Abstract
Oral mucosa is the target tissue for many microorganisms involved in periodontitis and other infectious diseases affecting the oral cavity. Three-dimensional (3D) in vitro and ex vivo oral mucosa equivalents have been used for oral disease modeling and investigation of the mechanisms of oral bacterial and fungal infections. This review was conducted to analyze different studies using 3D oral mucosa models for the evaluation of the interactions of different microorganisms with oral mucosa. In this study, based on our inclusion criteria, 43 articles were selected and analyzed. Different types of 3D oral mucosa models of bacterial and fungal infections were discussed in terms of the biological system used, culture conditions, method of infection, and the biological endpoints assessed in each study. The critical analysis revealed some contradictory reports in this field of research in the literature. Challenges in recovering bacteria from oral mucosa models were further discussed, suggesting possible future directions in microbiomics, including the use of oral mucosa-on-a-chip. The potential use of these 3D tissue models for the evaluation of the effects of antiseptic agents on bacteria and oral mucosa was also addressed. This review concluded that there were many aspects that would require optimization and standardization with regard to using oral mucosal models for infection by microorganisms. Using new technologies—such as microfluidics and bioreactors—could help to reproduce some of the physiologically relevant conditions and further simulate the clinical situation.
Impact statement
Tissue-engineered or commercial models of the oral mucosa are very useful for the study of diseases that involve the interaction of microorganisms and oral epithelium. In this review, challenges in recovering bacteria from oral mucosa models, the potential use of these three-dimensional tissue models for the evaluation of the effects of antiseptic agents, and future directions in microbiomics are discussed.
Keywords: 3D tissue models, bacterial infection, biofilm, candidiasis, engineered oral mucosa, microbiomics, oral mucosa models
Introduction
The oral cavity contains a large number of microorganisms, most of which are part of the normal flora and have a commensalism relationship with the host tissues. In this diverse population of microorganisms, there are some opportunistic and also nonresident species, which can cause diseases.1 Oral mucosa is one of the barriers in the oral cavity with an important role in inhibition of microorganism's colonization. It consists of the epithelium—including stratified and differentiated keratinocytes—and the connective tissue layer, containing predominantly fibroblasts.2 Even though there is a harsh exposure to different microorganisms like Streptococci, Actinobacillus, Porphyromonas, Tannerella, Fusobacterium, Prevotella, Campylobacter, Eikenella, and Treponema species, the oral mucosa limits microflora colonization and protects the oral cavity from invasion of microorganisms with high turnover and shedding, and secretion of different types of cytokines and antimicrobial proteins, like defensins.3 However, in certain conditions, breakdown of homeostasis in the normal flora would result in change of commensalism relationship of normal flora to parasitism, increase in the number of opportunistic microorganisms, and invasion into the underlying tissues, leading to disease development.4 In periodontal diseases, invasion of oral epithelial cells by pathogens (like Porphyromonas gingivalis or Fusobacterium nucleatum), their survival and proliferation in the epithelial tissue, and their penetration to connective tissue cause some immune responses that have key roles in periodontal breakdown.5 In oral mucositis, following chemotherapy and radiotherapy in some patients, oral tissues encounter damage and pathogens can penetrate tissues and cause infection.6 In candidiasis, invasion of oral epithelium by Candida albicans—especially in immunocompromised patients—is responsible for infection.7
Study of the mechanism of disease development in periodontal tissue or infection of oral mucosa by fungi or bacteria—which leads to periodontal disease, mucositis, stomatitis, candidiasis, or other mucosal infections—requires in vitro tissue culture models containing microorganisms to simulate the in vivo situation. Although two-dimensional (2D) monolayer cell culture systems contributed to the progress of our knowledge of oral microbiome, a multilayer epithelium, which works as a barrier against pathogen invasion and synergistic effects of fibroblasts and keratinocytes on secretion of cytokines, is missing from the monolayer cell culture systems.2,8,9 Degradation of epithelial layer, direct exposure of the connective tissue to the oral biofilm, and active participation of fibroblasts in bacterially induced inflammation are some of the limitations of in vitro multilayer epithelium models.10,11 Mimicking the in vivo condition requires models that reflect native tissue and their interactions with pathogens. For this purpose, many researchers use different types of oral mucosa equivalents as a relevant in vitro tool to investigate the interaction of microorganisms with oral mucosa, the process of epithelial layer's damage, and initial steps of infection, as well as treatment approaches.12
Isolation and expansion of epithelial and fibroblast cells from gingiva, buccal or palatal mucosa, seeding and culture of fibroblast in a suitable substrate, and finally, seeding of epithelial cells onto the engineered connective tissue layer is a common procedure for engineering of oral mucosa models. There are also commercially available oral mucosa models, which can be used for microbiological studies. Engineered or commercial models of oral mucosa are very useful for the study of diseases that involve interaction of microorganisms and oral epithelium.13 Reducing animal experiments is one of the most advantages of using tissue-engineered models in microbiology.14 This aspect is also considered in skin tissue engineering, using skin substitutes for in vitro infection modes, and engineering of intestinal functional models for application in food microbiology.15,16 Interaction of oral microbiomes with other microbiomes in various sites of human body, their implications in systemic pathologies (like esophageal cancer, colorectal cancer, pancreatic cancer, and inflammatory diseases such as atherosclerosis, pneumonia, heart diseases, and rheumatoid arthritis), and its relationship to diabetes and Alzheimer's disease highlight the importance of engineering in vitro models that mimic oral cavity situation for better disease diagnosis and treatment.17–20
Two review articles have been published thus far that investigate in vitro and in vivo model systems' potential for studying the human microbiome, but not oral mucosa equivalents. Coenye and Nelis drew attention to the tools that could be used for understanding medically relevant biofilms, while Werlang et al. investigated the requirement of mucin mimetics for in vitro culture systems and modulation of microbial community structure.13,21 The goal of this study was to answer the focused questions: what are the methods used for oral mucosa infection and which microorganisms are usually used for infection? Furthermore, the in vitro biological endpoint assessed as the outcome of the oral mucosa models' infection was evaluated.
Materials and Methods
The defined question of the study was used for the extraction of keywords. PubMed and Scopus databases were searched for the period time of 2000–2020 using the following separated or combined keywords: 3d oral mucosa, engineered oral mucosa, oral mucosa models, oral mucosa equivalents, bacterial infection, microbiology, microorganism, microbiota, Candida albicans, Porphyromonas, Fusobacterium, candidiasis, periodontal diseases, periodontitis, Streptococcus, and biofilms. Only English-language articles in which commercialized oral mucosa or full-thickness oral mucosa models were used for infection with one or multispecies bacteria were included. Studies on the interaction of microorganisms with monolayer cell cultures, epithelial cell 2D cultures, or epithelial cell sheets with lack of fibroblasts were excluded. Articles on the investigation of oral mucosa models for other purposes like biocompatibility of dental materials, assessment of radiotherapy-induced mucositis, or cytotoxic evaluation of oral antiseptics were excluded as well. The bibliography of selected articles was checked to identify other relevant articles. The classification of articles was according to the bacterial strain used, culture condition, oral mucosa model, time of contact between microorganism and oral mucosa model, infection evaluation, and results. Finally, 43 articles were selected for the final analysis and review.
Results
Methods of oral mucosa infection
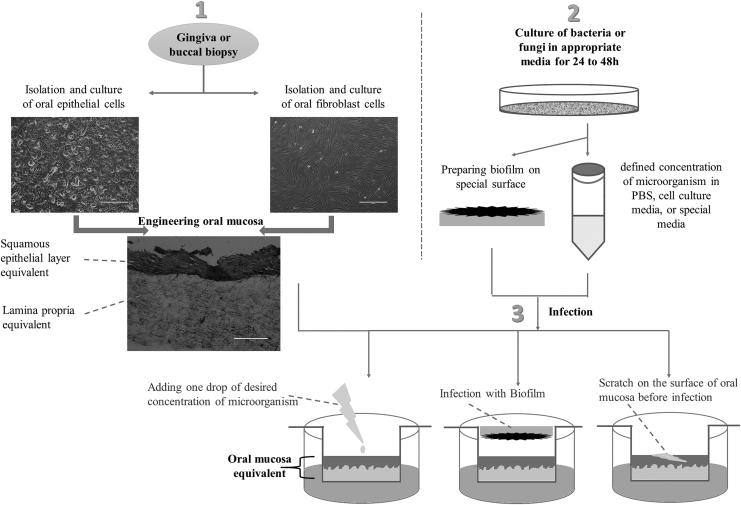
For infection of oral mucosa models, bacteria or fungi are cultured in an appropriate broth for 24–48 h, and after centrifugation, the suspension of bacteria in appropriate media—such as phosphate-buffered saline, cell culture media, or special media of microorganism—at a defined concentration is prepared. Oral mucosa is washed in antibiotic-free medium (24, 48, or 72 h before infection). Then, the desired concentration of microorganisms (respecting multiplicity of infection [MOI] of 100 bacteria per surface cell) in limited amount of appropriate media (20–50 μL) is added onto the surface of epithelial layer (center of oral mucosa model). After incubation of infected and noninfected tissues (control group) at 37°C/5% CO2 for different time points (24-, 48-, or 72-h incubation), the models are ready for analysis. The other option is producing biofilm of bacteria before infection.22–24 Also, one of the possibilities that should be considered in in vitro microbiological studies is producing damage to epithelial layer to provide a route for microbial invasion, as it occurs in some pathological conditions of the oral cavity.22,25 Figure 1 shows the different steps and methods of oral mucosa infection.
FIG. 1.
Steps and methods of oral mucosa infection.
Candida
In the oral cavity, 85 species of fungi exist—one of the most important being Candida. Denture stomatitis and candidiasis are infections related to fungus, specially, Candida albicans. Although this microorganism is a part of commensal flora and is found normally in healthy individuals, because of its opportunistic nature, its colonization could switch it to a pathogen in some patients (like elderly or immunocompromised hosts). Attachment of the yeast to mucosal cells by adhesins and invasion of cells by yeast–hyphal transition would result in mucosal inflammation. C. albicans is the most abundant yeast species in oral cavity, yet other species like C. glabrata or C. famata could co-infect with C. albicans, which can make the treatment more difficult. Even though single colonization of the cavity with C. albicans is possible, some other microorganisms—like oral Streptococci or Staphylococci—could help Candida in the production of biofilm. Coaggregation of these microorganisms as the primary colonizers of oral biofilm with Candida could enhance its filamentation and increase its pathogenicity.26,27
Table 1 shows the studies related to infection of oral mucosa models with Candida species alone or in association with other bacteria.
Table 1.
Studies Related to Infection of Oral Mucosa Models with Candida Species Alone or in Association with Other Bacteria
| Authors | Bacteria strain | Culture condition | Oral mucosa model | Time of contact between Candida and Mucosa | Assays | Results |
|---|---|---|---|---|---|---|
| Claveau et al.28 | Original clinical isolate (Candida-associated stomatitis) | 107C. albicans/mL of PBS (105/cm2) | EHOM:NOKs seeded on the collagen-embedded NOFs | 2, 4, 8, 24 h | RT-PCR, Western blotting, Zymography, ELISA | Candida increase expression of laminin-5, type IV collagen, MMP-2 and MMP-9 genes; decrease type 2 matrix metalloproteinase tissue inhibitors (TIMP-2) by oral epithelial cells |
| Mostefaoui et al.29 | C. albicans and Streptococcus salivarius (ATCC 25975) | Live and killed C. albicans (105/cm2) or S. salivarius (106/cm2) | EHOM:NOKs seeded on the collagen-embedded NOFs | 2, 4, 8, 24, 48 h | Epithelial cell viability, Masson trichrome staining, RT-PCR, ELISA | C. albicans or S. salivarius, induce release of proinflammatory mediators (IL-6, IL-8 and TNF-a) by oral epithelial cells (more efficiency of S. salivarius) |
| Mostefaoui et al.30 | Original clinical isolate (Candida-associated stomatitis) | Live and heat-inactivated C. albicans: 108C. albicans/mL (105/cm2) | EHOM:NOKs seeded on the collagen-embedded NOFs | 2, 4, 8, 24, 48 h | RT-PCR, epithelial cell viability, ELISA, Western blotting, bacteria count, H&E | Increased expression of IL-1b by oral epithelial cells in early stages of infection with live C. albicans |
| Green et al.31 | C. albicans strains: SC5314, B311 (ATCC 32354), GDH2346, and M61 | 50 μL C. albicans/PBS suspension (2 × 106 cells, 2 × 105 cells, or 2 × 104 cells/RHE model) | RHE (SkinEthic, Nice, France) (TR146 cell lines cultured on polycarbonate filters) | 12, 24, 36, 48 h | RT-PCR, SEM | Consistent detection of ALS genes in the Candida over time with progress destruction of the RHE |
| Schaller et al.32 | Clinical C. Albicans wild-type strain SC5314 | 50 μL C. albicans/PBS suspension (2 × 106 cells total) | RHE (SkinEthic, Nice, France). (TR146 cultured on polycarbonate filters) Supplemented with PMN | 12, 24 h | LDH, killing assay, qRT-PCR, FACS | Increase expression of IL-8 and GM-CSF, and chemoattraction of PMNs following infection |
| Tardif et al.33 | C. albicans LAM-1 (serotype A) | (1.5 × 106/cm2) seeded onto the EHOMs using sterile swab | EHOM:NOKs seeded on the collagen-embedded NOFs | 2, 4, 6, 12, 24, 48 h | Spectro-Photometric Analysis, RT-PCR, Western blotting, ELISA | Increased secretion of IL-18 and IFNγ in response to C. albicans |
| Dongari-Bagtzoglou and Kashleva34 | C. albicans strains: SC5314, efg1/efg1/cph1/cph1, rbt4/rbt4, rim101/rim101 | 50 μL live Candida/KSFM (106 organisms/insert) (MOI of 1:1 fungal to surface epithelial cells), or 4 mm diameter agar slices containing 103 yeast/mL on top of the epithelial layer | EpiOral (GIN-100, MaTek, Ashland, MA), NOKs over submucosa (containing NOFs), OKF6/TERT-2 cells over submucosa | 48 h | ELISA, LDH assay | Strain of Candida used for infection of oral mucosa influences the level of tissue invasion and damage infect oral epithelia |
| Samaranayake et al.35 | PL+ and PL−C. albicans isolates | — | RHOE (Skinethic, Nice, France) | 12, 24, 48 h | PASS, Genomic PCR | Expression of phospholipase gene in Candida influences its growth and invasion in the RHOE model |
| Zakrzewski and Rouabhia36 | Clinical C. albicans (Candida-associated stomatitis) | 107 cells/mL in PBS | Nonkeratinized and keratinized EHOM (NOKs seeded on the collagen embedded NOFs) | 2, 4, 8, 24 h | H&E, C. albicans count, Western blotting, IHC | Higher morphological change of C. albicans on nonkeratinized mucosa and significant disorganization of this mucosa following contact with C. albicans |
| Villar et al.37 | 12 strains of C. albicans | 1 × 105C. albicans cells in 100 μL of airlift medium | EHOM (NOKs seeded on the collagen-embedded NOFs) | 17–48 h | IHC, CLSM, TEM | Degradation of E-cadherin in epithelial cells by C. albicans facilitates its penetration in mucosal tissues |
| Ohnemus et al.38 | C. albicans strain ATCC 10231 | 105 CFU C. albicans diluted in 2 μL PBS | Ex vivo PMOCM | 24 h infection, 48 or 96 h treatment with nystatin | Evaluation of fungal growth, agar diffusion method, H&E, PASS | Equal efficiency of different dosage of Nystatin (230, 100, 20 IU) in C. albicans infection |
| Lermann and Morschhauser39 | C. albicans strains | Infection of RHOE with 5 × 105C. albicans cells. | RHOE (Skinethic Lab, Nice, France) | 48 h | Light microscopy and staining, LDH activity, PCR | Invasion of RHE by C. albicans is not dependent to expression of the SAP1–SAP6 genes |
| Decanis et al.40 | C. albicans isolated from Candida-associated candidiasis | Adjusted to 107/mL (106/cm2) | EHOM: OKF6/TERT-2 cells seeded on the collagen embedded NOFs | 4, 24 h | qRT-PCR, ELISA | Increase of epithelial cell defense against C. albicans infection by using farnesol |
| Bahri et al.41 | C. albicans (ATCC 10231) as a reference species, C. famata was isolated from water (various sites in the Mediterranean Sea) | Adjusted to 107/mL (106/cm2) | EHOM:NOKs seeded on the collagen embedded NOFs | 24 h | H&E, qRT-PCR | C. famata activate local defenses of human epithelial cells |
| Diaz et al.42 | Candida albicans SC5314, Streptococcus oralis 34 (provided by P.E. Kolenbrander), Streptococcus gordonii Challis CH1 (provided by J. M. Tanzer) and Streptococcus sanguinis SK36 (ATCC BAA-1455) | 106 cells of C. albicans or 107 cells of S. oralis or a combination of both organisms in 500 μL of salivary medium for biofilm formation | Immortalized human oral keratinocyte cell line (OKF6/TERT-2) seeded on collagen type I-embedded fibroblasts (3T3 fibroblasts) | 4, 16, 24 h | CLSM, IF, FISH, RT-PCR | Stimulation of biofilm formation of Streptococci in presence of C. albicans, increased invasion of oral mucosa by C. albicans in presence of Streptococci |
| Yadev et al.43 | C. albicans wild-type strain (CAF2–1) | 5 × 107 CFU/mL (100 μL: 5 × 106 CFU) | RHOE (Skinethic Lab, Nice, France), EpiOral (GIN-100, MaTek,Ashland,MA), FTOM (NOKs seeded on the collagen embedded NOFs) | 24 h | ELISA, IHC, PASS | Similar damage in all models following infection; more cytokine release in FTOM |
| Rouabhia et al.44 | Strains of Candida albicans: CAI4 wild-type, Δipt1 mutant, IPT1 revertant | 107/mL in PBS (105 cells/cm2) | EHOM:NOKs seeded on the collagen embedded NOFs | 24 h | qRT-PCR, ELISA | Reduced adhesion of Candida to epithelial cells in strains with disrupted IPT1 gene |
| Silva et al.45 | Six clinical isolates of C. glabrata, recovered from the oral cavity (strains D1 and AE2), vagina (strains 534784 and 585626) and urinary tract (strains 562123 and 513100); reference strain of C. glabrata (ATCC 2001) | 2 × 106 cells/mL (infected only with C. glabrata, or simultaneously with C. glabrata and C. albicans) | RHOE (Skinethic Lab, Nice, France) | 12 h | PNA FISH, CLSM, LDH activity | Increased invasiveness of C. glabrata and increased LDH release by the RHOE in presence of C. albicans |
| Semlali et al.46 | C. albicans (SC5314) | 106 cells in 200 μL of Sabouraud dextrose broth | EHOM:NOKs seeded on the collagen embedded NOFs | 24 h | qRT-PCR, Western blot, ELISA | No toxicity of KSL-W on epithelial cells and decrease of Candida virulence in its presence |
| Rouabhia et al.47 | Candida strains: CAF2-parental strain, RML1, RML2, RML3, RML4 | 104 cells/cm2 in a serum-free, antifungal-free DMEM medium | EHOM:NOKs seeded on the collagen embedded NOFs | 24 h | H&E, LDH assay, qRT-PCR, Western blot | Evidence on active role of ECM33 gene in biofilm formation and tissue damage of Candida |
| Whiley et al.7 | Denture stomatitis strain NCYC 1467, strain AC-1 from the saliva of a healthy subject, NCPF 8112 from vaginal candidosis, NCYC 1472 from an asymptomatic cervical smear | 4 × 107 CFU/mL: 50 μL = 2 × 106 CFU) | Models of human buccal and vaginal epithelia (SkinEthic Lab, Nice, France) | 4, 12, 24 h | MTT, ELISA, H&E, PAS, PL assay, SAP assay | Different response of oral and vaginal epithelial cells to C. albicans |
| de Carvalho Dias et al.12 | C. albicans SC5314 and S. aureus ATCC25923 | 1 × 107 cells/mL in RPMI 1640 | ROMT (NOK-si seeded on the collagen-embedded fibroblast cell line) | 8, 16 h | H&E, LDH assay | Synergistic interaction of C. albicans and S. aureus in tissue damage and depth of infection in ROMT |
| Sobue et al.48 | C. albicans strain SN425, C. glabrata strain GDH2269, S. oralis 34 (provided by Dr. P. Kolenbrander), and S. mitis 49456 | 20 μL media containing 106 fungal (C. albicans or C. glabrata) or 107 bacterial (S. oralis or S. mitis) cells | Keratinocyte cell line (SCC15) seeded on collagen-embedded fibroblasts (3T3 cell line) pretreated with 5-FU for mucosal injury | 6–16 h | IF, FISH, ELISA | Intensification of the inflammatory response, but not significant effect on fungal or bacterial biofilm by using 5-FU |
| Morse et al.49 | C. albicans ATCC 90028, S. sanguinis ATCC 10556, S. gordonii ATCC 10558, Actinomyces viscosus ATCC 15987, and A. odontolyticus NCTC 9935) | Single or mixed-species biofilm grown on PMMA coupons inverted and placed in direct contact with the OMMs | RHOE, EpiOral, FTOM: TR146 or FNB6 keratinocytes seeded on collagen-embedded NOFs | 12 h | H&E, Real-time qPCR, LDH activity | Increase in LDH activity and damage by C. albicans-only and mixed-species biofilms, higher extent of damage in FTOM |
| Bertolini et al.50 | C. albicans SC5314 and 529L, C. albicans tup1Δ/Δ homozygous deletion mutant, E. faecalis OG1RF | 106 cells of C. albicans SC5314, 107 cells of E. faecalis, or a combination | SCC15 oral keratinocytes seeded on collagen-embedded fibroblasts (3T3) pretreated with 5-FU for mucosal injury | 20 h | CFU determinations, immuno-FISH | Pronounced fungal invasion in 5-FU-treated tissues infected with both organisms |
5-FU, 5-fluorouracil; ATCC, American type culture collection; CFU, colony-forming unit; CLSM, confocal laser scanning microscopy; DMEM, Dulbecco's Modified Eagle Medium; EHOM, engineered human oral mucosa; ELISA, enzyme-linked immunosorbent assay; FACS, fluorescence-activated cell sorting; FISH, fluorescent in situ hybridization; FTOM, full-thickness oral mucosa; H&E, hematoxylin and eosin; IF, immunofluorescence; IFN, interferon; IHC, immunohistochemistry; IL, interleukin; LDH, lactate dehydrogenase; MOI, multiplicity of infection; MTT, (dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide); NOKs, normal oral keratinocytes; NOK-si, immortalized normal human oral keratinocytes; NOFs, normal oral fibroblasts; OMMs, oral mucosal models; PASS, periodic acid Schiff staining; PBS, phosphate-buffered saline; PMOCM, pig mucosa organ culture model; PL−, undetectable phospholipase activity; PL+, phospholipase positive; PMMA, poly-methyl methacrylate; PMN, polymorphonuclear leukocyte; PNA FISH, peptide nucleic acid fluorescent in situ hybridization; qRT-PCR, quantitative reverse transcription-polymerase chain reaction; RHE, reconstituted human epithelium; ROMT, reconstituted oral mucosa tissue; RHOE, reconstituted human oral epithelium; SAP, secreted aspartyl proteinase; SEM, scanning electron microscopy; TEM, transmission electron microscopy; TNF, tumor necrosis factor.
Porphyromonas gingivalis
Although periodontitis is a multifactorial disease, an abundance of bacteria (like P. gingivalis and Aggregatibacter actinomycetemcomitans) and lower levels of some other bacteria in the oral cavity of patients with periodontitis show important interaction of these bacteria with the host. The Gram-negative, anaerobic bacterium P. gingivalis is considered the main agent in etiology of periodontitis. This bacterium has the ability to invade oral mucosa cells, which result in its escape from therapeutic and host immune agents. This bacterium produces dental plaque biofilm in combination with primary (Streptococci) and secondary colonizers (Fusobacterium).51,52
The studies related to the infection of oral mucosa models with Porphyromonas alone or in association with other bacteria are summarized in Table 2.
Table 2.
Studies Related to the Infection of Oral Mucosa Models with Porphyromonas Alone or in Association with Other Bacteria
| Authors | Bacteria strain | Culture condition | Oral mucosa model | Time of contact between P. gingivalis and mucosa | Assays | Results |
|---|---|---|---|---|---|---|
| Andrian et al.5 | P. gingivalis ATCC 33277 and the derivative gingipain-null mutant KDP128 | 106 and 109 bacteria (ATCC 33277 or KPD128)/mL in DMEH, incubated in an anaerobic chamber | EHOM (primary epithelial and fibroblasts cells in collagen) | 24 h | TEM, ELISA | Higher penetration of nonmutant form in lamina propria; high secretion of cytokines from oral mucosa models after infection |
| Kimball et al.25 | P. gingivalis (ATCC 33277 or strain 861), S. gordonii DL-1, and Fusobacterium nucleatum ATCC 25586 | 6 × 106 bacteria in 10–50 μL bacterial growth medium (MOI of 100:1 bacteria per surface cell) | EpiOralTM (MatTek Corporation, Ashland, MA) | 24–72 h | H&E, IHC, qRT-PCR | Increase of hBD2 expression after infection |
| Andrian et al.53 | P. gingivalis ATCC 33277 or its derivative gingipain-null mutant (KDP128) | 100 μL of 109 bacteria/mL in DMEH, in an anaerobic chamber | EHOM (primary epithelial and fibroblasts cells in collagen) | 4, 8, 24 h | RT-PCR, ELISA | Increase activation of TIMP-2 and expression of MMP-2 and MMP-9 by oral mucosa following infection |
| Wayakanon et al.54 | Clinical strains (A245br) of P. gingivalis | MOI = 100 | OMM (NOK or TR146 cells on collagen containing NOFs) | 18 h | Bacteria count, IHC | Reduced number of intracellular P. gingivalis in presence of polymersome-encapsulated metronidazole or doxycycline |
| Belibasakis et al.55 | P. gingivalis ATCC 33277T, Campylobacter rectus (OMZ 697), F. nucleatum (OMZ 596), Prevotella intermedia ATCC 25611T, Tannerella forsythia OMZ1047, Treponema denticola ATCC 35405T, Veillonella dispar ATCC 17748T, Actinomyces oris (OMZ 745), S. anginosus (OMZ 871), and S. oralis SK 248 (OMZ 607) | 10-species “subgingival” biofilm model grown on sintered hydroxyapatite discs placed onto OMM | EpiGing, (MatTek, Ashland, MA) | 3–24 h | qPCR, LDH activity, ELISA | Upregulation of IL-8 gene expression and secretion after 3 h in both biofilms, in the presence of the “red complex” |
| Pinnock et al.56 | P. gingivalis strains NCTC 11834 and W50 | MOI of 100 (monolayer) or 2 × 107 cells/300 mL | OMMs with either NOK or the H357 cell line on collagen containing NOFs | 1.5 or 4 h | Antibiotic protection assay, IF, IHC, chemokine array | Higher intracellular survival of P. gingivalis in mucosal models compared with monolayer cultures |
| Thurnheer et al.57 | P. gingivalis ATCC 33277T, S. oralis SK248 S. anginosus ATCC 9895, Actinomyces oris (OMZ 745), F. nucleatum subsp. Nucleatum OMZ 598, Veillonella dispar ATCC 17748T, Campylobacter rectus OMZ 698, Prevotella intermedia ATCC 25611T, T. forsythia OMZ 1047, and Treponema denticola ATCC 35405 | Subgingival biofilm formed on hydroxyapatite discs put upside-down on the OMM | EpiGing (MatTek, Ashland, MA) | 24, 48 h | IF, CLSM, SEM, histological staining | Colonization of OMM by “red-complex” species, a colonization of Streptococci on the gingival epithelia, in the absence of all three “red complex” bacteria from the biofilm |
| Bao et al.58 | Porphyromonas gingivalis W50 (OMZ 308), Prevotella intermedia ATCC 25611T, A. actinomycetemcomitans JP2 (OMZ 295), Campylobacter rectus (OMZ 398), Veillonella dispar ATCC 17748T, F. nucleatum subsp. Nucleatum (OMZ 598), S. oralis SK248 (OMZ 607), Treponema denticola ATCC 35405T, Actinomyces oris (OMZ 745), S. anginosus ATCC 9895, and Tannerella forsythia (OMZ 1047) | 11-species biofilm formed on hydroxyapatite discs co-cultured with the OMM in the bioreactor | Immortalized epithelial cells, fibroblasts, and a monocytic cell line perfused through 3D collagen scaffold into the bioreactor | 24 h | Proteomic, LC-MS/MS analysis, gene ontology (GO) analysis | Identification of 896 proteins in the supernatant and 3363 proteins in the biofilm lysate, significant regulation of the levels of F. nucleatum, Actinomyces oris, and Campylobacter rectus proteins |
| Bao et al.59 | Porphyromonas gingivalis W50 (OMZ 308), Prevotella intermedia ATCC 25611T, A. actinomycetemcomitans JP2 (OMZ 295), Campylobacter rectus (OMZ 398), Veillonella dispar ATCC 17748T, F. nucleatum subsp. Nucleatum (OMZ 598), S. oralis SK248 (OMZ 607), Treponema denticola ATCC 35405T, Actinomyces oris (OMZ 745), S. anginosus ATCC 9895, and Tannerella forsythia (OMZ 1047) | 11-species biofilm formed on hydroxyapatite discs co-cultured with the OMM in the bioreactor (37°C, 2% O2 and 5% CO2) | Immortalized epithelial cells (HGEK-16), fibroblasts (GFB-16), and a monocytic cell line perfused through 3D collagen scaffold into the bioreactor | 24 h | qPCR, quantification of cytokine secretion, Masson's Trichrome Staining, SEM | Reduced growth of Campylobacter rectus, Actinomyces oris, S. anginosus, Veillonella dispar, and P. gingivalis in the presence of OMM; upregulation of cytokine release in cell culture supernatants in presence of the biofilm |
| Bugueno et al.60 | P. gingivalis strain 33277 | MOI = 100 | 3D microtissue of TERT-2 OKF-6 cell line on 3D spheroid of NOFs | 2–24 h | Antibiotic Protection Assay, qRT-PCR, IF, SEM, TEM | Invasion of the fibroblastic core and increased apoptosis after infection |
| Brown et al.61 | P. gingivalis W83, S. mitis NCTC 12261, S. intermedius 20753, S. oralis NTCC 11427, F. nucleatum ATCC 10596, F. spp. vincentii DSM 19507, Act. naeslundii DSM 17233, Veillonella NCTC 11831, Prevotella intermedia DSM 20706, and A. actinomycetemcomitans ATCC 43718 | Three multispecies oral biofilms representative of a “health associated” (3 species), “gingivitis-associated,” (7 species), and “periodontitis associated” (10 species) grown on coverslips attached to the underside of inserts, and then placed into inserts containing the HGE tissue | HGE (Episkin, Skinethic, Lyon, France) + PBMC/CD14 + monocytes | 1–2 days | H&E, LDH assay, qRT-PCR, ELISA | High viability of HGE exposed to all multispecies biofilms, more differential inflammatory response in immune cells cultured with epithelium stimulated by “gingivitis-associated” biofilm |
3D, three dimensional; PBMCs, peripheral blood mononuclear cells; HGE, human gingival epithelium; MS, mass spectrometry.
Other microorganisms
In the oral cavity, some bacteria are involved in pathogenesis of dental caries (Gram-positive Streptococcus mutans), while others are responsible for periodontal diseases (Gram-negative Actinobacillus actinomycetemcomitans and F. nucleatum). Bacteria in the oral cavity—and especially in dental plaque—often interact with each other and are associated together in the procedure of disease progression. It is important to consider primary and second colonizers, as well as the third colonizers.
The studies considering infection of oral mucosa models with microorganisms other than Candida and Porphyromonas are summarized in Table 3.
Table 3.
Studies Considering Infection of Oral Mucosa Models with Microorganisms Other Than Candida and Porphyromonas
| Authors | Bacteria strains | Culture condition | Oral mucosa model | Time of contact between biofilm and mucosa | Assays | Results |
|---|---|---|---|---|---|---|
| Gursoy et al.23 | Two strains of F. nucleatum: ATCC25586 and AHN9508 (clinical oral isolate) | Two groups: anaerobically grown biofilm on a semipermeable membrane placed upside-down on OCC, 10 μL (3 × 106 CFUs/PBS) of planktonic bacteria | HaCaT epithelial cells grown on a fibroblast collagen matrix (OCC model) | 24 h | H&E, Ki-67, PASS, LDH release | Invasion of the collagen matrix by one of the strains; more cytotoxicity and invasiveness of biofilm in comparison to planktonic bacteria |
| Dabija-Wolter et al.62 | Four strains of F. nucleatum: ATCC 10953, ATCC 25586, and two other clinical isolates: AHN 8158 and MRC-23 | 5 × 107 unstained or FITC-labeled F. nucleatum in 20–30 μL FAD medium, in anaerobic atmosphere for 3 h and then at 37°C in aerobic conditions | 3D engineered models of human gingiva using primary gingival keratinocytes and fibroblasts | 24, 48 h | CLSM, IHC, qRT-PCR | Penetration of F. nucleatum to gingival epithelium without causing permanent damage |
| Pollanen et al.63 | F. nucleatum (ATCC) 25586 | Biofilm grown on semipermeable nitrocellulose membranes placed on OMM | HaCaT cells seeded on collagen fibroblast gels and a tooth piece placed on top | ≤24 h | IHC | Epithelial migration and altered epithelial proliferation pattern |
| De Ryck et al.22 | Microbiota derived from a swab of the inner cheek | Microbiota grown on an agar/mucin layer positioned on top of oral mucosa | TR146, HaCaT, or normal keratinocyte cells grown on collagen layer containing NIH-3T3 fibroblasts | 72 h | Oral scratch assay, Pyrosequencing, PCR-DGGE analysis, live/dead staining, flow cytometry, SCFA, MTT, SRB, LDH, Western blot, lactate analysis, Van Gieson, Alcian Blue, E-cadherin, Ki67, H&E | Reduced healing in the presence of microbiota, no reduction of the proliferation index, no increase of apoptotic or necrotic cells |
| Buskermolen et al.64 | Three biofilm types: commensal, gingivitis, and cariogenic | 10 μL of 105, 106, or 107 CFUs/equivalent diluted in HBSS | Immortalized human keratinocyte (KC-TERT) and fibroblast (Fib-TERT) embedded in collagen hydrogel | 24 h | IHC, FISH, fluorescence resonance energy transfer, ELISA | Increased expression of elafin, secretion of the antimicrobial cytokine and inflammatory cytokines in the gingiva epithelium |
| Shang et al.24 | From healthy human saliva, consists of typical commensal genera Granulicatella and major oral microbiota genera Veillonella and Streptococcus | 107 CFU of biofilm cells diluted in 10 μL HBSS, dripped onto the surface of the RHG | RHG: immortalized human keratinocyte (KC-TERT) and fibroblast (Fib-TERT)-populated hydrogel | 1, 2, 4, or 7 days | ELISA, RT-PCR, CFU count, H&E, FISH | Increased epithelial thickness, stratification, keratinocyte proliferation, and production of anti-microbial proteins in biofilm exposed RHG |
| Rahimi et al.65 | Streptococcus mutans (strain UA-159) | Injection of 2 μL of bacterial solution (with optical density between 0.2 and 0.3) into the keratinocyte-containing channel of the device | Microfluidic mucosal model-on-a-chip: fibroblast cell line-laden collagen, followed by a keratinocyte cell line (Gie-No3B11) layer | 24 h | DiI fluorescence staining, TEER | Some infiltration in collagen layer, lower TEER after bacterial exposure |
| Shang et al.66 | Commensal, gingivitis, or cariogenic biofilms from human healthy saliva | Biofilms cultured in the AAA model diluted as 1 × 107 CFU biofilm cells in 10 μL HBSS | RHG: keratinocyte (KC-TERT, OKG4/bmi1/TERT) on collagen-embedded fibroblast (Fib-TERT) | 24 h | FISH, H&E, RT-PCR, Western blotting | Upregulation of gene expression involved in TLR signaling by commensal biofilm, and suppression of some by cariogenic biofilm; no significant damaging effect on RHG morphology |
| Ingendoh-Tsakmakidis et al.67 | Biofilm of S. oralis (DSM 20627) on polyethersulfone membrane, biofilm of A. actinomycetemcomitans JP2 strain on coverslip | S. oralis or A. actinomycetemcomitans biofilm facing the peri-implant oral mucosa model with direct contact to titanium disk | Peri-implant oral mucosa model assembly: OKF6/TERT-2 seeded on titanium disks-HGF-collagen matrix | 24 h | Microarray data analysis, ELISA, IHC | Induction of a protective stress response by S. oralis. downregulation of genes involved in inflammatory response by A. actinomycetemcomitans |
| Beklen et al.68 | A. actinomycetemcomitans strain D7S | A. actinomycetemcomitans biofilm cultured on porous filter discs added on top of OMM | Immortalized human gingival keratinocyte cells seeded on fibroblast-collagen matrix | 24 h | IHC, TEM | Thick necrotic layer and decrease of keratin expression in epithelium following infection |
AAA-model, Amsterdam active attachment model; AHN, anaerobe Helsinki negative; DGGE, denaturing gradient gel electrophoresis; FITC, fluorescein-isothiocyanate; HaCaT, human adult low-calcium high-temperature; HBSS, Hank's Balanced Salt Solution; KC-TERT, telomerase reverse transcriptase-immortalized human keratinocyte; OCC, organotypic cell culture; RHG, reconstructed human gingiva; SCFA, short-chain fatty acid; SRB, sulforhodamine B colorimetric assay; TEER, transepithelial electrical resistance; TLR, toll-like receptor.
Discussion
Monolayer culture of epithelial cells is considered to be a deficient model to study the interaction of pathogenic bacteria with host cells. In contrast, the potential of 3D models of human oral mucosa for histological analysis of the process of infection—and observation of the tissue invasion—makes these models very relevant and informative for microbiomics.69 In this study, we summarized the studies using 3D models of oral mucosa optimized for fungal pathogenesis and bacterial-derived oral infections. It seems that there are many aspects that require optimization and standardization with regard to using oral mucosal models (OMMs) for infection by microorganisms.
Equivalents of oral mucosa
Engineered oral mucosa includes a connective tissue layer containing fibroblasts as lamina propria covered by epithelium containing epithelial cells.8,70 The substrate used for cell culture in most of the engineered oral mucosa models used in this review was collagen. Ease of extraction and manipulation, reproducibility, and high growth of epithelial cells on its surface are the reasons for choosing this material to load fibroblast cells.71 The potential role of the scaffold as a barrier against infection has been mentioned by researchers.72 However, with advancing tissue engineering, scaffold-free approaches are now starting to be utilized in engineering of oral mucosa.73 One study prepared a 3D spheroid model of oral mucosa by hanging-drop method and infected it with P. gingivalis.60 However, lack of keratinization is a limitation of this micro-tissue model.
Cells used for oral mucosa models include primary cells—NOKs (human-derived normal oral keratinocyte cells from oral mucosa) or cell lines such as TR146 (oral squamous cell carcinoma cell line), HaCaT (immortalized keratinocyte cell line), H357 (cell line from squamous cell carcinoma of the tongue), OKF6/TERT-2, 20 (normal oral epithelial cell line, immortalized by forced expression of telomerase), and Gie-No3B11 (immortalized gingival keratinocytes). Upregulation of genes in tumor-derived cells suggests more suitability of normal or immortalized cells for OMM production.3 On the other hand, primary cells have short life span, and their growth rate and response to infection are different based on various donors.34
The engineered oral mucosa for investigation of oral microbiomics has been used since 2004.5,28,30,31 Based on this review, 29 studies used engineered oral mucosa, while 14 studies used commercialized models. Reconstituted human oral epithelium (RHOE, SkinEthic) model is a multilayered epithelium consisting of TR146 cells on a polycarbonate transwell insert. EpiOral (MaTek) is based on primary oral keratinocytes grown in Millipore Millicell inserts. Although these models are inexpensive, easily handled, and reproducible, the absence of fibroblast-embedded collagen layer in both of these models raises concerns about their reliability. Mimicking steps of keratinocyte differentiation requires their culture on a connective tissue layer.74 More cytokine release and expression of defensin from full-thickness engineered oral mucosa in comparison to split-thickness models suggest that they are better representative of in vivo conditions.43 Among articles reviewed in this study, only one study used porcine ex vivo oral mucosa model based on structural similarity to human oral mucosa.38
In native oral mucosa, many other cells besides fibroblasts and epithelial cells exist, including immune cells, endothelial cells, and melanocytes.75 Presence of neutrophils within biofilms was confirmed in different studies.76,77 In this review, one study used RHOE supplemented with polymorphonuclear leukocytes to study oral candidiasis.32 Another study used co-culture of immune cells (peripheral blood mononuclear cells and CD14+ monocytes), human gingival epithelium (Skinethic), and multispecies biofilms.61 Bao et al. used a monocytic cell line in their oral mucosa-infected model.59 Interaction of oral epithelial cells with immune cells in response to infection has been reported in many studies.78–80 To simulate the in vivo situation as closely as possible, engineering of more complex oral mucosa models that are vascularized or contain immune cells would be indicated for microbiomics.
Oral mucosa infection
Long-term co-culture of bacteria and oral mucosa model is challenging, because each of them requires different culture media. Time of infection of oral mucosa with pathogen microorganisms in different studies varies between 1.5 and 48 h. De Ryck et al. used 72-h bacterial exposure of oral mucosa model.22 Determination of time course of infection is important in different bacteria, because some microorganisms, like P. gingivalis, need anaerobic incubation, which compromises epithelial viability after 24 h.81 Contact of C. albicans with epithelium after 8 and 24 h causes tissue disorganization as well,30 but visible damage caused by S. salivarius is reported after 48 h contact.30 Shang et al. showed that commensal oral microbiota from healthy saliva could be in contact with oral mucosa model for 7 days.24
MOI used in most studies was 100. Groeger et al., reported no difference in the transepithelial electrical resistance at an MOI of 100, even after 48 h.82 Higher MOI could result in destruction of cell–cell contacts.
Another aspect of oral mucosa infection is the atmosphere of culture for producing optimum results. While Candida and Streptococcus could grow in aerobic conditions, Fusobacterium and P. gingivalis require an anaerobic atmosphere. However, prolonged incubation of oral mucosa model in this condition destroys its structure.81 Researchers showed that there is no significant difference in bacterial viability between anaerobic and aerobic incubation over 4-h infection of oral mucosa model.83 Gursoy et al. also showed that bacterial viability does not alter after change of the environment from anaerobic to aerobic.23
Beside oxygen, the effect of temperature on the growth of bacteria is important. Although the temperature of body is about 37°C, increase of temperature in some conditions—like inflammation in periodontitis—is reported, which must be considered in future studies. Dynamic environment of the oral cavity and shear forces by saliva also should be considered in infection of oral mucosa. In the study by Bao et al., a closed dynamic perfusion bioreactor system was used for the creation of continuous sheer forces.59 Mimicking temperature, atmosphere, and shear stress of the natural environment and simulating the environment of periodontal pocket or oral cavity are now possible by using bioreactors.
Biofilm versus non-biofilm design
Most studies concerning microbiomics of oral mucosa used single species and planktonic bacteria (non-biofilm design). Buskermolen used saliva-derived commensal and pathogenic biofilms for oral mucosa exposure,64 and Shang et al. used multispecies commensal biofilm,24 both from healthy human saliva. While these two studies used 10 μL of determined concentration of oral biofilm, De Ryck et al. used oral biofilm derived from swabs wiped along the inner cheek and after growth of this biofilm on an agar/mucin layer, it was placed on top of oral mucosa model with no direct contact.22 Gursoy et al. in their study by placing a biofilm of F. nucleatum on top of OMM, investigated direct contact between single-species biofilm and oral mucosa.23 Using poly-methyl methacrylate and hydroxyl apatite disc for producing oral biofilm before contact with oral mucosa has also been proposed in other studies.49,59 Microorganisms in the oral cavity have an affinity to form multispecies biofilm, and the behavior of them in a biofilm-embedded by matrix is very different from their planktonic form. Higher resistance of bacteria in biofilm to antibacterial agents and different gene expression by them highlight the importance of in vitro biofilm design.84
Another relevant aspect to consider in producing biofilm is the role of saliva containing mucin and acquired pellicle. Only one study used saliva as supplement of biofilm growth medium.42 Using natural or artificial saliva rather than culture media in co-culture of bacteria-OMM is a possible option for mimicking the condition of the oral cavity.85
Survival and penetration of microorganism in oral mucosa model
Survival of microorganisms in oral epithelial cells over different time periods was investigated in different studies. Studies related to C. albicans showed that transformation to the hyphal form, which begins 8 h after infection, could result in the decrease of colony-forming units (CFUs).30 Yeast transition is reduced in keratinized form of oral mucosa in comparison to nonkeratinized form.36 Although Samaranayake et al. reported no penetration of C. albicans into the connective tissue layer at 48 h,35 Whiley et al. and Dongari-Bagtzoglou and Kashleva showed that penetration into the submucosa was dependent on the strain used for infection.7,34 Association of C. albicans with other microorganisms, like Staphylococcus aureus or S. oralis, could result in deeper invasion into subepithelial collagen matrix.12,42 Hyphal transformation was not detectable in C. famata; however, its penetration to the lamina propria of the oral mucosa model was reported after 24 h of infection.41 Invasion of F. nucleatum to collagen matrix is also strain dependent and is enhanced in the biofilm form of F. nucleatum compared to the planktonic form.23 P. gingivalis penetration into the connective tissue has been demonstrated.54 Andrian et al. showed the contribution of P. gingivalis gingipains in its potency to penetrate the connective tissue.5 Pinnock et al. reported that submerged OMM with a thin epithelium allows penetration of bacteria into the connective tissue, while airlifted OMM with thicker epithelium prohibits its penetration to lamina propria. They also showed that the viability of this bacterium in OMM decreases over time.56
While almost all studies showed disorganization of epithelial layer after infection with pathogenic bacteria, Shang et al. reported higher epithelial thickness and keratinocyte proliferation in oral mucosa models exposed to biofilm that was composed of multispecies commensal microorganisms from healthy human saliva after 7 days.24 It seems that commensal oral bacteria act as an antagonist against potential pathogens and help in maintenance of oral mucosa health.9
Recovering bacteria from OMM
Different methods have been used for the release of bacteria from the infected oral mucosa models. One method is using tissue dissociator for dissociation of tissue, following by sonication.24 The second method is using homogenizer, lysing the keratinocyte plasma membrane, and robustly pipetting to release intracellular bacteria.54,56 One other option is treating tissue with lysis buffer and strictly mixing it.36 Scraping, or using the cycle of sonication and vortexing, was also suggested by Heersink.86 Hamilton et al., in their study of different methods of collecting biofilm cells from surfaces, emphasized the importance of using similar methods of harvesting biofilm for acceptable result of comparison.87
Further consideration in this step is the possible disorganization of epithelial cells over time and release of cells containing bacteria in culture media, which could result in false report of reduction of bacteria over time. Standardization of the techniques used for recovering bacteria from OMM is very important.
Methods of evaluation of infected OMM
Extent of bacteria proliferation or oral mucosa damage can be evaluated by different methods. Most of studies use qualitative/semiquantitative analyses for description of oral mucosa infection. Histology staining, (dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide) (MTT) and lactate dehydrogenase activity measurement were the most common methods for analysis of epithelial cell damage.7,22,39,76
Conventional plate count and CFU-based quantitation, which have been used in different studies, only consider the number of bacteria on the surface of OMM and not the bacteria in deeper layer. Also, this method is not useful for viable but nonculturable organisms.24,30,36,54 Alternative methods like crystal violet staining and resazurin staining can be used for biofilm research.88 Five studies used confocal laser scanning microscopy (CLSM) to investigate various aspects of microbial biofilm formation.37,42,45,57,62 One other approach for visualization of biofilm is using fluorescein isothiocyanate-labeled bacteria and flow cytometry.22,62 Flow cytometric cell sorting is also a useful tool for separation of bacteria.32 Because of concerns regarding dissociation of biofilm during handling and preparation for staining, Pittman et al. proposed using low-melting agarose on the surface of infected oral mucosa.89
One of the best quantitative methods for evaluation of barrier integrity of cells is the measurement of transepithelial electrical resistance (TEER)/transendothelial electrical resistance.65 This noninvasive method can reflect changes in tight junction proteins. Reduced TEER of keratinocytes after infection with bacteria was reported in several studies.3,90 When using TEER for comparing different models, it is important to consider the influencing parameters—like porosity and material of the model, and the medium used for the measurement.91
Fluorescent in situ hybridization (FISH) is also a useful technique that was used in seven studies for detection of microorganisms in OMMs.24,42,45,48,50,64,66 Combination of different methods, like FISH and CLSM, could help to better determine interaction between oral mucosa and biofilm.92
Effect of antibacterial agents
OMMs are suitable and relevant in vitro test systems for evaluating antibacterial products. The effect of an antibacterial agent on bacteria should be considered in combination with its biosafety for oral tissues. Effect of different dosage of a commercially available topical Nystatin suspension on an ex vivo model of oral mucosa infected with Candida was studied by Ohnemus et al.38 They proved that, while a dosage of 0.25 IU Nystatin was efficient in agar diffusion model, it had no confirmed activity at dosage of 10 and 0.1 IU on infected oral mucosa, suggesting the closer properties of OMM to the in vivo situation.38 Biocompatibility of synthetic antimicrobial decapeptide KSL-W and its antibacterial effects against C. albicans was investigated by Semlali et al. using OMM. They showed its safety for epithelial cells and its negative effect on the growth of Candida.46 Wayakanon et al. investigated the effect of metronidazole-, doxycycline-, and gentamicin-encapsulated polymersome on biocompatibility of keratinocyte cells and reduction of intracellular P. gingivalis load in OMMs.54 Effects of plasma treatment on reduction of the biofilm of C. albicans and Staphylococcus aureus without toxic effects on OMM have also been reported by Delben et al.93
Considering the importance of quorum-sensing and presence of adhesins for adhesion of bacteria to mucosal surfaces, future antibacterial approaches could be focused on the alteration of quorum-sensing or blocking of adhesins in combination with stimulation of defensin release from OMM. Finally, using oral mucosa-on-a-chip could be very helpful to study the reciprocal effects of antibacterial agents on bacteria and oral mucosa.65
Conclusion
Invasion of oral bacteria to tissue-engineered oral mucosa is dependent on the strains of bacterium and can be influenced by the type of cells and culture conditions used. The methods used for tissue processing and assessment of the effects of bacteria on oral mucosa can be potentially invasive and may alter the cells or bacteria. Therefore, data reported in the literature regarding invasion of oral mucosa by bacteria must be interpreted with caution.
Although OMMs are more relevant and more informative than monolayer cultures of epithelial cells, they lack some other types of cells present in the normal human oral mucosa. Other limitations of OMMs include nonconstant desquamation, absence of saliva consisting mucin, deficiency in the number of present bacteria and immune responses, and static environment, which make it difficult to extrapolate the data from the in vitro experiments to the clinical situation. Using new technologies, such as microfluidics and bioreactors, could help to reproduce some of these physiologically relevant conditions.
Acknowledgments
This research is supported by National Institute of Dental and Craniofacial Research (NIDCR) of the National Institutes of Health (NIH) under award number R15DE027533.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure Statement
No competing financial interests exist.
Funding Information
This study was funded by the National Institute of Dental & Craniofacial Research (NIDCR) of the National Institutes of Health (NIH) (award no. R15DE027533).
References
- 1. Rouabhia M. Interactions between host and oral commensal microorganisms are key events in health and disease status. Can J Infect Dis Med Microbiol 13, 47, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Groeger S.E., and Meyle J.. Epithelial barrier and oral bacterial infection. Periodontology 2000 69, 46, 2015 [DOI] [PubMed] [Google Scholar]
- 3. Bierbaumer L., Schwarze U.Y., Gruber R., and Neuhaus W.. Cell culture models of oral mucosal barriers: a review with a focus on applications, culture conditions and barrier properties. Tissue Barriers 6, 1479568, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mosaddad S.A., Tahmasebi E., Yazdanian A., et al. Oral microbial biofilms: an update. Eur J Clin Microbiol Infect Dis 38, 2005, 2019 [DOI] [PubMed] [Google Scholar]
- 5. Andrian E., Grenier D., and Rouabhia M.. In vitro models of tissue penetration and destruction by Porphyromonas gingivalis. Infect Immun 72, 4689, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vanhoecke B., De Ryck T., Stringer A., Van de Wiele T., and Keefe D.. Microbiota and their role in the pathogenesis of oral mucositis. Oral Dis 21, 17, 2015 [DOI] [PubMed] [Google Scholar]
- 7. Whiley R.A., Cruchley A.T., Gore C., and Hagi-Pavli E.. Candida albicans strain-dependent modulation of pro-inflammatory cytokine release by in vitro oral and vaginal mucosal models. Cytokine 57, 89, 2012 [DOI] [PubMed] [Google Scholar]
- 8. Moharamzadeh K., Colley H., Murdoch C., et al. Tissue-engineered oral mucosa. J Dent Res 91, 642, 2012 [DOI] [PubMed] [Google Scholar]
- 9. Dickinson B.C., Moffatt C.E., Hagerty D., et al. Interaction of oral bacteria with gingival epithelial cell multilayers. Mol Oral Microbiol 26, 210, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Belibasakis G.N., Bao K., and Bostanci N.. Transcriptional profiling of human gingival fibroblasts in response to multi-species in vitro subgingival biofilms. Mol Oral Microbiol 29, 174, 2014 [DOI] [PubMed] [Google Scholar]
- 11. Belibasakis G.N., Bostanci N., and Reddi D.. Regulation of protease-activated receptor-2 expression in gingival fibroblasts and Jurkat T cells by Porphyromonas gingivalis. Cell Biol Int 34, 287, 2010 [DOI] [PubMed] [Google Scholar]
- 12. de Carvalho Dias K., de Sousa D.L., Barbugli P.A., Cerri P.S., Salih V.M., and Vergani C.E.. Development and characterization of a 3D oral mucosa model as a tool for host-pathogen interactions. J Microbiol Methods 152, 52, 2018 [DOI] [PubMed] [Google Scholar]
- 13. Coenye T., and Nelis H.J.. In vitro and in vivo model systems to study microbial biofilm formation. J Microbiol Methods 83, 89, 2010 [DOI] [PubMed] [Google Scholar]
- 14. Schenke-Layland K., and Nerem R.M.. In vitro human tissue models—moving towards personalized regenerative medicine. Adv Drug Deliv Rev 63, 195, 2011 [DOI] [PubMed] [Google Scholar]
- 15. Groeber F., Holeiter M., Hampel M., Hinderer S., and Schenke-Layland K.. Skin tissue engineering—in vivo and in vitro applications. Adv Drug Deliv Rev 63, 352, 2011 [DOI] [PubMed] [Google Scholar]
- 16. Cencic A., and Langerholc T.. Functional cell models of the gut and their applications in food microbiology—a review. Int J Food Microbiol 141(Suppl 1), S4, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gao L., Xu T., Huang G., Jiang S., Gu Y., and Chen F.. Oral microbiomes: more and more importance in oral cavity and whole body. Protein Cell 9, 488, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jia G., Zhi A., Lai P.F.H., et al. The oral microbiota—a mechanistic role for systemic diseases. Br Dent J 224, 447, 2018 [DOI] [PubMed] [Google Scholar]
- 19. Lu M., Xuan S., and Wang Z.. Oral microbiota: a new view of body health. Food Sci Hum Wellness 8, 8, 2019 [Google Scholar]
- 20. Sudhakara P., Gupta A., Bhardwaj A., and Wilson A.. Oral dysbiotic communities and their implications in systemic diseases. Dent J 6, 10, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Werlang C., Cárcarmo-Oyarce G., and Ribbeck K.. Engineering mucus to study and influence the microbiome. Nat Rev Mater 4, 134, 2019 [Google Scholar]
- 22. De Ryck T., Grootaert C., Jaspaert L., et al. Development of an oral mucosa model to study host-microbiome interactions during wound healing. Appl Microbiol Biotechnol 98, 6831, 2014 [DOI] [PubMed] [Google Scholar]
- 23. Gursoy U.K., Pollanen M., Kononen E., and Uitto V.J.. Biofilm formation enhances the oxygen tolerance and invasiveness of Fusobacterium nucleatum in an oral mucosa culture model. J Periodontol 81, 1084, 2010 [DOI] [PubMed] [Google Scholar]
- 24. Shang L., Deng D., Buskermolen J.K., et al. Multi-species oral biofilm promotes reconstructed human gingiva epithelial barrier function. Sci Rep 8, 16061, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kimball J.R., Nittayananta W., Klausner M., Chung W.O., and Dale B.A.. Antimicrobial barrier of an in vitro oral epithelial model. Arch Oral Biol 51, 775, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chevalier M., Ranque S., and Precheur I.. Oral fungal-bacterial biofilm models in vitro: a review. Med Mycol 56, 653, 2018 [DOI] [PubMed] [Google Scholar]
- 27. Negrini T.C., Koo H., and Arthur R.A.. Candida-bacterial biofilms and host-microbe interactions in oral diseases. Adv Exp Med Biol 1197, 119, 2019 [DOI] [PubMed] [Google Scholar]
- 28. Claveau I., Mostefaoui Y., and Rouabhia M.. Basement membrane protein and matrix metalloproteinase deregulation in engineered human oral mucosa following infection with Candida albicans. Matrix Biol 23, 477, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Mostefaoui Y., Bart C., Frenette M., and Rouabhia M.. Candida albicans and Streptococcus salivarius modulate IL-6, IL-8, and TNF-alpha expression and secretion by engineered human oral mucosa cells. Cell Microbiol 6, 1085, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Mostefaoui Y., Claveau I., and Rouabhia M.. In vitro analyses of tissue structure and interleukin-1beta expression and production by human oral mucosa in response to Candida albicans infections. Cytokine 25, 162, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Green C.B., Cheng G., Chandra J., Mukherjee P., Ghannoum M.A., and Hoyer L.L.. RT-PCR detection of Candida albicans ALS gene expression in the reconstituted human epithelium (RHE) model of oral candidiasis and in model biofilms. Microbiology 150, 267, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Schaller M., Boeld U., Oberbauer S., Hamm G., Hube B., and Korting H.C.. Polymorphonuclear leukocytes (PMNs) induce protective Th1-type cytokine epithelial responses in an in vitro model of oral candidosis. Microbiology 150, 2807, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Tardif F., Goulet J.P., Zakrzewski A., Chauvin P., and Rouabhia M.. Involvement of interleukin-18 in the inflammatory response against oropharyngeal candidiasis. Med Sci Monit 10, BR239, 2004 [PubMed] [Google Scholar]
- 34. Dongari-Bagtzoglou A., and Kashleva H.. Development of a novel three-dimensional in vitro model of oral Candida infection. Microb Pathog 40, 271, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Samaranayake Y.H., Dassanayake R.S., Cheung B.P., et al. Differential phospholipase gene expression by Candida albicans in artificial media and cultured human oral epithelium. APMIS 114, 857, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Zakrzewski A., and Rouabhia M.. Engineered keratinized oral mucosa decreased C. albicans transition through the production of keratins 10, 14, 16, and 19 by oral epithelial cells. Open Mycol J 1, 1, 2007 [Google Scholar]
- 37. Villar C., Kashleva H., Nobile C., Mitchell A., and Dongari-Bagtzoglou A.. Mucosal tissue invasion by Candida albicans is associated with E-cadherin degradation, mediated by transcription factor Rim101p and protease Sap5p. Infect Immun 75, 2126, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ohnemus U., Willers C., Bubenheim M., et al. An ex-vivo oral mucosa infection model for the evaluation of the topical activity of antifungal agents. Mycoses 51, 21, 2008 [DOI] [PubMed] [Google Scholar]
- 39. Lermann U., and Morschhauser J.. Secreted aspartic proteases are not required for invasion of reconstituted human epithelia by Candida albicans. Microbiology 154, 3281, 2008 [DOI] [PubMed] [Google Scholar]
- 40. Decanis N., Savignac K., and Rouabhia M.. Farnesol promotes epithelial cell defense against Candida albicans through toll-like receptor 2 expression, interleukin-6 and human beta-defensin 2 production. Cytokine 45, 132, 2009 [DOI] [PubMed] [Google Scholar]
- 41. Bahri R., Saidane-Mosbahi D., and Rouabhia M.. Candida famata modulates toll-like receptor, beta-defensin, and proinflammatory cytokine expression by normal human epithelial cells. J Cell Physiol 222, 209, 2010 [DOI] [PubMed] [Google Scholar]
- 42. Diaz P.I., Xie Z., Sobue T., et al. Synergistic interaction between Candida albicans and commensal oral streptococci in a novel in vitro mucosal model. Infect Immun 80, 620, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yadev N.P., Murdoch C., Saville S.P., and Thornhill M.H.. Evaluation of tissue engineered models of the oral mucosa to investigate oral candidiasis. Microb Pathog 50, 278, 2011 [DOI] [PubMed] [Google Scholar]
- 44. Rouabhia M., Mukherjee P.K., Lattif A.A., Curt S., Chandra J., and Ghannoum M.A.. Disruption of sphingolipid biosynthetic gene IPT1 reduces Candida albicans adhesion and prevents activation of human gingival epithelial cell innate immune defense. Med Mycol 49, 458, 2011 [DOI] [PubMed] [Google Scholar]
- 45. Silva S., Henriques M., Hayes A., Oliveira R., Azeredo J., and Williams D.W.. Candida glabrata and Candida albicans co-infection of an in vitro oral epithelium. J Oral Pathol Med 40, 421, 2011 [DOI] [PubMed] [Google Scholar]
- 46. Semlali A., Leung K.P., Curt S., and Rouabhia M.. Antimicrobial decapeptide KSL-W attenuates Candida albicans virulence by modulating its effects on toll-like receptor, human beta-defensin, and cytokine expression by engineered human oral mucosa. Peptides 32, 859, 2011 [DOI] [PubMed] [Google Scholar]
- 47. Rouabhia M., Semlali A., Chandra J., Mukherjee P., Chmielewski W., and Ghannoum M.A.. Disruption of the ECM33 gene in Candida albicans prevents biofilm formation, engineered human oral mucosa tissue damage and gingival cell necrosis/apoptosis. Mediators Inflamm 2012, 398207, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sobue T., Bertolini M., Thompson A., Peterson D.E., Diaz P.I., and Dongari-Bagtzoglou A.. Chemotherapy-induced oral mucositis and associated infections in a novel organotypic model. Mol Oral Microbiol 33, 212, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Morse D.J., Wilson M.J., Wei X., et al. Denture-associated biofilm infection in three-dimensional oral mucosal tissue models. J Med Microbiol 67, 364, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bertolini M., Ranjan A., Thompson A., et al. Candida albicans induces mucosal bacterial dysbiosis that promotes invasive infection. PLoS Pathog 15, e1007717, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fiorillo L., Cervino G., Laino L., et al. Porphyromonas gingivalis, periodontal and systemic implications: a systematic review. Dent J (Basel) 7, pii:, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mysak J., Podzimek S., Sommerova P., et al. Porphyromonas gingivalis: major periodontopathic pathogen overview. J Immunol Res 2014, 476068, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Andrian E., Mostefaoui Y., Rouabhia M., and Grenier D.. Regulation of matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases by Porphyromonas gingivalis in an engineered human oral mucosa model. J Cell Physiol 211, 56, 2007 [DOI] [PubMed] [Google Scholar]
- 54. Wayakanon K., Thornhill M.H., Douglas C.W., et al. Polymersome-mediated intracellular delivery of antibiotics to treat Porphyromonas gingivalis-infected oral epithelial cells. FASEB J 27, 4455, 2013 [DOI] [PubMed] [Google Scholar]
- 55. Belibasakis G.N., Thurnheer T., and Bostanci N.. Interleukin-8 responses of multi-layer gingival epithelia to subgingival biofilms: role of the “red complex” species. PLoS One 8, e81581, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pinnock A., Murdoch C., Moharamzadeh K., Whawell S., and Douglas C.W.. Characterisation and optimisation of organotypic oral mucosal models to study Porphyromonas gingivalis invasion. Microbes Infect 16, 310, 2014 [DOI] [PubMed] [Google Scholar]
- 57. Thurnheer T., Belibasakis G.N., and Bostanci N.. Colonisation of gingival epithelia by subgingival biofilms in vitro: role of “red complex” bacteria. Arch Oral Biol 59, 977, 2014 [DOI] [PubMed] [Google Scholar]
- 58. Bao K., Belibasakis G.N., Selevsek N., Grossmann J., and Bostanci N.. Proteomic profiling of host-biofilm interactions in an oral infection model resembling the periodontal pocket. Sci Rep 5, 15999, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bao K., Papadimitropoulos A., Akgül B., Belibasakis G.N., and Bostanci N.. Establishment of an oral infection model resembling the periodontal pocket in a perfusion bioreactor system. Virulence 6, 265, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bugueno I.M., Batool F., Keller L., Kuchler-Bopp S., Benkirane-Jessel N., and Huck O.. Porphyromonas gingivalis bypasses epithelial barrier and modulates fibroblastic inflammatory response in an in vitro 3D spheroid model. Sci Rep 8, 14914, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Brown J.L., Johnston W., Delaney C., et al. Biofilm-stimulated epithelium modulates the inflammatory responses in co-cultured immune cells. Sci Rep 9, 1, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Dabija-Wolter G., Sapkota D., Cimpan M.R., Neppelberg E., Bakken V., and Costea D.E.. Limited in-depth invasion of Fusobacterium nucleatum into in vitro reconstructed human gingiva. Arch Oral Biol 57, 344, 2012 [DOI] [PubMed] [Google Scholar]
- 63. Pollanen M.T., Gursoy U.K., Kononen E., and Uitto V.J.. Fusobacterium nucleatum biofilm induces epithelial migration in an organotypic model of dento-gingival junction. J Periodontol 83, 1329, 2012 [DOI] [PubMed] [Google Scholar]
- 64. Buskermolen J.K., Janus M.M., Roffel S., Krom B.P., and Gibbs S.. Saliva-derived commensal and pathogenic biofilms in a human gingiva model. J Dent Res 97, 201, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rahimi C., Rahimi B., Padova D., et al. Oral mucosa-on-a-chip to assess layer-specific responses to bacteria and dental materials. Biomicrofluidics 12, 054106, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Shang L., Deng D., Buskermolen J.K., et al. Commensal and pathogenic biofilms alter toll-like receptor signaling in reconstructed human gingiva. Front Cell Infect Microbiol 9, 282, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ingendoh-Tsakmakidis A., Mikolai C., Winkel A., et al. Commensal and pathogenic biofilms differently modulate peri-implant oral mucosa in an organotypic model. Cell Microbiol [Epub ahead of print]; DOI: 10.1111/cmi.13078, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Beklen A., Torittu A., Ihalin R., and Pöllänen M.. Aggregatibacter actinomycetemcomitans biofilm reduces gingival epithelial cell keratin expression in an organotypic gingival tissue culture model. Pathogens 8, 278, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Schaller M., Sander C.A., Korting H.C., Mailhammer R., Grassl G., and Hube B.. Infection of human oral epithelia with Candida species induces cytokine expression correlated to the degree of virulence. J Invest Dermatol 118, 652, 2002 [DOI] [PubMed] [Google Scholar]
- 70. Moharamzadeh K., Brook I.M., Van Noort R., Scutt A.M., and Thornhill M.H.. Tissue-engineered oral mucosa: a review of the scientific literature. J Dent Res 86, 115, 2007 [DOI] [PubMed] [Google Scholar]
- 71. Kinikoglu B., Damour O., and Hasirci V.. Tissue engineering of oral mucosa: a shared concept with skin. J Artif Organs 18, 8, 2015 [DOI] [PubMed] [Google Scholar]
- 72. Liu J., Bian Z., Kuijpers-Jagtman A., and Von den Hoff J.. Skin and oral mucosa equivalents: construction and performance. Orthod Craniofac Res 13, 11, 2010 [DOI] [PubMed] [Google Scholar]
- 73. Nishiyama K., Akagi T., Iwai S., and Akashi M.. Construction of vascularized oral mucosa equivalents using a layer-by-layer cell coating technology. Tissue Eng Part C Methods 25, 262, 2019 [DOI] [PubMed] [Google Scholar]
- 74. Groeger S., and Meyle J.. Oral mucosal epithelial cells. Front Immunol 10, 208, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kinikoglu B., Auxenfans C., Pierrillas P., et al. Importance of cell interactions in tissue engineering of full-thickness oral mucosa. Abstract presented at the Tissue Engineering and Regenerative Medicine International Society Europe Meeting, Galway, Ireland, 2010 [Google Scholar]
- 76. Dongari-Bagtzoglou A. Pathogenesis of mucosal biofilm infections: challenges and progress. Expert Rev Anti-Infect Ther 6, 201, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Walker T.S., Tomlin K.L., Worthen G.S., et al. Enhanced Pseudomonas aeruginosa biofilm development mediated by human neutrophils. Infect Immun 73, 3693, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Galicia J.C., Benakanakere M.R., Stathopoulou P.G., and Kinane D.F.. Neutrophils rescue gingival epithelial cells from bacterial-induced apoptosis. J Leukoc Biol 86, 181, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Gonzalez O.A., Ebersole J.L., and Huang C.B.. Supernatants from oral epithelial cells and gingival fibroblasts modulate human immunodeficiency virus type 1 promoter activation induced by periodontopathogens in monocytes/macrophages. Mol Oral Microbiol 25, 136, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Yin L., Chino T., Horst O.V., et al. Differential and coordinated expression of defensins and cytokines by gingival epithelial cells and dendritic cells in response to oral bacteria. BMC Immunol 11, 37, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Nagaraj N.S., Vigneswaran N., and Zacharias W.. Hypoxia-mediated apoptosis in oral carcinoma cells occurs via two independent pathways. Mol Cancer 3, 38, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Groeger S., Doman E., Chakraborty T., and Meyle J.. Effects of Porphyromonas gingivalis infection on human gingival epithelial barrier function in vitro. Eur J Oral Sci 118, 582, 2010 [DOI] [PubMed] [Google Scholar]
- 83. Pinnock A. A Study of the Invasion and the Cellular Response of an In Vitro 3D Oral Mucosal Model by Porphyromonas gingivalis [Ph.D thesis]. Department of Oral and Maxillofacial Pathology, University of Sheffield, Sheffield, United Kingdom, 2012 [Google Scholar]
- 84. Brown J.L., Johnston W., Delaney C., et al. Polymicrobial oral biofilm models: simplifying the complex. J Med Microbiol 68, 1573, 2019 [DOI] [PubMed] [Google Scholar]
- 85. Seo S.-H., Han I., Lee H.S., et al. Antibacterial activity and effect on gingival cells of microwave-pulsed non-thermal atmospheric pressure plasma in artificial saliva. Sci Rep 7, 8395, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Heersink J. Basic biofilm analytical methods. In: Hamilton M., Heersink J., Buckingham-Meyer K., and Goeres D., eds. The Biofilm Laboratory: Step-By-Step Protocols for Experimental Design, Analysis, and Data Interpretation. Bozeman, MT: Cytergy Publishing, 2003, p. 16 [Google Scholar]
- 87. Hamilton M.A., Buckingham-Meyer K., and Goeres D.M.. Checking the validity of the harvesting and disaggregating steps in laboratory tests of surface disinfectants. J AOAC Int 92, 1755, 2009 [PubMed] [Google Scholar]
- 88. Azevedo N.F., Lopes S.P., Keevil C.W., Pereira M.O., and Vieira M.J.. Time to “go large” on biofilm research: advantages of an omics approach. Biotechnol Lett 31, 477, 2009 [DOI] [PubMed] [Google Scholar]
- 89. Pittman K.J., Robbins C.M., Stubblefield B.A., and Gilbert E.S.. Agarose stabilization of fragile biofilms for quantitative structure analysis. J Microbiol Methods 81, 101, 2010 [DOI] [PubMed] [Google Scholar]
- 90. Gröger S., Michel J., and Meyle J.. Establishment and characterization of immortalized human gingival keratinocyte cell lines. J Periodont Res 43, 604, 2008 [DOI] [PubMed] [Google Scholar]
- 91. Srinivasan B., Kolli A.R., Esch M.B., Abaci H.E., Shuler M.L., and Hickman J.J.. TEER measurement techniques for in vitro barrier model systems. J Lab Autom 20, 107, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Daims H., and Wagner M.. In situ techniques and digital image analysis methods for quantifying spatial localization patterns of nitrifiers and other microorganisms in biofilm and flocs. Methods Enzymol 496, 185, 2011 [DOI] [PubMed] [Google Scholar]
- 93. Delben J.A., Zago C.E., Tyhovych N., Duarte S., and Vergani C.E.. Effect of atmospheric-pressure cold plasma on pathogenic oral biofilms and in vitro reconstituted oral epithelium. PLoS One 11, e0155427, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]