ABSTRACT
Transgenic crops that produce Bacillus thuringiensis (Bt) toxins are effective tools for controlling lepidopteran pests. However, the degree of susceptibility to Bt toxins differs among various pest species due to relatively narrow spectrum and high selectivity of such toxins. Bt corn hybrids for Chinese market were designed to target Asian corn borer Ostrinia furnacalis (Guenée), while their efficacy against other lepidopteran pests are not well defined, such as Conogethes punctiferalis (Guenée), Helicoverpa armigera (Hübner), Agrotis ypsilon (Rottemberg), and Mythimna separata (Walker), which are also important lepidopteran pests on corn in the Huang-Huai-Hai Summer Corn Region of China. To determine what type of Bt corn is suitable for this region, the efficacy of five Bt toxins, i.e., Cry1Ab, Cry1Ac, Cry1F, Cry2Ab, and Vip3A, to these five lepidopteran species was evaluated in laboratory. Both O. furnacalis and C. punctiferalis showed similar high susceptibility to all five Bt toxins. A. ypsilon and M. separate were less sensitive to Cry1Ab and Cry1Ac than the other species. H. armigera, A. ypsilon and M. separate were less sensitive to Cry1F than O. furnacalis and C. punctiferalis. H. armigera was more sensitive to Cry2Ab than other tested species. All five species were equally sensitive to Vip3A, though their LC50s were all relatively higher. These findings suggest that the first generation Bt corn expressing single Cry1 toxin should not be the first choice because of the potential risk of control failure or less efficacy against H. armigera, A. ypsilon or M. separate. The second-generation Bt corn expressing Cry1 and Cry2 toxins, or the third generation Bt corn expressing Cry1, Cry2 and Vip3A toxins might produce better protection of corn in the Huang-Huai-Hai Summer Corn Region of China.
KEYWORDS: Bt toxin, corn pests, patterns of susceptibility, bioassay
Introdction
Transgenic Bt (Bacillus thuringiensis) crops expressing specific insecticidal proteins are a great success for controlling major agricultural insect pests with a total acreage of 191.7 million ha in 2018, increased ~113-fold since its first commercialization in 1996.1 Area-wide benefits have been created by adaptation of transgenic crops through both yield increase and reduction of chemical pesticide sprays, especially during seasons of moderate to severe insect pest infestations.2–4 Additional gain of 186.1 billion US dollars in farmer income was generated by Bt crops during the past two decades.1
The first Bt corn expressing Cry1Ab toxin was commercially planted in 1996 in the United States to control the European corn borer, Ostrinia nubilalis (Hübner), one of the most destructive pests in the U.S. corn belt.5 In 2017, a total of 29.44 million ha of Bt corn was planted in U.S. with an adoption rate of 81.26%.1 Among many events, Cry1Ab, Cry1Ac, and Cry1F are usually expressed in single or pyramided Bt corns, while Cry2Ab and Vip3A are available only as stacked traits. Plants containing two different Bt proteins with dissimilar binding sites have a broader efficacy spectrum and a potential to delay pest resistance development more effectively than single Bt crops, thus are widely adopted in recent years.6,7 In addition to the U.S., Brazil, Argentina, Canada, South Africa, Uruguay, Spain, Honduras, Chile, Egypt, Romania, Slovakia, Czech Republic, Portugal, and Philippines also planted large areas of Bt corn by 2018.1
Unlike traditional broad-spectrum chemical insecticides, Bt has selective toxicity for a certain group of insects, i.e., mainly lepidopterans, and the toxicity level can vary greatly between different species. For example, the Cry1Ab corn in Europe exhibits excellent efficacy against the two key lepidopteran pests, O. nubilalis and the Mediterranean corn borer Sesamia nonagrioides Lefèbvre (Lepidoptera: Noctuidae),8,9 but has a much lower efficacy against the other two secondary lepidopteran pests, i.e., the true armyworm Mythimna unipuncta Haworth and the corn earworm Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae).10,11 In Canada, the Cry1Ab corn has effectively controlled O. nubilalis and other lepidopteran pests, such as the fall armyworm Spodoptera frugiperda (Smith), and the corn earworm H. zea (Boddie), but showed limited ability of controlling the western bean cutworm Striacosta albicosta (Smith).12,13 Thus, planting Bt crops has a potential risk of secondary insect pests becoming key pests in such scenario.14
Although Bt corn has not been commercialized in China yet, it is a promising technology to be considered for corn pest control in future. There are 7 corn production regions in China. They are Northeast Spring Corn Region (NeSCR), North Spring Corn Region (NSCR), Huang-Huai-Hai Summer Corn Region (HHHSCR), Southeast Hilly Corn Region (SeHCR), Southwest Hilly Corn Region (SwHCR), Northwest Inland Corn Region (NwICR), and Qing-Zang Plateau Corn Region (QZPCR).15 The HHHSCR is the largest region and accounts for up to 40% of the total corn cultivation acreage in China, followed by the NeSCR, the second largest region which accounts for 25% of the total acreage. The pest profiles of these production regions are different. In the NeSCR, Asian corn borer Ostrinia furnacalis (Guenée) is the only major pest species on corn, but in the HHHSCR, additional species including the yellow peach moth Conogethes punctiferalis (Guenée), H. armigera (Hübner), black cutworm Agrotis ypsilon (Rottemberg), and oriental armyworm Mythimna separata (Walker) are also considered as major lepidopteran pests of corn.16 Therefore, the efficacy of different types of Bt corns against various major corn pests should be characterized before commercialization.
O. furnacalis, a sibling species of O. nubilalis, is the most destructive corn pest in China. The larvae feed on leaves, silks, tassels, and also bore into cornstalks and ears, which can cause approximately 10% grain yield losses.17–20 In addition, infested ears aggravate the occurrence of corn ear rot and dramatically decrease the grain quality. Previous studies showed that O. nubilalis and O. furnacalis had similar sensitivity to Cry1Aa, Cry1Ab, Cry1Ac, Cry1Ba, and Cry1F toxins.21 In addition to laboratory bioassays, field experiments also showed that O. furnacalis was susceptible to Bt corn and Bt cotton expressing Cry1Ab or Cry1Ac.22–25 These results are useful references for regulatory decisions regarding the use of transgenic corn in areas where O. furnacalis is a major pest in China. However, information on the susceptibility of other major insect pests of corn to Bt toxins is limited.
C. punctiferalis, is a polyphagous pest damaging multiple crops, such as peach, chestnut, sunflower, sorghum, and corn.26–28 Similar to O. nubilalis, C. punctiferalis larvae mainly feed on silks and then bore into ears and tunnel inside, causing fungal infections and ear contamination in addition to the physical damage. In recent years, this pest has increased its population abundance and becomes a major pest on corn in HHHSCR, and its damage is even greater than that of O. nubilalis in some locations within the region.16,29–31
H. armigera is an omnivorous pest causing serious damage to not only cotton but also wheat, sorghum, tobacco, pepper, and specifically the reproductive organs of corn. Since corn is the major summer crop in HHHSCR, the fourth generation of H. armigera would feed mainly on this crop and has become a serious pest.32,33 The larvae of H. armigera concentrate at the top of ears feeding on silks during its early stages, thus affecting pollination. They also bore into grains resulting in extensive accumulations of feces and mildew, which can easily contaminate grains, and thus seriously reduce the grain quality.32
A. ypsilon is one of the most important underground pests. The larvae hide in the soil and feed on the stems of corn seedlings, which results in damaged growth points and plant death.34 This pest is very difficult to control by spraying chemical insecticides due to high resistance and its nocturnal feeding behavior.35
M. separate is a well-known long-distance migrant pest of many crops and is widely distributed throughout northern and southern China.36,37 It is a sporadic pest of corn, wheat, sugarcane, and other crops. Because of polyphagous, migratory, and sporadic nature of this pest, sudden outbreaks often occur in some areas, consuming all available leaves and causing serious yield losses.
In the present study, laboratory bioassays were conducted to evaluate the efficacy of Cry1Ab, Cry1Ac, Cry1F, Cry2Ab, and Vip3A toxins against O. furnacalis, C. punctiferalis, H. armigera, A. ypsilon, and M. separate, thus to provide technical suggestions for commercialization of Bt corn with specific focus on the HHHSCR of China.
Materials and Methods
Insects
A Bt-susceptible colony of O. furnacalis was established from a collection of approximately 200 adults trapped by a 1000 W search light trap during late May and early June 2015 in Yuanyang County of Henan province (35.13°N, 113.41°E). The eggs were collected using wax paper which was changed daily in a large adult rearing box (40 × 30 × 25 cm3). The hatched larvae were reared using a modified artificial diet based on the ingredients described in Song et al.38
A Bt-susceptible strain of C. punctiferalis was established from approximately 300 larvae collected from sorghum fields during late September 2016 in Yuanyang County. The initial 12 generations of this colony were kept on corn seedlings with chestnuts as oviposition substitute, and then switched to artificial diet with major ingredients of chestnut powder, wheat germ and soybean powder (unpublished data).
A Bt-susceptible H. armigera colony maintained in the laboratory was established from a field collection of approximately 200 larvae from non-Bt corn in Yuanyang County in 2016. The larvae were reared for over 20 generations using artificial diet based on corn and soybean powder as described in Liang et al.39
A Bt-susceptible laboratory population of A. ypsilon was established from a field collection of more than 200 moths from Yuanyang County during May 2015. A meridic diet specific to A. ypsilon was used for larval rearing.40
A Bt-susceptible laboratory population of M. separata was established from a field collection of more than 300 larvae from non-Bt corn in Lingbao County of Henan province (34.61°N, 110.80°E) in 2016. A meridic diet specific to M. separata was used for larval rearing.41
All adults were maintained with 10% sugar solution, and all colonies were reared under laboratory conditions with a temperature of 25–28°C, relative humidity of 60–80%, and photoperiod of L:D of 16:8 h. All colonies had been maintained in the absence of Bt selection for at least 1–2 years before testing. It should be noted that Bt corn has not been commercially planted in China except for very few rigorously supervised experimental plots. Corn pest control has heavily relied on spaying chemical pesticides with little use of Bt formulations in the regions where collections were made to establish the colonies of the five pest species used in this study. Although Cry1Ac transgenic cotton, the major host of H. armigera, has been widely planted in this region, the acreage is shrinking and the field population of H. armigera remains very susceptible to Cry1Ac.42 Hence, potential selection for Bt resistance alleles is very weak and susceptibility data of Bt toxins collected on these species should represent the baseline level.
Bt Protein Preparation
Cry1Ab, Cry1Ac, and Cry1F 98% purified proteins were produced and provided by Marianne P. Carey, Case Western Reserve University (Ohio, U.S.). Bioassay solutions of these proteins were prepared by dissolving them in 50 mM 3-(cyclohexylamino) propanesulfonic acid (CAPS), pH 10.5 buffer. Cry2Ab and Vip3A were purchased from Beijing General Pest Biotech Research Co. Ltd. (Beijing, China). The Cry2Ab protein sample was delivered as a purified crystal solution of 1.5 mg/ml concentration. The protein crystals were solubilized by incubation in 0.1 M Na2CO3, pH 10.5. The purified Vip3A protein was delivered as a dry powder and freshly dissolved in distilled water before bioassay. All protein samples were stored in a freezer at −80°C.
Bioassays
All bioassays were conducted during April 2017 to July 2018 using one testing procedure (diet-overlay). Approximately, 1.5 ml of liquefied diet was poured into each well (13 mm in depth and 16 mm in diameter, 2 cm2 surface area) of a 24-well plate. After the diet solidified, 40 µl of Bt protein solutions of seven to nine concentrations or control (distilled water and buffer contains 0.1% (v/v) Triton X-100) was added onto the diet surface in each well using a pipette. When all wells on a plate were treated, the plate was tilted from front to back and from left to right to ensure that the protein solution was evenly distributed on the diet surface. After the diet surface dried, one neonate (0 to 24-h-old) was transferred to each well with a fine brush (24 neonates per concentration). After infestation, the plate was covered with a sheet of blow-molded paper pad, then fastened with 2 rubber bands to prevent escape. The infested plates were placed in a climatic chamber at 27 ± 1°C, L:D of 16:8 h, and RH of 60 ± 10%. The bioassays were repeated three times for each species and each toxin. The sample size (total numbers of neonates) are listed in Table 1. Mortality was recorded at 7 days after treatment. Larvae that remained as first instar throughout the experiment were counted as dead.
Table 1.
Lethal concentrations of five Bacillus thuringiensis toxins to Ostrinia furnacalis, Conogethes punctiferalis, Agrotis ypsilon, Mythimna separata, Helicoverpa armigera.
| Insect species | Bt Toxin | na | Slope(±SE) | LC50(95%FL)b | LC90(95%FL)b | χ2c | df |
|---|---|---|---|---|---|---|---|
| Ostrinia furnacalis | Cry1Ab | 432 | 1.62 ± 0.17 | 2.11(1.64–2.62) | 13.06(9.53–20.38) | 7.77 | 4 |
| Cry1Ac | 432 | 1.64 ± 0.19 | 1.70(1.25–2.19) | 10.29(7.49–16.26) | 7.27 | 5 | |
| Cry1F | 432 | 1.43 ± 0.19 | 4.61(3.48–6.04) | 36.52(22.67–78.80) | 9.13 | 4 | |
| Cry2Ab | 504 | 1.39 ± 0.14 | 134.87(104.77–171.34) | 1127.83(762.33–1957.88) | 10.62 | 5 | |
| Vip3A | 576 | 0.51 ± 0.09 | 328.44(183.99–660.54) | 104243.05(20067.75–2669993.94) | 9.27 | 6 | |
| Conogethes punctiferalis | Cry1Ab | 432 | 1.09 ± 0.16 | 3.41(2.20–4.96) | 50.62(27.56–137.89) | 8.54 | 4 |
| Cry1Ac | 504 | 1.27 ± 0.11 | 8.62(6.90–11.02) | 87.43(55.88–161.90) | 8.27 | 5 | |
| Cry1F | 504 | 1.25 ± 0.17 | 10.03(6.79–14.26) | 107.17(62.39–251.50) | 3.93 | 5 | |
| Cry2Ab | 504 | 1.12 ± 0.16 | 126.70(83.81–180.59) | 1762.68(961.45–4919.07) | 10.92 | 5 | |
| Vip3A | 576 | 0.95 ± 0.10 | 406.36(291.52–584.47) | 9158.11(4643.12–25184.36) | 6.48 | 6 | |
| Helicoverpa armigera | Cry1Ab | 432 | 1.24 ± 0.16 | 3.52(2.62–4.66) | 48.27(22.85–223.72) | 9.36 | 4 |
| Cry1Ac | 504 | 1.07 ± 0.14 | 7.33(5.34–10.12) | 191.10(86.45–753.18) | 8.56 | 5 | |
| Cry1F | 576 | 1.40 ± 0.12 | 1513.22(1206.02–1887.17) | 12497.37(8724.82–20238.11) | 9.47 | 7 | |
| Cry2Ab | 360 | 1.04 ± 0.14 | 70.39(39.47–106.65) | 1210.81(724.61–2678.95) | 9.51 | 6 | |
| Vip3A | 576 | 0.98 ± 0.11 | 468.07(333.76–649.50) | 9619.37(5322.10–2295.92) | 11.17 | 6 | |
| Agrotis ypsilon | Cry1Ab | 480 | 1.84 ± 0.34 | 762.61(530.76–1006.73) | 3779.38(2477.67–8473.58) | 10.73 | 6 |
| Cry1Ac | 480 | 1.33 ± 0.12 | 1090.42(855.09–1381.42) | 10022.41(7194.69–82230.30) | 11.87 | 6 | |
| Cry1F | 720 | 0.68 ± 0.07 | 1027.84(695.06–1544.08) | 79361.96(33296.01–290783.82) | 12.66 | 8 | |
| Cry2Ab | 420 | 1.348 ± 0.13 | 367.38(279.49–466.63) | 3352.74(2337.07–5521.13) | 10.83 | 5 | |
| Vip3A | 504 | 1.32 ± 0.14 | 352.81(273.79–453.77) | 3287.45(2095.47–6375.52) | 8.23 | 5 | |
| Mythimna separata | Cry1Ab | 432 | 1.01 ± 0.15 | 119.24(82.90–164.50) | 2250.03(1152.64–7167.31) | 5.36 | 4 |
| Cry1Ac | 480 | 0.61 ± 0.10 | 132.25(67.50–217.81) | 16821.22(5669.80–128957.09) | 9.04 | 6 | |
| Cry1F | 624 | 0.84 ± 0.09 | 812.18(564.24–1141.42) | 27304.07(14350.18–69978.33) | 8.74 | 7 | |
| Cry2Ab | 432 | 1.57 ± 0.18 | 379.21(293.81–480.88) | 2487.17(1691.91–4415.81) | 4.73 | 4 | |
| Vip3A | 480 | 0.98 ± 0.11 | 336.61(237.57–467.64) | 6744.22(3812.70–15452.58) | 6.18 | 6 |
atotal number of larvae tested in the bioassay.
bng of Bt toxin/cm2 of treated artificial diet surface with 95% fiducial limits in parentheses.
cChi-square goodness-of-fit test indicates all probit models were good fit (P > 0.05).
Data Analysis
The mortalities of each species and each toxin showed no significant difference among the three repeats, thus the data were pooled for probit analysis to estimate the median lethal concentration (LC50) and the slope of the regression using Polo-plus progra.43 The goodness of fit was tested with Chi-square test. The comparisons of the LC50s (susceptibility) were based on their 95% fiducial limits. Significant difference was declared when the 95% fiducial limits was not overlapping. To compare the pattern of relative tolerance of each insect species to different Bt toxins, a tolerance ratio (TR) was calculated using the LC50s of O. furnacalis as the reference (denominator) for each type of Bt toxin.
Results
Susceptibility of Five Insect Species to Five Bt Toxins
The probit analysis results of O. furnacalis, C. punctiferalis, H. armigera, A. ypsilon, and M. separate for each Bt toxin are shown in Table 1. Chi-square tests indicated that all probit models were good fit for the mortality data (Table 1). For a given toxin, significant differences were present among the tested species. The LC50 values of Cry1Ac, Cry1Ab, and Cry1F for O. furnacalis were 1.70, 2.11, and 4.61 ng/cm2, respectively. This indicated that O. furnacalis was highly susceptible to these three Cry1 toxins. In contrast, the LC50s of Cry2Ab and Vip3A for O. furnacalis were 134.87 and 328.44 ng/cm2, respectively, which indicated that O. furnacalis was more tolerant to Cry2Ab and Vip3A than to Cry1 toxins. Similarly, C. punctiferalis was highly susceptible to the three Cry1 toxins but more tolerant to Cry2Ab and Vip3A. H. armigera had a different trend. It was highly susceptible to Cry1Ab (LC50 = 3.52 ng/cm2) and Cry1Ac (LC50 = 7.33 ng/cm2), but not to Cry1F (LC50 = 1513.22 ng/cm2). Additionally, it was relative more susceptible to Cry2Ab (LC50 = 70.39 ng/cm2) compared to Cry1F. A. ypsilon was not susceptible to the three Cry1 toxins with LC50 values ranged from 762.61 to 1090.42 ng/cm2, while M. separate was moderately susceptible to Cry1Ab, Cry1Ac, Cry2Ab, and Vip3A with LC50 values ranging from 119.24 to 379.21 ng/cm2.
Patterns of Susceptibility
Based on the LC50 values and 95% fiducial limits (Table 1), the order of susceptibility to Cry1Ab was as follows: O. furnacalis, C. punctiferalis, H. armigera > M.separata > A. ypsilon; the order of susceptibility to Cry1Ac: O. furnacalis > C. punctiferalis, H. armigera > M. separata > A. ypsilon; the order of susceptibility to Cry1F: O. furnacalis > C. punctiferalis > M. separata, A. ypsilon > H. armigera; and the order of susceptibility to Cry2Ab: O. furnacalis, C. punctiferalis, H. armigera > M. separata, A. ypsilon. The susceptibility to Vip3A was similar among the five species.
The TR values show that the susceptibility profile of C. punctiferalis to different Bt toxins was similar to that of O. furnacalis. Both species were highly susceptible to Cry1Ab, Cry1Ac, and Cry1F, but relatively tolerant to Cry2Ab and to Vip3A (Fig. 1). In contrast, H. armigera was less susceptible to Cry1F, but similar compared with O. furnacalis and C. punctiferalis to other toxins. A. ypsilon and M. separata were less susceptible to the three tested Cry1 toxins, while A. ypsilon exhibited obvious tolerance to Cry1Ac, which is much different from O. furnacalis and C. punctiferalis.
Figure 1.
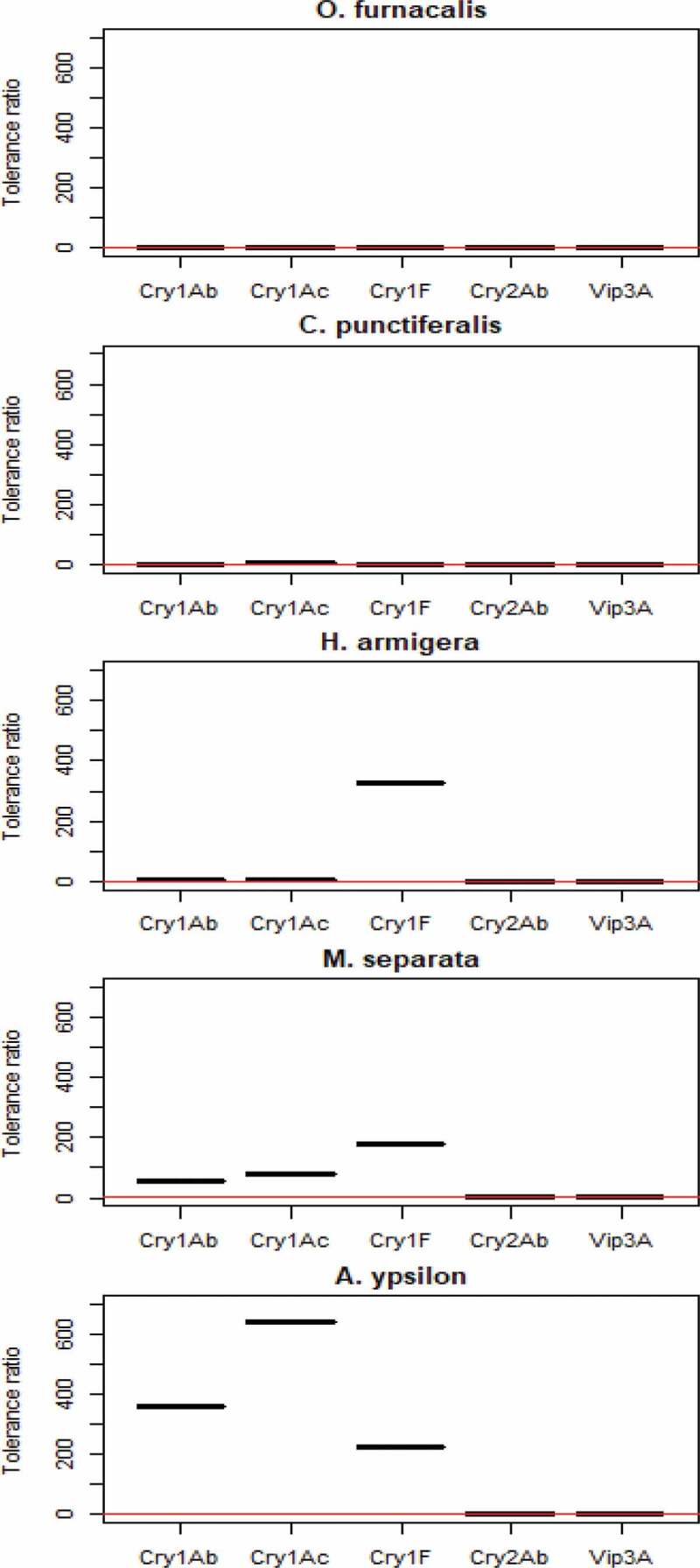
Tolerance ratio C. punctiferalis, H. armigera, A. ypsilon and M. separata relative to O. furnacalis for five Bt toxins (LC50 ratios of C. punctiferalis, H. armigera, A. ypsilon and M. separata relative to O. furnacalis)
Discussion
Until now, no universal artificial diet is available for rearing different lepidopteran insect species, thus in our study artificial diets of different ingredients were used for different species. Other than this uncontrollable factor, the bioassay procedure and Bt proteins were handled the same as much as possible. Fortunately, all neonates showed a similar feeding behavior while being reared on artificial diets – initially scraped the available surface of the diet before tunneling. This behavior similarity ensured that different species took up approximately equal amount of Bt toxins, and enabled valid comparisons among the species to the best possible degree.
In this study, we quantified the differences of susceptibilities to different Bt toxins among the five main lepidopteran pests damaging summer corn in the HHHSCR of China. The results suggested that O. furnacalis and C. punctiferalis had very similar susceptibility patterns to the five Bt toxins, while H. armigera, A. ypsilon, and M. separate showed different susceptibility patterns. This information is critical for making decision on which type of Bt corn to be chosen for commercialization in the region. Such basic knowledge also is valuable for decision making in other regions or countries where the tested pests are distributed.
The first generation Bt maize varieties expressing only a single toxin (Cry1Ab or Cry1F) were commercially planted in 1996 in the USA to control the main lepidopteran pest, O. nubilalis, and other stalk-boring pests.44 Large area planting of these hybrid maize varieties have reduced the overall population of O. nubilalis.45 However, in the meantime a secondary pest S. albicosta has increased and become a major lepidopteran pest in some areas of the Corn Belt in the US and Canada due to low susceptibility of this species to most transgenic maize expressing Cry1Ab.13,46–48 In Europe, Bt corn expressing Cry1Ab toxin exhibits high efficacy against two primary lepidopteran borers, S. nonagrioides and O. nubilalis,10,49 but low efficacy against several secondary pests, such as S. albicosta and M. unipuncta,50,51 and similarly the latter two species are becoming more common in some European countries.52 To overcome this phenomenon, stacked Bt corn hybrids targeting multiple species expressing combinations of Cry1Ab, Cry1F, Cry1A.105, Cry2Ab and/or Vip3A became available more recently.53 Comparing with single-toxin hybrids, the stacked hybrids have advantages of reducing crop damage, improving control of individual pest species, broadening control spectrum, and reducing production of resistance phenotypes in a given population.54,55 These transgenic crops, including Cry1Ab+Cry1F, Cry1F+Cry2Ab, and Cry1Ab+Cry1F+Vip3A, can control or suppress a range of insect pests, including H. zea and S. frugiperda.56 Because pyramided and/or stacked Bt crops can enhance resistance management as well as pest control, they are expected to become more dominant in the future. The results of this study provide good guidance to seed developers for proper configuration of Bt toxins.
Developing proper Bt corn hybrid varieties for targeted production regions are essential to commercial utilization of transgenic Bt corn. O. furnacalis, C. punctiferalis, and H. armigera are three serious and common pests in the HHHSCR of China. M. separate and A. ypsilon are both of long distance migration pests, commonly occurring in this region. In view of these five major lepidopteran pests exhibited different degrees of susceptibility to different Bt toxins (Table 1, Fig. 1), planting transgenic corn expressing single Cry1Ab or Cry1Ac toxins might be highly efficacious against O. furnacalis, C. punctiferalis, H. armigera, and M. separate, but might not be effective against A. ypsilon. Similarly, planting Bt corn expressing single Cry1F toxin might be able to control O. furnacalis and C. punctiferalis, but might not control H. armigera, M. separate and A. ypsilon. Under these scenarios, planting corn cultivars that produced two or more Bt proteins, such as Cry1Ab (Cry1Ac) + Cry2Ab, Cry1Ab (Cry1Ac) + Vip3A, or Cry1F+ Cry2Ab (Vip3A) may be necessary in order to achieve satisfactory control. The finding of this study suggests that the first generation Bt corn expressing single Cry1 toxin should not be the first choice because of the potential risk of failure or less efficacious to control H. armigera, A. ypsilon or M. separate. The second-generation Bt corn expressing pyramided Cry1 and Cry2 toxins, or the third generation Bt corn expressing stacked Cry1, Cry2 and Vip3A toxins might produce better protection to corn and delay resistance evolution of target pests in the HHHSCR of China.
A brand new threat to corn in China is S. frugiperda, which invaded in 2019.57,58 Currently, this species has been detected in 21 provinces in SeHCR, SwHCR, and HHHSCR.59 S. frugiperda has been a severe problem in North America and Latin America where it attacks multiple crops and has developed resistance to Cry1F, Cry1Ab, and Cry2Ab corns.60–65 Fortunately, the invaded S. frugiperda population in China is highly susceptible to Cry1Ab, Cry1F, and Vip3A.66 Thus, planting corns of Cry1Ab (Cry1Ac) + Cry2Ab, Cry1Ab (Cry1Ac) + Vip3A, or Cry1F + Cry2Ab (Vip3A) in HHHSCR can also control this pest. However, since this invaded population might have originated from North America,67 it is possible that lower frequency resistance alleles might have been carried in already. Therefore, characterization of resistance allele frequency in this S. frugiperda population to different Bt proteins is necessary for resistance management in future.
In the present study, only laboratory bioassays with purified proteins were conducted to compare the pattern of susceptibility among the main lepidopteran pests. Without directly comparing the expression level of each protein in actual Bt corn events and plant tissue consumption by each pest, these results provide a starting point to determine what type of Bt corn is suitable for different corn production regions, and enable better preparation for transgenic Bt corn being authorized for commercial planting in China. In addition, Bt toxin baseline susceptibility data among main corn pest populations were documented. This broadens the database to model and assess the potential of resistance evolution to different Bt crops in combination with different pest species. Currently, the high-dose/refuge strategy is the most commonly recommended strategy for delaying resistance evolution. With this strategy, insects that feed on Bt maize are exposed to an extremely high dose of toxin, which makes insect resistance alleles functionally recessive. Since the toxicity of each protein varies depending on insect species, determination of efficacy of each Bt corn trait against all above lepidopteran pests to define a high dose should be systematically evaluated as we did in this study and in future.
Funding Statement
This research was supported by the Key Project for Breeding Genetically Modified Organisms [No.2016ZX08012004-007, No.2019ZX08012004-004] and the national key research and development program of China [2019YFD0300105, 2016YFD0300705, 2018YFD0200605] sponsored by the Ministry of Science and Technology of China.
References
- 1.International Service for the Acquisition of Agri-Biotech Applications (ISAAA) . 2018. ISAAA publications. http://www.isaaa.org/resources/publications/briefs/54/default.asp
- 2.Wu KM, Lu YH, Feng HQ, Jiang YY, Zhao JZ.. Suppression of cotton bollworm in multiple crops in China in areas with Bt toxin-containing cotton. Science. 2008;321(5896):1676‒1678. doi: 10.1126/science.1160550. [DOI] [PubMed] [Google Scholar]
- 3.Hutchison WD, Burkness EC, Mitchell PD, Moon RD, Leslie TW, Fleischer SJ, Abrahamson M, Hamilton KL, Steffey KL, Gray ME, et al. Area wide suppression of European corn borer with Bt maize reaps savings to Non-Bt maize growers. Science. 2010;330(6001):222‒225. doi: 10.1126/science.1190242. [DOI] [PubMed] [Google Scholar]
- 4.Dively GP, Venugopal PD, Bean D, Whalen J, Holmstrom K, Kuhar TP, Doughty HB, Patton T, Cissel W, Hutchison WD. Regional pest suppression associated with widespread Bt maize adoption benefits vegetable growers. Proc Natl Acad Sci U S A. 2018;115(13):3320‒3325. doi: 10.1073/pnas.1720692115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mason CE, Rice ME, Calvin DD, Van Duyn JW, Showers WB, Hutchinson WD, Witkowski JF, Higgins RA, Onstad DW, Dively GP. European corn borer ecology and management. Ames: Iowa State University. North Central Regional Publication, Publication 327; 1996. p. 57. [Google Scholar]
- 6.Zhao JZ, Cao J, Collins HL, Bates SL, Roush RT, Earle ED, Shelton AM. Concurrent use of transgenic plants expressing a single and two Bacillus thuringiensis genes speeds insect adaptation to pyramided plants. Proc Natl Acad Sci USA. 2005;102:8426‒8430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.James C. Global Status of Commercialized Biotech/GM Crops in 2017: Biotech Crop Adoption Surges as Economic Benefits Accumulate in 22 Years. The International Service for the Acquisition of Agri-biotech Applications. Ithaca (NY): ISAAA; 2017. [Google Scholar]
- 8.Barry BD, Darrah LL, Huckla DL, Antonio AQ, Smith GS, O’Day MH. Performance of transgenic corn hybrids in Missouri for insect control and yield. J Econ Entomol. 2000;93:993‒999. [DOI] [PubMed] [Google Scholar]
- 9.Pérez-Hedo M, Albajes R, Eizaguirre M. Modification of hormonal balance in larvae of corn borer Sesamia nonagrioides (Lepidoptera: noctuidae) due to sublethal Bacillus thuringiensis protein ingestion. J Econ Entomol. 2011;104:853‒861. [DOI] [PubMed] [Google Scholar]
- 10.Eizaguirre M, Madeira F, Lopez C. Effects of Bt maize on nontarget lepidopteran pests. IOBC/WPRS Bull. 2010;52:49–55. [Google Scholar]
- 11.Pérez-Hedo M, Marques T, López C, Eizaguirre M. Determination of the Cry1Ab toxin in Helicoverpa armigera larvae fed on diet containing lyophilized Bt leaves. IOBC/WPRS Bull. 2011;73:75–81. [Google Scholar]
- 12.Catangui MA, Berg RK. Western bean cutworm, Striacosta albicosta (Smith) (Lepidoptera: noctuidae), as a potential pest of transgenic Cry1Ab Bacillus thuringiensis corn hybrids in South Dakota. Environ Entomol. 2006;35:1439–52. doi: 10.1093/ee/35.5.1439. [DOI] [Google Scholar]
- 13.Eichenseer H, Strohbehn R, Burks JC. Frequency and severity of western bean cutworm (Lepidoptera: noctuidae) ear damage in transgenic corn hybrids expressing different Bacillus thuringiensis Cry toxins. J Econ Entomol. 2008;101:555–63. doi: 10.1093/jee/101.2.555. [DOI] [PubMed] [Google Scholar]
- 14.Catarino R, Ceddia G, Areal F, Parisey N, Park J. Managing maize under pest species competition: is Bt (Bacillus thuringiensis) maize the solution? Ecosphere. 2016;7(6):e01340. doi: 10.1002/ecs2.1340. [DOI] [Google Scholar]
- 15.He KL, Wang ZY, Wen LP, Bai SX, Ma X, Yao Z. Determination of baseline susceptibility to Cry1Ab protein for Asian corn borer (Lepidoptera: crambidae). J Appl Entomol. 2005;129:407–12. doi: 10.1111/j.1439-0418.2005.00989.x. [DOI] [Google Scholar]
- 16.Shi J, Wang ZY, He KL. Changes and occurrence trend of corn diseases and insect pests in Huang-HuaiHai summer corn regions. Plant Prot. 2005;31:63–65. [Google Scholar]
- 17.Wen LP, Wang ZY, Ye ZH. Yield losses and economic threshold of Asia corn borer, Ostrinia furnacalis: guenee on corn. Sci Agric Sin. 1992;25:44–49. [Google Scholar]
- 18.He K, Zhou DR, Wang ZY. Study on the damage and control tactics of Asian corn borer Ostrinia furnacalis: guenéé in sweet corn field. Acta Phytophyl Sin. 2002;29:199–204. [Google Scholar]
- 19.Wang ZC, Qian HI, Dong H, Wang JH, Wang ZY. Studies on the damage degree and yield loss by Asian corn borer. Plant Prot. 2008;34:112–15. [Google Scholar]
- 20.Chen RZ, Klein MG, Sheng CF, Li Y, Shao DX, Li QY. Use of pheromone timed insecticide applications integrated with mating disruption or mass trapping against Ostrinia furnacalis (Génuéé) (Lepidoptera: pyralidae) in sweet corn. Environ Entomol. 2013;42:1390–99. doi: 10.1603/EN13143. [DOI] [PubMed] [Google Scholar]
- 21.Tan SY, Cayabyab BF, Alcantara EP, Ibrahim YB, Huang FN, Blankenship EE, Siegfried BD. Comparative susceptibility of Ostrinia furnacalis, Ostrinia nubilalis and Diatraea saccharalis (Lepidoptera: crambidae) to Bacillus thuringiensis Cry1 toxins. Crop Prot. 2011;30:1184–89. doi: 10.1016/j.cropro.2011.05.009. [DOI] [Google Scholar]
- 22.He KL, Wang ZY, Wen LP, Bai SX, Zhou DR, Zhu QR. Field evaluation of the Asian corn borer control in hybrid of transgenic maize event MON810. Agric Sci China. 2003;2:1363–68. [Google Scholar]
- 23.He KL, Wang ZY, Zhou DR, Wen LP, Song YY, Yao ZY. Evaluation of transgenic Bt corn for resistance to the Asian corn borer (Lepidoptera: pyralidae). J Econ Entomol. 2003;96:935‒940. [DOI] [PubMed] [Google Scholar]
- 24.He KL, Wang ZY, Bai SX, Zheng L, Wang Y. Field efficacy of transgenic cotton containing single and double toxin genes against the Asian corn borer (Lepidoptera: pyralidae). J Appl Entomol. 2004;128:710‒715. [Google Scholar]
- 25.He KL, Wang ZY, Bai SX, Zheng L, Wang YB, Cui HY. Efficacy of transgenic Bt cotton for resistance to the Asian corn borer (Lepidoptera: crambidae). Crop Prot. 2006;25:167‒173. [Google Scholar]
- 26.Wei HJ. A key insect pest of sunflower – yellow peach moth. Chin Bull Entomol. 1956;2:78. [Google Scholar]
- 27.Wang YH, Pan XX, Cheng KL, Huang F, Wang ZM, Liu MX, Liu XY, Zhang CW. Community composition and distribution of insect pests on sorghum panicles in Sichuan. J Southwest Agric Univ. 1991;13:569–74. [Google Scholar]
- 28.Wu JD, Zhu YJ, Zhu GL, Zhu CH. Occurrence and control of yellow peach moth on chestnut. Hebei Fruits. 1999;17–18. [Google Scholar]
- 29.Wang ZY, He KL, Shi J, Ma SY. Analysis of the heavily occurrence trend of the yellow peach borer in corn and its management strategy. Plant Prot. 2006;32:67–69. [Google Scholar]
- 30.Wang ZY, Wang XM. To strength researches on the occurrence of maize pests and related control techniques for ensuring the maize production security.Acta Phytophyl. Sin. 2015;42:865‒868. [Google Scholar]
- 31.Liu Y, Li RR, He KL, Bai SX, Zhang TT, Cong B, Wang ZY. Effects of Conogethes punctiferalis (Lepidopteran: crambidae) infestation on the occurrence of Fusarium ear rot and the yield loss of spring corn. Acta Entomol Sin. 2017;60:576‒581. [Google Scholar]
- 32.Guo YY. The research of cotton bollworm. Beijing: China’s agriculture press; 1998. [Google Scholar]
- 33.Wang ZY, He KL, Wen LP, Zhang GY, Zheng L. Spatial-temporal distributions of cotton bollworm eggs on summer corn seeded at different times in North China. Sci Agric Sin. 2001;34:153‒156. [Google Scholar]
- 34.Cui JH. Discussion on the occurring and control of the black cutworm, Agrotis ypsilon (Rottemberg). J Henan For Sci Technol. 2004;12:50‒51. [Google Scholar]
- 35.Li Q, Fan Y, Zhang GA. Investigation of damage by the black cutworm, Agrotis ypsilon (Rottermberg) and its control with insecticide in corn fields of Northwest of Qian. Bull Anhui Agric Sci. 2008;14:172‒173. [Google Scholar]
- 36.Zeng J, Jiang YY, Liu J. Analysis of the armyworm outbreak in 2012 and suggestions of monitoring and forecasting. Plant Prot. 2013;39:117‒121. [Google Scholar]
- 37.Jiang XF, Zhang L, Cheng YX, Luo LZ. Current status and trends in research on the oriental armyworm, Mythimna separata (Walker) in China. Chin J Appl Entomol. 2014;51:881‒889. [Google Scholar]
- 38.Song YY, Zhou DR, He KL. Studies of mass rearing of Asian corn borer: development of a satisfactory non-agar semi-artificial diet and its use. Acta Phytophyl Sin. 1999;26:324‒328. [Google Scholar]
- 39.Liang GM, Tan WJ, Guo YY. An improved artificial rearing technique for Helicoverpa armigera. Plant Prot. 1999;25:15‒17. [Google Scholar]
- 40.Zhang YY, Lu Q, Gu SH, Lu YH, Wu KM. 2009. The artificial diet for rearing Agrotis ypsilon and its application: China. Patent: CN101584411A.
- 41.Jiang SJ, Luo LZ. 2012. The artificial diet for rearing Mythimna separata: China. Patent: CN101856085B
- 42.Li GP, Gao LN, Huang JR, Ji TJ, Huang B, Tian CH, Lu YH, Feng HQ. Frequency of Bt resistant alleles in wild cotton bollworm populations. Chin J Appl Entomol. 2018;55:49–54. [Google Scholar]
- 43.LeOra Software . PoloPlus: a user’s guide to Probit or Logit analysis. Berkeley (CA): LeOra Software; 2003. [Google Scholar]
- 44.Koziel MG, Beland GL, Bowman C, Carozzi NB, Crenshaw R, Crossland L, Dawson J, Desai N, Hill M, Kadwell S, et al. Field performance of elite transgenic maize plants expressing an insecticidal protein derived from Bacillus thuringiensis. Nat Biotech. 1993;11:194–200. doi: 10.1038/nbt0293-194. [DOI] [Google Scholar]
- 45.Hutchison WD, Burkness EC, Mitchell PD, Moon RD, Leslie TW, Fleischer SJ, Abrahamson M, Hamilton KL, Steffey KL, Gray ME, et al. Raun ES: area wide suppression of European corn borer with Bt maize reaps saving to non-Bt maize growers. Science. 2015;330:222‒225. [DOI] [PubMed] [Google Scholar]
- 46.Dorhout DL, Rice ME. Intraguild competition and enhanced survival of western bean cutworm (Lepidoptera: noctuidae) on transgenic Cry1Ab (MON810) Bacillus thuringiensis corn. J Econ Entomol. 2010;103:54–62. doi: 10.1603/EC09247. [DOI] [PubMed] [Google Scholar]
- 47.Lindroth E, Hunt TE, Skoda SR, Culy MD, Lee D, Foster JE. Population genetics of the western bean cutworm (Lepidoptera: noctuidae) across the United States. Ann Entomol Soc Am. 2012;105:685–92. doi: 10.1603/AN11084. [DOI] [Google Scholar]
- 48.Michel AP, Krupke CH, Baute TS, Difonzo CD. Ecology and management of the western bean cutworm (Lepidoptera: noctuidae) in corn and dry beans. J Integr Pest Manage. 2010;1:1–10. doi: 10.1603/IPM10003. [DOI] [Google Scholar]
- 49.González-Núñez M, Ortego F, Castañera P. Susceptibility of Spanish populations of the corn borers Sesamia nonagrioides (Lepidoptera: noctuidae)and Ostrinia nubilalis (Lepidoptera: crambidae) to a Bacillus thuringiensis endotoxin. J Econ Entomol. 2000;93:459–63. doi: 10.1603/0022-0493-93.2.459. [DOI] [PubMed] [Google Scholar]
- 50.Gray ME, Sappington TW, Miller NJ, Moeser J, Bohn MO. Adaptation and invasiveness of western corn rootworm: intensifying research on a worsening pest. Annu Rev Entomol. 2009;54:303–21. doi: 10.1146/annurev.ento.54.110807.090434. [DOI] [PubMed] [Google Scholar]
- 51.Pérez-Hedo M, López C, Albajes R, Eizaguirre M. Low susceptibility of non-target Lepidopteran maize pests to the Bt protein Cry1Ab. Bull Entomol Res. 2012;102(6):737–43. doi: 10.1017/S0007485312000351. [DOI] [PubMed] [Google Scholar]
- 52.Catarino R, Ceddia G, Areal FJ, Park J. The impact of secondary pests on Bacillus thuringiensis (Bt) crops. Plant Biotechnol J. 2015;13:601‒612. [DOI] [PubMed] [Google Scholar]
- 53.Carrière Y, Crickmore N, Tabashnik BE. Optimizing pyramided transgenic Bt crops for sustainable pest management. Nat Biotechnol. 2015;33:161–68. doi: 10.1038/nbt.3099. [DOI] [PubMed] [Google Scholar]
- 54.Carrière Y, Fabrick JA, Tabashnik BE. Can pyramids and seed mixtures delay resistance to Bt crops? Trends Biotechnol. 2016;34:291–302. doi: 10.1016/j.tibtech.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 55.Storer NP, Thompson GD, Head GP. Application of pyramided traits against Lepidoptera in insect resistance management for Bt crops. GM Crops Food. 2012;3:154–62. doi: 10.4161/gmcr.20945. [DOI] [PubMed] [Google Scholar]
- 56.Bilbo TR, Reay-Jones FPF, Reisig DD, Musser FR, Greene JK. Effects of Bt corn on the development and fecundity of corn earworm (Lepidoptera: noctuidae). J Econ Entomol. 2018;111(5):2233‒2241. doi: 10.1093/jee/toy203. [DOI] [PubMed] [Google Scholar]
- 57.Jing DP, Guo JF, Jiang YY, Zhao JZ, Sethi A, He KL, Wang ZY. Initial detections and spread of invasive Spodoptera frugiperda in China and comparisons with other noctuid larvae in cornfields using molecular techniques. Insect Sci. 2020;27:780‒790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu QL, Jiang YY, Wu KM. Analysis of migration routes of the fall armyworm Spodoptera frugiperda (J.E. Smith) from Myanmar to China. Plant Prot. 2019;45:1‒6. [Google Scholar]
- 59.National Agricultural Technology Extension Service Center . 2019. The occurrence trend of maize pests and diseases in the second half of 2019 is predicted. Plant pathogen and pest information. 2019-7-26. https://www.natesc.org.cn/Html/2019_07_26/28092_151760_2019_07_26_459392.html
- 60.Blanco CA, Portilla M, Jurat-Fuentes JL, Sánchez JF, Viteri D, Vega-Aquino P, Terán-Vargas AP, Azuara-Domínguez A, López JD Jr, Arias R, et al. Susceptibility of Isofamilies of Spodoptera frugiperda (Lepidoptera: noctuidae) to Cry1Ac and Cry1Fa proteins of Bacillus thuringiensis. Southwest Entomol. 2010;35:409–15. doi: 10.3958/059.035.0325. [DOI] [Google Scholar]
- 61.Storer NP, Babcock JM, Schlenz M, Meade T, Thompson GD, Bing JW, Huckaba RM. Discovery and characterization of feld resistance to Bt maize: spodoptera frugiperda (Lepidoptera: noctuidae) in Puerto Rico. J Econ Entomol. 2010;103:1031–38. doi: 10.1603/EC10040. [DOI] [PubMed] [Google Scholar]
- 62.Li GP, Reisig DD, Miao J, Gould F, Huang FF, Feng HQ. Frequency of Cry1F non-recessive resistance alleles in North Carolina field populations of Spodoptera frugiperda (Lepidoptera: noctuidae). PLoS One. 2016;11:e0154492. doi: 10.1371/journal.pone.0154492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang FN, Qureshi JA, Head GP, Price PA, Levy R, Yang F, Niu Y. Frequency of Bacillus thuringiensis Cry1A.105 resistance alleles in field populations of the fall armyworm, Spodoptera frugiperda, in Louisiana and Florida. Crop Prot. 2016;83:83–89. doi: 10.1016/j.cropro.2016.01.019. [DOI] [Google Scholar]
- 64.Blanco CA, Chiaravalle W, Dalla-Rizza M, Farias JR, García-Degano MF, Gastaminza G, Mota-Sánchez M, Omoto C, Pieralisi BK, Rodríguez J, et al. Current situation of pests targeted by Bt crops in Latin America. Curr Opin Insect Sci. 2016;15:131–38. doi: 10.1016/j.cois.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 65.Chandrasena DI, Signorini AM, Abratti G, Storer NP, Olaciregui ML, Alves AP, Pilcher CD. Characterization of field-evolved resistance to Bacillus thuringiensis-derived Cry1F δ-endotoxin in Spodoptera frugiperda populations from Argentina. Pest Manag Sci. 2018;74:746–54. doi: 10.1002/ps.4776. [DOI] [PubMed] [Google Scholar]
- 66.Li GP, Ji TJ, Sun XX, Jiang YY, Wu KM, Feng HQ. Susceptibility evaluation of invaded Spodoptera frugiperda population in Yunan province to five Bt proteins. Plant Prot. 2019;45:15‒20. [Google Scholar]
- 67.Zhang L, Liu B, Jiang YY, Liu J, Wu KM, Xiao YT. Molecular characterization analysis of fall armyworm populations in China. Plant Prot. 2019;45:20‒27. [Google Scholar]


