Abstract
Background
Human immunodeficiency virus (HIV) drug resistance profiles are needed to optimize individual patient management and to develop treatment guidelines. Resistance profiles are not well defined among individuals on failing second-line antiretroviral therapy (ART) in low- and middle-income countries (LMIC).
Methods
Resistance genotypes were performed during screening for enrollment into a trial of third-line ART (AIDS Clinical Trials Group protocol 5288). Prior exposure to both nucleoside reverse transcriptase inhibitors (NRTIs) and non-NRTIs and confirmed virologic failure on a protease inhibitor–containing regimen were required. Associations of drug resistance with sex, age, treatment history, plasma HIV RNA, nadir CD4+T-cell count, HIV subtype, and country were investigated.
Results
Plasma HIV genotypes were analyzed for 653 screened candidates; most had resistance (508 of 653; 78%) to 1 or more drugs. Genotypes from 133 (20%) showed resistance to at least 1 drug in a drug class, from 206 (32%) showed resistance to at least 1 drug in 2 drug classes, and from 169 (26%) showed resistance to at least 1 drug in all 3 commonly available drug classes. Susceptibility to at least 1 second-line regimen was preserved in 59%, as were susceptibility to etravirine (78%) and darunavir/ritonavir (97%). Susceptibility to a second-line regimen was significantly higher among women, younger individuals, those with higher nadir CD4+ T-cell counts, and those who had received lopinavir/ritonavir, but was lower among prior nevirapine recipients.
Conclusions
Highly divergent HIV drug resistance profiles were observed among candidates screened for third-line ART in LMIC, ranging from no resistance to resistance to 3 drug classes. These findings underscore the need for access to resistance testing and newer antiretrovirals for the optimal management of third-line ART in LMIC.
Keywords: HIV-1 drug resistance, non-subtype B, second-line ART failure, resource-limited setting
Human immunodeficiency virus (HIV) drug resistance profiles at second-line antiretroviral therapy virologic failure in low- and middle-income countries ranged from no to multi-class resistance. HIV RNA levels were nondiscriminatory for extent of resistance or optimal selection of the next regimen.
Global access to antiretroviral therapy (ART) increased to 20.9 million individuals by mid-2017 (http://www.who.int/features/qa/71/en/). A population-based approach has been used in low- to middle-income countries (LMIC) to recommend those first- and second-line regimens expected to be most effective in suppressing viremia. It is not known whether a similar approach will be effective in individuals requiring third-line ART. Treatment failure of both first- and second-line regimens can be a result of several factors, including transmitted or acquired human immunodeficiency virus (HIV) drug resistance, inadequate drug exposure from suboptimal pharmacokinetics, medication nonadherence or intolerance, and interruptions in the drug supply. These factors can result in the incomplete suppression of viral replication, emergence of drug resistance, and transmission of drug-resistant HIV [1–3].
During the last decade, the World Health Organization recommended a second-line ART consisting of 2 nucleoside reverse transcriptase inhibitors (NRTIs; lamivudine [3TC] or emtricitabine with zidovudine or tenofovir [TDF]) and a protease inhibitor (PI) with pharmacological boosting using low-dose ritonavir (lopinavir/ritonavir [LPV/r] or atazanavir/ritonavir [ATV/r]). The use of these combinations varies by country. Recent studies of second-line regimens [4–7] have shown that despite frequent NRTI resistance mutations from the failure of first-line regimens, the recycling of NRTI with a pharmacologically boosted PI is effective at suppressing viremia in over 90% of participants for 48 weeks or longer. These findings indicate that HIV drug resistance from a first-line failure has less of an impact on the response to second-line, PI-based ART than previously expected.
By contrast, there are limited data on the efficacy of third-line ART regimens in LMIC and on the prevalence and impact of drug resistance from first- and second-line regimen failures on virologic responses to subsequent regimens. To address this need, AIDS Clinical Trials Group protocol A5288 was designed to assess an ART strategy for individuals failing second-line ART, in which ART regimens were determined based upon prior drug exposure, testing for confirmation of virologic failure, and an HIV drug resistance genotype analysis. We present here the resistance profiles obtained from individuals who were screened for enrollment into the A5288 study, and the associations between these profiles and clinical and laboratory characteristics.
METHODS
Study Design and Participants
A5288 was an open-label, Phase IV, prospective strategy study in LMIC for participants with 3 drug-class experiences or 3 drug-class resistances (NRTI, non-NRTI [NNRTI], and PI) who were on a failing PI-based, second-line regimen. Individuals were screened at clinical research sites in 10 countries: Brazil, Haiti, India, Kenya, Malawi, Peru, South Africa, Thailand, Uganda, and Zimbabwe between January 2013 to September 2015. The screening process occurred as follows: HIV RNA level measurements and CD4+ T-cell counts were performed and, if the HIV RNA level was confirmed to be above 1000 RNA copies/ml, then an HIV drug resistance test was performed and the antiretroviral (ARV) drug history was provided to the A5288 team to help determine the best regimen for third-line ART.
Human Immunodeficiency Virus–1 Drug Resistance Testing, Scoring, and Categorization
Real-time, population-based HIV drug resistance testing of HIV-1 protease and reverse transcriptase was performed using a laboratory-developed assay that was Division of AIDS (DAIDS) virology quality assessment–certified at Fundação Oswaldo Cruz, Brazil; Y.R. Gaitonde Centre for AIDS Research and Education, India; and, Bio Analytical Research Corporation South Africa/Lancet Laboratories, South Africa. Known HIV drug resistance mutations and scores were determined using the Stanford Drug Resistance Database (v6.2) [8] for all NRTIs, NNRTIs, and PIs (except for etravirine [ETR] and darunavir [DRV]). For ETR, a score above 2.5 was categorized as resistant [9]; for DRV, having ≥3 DRV mutations was categorized as resistant [10]. For the statistical analysis, resistance was defined as being in intermediate or high-level categories defined by Stanford or being in the resistant category for ETR and DRV. In a sensitivity analysis, resistance was defined as being only in the high-level resistance Stanford category or in the resistant category for ETR and DRV.
Drug Class Resistance and Susceptibility to Second-line Antiretroviral Therapy
NRTI class resistance was defined as having an intermediate or higher category of resistance, as determined by the Stanford database, to at least 1 of the NRTIs considered. NNRTI resistance and PI resistance were similarly defined. Resistance could be to a maximum of 3 drug classes (NRTI, NNRTI, and PI). Susceptibility to second-line ART was defined as having no intermediate or higher category of resistance, as determined by the Stanford database, to a commonly available, second-line, PI-containing regimen.
Human Immunodeficiency Virus–1 Subtype Analysis
HIV sequences were subtyped using the PHYLogeny Inference Package (PYLIP) dnadist program. If the subtype could not be assigned, the sequence was analyzed using the Los Alamos National Laboratory Recombinant Identification Program (LANL RIP) program.
Statistical Methods
Logistic regression was used to evaluate associations of resistance/susceptibility to second-line regimens with participant characteristics in univariable and multivariable models. The following predictor variables were considered: screening HIV RNA level (categorized as <4.00, 4.00–4.99, and ≥5.0 log10 copies/mL), nadir and CD4+T-cell counts (≤50, 51–100, 101–200, and > 200 cells/mm3), sex, age (18–29, 30–39, 40–49, and 50+ years), number of prior/ongoing NRTIs prescribed (≤3, 4, and ≥5 NRTIs), prior/ongoing NNRTI exposure (efavirenz [EFV] only, nevirapine [NVP] only, and both NVP and EFV) and prior/ongoing PI exposure. PI exposure was categorized as LPV only (51%), ATV only (27%), and both ATV and LPV (22%); each of these 3 PI exposure groups included some participants (<20%) who had also taken PIs other than LPV and ATV, including fosamprenavir (FPV), indinavir (IDV), nelfinavir (NFV), and saquinavir (SQV). We also considered 2 proxy variables for the duration of first-line and second-line ART: the number of weeks between the first and last NNRTI use (categorized into quartiles: <104 weeks, 104 to <188, 188 to <289, and ≥289 weeks), and the number of weeks between the last NNRTI use and the date of the genotype sample (categorized into quartiles: <90, 90 to <161, 161 to <260, and ≥260 weeks). Country and HIV subtypes were considered, but these 2 variables are so interlinked they were combined into a single variable reflecting dominant subtypes within countries: Brazil/subtype B, Haiti/B, Peru/B, Thailand/CRF01-AE, Kenya/A1, Uganda/A1, Kenya/D, Uganda/D, Malawi/C, South Africa/C, Zimbabwe/C, and India/C. Those participants not in these country/subtype combinations were included in a category of “any country/any other subtype.” Multivariable models were also fitted, including all predictor variables, first without and then with country/subtype, to allow an assessment of whether associations changed qualitatively.
To focus on those participants failing the standard second-line ART regimens used in LMIC settings, the analysis population excluded participants if they did not have NNRTI exposure (1 participant), if they had DRV or ETR exposure (5 participants), or if they did not have exposure to LPV/r and/or ATV/r (3 participants).
Statistical comparisons by country and by sex were evaluated using the Wilcoxon and Kruskal-Wallis tests for quantitative variables and the Chi-squared test for categorical variables. All analyses were performed using SAS version 9.2 (Cary, NC).
RESULTS
Countries, Subtypes, and Prior Antiretroviral Exposure
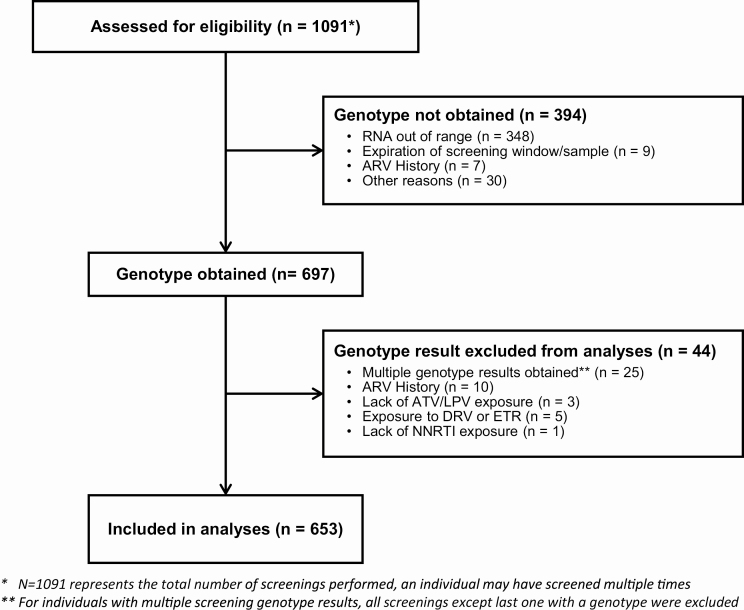
653 candidates screened for A5288 had plasma HIV RNA genotype results available and were included in this analysis (Figure 1). The 653 candidates were from 10 countries and had a median age of 41 years (quartiles: 36 to 47 years). The majority were male (53%). The median plasma HIV RNA level at time of screening was 4.5 log10 copies/ml (quartiles 4.0–5.1 log10 copies/ml) and the median nadir CD4+ T-cell count was 64 cells/mm3 (quartiles 25–141 cells/mm3; Table 1). Of the 653 candidates, 624 had full ART records available. All 624 had been exposed to an NRTI, with all having taken 3TC or emtricitabine; TDF had been taken by 84% and zidovudine by 77%. All 624 participants had prior exposure to an NNRTI. Only 1% of participants were still on an NNRTI (also with a PI) at the time of screening. Prior exposure to LPV/r was only reported in 51%, to ATV/r only in 27%, and to both LPV/r and ATV/r in 22%. Only 6% of participants had prior raltegravir exposure and none had exposure to other integrase inhibitors.
Figure 1.
Consort diagram. Abbreviations: ARV, antiretroviral; ATV, atazanavir; DRV, darunavir; ETR, etravirine; LPV, lopinavir; NNRTI, nonnucleoside reverse transcriptase inhibitor.
Table 1.
Characteristics of Participants, by Country
| Total, N = 653 | Haiti, n = 53 | Brazil, n = 57 | Peru, n = 23 | Kenya, n = 87 | Uganda, n = 97 | Malawi, n = 42 | South Africa, n = 96 | Zimbabwe, n = 26 | India n = 151 | Thailand, n = 21 | P Value | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, years | n | 653 | 53 | 57 | 23 | 87 | 97 | 42 | 96 | 26 | 151 | 21 | .001a |
| Median | 41 | 45 | 43 | 37 | 41 | 41 | 39 | 41 | 43 | 42 | 33 | ||
| Q1, Q3 | 36, 47 | 38, 53 | 37, 48 | 34, 43 | 32, 48 | 33, 50 | 32, 47 | 37, 47 | 34, 52 | 39, 46 | 22, 41 | ||
| Sex | Male | 343 (53%) | 27 (51%) | 27 (47%) | 15 (65%) | 34 (39%) | 47 (48%) | 17 (40%) | 31 (32%) | 14 (54%) | 119 (79%) | 12 (57%) | <.001b |
| Female | 310 (47%) | 26 (49%) | 30 (53%) | 8 (35%) | 53 (61%) | 50 (52%) | 25 (60%) | 65 (68%) | 12 (46%) | 32 (21%) | 9 (43%) | ||
| Subtype | C | 316 (48%) | 0 (0%) | 2 (4%) | 0 (0%) | 6 (7%) | 1 (1%) | 42 (100%) | 92 (96%) | 26 (100%) | 147 (97%) | 0 (0%) | <.001b |
| B | 127 (19%) | 53 (100%) | 51 (89%) | 22 (96%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1%) | 0 (0%) | 0 (0%) | 0 (0%) | ||
| A1 | 122 (19%) | 0 (0%) | 0 (0%) | 0 (0%) | 61 (70%) | 57 (59%) | 0 (0%) | 2 (2%) | 0 (0%) | 2 (1%) | 0 (0%) | ||
| D | 46 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 10 (11%) | 35 (36%) | 0 (0%) | 1 (1%) | 0 (0%) | 0 (0%) | 0 (0%) | ||
| CRF01_AE | 22 (3%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1%) | 21 (100%) | ||
| Other | 20 (3%) | 0 (0%) | 4 (7%) | 1 (4%) | 10 (11%) | 4 (4%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1%) | 0 (0%) | ||
| Screening HIV-1 RNA, log10 copies/mL | n | 652 | 53 | 56 | 23 | 87 | 97 | 42 | 96 | 26 | 151 | 21 | .075a |
| Median | 4.5 | 4.7 | 4.4 | 4.4 | 4.8 | 4.8 | 4.4 | 4.5 | 4.5 | 4.5 | 4.8 | ||
| Q1, Q3 | 4.0, 5.1 | 4.3, 4.9 | 4.0, 4.8 | 3.7, 4.9 | 4.0, 5.3 | 4.0, 5.3 | 3.7, 4.8 | 4.0, 5.0 | 4.0, 5.3 | 3.9, 5.1 | 4.0, 5.3 | ||
| Nadir CD4 count, cells/mm3 | n | 645 | 53 | 53 | 23 | 87 | 96 | 40 | 95 | 26 | 151 | 21 | <.001a |
| Median | 64 | 58 | 123 | 72 | 69 | 99 | 55 | 103 | 56 | 51 | 19 | ||
| Q1, Q3 | 25, 141 | 29, 141 | 24, 266 | 38, 151 | 16, 131 | 33, 167 | 8, 127 | 51, 183 | 19, 141 | 20, 89 | 6, 86 |
Abbreviations: HIV, human immunodeficiency virus; Q, quartile.
aKruskal-Wallis test.
bChi-square test.
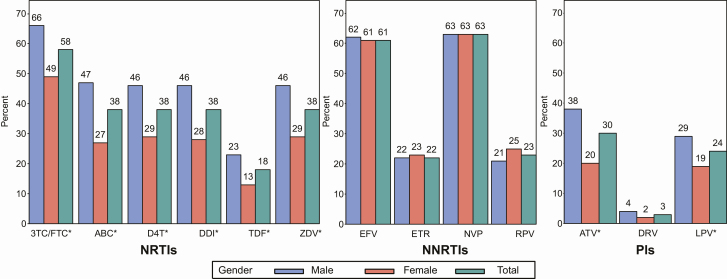
At the time of screening, TDF (67%) and 3TC (90%) were the most commonly used NRTIs, with either LPV/r (55%) or ATV/r (44%). The most common HIV subtype in the study population was C (48%; Table 1). There was no difference in the time on ART by sex (medians of 425 weeks for males vs 411 weeks for females; P = .44). Prior and ongoing ARV usage by sex was similar for the NRTI and integrase inhibitor classes and different for the NNRTI and PI classes. Specifically, males were more likely to have been exposed to EFV (64% for males vs 48% for females; P < .001) and ATV/r (57% for males vs 40% for females; P < .001), while females had more exposure to NVP (61% for males vs 70% for females; P = .01) and LPV/r (65% for males vs 81% for females; P < .001).
Human Immunodeficiency Virus–1 Drug Resistance Profiles
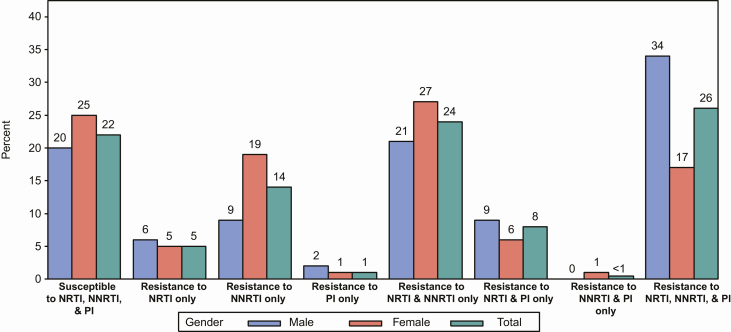
Of the 653 genotype results analyzed, 78% had resistance to at least 1 drug, but the remaining 22% had no drug resistance (ie, no intermediate or higher resistance to any drug) despite having a history of failing first-line ART and being on a failing second-line regimen (Table 2). The analysis showed that 62% had resistance (intermediate or higher) to 1 or more NRTI, 64% to 1 or more NNRTI, and 35% to 1 or more PI. Also, 24% had resistance to at least 1 drug in the NRTI class and at least 1 drug in the NNRTI class, and 26% had resistance to at least 1 drug in each of the 3 drug classes (NRTI, NNRTI, and PI; Figure 2). Importantly, a slight majority (59%) showed susceptibility to a least 1 PI-containing second-line regimen (defined as 2 NRTIs and either LPV/r or ATV/r; Table 2) and a large majority were susceptible or had only low-level resistance to DRV/r (97%) and ETR (78%; Figure 3).
Table 2.
Human Immunodeficiency Virus–1 Drug Resistance by Country and Antiretroviral Class
| HIV Drug Resistancea | Total, N = 653 | Haiti, n = 53 | Brazil, n = 57 | Peru, n = 23 | Kenya, n = 87 | Uganda, n = 97 | Malawi, n = 42 | South Africa, n = 96 | Zimbabwe, n = 26 | India n = 151 | Thailand, n = 21 | P Valueb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Resistance to any ARV | 508 (78%) | 37 (70%) | 36 (63%) | 15 (65%) | 67 (77%) | 77 (79%) | 30 (71%) | 77 (80%) | 22 (85%) | 130 (86%) | 17 (81%) | .022 |
| Resistance to NRTI | 407 (62%) | 28 (53%) | 31 (54%) | 8 (35%) | 52 (60%) | 63 (65%) | 23 (55%) | 59 (61%) | 19 (73%) | 110 (73%) | 14 (67%) | .012 |
| Resistance to NNRTI | 416 (64%) | 32 (60%) | 27 (47%) | 10 (43%) | 60 (69%) | 53 (55%) | 27 (64%) | 65 (68%) | 19 (73%) | 113 (75%) | 10 (48%) | .001 |
| Resistance to PI | 229 (35%) | 6 (11%) | 19 (33%) | 2 (9%) | 31 (36%) | 44 (45%) | 13 (31%) | 19 (20%) | 16 (62%) | 70 (46%) | 9 (43%) | <.001 |
| No resistance to any drug class | 145 (22%) | 16 (30%) | 21 (37%) | 8 (35%) | 20 (23%) | 20 (21%) | 12 (29%) | 19 (20%) | 4 (15%) | 21 (14%) | 4 (19%) | <.001 |
| Resistance to ≥1 drug in 1 drug class | 133 (20%) | 12 (23%) | 8 (14%) | 10 (43%) | 16 (18%) | 21 (22%) | 8 (19%) | 23 (24%) | 3 (12%) | 28 (19%) | 4 (19%) | |
| Resistance to ≥1 drug in 2 drug classes | 206 (32%) | 21 (40%) | 15 (26%) | 5 (22%) | 26 (30%) | 29 (30%) | 11 (26%) | 42 (44%) | 6 (23%) | 41 (27%) | 10 (48%) | |
| Resistance to ≥1 drug in 3 drug classes | 169 (26%) | 4 (8%) | 13 (23%) | 0 (0%) | 25 (29%) | 27 (28%) | 11 (26%) | 12 (13%) | 13 (50%) | 61 (40%) | 3 (14%) | |
| Susceptible to second-line ART, 2 NRTIs + ATV/r or LPV/r | 384 (59%) | 36 (68%) | 38 (67%) | 19 (83%) | 52 (60%) | 48 (49%) | 25 (60%) | 72 (75%) | 10 (38%) | 71 (47%) | 13 (62%) | <.001 |
Data are for intermediate or higher-level resistance. The ARV classes were NRTI, NNRTI, or PI.
Abbreviations: 3TC, lamivudine; ABC, abacavir; ART, antiretroviral treatment; ARV, antiretroviral; ATV/r, ritonavir-boosted atazanavir; FTC, emtricitabine; HIV, human immunodeficiency virus; LPV/r, ritonavir-boosted lopinavir; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; TDF, tenofovir; ZDV, zidovudine.
aHIV drug resistance was determined for all NRTIs, NNRTIs, and PIs currently used in low- and middle-income countries and, except for etravirine and darunavir, was classified into 5 distinct categories: susceptible, potential low-level resistance, low-level resistance, intermediate resistance, and high-level resistance. For analyses and presentation, resistance was then defined as being in the intermediate or higher categories, while susceptible was defined as being in the low-level or lower categories. Susceptibility to second-line ART was defined as having no intermediate or higher category of resistance to a commonly available, second-line, PI-containing regimen, so specifically being in the susceptible, potential low-level resistance or low-level resistance categories for at least 2 NRTIs among 3TC (or FTC), ABC, ZDV, and TDF, and for 1 or both of ATV/r and LPV/r.
bChi-square test.
Figure 2.
Percentage of candidates with intermediate or higher-level resistance to various drug class (NRTI, NNRTI, and PI) categories. Abbreviations: NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
Figure 3.
Percentage of participants by sex with intermediate/higher resistance to specific drugs in the NRTI, NNRTI, and PI drug classes. Drugs labelled with an asterisk were significantly different by sex, with P values < .005. Abbreviations: 3TC, lamivudine; ABC, abacavir; ATV, atazanavir; D4T, stavudine; DDI, didanosine; DRV, darunavir; EFV, efavirenz; ETR, etravirine; FTC, emtricitabine; LPV, lopinavir; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; NVP, nevirapine; PI = protease inhibitor; RPV, rilpivirine; TDF, tenofovir; ZDV, zidovudine.
The most common NRTI mutation was M184V/I (57% of candidates), followed by thymidine analogue mutations at codons 215 (26%), 67 (22%), 41 (20%), 70 (18%), and 219 (18%). Mutations at codon K65R occurred at a very low frequency (3%). The most frequent NNRTI mutations were at codons K103 (34%), G190 (19%), and Y181 (15%), and the most frequent major PI mutations were at codons M46 (21%), A71 (21%), V82 (21%), and I54 (20%). PI-associated resistance was most common in the participants exposed only to ATV/r (46%), compared those exposed to LPV/r alone (30%) or to both LPV/r and ATV/r (34%; P = .002).
Factors Associated with the Extent of Human Immunodeficiency Virus–1 Drug Resistance
Given the highly diverse resistance profiles, we sought to evaluate associations in both univariate and multivariate models between variables at screening (HIV RNA; nadir CD4+ T-cell count; sex; age; number or type of prior/ongoing NRTI, NNRTI, or PI exposure; ART duration variables; and country/subtype) and resistance by drug class. We also analyzed associations with susceptibility to a second-line ART regimen. Detailed results of these analyses are shown in Supplementary Tables S1–4, and the most important findings are summarized below.
Sex
Sex was found to be associated with differences in resistance profiles in both univariable and multivariable analyses (Supplementary Tables S1–4). The median duration of time on ART was similar by sex; however, more men had resistance to at least 1 drug in the NRTI and PI classes, but not the NNRTI class, compared to women (NRTI, 69% vs 55%, respectively [P < .001]; PI, 45% vs 24%, respectively [P < .001]; NNRTI, 64% vs 64%, respectively [P = .94]). More men (34%) than women (17%) had resistance to at least 1 drug in each of the 3 drug classes (P < .001).
Susceptibility to a Second-line Antiretroviral Therapy Regimen
Similar models were used to assess associations between participant characteristics and susceptibility to a second-line regimen, defined as susceptibility to at least 2 NRTIs and either LPV/r or ATV/r (Supplementary Table S4). In univariable analyses, higher odds of susceptibility to a second-line regimen were associated with higher nadir CD4+ T-cell counts (P = .004), female sex (P < .001), lower age (P = .023), prior exposure to EFV only (versus NVP only or both NVP and EFV; P < .001), and prior exposure to LPV/r only or to both ATV/r and LPV/r versus ATV/r only (P < .001). The lower susceptibility to a second-line regimen among individuals exposed only to ATV/r primarily reflected an inability to identify 2 susceptible NRTIs (54% of individuals in this group vs 25% among individuals exposed to LPV/r only and 35% among individuals exposed to both LPV/r and ATV/r). Similarly, lower susceptibility to a second-line regimen among individuals exposed to NVP with or without exposure to EFV primarily reflected an inability to identify 2 susceptible NRTIs (46% and 39% of individuals in the 2 NVP groups, respectively, versus 22% among individuals exposed only to EFV). There was also significant variation in the odds of susceptibility by country/subtype (P < .001). These associations persisted in multivariable models, although the CD4+ T-cell nadir was not statistically significant in the model without an adjustment for country/subtype (P = .059).
Sensitivity analyses that used the screening CD4+ T-cell count, rather than the nadir CD4+ T-cell count, and that did not include intermediate resistance scores in the resistant category did not substantially alter the associations found.
DISCUSSION
This multi-center study describes the HIV drug resistance profiles of individuals failing second-line ART at urban centers from 10 LMICs across 3 continents. The resistance patterns found were extremely diverse: 22% had no resistance, whereas 32% and 26% had resistance to at least 1 drug in 2 or 3 drug classes, respectively. These findings are distinct from what has been reported after first-line ART failure, where the majority of individuals (75–95%) [1, 4] have at least 1 NRTI mutation (usually M184I/V) and an NNRTI mutation. In the current study, only 24% exhibited this pattern of having only NRTI and NNRTI resistance (Figure 2). Put differently, resistance patterns after first-line treatment failure are largely predictable, whereas those after second-line therapy failure do not appear to be. This is likely the case because of heterogeneity in first- and second-line regimens, the duration of therapy, and adherence to or tolerance of the prescribed regimen at the time of screening. These important findings suggest that access to resistance testing may be needed to optimally guide third-line treatment strategies in LMIC settings.
A slight majority (59%) of the participants were still susceptible to a standard second-line regimen. This finding has been observed among individuals experiencing virologic failure in clinical trials of second-line ART, suggesting that poor adherence or intolerability to second-line regimens is a common mechanism of regimen failure, rather than only HIV drug resistance [5–7]. In the current study, 41% were not susceptible to second-line regimens, which underscores the complexity of managing second-line ART failure. In a multivariable analysis, a higher nadir CD4+ T-cell count, but not plasma HIV RNA, discriminated modestly between those with and without susceptibility to second-line regimens, although the clinical utility of the observed CD4+ T-cell count differences is uncertain. We also found reasonably large differences in susceptibility to a second-line regimen according to the types of PIs and NNRTIs individuals had previously taken. These differences primarily reflected higher proportions of individuals exposed to ATV/r only and of individuals exposed to NVP (with or without EFV) in whom it was not possible to identify 2 susceptible NRTIs. While these associations might reflect differences in drug potency, other unmeasured factors might explain these findings.
The moderate frequency of PI resistance observed (35%) is within the range observed in prior reports [11]. These studies have observed PI resistance in 7–22% of individuals failing second-line ART in the public sector, increasing to 47% in the private sector in South Africa [11–13]. The reason for this large difference in PI resistance across sectors is unknown, but may be related to adherence or tolerance to second-line regimens among different populations, as well as different uses of other ARVs. Although recent studies suggest that PI resistance is increasing over time, the majority of participants screened for A5288 did not have resistance mutations in protease. It is possible that mutations decreasing PI susceptibility are occurring in other regions of HIV genome, such as gag or env, that we did not assay. Several studies have documented the development of compensatory mutations in gag [14–17] that result in resistance to PIs; however, these gag mutations generally develop after mutations in the protease region appear [18]. The studies of env have not determined the key mutations that could confer PI resistance, but in vitro work by Rabi and colleagues [19] has shown that there is reduced growth of viral clones containing env genes derived from individuals on failing PI-containing regimens. Sequencing of the gag and env regions in second-line failures should be undertaken to further investigate the observed lack of major PI resistance mutations.
Given that medication intolerance is a reason for failures of second-line ART, it is vital that regimens that are better tolerated and easier to take be identified. Along these lines, a single-tablet regimen containing dolutegravir, TDF, and 3TC is being considered for first-line ART, as well as for second-line ART when not used in first-line regimens, although a recent report of neural tube defects in infants of women receiving dolutegravir during conception has given pause to the wide adoption of this single-tablet regimen [20].
Although no association was observed between screening plasma HIV RNA level and drug resistance in a multivariable analysis adjusted for country, there was some evidence of modest associations between screening plasma HIV RNA levels and resistance to specific drug classes. Among participants with HIV-1 RNA levels <4.00 log10 copies/mL, 69% had intermediate or higher-level resistance to at least 1 NRTI, compared with 61% among participants with HIV-1 RNA levels ≥5.00 log10 copies/mL, a difference which persisted in a multivariable analysis including an adjustment for country/subtype (adjusted odds ratio, 1.83; 95% confidence interval, 1.10–3.03; Supplementary Table S1). In contrast, the reverse association was observed for PI resistance: 32% with HIV-1 RNA levels <4.00 log10 versus 43% with HIV-1 RNA levels ≥5.00 log10 copies/mL had resistance to at least 1 PI (adjusted odds ratio, 0.58; 95% confidence interval, 0.36–0.97; Supplementary Table S3). It is important to highlight this, as the plasma HIV RNA level is often considered in LMICs to be an indicator of resistance because of the potential effects of HIV drug resistance mutations on viral fitness; that is, the lower the HIV RNA level, the more likely resistance will be present. The data in the current study shows that these associations may be modest in strength and inconsistent in direction among drug classes.
Important sex differences in HIV drug resistance were identified in the current study. Men were more likely than women to have reduced susceptibility to all currently prescribed NRTIs and PIs. This novel finding suggests possible sex differences in access to ART or differences in drug pharmacokinetics, medication tolerance, or levels of adherence. Alternatively, men may be more likely to take ARVs intermittently, resulting in greater resistance.
In conclusion, the screening of candidates for a study (A5288) of third-line ART showed highly divergent resistance patterns. More than half of individuals (59%) remained susceptible to a second-line, PI-containing regimen, but there was frequent resistance to at least 1 drug in each of 2 or 3 drug classes. These divergent resistance profiles could only be clearly differentiated by HIV drug resistance testing, which has been shown to increase survival and be a cost-effective approach for guiding third-line ART [21]. Our findings support increased access to resistance testing or the need for access to newer ARV drugs that are highly effective in suppressing viremia despite prior resistance to 2 or 3 drug classes.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. C. L. W. contributed to the study design, virological resistance studies, review of all resistance data and interpretation, and manuscript drafting and revision. M. D. H. contributed to the study design, data analysis and interpretation, and manuscript drafting and revision. J. R. contributed to the figures, data analysis and interpretation, and manuscript drafting and revision. R. V., C. S dJ., and S. S. contributed to the virological resistance studies and manuscript revision. M. vS. and R. M. contributed to participant recruitment, data collection and interpretation, and manuscript revision. R. S., E. H., and R. G. contributed to the study design, data interpretation, and manuscript revision. P. M. contributed to the study design, participant recruitment and data collection, and manuscript revision. L. W. contributed to the data management and interpretation and manuscript revision. C. G. and A. C. C. contributed to the literature search, study design, data interpretation, and manuscript revision. B. G. contributed to the literature search, study design, participant recruitment, data collection and interpretation, and manuscript revision. J. W. M. contributed to the study design, virological resistance studies, data interpretation, and manuscript drafting and revision.
Acknowledgments. The authors thank all AIDS Clinical Trials Group (ACTG) A5288 study volunteers for their participation; Dimagi, the other members of the A5288 team, the genotyping laboratories (Bio Analytical Research Corporation South Africa/Lancet Dr Raquel Viana; Y.R. Gaitonde Centre for AIDS Research and Education Dr Saravanan; Fundação Oswaldo Cruz Carlos Silva de Jesus) for the primary endpoint and the genotyping; and the staff at the sites and grants supporting their work.
Disclaimer. The views in this paper do not necessarily represent those of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases.
Financial support. This work was funded by the National Institute of Allergy and Infectious Diseases (award numbers UM1 AI068636, UM1 AI068634 [AIDS Clinical Trials Group (ACTG) Statistical and Data Management Center], UM1 AI069423, and UM1 AI069481); AbbVie, Gilead Sciences, Janssen Pharmaceuticals, and Merck & Company provided the study drugs.
Potential conflicts of interest. J. W. M. is a consultant for Gilead Sciences, Bristol-Myers Squibb, Merck, and Xi’an Yufan Biotechnologies, has received research grants to the University of Pittsburgh from Gilead Sciences and Janssen Pharmaceuticals, and owns share options in Co-Crystal Pharma, Inc., which are unrelated to the current study; he also has Patent #: 8 815 829 pending. C. L. W. is a consultant for the international partnership for microbicides, has received honorariums for training clinicians in human immunodeficiency virus (HIV)-1 drug resistance from AbbVie, MSD, Mylan, Abbott, and Right-to-Care and served as an expert on the Stanford HIV drug resistance algorithm review for Celera, which are unrelated to the current study. A. C. C. has been a member of a Merck & Co. Data Safety and Monitoring Committee and received research grants to the University of Washington from Bristol-Myers-Squibb, which are unrelated to the current study. R. G. reports personal fees from Pfizer, outside the submitted work. R. V. reports speaker agreements form Johnson and Johnson, Mylan, and AbbVie, outside the submitted work. M. D. H. reports grants from the National Institutes of Health (NIH) outside the submitted work. C. G. is an NIH employee. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Wallis CL, Mellors JW, Venter WD, Sanne I, Stevens W. Varied patterns of HIV-1 drug resistance on failing first-line antiretroviral therapy in South Africa. J Acquir Immune Defic Syndr 2010; 53:480–4. [DOI] [PubMed] [Google Scholar]
- 2. Wallis CL, Godfrey C, Fitzgibbon JE, Mellors JW. Key factors influencing the emergence of human immunodeficiency virus drug resistance in low- and middle-income countries. J Infect Dis 2017; 216:851–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hamers RL, Rinke de Wit TF, Holmes CB. HIV drug resistance in low-income and middle-income countries. Lancet HIV 2018; 5:e588–96. [DOI] [PubMed] [Google Scholar]
- 4. Harrison L, La Rosa A, Viana RV, et al. Is resistance testing of value after first-line ART failure in resource limited settings? Insights from AIDS Clinical Trials Group (ACTG) 5273. Global Antiviral Journal. 2016; 12(Suppl 1):A37. [Google Scholar]
- 5. Boyd MA, Kumarasamy N, Moore CL, et al. ;. SECOND-LINE: A Trial of 2 Options for Second Line Combination Antiretroviral Therapy Following Virological Failure of a Standard Non-nucleoside Reverse Transcriptase Inhibitor (NNRTI)+2N(t)RTI First Line Regimen (SECOND-LINE). Lancet 2013; 381:2091–9. [DOI] [PubMed] [Google Scholar]
- 6. Paton NI, Kityo C, Hoppe A, et al. ; Europe–Africa Research Network for Evaluation of Second-Line Therapy Trial Team. Assessment of second-line antiretroviral regimens for HIV therapy in Africa. N Engl J Med 2014; 371:234–47. [DOI] [PubMed] [Google Scholar]
- 7. La Rosa AM, Harrison LJ, Taiwo B, et al. ; AIDS Clinical Trials Group (ACTG) A5273 Study Group Raltegravir in second-line antiretroviral therapy in resource-limited settings (SELECT): a randomised, phase 3, non-inferiority study. Lancet HIV 2016; 3:e247–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu TF, Shafer RW. Web resources for HIV type 1 genotypic-resistance test interpretation. Clin Infect Dis 2006; 42:1608–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vingerhoets J, Nijs S, Tambuyzer L, Hoogstoel A, Anderson D, Picchio G. Similar predictions of etravirine sensitivity regardless of genotypic testing method used: comparison of available scoring systems. Antivir Ther 2012; 17:1571–9. [DOI] [PubMed] [Google Scholar]
- 10. de Meyer S, Dierynck I, Lathouwers E, et al. Phenotypic and genotypic determinants of resistance to darunavir: analysis of data from treatment-experienced patients in POWER 1,2,3 and DUET-1 and 2 [abstract 31]. Antiviral Therapy 2008; 13(Suppl 3):A33. [Google Scholar]
- 11. Boender TS, Hamers RL, Ondoa P, et al. Protease inhibitor resistance in the first 3 years of second-line antiretroviral therapy for HIV-1 in Sub-Saharan Africa. J Infect Dis 2016; 214:873–83. [DOI] [PubMed] [Google Scholar]
- 12. Wallis CL, Mellors JW, Venter WD, Sanne I, Stevens W. Protease inhibitor resistance is uncommon in HIV-1 subtype C infected patients on failing second-line lopinavir/r-containing antiretroviral therapy in South Africa. AIDS Res Treat 2011; 2011:769627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wallis CL, Viana R, St John EP, Glass A, Mellors JW. An in-depth resistance analysis of HIV-1 subtype C-infected patients failing a lopinavir/ritonavir (LPV/r) second-line regimen in the South African private sector. Antiviral Therapy 2012; 17(Suppl 1):A123. [Google Scholar]
- 14. Maguire MF, Guinea R, Griffin P, et al. Changes in human immunodeficiency virus type 1 Gag at positions L449 and P453 are linked to I50V protease mutants in vivo and cause reduction of sensitivity to amprenavir and improved viral fitness in vitro. J Virol 2002; 76:7398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gatanaga H, Suzuki Y, Tsang H, et al. Amino acid substitutions in Gag protein at non-cleavage sites are indispensable for the development of a high multitude of HIV-1 resistance against protease inhibitors. J Biol Chem 2002; 277:5952–61. [DOI] [PubMed] [Google Scholar]
- 16. Nijhuis M, van Maarseveen NM, Lastere S, et al. A novel substrate-based HIV-1 protease inhibitor drug resistance mechanism. PLOS Med 2007; 4:e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fun A, Wensing AM, Verheyen J, Nijhuis M. Human immunodeficiency virus Gag and protease: partners in resistance. Retrovirology 2012; 9:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mammano F, Petit C, Clavel F. Resistance-associated loss of viral fitness in human immunodeficiency virus type 1: phenotypic analysis of protease and gag coevolution in protease inhibitor-treated patients. J Virol 1998; 72:7632–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rabi SA, Laird GM, Durand CM, et al. Multi-step inhibition explains HIV-1 protease inhibitor pharmacodynamics and resistance. J Clin Invest 2013; 123:3848–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zash R, Makhema J, Shapiro RL. Neural-tube defects with dolutegravir treatment from the time of conception. N Engl J Med 2018; 379:979–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lorenzana SB, Hughes MD, Grinsztejn B, et al. Genotype assays and third-line ART in resource-limited settings: a simulation and cost-effectiveness analysis of a planned clinical trial. AIDS 2012; 26:1083–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.