Abstract
Background
concurrent declines in gait speed and cognition have been associated with future dementia. However, the clinical profile of ‘dual decliners’, those with concomitant decline in both gait speed and cognition, has not been yet described. We aimed to describe the phenotype and the risk for incident dementia of those who present with dual decline in comparison with non-dual decliners.
Methods
prospective cohort of community-dwelling older adults free of dementia at baseline. We evaluated participants’ gait speed, cognition, medical status, functionality, incidence of adverse events and dementia, biannually over 7 years. Gait speed was assessed with a 6-m electronic walkway and global cognition using the MoCA test. We compared characteristics between dual decliners and non-dual decliners using t-test, chi-square and hierarchical regression models. We estimated incident dementia using Cox models.
Results
among 144 participants (mean age 74.23 ± 6.72 years, 54% women), 17% progressed to dementia. Dual decliners had a 3-fold risk (HR: 3.12, 95%CI: 1.23–7.93, P = 0.017) of progression to dementia compared with non-dual decliners. Dual decliners were significantly older with a higher prevalence of hypertension and dyslipidemia (P = 0.002). Hierarchical regression models show that age and sex alone explained 3% of the variation in the dual decliners group. Adding hypertension and dyslipidemia increased the explained variation by 8 and 10%, respectively. The risk of becoming a dual decliner was 4-fold higher if hypertension was present.
Conclusion
older adults with a concurrent decline in gait speed and cognition represent a group at the highest risk of progression to dementia. Older adults with dual decline have a distinct phenotype with a higher prevalence of hypertension, a treatable condition.
Keywords: cognition, gait speed, cohort study, dementia, dual decliners, older people
Key points
Concurrent decline in both cognition and gait speed with ageing is associated with a 3-fold risk of progression to dementia.
Older adults with dual decline exhibit a simple four-factor phenotype, being older and having hypertension, dyslipidemia, and a high vascular index
Dual decline in older adults may be a distinct and easily recognised target for dementia prevention
Those with dual decline may represent a subgroup of older adults with a high vascular and metabolic component in their dementia risk.
Older adults with dual decline may benefit from prompt targeting their reversible dementia risk factors.
Introduction
Gait speed (i.e. walking performance) and cognition both decline with ageing and are associated with the development of adverse events, including future disability and mortality [1–3]. Studies have shown that the coexistence of gait and cognitive impairment is more prevalent in older adults at risk of dementia [4, 5]. Cognitive decline precedes progression to dementia by several years, and a decline in gait speed can precede cognitive decline by more than a decade [4, 6–10]. A recent study showed that older adults who exhibit a concomitant decline in both gait speed and cognition (‘dual decliners’) are at higher risk of progression to dementia [11]. However, the specific clinical characteristics or phenotype of dual decliners has not yet been described, and direct comparisons with non-dual decliners are lacking. This may have important clinical relevance, first, due to the simplicity of stratifying older adults in these two categories and, second, for the potential identification of reversible factors in the group with dual decline that may guide specific targeted interventions to delay progression to dementia.
Our main goal was to describe the risk for future dementia and the clinical characteristics of dual decliners compared with non-dual decliners in a cohort of community-dwelling older adults free of dementia at baseline. We postulated that dual decliners represent a distinct group with risk factors that could be potentially targeted for intervention.
Methods
Design
The Gait and Brain Study is a longitudinal prospective cohort study (NTC03020381) aimed to elucidate cognitive and motor predictors of dementia. Participants were recruited from the community and geriatric clinics and underwent a comprehensive baseline evaluation followed by twice-yearly assessments over 7 years of follow-up. Assessments included a battery of cognitive tests and quantitative gait performance testing. We systematically recorded the following adverse events during follow-up: falls, hospitalisations, progression to dementia and death. Participants were eligible if they were age 65 and older, English speaking and able to walk 10 m without a mobility aid. Exclusion criteria included severe gait disorder due to musculoskeletal disease or neurological motor deficit (e.g. severe osteoarthritis, lower limb amputation, Parkinson’s disease, major stroke with motor sequelae), the use of neuroleptics, major depression or clinician diagnosis of dementia using criteria from the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Revised (DSM-IV TR) [12].
Ethical approval was obtained from the University of Western Ontario Ethics Board for Health Sciences Research Involving Human Subjects. Signed informed consent was obtained at enrolment. Data collection occurred between September 2008 and August 2017. This study follows the STROBE guidelines for reporting cohort studies [13].
Medical, cognitive and gait assessments
Sociodemographic characteristics were measured at baseline. Comorbidities, chronic medications and activities of daily living (ADLs, Lawton Brody Scale) were collected in face-to-face interviews at each 6-month visit (Table 1). A vascular risk index was determined using a previously validated 7-point scale of vascular risk factors (i.e. hypertension, dyslipidemia) and diseases (i.e. coronary artery disease, stroke, diabetes mellitus, congestive heart failure and atrial fibrillation). The scores range from 1 to 7, with one point added for each risk factor present in an individual [14]. Global cognition was assessed using the Montreal Cognitive Assessment (MoCA) [15]. Three alternative versions of the MoCA were used in consecutive assessments to minimise learning effects [16]. The Clinical Dementia Rating (CDR) scale [17] was also performed at all visits to assist in dementia ascertainment. Gait performance was evaluated at all visits by quantifying gait speed (cm/s) using an electronic walkway (Zeno® Walkway/Protokinetics, Havertown, PA; 600 cm long × 90 cm) following a validated gait protocol [18–20]. Participants were asked to walk at their usual pace. Start and end points were marked on the floor 1 m from either end of the walkway to avoid recording acceleration and deceleration phases. Frailty was operationalised using the Fried and Walston criteria [21], which includes slow gait speed, low physical activity, weakness, shrinking/weight loss and exhaustion, as previously validated [22].
Table 1.
Demographic and clinical characteristics of 144 participants at baseline, as well as adverse events at follow-up stratified by dual decline and non-dual decline status
| Non-dual decliners (n = 120) | Dual decliners (N = 24) | P value | |
|---|---|---|---|
| Demographics | |||
| Age, mean (SD) | 73.60 (6.65) | 77.67 (6.15) | 0.006 |
| Women, n (%) | 72 (55%) | 12 (50%) | 0.663 |
| Number of years of education, mean (SD) | 13.59 (3.73) | 13.04 (2.70) | 0.467 |
| Number of comorbidities, mean (SD) | 5.16 (2.73) | 5.54 (3.13) | 0.540 |
| Number of medications, mean (SD) | 6.83 (3.90) | 6.37 (3.12) | 0.589 |
| ApoE4 (at least one allele), n (%) | 15 (35%) | 4 (50%) | 0.450 |
| ApoE4-E4, n (%) | 2 (5%) | 1 (13%) | 0.407 |
| BMI, mean (SD) | 27.05 (8.0) | 25.94 (3.19) | 0.512 |
| Vascular comorbidities | |||
| Hypertension, n (%) | 65 (50%) | 19 (79%) | 0.008 |
| Chronic heart failure, n (%) | 1 (1%) | 1 (4%) | 0.287 |
| Diabetes, n (%) | 20 (15%) | 5 (21%) | 0.546 |
| Smoking, n (%) | 25 (58%) | 5 (63%) | 1.000 |
| Heart attack, n (%) | 8 (6%) | 4 (17%) | 0.095 |
| Dyslipidemia, n (%) | 57 (51%) | 17 (74%) | 0.043 |
| Vascular risk index, mean (SD) | 1.43 (1.14) | 2.13 (1.10) | 0.009 |
| Other comorbidities | |||
| Osteoporosis, n (%) | 17 (13%) | 2 (8%) | 0.524 |
| Lung disease, n (%) | 10 (8%) | 1 (4%) | 1.000 |
| Osteoarthritis, n (%) | 41 (31%) | 9 (38%) | 0.636 |
| Cancer, n (%) | 46 (35%) | 6 (25%) | 0.481 |
| Hearing problems, n (%) | 50 (38%) | 9 (38%) | 0.929 |
| Diagnosed with depression, n (%) | 25 (19%) | 4 (17%) | 1.000 |
| Functionality | |||
| IADL score, mean (SD) | 7.70 (0.86) | 7.75 (0.71) | 0.872 |
| ADL score, mean (SD) | 6.00 (0.0) | 6.0 (0.0) | N/A |
| Gait speed (cm/s), mean (SD) | 113.03 (22.48) | 112.16 (21.26) | 0.861 |
| Cognitive performance | |||
| MoCA, mean (SD) | 24.76 (3.41) | 23.92 (3.30) | 0.267 |
| TMT A, mean (SD) | 49.67 (17.09) | 53.72 (21.43) | 0.538 |
| TMT B, mean (SD) | 129.22 (64.50) | 149.68 (82.06) | 0.411 |
| RAVLT 5 m delay recall, mean (SD) | 4.89 (3.09) | 5.60 (3.36) | 0.626 |
| Frailty | |||
| No frailty | 52 (40%) | 8 (33%) | 0.632 |
| Pre-frail | 69 (53%) | 15 (63%) | |
| Frail | 10 (8%) | 1 (4%) | |
| Adverse events during follow-up | |||
| Falls with injury, n (%) | 18 (15%) | 6 (25%) | 0.293 |
| Hospitalisation, n (%) | 19 (27%) | 5 (63%) | 0.098 |
| Deaths, n (%) | 2 (2%) | 2 (8%) | 0.114 |
| Two or more adverse events, n (%) | 63 (48%) | 24 (100%) | <0.001 |
| Incident Dementia, n (%) | 13 (11%) | 11 (46%) | <0.001 |
Abbreviations: IADL: Instrumental activities of daily living, scores range from 1 to 7; ADL: basic activities of daily living, scores range 1 to 6; RAVLT: Rey Auditory Verbal Learning Test; TMT A&B: Trail Making Tests A and B; *P-value determined using Chi square (X2) or Student t-tests, as deemed appropriate; statistically significant values are in bold.
Ascertainment of cognitive and gait declines
Cognitive decline was operationalised as a decrease of at least two points in MoCA scores between baseline and the final assessment, as previously validated [23, 24]. Gait decline was operationalised as a reduction equal to or greater than 10 cm/s in gait speed between baseline and the final assessment, as previously validated [25]. Participants who developed during follow-up cognitive and gait decline were categorised as dual decliners, and participants who had only cognitive or gait speed decline, or neither, were categorised as non-dual decliners.
Outcome measure
Incident dementia was the main outcome, determined by a clinician investigator, a geriatrician with expertise in dementia assessment and management, during follow-up visits using DSM-IV TR criteria and when global CDR progressed to one or higher. DSM-IV TR and CDR were assessed by independent raters. The geriatrician was blinded to gait assessment and to CDR, but not blinded to MoCA scores. Cognitive testing and CDR rating were performed by certified research assistants. The type of dementia was established using standardised criteria for Alzheimer’s disease (ad) dementia [26], frontotemporal dementia [27], Lewy body dementia [28] and vascular dementia (VaD) [29], only for descriptive purposes. All subtypes were analysed together as a general outcome of conversion to dementia.
Data analysis
Demographic and clinical characteristics comparing dual decliners and non-dual decliners were summarised using either means and standard deviations or frequencies and percentages, as appropriate. Statistically significant differences were determined using t-tests or chi-square tests. Cox proportional hazards regression modelling was used to estimate the risk of progression to dementia of dual decliners compared with non-dual decliners. Models were adjusted for covariates including baseline gait speed and cognitive status (MoCA score), age, sex, number of comorbidities and years of education. As a sensitivity analysis, we also adjusted for specific comorbidities that were deemed potential confounders for increasing dementia risk, such as dyslipidemia, vascular risk index and hypertension. Hierarchical logistic regression was used to estimate with odds ratios the statistically independent contribution of each risk factor. Adjustments to these models were made for vascular index since it showed significant differences between dual decliners and non-dual decliners at baseline. Statistical significance was set at P < 0.05 (two-sided), and analyses were conducted using SPSS (v23.0, IBM Corporation, Chicago, IL).
Results
Participant characteristics
One hundred and forty-four participants aged 65 and older (mean age 74.23 ± 6.72; 54% women) had full data for all biannual assessments with a mean follow-up of 28 months (range, 6–72 months). During follow-up, 24 participants (17%) progressed to dementia, 19 (86.4%) to Alzheimer’s disease dementia, 1 (4.5%) to Vascular dementia and 2 (9.1%) to Lewy body dementia.
Characteristics of dual decliners
Table 1 presents the demographic and clinical characteristics of dual and non-dual decliners. There were no significant baseline differences between the groups for sex, years of education, number of comorbidities, number of medications taken, frailty status and functionality. Dual decliners were older and had more comorbidities related to cardiovascular disease, a high vascular index and a significantly higher prevalence of dyslipidemia and hypertension, even after adjustment for age. Dual decliners had lower MoCA scores and carried at least one ApoE4 allele, although these differences were not significant. During follow-up, dual decliners experienced significantly higher incidence of two or more adverse clinical events and more progression to dementia. Other clinical events such as hospitalisations, falls with injuries and mortality had also higher prevalence in dual decliners, but these differences were not significant.
Associations with incident dementia
Only 11% of non-dual decliners participants progressed to dementia (n = 13, out of 120), whereas 46% of dual decliners progressed to dementia (n = 11, out of 24). Figure 1 graphs these results with the prevalence of comorbidities and the proportion of participants that progressed to dementia, in the two groups.
Figure 1.
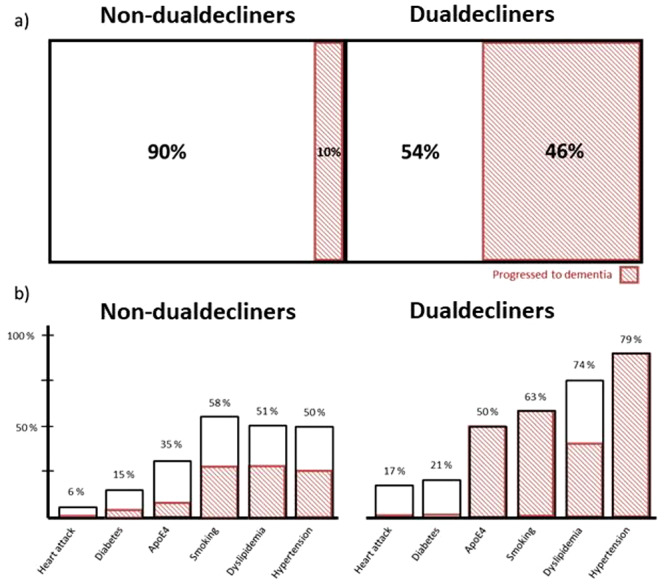
Differences between the dual and non-dual decliners groups. (a) The proportion of participants that progressed to dementia (shaded) between the groups. (b) The proportion of participants that present different comorbidities between the groups, as well as the proportion of individuals that had those comorbidities and progressed to dementia (shaded).
Dual decliners had a 3-fold increased risk (HR: 3.48, 95%CI: 1.55–7.81, P = 0.002) of progression to dementia compared with non-dual decliners (Figure 2). Appendix 1 in the Supplementary data shows the risk of dementia stratified by the two groups. When adjusted by baseline MoCA and gait speed (Model 1), dual decliners still had a 3-fold risk of progression to dementia (HR: 3.18, 95% CI: 1.40–7.25, P = 0.006). Model 2 and 3 show a two and 3-fold risk when additional adjustments were done by age and sex (HR: 2.77, 95% CI: 1.19–6.43, P = 0.018) and age, sex, comorbidities and years of education (HR: 3.12, 95% CI: 1.23–7.93, P = 0.017), respectively.
Figure 2.
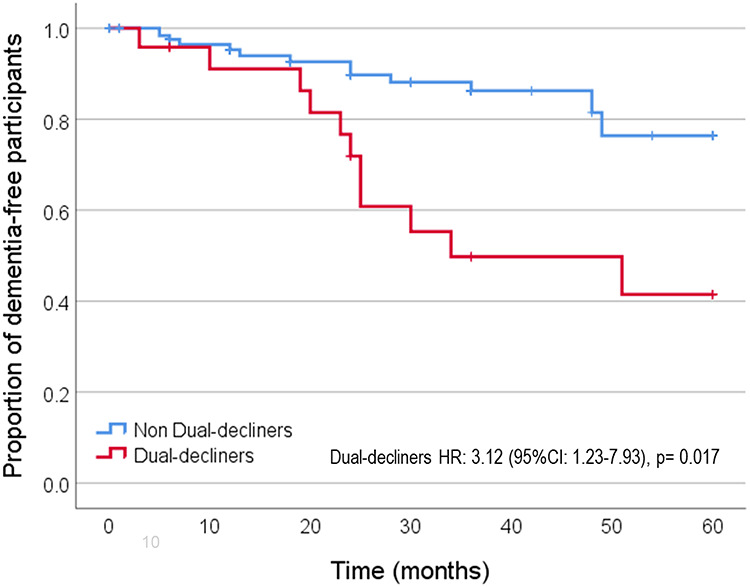
Kaplan–Meier curve showing the proportion of dementia-free participants during follow-up between dual and non-dual decliners (Model 3, fully adjusted).
When the Cox models were adjusted by dyslipidemia and vascular index, the risk of progression to dementia was attenuated but remained significant (HR: 2.40, 95% CI: 1.01–5.69, P = 0.048, sensitivity analysis, Appendix 1b in the Supplementary data). However, when the model was adjusted for hypertension, the associations were no longer significant (HR: 1.97, 95% CI: 0.74–5.21, P = 0.173, sensitivity analysis, Appendix 1b in the Supplementary data). Although hypertension and dyslipidemia are included in the vascular index, they were included as covariates because of their strong association with dual decliners and their low pairwise correlation (<0.8).
Risk factors for dual decliners
Hierarchical logistic regression models were used to estimate the contribution of selected covariates—those that showed a significant difference between groups—in describing the dual decliner phenotype (Appendix 2 in the Supplementary data). Age and sex alone explained almost 3% of the variation in the dual decliners group. Adding hypertension to the model increased the explained variation to almost 8% and adding dyslipidemia increased it to almost 10%. Moreover, the risk of becoming a dual decliner was almost 4-fold higher if the participant had hypertension and 2-fold for dyslipidemia, whereas age and sex did not contribute any significant risk to the models (Appendix 2 in the Supplementary data).
Discussion
This study prospectively demonstrated that a decline in both cognitive performance and gait speed in older adults (dual decliners) over an average of 28 months of follow-up is independently associated with a 3-fold higher risk of progression to dementia compared to those without dual decline. Phenotypically, dual decliners were older and had more comorbidities related to cardiovascular diseases with a higher prevalence of hypertension and dyslipidemia.
Dual decline in gait speed and global cognition affected almost half of the participants who progressed to dementia versus only 10% of non-dual decliners, suggesting that it might represent a recognisable prodromal phase of dementia. A parsimonious explanation is that cognitive and gait functions are not true causal risk factors for one another but rather that both are affected by a common underlying pathophysiology. Thus, older adults who manifest both cognitive and gait speed declines might have a greater burden of a shared underlying pathology. Operationally, modelling both declines together provided simplicity in our study, and from a diagnostic perspective, our results suggest that the prediction of dementia can be improved by combining cognitive and gait decline, which can easily be done clinically with minimal cost and time.
It has been proposed that this concurrent dual decline is related to specific mechanisms including vascular damage, chronic inflammation, neurodegeneration and micronutrient deficiencies [11, 30, 31]. Recently, it has been suggested that frailty may mediate the association between brain neuropathology and impending dementia [32]. In our cohort, pre-frailty was more common in dual decliners, although not significantly. Thus, this phenotype can represent an early stage in the continuum before frailty can be fully expressed. Aligned with these potential mechanisms proposed, we were able to detect important clinical characteristics in the dual decliner group, such as a higher vascular index score with a higher prevalence of chronic hypertension and dyslipidemia. Our hierarchical models showed that hypertension and dyslipidemia explained most variation in the outcome, denoting the potential key role of vascular risk factors in this phenotype.
The Canadian Study of Health and Aging has also shown an increased progression to dementia among subjects with hypertension [33]. Similarly, the Cardiovascular Health Study and the Mobilize Boston Study has shown that the presence of vascular risk factors and hypertension in community older adults is cross-sectionally associated with slowing gait and with low cognition, executive function and low mood [34–36]. Thus, dual decliners may represent a subgroup with higher vascular components in their dementia risk. Emerging evidence shows that the most common form of clinical dementia, late-onset ad, presents mixed pathology with significant vascular pathology [37].
Mechanistically, our results provide empirical evidence for the hypothesis that concomitant declines in gait speed and cognition may result from vascular burden in shared brain neural substrates [3]. High-level cognitive abilities like attention, executive function and memory rely on specific brain regions and their connections [2, 38, 39] that also regulate planning and monitoring goal-directed behaviour, including gait [3, 40]. Anatomically, concurrent cognitive and gait deficits have been attributed to damage to the fronto-parietal and cingulate cortical areas and striatal and hippocampal networks [2, 3] including the hippocampus itself [2, 41]. Because of their watershed vascularisation, frontal and subcortical neuronal networks are susceptible to vascular risk factors, microvascular disease and blood oxygenation, resulting in white matter hyperintensities [3]. These hyperintensities may reflect ischemia due to occlusive lesions of deep penetrating arteries; however, they possibly have heterogeneous aetiologies including blood–brain barrier dysfunction, inflammation and immunological mechanisms [42].
Our findings have clinical relevance for detecting early signs of an underlying dementia. Older adults with dual decline in cognition and gait speed should receive further attention to address their cardiovascular and metabolic risk factors to potentially attenuate their risk of dementia. In light of the findings from the SPRINT MIND [43] study that showed that intensive control of blood pressure in older adults with hypertension can reduce progressions to mild cognitive impairment and dementia, our results support the rationale for hypertension to be intensively targeted in dual decliners, to temper their dementia risk [43]. Noteworthy, aggressive treatments for hypertension in older adults are not widely recommended since they may increase the risk of postural hypotension, which may cause subtle brain damage and cognitive impairment. However, the benefits of intensive blood pressure treatment may outweigh the harms in dual decliners. This approach may help to appropriately target patients for hypertension-intensive treatment when the goal is improving brain health and cognition. Future studies are needed to test this hypothesis.
Our study is not free of limitations. Participants were mainly recruited from geriatric medicine clinics, and, thus, our results are only generalisable to clinic-based populations. Replication of our findings in general population samples is required. The low number of participants who progressed to dementia may also affect generalisability. Although the requirement of serial gait speed measures to detect decline currently represents a practical limitation, this may change if gait speed testing becomes routinely available in clinics or through increased use of actigraphy sensors. At a minimum, gait speed can be adequately measured using a stopwatch [44]. Strengths include a well-characterised cohort purposely designed to assess cognitive and gait speed changes over close follow-up intervals to accurately detect early decline and to adequately monitor time to progression to dementia. We used a robust multivariable longitudinal modelling analysis adjusted for known covariates. We chose readily available tests, such as the MoCA and gait speed, due to their simplicity and good psychometric responsiveness. In addition this can facilitate future testing of the external validity of our results in larger samples.
In conclusion, concurrent dual decline in gait speed and cognition in older adults increases the risk of progression to dementia by three times. Dual decliners were older and had a higher prevalence of cardiovascular risk factors such as hypertension and dyslipidemia that, if managed, may temper their risk of progression to dementia. Identification of dual decliners may point to a group of older adults at heightened risk for future dementia who may benefit from prompt targeting of their potentially reversible risk factors.
Supplementary Material
Declaration of Conflicts of Interest
Dr M. Montero-Odasso is a member of the Executive and Board of the Canadian Geriatrics Society, an Associate Editor of the Journal of Alzheimer’s Disease and an Editorial Board Member of the Journal Gerontology Medical Sciences and Geriatrics. Dr. J Wells was a part-time employee for Pfizer pharmaceuticals and owns stock employee option.
Funding
Dr Montero-Odasso’s program in Gait and Brain Health is supported by grants from the Canadian Institutes of Health Research (CIHR; MOP 211220, PJT 153100), the Ontario Ministry of Research and Innovation (ER11–08–101), the Ontario Neurodegenerative Diseases Research Initiative (OBI 34739), the Canadian Consortium on Neurodegeneration in Aging (FRN CNA 137794) and the Department of Medicine Program of Experimental Medicine Research Award (POEM 768915), University of Western Ontario. He is the first recipient of the Schulich Clinician–Scientist Award. Drs Q. Tian and L. Ferrucci are supported by the Intramural Research Program of the NIH, National Institute on Aging.
References
- 1. Montero-Odasso M, Bherer L, Studenski S et al. Mobility and cognition in seniors. Report from the 2008 Institute of Aging (CIHR) mobility and cognition workshop. Can Geriatr J 2015; 18: 159–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rosso AL, Studenski SA, Chen WG et al. Aging, the central nervous system, and mobility. J Gerontol A Biol Sci Med Sci 2013; 68: 1379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Montero-Odasso M, Hachinski V. Preludes to brain failure: executive dysfunction and gait disturbances. Neurol Sci 2014; 35: 601–4. [DOI] [PubMed] [Google Scholar]
- 4. Montero-Odasso MM, Barnes B, Speechley M et al. Disentangling cognitive-frailty: results from the gait and brain study. J Gerontol A Biol Sci Med Sci 2016; 71: 1476–82. [DOI] [PubMed] [Google Scholar]
- 5. Verghese J, Wang C, Lipton RB, Holtzer R. Motoric cognitive risk syndrome and the risk of dementia. J Gerontol A Biol Sci Med Sci 2013; 68: 412–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buracchio T, Dodge HH, Howieson D, Wasserman D, Kaye J. The trajectory of gait speed preceding mild cognitive impairment. Arch Neurol 2010; 67: 980–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Callisaya ML, Blizzard CL, Wood AG, Thrift AG, Wardill T, Srikanth VK. Longitudinal relationships between cognitive decline and gait slowing: the Tasmanian study of cognition and gait. J Gerontol A Biol Sci Med Sci 2015; 70: 1226–32. [DOI] [PubMed] [Google Scholar]
- 8. Mielke MM, Roberts RO, Savica R et al. Assessing the temporal relationship between cognition and gait: slow gait predicts cognitive decline in the Mayo Clinic study of aging. J Gerontol A Biol Sci Med Sci 2013; 68: 929–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dumurgier J, Artaud F, Touraine C et al. Gait speed and decline in gait speed as predictors of incident dementia. J Gerontol A Biol Sci Med Sci 2017; 72: 655–61. [DOI] [PubMed] [Google Scholar]
- 10. Kueper JK, Speechley M, Lingum NR, Montero-Odasso M. Motor function and incident dementia: a systematic review and meta-analysis. Age Ageing 2017; 46: 729–738. doi: 10.1093/ageing/afx084. [DOI] [PubMed] [Google Scholar]
- 11. Montero-Odasso M, Speechley M, Muir-Hunter SW et al. Motor and cognitive trajectories before dementia: results from gait and brain study. J Am Geriatr Soc 2018; 66: 1676–1683. doi: 10.1111/jgs.15341. [DOI] [PubMed] [Google Scholar]
- 12. Association AP Diagnostic and Statistical Manual of Mental Disorders 4th ed. - Text Revision; [DSV-IV-TR Washington, DC: Author, 2000 2000. [Google Scholar]
- 13. Elm E, Altman DG, Egger M et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Rev Esp Salud Publica 2008; 82: 251–9. [DOI] [PubMed] [Google Scholar]
- 14. Villeneuve S, Belleville S, Massoud F, Bocti C, Gauthier S. Impact of vascular risk factors and diseases on cognition in persons with mild cognitive impairment. Dement Geriatr Cogn Disord 2009; 27: 375–81. [DOI] [PubMed] [Google Scholar]
- 15. Nasreddine ZS, Phillips NA, Bedirian V et al. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005; 53: 695–9. [DOI] [PubMed] [Google Scholar]
- 16. Costa AS, Fimm B, Friesen P et al. Alternate-form reliability of the Montreal cognitive assessment screening test in a clinical setting. Dement Geriatr Cogn Disord 2012; 33: 379–84. [DOI] [PubMed] [Google Scholar]
- 17. Morris JC. The clinical dementia rating (CDR): current version and scoring rules. Neurology 1993; 43: 2412–4. [DOI] [PubMed] [Google Scholar]
- 18. Montero-Odasso M, Casas A, Hansen KT et al. Quantitative gait analysis under dual-task in older people with mild cognitive impairment: a reliability study. J Neuroeng Rehabil 2009; 6: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Montero-Odasso M, Almeida QJ, Bherer L et al. Consensus on shared measures of mobility and cognition: from the Canadian consortium on Neurodegeneration in aging (CCNA). J Gerontol A Biol Sci Med Sci 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cullen S, Montero-Odasso M, Bherer L et al. Guidelines for gait assessments in the Canadian consortium on Neurodegeneration in aging (CCNA). Can Geriatr J 2018; 21: 157–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fried LP, Tangen CM, Walston J et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56: M146–56. [DOI] [PubMed] [Google Scholar]
- 22. Montero-Odasso M, Muir SW, Hall M et al. Gait variability is associated with frailty in community-dwelling older adults. J Gerontol A Biol Sci Med Sci 2011; 66: 568–76. [DOI] [PubMed] [Google Scholar]
- 23. Julayanont P, Brousseau M, Chertkow H, Phillips N, Nasreddine ZS. Montreal cognitive assessment memory index score (MoCA-MIS) as a predictor of conversion from mild cognitive impairment to Alzheimer's disease. J Am Geriatr Soc 2014; 62: 679–84. [DOI] [PubMed] [Google Scholar]
- 24. Krishnan K, Rossetti H, Hynan LS et al. Changes in Montreal cognitive assessment scores over time. Assessment 2017; 24: 772–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc 2006; 54: 743–9. [DOI] [PubMed] [Google Scholar]
- 26. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology 1984; 34: 939–44. [DOI] [PubMed] [Google Scholar]
- 27. Rascovsky K, Hodges JR, Knopman D et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011; 134Pt 9: 2456–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McKeith IG. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. J Alzheimers Dis 2006; 9: 417–23. [DOI] [PubMed] [Google Scholar]
- 29. Chui HC, Victoroff JI, Margolin D, Jagust W, Shankle R, Katzman R. Criteria for the diagnosis of ischemic vascular dementia proposed by the state of California Alzheimer's disease diagnostic and treatment Centers. Neurology 1992; 42: 473–80. [DOI] [PubMed] [Google Scholar]
- 30. Montero-Odasso M, Pieruccini-Faria F, Bartha R et al. Motor phenotype in neurodegenerative disorders: gait and balance platform study design protocol for the Ontario neurodegenerative research initiative (ONDRI). J Alzheimers Dis 2017; 59: 707–721. doi: 10.3233/JAD-170149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. De Cock AM, Perkisas S, Verhoeven V, Vandewoude M, Fransen E, Remmen R. The impact of cognitive impairment on the physical ageing process. Aging Clin Exp Res 2018; 30: 1297–306. [DOI] [PubMed] [Google Scholar]
- 32. Wallace LMK, Theou O, Godin J, Andrew MK, Bennett DA, Rockwood K. Investigation of frailty as a moderator of the relationship between neuropathology and dementia in Alzheimer's disease: a cross-sectional analysis of data from the rush memory and aging project. Lancet Neurol 2019; 18: 177–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Oveisgharan S, Hachinski V. Hypertension, executive dysfunction, and progression to dementia: the Canadian study of health and aging. Arch Neurol 2010; 67: 187–92. [DOI] [PubMed] [Google Scholar]
- 34. Sorond FA, Galica A, Serrador JM et al. Cerebrovascular hemodynamics, gait, and falls in an elderly population: MOBILIZE Boston study. Neurology 2010; 74: 1627–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hajjar I, Quach L, Yang F et al. Hypertension, white matter hyperintensities, and concurrent impairments in mobility, cognition, and mood: the cardiovascular health study. Circulation 2011; 123: 858–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hajjar I, Yang F, Sorond F et al. A novel aging phenotype of slow gait, impaired executive function, and depressive symptoms: relationship to blood pressure and other cardiovascular risks. J Gerontol A Biol Sci Med Sci 2009; 64: 994–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hachinski V. Dementia: paradigm shifting into high gear. Alzheimers Dement 2019; 15: 985–94. [DOI] [PubMed] [Google Scholar]
- 38. Montero-Odasso M, Verghese J, Beauchet O, Hausdorff JM. Gait and cognition: a complementary approach to understanding brain function and the risk of falling. J Am Geriatr Soc 2012; 60: 2127–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Montero-Odasso M, Oteng-Amoako A, Speechley M et al. The motor signature of mild cognitive impairment: results from the gait and brain study. J Gerontol A Biol Sci Med Sci 2014; 69: 1415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sorond FA, Cruz-Almeida Y, Clark DJ et al. Aging, the central nervous system, and mobility in older adults: neural mechanisms of mobility impairment. J Gerontol A Biol Sci Med Sci 2015; 70: 1526–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wennberg AM, Savica R, Mielke MM. Association between various brain pathologies and gait disturbance. Dement Geriatr Cogn Disord 2017; 43: 128–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gouw AA, Seewann A, Flier WM et al. Heterogeneity of small vessel disease: a systematic review of MRI and histopathology correlations. J Neurol Neurosurg Psychiatry 2011; 82: 126–35. [DOI] [PubMed] [Google Scholar]
- 43. Kjeldsen SE, Narkiewicz K, Burnier M, Oparil S. Intensive blood pressure lowering prevents mild cognitive impairment and possible dementia and slows development of white matter lesions in brain: the SPRINT memory and cognition IN decreased hypertension (SPRINT MIND) study. Blood Press 2018; 27: 247–8. [DOI] [PubMed] [Google Scholar]
- 44. Cullen S, Montero-Odasso M, Bherer L et al. Guidelines for gait assessments in the Canadian consortium on Neurodegeneration in aging (CCNA). Can Geriatr J 2018; 21: 157–165. doi: 10.5770/cgj.21.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


