Abstract
Background: Having a pregnancy complicated by hypertensive disorders of pregnancy (HDP) and/or having a small or preterm baby put a woman at risk for later cardiovascular disease (CVD). It is uncertain if higher maternal CVD risk factors (reflected by increased peripartum CVD biomarker levels) account for this risk, or if experiencing a complicated pregnancy itself increases a woman's CVD risk (reflected by an increase in biomarker trajectories from early pregnancy to postpartum).
Methods: We conducted a secondary analysis of an 8-week mindful eating and stress reduction intervention in 110 pregnant women. We used mixed linear regression analysis to compare CVD biomarker levels and trajectories, between women with and without a CVD-related pregnancy complication (including HDP [gestational hypertension or preeclampsia] or having a small for gestational age [<10th percentile] or preterm [<37 weeks] baby), at three times: (1) 12–20 weeks of gestation, (2) 3 months postpartum, and (3) 9 months postpartum. CVD biomarkers studied included serum glucose, insulin, homeostasis model assessment of insulin resistance (HOMA-IR), body mass index (BMI), blood pressure (BP), interleukin-6 (IL-6), tumor necrosis factor, and lipids. We adjusted for age, maternal smoking, prepregnancy BMI, BP, age × time, and BMI × time.
Results: Women had a mean age of 28 years (standard deviation [SD] 6), mean prior pregnancies of 0.8 (SD 1.0), and 22 women had one or more CVD-related pregnancy complications. HOMA-IR, diastolic BP, triglyceride, high-density lipoprotein cholesterol, and IL-6 average levels, but not trajectories, differed among women with complicated versus normal pregnancy (all p values were ≤0.04). Peripartum glucose and systolic BP trajectories were statistically greater in complicated versus normal pregnancies (p values were 0.008 and 0.01, respectively).
Conclusion: We conclude that the experience of a complicated pregnancy in addition to elevated CVD risk factor levels may both increase a woman's risk of future CVD.
ClinicalTrials.gov Identifier: NCT01307683.
Keywords: adverse pregnancy outcomes, cardiovascular biomarkers, trajectory, blood pressure, insulin resistance, lipids
Introduction
Pregnancy has been referred to as a maternal cardiometabolic stress test,1 such that if a woman has a pregnancy complicated by hypertensive disorders of pregnancy (HDP; including pre-/eclampsia or gestational hypertension), delivery of a small for gestational age (SGA), or preterm infant, this “unmasks” a 1.5 to 3-fold increased risk of developing later hypertension and cardiovascular disease (CVD).2
Preconception CVD risk factors explain some but not necessarily all of the association between key pregnancy complications and later CVD.3,4 Specifically, high triglycerides before conception3 and insulin resistance during pregnancy5 are associated with preeclampsia onset. Dyslipidemia is associated with the onset of both preeclampsia and preterm delivery.6–8 In addition to having elevated early pregnancy cardiometabolic risk profiles, we hypothesized that a having complicated pregnancy may alter a woman's cardiometabolic profile.
Indeed, in complicated pregnancies, biologic alterations resulting from abnormal placentation affect several cardiometabolic disease pathways. Defective placental implantation into the maternal uterine myometrium characterizes HDP, and intrauterine growth restriction resulting in SGA babies and preterm delivery.9,10 This abnormal placentation is characterized by adverse vascular remodeling of spiral arteries and presence of pathological obstructive lesions within placental spiral arteries.9 This in turn results in abnormalities of vascular smooth muscle, inflammation, and oxidative stress.11,12 Several of these pathophysiologic features are also well-known pathways/features of CVD (i.e., vascular remodeling, inflammation, oxidative stress, and obstructive arterial lesions).13
Key CVD-related pregnancy complications also individually predict the incidence of later CVD risk factors.14–17 HDP are independently associated with an increased calculated 10-year Framingham CVD risk.14 Several cardiometabolic disease pathways, including glucose intolerance, inflammation, adiposity, lipids, and blood pressure (BP), are altered during the course of pregnancy.18
One prior investigation demonstrated that HDP were associated with later BP,15 lipids and insulin14 and a separate investigation demonstrated HDP but not preterm delivery were associated with later increases in inflammatory markers (elevated C- reactive protein and IL-6).17 SGA and preterm delivery were associated with higher BP later in life among mothers.14–16 One prior study demonstrated that BP trajectories are increased from early to late pregnancy among women with HDP compared with normal pregnancy.19 To extend prior findings that key CVD-related pregnancy complications are related to later life elevations in CVD biomarkers, we specifically sought to investigate whether both level and trajectory of cardiometabolic biomarkers were elevated in a complicated versus normal pregnancy, from the intrapartum to postpartum period.
We hypothesized that CVD biomarker levels and trajectories measured during early pregnancy through the postpartum period would be higher in complicated versus normal pregnancy. We tested this hypothesis among 110 women who participated in the University of California San Francisco Maternal Adiposity Metabolism and Stress (MAMAS) study20 with measured CVD biomarkers at two time points, during pregnancy and at 9 months after delivery.
Methods
Study sample
We studied 110 women in the intervention arm of the MAMAS study of an 8-week mindful eating and stress reduction intervention.20,21 Inclusion criteria for this study included English-speaking pregnant women aged 18–45 with self-reported prepregnancy body mass index (BMI) in the overweight or more category (i.e., BMI 25–41 kg/m2), with singleton pregnancies, and household income <500% of the federal poverty level. The study protocol and participant recruitment have been previously described.20
Exposure measurements
CVD biomarker measurements were taken at three times points: (1) between 12 and 20 weeks of gestational age, (2) 3 months postpartum, and (3) 9 months postpartum. A single BP measurement was taken during the three face-to-face study visits by an automated upper arm cuff and was done seated after a 5-minute rest. Serum was obtained for serum glucose, insulin, homeostasis model assessment of insulin resistance (HOMA-IR), interluekin-6 (IL-6), tumor necrosis factor (TNF), total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides. Laboratory procedures for measurement of inflammatory biomarkers used standardized techniques, and measurement protocols have been previously described in detail.22 Insulin was assayed with a radioimmunoassay kit using an I125-iodinated insulin tracer, anti-human insulin-specific antibody, and human insulin standards from Linco Research, Inc. (St. Charles, MO). Glucose was measured enzymatically (glucose oxidase) with an automated YSI 2300 analyzer from YSI Life Sciences (Yellow Spring, OH). Insulin resistance was assessed by HOMA-IR from values obtained from plasma glucose and insulin assays.23 Total cholesterol, LDL- and HDL-cholesterol, and triglycerides were determined on fasting samples with the Beckman Coulter Synchron LX (Fullerton, CA).
CVD-related pregnancy complications
A woman was considered to have a CVD-related pregnancy complication if she experienced any of the following: HDP [defined (per prior American College of Obstetrics and Gynecology definitions that were in place at the time of the MAMAS study implementation),24 new-onset hypertension in pregnancy with proteinuria (preeclampsia) or without proteinuria (gestational hypertension)], delivering an SGA baby (defined as <10th percentile of weight for gestational age based on U.S. growth curves), or preterm delivery (any delivery at a gestational age <37 weeks). Given the common vascular underpinnings of these pregnancy complications13 and to maximize statistical power to detect differences, we combined preterm delivery, delivering an SGA baby, and HDP into a single CVD-related pregnancy complication exposure group.9
Description of covariates
Prepregnancy weight and height were ascertained from the medical records for the vast majority of participants and if missing was asked on a follow-up survey of MAMAS study participants. Smoking was obtained from the study medical records and follow-up surveys.
Statistical methods
We performed descriptive statistics, including n's, means, and percentages among women with and without pregnancy complications. Differences in the group-specific biomarker trajectories were assessed using linear mixed models (LMMs), using random effects to account for within-subject correlation of the three repeated measures. LMMs allow statistical testing of differences in both the average biomarker level (averaged across time points studied) and testing for differences in biomarker trajectories. The dichotomous group categories included (1) women with CVD-related pregnancy complications and (2) women without CVD-related pregnancy complications.
Cardiometabolic disease biomarkers studied included BP, serum glucose, insulin, HOMA-IR, total cholesterol, LDL, HDL, triglycerides, log-transformed IL-6, and TNF. Covariates for multivariable adjustment included age, gestational diabetes mellitus, current smoking, prepregnancy BMI, BP, age × time, and BMI × time. The interaction terms were included to account for time varying effects of age and BMI over time. All of the covariates were also entered as time-dependent variables in multivariable models. We plotted crude and multivariable adjusted plots of biomarker at the three time points during and postpregnancy in normal and abnormal pregnancy. A biomarker level or trajectory was considered significantly different between women with and without a pregnancy complication if the p value was <0.05 for the main effect or the multiplicative interaction term between pregnancy complication and time, respectively. In secondary analysis, we jointly included all biomarkers that demonstrated a statistically significant trajectory (in the primary analysis) in a multivariable model. We used SAS version 9.4 (SAS Institute, Inc., Cary, NC) for all analyses.
Ethical approval for this study was provided by the University of California San Francisco Institutional Review Board (formerly called the Center for Human Research).
Results
Characteristics of study participants
Table 1 summarizes the characteristics of study participants. In the MAMAS intervention study, women had a mean age of 28 years (standard deviation [SD] 6), mean prior pregnancies of 0.8 (SD 1.0), 13% were white, 36% African American, and 32% Latina. There were 22 women who experienced one or more CVD-related pregnancy complications. These included 18 women with preeclampsia/eclampsia, 15 women who delivered an SGA baby, and 8 women with preterm delivery (<37 weeks). There were 10 women who experienced more than one CVD-related pregnancy complication, (including 7 women with 2 pregnancy complications and 3 women with 3 pregnancy complications). Among all women in the MAMAS study, mean early pregnancy systolic BP was 111 mmHg (SD 10.8 mmHg) and mean diastolic BP was 63 mmHg (8.4 mmHg).
Table 1.
Baseline Characteristics: MAMAS Intervention Study, by Pregnancy Complication Status and Among All Study Participants
| No pregnancy complication (n = 88) | Pregnancy complication (n = 22) | All participants (n = 110) | |
|---|---|---|---|
| Age (years), mean (SD) | 27.8 (5.8) | 28.2 (5.7) | 27.8 (5.7) |
| Race/ethnicity, n (%) | |||
| White | 10 (11.5) | 4 (18.2) | 14 (12.8) |
| African American | 30 (34.5) | 9 (41) | 39 (35.8) |
| Latino | 28 (32.2) | 7 (31.8) | 35 (32.1) |
| Other/multiracial | 19 (21.8) | 2 (9.1) | 21 (19.2) |
| Education, n (%) | |||
| <12 years | 9 (10.2) | 1 (4.6) | 10 (9.1) |
| High school graduate/GED | 20 (22.7) | 10 (45.5) | 30 (27.3) |
| Any college or vocational training | 46 (52.3) | 10 (45.5) | 56 (50.9) |
| College graduate or higher | 13 (14.8) | 1 (4.6) | 14 (12.7) |
| Marital status, n (%) | |||
| Married or in committed relationship | 63 (71.6) | 11 (50) | 74 (67.3) |
| Single, separated, or divorced | 25 (28.4) | 11 (50) | 36 (32.7) |
| Household income, mean (SD) | $26,612 ($23,078) | $17,684.5 ($18,813) | $24,723 ($22,459) |
| No. of previous children, mean (SD) | 0.89 (0.84) | 0.50 (0.86) | 0.8 (1.0) |
| Nulliparous, n (%) | 41 (46.6) | 14 (63.6) | 55 (50) |
| Prepregnancy BMI, kg/m2, mean (SD) | 30.1 (29.2) | 31.5 (29.6) | 30.4 (4.3) |
| Prepregnancy weight status, n (%) | |||
| Normal or overweight | 48 (57.8) | 10 (45.5) | 58 (55.2) |
| Class I obese | 23 (27.7) | 7 (31.8) | 30 (28.6) |
| Class II obese | 12 (14.5) | 5 (22.7) | 17 (16.2) |
| Smoking status, n (%) | |||
| Current smoker | 3 (3.6) | 2 (10) | 5 (4.8) |
| Former smoker | 36 (42.9) | 8 (40) | 44 (42.3) |
| Never smoker | 45 (53.6) | 10 (50) | 55 (52.9) |
| Leisure time physical activity, n (%) | |||
| Inactive or light activity | 47 (57.3) | 11 (55) | 58 (56.9) |
| Moderate or vigorously around three times/week | 18 (22.0) | 3 (15) | 21 (20.6) |
| Moderate or vigorously on most days | 17 (20.7) | 6 (30) | 23 (22.6) |
Pregnancy complications include HDP (including pre-/eclampsia or gestational hypertension), preterm delivery, or delivery of an SGA infant.
BMI, body mass index; GED, General education diploma; HDP, hypertensive disorders of pregnancy; MAMAS, Maternal Adiposity Metabolism and Stress study; SD, standard deviation; SGA, small for gestational age.
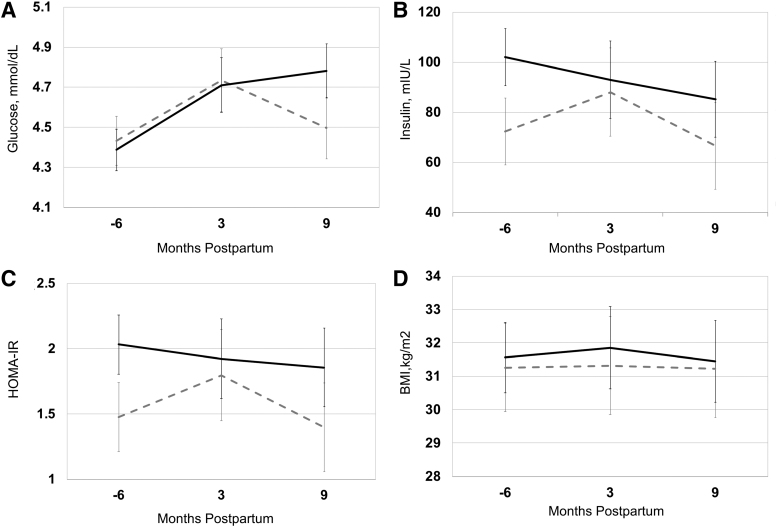
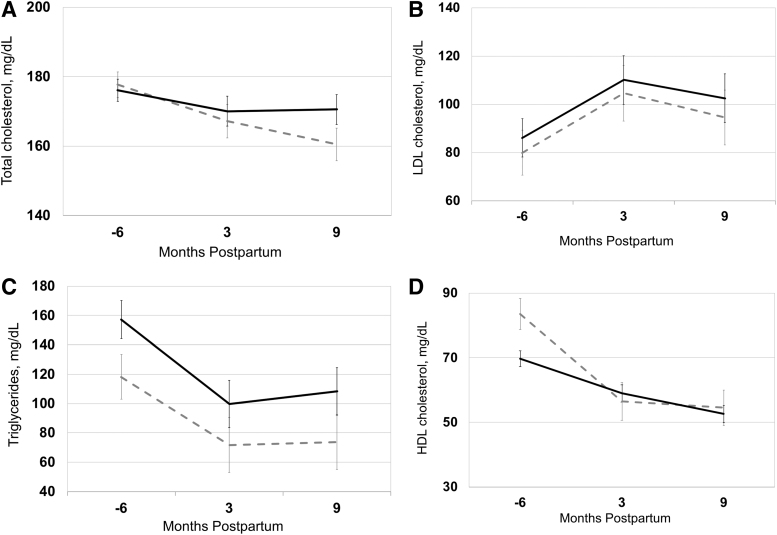
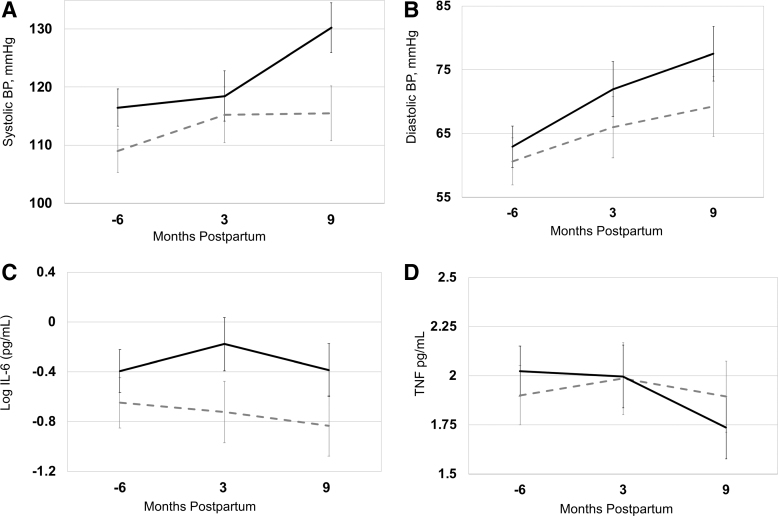
The Figures 1–3 (A–D) and Table 2 show the levels and trajectories of biomarkers relative to months postdelivery.
FIG. 1.
(A–D) Glucose, insulin, HOMA-IR, and BMI levels and trajectories in pregnancy and postpartum in normal pregnancy (dashed gray line, n = 88) compared with CVD-related pregnancy complications (black solid line, n = 22). Data points represent adjusted means with standard error bars. Mixed model linear regression was used to test differences in levels and trajectory between two groups. The horizontal axis represents months relative to delivery and −6 refers to 12–20 weeks of gestation. BMI, body mass index; CVD, cardiovascular disease; HOMA-IR, homeostasis model assessment of insulin resistance.
FIG. 3.
(A–D) Lipid levels and trajectories in pregnancy and postpartum, in normal pregnancy (dashed gray line, n = 88) compared with CVD-related pregnancy complications (black solid line, n = 22). Data points represent adjusted means with standard error bars. Mixed model linear regression was used to test differences in levels and trajectory between two groups. The horizontal axis represents months relative to delivery and −6 refers to 12–20 weeks of gestation.
Table 2.
Adjusted Least Square Means for CVD Biomarkers at Time Points Relative to Delivery, and According to CVD-Related Pregnancy Complication Status, in the MAMAS Study
| Time relative to delivery, (months) | No pregnancy complication | Pregnancy complication | p | |
|---|---|---|---|---|
| Glucose (mmol/L) | −6 | 4.43 | 4.39 | p (level) = 0.42 |
| p (trajectory) = 0.008 | ||||
| 3 | 4.73 | 4.71 | ||
| 9 | 4.5 | 4.78 | ||
| Insulin (mIU/L) | −6 | 72.29 | 102.02 | p (level) = 0.06 |
| p (trajectory) = 0.24 | ||||
| 3 | 88.06 | 93.01 | ||
| 9 | 66.59 | 85.19 | ||
| HOMA-IR | −6 | 1.48 | 2.03 | p (level) = 0.04 |
| p (trajectory) = 0.32 | ||||
| 3 | 1.8 | 1.92 | ||
| 9 | 1.4 | 1.86 | ||
| BMI (kg/m2) | −6 | 31.26 | 31.56 | p (level) = 0.76 |
| p (trajectory) = 0.90 | ||||
| 3 | 31.32 | 31.85 | ||
| 9 | 31.22 | 31.45 | ||
| SBP, mmHg | −6 | 109.02 | 116.48 | p (level) = 0.001 |
| p (trajectory) = 0.02 | ||||
| 3 | 115.23 | 118.47 | ||
| 9 | 115.5 | 130.21 | ||
| DBP, mmHg | −6 | 60.6 | 62.93 | p (level) = 0.01 |
| p (trajectory) = 0.13 | ||||
| 3 | 65.98 | 71.95 | ||
| 9 | 69.23 | 77.51 | ||
| Log (IL-6) (pg/mL) | −6 | −0.65 | −0.4 | p (level) = 0.01 |
| p (trajectory) = 0.21 | ||||
| 3 | −0.72 | −0.18 | ||
| 9 | −0.83 | −0.39 | ||
| TNF (pg/mL) | −6 | 1.9 | 2.02 | p (level) = 0.93 |
| p (trajectory) = 0.06 | ||||
| 3 | 1.99 | 2 | ||
| 9 | 1.89 | 1.73 | ||
| Total cholesterol (mg/dL) | −6 | 177.67 | 176.09 | p (level) = 0.62 |
| p (trajectory) = 0.33 | ||||
| 3 | 167.14 | 170 | ||
| 9 | 160.42 | 170.51 | ||
| LDL cholesterol (mg/dL) | −6 | 79.93 | 86.06 | p (level) = 0.36 |
| p (trajectory) = 0.95 | ||||
| 3 | 104.62 | 110.08 | ||
| 9 | 94.55 | 102.51 | ||
| Triglycerides (mg/dL) | −6 | 118.13 | 157.26 | p (level) = 0.005 |
| p (trajectory) = 0.68 | ||||
| 3 | 71.67 | 99.64 | ||
| 9 | 73.57 | 108.27 | ||
| HDL (mg/dL) | −6 | 80.8 | 64.06 | p (level) = 0.04 |
| p (trajectory) = 0.15 | ||||
| 3 | 47.35 | 45.64 | ||
| 9 | 50.73 | 45.63 |
For time relative to delivery: −6 refers to 12–20 weeks of gestation. Pregnancy complications include HDP (including pre-/eclampsia or gestational hypertension), preterm delivery, or delivery of an SGA infant.
CVD, cardiovascular disease; DBP, diastolic blood pressure; HDL, high-density lipoprotein; HOMA-IR, homeostasis model assessment of insulin resistance; IL-6, interleukin-6; LDL, low-density lipoprotein; SBP, systolic blood pressure; TNF, tumor necrosis factor.
Measures of insulin resistance
In mixed linear models, glucose trajectory (but not level) was significantly higher from early pregnancy to 9 months postpartum among women with a CVD-related pregnancy complication (p = 0.008) (Fig. 1A). Insulin levels were visually higher among women with CVD-related pregnancy complications versus normal pregnancy and this was of borderline statistical significance (p = 0.06) (Fig. 1B). Insulin trajectory did not significantly differ among women with CVD-related pregnancy complications versus normal pregnancy (Fig. 1B). HOMA-IR levels (p = 0.04) but not trajectory were significantly higher among women with a CVD-related pregnancy complication versus normal pregnancy (Fig. 1C).
Body mass index
Neither the levels nor trajectory for BMI differed in complicated versus normal pregnancies (Fig. 1D).
Blood pressure
Systolic BP levels (p = 0.001) and trajectory (p = 0.01) were both significantly higher from early pregnancy to 9 months postpartum among women with a CVD-related pregnancy complication (Fig. 2A). Diastolic BP levels (p = 0.01) (but not trajectory) were higher among women with a CVD-related pregnancy complication versus uncomplicated pregnancy (Fig. 2B).
FIG. 2.
(A–D) Systolic blood pressure, diastolic blood pressure, IL-6, and TNF levels and trajectories in pregnancy and postpartum, in normal pregnancy (dashed gray line, n = 88) compared with CVD-related pregnancy complications (black solid line, n = 22). Data points represent adjusted means with standard error bars. Mixed model linear regression was used to test differences in levels and trajectory between two groups. The horizontal axis represents months relative to delivery and −6 refers to 12–20 weeks of gestation. IL-6, interleukin-6; TNF, tumor necrosis factor.
Inflammatory biomarkers
IL-6 levels (p = 0.01) but not trajectory were higher among women with CVD-related pregnancy complications (Fig. 2C). TNF-alpha levels did not differ (Fig. 2D). However, TNF-alpha trajectory visually differed such that TNF-alpha levels were higher in first trimester and dropped to relatively lower levels at 9 months postpartum in women with CVD-related pregnancy complications compared with women with normal pregnancy and this was of borderline statistical significance (p = 0.06) (Fig. 2D).
Lipids
Neither levels nor trajectory of total cholesterol or LDL was statistically different in women with a CVD-related pregnancy complication (Figs. 3A, B). Triglyceride levels (p = 0.005) (but not trajectory) were higher among women with CVD-related pregnancy complications (Fig. 3C). HDL cholesterol levels (p = 0.04) but not trajectory were higher among women with CVD-related pregnancy complications (Fig. 3D).
Secondary analysis
In secondary analysis of glucose trajectories, we additionally adjusted for systolic BP level and systolic BP × time. Glucose trajectories remained significantly elevated among women with a pregnancy complication versus women with uncomplicated pregnancy (p = 0.03). In models considering systolic BP trajectories, we additionally adjusted for glucose and glucose × time and results were similar (p value for difference in systolic BP trajectory = 0.04).
Discussion
Summary of key findings
We demonstrate that the trajectories of systolic BP and glucose were increased from early pregnancy until 9 months postpartum among women with one or more CVD-related pregnancy complications. In addition, our findings demonstrate that the levels of diastolic BP, HOMA-IR, triglycerides, HDL, and IL-6 were higher among women with a CVD-related pregnancy complication. Our findings were not driven by baseline BMI or peripartum changes in BMI. Taken together, we find evidence that both early pregnancy CVD biomarker levels and peripartum CVD biomarker trajectories are increased among women with CVD-related pregnancy complications.
Our findings that biomarkers for which levels alone differed suggest that for some cardiometabolic disease pathways (such as inflammation or dyslipidemia), women who develop CVD-related pregnancy complications have higher early pregnancy CVD risk factor levels that remain proportionally elevated in the postpartum period. In contrast, the biomarkers for which we found that trajectories differed among women with CVD-related pregnancy complications versus uncomplicated pregnancy (systolic BP and glucose), our results demonstrated even higher postpartum biomarker levels than would be expected based on the early pregnancy biomarker level elevations. This raises the possibility that experiencing the adverse pregnancy outcome may activate specific biologic pathways that result in postpartum accelerations of systolic BP and glucose compared with experiencing an uncomplicated pregnancy.
Glucose and insulin resistance
Insulin resistance in normal pregnancy is likely mediated by several mechanisms, including increased maternal adiposity, placental lactogen, and human chorionic gonadotropin.25 Metabolic syndrome and insulin resistance in the absence of gestational diabetes are related to the onset of HDP26 and medically indicated preterm birth.27,28 We found that trajectories of glucose and levels of insulin and insulin resistance (as measured by HOMA-IR) were increased in CVD-related pregnancy complications versus normal pregnancy. This is a significant finding that both extends and compliments a prior study that highlights that each degree of late second/early third trimester gestational glucose intolerance, as measured by oral glucose tolerance testing, predicts distinct trajectories of β cell function, insulin sensitivity, and glycemia in the first 3 years postpartum.29 Our findings suggest that vascular complications of pregnancy can additionally increase a woman's early pregnancy to late postpartum glucose trajectory, even when accounting for the presence of gestational diabetes mellitus (via multivariable adjustment). We postulate that this may in turn elevate her future risk of developing diabetes and possibly CVD through well-delineated accepted pathways of insulin resistance with CVD development.
Blood pressure
Women diagnosed with preeclampsia or gestational hypertension consistently have higher systolic and diastolic BP both antepartum24 and postpartum.30 Data from the Avon Longitudinal Study demonstrated increases in systolic and diastolic BP trajectories after 18 weeks and faster increases after 30 weeks of gestation in women with preeclampsia and gestational hypertension.19 Still, it is uncertain whether this BP rise persists postpartum or whether it reverts to early pregnancy or prepregnancy levels. Preeclampsia is a known risk factor for later high BP.15,30 Whether this increased later high BP is entirely mediated by a woman's prepregnancy levels of BP has not been certain. Our data demonstrating increased trajectories of systolic BP in women with complicated pregnancies (compared with normal pregnancies) suggest that a complicated pregnancy may serve to accelerate the rate of rise of high BP in women having CVD-related pregnancy complication. Notably, our data demonstrate that the BP increase persists in all women, but is more exaggerated at 9 months postpartum among women with CVD-related pregnancy complications compared with normal pregnancies. Mechanisms underlying increased BP trajectories should be studied in future investigations. Furthermore, CVD-related pregnancy complications should alert providers to refer patients early to medical providers during the postpartum setting for more aggressive risk factor screening and modification.
Inflammation
Increased inflammation is related to CVD-related pregnancy outcomes.31 Amniotic C-reactive protein, a biomarker of systemic inflammation, is elevated among women who deliver preterm (<34 weeks) compared with term, reflecting a key role of inflammation in very preterm birth.32 The interplay between increased inflammation (as reflected by increased TNF-alpha) in hypertensive pregnancies may underlie associated increased maternal insulin resistance,33 demonstrating the complex interplay among several cardiometabolic pathways in CVD-related pregnancy complications. Our study demonstrated a significantly higher level of IL-6 among women experiencing a CVD-related pregnancy complication. Our data did not, however, demonstrate significantly different trajectories of the biomarkers IL-6 or TNF-alpha, although we may have been limited by statistical power to detect differences.
Dyslipidemia
Maternal hypertriglyceridemia but not HDL cholesterol is associated with preterm delivery.34 Both high triglycerides and low HDL are related to the onset of preeclampsia.4 Our findings demonstrated that pregnancy and postpartum triglycerides and HDL levels, but not trajectories, were increased in women experiencing CVD-related pregnancy complications versus normal pregnancy. Lifestyle modification, including dietary changes and exercise measures, can lower triglycerides and raise HDL cholesterol levels in the general population, however, these interventions have not been systematically tested to determine if they would prevent peripartum levels of lipids and/or prevent CVD-related pregnancy complications.
Strengths and limitations
Our study had the strength of directly collected biomarker measurements at three serial time points in a diverse group of women. An important limitation was the lack of preconception risk factors and prior pregnancy complication data. Although we could not assess this earlier time point for our analysis that aimed at assessing differences in level and trajectory of CVD biomarkers, the lack of preconception biomarkers would not have affected our results demonstrating that systolic BP and glucose trajectories differed in women during the three time points we did study. Adjusting for preconception biomarkers was not the aim of our study, but rather studying differences in the level and trajectories of these biomarkers during and after pregnancy; therefore, preconception biomarkers would have added a relevant extra early time point to our study.
Due to the relatively small numbers of study participants, we did not exclude women with gestational diabetes mellitus from either the group with pregnancy complications or the reference group. This could have possibly led to attenuation of our estimates toward the null value (particularly our glucose, HOMA-IR, and insulin results). Given that we did not have a pregnancy complication history for many of the parous women in our study, this likely may have led to a possible underestimation of the impact of pregnancy complications on postpregnancy biomarkers as some of the women with uncomplicated study pregnancies (“unexposed”) may have had a history of pregnancy complications in previous pregnancies that we did not capture. Our study had a small sample size with limited statistical power to explore individual pregnancy complications. We did not collect information on chronic kidney disease or rheumatologic diseases. Furthermore, our study population of only overweight and obese women may limit generalizability of these findings.
Clinical and public health implications
With more than four million women giving birth in the United States each year, and with ∼85% of women undergoing a pregnancy during her lifetime, the peripartum period represents a window of health care opportunity for the majority of women in the United States. For instance, the vast majority of women utilize health care in the late postpartum period (2 months to 2 years postpartum).35 Identifying women at risk for chronic disease and beginning prevention efforts during the postpartum period could be highly impactful. Knowing when a woman's risk of cardiometabolic disease increases is therefore an important public health problem. Future studies aimed at uncovering the specific biologic mechanisms through which CVD-related pregnancy complications alter cardiometabolic rick trajectories may lend important insights into the biology of pregnancy and potentially novel cardiometabolic pathways.
Conclusions
BP and glucose levels are not only higher but also have higher peripartum trajectories in women having CVD-related pregnancy complications, including HDP, preterm delivery, and delivering an SGA baby. Specific biologic mechanisms that lead to adverse cardiometabolic maladaptation in women with CVD-related pregnancy complications, including long-term cardiovascular effects of placental insufficiency, should be investigated in future studies. This study (1) identifies some biomarker levels and trajectories that differ between women with and without pregnancy complications, which are apparent before the development of those complications and (2) identifies potential biomarker pathways through which the increased CVD risk among women with a history of pregnancy complications may be mediated.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
NIH/NHLBI 1U01HL09797301.
References
- 1. Sattar N, Greer IA. Pregnancy complications and maternal cardiovascular risk: Opportunities for intervention and screening? BMJ 2002;325:157–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Edstedt Bonamy A-K, Parikh NI. Predicting women's future cardiovascular health from pregnancy complications. Curr Cardiovasc Risk Rep 2013;7:173–182 [Google Scholar]
- 3. Harville EW, Viikari JS, Raitakari OT. Preconception cardiovascular risk factors and pregnancy outcome. Epidemiology 2011;22:724–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baumfeld Y, Novack L, Wiznitzer A, et al. . Pre-conception dyslipidemia is associated with development of preeclampsia and gestational diabetes mellitus. PLoS One. 2015;10:e0139164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hauth JC, Clifton RG, Roberts JM, et al. . Maternal insulin resistance and preeclampsia. Am J Obstet Gynecol 2011;204:327..e321–e326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Magnussen EB, Vatten LJ, Myklestad K, Salvesen KÅ, Romundstad PR. Cardiovascular risk factors prior to conception and the length of pregnancy: Population-based cohort study. Am J Obstet Gynecol 2011;204:526.. e521–e526, e528. [DOI] [PubMed] [Google Scholar]
- 7. Magnussen EB, Vatten LJ, Lund-Nilsen TI, Salvesen KA, Davey Smith G, Romundstad PR. Prepregnancy cardiovascular risk factors as predictors of pre-eclampsia: Population based cohort study. BMJ 2007;335:978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Romundstad PR, Davey Smith G, Nilsen TI, Vatten LJ. Associations of prepregnancy cardiovascular risk factors with the offspring's birth weight. Am J Epidemiol 2007;166:1359–1364 [DOI] [PubMed] [Google Scholar]
- 9. Brosens I, Pijnenborg R, Vercruysse L, Romero R. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am J Obstet Gynecol 2011;204:193–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kalafat E, Thilaganathan B. Cardiovascular origins of preeclampsia. Curr Opin Obstet Gynecol 2017;29:383–389 [DOI] [PubMed] [Google Scholar]
- 11. Sultana Z, Maiti K, Aitken J, Morris J, Dedman L, Smith R. Oxidative stress, placental ageing-related pathologies and adverse pregnancy outcomes. Am J Reprod Immunol 2017;77:e12653. [DOI] [PubMed] [Google Scholar]
- 12. Thornburg KL, O'Tierney PF, Louey S. Review: The placenta is a programming agent for cardiovascular disease. Placenta 2010;31 Suppl:S54–S59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lane-Cordova AD, Khan SS, Grobman WA, Greenland P, Shah SJ. Long-term cardiovascular risks associated with adverse pregnancy outcomes. J Am Coll Cardiol 2019;73:2106. [DOI] [PubMed] [Google Scholar]
- 14. Fraser A, Nelson SM, Macdonald-Wallis C, et al. . Associations of pregnancy complications with calculated cardiovascular disease risk and cardiovascular risk factors in middle age: The Avon Longitudinal Study of parents and children. Circulation 2012;125:1367–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Parikh NI, Norberg M, Ingelsson E, et al. . Association of pregnancy complications and characteristics with future risk of elevated blood pressure: The Vasterbotten Intervention Program. Hypertension 2017;69:475–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Perng W, Stuart J, Rifas-Shiman SL, Rich-Edwards JW, Stuebe A, Oken E. Preterm birth and long-term maternal cardiovascular health. Ann Epidemiol 2015;25:40–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tanz LJ, Stuart JJ, Missmer SA, et al. . Cardiovascular biomarkers in the years following pregnancies complicated by hypertensive disorders or delivered preterm. Pregnancy Hypertens 2018;13:14–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kaaja RJ, Greer IA. Manifestations of chronic disease during pregnancy. JAMA 2005;294:2751–2757 [DOI] [PubMed] [Google Scholar]
- 19. Macdonald-Wallis C, Lawlor DA, Fraser A, May M, Nelson SM, Tilling K. Blood pressure change in normotensive, gestational hypertensive, preeclamptic, and essential hypertensive pregnancies. Hypertension 2012;59:1241–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vieten C, Laraia BA, Kristeller J, et al. . The mindful moms training: Development of a mindfulness-based intervention to reduce stress and overeating during pregnancy. BMC Pregnancy Childbirth 2018;18:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Headen I, Laraia B, Coleman-Phox K, Vieten C, Adler N, Epel E. Neighborhood typology and cardiometabolic pregnancy outcomes in the maternal adiposity metabolism and stress study. Obesity (Silver Spring) 2019;27:166–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zota AR, Geller RJ, Romano LE, et al. . Association between persistent endocrine-disrupting chemicals (PBDEs, OH-PBDEs, PCBs, and PFASs) and biomarkers of inflammation and cellular aging during pregnancy and postpartum. Environ Int 2018;115:9–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004;27:1487–1495 [DOI] [PubMed] [Google Scholar]
- 24. American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy. Hypertension in pregnancy: Report of the American College of Obstetricians and Gynecologists' Task Force on Hypertension in Pregnancy. Obstet Gynecol 2013;122:1122–1131 [DOI] [PubMed] [Google Scholar]
- 25. Abhari FR, Ghanbari Andarieh M, Farokhfar A, Ahmady S. Estimating rate of insulin resistance in patients with preeclampsia using HOMA-IR index and comparison with nonpreeclampsia pregnant women. Biomed Res Int 2014;2014:140851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kaaja R. Insulin resistance syndrome in preeclampsia. Semin Reprod Endocrinol 1998;16:41–46 [DOI] [PubMed] [Google Scholar]
- 27. Hooijschuur MC, Ghossein-Doha C, Al-Nasiry S, Spaanderman ME. Maternal metabolic syndrome, preeclampsia, and small for gestational age infancy. Am J Obstet Gynecol 2015;213:370..e371–e377. [DOI] [PubMed] [Google Scholar]
- 28. Temming LA, Tuuli MG, Stout MJ, Macones GA, Cahill AG. Maternal and perinatal outcomes in women with insulin resistance. Am J Perinatol 2016;33:776–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kramer CK, Swaminathan B, Hanley AJ, et al. . Each degree of glucose intolerance in pregnancy predicts distinct trajectories of beta-cell function, insulin sensitivity, and glycemia in the first 3 years postpartum. Diabetes Care 2014;37:3262–3269 [DOI] [PubMed] [Google Scholar]
- 30. Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: Systematic review and meta-analysis. BMJ 2007;335:974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Triggianese P, Perricone C, Chimenti MS, De Carolis C, Perricone R. Innate immune system at the maternal-fetal interface: Mechanisms of disease and targets of therapy in pregnancy syndromes. Am J Reprod Immunol 2016;76:245–257 [DOI] [PubMed] [Google Scholar]
- 32. Ghezzi F, Franchi M, Raio L, et al. . Elevated amniotic fluid C-reactive protein at the time of genetic amniocentesis is a marker for preterm delivery. Am J Obstet Gynecol 2002;186:268–273 [DOI] [PubMed] [Google Scholar]
- 33. Solomon CG, Seely EW. Brief review: Hypertension in pregnancy: A manifestation of the insulin resistance syndrome? Hypertension 2001;37:232–239 [DOI] [PubMed] [Google Scholar]
- 34. Moayeri M, Heida KY, Franx A, Spiering W, de Laat MW, Oudijk MA. Maternal lipid profile and the relation with spontaneous preterm delivery: A systematic review. Arch Gynecol Obstet 2017;295:313–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bryant A, Blake-Lamb T, Hatoum I, Kotelchuck M. Women's use of health care in the first 2 years postpartum: Occurrence and correlates. Matern Child Health J 2016;20:81–91 [DOI] [PubMed] [Google Scholar]