Abstract
Objective:
To review and discuss the literature on the role of thalamic structure and function in migraine.
Discussion:
The thalamus holds an important position in our understanding of allodynia, central sensitization and photophobia in migraine. Structural and functional findings suggest abnormal functional connectivity between the thalamus and various cortical regions pointing towards an altered pain processing in migraine. Pharmacological nociceptive modulation suggests that the thalamus is a potential drug target.
Conclusion:
A critical role for the thalamus in migraine-related allodynia and photophobia is well established. Additionally, the thalamus is most likely involved in the dysfunctional pain modulation and processing in migraine, but further research is needed to clarify the exact clinical implications of these findings.
Keywords: Migraine, thalamus, sensitization, allodynia, photophobia, functional connectivity, pain processing
Introduction
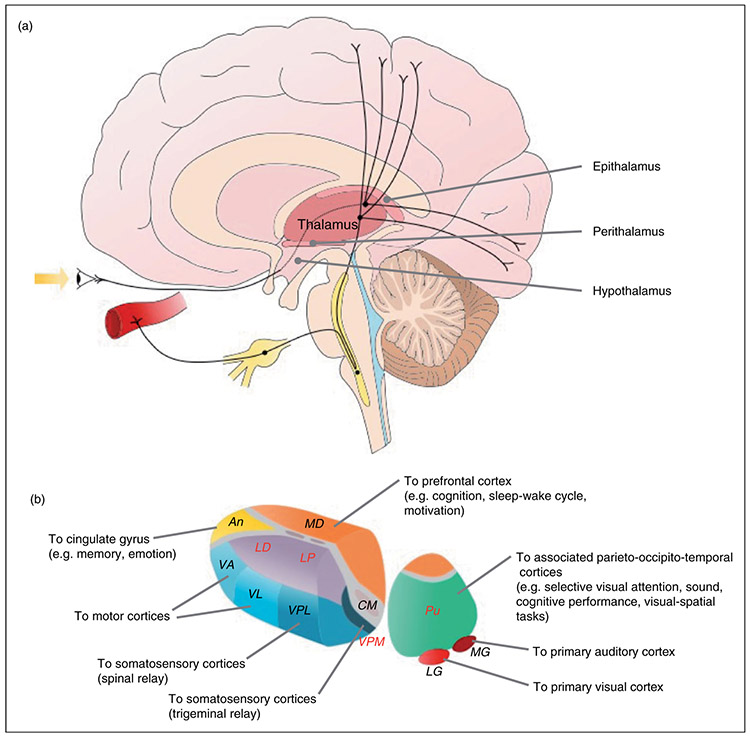
The thalamus is a nuclear complex located in the central deep brain structure of diencephalon between the midbrain and cortex. The thalamic nuclei reside in: dorsal thalamus, ventral thalamus (perithalamus), epithalamus, and hypothalamus (1). The dorsal thalamus (hereinafter referred to as thalamus) and hypothalamus are structures of interest in migraine.
The principal function of the thalamus is relaying and modulating the sensory and motor information between the peripheral nervous system and numerous cortical regions, including pain regulation, and is further involved in regulating the sleep–wake cycle, awareness, cognitive behaviors such as memory, attention and decision-making, and the modulation of visual information (1-3) (Figure 1). It also plays an important role in the cortico-cortical communication through transthalamic transfer of information between cortical areas (4). The focus of this paper is the sensory and nociceptive function in migraine, which primarily involves the lateral and posterior region of the thalamus. The role of the hypothalamus in migraine is discussed separately in this special issue.
Figure 1.
Schematic presentation of the thalamus and its nuclei in relation to migraine. (A) Schematic presentation of the thalamic projections of the ascending trigeminovascular and photophobia pathway in migraine. (B) Overview of the projections from the thalamic nuclei to various cortical regions. The thalamic trigeminovascular neurons project from VPM, posterior nucleus and LD/LP to, for example, the somatosensory, auditory, visual and parietal association cortices. The thalamic photosensitive trigeminovascular neurons project from the posterior nucleus and LD/LP to the somatosensory, motor, visual, parietal association and retrosplenial cortices. An: anterior nucleus; CM: centromedial nucleus; LD: lateral dorsal nucleus; LG: lateral geniculate nucleus; LP: lateral posterior nucleus; MD: mediodorsal nucleus; MG: medial geniculate nucleus; Pu: pulvinar; VA: ventral anterior nucleus; VL: ventral lateral nucleus; VPL: ventral posterolateral nucleus; VPM: ventral posteromedial nucleus. The red labels are nuclei, which are likely involved in the migraine pathophysiology.
In migraine pathogenesis, the role of the thalamus is considered as the relay center for ascending nociceptive information, via the trigeminovascular pain pathway, from lower brain areas to various cortical regions. Recent research, including translational studies, has revealed an expanding spectrum of additional structural and functional roles of the thalamus in migraine which could provide a better understanding of the migraine pathophysiology. The advantages of structural and functional magnetic resonance imaging (MRI) techniques have contributed to these findings.
The scope of this review is to provide an overview of the current understanding of the thalamus in migraine based on a selection of the existing research.
Structural studies
A large multi-center study used high-resolution T1-weighted MRI scans to investigate morphological changes in the thalamus of 131 migraine patients (29% with aura) compared to healthy controls (5). In migraine patients, the volume of several thalamic nuclei was reduced, including the central nuclear complex, anterior nucleus, and lateral dorsal nucleus. These nuclei are closely connected to the limbic system, suggesting abnormal processing of the affective and cognitive components of pain (5). Another study investigated microstructural and myelin content changes, and iron accumulation in the thalamus between migraine with and without aura patients (6). Compared to both migraine without aura patients and healthy controls, the T1 relaxation time was shorter in several thalamic nuclei in migraine with aura, including the anterior and lateral dorsal nuclei, which points to microstructural changes (6), supporting the previous study findings (5). Compared to controls, only the T1 relaxation time in the right thalamus was shorter for migraine with aura patients, translating to unilateral microstructural changes (6). Regional analysis revealed microstructural changes in the ventral posterolateral and ventral posteromedial (VPM) thalamic nuclei as well (6), which are functional relay stations for pain processing (1). However, there were no changes in the overall volume of the thalamus in migraine compared to controls (6). These findings may reflect abnormal cortical excitability control, which could increase the susceptibility to cortical spreading depression and visual aura (6). Neither of the two studies found an association of structural changes to disease duration or headache frequency.
Functional studies
One resting state functional MRI (fMRI) study investigated the thalamic functional connectivity of migraine patients without aura during attacks with right-sided (n=7), left-sided (n=6), or bilateral migraine (n=4) headache compared to their interictal state (7). While the study reported both increased and decreased functional connectivity between the right thalamus and various pain modulating and pain encoding cortical areas during migraine attacks, there was no altered connectivity between the left thalamus and other brain regions (7). The authors suggested that the altered functional connectivity of the right thalamus might be due to a usually higher norepinephrine concentration in the right side compared to the left (8). This is relevant as norepinephrine is involved in intrinsic pain control (9) and right thalamic lesions are more often associated with pain syndromes (10,11).
Two resting state fMRI studies investigated the thalamic functional connectivity in the interictal state of migraine without aura patients compared to healthy controls (12,13). The first study reported increased activity only in the right thalamus and increased connectivity between the right thalamus and bilateral caudate nuclei in patients compared to controls (12). The findings could be associated with functional impairments of pain processing (12). The study found no correlation between activation of the right thalamus at rest and disease duration or attack frequency (12). The usual pain side location during attacks was not reported and therefore it was not possible to determine whether the interictally increased right thalamic connectivity to the caudate nuclei was independent of the pain side, as previously reported during spontaneous attacks. The second study reported abnormal connectivity between the posterior thalami and various prefrontal cortical areas in the interictal state, suggesting that pain modulation is disrupted in migraine (13). Thus, these studies support the notion of abnormal nociceptive processing involving the thalamus in migraine.
Another resting state fMRI study compared migraine without aura patients with allodynia to patients without allodynia and to healthy controls (14). The functional connectivity was changed in migraine patients with allodynia between the two bilateral posterior thalami, and brain regions involved in emotional-cognitive pain processing and regulation (i.e. limbic, parieto-occipital and temporoparietal brain regions, and the medial prefrontal cortex) (14). Altogether, the findings suggest that a complex, multi-dimensional, pain processing network, integrating ascending and descending pathways at the level of the thalamus, is dysfunctional in migraine patients with allodynia (14).
Functional connectivity of the thalamus was also investigated in combination with diffusion tensor imaging in migraine without aura patients during (15) and between spontaneous attacks (16). While the fractional anisotropy was increased bilaterally in the thalamus of migraine patients between attacks (16), it remained unchanged during attacks (15), as compared to controls, suggesting interictal reduction of thalamic activity. The functional connectivity was decreased interictally between the default mode network, and the visuo-spatial system and medial visual cortical areas (16). Furthermore, the functional connectivity within the visuo-spatial system and medial visual cortical areas was negatively correlated with the bilaterally increased fractional anisotropy in the thalamus (16). Altogether, these findings suggest that reduced thalamic activity between attacks could lead to a reduced activation of the visuo-spatial system and medial visual cortical areas, which further deactivate the default mode network, supporting the hypothesis of a general dysfunctional multi-sensory information processing and integration in migraine (16).
During migraine without aura, the functional connectivity between the executive and dorso-ventral attention networks was decreased as compared to controls, but did not correlate to the ictally normal fractional anisotropy of the thalamus bilaterally (15). This suggests that the attention network is reduced to compensate for the ictal sensory overload, possibly because of the increased thalamic activity during an attack, which is a recovery from the abnormally decreased thalamic activity between attacks (15,16). Reduction of attentional network activity might explain the ictal occurrence of cognitive impairment, which is a recognized feature of migraine attacks (17,18) that may worsen with increasing attack frequency (15).
An fMRI study showed that migraine patients may be hypersensitive to aversive or negative emotional stimuli based on findings of increased neuronal activation of both nociceptive and emotional processing structures, including the thalamus, posterior cingulate cortex, caudate nucleus, and amygdala, after visual aversive or negative emotional stimulation (19). The increased activation of those structures can potentially amplify the attack severity and risk of chronification (19).
Another study, using H215O-PET in five episodic migraine patients, found unilateral thalamic activation during spontaneous attacks, which was strictly contralateral to the pain side, thereby following the trigeminal nociceptive pathway, as well as dorsolateral brainstem activation to the left side during right-sided migraine attacks (20). The hyperperfusion of the thalamus, contralateral to pain location, is interesting in light of the resting state fMRI studies reporting right-sided activation and altered connectivity to other brain regions regardless of pain side (7,12).
One xenon-enhanced computed tomographic (Xe CT-CBF) study investigated migraine with and without aura patients during and between attacks (21). Hyperperfusion was observed during attacks in cortex, thalamus, basal ganglia, and subcortical white matter (21). The largest increase was observed bilaterally in the thalamus during spontaneous attacks as compared to the interictal state (21). In contrast, an arterial spinlabeling MR study showed hypoperfusion in bilateral thalamic areas and hypothalamus during one spontaneous migraine attack without aura when compared to the interictal state (22). The hypoperfusion improved after treatment with rizatriptan (22), advocating involvement of thalamic activity in migraine attacks.
Finally, neurophysiological studies (23-25) and an fMRI study (26) suggest a dysfunctional connectivity between thalamus and cortical regions (23-25). Thalamocortical dysrhythmia has been hypothesized to be the underlying cause of abnormal sensory processing in migraine patients (27), where the hub of the arrhythmic activity was suggested to be the medial dorsal nucleus of the thalamus (26). Such alteration may involve lack of habituation of responses to sensory input between attacks, followed by a progression to a normal, synchronized state during attacks (27). In this respect, thalamocortical synchronization appears to be highly influenced by a vast, converging network of neurotransmitters and/or neuropeptides originating in brainstem (glutamate, serotonin, and noradrenalin), reticular thalamic (γ-aminobutyric acid (GABA)) and hypothalamic nuclei (dopamine, histamine, orexin, and melanin-containing hormone) (28). Consequently, thalamic activity can be selectively enhanced or inhibited by a variety of physiological conditions including wakefulness, food intake, attention, and stress, among others (28). This network of converging inputs may also potentially affect the functional connectivity between the thalamus and other brain regions involved in pain modulation and processing, as reported in the resting state fMRI studies previously described (12,13,16). Moreover, projection of thalamic neurons to multiple cortical areas with diverse functions may also account for some of the common neurological disturbances during migraine (2).
Two proton MR spectroscopy studies reported decreased concentration of N-acetyl-aspartate in the thalamus. One study reported a bilateral decrease, which was lower in the right thalamus (29), while the other study reported a decrease in the left thalamus only (30). N-acetyl-aspartate is commonly regarded as a marker of neuronal loss (31). However, the decrease of N-acetyl-aspartate observed in those studies could also suggest a subsided mitochondrial dysfunction in the thalamus leading to an abnormal energy metabolism in migraine, thus increasing the susceptibility to attacks (31,32). Moreover, one genetic study reported a higher prevalence of mitochondrial DNA mutations in migraine patients as compared to controls, suggesting an association between mitochondrial dysfunction and susceptibility to migraine (33).
Translational functional studies
Thalamus and allodynia
Cutaneous allodynia (i.e. pain resulting from an innocuous stimulus to normal skin or scalp) is a clinically relevant, objective parameter that can be measured in clinical settings using quantitative sensory testing (34,35). Using this method, a study reported cutaneous allodynia in 79% of the migraine patients ictally (34). In a population-based study, more than 63% of migraine patients reported allodynia while performing activities such as hair combing, showering, heat or cold exposure, and wearing necklaces or rings during migraine attacks (36). Cutaneous allodynia in migraine is thought to be a consequence of sequential activation and sensitization of neurons along the trigeminovascular pathway. Initial activation and sensitization of first-order neurons in the trigeminal ganglion leads to subsequent sensitization of second-order neurons in the spinal cord mediating ipsilateral cephalic allodynia (37). As time passes from the onset of an attack, sensitization of first and second-order neurons may spread upstream and cause central sensitization of third-order neurons in the thalamus, mediating thus contralateral and extracranial allodynia (37). Supporting evidence for allodynia in migraine emerged from studies in rats, where noxious stimulation of the dura was used to reveal ascending projections from brainstem trigeminovascular neurons to higher-order neurons in the posterior and VPM thalamic nuclei (38). Sensitization of dura-sensitive neurons within these thalamic nuclei was observed when mechanical and thermal stimuli were applied to the cranial and extracranial skin (35), suggesting that sensory inputs from the meninges and skin converge at this level as well (35). This clinical and preclinical evidence supports the notion that the thalamus and central sensitization mechanisms are significant contributors to the occurrence of extracranial allodynia accompanying headaches.
Migraine patients, with a history of extracranial allodynia during attacks, were MRI scanned at baseline and during a spontaneous unilateral migraine attack by fMRI using blood oxygenation level-dependent (BOLD) contrast. The thalamic activity, combined with mechanical and thermal stimulation of the ipsilateral dorsal hand, was investigated (35). The patients reported mechanical and thermal extracranial allodynia during attacks and the BOLD signal in thalamus was larger during both mechanical and thermal stimulation, compared to the migraine- and allodynia-free state. These findings further support the hypothesis that allodynia and thermal hyperalgesia in extracranial skin areas during migraine attacks are most likely associated with sensitization of thalamic neurons hosting convergence of sensory inputs from meninges and skin of the head and body (35).
Thalamus and photophobia
An important feature of migraine is photophobia, an experience of pain or discomfort to light. The quest for clarification of this phenomenon and its association to headache yields the study of the neuronal projections of the retino-thalamo-cortical pathway (3,39,40). Studies reveal that the migraine photophobia most likely originates in the non-image-forming retinal pathway and is modulated at the level of thalamus due to convergence of the retinal axons and dura-sensitive thalamic neurons (3,39,40). Furthermore, a large translational study suggested that varying photophobic response to different colors of light can involve activation of the cone-mediated retinal pathway in the beginning of the non-image-forming visual pathway (40). The study additionally revealed that the visual sensitization is regulated and fine-tuned in thalamus, with green light exhibiting a possible soothing effect (40). The role of photophobia in migraine is further elaborated in a separate section of this special issue (41).
Pharmacological modulation of nociceptive transmission in thalamus
Several in vivo electrophysiological studies investigated the effects of acute and preventive migraine medication on VPM thalamic neurons in rats (42-46). The studies used electrical stimulation of the superior sagittal sinus to produce intracranial pain to simulate migraine headache and to locate trigeminovascular neurons in the VPM. Altogether, the findings showed that VPM may be a site of nociceptive modulation by triptans (42), calcitonin gene-related peptide receptor antagonists (46), and the migraine preventives beta blocker propranolol (via β1-adrenoceptor antagonist activity) (43) as well as valproate (presumably via GABA receptors), but not gabapentin (44). These studies suggest that the thalamus is a potential pharmacological target for the preventive treatment. However, it should be noted that the reported pharmacological effects of the substances in rodents may differ when administered to humans. Moreover, the drugs were administered iontophoretically, which may have little relevance to the actual effect in treating migraine as the blood–brain barrier is artificially bypassed. Nevertheless, these results suggest that migraine preventive drugs may work by decreasing the firing threshold of thalamic neurons. In rats, microiontophoresis and intravenous administration of topiramate, a GluK1-kainate receptor antagonist, inhibited VPM trigeminovascular neurons (45), supporting the role of glutamatergic mechanisms at the thalamic level in the migraine pathophysiology (47). As migraine-related nociceptive information can also reach the cortex via brainstem nuclei such as the parabrachial nuclear complex (48,49) and diencephalic hypothalamic nuclei (39), among other brain regions, prophylactic migraine drugs, thought to act on the thalamus, can indirectly modulate the cortex when normalizing thalamic activity.
Conclusion and perspectives
The thalamus is one of the main higher-order structures in the CNS relaying ascending nociceptive information from the peripheral nervous system to the cortex; it plays, nevertheless, a variety of additional roles in the development of migraine and migraine-associated symptoms. Structural and functional imaging studies have revealed increased neuronal activation in the thalamus during migraine attacks and yield an overall abnormal functional connectivity in the thalamocortical limb of the trigeminovascular pathway, suggesting dysfunctional pain processing in migraine. Moreover, metabolic changes in the thalamus suggest a component of mitochondrial dysfunction in migraine. Finally, thalamic involvement in migraine features, such as cutaneous allodynia and photophobia, is well supported by translational studies.
However, further research is needed to improve our understanding of these findings in relation to migraine pathophysiology: Are the reported thalamic abnormalities in migraine the predisposing cause or secondary as a result of the disorder? Moreover, the exact translation of the findings to establish their clinical significance and their implication in the development of new treatment options is still lacking, but the ever-developing neuroimaging techniques may provide a source for progress in our understanding of the thalamus in migraine.
Clinical implications.
Structural and functional thalamic findings point to abnormal pain processing and modulation in migraine.
The thalamus holds an important role in the development of central sensitization and allodynia in migraine.
Visual and pain perception involves regulation in the thalamus before transmission to the cortex.
Thalamus may be a potential pharmacological target for preventive treatment options in migraine.
Acknowledgments
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Lundbeck Foundation [grant number R155-2014-171] and Research Foundation of Rigshospitalet [grant number E-23327-02]. The funding sources played no role in the preparation or publication of this article.
Footnotes
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: MA reports personal fees from Alder BioPharmaceuticals, Allergan, Amgen, Eli Lilly, Novartis and Teva. MA acted as principal investigator in the following clinical trials: Alder ALD403-CLIN-011 (Phase 3b), Amgen 20120178 (Phase 2), 20120295 (Phase 2), 20130255 (OLE), 20120297 (Phase 3), 20150308 (Phase 2a), Electrocore GM- 11 gamma-Core-R, Novartis CAMG334a2301 (Phase 3b), Teva TV48125-CNS-30068 (Phase 3). MA has no ownership interest and does not own stocks of any pharmaceutical company. MA serves as associated editor of Cephalalgia and co-editor of the Journal of Headache and Pain. MA is President-elect of the International Headache Society and General Secretary of the European Headache Federation. The remaining authors report no conflicts of interest.
References
- 1.Herrero MT, Barcia C and Navarro JM. Functional anatomy of thalamus and basal ganglia. Childs Nerv Syst 2002; 18: 386–404. [DOI] [PubMed] [Google Scholar]
- 2.Noseda R, Jakubowski M, Kainz V, et al. Cortical projections of functionally identified thalamic trigeminovascular neurons: Implications for migraine headache and its associated symptoms. J Neurosci 2011; 31: 14204–14217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noseda R, Kainz V, Jakubowski M, et al. A neural mechanism for exacerbation of headache by light. Nat Neurosci 2010; 13: 239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sherman SM and Guillery RW. Distinct functions for direct and transthalamic corticocortical connections. J Neurophysiol 2011; 106: 1068–1077. [DOI] [PubMed] [Google Scholar]
- 5.Magon S, May A, Stankewitz A, et al. Morphological abnormalities of thalamic subnuclei in migraine: A multi-center MRI study at 3 Tesla. J Neurosci 2015; 35: 13800–13806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Granziera C, Daducci A, Romascano D, et al. Structural abnormalities in the thalamus of migraineurs with aura: A multiparametric study at 3 T. Hum Brain Mapp 2014; 35: 1461–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amin FM, Hougaard A, Magon S, et al. Altered thalamic connectivity during spontaneous attacks of migraine without aura: A resting-state fMRI study. Cephalalgia 2018; 38: 1237–1244. [DOI] [PubMed] [Google Scholar]
- 8.Oke A, Keller R, Mefford I, et al. Lateralization of norepinephrine in human thalamus. Science 1978; 200: 1411–1413. [DOI] [PubMed] [Google Scholar]
- 9.Pertovaara A Noradrenergic pain modulation. Prog Neurobiol 2006; 80: 53–83. [DOI] [PubMed] [Google Scholar]
- 10.Nasreddine ZS and Saver JL. Pain after thalamic stroke: Right diencephalic predominance and clinical features in 180 patients. Neurology 1997; 48: 1196–1199. [DOI] [PubMed] [Google Scholar]
- 11.Schmahmann JD. Vascular syndromes of the thalamus. Stroke 2003; 34: 2264–2278. [DOI] [PubMed] [Google Scholar]
- 12.Xue T, Yuan K, Cheng P, et al. Alterations of regional spontaneous neuronal activity and corresponding brain circuit changes during resting state in migraine without aura. NMR Biomed 2013; 26: 1051–1058. [DOI] [PubMed] [Google Scholar]
- 13.Wang T, Zhan W, Chen Q, et al. Altered resting-state ascending/descending pathways associated with the posterior thalamus in migraine without aura. Neuroreport 2016; 27: 257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang T, Chen N, Zhan W, et al. Altered effective connectivity of posterior thalamus in migraine with cutaneous allodynia: A resting-state fMRI study with Granger causality analysis. J Headache Pain 2016; 17: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coppola G, Di Renzo A, Tinelli E, et al. Thalamocortical network activity during spontaneous migraine attacks. Neurology 2016; 87: 2154–2160. [DOI] [PubMed] [Google Scholar]
- 16.Coppola G, Di Renzo A, Tinelli E, et al. Thalamo-cortical network activity between migraine attacks: Insights from MRI-based microstructural and functional resting-state network correlation analysis. J Headache Pain 2016; 17: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gil-Gouveia R, Oliveira AG and Martins IP. Cognitive dysfunction during migraine attacks: A study on migraine without aura. Cephalalgia 2015; 35: 662–674. [DOI] [PubMed] [Google Scholar]
- 18.Farmer K, Cady R, Bleiberg J, et al. A pilot study to measure cognitive efficiency during migraine. Headache 2000; 40: 657–661. [DOI] [PubMed] [Google Scholar]
- 19.Wilcox SL, Veggeberg R, Lemme J, et al. Increased functional activation of limbic brain regions during negative emotional processing in migraine. Front Hum Neurosci 2016; 10: 366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Afridi S, Giffin N, Kaube H, et al. A positron emission tomographic study in spontaneous migraine. Arch Neurol 2005; 62: 1270–1275. [DOI] [PubMed] [Google Scholar]
- 21.Kobari M, Meyer JS, Ichijo M, et al. Hyperperfusion of cerebral cortex, thalamus and basal ganglia during spontaneously occurring migraine headaches. Headache 1989; 29: 282–289. [DOI] [PubMed] [Google Scholar]
- 22.Kato Y, Araki N, Matsuda H, et al. Arterial spin-labeled MRI study of migraine attacks treated with rizatriptan. J Headache Pain 2010; 11: 255–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coppola G, De Pasqua V, Pierelli F, et al. Effects of repetitive transcranial magnetic stimulation on somatosensory evoked potentials and high frequency oscillations in migraine. Cephalalgia 2012; 32: 700–709. [DOI] [PubMed] [Google Scholar]
- 24.Coppola G, Vandenheede M, Di Clemente L, et al. Somatosensory evoked high-frequency oscillations reflecting thalamo-cortical activity are decreased in migraine patients between attacks. Brain 2005; 128: 98–103. [DOI] [PubMed] [Google Scholar]
- 25.Coppola G, Ambrosini A, Di Clemente L, et al. Interictal abnormalities of gamma band activity in visual evoked responses in migraine: An indication of thalamocortical dysrhythmia? Cephalalgia 2007; 27: 1360–1367. [DOI] [PubMed] [Google Scholar]
- 26.Hodkinson DJ, Wilcox SL, Veggeberg R, et al. Increased amplitude of thalamocortical low-frequency oscillations in patients with migraine. J Neurosci 2016; 36: 8026–8036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Tommaso M, Ambrosini A, Brighina F, et al. Altered processing of sensory stimuli in patients with migraine. Nat Rev Neurol 2014; 10: 144–155. [DOI] [PubMed] [Google Scholar]
- 28.Noseda R, Borsook D and Burstein R. Neuropeptides and neurotransmitters that modulate thalamo-cortical pathways relevant to migraine headache. Headache 2017; 57(Suppl 2): 97–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohamed RE, Aboelsafa AA and Al-Malt AM. Interictal alterations of thalamic metabolic concentration ratios in migraine without aura detected by proton magnetic resonance spectroscopy. Egypt J Radiol Nucl Med 2013; 44: 859–870. [Google Scholar]
- 30.Gu T, Ma X-X, Xu Y-H, et al. Metabolite concentration ratios in thalami of patients with migraine and trigeminal neuralgia measured with 1H-MRS. Neurol Res 2008; 30: 229–233. [DOI] [PubMed] [Google Scholar]
- 31.Clark JB. N-acetyl aspartate: A marker for neuronal loss or mitochondrial dysfunction. Dev Neurosci 1998; 20: 271–276. [DOI] [PubMed] [Google Scholar]
- 32.Younis S, Hougaard A, Vestergaard MB, et al. Migraine and magnetic resonance spectroscopy: A systematic review. Curr Opin Neurol 2017; 30: 246–262. [DOI] [PubMed] [Google Scholar]
- 33.Guo S, Esserlind A, Andersson Z, et al. Prevalence of migraine in persons with the 3243A>G mutation in mitochondrial DNA. Eur J Neurol 2016; 23: 175–181. [DOI] [PubMed] [Google Scholar]
- 34.Burstein R, Yarnitsky D, Goor-Aryeh I, et al. An association between migraine and cutaneous allodynia. Ann Neurol 2000; 47: 614–624. [PubMed] [Google Scholar]
- 35.Burstein R, Jakubowski M, Garcia-Nicas E, et al. Thalamic sensitization transforms localized pain into widespread allodynia. Ann Neurol 2010; 68: 81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lipton RB, Bigal ME, Ashina S, et al. Cutaneous allodynia in the migraine population. Ann Neurol 2008; 63: 148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burstein R, Cutrer MF and Yarnitsky D. The development of cutaneous allodynia during a migraine attack Clinical evidence for the sequential recruitment of spinal and supraspinal nociceptive neurons in migraine. Brain 2000; 123: 1703–1709. [DOI] [PubMed] [Google Scholar]
- 38.Burstein R, Yamamura H, Malick A, et al. Chemical stimulation of the intracranial dura induces enhanced responses to facial stimulation in brain stem trigeminal neurons. J Neurophysiol 1998; 79: 964–982. [DOI] [PubMed] [Google Scholar]
- 39.Maleki N, Becerra L, Upadhyay J, et al. Direct optic nerve pulvinar connections defined by diffusion MR tractography in humans: Implications for photophobia. Hum Brain Mapp 2012; 33: 75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noseda R, Bernstein CA, Nir RR, et al. Migraine photophobia originating in cone-driven retinal pathways. Brain 2016; 139: 1971–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noseda R, Copenhagen D, Burstein R. Current understanding of photophobia, visual networks and headaches. Cephalalgia 2019; 39: 1623–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shields KG and Goadsby PJ. Serotonin receptors modulate trigeminovascular responses in ventroposteromedial nucleus of thalamus: A migraine target? Neurobiol Dis 2006; 23: 491–501. [DOI] [PubMed] [Google Scholar]
- 43.Shields KG and Goadsby PJ. Propranolol modulates trigeminovascular responses in thalamic ventroposteromedial nucleus: A role in migraine? Brain 2005; 128: 86–97. [DOI] [PubMed] [Google Scholar]
- 44.Andreou AP, Shields KG and Goadsby PJ. GABA and valproate modulate trigeminovascular nociceptive transmission in the thalamus. Neurobiol Dis 2010; 37: 314–323. [DOI] [PubMed] [Google Scholar]
- 45.Andreou AP and Goadsby PJ. Topiramate in the treatment of migraine: A kainate (glutamate) receptor antagonist within the trigeminothalamic pathway. Cephalalgia 2011; 31: 1343–1358. [DOI] [PubMed] [Google Scholar]
- 46.Summ O, Charbit AR, Andreou AP, et al. Modulation of nocioceptive transmission with calcitonin gene-related peptide receptor antagonists in the thalamus. Brain 2010; 133: 2540–2548. [DOI] [PubMed] [Google Scholar]
- 47.Hoffmann J and Charles A. Glutamate and its receptors as therapeutic targets for migraine. Neurotherapeutics 2018; 15: 361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cechetto DF, Standaert DG and Saper CB. Spinal and trigeminal dorsal horn projections to the parabrachial nucleus in the rat. J Comp Neurol 1985; 240: 153–160. [DOI] [PubMed] [Google Scholar]
- 49.Saito H, Katagiri A, Okada S, et al. Ascending projections of nociceptive neurons from trigeminal subnucleus caudalis: A population approach. Exp Neurol 2017; 293: 124–136. [DOI] [PubMed] [Google Scholar]