Abstract
Background
we investigated whether two frailty tools predicted mortality among emergency department (ED) patients referred to internal medicine and how the level of illness acuity influenced any association between frailty and mortality.
Methods
two tools, embedded in a Comprehensive Geriatric Assessment (CGA), were the clinical frailty scale (CFS) and a 57-item deficit accumulation frailty index (FI-CGA). Illness acuity was assessed using the Canadian Triage and Acuity Scale (CTAS). We examined all-cause 30-day and 6-month mortality and time to death.
Results
in 808 ED patients (mean age ± SD 80.8 ± 8.8, 54.4% female), the mean FI-CGA score was 0.44 ± 0.14, and the CFS was 5.6 ± 1.6. A minority (307; 38%) were classified as having high acuity (CTAS: 1–2). The 30-day mortality rate was 17%; this increased to 34% at 6 months. Compared to well patients with low acuity, the risk of 30-day mortality was 22.5 times (95% CI: 9.35–62.12) higher for severely frail patients with high acuity; 53% of people with very severe frailty (CFS = 8) and high acuity died within 30 days. When acuity was low, the risk for 30-day mortality was significantly higher only among those with very high levels of frailty (CFS 7–9, FI-CGA > 0.5). When acuity was high, even lower levels of frailty (CFS 5–6, FI-CGA 0.4–0.5) were associated with higher 30-day mortality.
Conclusions
across levels of frailty, higher acuity increased mortality risk. When acuity was low, the risk was significant only when the degree of frailty was high, whereas when acuity was high, even lower levels of frailty were associated with greater mortality risk.
Keywords: frailty, mortality, patient acuity, survival analysis, emergency department, older people
Key Points
The frailty index and the Clinical Frailty Scale are strongly associated with mortality, especially in the short term.
The risk of 30-day mortality is the highest among severely frail patients with high acuity.
Higher acuity increases mortality risk across levels of frailty.
When acuity is low, the mortality risk is significant in patients with high levels of frailty.
When acuity is high, the mortality risk is significant even in lower levels of frailty.
Introduction
Frailty is a state of increased vulnerability to adverse outcomes among people of the same age [1, 2]. It is strongly associated with increased risks of death and of worsening health status, notably among older patients with acute medical conditions [3–7]. This relationship is less studied among patients in the emergency department (ED) setting [7–10], despite its implications for appropriate care planning [11, 12].
The increased risk experienced by frail older adults who are acutely ill comes not just from their frailty, but from the severity of their illness [7, 13, 14]. Both frailty and illness acuity can add information about risk [5, 6, 13]. Screening and assessment measures include formal acuity-based measures (e.g. Emergency Department Medical Early Warning Score) and a variety of measures of critical illness in patients admitted to specialized intensive treatment units (ITUs) [15]. Typically ITU personnel administer the latter, even in the ED. Laboratory data assembled in a frailty index have also been employed as an illness acuity measure [5].
The objectives of this study were to examine (i) whether two frailty tools embedded in a Comprehensive Geriatric Assessment (CGA) form—the clinical frailty scale (CFS) and a deficit accumulation frailty index (FI-CGA)—predicted mortality among acutely ill older patients (65+ years) referred to internal medicine and (ii), if so, how the level of illness acuity influenced the association between frailty and mortality.
Methods
Study design and data
This is a retrospective study of patients (N = 1024) seen in the ED by one of us (KR) having been referred to internal medicine; compared against billing records, approximately 65–70% of all such patients are included here. KR supervised the CGA and the CFS scoring, which were completed for all patients to assess their health and social status and to assist with treatment planning (Figure S1 in the Supplementary data). The database was linked to vital statistics for all-cause mortality data following the CGA assessment. Mortality data were only available from January 2009 to December 2017, allowing complete information on 808 ED patients; 20 patients were included twice—the average time between the visits was 214 (SD ±328) days (Figure S2 in the Supplementary data).
Frailty tools
The FI-CGA was constructed using 57 health-related items (Table S1 in the Supplementary data). The FI-CGA follows the deficit accumulation approach, which suggests that the more deficits a person has, the more likely that person is to be frail [2, 16, 17]. We used the FI-CGA to measure the current health state of the patient at admission; this chiefly consists in the items from the right-hand side in the CGA, and all items on the problem list (Figure S1 in the Supplementary data). Note that inasmuch as the patients have come to hospital, at least one new problem was added to each list. For example, ‘acute exacerbation of chronic obstructive pulmonary disease’ could be the reason for referral (RFR) and listed first, and COPD, as the chronic condition, could be listed second. That same convention holds for acute exacerbations of other chronic illnesses that prompt presentation to the ED. Current worsening is also captured within the CGA by features such as delirium, recent mobility impairment, falls, functional decline or incontinence. The FI-CGA scores were calculated by dividing the summed deficits by the total number of items. This yields FI-CGA scores that potentially can range from 0 to 1; higher values indicate greater frailty.
The CGA assessment form includes the CFS (Figure S1 in the Supplementary data; far right columns; one is for the patient (‘Pt’), and the other is to estimate caregiver (‘CG’) health). The CFS summarizes the overall level of fitness or frailty of an older adult at baseline. Originally, the CFS was introduced to summarize assessment carried out by physicians from disparate disciplines who had received at least some notional training in the CGA rubric [18]. It is employed that way here, after the CGA had been reviewed by KR as attending physician on the General Internal Medicine-ED consult service. The CFS is now also commonly used as a screening tool. That too happened here, in evaluating the health of the main caregiver; caregivers of patients in long-term care facilitates are typically scored as CFS = 2. Otherwise, the CFS is a measure of patients’ pre-admission health state; for ED patients this, by a convention validated in an earlier study [19], is 2 weeks prior to admission. The CFS ranges from 1 to 9, and as with the FI-CGA, higher values indicate greater frailty (1 = very fit, 2 = well, 3 = managing well, 4 = very mildly frail (previously ‘apparently vulnerable’), 5 = mildly frail, 6 = moderately frail, 7 = severely frail, 8 = very severely frail and 9 = terminally ill). Category 9 is assigned for people who are terminally ill. An CFS score of 9 is the only case in which the current state trumps the baseline state, in that these terminally ill patients might have been operating at various frailty levels at baseline.
Acuity
We used the Canadian Triage and Acuity Scale (CTAS) to assess illness acuity [7, 20]. The CTAS is a descriptive 5-point triage scale, where level 1 is resuscitation, 2 is emergent, 3 is urgent, 4 is less urgent and 5 is non-urgent. Due to small sample size for people with scores 1 (N = 18), 4 (N = 24) and 5 (N = 2), we grouped patients with CTAS levels 1–2 as high acuity and those with CTAS level 3–5 as low acuity.
Mortality
The primary outcome variable was all-cause mortality following the CGA assessment. We matched participants against death records from vital statistics; time to death was recorded in months. For time to death analysis, we used 1-, 2- and 5-year mortality as the outcomes. Given their common use clinically, we also analysed the probability of death within 30 days and within 6 months from the CGA assessment.
Statistical analysis
All analyses were performed in Stata version 15.0 (Stata Corp., College Station, TX). We report descriptive statistics as means (SD) and medians (inter quartile range) for continuous variables and as proportions for categorical variables. Survival analysis was used to analyse time to death outcomes, including graphical presentations of survival probabilities using Kaplan Meier curves and estimation of the multivariate Cox regression model parameters. The assumption of proportionality of hazards was assessed graphically, with no evidence of violation (all P > 0.10). We analysed the association of the FI-CGA and CFS independently and combined with the acuity score; we combined the FI-CGA and CFS scores with acuity, creating 10- and 8-category frailty-acuity variables, respectively. We used logistic regression models to analyse the relationship between binary outcomes and independent variables. We calculated predicted probabilities at different levels of FI-CGA and CFS using the estimates from logistic models using the margins command in Stata [21]. We plotted the predicted probabilities (average predictive margins) using the marginsplot command in Stata. The predictive margins were calculated by averaging the predictions from the fitted models at the fixed values FI-CGA and CFS for each individual in the sample [21, 22]. Statistical significance was set at P < 0.05 and all tests were two-sided.
Ethics approval
Individuals whose data are included in the GPID database have provided written informed consent, or consent has been obtained from their substitute decision-makers, to allow their data to be recorded and used for research purposes. The study protocol was reviewed and approved by the Nova Scotia Health Authority Research Ethics Board (NSHA-REB File No. 1022792). Linkages with Nova Scotia Department of Health and Wellness datasets were approved by the NSHA-REB and by the Health Data Nova Scotia (HDNS) Data Access Committee (File 2017-OAT-001).
Results
The mean (SD) age was 80.8 (8.3) years; 455 patients (56%) were female (Table 1). Many patients (307; 38%) were classified as low acuity (CTAS: 1–2); this proportion was similar across levels of frailty (Tables S2 and S3 in the Supplementary data). The mean (SD) of FI-CGA was 0.45 (SD: 0.14) with 118 (14.7%) having FI-CGA scores more than 0.6. The FI-CGA was normally distributed (Figure S3 in the Supplementary data). Individual ages were weakly but significantly related to the FI-CGA scores (Figure S5 in the Supplementary data). The mean (SD) of the CFS was 5.6 (1.6), and 192 (23.7%) of the patients were severely frail (CFS: 7–9) (Table 1).
Table 1.
Characteristics of the patients included in the study
| Mean (±SD) | N (%) | Median | IQR | Min | Max | |
|---|---|---|---|---|---|---|
| Age | 80.8 (8.3) | 81 | 13 | 57 | 102 | |
| Female | 455 (56.3) | |||||
| N medications | 6.9 (3.8) | 6 | 5 | 0 | 25 | |
| N comorbidities | 8.8 (3.3) | 9 | 5 | 1 | 23 | |
| CTAS (1–5) | 2.6 (0.6) | 3 | 1 | 1 | 5 | |
| CTAS groups | ||||||
| High acuity (CTAS:1–2)a | 307 (38.1) | |||||
| Low acuity (CTAS:3–5)a | 499 (61.9) | |||||
| FI-CGA (0–1) | 0.44 (0.14) | 0.45 | 0.21 | 0.04 | 0.79 | |
| FI-CGA groups | ||||||
| FI-CGA:0–0.2a | 44 (5.5) | |||||
| FI-CGA:0.2–0.3 | 82 (10.2) | |||||
| FI-CGA:0.3–0.4 | 164 (20.4) | |||||
| FI-CGA:0.4–0.5 | 216 (26.9) | |||||
| FI-CGA:0.5–0.6 | 178 (22.1) | |||||
| FI-CGA:0.6+a | 118 (14.7) | |||||
| CFS (1–9) | 5.6 (1.6) | 5 | 1 | 1 | 9 | |
| CFS groups | ||||||
| Fit and well (1–3)a | 78 (9.7) | |||||
| Very mildly frail (4) | 113 (13.9) | |||||
| Mildly frail (5) | 218 (26.9) | |||||
| Moderately frail (6) | 207 (25.6) | |||||
| Severely frail (7) | 85 (10.6) | |||||
| Very severely frail (8) | 38 (4.7) | |||||
| Terminally ill (9) | 69 (8.5) | |||||
| 30-day mortality | 139 (17.2) | |||||
| 6-month mortality | 276 (34.2) |
aCombined due to low sample size.
Overall, 17% (95% CI: 0.15–0.20) and 34% (95% CI: 0.31–0.38) of the patients died within 30 days and 6 month of the CGA assessment, respectively. Mortality rates were higher among people with higher level of frailty and acuity (Figure 1; Figure S6 in the Supplementary data). For example, among mildly frail patients (CFS 5), 8.6% (95% CI: 0.05–0.14) of the patients with low acuity (CTAS 3–5) and 14.3% (95% CI: 0.08–0.24) of those with high acuity (CTAS 1–2) died within 30 days. Among very severely frail patients (CFS 8), 33.3% (95% CI: 0.16–0.57) of the patients with low acuity and 52.9% (95% CI: 0.28–0.76) of those with high acuity died within 30 days (Figure 1).
Figure 1.
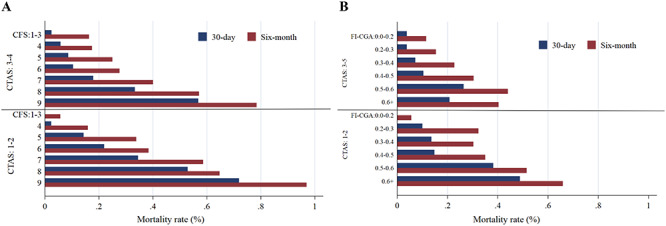
30-day and 6-month mortality rates by CTAS and frailty [CFS (Panel a), FI-CGA (Panel b)]. Note: People with CFS 1–3 and FI-CGA 0–0.02 and 0.6+ were combined due to low sample size
When frailty was examined independently, using either the CFS or the FI-CGA, the probability of mortality increased with higher levels of frailty (Tables S4 and S5, Figure S6 and S7 in the Supplementary data) as well as when combined with acuity (Figure 3). The association with 30-day mortality was stronger than in the longer term. For example, the likelihood of 30-day mortality was about 22 (OR: 22.50, 95% CI: 9.35–62.12) times higher for the severely frail patients with high acuity compared to well patients with low acuity, while it was 14 (OR: 14.50, 95% CI: 8.32–29.07) times higher for 6-month mortality (Figure 2). Similarly, the HR was 7.47 (95% CI: 4.75–11.77) for 1-year mortality, whereas it was 4.73 (95% CI: 3.45–6.48) for 5-year mortality when patients were severely frail with high acuity (Figure 3).
Figure 3.
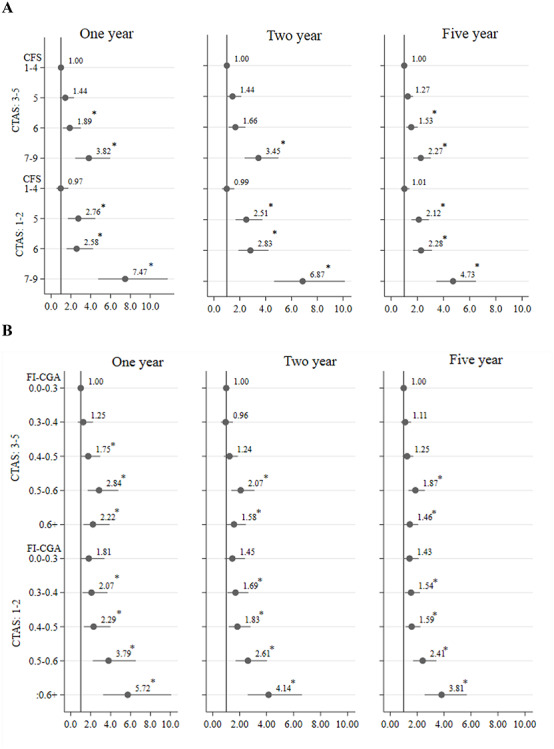
Hazard ratios for mortality based on acuity (CTAS) and frailty [CFS (panel a), FI-CGA (panel b)]. Note: People with CFS 1–4 and 7–9 and FI-CGA 0–0.03 and 0.6+ were combined due to low sample size; *P < 0.05.
Figure 2.
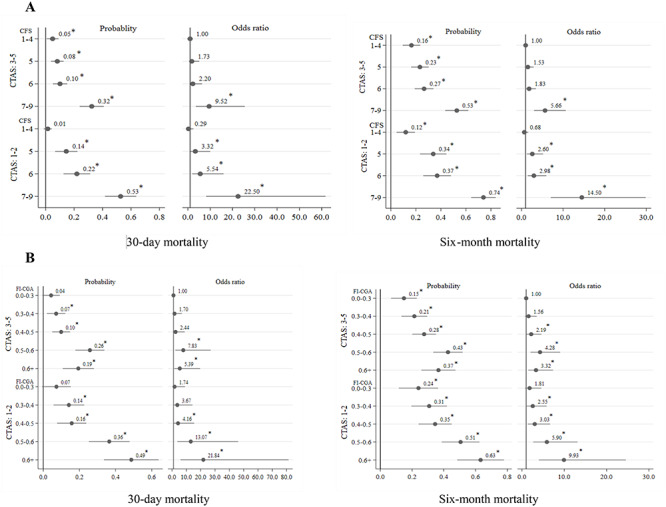
Predicted probabilities and odds ratio for mortality based on acuity and frailty [CFS (Panel a), FI-CGA (Panel b)]. Note: People with CFS 1–4 and 7–9 and FI-CGA 0–0.03 and 0.6+ were combined due to low sample size; *P < 0.05.
Acuity also influenced the association of frailty with mortality. When acuity was low, the risk for 30-day mortality was significant only among those with very high levels of frailty (CFS 7–9, FI-CGA > 0.5). When acuity was high, even lower levels of frailty (CFS 5–6, FI-CGA 0.4–0.5) were associated with a higher risk of 30-day mortality (Figure 2). The findings were also similar regarding the impact of acuity on the relationship of frailty with 1–5 years mortality (Figure 3).
Kaplan–Meier analysis showed that age did not much discriminate survival probability (Figure S8, Panel A in the Supplementary data), whereas acuity (Figure S8, Panel B in the Supplementary data) and frailty did (Figure S8, Panels C and D in the Supplementary data). Severely frail patients with high acuity had the lowest chances of survival (Figure 4). For example, the probability of surviving up to 40 months was only about 8% for the patients with FI-CGA > 0.6 and high acuity and 10% for patients with 7–9 CFS and high acuity (Figure 4).
Figure 4.
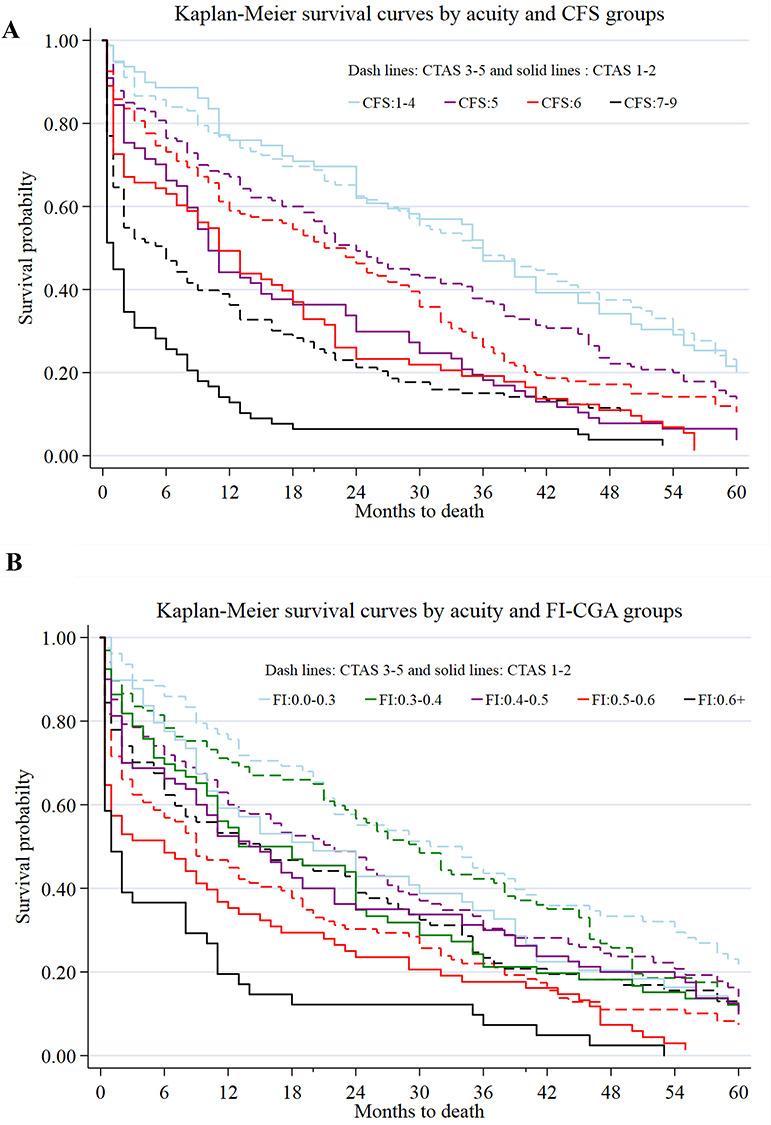
Survival probability by CTAS and frailty [CFS (panel a), FI-CGA (panel b)]. Note: People with CFS 1–4 scores were combined due to low sample size, as were those with CFS scores 7–9, and FI-CGA 0–0.03 and 0.6+.
Discussion
We explored how the severity of acute illness and the degree of frailty interact and how that interaction might be assessed by a geriatrician in the initial evaluation of a patient in an ED setting. The data suggest that a combination of an acuity/severity measure (which can be either at triage/screening or arising from a CGA) and a frailty measure (potentially a frailty screen measure, or as here, informed by the CGA) gives better information than either alone. Here, for the most part, the risks arising from the two are more than additive. Importantly, severely–very severely frail patients who are acutely unwell bear a substantial 30-day mortality rate (of 35–55%).
Frailty and acuity each merit formal consideration in the stratification of risk. As with the severity of an acute illness, the degree of frailty matters. A recent report offers another insight into stratifying risk based on illness severity and frailty through the use of a frailty index based solely on routinely gathered laboratory data [5]. Here we extend some seminal work from Cambridge on the synergy between illness severity and frailty in increasing risk [6].
The CFS and the baseline information in the CGA allow a routine means of understanding baseline function. This is crucial in understanding illness severity, often signalled by greater 2-week decline in high-order measures such as cognition, mobility and function. Information about baseline function is also essential to the care plan. Care planning must consider whether recovery to baseline is first likely and how that might fit with patient and carer preferences. Exactly how to optimize data collection and its incorporation into care planning from the start of the patient’s admission remains a compelling challenge. For this we are evaluating whether the Pictorial Fit-Frail Scale [23–25], which is being made widely available (specifically including to non-geriatricians), might facilitate the initial assessment of baseline function from informants.
Our study has important limitations. These are all patients from a single Canadian tertiary care hospital who were consulted to internal medicine, when the internist covering happened to be a geriatrician. About 30–35% of the patients referred to internal medicine were not included in the database. The main reasons were patients could not provide consent and substitute decision-makers were not available, patients were admitted from long-term care (LTC) institutions where a modified version of the CGA had already been completed as part of the LTC records and patients were seen under regulations of the Adult Protection Act (as adults in need of protection, who do not have substitute decision-makers) and a small number who refused consent. Since we did not have consent for these patients, we could not extract any demographic or clinical information to compare them with the patients who were included.
The CFS as used here summarized a CGA; it was not used as a screening tool. Even so, these data offer some insight into how the combination of an acuity measure and a CFS might better predict risk. We are careful here to note that this is a separate inquiry for which the current study offers hypotheses, not their tests. All this too we hope to address in other settings, at a larger scale. For example, it is a project that might be suited to the Acute Frailty Network [26]. Similarly, we do not have data on patient status other than vital status. An important question in many contexts is not the chance that a given patient will be alive, but the extent to which acute changes in cognition or function or mobility are likely to recover in those respects. The across-the-board decline seen with those high-order functions may represent an instance of increased cross-correlation, reflecting greater mutual dependence with loss of resilience [27, 28]. These considerations are motivating additional inquires by our group. Finally, reflecting the real-world nature of the evidence, we do not have confirmation of the exact degree of frailty at the baseline state. For the most part, this information comes from a history given by a knowledgeable informant. Other designs, such as the DELPHIC study, are better suited to that [29].
Conclusion
This study has combined the severity of acute illness with baseline frailty to predict mortality among older ED patients seen by a geriatrician. Our findings show that both the CFS and FI-CGA were strongly associated with short-term mortality among older ED patients referred to internal medicine. We found that higher severity of acute illness increased mortality risk across the levels of frailty and that when acuity was high, even lower levels of frailty were associated with greater mortality risk. Therefore, it is important to consider the interaction between frailty and illness severity to develop treatment plan in acute care. Using routine laboratory test data, future work should aim to refine the assessment of illness acuity for older patients in ED.
Supplementary Material
Acknowledgements:
The data (or portions of the data) used in this paper were made available by Health Data Nova Scotia of Dalhousie University. Although this research is based on data obtained from the Nova Scotia Department of Health and Wellness, the observations and opinions expressed are those of the authors and do not represent those of either Health Data Nova Scotia or the Department of Health and Wellness.
Declaration of Conflict: of Interest
Kenneth Rockwood has asserted copyright of the Clinical Frailty Scale through Dalhousie University. Use is free for research, education or not-for-profit care. (Users are asked not to change it or charge for its use.) In addition to academic and hospital appointments, he is President and Chief Science Officer of DGI Clinical, which in the last 5 years, has contracts with pharma and device manufacturers (Baxter, Baxalta, Biogen, Shire, Hollister, Nutricia, Roche, Otsuka) on individualized outcome measurement. In 2017 he attended an advisory board meeting with Lundbeck. He is an Associate Director of the Canadian Consortium on Neurodegeneration in Aging, which is funded by the Canadian Institutes of Health Research (CAN-137794), with additional funding from the Alzheimer Society of Canada and several other charities. He receives research support through grants from the Canadian Institutes of Health Research, the Canadian Frailty Network, the Nova Scotia Health Research Foundation, the Nova Scotia Health Authority Research Fund, the Dalhousie Medical Research Fund as the Kathryn Allen Weldon Professor of Alzheimer Research and the Fountain Family Innovation Fund of the QEII Health Science Centre Foundation.
Declaration of Funding:
This study is funded by the Canadian Frailty Network (CFN), TG2015-24, and the Nova Scotia Health Research Foundation (NSHRF), CAT-2016-1101. However, the interpretation and conclusion of this study are those of the authors alone.
References
- 1. Vaupel JW, Manton KG, Stallard E. The impact of heterogeneity in individual frailty on the dynamics of mortality. Demography 1979; 16: 439–54. [PubMed] [Google Scholar]
- 2. Rockwood K, Howlett SE. Age-related deficit accumulation and the diseases of ageing. Mech Ageing Dev 2019; 180: 107–16. [DOI] [PubMed] [Google Scholar]
- 3. Theou O, Squires E, Mallery K et al. What do we know about frailty in the acute care setting? A scoping review. BMC Geriatr 2018; 18: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Klausen HH, Petersen J, Bandholm T et al. Association between routine laboratory tests and long-term mortality among acutely admitted older medical patients: a cohort study. BMC Geriatr 2017; 17: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ellis HL, Wan B, Yeung M et al. Complementing chronic frailty assessment at hospital admission with an electronic frailty index (FI-Laboratory) comprising routine blood test results. CMAJ 2020; 192: E3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Romero-Ortuno R, Wallis S, Biram R, Keevil V. Clinical frailty adds to acute illness severity in predicting mortality in hospitalized older adults: an observational study. Eur J Intern Med 2016; 35: 24–34. [DOI] [PubMed] [Google Scholar]
- 7. Mowbray F, Brousseau AA, Mercier E, Melady D, Émond M, Costa AP. Examining the relationship between triage acuity and frailty to inform the care of older emergency department patients: findings from a large Canadian multisite cohort study. Can J Emerg Med 2020; 22: 74–81. [DOI] [PubMed] [Google Scholar]
- 8. Jørgensen R, Brabrand M. Screening of the frail patient in the emergency department: a systematic review. Eur J Intern Med 2017; 45: 71–3. [DOI] [PubMed] [Google Scholar]
- 9. Hubbard RE, Peel NM, Samanta M, Gray LC, Mitnitski A, Rockwood K. Frailty status at admission to hospital predicts multiple adverse outcomes. Age Ageing 2017; 46: 801–6. [DOI] [PubMed] [Google Scholar]
- 10. Brousseau A-A, Dent E, Hubbard R et al. Identification of older adults with frailty in the emergency department using a frailty index: results from a multinational study. Age Ageing 2018; 47: 242–8. [DOI] [PubMed] [Google Scholar]
- 11. Theou O, Campbell S, Malone ML, Rockwood K. Older adults in the emergency department with frailty. Clin Geriatr Med 2018; 34: 369–86. [DOI] [PubMed] [Google Scholar]
- 12. Murphy A, Briggs S. The accuracy of frailty scoring and uptake of comprehensive geriatric assessment (CGA) in patients living with frailty admitted through the emergency department (ED) in Wythenshawe hospital. Age Ageing 2020; 49(Supplement 1): i11–3. [Google Scholar]
- 13. Chong E, Ho E, Baldevarona-Llego J et al. Frailty in hospitalized older adults: comparing different frailty measures in predicting short- and long-term patient outcomes. J Am Med Dir Assoc 2018; 19: 450–7.e3. [DOI] [PubMed] [Google Scholar]
- 14. Chua XY, Toh S, Wei K, Teo N, Tang T, Wee SL. Evaluation of clinical frailty screening in geriatric acute care. J Eval Clin Pract 2020; 26: 35–41. [DOI] [PubMed] [Google Scholar]
- 15. Muscedere J, Waters B, Varambally A et al. The impact of frailty on intensive care unit outcomes: a systematic review and meta-analysis. Intensive Care Med 2017; 43: 1105–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr 2008; 8: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal 2001; 1: 323–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rockwood K, Song X, MacKnight C et al. A global clinical measure of fitness and frailty in elderly people. Can Med Assoc J 2005; 173: 489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jarrett PG, Rockwood K, Carver D, Stolee P, Cosway S. Illness presentation in elderly patients. Arch Intern Med 1995; 155: 1060–4. [PubMed] [Google Scholar]
- 20. Elkum NB, Barrett C, Al-Omran H. Canadian emergency department triage and acuity scale: implementation in a tertiary care center in Saudi Arabia. BMC Emerg Med 2011; 11: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Muller CJ, MacLehose RF. Estimating predicted probabilities from logistic regression: different methods correspond to different target populations. Int J Epidemiol 2014; 43: 962–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cameron AC, Trivedi P. Regression Analysis of Count Data. Cambridge: Cambridge University Press, 2013. [Google Scholar]
- 23. Theou O, Andrew M, Ahip SS et al. The pictorial fit-frail scale: developing a visual scale to assess frailty. Can Geriatr J 2019; 22: 64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McGarrigle L, Squires E, Wallace LMK et al. Investigating the feasibility and reliability of the pictorial fit-frail scale. Age Ageing 2019; 48: 832–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wallace LMK, McGarrigle L, Rockwood K, Andrew MK, Theou O. Validation of the pictorial fit-frail scale in a memory clinic setting. Int Psychogeriatr 2019: 1–10. [Epub ahead of print 29 September 2019]. [DOI] [PubMed] [Google Scholar]
- 26. Conroy S. The acute frailty network—supporting people with frailty and urgent care needs to get home sooner and healthier. Futur Health J 2019; 6: 109–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rutenberg AD, Mitnitski AB, Farrell SG, Rockwood K. Unifying aging and frailty through complex dynamical networks. Exp Gerontol 2018; 107: 126–9. [DOI] [PubMed] [Google Scholar]
- 28. Gijzel SMW, Van De Leemput IA, Scheffer M, Roppolo M, Olde Rikkert MGM, Melis RJF. Dynamical resilience indicators in time series of self-rated health correspond to frailty levels in older adults. Journals Gerontol-Ser A Biol Sci Med Sci 2017; 72: 991–6. [DOI] [PubMed] [Google Scholar]
- 29. Davis D, Richardson S, Hornby J et al. The delirium and population health informatics cohort study protocol: ascertaining the determinants and outcomes from delirium in a whole population. BMC Geriatr 2018; 18: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


