Abstract
The COVID-19 pandemic, the result of severe acute respiratory syndrome (SARS)-CoV-2, is a major cause of worldwide mortality with a significant cardiovascular component. While a number of different cardiovascular histopathologies have been reported at postmortem examination, their incidence is unknown, due to limited numbers of cases in any given study. A literature review was performed identifying 277 autopsied hearts across 22 separate publications of COVID-19 positive patients. The median age of the autopsy cohort was 75 and 97.6% had one or more comorbidities. Initial review of the data indicate that myocarditis was present in 20 hearts (7.2%); however, closer examination of additional reported information revealed that most cases were likely not functionally significant and the true prevalence of myocarditis is likely much lower (<2%). At least one acute, potentially COVID-19-related cardiovascular histopathologic finding, such as macro or microvascular thrombi, inflammation, or intraluminal megakaryocytes, was reported in 47.8% of cases. Significant differences in reporting of histopathologic findings occurred between studies indicating strong biases in observations and the need for more consistency in reporting. In conclusion, across 277 cases, COVID-19-related cardiac histopathological findings, are common, while myocarditis is rare.
Keywords: COVID-19, SARS-CoV-2, autopsy, myocarditis, heart, inflammation
1. Background
The severe acute respiratory syndrome (SARS)-CoV-2 coronavirus causing COVID-19 was initially detected in Wuhan, China at the end of 2019 and has now caused a global pandemic resulting in the loss of over 1 million lives [1]. The initial clinical reports described a severe pneumonia and cardiovascular disease, although most organs were reported to be involved in some capacity. The cardiovascular morbidity was based on clinical, radiological, and laboratory measurements [2]. As autopsies were generally not performed, or at least not published, from the earliest Wuhan experience, many of the concepts concerning SARS-CoV-2 and cardiovascular disease were made in the absence of histopathological review.
Among the commonly described cardiovascular complications of SARS-CoV-2 were myocarditis, stroke, other thrombotic events, and myocardial infarction [3,4]. Later cardiovascular concerns included a systemic inflammatory response involving the endothelium and a rare, but serious, multisystem inflammatory syndrome in children that involved the entire vasculature [5].
Potentially concerning cardiovascular disease data has also come from early COVID-19 survivor studies. One study suggested that after 2+ months post-SARS-CoV-2 positivity that 78% of survivors had lingering heart disease, of which 60% had myocarditis [6]. A preprint, also looking at health-care workers >2 months after disease resolution suggested rates of myocarditis, myopericarditis, and pericarditis at 26%, 11%, and 4% respectively. Both studies relied on cardiac magnetic resonance imaging (MRI) and hsTnT for these diagnoses [7].
All of these clinical and radiological findings would benefit from the definitive diagnoses available through complete and well-performed postmortem examinations. Fortunately, there have been a number of small to medium sized autopsy case series that have documented cardiovascular diseases. While the depth and quality of reporting may vary across studies, a wide range of histopathologies have been described in these COVID-19 fatalities, from which general concepts of the disease can be learned. However, when findings are reported in small numbers of samples, it has not been possible to determine the true frequencies of their occurrence. For that, ideally hundreds of cases should be used. This manuscript is an attempt to collect the cardiovascular findings summed from all current reported autopsy series, such that reasonable rates of various histopathologic diagnoses can be obtained based on a significant sample size. This data demonstrates a wide collection of histopathologies, a concerning study-to-study difference on what histopathologies are reported, and overall low rates of myocarditis.
2. Material and methods
2.1. Cohort collection
A literature review was performed of the terms “COVID-19” and “autopsy” or “postmortem” at both Google Scholar and PubMed limited to the year 2020 searched before September 24, 2020. Additional COVID-19 autopsy manuscripts were identified based on the citations of these manuscripts. This resulted in 22 studies with at least 2 autopsies reported (Supplemental Table 1) [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29]. Individual case reports were excluded. Epidemiological, clinical, and autopsy finding data, as available, was compiled into a single table (Supplemental Table 2). Like terms were merged (e.g., chronic renal failure, end stage renal disease, and renal insufficiency) across the cohorts as disease descriptions were variable by study. The term “heart disease” captured atrial fibrillation, ischemic cardiomyopathy, heart failure, coronary stenting, valvular disease, and other rarer entities. Similar terms “apoptotic bodies,” “single cell ischemia,” and “single cell necrosis” were collapsed into “single cell ischemia.”
Two studies utilized 12 of the same patients and this data was reported through the larger study [11,16]. Three SARS-CoV-2 viral PCR negative, but presumed COVID-19+ cases, were excluded from one study [17], but in a second study a “nondefinitive” SARS-CoV-2 could not be removed among 10 autopsies as the data was not available per case [14]. In one series, a heart was not evaluated in one autopsy and that case was removed [15]. Days from diagnosis to death were variably reported and in some reports, only hospitalized days were noted. Thus, the longest time course for each subject was included, when reported, but likely undercounts the full-time course of disease.
Autopsies were considered complete if either the authors reported as such (“complete” or “complete, no brain”) or if organ findings were described from above and below the diaphragm in the report. This was to compare against “in situ” or “core biopsy” types of autopsy.
2.2. Data organization and adjudication
The data reporting was variable by study and an attempt to reach the corresponding author of many studies was made to determine if additional epidemiological and clinical information clarification (e.g., sex, body mass index (BMI), postmortem interval, and heart weight) could be provided to improve the strength of the dataset. Additionally, some reports that represented extreme outliers for the frequency of certain cardiovascular findings and an attempt was made to adjudicate, with the corresponding author, an attempt to properly report on the case findings.
2.3. Statistics
A 2-sided Fisher's Exact Test, performed in R (version 3.5.3), was used to determine skew in reporting of myocarditis cases across publications. A 2-tailed t test (assuming unequal variances), performed in Excel, was performed on BMI data.
3. Results
3.1. Patient demographics
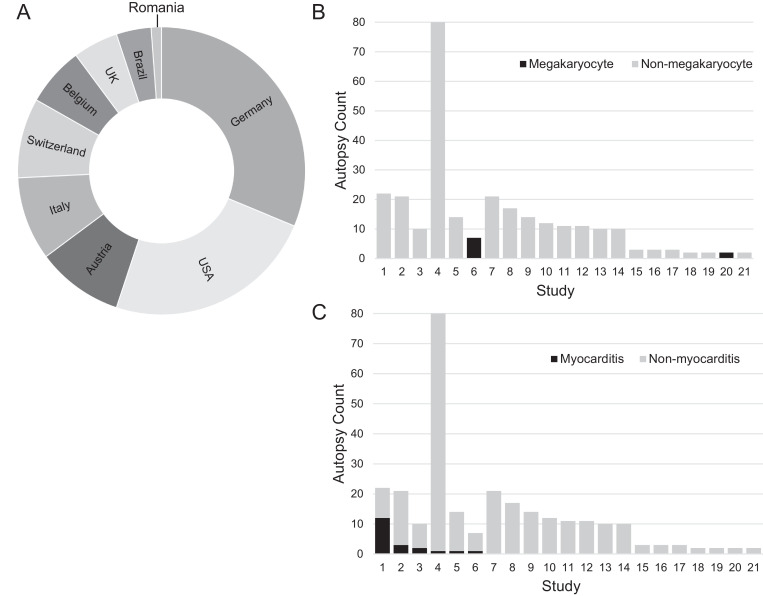
Autopsy cases were obtained from 22 separate manuscripts spanning the experiences from 10 countries (Fig. 1 A). The number of cases per manuscript ranged from 2 to 80 (median 10.5) and 7 of these manuscripts covering 165 subjects were reported as “consecutive autopsies” series, removing potential selection biases (Table 1 ). There were 293 total cases but 16 were removed (including the duplication of an entire publication) for a final cohort of 277 hearts. The cohort was 172 men (62%) and 105 women with a median age of 75 (range 22-97). Of the autopsies where completeness was reported or could be inferred, 18 were minimally invasive, 4 were partial and 221 were complete (± brain). The median number of days from diagnosis to fatality was 10 (range 1-51 days).
Fig. 1.
Autopsy cohort information. (A) Relative number of the 277 autopsy cases from each country (the distribution of cases from a consortium could not be determined, but included the Netherlands, Italy and USA). (B) The presence of intravascular megakaryocytes are only described in 2 cases. (C) Although myocarditis was diagnosed in multiple studies, the overall prevalence was significantly skewed due to 12 reports in one study.
Table 1.
Demographic and histopathologic findings
| Study characteristics | Median/Count | Percent | Available data points |
|---|---|---|---|
| Autopsied hearts | 277 | ||
| Reports | 22 | ||
| Consecutive autopsies | 165 | 73.0% | 226 |
| Demographics | |||
| Male | 172 | 62.1% | 277 |
| Age | 75 (range 22-97) | 254 | |
| BMI | 26.9 (range 14.9-59) | 139 | |
| Disease length1 | 10 days (range 1-51) | 167 | |
| Heart disease | 153 | 55.2% | 277 |
| Hypertension | 152 | 54.9% | 277 |
| Diabetes2 | 89 | 32.1% | 277 |
| Obesity | 44 | 15.9% | 277 |
| COPD/emphysema | 70 | 25.3% | 277 |
| Renal disease | 51 | 18.4% | 277 |
| Malignancy | 44 | 15.9% | 277 |
| Sleep apnea | 12 | 4.3% | 277 |
| Dementia | 34 | 12.3% | 277 |
| Cardiovascular findings | |||
| Heart weight | 483 gm (range 250-1070) | 73 | |
| Cardiac amyloidosis | 11 | 4.0% | 277 |
| Myocarditis | 20 | 7.2% | 277 |
| Pericarditis | 19 | 6.9% | 277 |
| Nonmyocarditis inflammation | 35 | 12.6% | 277 |
| Single cell ischemia | 38 | 13.7% | 277 |
| Small vessel thrombi | 30 | 10.8% | 277 |
| Macrothrombi | 53 | 19.1% | 277 |
| Intravascular megakaryocytes | 9 | 3.2% | 277 |
| Acute myocardial infarction | 13 | 4.7% | 277 |
Key
Mimimal time from diagnosis/symptoms to death.
Both type I and type II.
3.2. Cardiovascular co-morbidity data
A number of co-morbidities were common in this SARS-CoV-2+ cohort. Hypertension was reported in 152 individuals. Diabetes (predominately type II) was reported in 89 subjects. There were 44 reports of obesity, however, 53 subjects had a BMI of ≥30 (38%). The median BMI was 26.9, but varied significantly between US cases (34.05) and non-US cases (25.2, P=.0001, t test). All other forms of heart disease including cardiomyopathies, atrial fibrillation, coronary artery disease, and prior heart surgeries were collapsed into one group, of which 153 subjects (55%) reported at least one of these co-morbidities (Table 1).
3.3. Noncardiovascular co-morbidity data
Common noncardiovascular co-morbidities in this autopsy cohort included obstructive pulmonary disease (COPD)/emphysema (n=70), chronic renal disease (n=51), malignancy (n=44), sleep apnea (n=12), and dementia (n=34). Less frequent co-morbidities included Down Syndrome (n=3), HIV/AIDS (n=3), and lupus (n=1). Only 6 individuals had no reported co-morbidities (average age 52.3).
3.4. Cardiovascular autopsy findings
Heart weights were available on 73 hearts (26%) with the median weight being 483 gm (range 250-1070 gm). Although chronic non-COVID-19-related changes such as fibrosis and scarring were reported for many autopsies, those results are not given here. Acute myocardial infarctions were reported in 13 cases (4.7%). Myocarditis was reported in 20 cases (7.2%), pericarditis in 19 cases (6.9%), nonmyocarditis inflammation in 35 cases (12.6%), single cell ischemia in 38 cases (13.7%), small vessel thrombi in 30 cases (10.8%), intravascular megakaryocytes in 9 cases (3.2%), and amyloidosis in 11 cases (4%). In most of the reports there was very little description of these entities beyond stating their presence. For example, the single cell ischemia was described variably as “scattered individual cell myocyte necrosis,” “myocytes damage,” and “focal necrosis.” [15,20,27,28]. A macrovascular thrombus, occurring anywhere throughout the vasculature (deep vein thrombosis, pulmonary embolism, mural thrombus, cardiac vein, etc.) was noted in 53 cases (19.1%). At least one COVID-19-related cardiovascular histopathologic finding (all of the above except amyloidosis) was reported in 47.8% of cases where this could be determined. In comparing the 22 different reports, it became clear that while some histopathologic entities were seen/reported by multiple groups, many entities were reported at high levels specifically in one cohort. This unusual skewing was further evaluated.
3.5. Differences in cardiovascular findings by publication
Nine different histopathologic cardiovascular findings, predominately acute and assumed COVID-19-related, were evaluated across the 22 studies, and most demonstrated observer/reported based skewing of frequencies. The most extreme example of skewing is the presence of intraluminal megakaryocytes. Megakaryocytes within small vessels were noted in all 7 autopsy hearts of one study [22] and both hearts of a second study [18], but were not noted in any of the other 20 studies (100% vs 0%; Fig. 1B). Of 19 pericarditis cases, 13 were reported from one study [28]. Myocarditis was reported across 6 studies. However, of the 20 total cases of myocarditis, 12 were reported in a single study of 22 subjects (55% of that study; Fig. 1C) [28]. This skew in reporting was significant (P=2.3e-07, Fisher exact test, 2-sided), as an unbiased distribution of myocarditis cases, based on a rate of 7.2% would not predict that many cases from a single cohort. Amyloidosis, albeit not the result of COVID-19, was a more homogeneous finding, being reported in 5 separate studies with the highest incidence in any study being 28.5% (6 of 21 cases).
3.6. Analysis of myocarditis reporting
Due to the enormous interest in COVID-19 myocarditis, specific attention to this entity is provided. Six manuscripts reported at least one case as having myocarditis. One manuscript [11] contained 12 autopsy cases and reported “lymphocytic myocarditis was seen in the right ventricle” for one case with an accompanying picture showing one focus of notable inflammation. The cause of death was listed as pulmonary embolism and pneumonia. A second manuscript [22] contained 7 subjects and one subject “had a focal acute lymphocytic epimyocarditis.” Of note, “multiple additional sections of cardiac tissue were taken without further evidence of inflammation.” The authors suggested this finding may be nonspecific and not a cause of cardiac dysfunction. A third manuscript [19] contained 14 patients and in one “myocarditis was present with aggregates of lymphocytes surrounding necrotic myocytes.” Figures demonstrated foci of modest lymphocytic infiltrate with a damaged myocyte. Of note, that patient also tested positive for influenza A. A fourth manuscript [14] described “mild lymphomononuclear myocarditis” in 2 of 10 cases. These hearts were evaluated by a single 14 gauge TruCut needle biopsy without any images of the infiltrate provided. A fifth manuscript [28] reported myocarditis in 12 of 22 cases in tabular form without description in the text. An accompanying figure legend states “active myocarditis is characterized by mononuclear, predominantly lymphocytic infiltrate, associated with focal myocytes necrosis” and the 2 images show, at most, scant inflammation and a possible single cell necrosis. A sixth manuscript [29] reported myocarditis in 3 of 21 cases. The authors defined myocarditis as “the presence of an inflammatory infiltrate associated with myocyte injury not due to some other cause, which was present in multiple foci.” Multiple images were provided and demonstrated significant and diffuse inflammation in 2 cases and more modest inflammation in the third case, but with myocyte injury.
4. Discussion
The summed findings of 277 cardiac autopsy reports from 22 studies demonstrate modest frequencies of COVID-19-related cardiac histopathologies. This is in marked contrast to the high rate of pulmonary findings in autopsies such as diffuse alveolar damage and acute lung injury [29]. The most commonly reported cardiac findings were a nonmyocarditis inflammatory infiltrate and single cell ischemia occurring in 12.6% and 13.7% of cases respectively. Acute myocardial infarctions were noted in 4.7% of individuals and myocarditis was reported in 7.2% of cases.
In evaluating these 22 different reports, an important factor in the frequency of these histopathologies is the low level of consistency of reporting across studies and lack of a common COVID-19 presentation. Some of this relates to different autopsy methodologies used, particularly for noncardiac thrombus identification. However, extremely skewed rates of reporting for megakaryocytes in small vessels or the claim of myocarditis, indicate a need for a more rigorous approach to categorizing heart findings. The attached checklist is designed to aid pathologists in assessing heart pathologies that have been described in COVID-19 autopsies (Supplemental Table 3). It will be useful for all institutions to report positively or negatively on unusual and subtle findings such as microvascular thrombi or intravascular megakaryocytes. It is unknown how frequently these entities were not considered or missed and having a routine reporting approach may result in large changes in the frequency of some subtle findings. Regardless, across these 277 cases, no distinct histopathology of COVID-19 has yet emerged.
The low incidence of COVID-19 myocarditis across this large series stands in sharp contrast to the reports of COVID-19 survivors having a myocarditis rate of 60% as determined by cardiac MRI. Even this reported rate of 7.2% is likely a significant overstatement of the true incidence of fatal COVID-19. A desire by pathologists to identify myocarditis might have played a role. The first COVID-19 autopsy series appeared in early April, 2020. Prior to that, our understanding of cardiovascular pathology was based upon clinical and radiological findings. Those reports indicated frequent cases of myocarditis, even fulminant myocarditis [30,31]. Thus, when postmortem examinations began to occur, there was a strong desire by pathologists to validate that clinical finding and declare any inflammation as myocarditis. Unfortunately, or perhaps fortunately, it has not been the case that myocarditis is occurring at that expected high frequency, and other primary or secondary cardiovascular injuries seem more likely culprits. The descriptions of insignificant inflammation as “myocarditis” across these autopsies may have been an attempt to satisfy this expectation of disease, rather than represent a true viral myocarditis of the extent that can cause real cardiac injury or death.
Myocarditis is generally defined as the presence of an inflammatory infiltrate with adjacent myocyte injury. That infiltrate is lymphocyte predominant, particularly with viral infections. Occasionally the dominant cell type of myocarditis can be eosinophils or rarely neutrophils. There is no formal definition of myocarditis for autopsy studies thus a wide range of histopathologies can be included under the umbrella designation of “myocarditis.” The Dallas criteria to diagnose myocarditis were developed for endomyocardial biopsies although they have been used in the setting of autopsy [32]. It is thought that many elderly hearts contain small collections of tissue lymphocytes. These small foci of inflammatory cells are not sufficient evidence of myocarditis without necrosis [33]. There is a chance of identifying one of these sites on any random section and these should not be overcalled as myocarditis. That is likely one reason a distinction was made by some of the authors between calling their findings myocarditis and nonmyocarditis inflammation. A myocarditis that is sufficient to cause cardiac dysfunction is expected to be diffuse or multifocal with a significant amount of infiltrate and myocyte injury. Of note, a reinvestigation of autopsy-reported myocarditis deaths in Finland, using the Dallas criteria adjudicated only 32% (46 of 142) of the cases as true myocarditis, indicating a significant over-diagnosis rate [32].
There were 20 reports of myocarditis in this cohort. However, the evidence supporting 16 of these are questionable. If those cases were reported as nonspecific inflammatory infiltrates [33], the incidence of myocarditis falls to 1.4%. Even among these remaining 4, it is not clear from the manuscripts that COVID-19 myocarditis was determined as the cause of death [29]. Considering the SARS pandemic (>8,000 infected) caused no known myocarditis cases [34] and the MERS epidemic (>2,000) caused only a single MRI-diagnosed case of myocarditis [35], this seems to be a rate more typical of severe coronaviruses. Even a low myocarditis rate of ∼1.4% among fatal cases would still predict hundreds of thousands of worldwide cases of myocarditis in severe COVID-19 due to the enormous numbers of infected individuals. Low rates of myocarditis do not indicate that SARS-CoV-2 individuals are not having cardiovascular problems, but rather those complications are likely due to other stressors such as endothelial cell activation, cytokine storms, or electrolyte imbalances. It will be incumbent on our colleagues in radiology to better interpret the meaning of cardiac MRI changes and other study data in light of this low incidence of histopathologic myocarditis [6,36].
There are multiple limitations to this study. As stated, a range of histopathologic and clinical descriptions were used by the different authors and some collapsed terminology may not be directly the same between manuscripts. The study is reliant on each pathologist adequately evaluating the autopsy hearts, which may have been secondary considerations in some studies. Consistent with this concern, the one study authored by members of the two main cardiovascular pathology societies identified significantly more subtle histopathology than many other studies [29]. As studies focused on different aspects of the disease, some data points were missing or collapsed across multiple individuals. Only 142 cases were of “consecutive study” design, thus some biases known (e.g., nonacute lung injury only cases) and unknown may impact on the autopsies included. This study did not include any cases of multisystem inflammatory syndrome in children, which by case report [37] and personal experience, has an exuberant endotheliitis/myocarditis picture. This analysis did not aggregate ultrastructural (electron microscopy) findings or rates of positive viral PCR on heart tissue. Finally, if only a handful of the “myocarditis” cases are to be believed as symptomatic COVID-19, hundreds of additional cases will be needed to further clarify the incidence rate of “COVID-19 myocarditis.”
In conclusion, there is, as yet, no specific, reproducible histopathology of COVID-19 myocardial injury at autopsy and rates of fatal myocarditis are low.
Declaration of Competing Interest
The authors report no conflicts of interest.
Acknowledgments
The authors thank Joseph J. Maleszewski for helpful comments.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.carpath.2020.107300.
Appendix. Supplementary materials
References
- 1.Wolfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Muller MA, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 2.Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(7):811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klok FA, Kruip M, van der Meer NJM, Arbous MS, Gommers D, Kant KM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riollano-Cruz M, Akkoyun E, Briceno-Brito E, Kowalsky S, Posada R, Sordillo EM, et al. Multisystem inflammatory syndrome in children (MIS-C) related to COVID-19: a New York City experience. J Med Virol. 2020:1–10. doi: 10.1002/jmv.26224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from Coronavirus Disease 2019 (COVID-19) JAMA Cardiol. 2020:E1–E9. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eiros R, Barreiro-Perez M, Martin-Garcia A, Almeida J, Villacorta E, Perez-Pons A, et al. Pericarditis and myocarditis long after SARS-CoV-2 infection: a cross-sectional descriptive study in health-care workers. medRxiv. 2020 doi: 10.1101/2020.07.12.20151316. https://doi.org/ [DOI] [Google Scholar]
- 8.Barton LM, Duval EJ, Stroberg E, Ghosh S, Mukhopadhyay S. COVID-19 autopsies, Oklahoma, USA. Am J Clin Pathol. 2020;153(6):725–733. doi: 10.1093/ajcp/aqaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menter T, Haslbauer JD, Nienhold R, Savic S, Hopfer H, Deigendesch N, et al. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology. 2020;77(2):198–209. doi: 10.1111/his.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wichmann D, Sperhake JP, Lutgehetmann M, Steurer S, Edler C, Heinemann A, et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med. 2020;173(4):268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lax SF, Skok K, Zechner P, Kessler HH, Kaufmann N, Koelblinger C, et al. Pulmonary arterial thrombosis in COVID-19 with fatal outcome: results from a prospective, single-center, clinicopathologic case series. Ann Intern Med. 2020;173(5):350–361. doi: 10.7326/M20-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buja LM, Wolf DA, Zhao B, Akkanti B, McDonald M, Lelenwa L, et al. The emerging spectrum of cardiopulmonary pathology of the coronavirus disease 2019 (COVID-19): Report of 3 autopsies from Houston, Texas, and review of autopsy findings from other United States cities. Cardiovasc Pathol. 2020;48 doi: 10.1016/j.carpath.2020.107233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nunes Duarte-Neto A, de Almeida Monteiro RA, da Silva LFF, Malheiros D, de Oliveira EP, Theodoro Filho J, et al. Pulmonary and systemic involvement of COVID-19 assessed by ultrasound-guided minimally invasive autopsy. Histopathology. 2020;77(2):186–197. doi: 10.1111/his.14160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fox SE, Akmatbekov A, Harbert JL, Li G, Quincy Brown J, Vander Heide RS. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med. 2020;8(7):681–686. doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edler C, Schroder AS, Aepfelbacher M, Fitzek A, Heinemann A, Heinrich F, et al. Dying with SARS-CoV-2 infection-an autopsy study of the first consecutive 80 cases in Hamburg, Germany. Int J Legal Med. 2020;134(4):1275–1284. doi: 10.1007/s00414-020-02317-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Youd E, Moore L. COVID-19 autopsy in people who died in community settings: the first series. J Clin Pathol. 2020:1–5. doi: 10.1136/jclinpath-2020-206710. [DOI] [PubMed] [Google Scholar]
- 18.Tombolini A, Scendoni R. SARS-CoV-2-related deaths in routine forensic autopsy practice: histopathological patterns. Int J Legal Med. 2020;134(6):2205–2208. doi: 10.1007/s00414-020-02354-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bradley BT, Maioli H, Johnston R, Chaudhry I, Fink SL, Xu H, et al. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. Lancet. 2020;396(10247):320–332. doi: 10.1016/S0140-6736(20)31305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fox SE, Li G, Akmatbekov A, Harbert JL, Lameira FS, Brown JQ, et al. Unexpected features of cardiac pathology in COVID-19 Infection. Circulation. 2020;142(11):1123–1125. doi: 10.1161/CIRCULATIONAHA.120.049465. [DOI] [PubMed] [Google Scholar]
- 21.Grimes Z, Bryce C, Sordillo EM, Gordon RE, Reidy J, Paniz Mondolfi AE, et al. Fatal pulmonary thromboembolism in SARS-CoV-2-infection. Cardiovasc Pathol. 2020;48 doi: 10.1016/j.carpath.2020.107227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rapkiewicz AV, Mai X, Carsons SE, Pittaluga S, Kleiner DE, Berger JS, et al. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: A case series. EClinicalMedicine. 2020;24 doi: 10.1016/j.eclinm.2020.100434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grosse C, Grosse A, Salzer HJF, Dunser MW, Motz R, Langer R. Analysis of cardiopulmonary findings in COVID-19 fatalities: High incidence of pulmonary artery thrombi and acute suppurative bronchopneumonia. Cardiovasc Pathol. 2020;49 doi: 10.1016/j.carpath.2020.107263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Remmelink M, De Mendonca R, D'Haene N, De Clercq S, Verocq C, Lebrun L, et al. Unspecific post-mortem findings despite multiorgan viral spread in COVID-19 patients. Crit Care. 2020;24(1):495. doi: 10.1186/s13054-020-03218-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanley B, Naresh KN, Roufosse C, Nicholson AG, Weir J, Cooke GS, et al. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. Lancet Microbe. 2020;1(6):e245–e253. doi: 10.1016/S2666-5247(20)30115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oprinca GC, Muja LA. Postmortem examination of three SARS-CoV-2-positive autopsies including histopathologic and immunohistochemical analysis. Int J Legal Med. 2020:1–11. doi: 10.1007/s00414-020-02406-w. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Michele S, Sun Y, Yilmaz MM, Katsyv I, Salvatore M, Dzierba AL, et al. Forty postmortem examinations in COVID-19 Patients. Am J Clin Pathol. 2020:1–14. doi: 10.1093/ajcp/aqaa156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Falasca L, Nardacci R, Colombo D, Lalle E, Di Caro A, Nicastri E, et al. Post-mortem findings in Italian patients with COVID-19 - a Descriptive full autopsy study of cases with and without co-morbidities. J Infect Dis. 2020:1–9. doi: 10.1093/infdis/jiaa578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Basso C, Leone O, Rizzo S, De Gaspari M, van der Wal AC, Aubry MC, et al. Pathological features of COVID-19-associated myocardial injury: a multicentre cardiovascular pathology study. Eur Heart J. 2020:1–9. doi: 10.1093/eurheartj/ehaa664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ho JS, Sia CH, Chan MY, Lin W, Wong RC. Coronavirus-induced myocarditis: a meta-summary of cases. Heart Lung. 2020;49(6):681–685. doi: 10.1016/j.hrtlng.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu H, Ma F, Wei X, Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J. 2020 doi: 10.1093/eurheartj/ehaa190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kyto V, Saukko P, Lignitz E, Schwesinger G, Henn V, Saraste A, et al. Diagnosis and presentation of fatal myocarditis. Hum Pathol. 2005;36(9):1003–1007. doi: 10.1016/j.humpath.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 33.Basso C, Aguilera B, Banner J, Cohle S, d'Amati G, de Gouveia RH, et al. Guidelines for autopsy investigation of sudden cardiac death: 2017 update from the Association for European Cardiovascular Pathology. Virchows Arch. 2017;471(6):691–705. doi: 10.1007/s00428-017-2221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oudit GY, Kassiri Z, Jiang C, Liu PP, Poutanen SM, Penninger JM, et al. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Invest. 2009;39(7):618–625. doi: 10.1111/j.1365-2362.2009.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alhogbani T. Acute myocarditis associated with novel Middle east respiratory syndrome coronavirus. Ann Saudi Med. 2016;36(1):78–80. doi: 10.5144/0256-4947.2016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maleszewski JJ, Young PM, Ackerman MJ, Halushka MK. An urgent need for studies of the late effects of SARS-CoV-2 on the cardiovascular system. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.051362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fox SE, Lameira FS, Rinker EB, Vander Heide RS. Cardiac endotheliitis and multisystem inflammatory syndrome After COVID-19. Ann Intern Med. 2020:1–3. doi: 10.7326/L20-0882. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.