0.4 | 7.3 ± 0.1 | 4.4 ± 0.1 | 7.6 ± 0.1 | 13.5 ± 0.2 | |
| Excrements | WB | 3.0 ± 0.1 | 3.1 ± 0.1 | 6.3 ± 0.1 | 2.6 ± 0.1 | 6.0 ± 0.1 | 9.9 ± 0.2 | 4.2 ± 0.1 | 2.2 ± 0.1 | 3.9 ± 0.1 | |
| Excrements | LF | 8.0 ± 0.1 | 16.3 ± 0.1 | 25.4 ± 0.3 | 2.1 ± 0.1 | 33.7 ± 0.1 | 8.7 ± 0.1 | 5.3 ± 0.1 | 8.5 ± 0.1 | 14.5 ± 0.1 | |
| Excrements | LF + WB | 6.6 ± 0.1 | 13.1 ± 0.1 | 19.4 ± 0.4 | 2.2 ± 0.1 | 28.1 ± 0.5 | 8.1 ± 0.1 | 4.6 ± 0.1 | 6.9 ± 0.1 | 11.6 ± 0.2 | |
| Insect | 15°C | WB | 31.9 ± 0.8 | 21.8 ± 0.1 | 40.9 ± 0.1 | 5.8 ± 0.1 | 54.5 ± 0.7 | 24.0 ± 0.6 | 14.0 ± 0.2 | 19.8 ± 0.4 | 33.0 ± 0.8 |
| Insect | 20°C | WB | 32.7 ± 0.7 | 23.1 ± 0.3 | 41.7 ± 0.3 | 5.4 ± 0.1 | 58.8 ± 0.2 | 25.4 ± 0.4 | 15.0 ± 0.1 | 20.4 ± 0.4 | 33.9 ± 0.6 |
| Insect | 25°C | WB | 36.7 ± 0.5 | 25.6 ± 0.4 | 45.6 ± 0.7 | 6.4 ± 0.1 | 61.7 ± 0.2 | 27.0 ± 0.4 | 16.0 ± 0.1 | 21.8 ± 0.1 | 36.3 ± 0.9 |
| Insect | 15°C | LF | 40.7 ± 0.8 | 28.4 ± 0.1 | 45.6 ± 0.4 | 6.7 ± 0.1 | 44.1 ± 0.1 | 31.4 ± 0.5 | 17.4 ± 0.3 | 23.9 ± 0.3 | 39.2 ± 0.4 |
| Insect | 20°C | LF | 37.3 ± 0.3 | 28.0 ± 0.4 | 47.6 ± 0.7 | 4.2 ± 0.1 | 61.6 ± 1.4 | 27.0 ± 0.1 | 16.8 ± 0.1 | 25.1 ± 0.6 | 38.1 ± 0.9 |
| Insect | 25°C | LF | 42.5 ± 0.9 | 27.4 ± 0.1 | 45.2 ± 0.1 | 4.1 ± 01 | 63.3 ± 0.4 | 30.3 ± 0.7 | 16.5 ± 0.3 | 24.8 ± 0.5 | 38.9 ± 0.2 |
| Insect | 15°C | LF + WB | 35.0 ± 0.1 | 24.8 ± 0.5 | 39.7 ± 0.1 | 4.2 ± 0.1 | 57.7 ± 1.2 | 26.3 ± 0.5 | 13.9 ± 0.2 | 21.7 ± 0.4 | 35.1 ± 0.6 |
| Insect | 20°C | LF + WB | 36.3 ± 0.5 | 24.7 ± 0.1 | 44.2 ± 0.1 | 4.1 ± 0.1 | 59.7 ± 1.0 | 27.1 ± 0.3 | 15.6 ± 0.1 | 23.3 ± 0.3 | 37.6 ± 0.4 |
| Insect | 25°C | LF + WB | 36.6 ± 0.7 | 25.0 ± 0.3 | 42.6 ± 0.3 | 4.2 ± 0.1 | 59.6 ± 0.3 | 27.6 ± 0.2 | 15.6 ± 0.3 | 22.5 ± 0.3 | 36.4 ± 0.4 |
WB –Wheat bran, LF = lentil flour.
Table 2.
Representation of individual content of amino acids (average ± standard error) dried material in T. molitor in the dependency on the feed and temperature: second part
| Sample type | Temp | Feed used | Amino acid [g kg−1] | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LYS | MET | PHE | PRO | SER | THR | TYR | VAL | |||
| Feed | WB | 6.1 ± 0.1 | 1.7 ± 0.1 | 5.9 ± 0.1 | 8.1 ± 0.2 | 5.9 ± 0.1 | 5.2 ± 0.1 | 3.3 ± 0.1 | 7.1 ± 0.1 | |
| Feed | LF | 10.8 ± 0.2 | 1.7 ± 0.1 | 9.2 ± 0.1 | 6.4 ± 0.1 | 8.2 ± 0.2 | 7.0 ± 0.1 | 4.7 ± 0.1 | 9.2 ± 0.1 | |
| Excrements | WB | 2.4 ± 0.1 | 0.9 ± 0.1 | 2.2 ± 0.1 | 2.7 ± 0.1 | 2.8 ± 0.1 | 2.7 ± 0.1 | 1.6 ± 0.1 | 3.0 ± 0.1 | |
| Excrements | LF | 12.4 ± 0.2 | 1.5 ± 0.1 | 9.7 ± 0.1 | 7.1 ± 0.1 | 9.1 ± 0.2 | 7.7 ± 0.1 | 4.7 ± 0.1 | 10.1 ± 0.1 | |
| Excrements | LF + WB | 9.3 ± 0.1 | 1.4 ± 0.1 | 8.0 ± 0.1 | 6.8 ± 0.1 | 7.4 ± 0.1 | 6.1 ± 0.1 | 3.9 ± 0.1 | 8.0 ± 0.4 | |
| Insect | 15°C | WB | 23.4 ± 0.4 | 6.6 ± 0.1 | 16.1 ± 0.3 | 33.8 ± 0.1 | 19.1 ± 0.3 | 19.0 ± 0.1 | 22.6 ± 0.1 | 28.6 ± 0.6 |
| Insect | 20°C | WB | 23.9 ± 0.4 | 7.0 ± 0.1 | 17.1 ± 0.4 | 33.9 ± 0.2 | 20.1 ± 0.2 | 20.1 ± 0.4 | 27.3 ± 0.4 | 30.1 ± 0.2 |
| Insect | 25°C | WB | 25.8 ± 0.5 | 7.3 ± 0.2 | 17.7 ± 0.1 | 34.3 ± 0.3 | 21.2 ± 0.3 | 20.6 ± 0.2 | 25.9 ± 0.4 | 33.0 ± 0.4 |
| Insect | 15°C | LF | 26.1 ± 0.5 | 5.8 ± 0.1 | 18.1 ± 0.1 | 43.5 ± 0.6 | 20.5 ± 0.4 | 22.0 ± 0.4 | 24.9 ± 0.5 | 36.3 ± 0.3 |
| Insect | 20°C | LF | 26.6 ± 0.5 | 4.4 ± 0.1 | 17.8 ± 0.1 | 40.5 ± 0.2 | 20.0 ± 0.2 | 22.3 ± 0.3 | 23.5 ± 0.1 | 34.5 ± 0.6 |
| Insect | 25°C | LF | 25.5 ± 0.4 | 4.0 ± 0.1 | 18.2 ± 0.2 | 36.7 ± 0.2 | 20.1 ± 0.2 | 19.5 ± 0.1 | 25.2 ± 0.4 | 32.8 ± 0.5 |
| Insect | 15°C | LF + WB | 23.5 ± 0.3 | 4.1 ± 0.1 | 14.9 ± 0.1 | 35.7 ± 0.6 | 17.8 ± 0.2 | 18.5 ± 0.1 | 19.6 ± 0.4 | 30.5 ± 0.7 |
| Insect | 20°C | LF + WB | 26.3 ± 0.4 | 4.4 ± 0.1 | 17.9 ± 0.3 | 33.0 ± 0.6 | 19.7 ± 0.3 | 21.0 ± 0.3 | 22.9 ± 0.5 | 35.0 ± 0.6 |
| Insect | 25°C | LF + WB | 25.5 ± 0.2 | 4.8 ± 0.1 | 17.8 ± 0.1 | 32.1 ± 0.3 | 19.5 ± 0.4 | 20.1 ± 0.1 | 25.0 ± 0.5 | 32.3 ± 0.1 |
LF = lentil flour; WB = wheat bran.
Table 3.
Statistically significant difference (P < 0.05) between content of individual amino acids in dependency on the feed at constant temperature
| Temp | Feed | Amino acid | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ALA | ARG | ASP | CYS | GLU | GLY | HIS | ILE | LEU | LYS | MET | PHE | PRO | SER | THR | TYR | VAL | ||
| 15°C | WB vs LF + WB | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | * | NS | NS | NS | NS | NS | NS |
| WB vs LF | * | * | NS | NS | NS | * | NS | * | * | NS | NS | NS | * | NS | NS | NS | * | |
| LF + WB vs LF | NS | NS | * | * | * | NS | * | NS | NS | NS | NS | * | NS | * | * | * | NS | |
| 20°C | WB vs LF + WB | NS | NS | NS | * | NS | NS | NS | NS | NS | NS | * | NS | NS | NS | NS | NS | * |
| WB vs LF | * | * | * | * | NS | * | * | * | * | * | * | NS | NS | NS | * | NS | NS | |
| LF + WB vs LF | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | * | NS | NS | NS | NS | |
| 25°C | WB vs LF + WB | NS | NS | NS | * | NS | NS | NS | NS | NS | NS | NS | NS | NS | * | NS | NS | NS |
| WB vs LF | NS | NS | NS | * | NS | * | NS | * | NS | NS | * | * | NS | NS | * | NS | NS | |
| LF + WB vs LF | NS | * | NS | NS | * | NS | NS | NS | NS | NS | NS | NS | * | NS | NS | NS | NS | |
LF = lentil flour; WB = wheat bran.
*Statistically significant dependence (P < 0.05); NS = not significant.
Table 4.
Statistically significant difference (P < 0.05) between content of individual amino acids in dependency on the temperature using the same feed
| Feed | Temp | Amino acid | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ALA | ARG | ASP | CYS | GLU | GLY | HIS | ILE | LEU | LYS | MET | PHE | PRO | SER | THR | TYR | VAL | ||
| WB | 15 vs 20°C | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | * | NS |
| 15 vs 25°C | * | * | * | NS | * | * | * | * | * | * | * | * | NS | * | * | NS | * | |
| 20 vs 25°C | NS | NS | NS | * | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | |
| LF | 15 vs 20°C | NS | NS | NS | * | NS | * | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| 15 vs 25°C | NS | * | NS | * | * | NS | NS | NS | NS | NS | * | NS | * | NS | NS | NS | * | |
| 20 vs 25°C | * | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | * | * | NS | |
| LF + WB | 15 vs 20°C | NS | NS | * | NS | NS | NS | NS | NS | * | * | NS | NS | NS | * | * | NS | * |
| 15 vs 25°C | NS | NS | NS | NS | NS | NS | * | NS | NS | NS | * | * | * | NS | NS | * | NS | |
| 20 vs 25°C | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | |
LF = lentil flour; WB = wheat bran.
*Statistically significant dependence (P < 0.05); NS = not significant.
The dependency of the amino acid content on the feed and temperature cannot be generalized. It is always necessary to evaluate the dependency individually for each amino acid. However, in general, it can be said that the content of amino acids depends on the feed, especially for lower temperatures (sum of amino acids for 15°C for feed WB—415 g kg−1, LF + WB—423 g kg−1, LF—475 g kg−1).
The largest difference between the observed maximum and minimum content value depending on the temperature and the feed for individual amino acids was found to be up to 19.2 g kg−1 for Glu and 11.4 g kg−1 for Pro and for the essential amino acids, up to 7.7 g kg−1 for Val, 6.6 g kg−1 for Arg, and 6.2 g kg−1 for Leu.
It can be said that Glu, Cys, and Met play an important role in the nutrition of the larvae. Met is the amino acid for which the statistically significant differences were generally confirmed at both the constant temperature and the constant feed. The highest content was found in WB feeding, independent of temperature. When feeding WB and the combination of LF + WB, the content of Met increased with temperature, but the feeding of LF decreased with temperature.
The highest Glu content was generally found at 25°C and decreased with decreasing temperature. For the LF + WB feed combination, this reduction was indicated by average value, but was not statistically demonstrated (H (2, N = 12) = 1.50; P = 0.47). No trend was found for feed dependence, but it was found that feed dependence did not change significantly at 20°C (H (2, N = 12) = 2.92; P = 0.23).
In the case of Cys, statistically significant differences were found, but without any general trend (increasing or decreasing tendencies). Due to the effect of feed at a constant temperature, as the protein content in the diet increased, the Cys content (between WB and LF + WB) decreased. With a further increase in protein content (between LF + WB and LF feed), the Cys content did not change at 20 and 25°C or increased at 15°C. No statistically significant changes (H (2, N = 18) = 1.70; P = 0.42) were observed when monitoring the effect of temperature on LF + WB constant feed and the value of Cys content was independent of temperature. For LF feed, the values were the same for 20 and 25°C as for LF + WB feed, but for 15°C the value increased by 60%. In addition to this increase, the highest Cys values were set for WB feed regardless of the temperature.
The characteristics of the other amino acids can be deduced from Tables 1–4.
Fat Content Determination
For each experimental group, the fat content in DM was evaluated and the results are shown in Fig. 3. The dependency of the fat content on the temperature while feeding bran was evaluated as statistically significant (H (2, N = 9) = 7.20; P = 0.03). A similar trend was also observed in the group fed by lentil flour exclusively. Absolute values of the fat content at the same temperature were lower (up to 10% lower) in the group fed by lentil flour than in the group fed by wheat bran. Nevertheless, the dependency of the fat content on the temperature on feeding with lentil flour was evaluated as statistically insignificant (H (2, N = 9) = 5.47; P = 0.07). The situation was similar in the group fed by the mixture of lentil flour and wheat bran, where the dependency of the fat content on the temperature was evaluated as not statistically significant (H (2, N = 9) = 5.60; P = 0.06). However, the trend was different in this group. The fat content first dropped and then the mean value remained approximately at the same level.
Fig. 3.
Dependency of the fat content (average ± standard error) in T. molitor on the temperature and feed. The ‘*’ on the column indicates a significant difference (P < 0.05, Kruskal–Wallis test, Mann–Whitney post hoc tests) for constant temperature. The ‘+’ on the column indicates a significant difference (P < 0.05, Kruskal–Wallis test, Mann–Whitney post hoc tests) for constant feed. The fat content was measured three times.
The dependency of the fat content on the feed at 15°C (H (2, N = 9) = 6.54; P = 0.04), 20°C (H (2, N = 9) = 7.20; P = 0.03), and 25°C (H (2, N = 9) = 6.49; P = 0.04) was evaluated as statistically significant. To gain the highest fat content in the low and the high temperature, the optimal feed was a mixture of wheat bran and lentil flour. At 20°C the highest fat content was detected while feeding wheat bran and the trend was observed showed that with a decrease in the content of carbohydrates in the feed, the fat content in the evaluated species decreased.
Fatty Acid Profile Determination
The dependency of the fatty acid profile of T. molitor on the temperature and feed in % of total fatty acids is shown in Table 5.
Table 5.
Dependency of the fatty acid profile in dried material of T. molitor on the temperature and feed (% of total fatty acids (average ± standard error))**
| WB | LF + WB | LF | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 15°C | 20°C | 25°C | 15°C | 20°C | 25°C | 15°C | 20°C | 25°C | |
| C12:0 | 0.1 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | <LOD | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.3 ± 0.1 |
| C13:0 | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | 0.1 ± 0.1 |
| C14:0 | 1.7 ± 0.1 | 3.0 ± 0.1 | 2.0 ± 0.1 | 2.1 ± 0.1 | 2.4 ± 0.1 | 2.6 ± 0.1 | 2.7 ± 0.1 | 2.7 ± 0.1 | 4.0 ± 0.1 |
| C15:0 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0.1 | <LOD | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.2 ± 0.1 |
| C16:0 | 15.6 ± 0.1 | 20.1 ± 0.1 | 12.6 ± 0.2 | 14.4 ± 0.1 | 13.4 ± 0.3 | 13.1 ± 0.1 | 11.7 ± 0.1 | 11.5 ± 0.1 | 12.7 ± 0.1 |
| C17:0 | 2.8 ± 0.1 | 2.2 ± 0.1 | 4.9 ± 0.2 | 0.4 ± 0.1 | 5.3 ± 0.1 | 5.4 ± 0.1 | 2.8 ± 0.1 | 2.8 ± 0.1 | 0.3 ± 0.1 |
| C18:0 | 7.8 ± 0.1 | 6.7 ± 0.1 | 9.6 ± 0.1 | 7.7 ± 0.1 | 7.9 ± 0.1 | 7.5 ± 0.1 | 7.6 ± 0.1 | 7.6 ± 0.1 | 6.3 ± 0.1 |
| Total SFA | 28.1 | 32.3 | 29.4 | 24.6 | 29.3 | 28.9 | 25.1 | 24.9 | 23.9 |
| C16:1 (cis-9) | 1.6 ± 0.1 | 1.7 ± 0.1 | 0.9 ± 0.1 | 1.5 ± 0.3 | 1.7 ± 0.1 | 1.3 ± 0.1 | 1.0 ± 0.1 | 1.2 ± 0.1 | 1.3 ± 0.1 |
| C18:1 (cis-9) | 22.8 ± 0.1 | 26.3 ± 0.1 | 27.6 ± 0.7 | 26.6 ± 0.1 | 26.4 ± 0.5 | 28.5 ± 0.1 | 28.1 ± 0.1 | 29.3 ± 0.1 | 35.9 ± 0.1 |
| C20:1 (cis-11) | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.8 ± 0.1 | 1.4 ± 0.1 | 1.1 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.1 ± 0.1 |
| Total MUFA | 25.3 | 28.9 | 29.3 | 29.5 | 29.2 | 30.8 | 30.1 | 31.5 | 38.3 |
| C18:2 (all-cis-9.12) | 39.5 ± 0.1 | 34.1 ± 0.1 | 40.1 ± 0.9 | 46.0 ± 0.1 | 36.4 ± 0.7 | 34.7 ± 0.1 | 42.0 ± 0.2 | 39.1 ± 0.2 | 37.8 ± 0.1 |
| C22:6 (all-cis-4.7.10.13.16.19) | 6.9 ± 0.3 | 4.6 ± 0.3 | 3.7 ± 0.1 | <LOD | 6.8 ± 0.5 | 5.4 ± 0.3 | 2.8 ± 0.5 | 4.4 ± 0.4 | <LOD |
| Total PUFA | 46.4 | 38.7 | 43.8 | 46.0 | 43.2 | 40.1 | 44.8 | 43.5 | 37.8 |
LF = lentil flour; LOD = limit of detection; WB = wheat bran.
Statistically significant dependence (H (2, N = 12) = 9.85; P = 0.01) of saturated fatty acids content on feed with a clear trend was found at 20°C. A statistically significant difference was also demonstrated for other variables on feed at the temperature 15°C (H (2, N = 12) = 9.85; P = 0.01) and 25°C (H (2, N = 12) = 8.00; P = 0.02), but the trend is no longer clear. The same is true for the temperature dependence of a constant feed. Again, a statistically significant difference was demonstrated in all cases (WB—H (2, N = 12) = 9.85; P = 0.01; LF + WB—H (2, N = 12) = 7.54; P = 0.0231; LF—H (2, N = 12) = 8.00; P = 0.02), but the trend was not that clear.
Similar to saturated fatty acids, the dependence of the content of monoenoic fatty acids on feed and temperature was demonstrated. In general, the content of monoenoic fatty acids increased with increasing temperature and the content of lentil flour in the feed. This increase was most evident in the dependence on feed at 25°C (statistically significant difference H (2, N = 12) = 9.85; P = 0.01), when there was an increase of 9%.
An important finding was the proof of the dependency of the polyene fatty acid content on the feed and the temperature. The general trend was often declining. An almost uniform decrease was found in the temperature dependence when feeding with mixtures of wheat bran and lentil flour. In this case, it decreased by 6% with an increase in temperature.
Individual fatty acids include significant unsaturated acids C18:1 (cis-9) and C18:2 (all-cis-9.12). For both acids, a statistically significant difference was demonstrated in all investigated cases of temperature and feed dependence (Tables 6 and 7). Generally, the C18:1 (cis-9) content increased with an increase in the content of the lentils in the diet, but the overall fat content decreased (with more than 50% of the lentils). It further increased with increasing temperature (up to 8%). In contrast to C18:1 (cis-9), no general trend was found for C18:2 (all-cis-9.12). An important characteristic, however, was the dependence of the C18:2 acid content (all-cis-9.12) on the temperature when feeding mixtures of lentil flour and wheat bran, where the acid content was reduced by up to 11% (statistically significant difference H (2, N = 12) = 9.85; P = 0.01). When feeding mixtures (lentils and bran), this fatty acid content was proved to be significantly temperature-dependent which signified that the amount of this essential acid can be influenced by the rearing conditions.
Table 6.
Statistically significant difference (P < 0.05) between content of individual fatty acid in dependency on the feed at constant temperature
| Temp | Feed | Fatty acid | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C12:0 | C13:0 | C14:0 | C15:0 | C16:0 | C17:0 | C18:0 | C16:1 (cis-9) | C18:1 (cis-9) | C20:1 (cis-11) | C18:2 (all-cis-9.12) | C22:6 (all-cis-4.7. 10.13.16.19) | ||
| 15°C | WB vs LF + WB | NS | NS | NS | NS | NS | NS | NS | NS | NS | * | * | * |
| WB vs LF | NS | NS | * | NS | * | NS | * | NS | * | NS | NS | NS | |
| LF + WB vs LF | * | NS | NS | * | NS | * | NS | NS | NS | NS | NS | NS | |
| 20°C | WB vs LF + WB | NS | NS | * | NS | NS | * | * | NS | NS | * | NS | NS |
| WB vs LF | * | NS | NS | * | * | NS | NS | * | NS | NS | * | NS | |
| LF + WB vs LF | NS | NS | NS | NS | NS | NS | NS | NS | * | NS | NS | * | |
| 25°C | WB vs LF + WB | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | * | NS |
| WB vs LF | * | NS | * | * | NS | NS | * | * | NS | * | NS | NS | |
| LF + WB vs LF | NS | NS | NS | NS | NS | * | NS | NS | NS | NS | NS | * | |
LF = lentil flour; WB = wheat bran.
*Statistically significant dependence (P < 0.05); NS = not significant.
Table 7.
Statistically significant difference (P < 0.05) between content of individual fatty acid in dependency on the temperature at constant feed
| Feed | Temp | Fatty acid | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C12:0 | C13:0 | C14:0 | C15:0 | C16:0 | C17:0 | C18:0 | C16:1 (cis-9) | C18:1 (cis-9) | C20:1 (cis-11) | C18:2 (all-cis-9.12) | C22:6 (all- cis-4.7.10.13.16.19) | ||
| WB | 15 vs 20°C | * | NS | * | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| 15 vs 25°C | NS | NS | NS | NS | NS | NS | NS | NS | * | * | NS | * | |
| 20 vs 25°C | NS | NS | NS | NS | * | * | * | * | NS | NS | NS | NS | |
| LF + WB | 15 vs 20°C | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | * |
| 15 vs 25°C | * | NS | * | * | * | * | NS | NS | NS | * | * | NS | |
| 20 vs 25°C | NS | NS | NS | NS | NS | NS | * | NS | * | NS | NS | NS | |
| LF | 15 vs 20°C | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| 15 vs 25°C | NS | NS | * | * | NS | * | * | * | * | * | * | NS | |
| 20 vs 25°C | * | NS | NS | NS | * | NS | NS | NS | NS | NS | NS | * | |
LF = lentil flour; WB = wheat bran.
*Statistically significant dependence (P < 0.05); NS = not significant.
Next, statistically significant difference (P < 0.05) between content of individual fatty acid in dependency on the temperature using the same feed is shown in Table 7.
Discussion
On a comparison basis with the works of van Broekhoven et al. (2015) and Oonincx et al. (2015), higher content of crude protein was detected. van Broekhoven et al. (2015) stated that the content of protein in T. molitor reared at 28°C did not change significantly (45–49%) in the dependency on the protein content in the feed (2–6%). However, when the content of fat in feed was changed, there were nutrition changes in the fat content and fatty acid profile. The trend may be different for species other than T. molitor. van Broekhoven et al. (2015) stated that the protein content in larvae of Zophobas morio (Z. morio) (Coleoptera: Tenebrionidae) (34–43%) reared at the same temperature and fed the same feed, was highly dependent on the protein content in the feed (12–39%). On the contrary, Oonincx et al. (2015), who reared insect at the same temperature (28°C) as van Broekhoven et al. (2015), added beet molasses into the feed and used another type of brewery yeast. His results showed the protein content in the range of 44–54% in dry matter, after the change of the protein content in the feed from 13 to 23%. The protein content in larvae corresponded with the protein content in the feed, both for low and high content of fat in the feed. Results in the present study corresponded with the results of Oonincx et al. (2015).
Nitrogenous substances increased with the content of lentil flour in the feed regardless of the breeding temperature. From the results obtained in the present work and from studies of other authors (e.g., van Broekhoven et al. 2015) it can be shown that the protein content of insects increases with the content of protein feed. In this way, flour can be obtained for the production of products for the special nutrition of athletes and in convalescence for the growth of muscle mass.
The edible insect contains a lot of nutritionally valuable amino acids, including a high content of essential amino acids that humans must receive in the diet (Paul et al. 2016). Native inhabitants in different parts of the world often compensate the lack of amino acids from other food by eating the edible insect; therefore, the nutrient intake of such combination of food is balanced (Bukkens 2005, van Huis et al. 2013), e.g., in Angola, the intake of these nutrients could be fulfilled by eating the termites of the Macrotermes subhyalinus (M. subhyalinus) (Isoptera: Termitidae) species (Sogbesan and Ugwumba 2008). From the human nutrition point of view, it is possible to modify the feed and the temperature to gain the expected results—suitable content of each amino acid. This finding can contribute to practical application in the food industry in the way of—extraction of amino acids and their subsequent supplementation into other commodities.
In general, glutamic acid was the most abundant, which in humans is used to build protein molecules and to transfer ammonia from the organs to the liver (Svačina 2010). When feeding wheat bran, the consumption of glutamic acid is more than 78% of the total amount of this acid in the feed. The second most utilized amino acid is arginine (75%). The only amino acid from this diet, whose content was higher in excrements than in the feed, was glycine.
After adding the lentil flour into the diet, the change occurs—cysteine and methionine become the most utilized amino acids. By including the protein feed in the form of lentil flour, the content of amino acids rises together with the rising content of this component in the feed. When feeding only the lentil flour, the content of amino acids, except for cysteine, methionine, and tyrosine, is higher in excrements than in the feed. For this reason, it is possible to evaluate the content of amino acids in the feed from the two points of view—not only considering the limiting amino acids but also evaluating the balance of the diet and species. According to the Wolf’s law of surplus amino acids (Svačina 2010) it is therefore useless to feed the high amount of these amino acids, because the larvae will pour them out in excrements and the effect of surplus amino acid is amplified. The reason may also be the anatomical structure of the analyzed species where no significant amounts of amino acids are required for the construction of muscle mass.
For rearing of insect, the mixed feed (mixture of wheat bran and lentil flour) is an advantageous combination regarding the amino acid content. The insect gets most of the amino acids in the required quantity. The content of individual amino acids in protein in T. molitor was detected, e.g., in the study of Zielinska et al. (2015), Bednářová et al. (2013), and Ravzanaadii et al. (2012), who mentioned the influence of developmental stage on the content of each amino acid. The content of lysine detected was 26.7 mg g−1 of protein in the larval stage (Zielińska et al. 2015), 29.06 mg g−1 of protein in the larval stage and 22.27 mg g−1 of protein in adult (Ravzanaadii et al. 2012). The detected lysine contents were lower in this work than in Zielińska et al. (2015) or Ravzanaadii et al. (2012), to whose work they approached. The content of methionine was 9.6 mg g−1 of protein in the larval stage (Zielińska et al. 2015), 6.72 mg g−1 of protein in the larval stage and 5.47 mg g−1 of protein in adult (Ravzanaadii et al. 2012). Ravzanaadii et al. (2012) in his study, which was dealing with the different content of specific amino acids in the dependence on the developmental stage, detected that the content of the basic amino acid glycine and other amino acids, e.g., tyrosine, leucine, valine, and alanine, was significantly different.
From a nutritional point of view, it was mainly focused on monitoring essential amino acids, which showed that their highest content was when fed with lentil flour at 15°C. Again, a commodity with a controlled amino acid composition can be obtained, which is then directly reflected in the biological value of the proteins. This could be advantageous in the case of the preparation of products for special nutrition such as metabolic disorders (e.g., monitoring of phenylalanine in the case of phenylketonuria). Because insects are normally fed with lysine-deficient bran, the present study also focused on the limiting amino acids lysine and methionine in the diet in order to balance these amino acids in the larval’s body (so far regardless of consumption).
Edible insects are characterized by the great variability not only in protein but also in the fat content. This variability depends on the season, stage of development, sex, environment, and nutrition. As reported by Li et al. (2013) and Rumpold and Schlüter (2013) the fat content of T. molitor larvae ranges from 6.4 to 43.1%. These values are in accordance with those measured in this work.
van Broekhoven et al. (2015) described in their study that with a change in the fat content in feed, the change of fat content and fatty acid profile occurs. Another insect species might not follow the same trend as a T. molitor. Study of van Broekhoven et al. (2015) reports the dependency of the fat content on the fat content in the feed for T. molitor and Z. morio. The main ingredients of their feed were brewer’s dough and brewery yeasts (Saccharomyces cerevisiae Meyen ex Hansen, Saccharomycetales: Saccharomycetaceae), bread leftovers, cookies leftover, and potato peels. On the contrary, Oonincx et al. (2015) who kept insect at the same temperature (28°C) as van Broekhoven et al. (2015), included root molasses in the diet and used a different species of brewery yeasts (Anheuser-Busch, Dommelen, The Netherlands). With a change of the fat content in the feed (1–15%) the change of the fat content in the larvae occurred within the range from 23 to 29%. However, the results were not directly proportional—the highest content of fat was detected in the larvae fed by the feed with the lowest content of fat and protein.
The previous study of Oonincx and van der Poel (2011) dealing with Locusta migratoria (L. migratoria) (Orthoptera: Acrididae) showed an impact of the rearing conditions on the nutritional value of this species. The study stated an important influence of the change in feed composition (change of the ratio of grass, wheat bran, and carrot) on the content of fat and protein in the body of the insect. The change in the fat content is from 18 to 25% in the penultimate instar and 19–30% in adult (with the growing amount of wheat bran and carrot the content raises). The inference was that with a carbohydrate diet, the fat content in insect may increase.
Comparing the results obtained with Oonincx et al. (2015) and van Broekhoven et al. (2015) the fat content found was lower than reported by Oonincx et al. (2015) and comparable to van Broekhoven et al. (2015). The results of van Broekhoven et al. (2015) showed an increase in the fat content of larvae while increasing the fat content of the diet. This trend was comparable to the values found in this article. From the results of van Broekhoven et al. (2015) it is also possible to find almost constant values of crude protein content with an increase in fat and protein in the diet. However, the results in this article prove a general consideration that with the increasing content of crude protein in the biological material the fat content decreases and vice versa.
This general view is supported by the results reported by Oonincx et al. (2015). However, its protein and carbohydrate results are highly dependent on the protein content of the diet. On the contrary, the effect of fat content in feed on the fat content of the larval body cannot be found in the results reported by Oonincx et al. (2015) to be traced. This is in line with the results reported in this article, for which great variability was also found.
The dependence of fat content in larvae on feed is in this work connected with another significant influence—the dependence on the temperature. In insect fed with wheat bran, the average content of fat was highest at 20°C (30%), Fig. 3, and dropped with increasing or decreasing temperature. It was reasoned out that with an increase in the temperature the analyzed insect did not need higher amounts of fat to ensure physiological processes. Fat body cells not only control the synthesis and utilization of energy reserves (fat and glycogen), but also synthesize most of the hemolymph proteins and circulating metabolites (Keeley 1985). Another key factor may be the activity of enzymes and hormones. Hormones (acydsteroids, juvenile hormone) can influence fat metabolism (Ravzanaadii et al. 2012).
Compared to other nutrients, fat is best affected by nutrition and therefore it was focused on obtaining a commodity with a high proportion of fat for energy nutrition and the use of fats for special applications (e.g., cosmetics, oil for technical purposes). On the other hand, there is a requirement from insect meal processors to insect farmers to focus their breeding on low-fat production.
The fatty acid content using the same feed was highly fluctuating in dependency on the temperature, as can be seen in Table 5. This influence cannot be complexly compared as many authors do not mention rearing temperature. The highest content of polyene fatty acids was achieved at 15°C and wheat bran diet. The closest to the recommended ratio of fatty acids SFA:MUFA:PUFA (0.8–1:1–1.2:1) was the rearing temperature 25°C and lentil flour diet (Dostálová et al. 2012). The highest content of monoenoic fatty acids was also achieved with this combination.
The content of the specific fatty acids varied depending on the feed and temperature, and for the most nutritionally important fatty acids, their content increased with the content of proteins in the diet. With a suitable choice of rearing conditions (25°C, lentils as feed) lower fat content and a stronger presence of essential fatty acids compared to trans fatty acids were achieved.
From the nutritional and technological point of view, the important acids are C18:0, C18:1 (cis-9), and C18:2 (all-cis-9.12) which have been found to have a significant dependency on the lentil flour content. With an increase in the content of lentil flour, neglecting the effect of temperature, the average fatty acid content increased. Table 5 also shows that no significant amounts of trans fatty acids were detected in the analyzed samples, but significant levels of essential fatty acids were found. Considering the fatty acid profile, a protein diet and a combination of protein and carbohydrate diet are preferable to diet based on carbohydrates alone. Similar results were measured by Oonincx et al. (2015). The presence of individual fatty acids in the T. molitor may vary, depending on the feed. Oonincx et al. (2015) reported that T. molitor was rich in C18:1 (cis-9), C18:2 (all-cis-9.12), and C16:0 for all diets tested. After adding carrots to the feed, the ratio of n-6:n-3 fatty acids changed. Changes in starch and protein content in the feed were reviewed by van Broekhoven et al. (2015) showing a change in n-6:n-3 fatty acid ratio of 19:1 to 32:1. The n-6 fatty acid content was significantly above the recommended value for human nutrition. Among the observed fatty acids reported by van Broekhoven et al. (2015) were palmitic acid ranging from 16.13 to 16.96 g/100 g of fat and stearic acid with a value of 2.64–2.97 g/100 g of fat, for which there was no significant effect of the change of carbohydrate and protein content in the feed on the fatty acid content. For some nutritionally important acids, there was a significant change in their content, e.g., oleic acid, from 39.78 g/100 g of fat for a high protein and a low starch content in the feed, up to 57.63 g/100 g of fat, in case of a low protein and high starch content in feed. Similarly, the change in feed resulted in linoleic acid (15.45–31.25 g/100 g of fat) and α-linolenic acid (0.67−1.48 g/100 g of fat) content changed. It is clear from the results that it is possible to increase or decrease the content of certain fatty acids by suitable feed choice.
The benefit of the work is the information that shows that by changing the feed composition for edible insect its nutritional value can be significantly affected. The influence of the temperature and the feed on the amino acid content of the T. molitor body cannot be assessed generally, it is always necessary to focus on the particular amino acid. In some cases, the present study demonstrated a statistically significant difference in their presentation of particular amino acid, depending on the temperature and the feed. Wu et al. (2020) found that T. molitor contains 19 amino acids. They claimed that the amount of essential amino acid composition in their samples was similar to a quantity that is recommended by FAO (2013). In comparison, in this research, most of the values were slightly higher than those reported in the research by Wu et al. (2020).
In the case of a carbohydrate diet, the fat content of insects may be increased. The highest fat content in insects can be achieved by a suitable rearing temperature, which was 20°C in this case. The content of the specific fatty acid varied depending on the feed and the temperature and for the most nutritionally important fatty acids, their content increased as a function of the content of proteins in the diet. Since the breeding temperature is an important factor affecting the development speed, Xu et al. (2012) investigated the optimum temperature for breeding T. molitor. They compared five different temperatures (20, 23, 26, 29, and 32°C) and their results showed that the T. molitor development was faster with the growing temperature. The faster development speed in different temperatures can suggest the change of their nutritive value. A suitable choice of rearing conditions (25°C, lentils as feed) can result in a higher proportion of crude protein, a lower fat content, and a greater proportion of essential fatty acids than trans fatty acids. For this reason, this species could serve as a prevention of noninfectious civilization diseases (cardiovascular diseases, diabetes mellitus, colon carcinoma). In addition to prevention, this species could be included not only in the rational diet, but also in the special diets such as for people with diabetes mellitus.
Wu et al. (2020) mentioned that insects contain not only fats and proteins, but bioactive substances as well, namely, alkaloids, flavonoids, terpenoids, and phenols. This could be a subject for future research, if bioactive substances depend on temperature and feed as well.
These findings, could in the future, allow the use of edible insects as a tool for the targeted nutritional supplement of a specific animal species and a targeted enrichment of a diet with the certain nutritional values for a particular group of consumers. It is believed that a suitably selected concentration of certain nutrients in insect feed could provide a specific diet to improve consumer health. The practical benefit of the present work therefore was based on the possibility of creating material for fortification of commercial products or food supplements for specific diets such as sports nutrition, occupations, and activities with high energy expenditure and also for nutrition for patients during convalescence, which contains flour from T. molitor, whose nutritional value can be controlled in the range of nitrogenous substances 546–657 g kg−1, fat 166–295 g kg−1, and total amino acids 415–475 g kg−1. The flour can be prepared by a controlled temperature-feeding regime of T. molitor in the temperature range of 15–25°C with a diet containing 50–100% by weight of lentil flour and/or 50–100% by weight of wheat bran for 28 d, followed by starving, killing, drying, and grinding the larvae.
Acknowledgments
This article was supported by the internal grant of Tomas Bata University in Zlín (IGA/FT/2020/010) and project Brno University of Technology in Brno (FEKT-S-20-6215).
References Cited
- Bednářová M, Borkovcová M, Mlček J, Rop O, and Zeman L. . 2013. Edible insects – species suitable for entomophagy under condition of Czech Republic. Acta Univ. Agric. Silvic. Mendel. Brun. 61: 587–593. [Google Scholar]
- van Broekhoven S, Oonincx D G, van Huis A, and van Loon J J. . 2015. Growth performance and feed conversion efficiency of three edible mealworm species (Coleoptera: Tenebrionidae) on diets composed of organic by-products. J. Insect Physiol. 73: 1–10. [DOI] [PubMed] [Google Scholar]
- Bukkens G F. 2005. Insects in the human diet: nutritional aspects, pp. 545–577. In Paoletti M G. (ed.), Ecological implications of minilivestock: potential of insects, rodents, frogs and snails. Science Publishers, Enfield, NH. [Google Scholar]
- Defoliart G R. 1992. Insects as human food. Crop Prot. 11: 395–399. [Google Scholar]
- Dostálová J, Dlouhý P, and Tláskal P. . 2012. Výživová doporučení pro obyvatelstvo České republiky Společnost pro výživu; http://www.vyzivaspol.cz/vyzivova-doporuceni-pro-obyvatelstvo-ceske-republiky/. [Google Scholar]
- Dreassi E, Cito A, Zanfini A, Materozzi L, Botta M, and Francardi V. . 2017. Dietary fatty acids influence the growth and fatty acid composition of the yellow mealworm Tenebrio molitor (Coleoptera: Tenebrionidae). Lipids. 52: 285–294. [DOI] [PubMed] [Google Scholar]
- (FAO) Food and Agriculture Organization of the United Nations 2013. Dietary protein quality evaluation in human nutrition: report of an FAO expert consultation. FAO, Rome, Italy. [Google Scholar]
- Grau T, Vilcinskas A, and Joop G. . 2017. Sustainable farming of the mealworm Tenebrio molitor for the production of food and feed. Z. Naturforsch. C. J. Biosci. 72: 337–349. [DOI] [PubMed] [Google Scholar]
- van Huis A. 2016. Edible insects are the future? Proc. Nutr. Soc. 75: 294–305. [DOI] [PubMed] [Google Scholar]
- van Huis A, van Itterbeeck J, Klunder H, Mertens E, Halloran A, Muir G, and Vantomme P. . 2013. Edible insects: future prospects for food and feed security. FAO UN, Forestry Department, Rome, Italy. [Google Scholar]
- ISO 1871:2009. Food and feed products-General guidelines for the determination of nitrogen by the Kjeldahl method. International Organization for Standardization, Geneva, Switzerland. [Google Scholar]
- Józefiak A, Kierończyk B, Rawski M, Mazurkiewicz J, Benzertiha A, Gobbi P, Nogales-Merida S, Świątkiewicz S, and Józefiak D. . 2018. Full-fat insect meals as feed additive – the effect on broiler chicken growth performance and gastrointestinal tract microbiota. J. Anim. Feed Sci. 27: 131–139. [Google Scholar]
- Keeley L L. 1985. Biochemistry and physiology of the insect fat body, pp. 211–248. In Kerkut G A and Gilbert L I (eds.), Comprehensive insect physiology, biochemistry and pharmacology. Pergamon, New York. [Google Scholar]
- Kim S Y, Park J B, Lee Y B, Yoon H J, Lee Y, and Kim N J. . 2015. Growth characteristics of mealworm Tenebrio molitor. J. Sericult. Entomol. Sci. 53: 1–5. [Google Scholar]
- Li L Y, Zhao Z R, and Liu H. . 2013. Feasibility of feeding yellow mealworm (Tenebrio molitor L.) in bioregenerative life support systems as a source of animal protein for humans. Acta Astronaut. 92: 103–109. [Google Scholar]
- Li L, Xie B, Dong C, Hu D, Wang M, Liu G, and Liu H. . 2015. Rearing Tenebrio molitor L. (Coleoptera: Tenebrionidae) in the “Lunar Palace 1” during a 105-day multi-crew closed integrative BLSS experiment. Life Sci. Space Res. (Amst). 7: 9–14. [DOI] [PubMed] [Google Scholar]
- Mancini S, Fratini F, Turchi B, Mattioli S, Dal Bosco A, Tuccinardi T, Nozic S and Paci G. . 2019. Former foodstuff products in Tenebrio Molitor rearing: effects on growth, chemical composition, microbiological load, and antioxidant status. Animals. 9. Article number 484, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mišurcová L, Buňka F, Ambrožová J V, Machů L, Samek D, and Kráčmar S. . 2014. Amino acid composition of algal products and its contribution to RDI. Food Chem. 151: 120–125. [DOI] [PubMed] [Google Scholar]
- Mlček J, Rop O, Borkovcova M, and Bednarova M. . 2014. A comprehensive look at the possibilities of edible insects as food in Europe - a review. Polish J. Food Nutr. Sci. 64: 147–157. [Google Scholar]
- Oonincx D G A B, and van der Poel A F B. . 2011. Effects of diet on the chemical composition of migratory locusts (Locusta migratoria). Zoo Biol. 30: 9–16. [DOI] [PubMed] [Google Scholar]
- Oonincx D G A B, van Broekhoven S, van Huis A, and van Loon J J A. . 2015. Feed conversion, survival and development, and composition of four insect species on diets composed of food by-products. PLoS One 10: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul A, Frederich M, Uyttenbroeck R, Hatt S, Malik P, Lebecque S, Hamaidia M, Miazek K, Goffin D, Willems L, . et al. 2016. Grasshoppers as a food source? A review. Biotechnol. Agron. Soc. 20: 337–352. [Google Scholar]
- Ramos-Elorduy J, Moreno J M P, Vázquez A I, Landero I, Oliva-Rivera H, and Camacho V H. . 2011. Edible Lepidoptera in Mexico: geographic distribution, ethnicity, economic and nutritional importance for rural people. J. Ethnobiol. Ethnomed. 7. Article number 2, 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravzanaadii N, Kim S H, Choi W H, Hong S J, and Kim N J. . 2012. Nutritional value of mealworm, Tenebrio molitor as food source. Int. J. Ind. Entomol. 25: 93–98. [Google Scholar]
- Rumpold B A, and Schlüter O K. . 2013. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 57: 802–823. [DOI] [PubMed] [Google Scholar]
- Ruschioni S, Loreto N, Foligni R, Mannozzi C, Raffaelli N, Zamporlini F, Pasquini M, Roncolini A, Cardinali F, Osimani A, . et al. 2020. Addition of olive pomace to feeding substrate affects growth performance and nutritional value of mealworm (Tenebrio Molitor L.) larvae. Foods. 9: 317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogari G, Amato M, Biasato I, Chiesa S, and Gasco L. . 2019. The potential role of insects as feed: a multi-perspective review. Animals 9: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogbesan A O, and Ugwumba A A A. . 2008. Nutritional evaluation of termite (Macrotermes subhyalinus) meal as animal protein supplements in the diets of Heterobranchus longifilis (Valenciennes, 1840) fingerlings. Turk. J. Fish. Aquat. Sci. 8: 149–157. [Google Scholar]
- Soxhlet F. 1871. Die gewichtsanalytische bestimmung des Milchfettes. Dinglers Polytech. J. 232: 461–465. [Google Scholar]
- Svačina Š. 2010. Poruchy metabolismu a výživy, 1st ed. Galén, Prague, Czech Republic. [Google Scholar]
- Wang H C, Liao H Y, and Chen H L. . 2012. Tenebrio small-scale ecological farming feasibility study. Adv. Mater. Res. 356–360: 267–270. [Google Scholar]
- Wu R A, Ding Q, Yin L, Chi X, Sun N, He R, Luo L, Ma H and Li Z. . 2020. Comparison of the nutritional value of mysore thorn borer (Anoplophora chinensis) and mealworm larva (Tenebrio molitor): amino acid, fatty acid, and element profiles. Food Chem. 323. Article number 126818, 1–8. [DOI] [PubMed] [Google Scholar]
- Xu S C, Gu M Z, Liu X W and Yang L L. . 2012. Experimental population life table of Tenebrio molitor at different temperatures. J. Henan Agric. Sci. 3: 85–89. [Google Scholar]
- Yi L, Lakemond C M M, Sagis L M C, Eisner-Schadler V, van Huis A, and van Boekel M A J S. . 2013. Extraction and characterisation of protein fractions from five insect species. Food Chem. 141: 3341–3348. [DOI] [PubMed] [Google Scholar]
- Zielińska E, Baraniak B, Karaś M, Rybczyńska K, and Jakubczyk A. . 2015. Selected species of edible insects as a source of nutrient composition. Food Res. Int. 77: 460–466. [Google Scholar]