Abstract
Gingival and periodontal diseases constitute a major distress in the field of dentistry. The greater part of the contributing and etiologic factors are diminished or treated with all forms of ozone. Ozone which is well known for its antimicrobial and tissue regenerative properties is indicated in all the stages of gingival and periodontal diseases. The aim of this study was to review literature on the effects of ozone as an adjunct to mechanical therapy in periodontitis patients. A search of literature was conducted to identify articles of ozone therapy in periodontitis published during the period from January 1, 2010, to July 30, 2018. PubMed, Medline, Embase, Cochrane, and Google Scholar search and hand searching of journals were conducted to identify relevant articles. The search strategy employed both dental subject headings and free-text terms. Out of a total of 123 studies that fit the initial inclusion criteria, 117 studies were further excluded. Only six studies were included in the meta-analyses. A high level of heterogeneity in the selected studies was found as demonstrated by Q-value of 10.241 and I2 value of 80.49%. However, the funnel plots showed symmetrical shape, with prevalence studies indicating absence of publication bias. Ozone therapy can be used effectively as an adjunct to scaling and root planing in the treatment of periodontitis.
Keywords: Aggressive periodontitis, chronic periodontitis, ozone therapy, scaling and root planing, subgingival debridement
Introduction
Periodontitis is a complex disease which is marked by microbial and host response interaction. Microorganisms have also been found to be associated with the commencement of disease in the subgingival environment.[1]
Ecological plaque hypothesis suggests that a disease is not only hindered by suppressing the presumptive pathogens alone but also by impeding with the factors responsible for the transition of plaque microflora from the commensal to a pathogenic relationship with the host. The critical event in ecological plaque hypothesis is collapse of microbial hemostasis and shifting the ecological balance of plaque back toward one that is compatible with dental health. Thus, preventing strategies include altering subgingival environment which includes application of oxygenating agents. An alternative approach by the suppression of subgingival bacteria is to inhibit their growth by changing the subgingival environment (anaerobic) to aerobic.[1]
Ozone which is present in atmosphere is an uncolored gas form of oxygen. It is formed as a result of exposure to ultraviolet (UV) rays on a combination of three atoms of oxygen. It is also formed by the action of lightening discharges and has the capacity to absorb harmful UV rays.[1]
In 1785, Van Marum noticed that the air next to his electrostatic machine acquired a characteristic odor when electric sparks were passed. “Ozone” was named by Schonbein in the year 1840. It is derived from the Greek word “Ozein” meaning odorant. At very low temperature, ozone which is a pale blue gas will be condensed to a deep blue liquid. It is unstable and quickly gives up the nascent oxygen to form oxygen. Hence, the powerful oxidizer, ozone, is routinely used in human medicine to kill bacteria and fungi and to inactivate viruses. The nascent oxygen released by the spontaneous breakdown of ozone combines with water molecules to form hydroxyl group which is a more powerful oxidizer.[1]
Ozone is a powerful oxidant with distinct antimicrobial activity and has the capacity to act as a metabolic and host immune modulator. Ozone has been used for sterilization of cavities, root canals, and periodontal pockets, in the treatment of early carious lesions; to enhance epithelial wound healing, such as that caused by ulceration and herpetic lesions; as a rinse for avulsed teeth; and as a denture cleaner. The routes of administration of ozone for both gaseous and aqueous forms are topical and regional.[2] It has been documented that aqueous ozone possesses a distinctive biocompatibility to fibroblasts, cementoblasts, and epithelial cells, suggesting aptness of its use against oral infectious diseases such as periodontal disease, apical periodontitis, and peri-implantitis.[3]
Ozone in periodontitis treatment is used as an adjunctive to scaling and root planing (SRP) as compared to SRP alone.
Ozone has a role to play several actions in the human body such as an immune-stimulating, an analgesic, an antihypoxic, a detoxicating, an antimicrobial, a bioenergetic, and a biosynthetic agent.[4]
Ozone therapy as a contemporary noninvasive method of treatment is gaining popularity; it is a strong oxidizing agent with a high antimicrobial power against oral microorganisms, without developing resistance neither for gaseous, nor for aqueous ozone. It is used widely in varying treatment methods in the field of medicine, dentistry, veterinary, food industry, and water treatment.
Despite these potential beneficial effects, a meta-analysis of the effectiveness of ozone therapy in treating periodontitis has not been reported by studies conducted on humans. Furthermore, given the results of various studies and the absence of any previous meta-analyses, there is a great need to assess the literature systematically. The aim of the present meta-analysis is to evaluate the role of ozone therapy in treating periodontitis.
Materials and methods
Search criteria
PubMed
Medline
Embase
Sci-hub
Cochrane
Science Direct
Google
2010–2018.
Focused question
How effective is ozone therapy as an adjunct in treating periodontitis?
Population: Individuals with chronic periodontitis (CP) and aggressive periodontitis
Intervention: Use of ozone therapy as an adjunct to mechanical SRP
Comparison: Between ozone therapy group and placebo/no treatment group
Outcome: Changes in pocket depth, clinical attachment level (CAL), bleeding on probing (BOP), Plaque Index (PI), and Gingival Index (GI)
Selection criteria.
Inclusion criteria
Publication of domestic and international peer-review literature
Articles written in English
Human randomized controlled trial
More than 6 weeks of follow-up
More than ten patients participating in the study
Articles reporting data on change of pocket depth, clinical attachment loss, GI, PI, and BOP.
Parameters used
Probing pocket depth (PPD), clinical attachment loss, GI, PI, BOP, Gingival Bleeding Index, and Sulcular Bleeding Index.
Exclusion criteria
In vitro studies
Animal studies
Retrospective cross-sectional studies.
Search strategy
A literature search was performed at Rajarajeswari Dental College and Hospital, Bengaluru, using electronic media in the following databases: Pubmed.com, Cochrane database, EBSCO, Medline, Google.com, Sci-hub, and Embase between January 1, 2010, and July 30, 2018, using the following terms: Ozone therapy, aggressive periodontitis, chronic periodontitis, ozone nanobubble water, chlorhexidine, and subgingival debridement.
Two reviewers independently performed article search. They first examined the titles and abstracts of articles searched and selected papers for full-text screening. K value was introduced to examine agreement.
Results
Our initial search resulted in the identification of 123 articles. Subsequently, 109 articles on the basis of title and abstract were excluded. Fourteen full-text article studies were considered to be potentially relevant for this review. Of these, we excluded five articles during full-text screening because they did not provide individual patient data or comparison between test and control groups.
In addition, we excluded two articles because they were review articles.
By the end of the search phase, we considered seven articles eligible; their data are the basis of review. Therefore, we cited the articles with shorter follow-up period. Figure 1 is a flowchart of studies assessed and excluded at various stages of the review.
Figure 1.
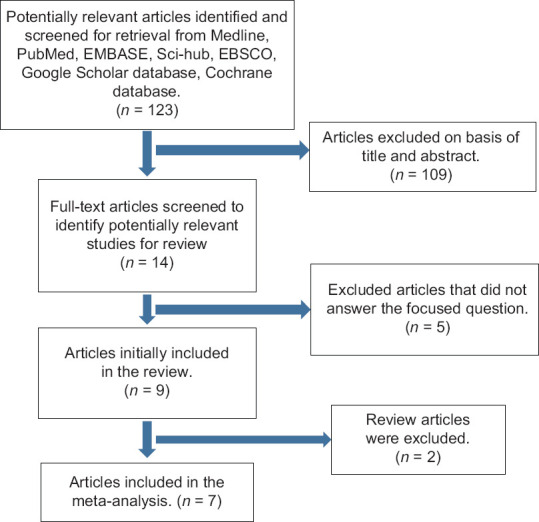
Flowchart of manuscript screened through the review process
Characteristics of the included studies
The seven included studies which fulfilled the eligibility criteria were clinical and either performed at universities or at oral health-care centers.
The number of participants ranged between 13 and 113 individuals and all were either CP or aggressive periodontitis patients. All individuals were adults and their age ranged between 20 and 72 years.
Most of the studies that fulfilled eligibility criteria were randomized controlled clinical trials. All the seven included studies for meta-analysis received SRP and subgingival application of ozone in different forms according to the test and control groups as divided by the examiner.
Outcome measures
The researchers observed changes in PPD, CAL, PI, GI, and BOP during the course of each study. Most of the investigators reported a statistically significant gain in CAL, PPD, BOP, PI, and GI.
In the meta-analysis of seven studies, comparing mechanical debridement alone and mechanical debridement + ozone-treated group, the results indicated a statistically significant reduction in PPD with the weighted mean difference of 0.03 (95% CI, −0.44, 0.50) and random effect model estimate of 0.0286. Reduction in PI, GI, and CAL was also seen with a weighted mean difference of −0.23 (95% CI, −0.64, 0.19), −0.45 (95% CI, −1.37, 0.46), and −0.26 (95% CI, −0.89, 0.37), respectively, with random effect model estimate of −0.226, −0.454, and −0.263, respectively.
Discussion
In all of the seven studies analyzed in this review, the results showed that application of ozone in topical form reduced clinical parameters such as GI, PI, PPD, and CAL [Table 1].
Table 1.
Characteristics of included studies
| Author | Purpose | Methods | Patients | Interventions and number of patients treated per group | Results | Sites and funding |
|---|---|---|---|---|---|---|
| 1. M. Y. M. Shoukheba et al. (2014) | To evaluate the effect of subgingival application of ozonated olive oil gel as an adjunct to SRP in aggressive periodontitis | Randomized controlled clinical trial, 6-month duration | Thirty patients with localized aggressive periodontitis (21 females and 9 males) whose ages ranged from 21 to 30 years were selected | Group I (control group): 15 patients received oral hygiene instructions and SRP of all teeth Group II (test group): 15 patients received oral hygiene instructions, SRP of all teeth in addition to subgingival application of an ozonated olive oil gel (Oxaktiv gel) |
Changes in PI, GI, BOP, probing depth, and CAL at 6 months | Patients were selected from Periodontology Department Clinic, Faculty of Dentistry, Tanta University |
| 2. Gianluca Sacco et al. (2016) | To evaluate the clinical and microbiological effectiveness of local oxygen-ozone therapy in the treatment of patients with chronic periodontal disease | Randomized clinical trial, 6-month duration | 113 patients aged over 30 years, with CP | Case group: 56 patients (25 males, 31 females) received mechanical therapy + local oxygen-ozone therapy Control group: 57 patients (17 males and 40 females) received MPT alone |
Significant reduction in probing depth and BOP | The study was conducted at the Institute of Clinical Dentistry at the University of Sassari, Italy |
| 3. Durga Kshitish and Vandana K Laxman (2010) | To evaluate and compare the effects of oral irrigation with ozonated water and 0.2% chlorhexidine on clinical parameters such as PI, GI, and Gingival Bleeding Index | Randomized, double-blind, crossover, split-mouth design, 18-day duration | Sixteen patients suffering from generalized CP and aggressive periodontitis, aged 20-60 years | The study period of 18 days was divided into two time intervals, i.e., baseline (0 day) to the 7th day, with a washout period of 4 days followed by a second time interval of 7 days | Reduction in PI, GI, and bleeding index was observed | University based |
| 4. Selcuk Yilmaz et al. (2013) | To evaluate the clinical and microbiological results of treatment with Er:YAG laser and topical gaseous ozone application as adjuncts to initial periodontal therapy in CP patients | Randomized controlled clinical trial, 3-month duration | Thirty systemically healthy CP patients between 37 and 67 years of age | Group 1: An adjunctive disinfection procedure was applied with an Er:YAG laser after SRP Group 2: Topical gaseous O3 was applied twice a week for 2 weeks after SRP Group 3: Only the initial periodontal therapy was performed, without any adjunctive procedure |
Reduction in PD and RAL was observed | University based and supported by the Department of Periodontology, Faculty of Dentistry, Yeditepe University |
| 5. Skurska A et al. | To compare the clinical status and salivary MMP levels after SRP alone or with ozonotherapy in patients with aggressive and CP | Randomized controlled clinical study, 2-month duration | Fifty-two generally healthy controls with CP or aggressive periodontitis (35 women and 17 men), aged 25-68 years | Group CP-S: 12 patients with CP (10 women and 2 men), aged 43-66 years, underwent SRP procedure Group CP-O: 25 patients with CP (18 women and 7 men), aged 39-68 years, underwent SRP in combination with ozonotherapy Group AP: 15 patients with aggressive periodontitis (7 women and 8 men), aged 25-44 years, underwent SRP in combination with ozonotherapy Control group (C): 14 generally healthy controls (10 women and 4 men), aged 41-80 years, with no periodontal lesions |
Significant reduction in PI, approximal PI, BOP, PPD, Sulcular Bleeding Index, and clinical attachment loss was observed | University based and supported by the institutional ethical committee |
| 6. R Al Habashneh, W. Alsalman, Y. Khader (2014) | To determine the clinical and biological effects of the adjunctive use of ozone in nonsurgical periodontal treatment | A randomized controlled clinical trial, 3-month duration | Forty-one patients with CP, aged 23-65 years | Group 1 (control group): 21 patients received nonsurgical treatment in addition to irrigation of pockets with distilled water Group 2 (test group): twenty patients received nonsurgical treatment in addition to irrigation of pockets with ozonated water |
Significant reduction in PPD, BOP, and clinical attachment loss was observed | University based supported by Dental Teaching Center at Jordan University of Science and Technology for treatment and follow-up |
| 7. Sae Hayakumo et al. (2013) | To evaluate the clinical and microbiological effects of NBW3 irrigation as an adjunct to subgingival debridement for periodontal treatment | Single-center, placebo-controlled, randomized trial, 2-month duration | Twenty-two healthy, nonsmoking controls, 6 females and 16 males aged 26-72 years | The patients in this study were subjected to one of the two treatment protocols: full-mouth mechanical debridement with tap water in a single visit (WATER) or full-mouth mechanical debridement with NBW3 in a single visit (NBW3) | Significant reduction in PPD, CAL, and BOP was observed | University based supported by the Ethics Committee of Tokyo Medical and Dental University, Tokyo, Japan (#234), and all patients signed a written informed consent form |
SRP: Scaling and root planing; CP: Chronic periodontitis; BOP: Bleeding on probing; PPD: Probing pocket depth; GI: Gingival Index; CAL: Clinical attachment level; MPT: Mechanical periodontal therapy; Er:YAG: Erbium: yttrium-aluminum-garnet; RAL: Relative attachment level; PD: Pocket depth; PI: Plaque Index; MMP: Matrix Metalloproteinase
In 2014, Shoukheba and Ali evaluated the effect of subgingival application of ozonated olive oil gel as an adjunct to SRP in aggressive periodontitis patients. The control group which consisted of 15 patients received oral hygiene instructions, SRP of all teeth, in addition to the subgingival application of an ozonated olive oil gel (Oxaktiv® gel). Clinical parameters such as PI, GI, BOP, and CAL were analyzed. The results of ozone irrigation showed improvement in all the clinical parameters in the ozone-treated group, which was maintained up to 6 months except for BOP up to 3 months.[2]
A prospective randomized clinical study was conducted by Gianluca Sacco in 2016 on 113 patients with periodontal disease. Local oxygen–ozone therapy was used in combination with traditional mechanical therapy versus the use of mechanical therapy alone in a group of patients with periodontal disease. After 6 months of root planing, the individuals who received oxygen–ozone therapy as an integral part of the periodontal treatment had significant stabilization of the clinical values. It was observed in particular that in both groups that the deepest pockets (PD > 6 mm) had the greatest decrease.[3]
Kshitish and Laxman developed a randomized, double-blind, crossover, split-mouth design. This split-mouth design was used in this study for subgingival irrigation of each half of the mouth with either ozone or chlorhexidine at different time intervals. The interpretation of clinical and microbial data is from baseline to the 7th day. The higher percentage of PI (12%), GI (29%), and Bleeding Index (26%) reduction was observed using ozone irrigation as compared to chlorhexidine.[4]
A study conducted by Yilmaz et al. evaluated the clinical and microbiological results of treatment with Er: yttrium aluminum garnet (YAG) laser and topical gaseous ozone application as adjuncts to initial periodontal therapy in CP patients. Thirty patients with CP were randomly divided into three parallel groups, each composed of ten individuals. Group 1: SRP + Er: YAG laser; Group 2: SRP + topical gaseous ozone; and Group 3: SRP alone. The clinical parameters were monitored at day 0 and day 90. Clinical parameters such as PI, SBI, PD, and relative attachment levels were measured at the end of the observation period; statistically significant improvements in clinical parameters were observed within each group.[5]
Al Habashneh et al.[6] determined the clinical and biological effects of the adjunctive use of ozone in nonsurgical periodontal treatment. Forty-one patients with CP were randomized to treatment with either subgingival SRP followed by irrigation with ozonated water for the test group or subgingival SRP followed by irrigation with distilled water irrigation for the control group. The parameters such as PI, GI, BOP, PPD, gingival recession, and clinical attachment loss were evaluated at baseline and at 3 months and statistically significant improvement was observed in the study parameters in both groups at baseline and 3 months, except for GI.
In a randomized study, Sae Hayakamo et al. evaluated the clinical and microbiological effects of NBW3 irrigation as an adjunct to subgingival debridement for periodontal treatment. Twenty-two patients were randomly assigned to one of the following two treatment groups: full-mouth mechanical debridement with tap water (WATER) or full-mouth mechanical debridement with NBW3 (NBW3). Full-mouth clinical measurements of PPD, CAL, and the percentage of BOP (%) were recorded at baseline and at 4 and 8 weeks after treatment. There were significant improvements in all the clinical parameters after 4 weeks in both groups. The reduction in the PPD and the clinical attachment gain after 4 and 8 weeks in the NBW3 group was significantly greater than that in the WATER group. BOP (%) was reduced by 15.69 and 8.98 at 4 weeks and 13.47 and 6.97 at 8 weeks in the NBW3 and WATER groups, respectively.[7]
Conclusion
Local ozone application can serve as a potential atraumatic, promising antimicrobial agent to treat periodontal disease nonsurgically, both for home care and professional practice. It may serve as a good tool during supportive periodontal therapy. Ozone may be considered as an alternative management strategy due to its powerful ability to inactivate microorganisms. Thus, subgingival ozone irrigation can be successfully used as an adjunct to periodontal treatment.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Meta-Analysis (Control)
Plaque Index at the end of the study
| Random-effects model (k=4) | ||||||
|---|---|---|---|---|---|---|
| Estimate | se | Z | P | CI lower bound | CI upper bound | |
| Intercept | −0.226 | 0.211 | −1.07 | 0.283 | −0.640 | 0.187 |
Note. Tau² estimator: Restricted maximum likelihood. CI: Confidence interval
| Heterogeneity statistics | |||||||
|---|---|---|---|---|---|---|---|
| Tau | Tau² | I² | H² | R² | df | Q | P |
| 0.207 | 0.0429 (SE=0.1453) | 24% | 1.316 | . | 3.000 | 3.883 | 0.274 |
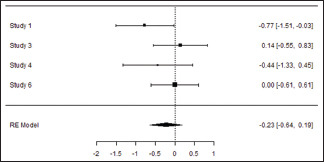
Forest plot
Publication bias assessment
| Fail-safe n analysis (file drawer analysis) | |
|---|---|
| Fail-safe n | P |
| 0.000 | 0.096 |
Note. Fail-safe n calculation using the Rosenthal approach
| Rank correlation test for funnel plot asymmetry | |
|---|---|
| Kendall’s Tau | P |
| −0.333 | 0.750 |
| Regression test for funnel plot asymmetry | |
|---|---|
| Z | P |
| −1.003 | 0.316 |
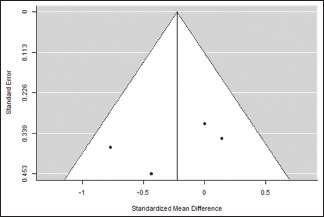
Funnel plot
Meta-analysis
Gingival Index at the end of the study
| Random-effects model (k=3) | ||||||
|---|---|---|---|---|---|---|
| Estimate | se | Z | P | CI lower bound | CI upper bound | |
| Intercept | −0.454 | 0.466 | −0.973 | 0.331 | −1.368 | 0.460 |
Note. Tau² Estimator: Restricted Maximum-Likelihood
| Heterogeneity Statistics | |||||||
|---|---|---|---|---|---|---|---|
| Tau | Tau² | I² | H² | R² | df | Q | P |
| 0.724 | 0.524 (SE=0.6527) | 80.49% | 5.125 | . | 2.000 | 10.241 | 0.006 |
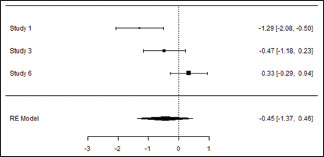
Forest Plot
Publication Bias Assessment
| Fail-Safe n Analysis (File Drawer Analysis) | |
|---|---|
| Fail-safe n | P |
| 2.000 | 0.022 |
Note. Fail-safe N Calculation Using the Rosenthal Approach
| Rank Correlation Test for Funnel Plot Asymmetry | |
|---|---|
| Kendall’s Tau | P |
| −1.000 | 0.333 |
| Regression Test for Funnel Plot Asymmetry | |
|---|---|
| Z | P |
| −3.200 | 0.001 |
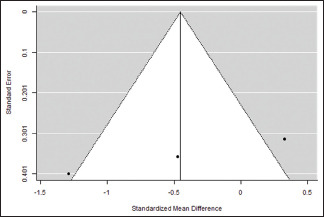
Funnel Plot
Meta-Analysis
Probing pocket depth at the end of the study
| Random-effects model (k=3) | ||||||
|---|---|---|---|---|---|---|
| Estimate | se | Z | P | CI Lower bound | CI Upper bound | |
| Intercept | 0.0286 | 0.241 | 0.119 | 0.905 | −0.443 | 0.500 |
Note. Tau² Estimator: Restricted maximum likelihood
| Heterogeneity statistics | |||||||
|---|---|---|---|---|---|---|---|
| Tau | Tau² | I² | H² | R² | df | Q | P |
| 0.291 | 0.0846 (SE=0.1792) | 48.22% | 1.931 | . | 2.000 | 3.943 | 0.139 |
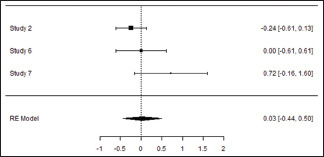
Forest plot
Publication bias assessment
| Fail-safe n analysis (file drawer analysis) | |
|---|---|
| Fail-safe n | P |
| 0.000 | 0.427 |
Note. Fail-safe n calculation using the Rosenthal approach
| Rank correlation test for funnel plot asymmetry | |
|---|---|
| Kendall’s Tau | P |
| 1.000 | 0.333 |
| Regression test for funnel plot asymmetry | |
|---|---|
| Z | P |
| 1.910 | 0.056 |
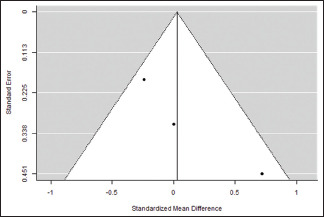
Funnel plot
Meta-Analysis
Clinical attachment loss at the end of the study
| Random-effects model (k=3) | ||||||
|---|---|---|---|---|---|---|
| Estimate | se | Z | P | CI Lower bound | CI Upper bound | |
| Intercept | −0.263 | 0.321 | −0.820 | 0.412 | −0.891 | 0.366 |
Note. Tau² estimator: Restricted maximum likelihood
| Heterogeneity statistics | |||||||
|---|---|---|---|---|---|---|---|
| Tau | Tau2 | I2 | H2 | R2 | df | Q | P |
| 0.384 | 0.1471 (SE=0.3117) | 47.43% | 1.902 | . | 2.000 | 3.840 | 0.147 |
Forest plot
Publication bias assessment
| Fail-safe n analysis (file drawer analysis) | |
|---|---|
| Fail-safe | P |
| 0.000 | 0.124 |
Note. Fail-safe n calculation using the Rosenthal approach
| Rank correlation test for funnel plot asymmetry | |
|---|---|
| Kendall's Tau | P |
| -1.000 | 0.333 |
| Regression test for funnel plot asymmetry | |
|---|---|
| Z | P |
| -0.645 | 0.519 |
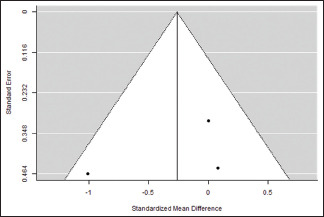
Funnel plot
References
- 1.Vinutha RS, Lakshmanan R. Ozone and Its Role in Periodontal Therapy-A Review.IOSR-JDMS 2014;13:107-10. S. 2014;13:107–10. S 2014;13: [Google Scholar]
- 2.Shoukheba MY, Ali S. The effects of subgingival application of ozonated olive oil gel in patient with localized aggressive periodontitis. A clinical and bacteriological study. Tanta Dent J. 2014;11:63–73. [Google Scholar]
- 3.Sacco G, Campus G. The treatment of periodontal disease using local oxygen-ozone. Ozone Ther. 2017;1:45–52. [Google Scholar]
- 4.Kshitish D, Laxman VK. The use of ozonated water and 0.2% chlorhexidine in the treatment of periodontitis patients: A clinical and microbiologic study. Indian J Dent Res. 2010;21:341–8. doi: 10.4103/0970-9290.70796. [DOI] [PubMed] [Google Scholar]
- 5.Yılmaz S, Algan S, Gursoy H, Noyan U, Kuru BE, Kadir T. Evaluation of the clinical and antimicrobial effects of the Er:YAG laser or topical gaseous ozone as adjuncts to initial periodontal therapy. Photomed Laser Surg. 2013;31:293–8. doi: 10.1089/pho.2012.3379. [DOI] [PubMed] [Google Scholar]
- 6.Al Habashneh R, Alsalman W, Khader Y. Ozone as an adjunct to conventional nonsurgical therapy in chronic periodontitis: a randomized controlled clinical trial. J Periodontal Res. 2015;50:37–43. doi: 10.1111/jre.12177. [DOI] [PubMed] [Google Scholar]
- 7.Hayakumo S, Arakawa S, Mano Y, Izumi Y. Clinical and microbiological effects of ozone nano-bubble water irrigation as an adjunct to mechanical subgingival debridement in periodontitis patients in a randomized controlled trial. Clin Oral Investig. 2013;17:379–88. doi: 10.1007/s00784-012-0711-7. [DOI] [PubMed] [Google Scholar]


