We read with great interest the recently published article by Abbasi-Oshaghi et al. that discussed the potential of repurposing FDA-approved drugs and other therapeutic strategies for managing COVID-19-associated deaths [1]. We would like to compliment, and add our analysis on another strategy that targets glutathione peroxidase 1 (GPX1) detoxifying system and the main protease (Mpro) of SARS-CoV-2 for treating COVID-19 patients. This is made possible by utilizing the GPX1-mimetic drug, ebselen.
The emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in China was accompanied by the need for developing specific therapeutics that can put an end to the ongoing pandemic. However, the development of a novel SARS-CoV-2-specific drug will take several years and cost billions because of the need to establish efficacy and long-term safety profiles. Therefore, the repurposing of already known drugs with well-established safety profiles can accelerate the evaluation process and shorten the gap between efficacy studies and their clinical utility. Recently, Jin et al. have assayed more than 10,000 compounds using a combination of structure-based virtual and high-throughput screening to identify efficient inhibitors of main protease (Mpro), a key enzyme of SARS-CoV-2. One of the six compounds that inhibited SARS-CoV-2 Mpro, ebselen (SPI-1005), also exhibited promising antiviral activity in cell-based assays [2]. Ebselen (2-phenyl-1,2-benzoisoselenazol-3(2H)-one) is an organoselenium compound with hydroperoxide- and peroxynitrite-reducing activity (Figure - 1 ). Biologically it acts as an enzyme mimetic, catalyzing the glutathione peroxidase reaction [3]. Ebselen has also been reported to possess antioxidant, anti-inflammatory, and cytoprotective properties [2].
Fig. 1.
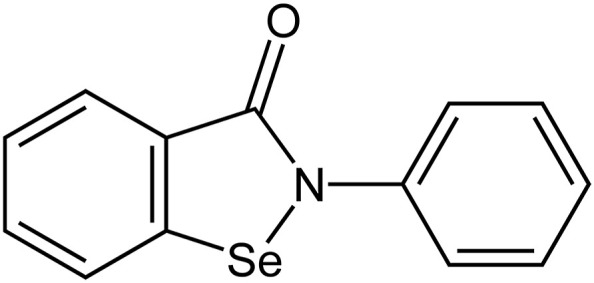
Chemical structure of ebselen, an organoselenium compound that mimics glutathione peroxidase activity.
Ebselen has been previously found to exhibit potent antiviral activity against many viruses including human immunodeficiency virus type 1 (HIV-1) [4], hepatitis C virus (HCV) [5], influenza A virus [6], and Zika virus [7]. The antiviral activity of ebselen against HIV-1 is mediated via the inhibition of HIV-1 capsid protein, which has a central role in the events leading to viral infection [8]. Furthermore, the in vitro studies have validated that the antiviral activity of ebselen can also be attributed to its ability to interferes with the binding of chromatin-associated host cell molecule and lens-epithelium-derived growth-factor (LEDGF/p75) to HIV-1 integrase resulting in failure of viral genome integration to the host cell DNA [4]. However, the antiviral action of ebselen against the hepatitis C virus (HCV) is mediated via the inhibition of NS3 helicase activity that is required for viral assembly and replication but does not inhibit the protease activity [5].
Apart from promising antiviral activity, ebselen also possess antioxidant, anti-inflammatory, antimalarial, anti-trypanosomal and cytoprotective properties indicating a broad spectrum of activity [2,9,10]. Interestingly, ebselen is also considered for the prevention of noise-induced hearing loss and bipolar disorder, currently being studied in a phase-2 trial [11,12]. Early research on ebselen documented that it has numerous targets in biological pathways with a distinct mechanism of action and can be utilized for treating different clinical conditions, attributing to its broad spectrum of activity [13].
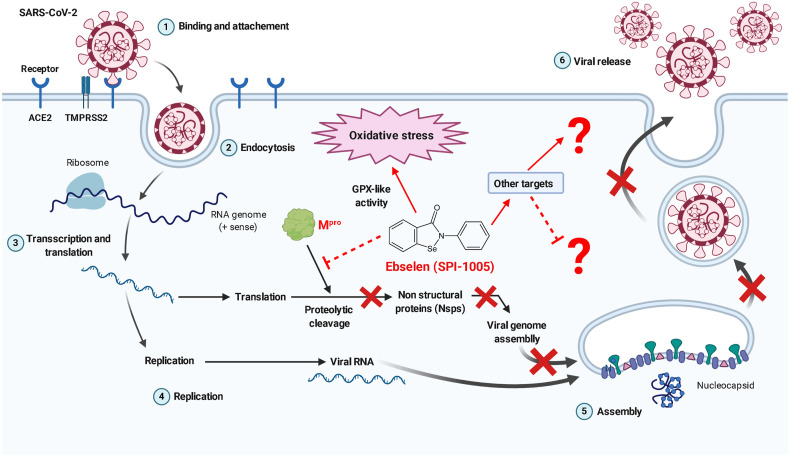
As an antiviral drug against SARS-CoV-2, ebselen acts through the inhibition of the main viral protease, Mpro [14]. The main protease is one of the crucial enzymes in the viral life cycle that plays a key role in viral replication and transcription process [15]. Inhibition of Mpro and subsequently, the nonstructural proteins (Nsps) arrests the process of viral assembly in the SARS-CoV-2 replication cycle, making it a promising drug for treating COVID-19 patients (Figure - 2 ). Studies using experimental animal models have demonstrated that ebselen, when given orally, effectively combats lung inflammation, possibly by inhibition of leukocyte infiltration, IL1β, TNFα, and inflammatory cytokines in the lungs [16]. Pre-treatment of mice with ebselen at the dose of 10 mg/kg given orally before intranasal inoculation and infection with influenza A virus (H3N2) have resulted in the significant reduction of virus titers, leukocyte count in bronchioalveolar lavage fluid, and expression of inflammatory cytokines [17]. Therefore, the anti-inflammatory activity exhibited by ebselen in the lungs can be utilized to treat COVID-19, as the primary target organ of SARS-CoV-2 is lungs. Further studies are required to evaluate the beneficial interaction of ebselen with the replication cycle of SARS-CoV-2.
Fig. 2.
Ebselen exhibits antiviral activity against SARS-CoV-2 via the inhibition of the main viral protease (Mpro).
Besides its direct antiviral activity against SARS-CoV-2, ebselen also possess excellent anti-inflammatory property owing to the thiol-mediated and peroxiredoxin-like inhibitory effects on inflammation. Ebselen is likely to exhibit potential effects in inflammatory conditions sequential to acute respiratory distress syndrome (ARDS), one of the primary reasons for mortality in COVID-19 patients [3]. Moreover, it is also reported that ebselen can correct the disturbances in iron homeostasis induced by stressful stimuli in rats [18]. The increased release of iron from the circulating ferritin in COVID-19 patients disturbs the iron homeostasis, thereby increasing the susceptibility to cytotoxicity and increased chances of mortality. Therefore, treatment with ebselen can further decrease the mortality rate in SARS-CoV-2 infection [19].
In the SARS-CoV-2 context, the proposed antiviral activity of ebselen identified based on crystallographic studies is mediated via the direct inhibition of the main protease through non-covalent binding with cysteine present in the active site of Mpro forming selenosulfide [2]. It has been also demonstrated that the ebselen at half-maximal effective concentration (EC50) value of 4.67 μM has exhibited inhibitory activity against SARS-CoV-2 in cell-based assays. Furthermore, the dose-response curve suggests it can penetrate the cell membranes and access their targets [2]. Even though the antiviral activity of ebselen is well documented based on in silico and in vitro studies, further investigation is warranted using in vivo studies with suitable animal models. Additional studies are also required to investigate whether ebselen affects capsid formation and viral replication in SARS-CoV-2, as in the case of HIV-1 or HCV [3].
It has also been proven that ebselen is capable of inhibiting liver injury induced by chemical and microbial stimuli [20]. One of the most common findings observed in severe cases of COVID-19 is liver injury [21]. Therefore, treatment with ebselen might also have added benefits in this particular aspect of the disease. Moreover, ebselen is also found to be effective in managing focal ischemic injury by decreasing IL-6 [22], which can protect SARS-CoV-2 infected patients with venous thrombosis and vascular injury [23]. Nevertheless, the findings of these previous studies give us hope for the therapeutic potential of ebselen in managing COVID-19. Further investigations are required using randomized clinical control trials before they can be included in any treatment regimen. Considering the therapeutic potential of ebselen in COVID-19, two major clinical trials have already been registered to evaluate the safety and efficacy of this repurposed drug in moderate and severe COVID-19 patients (Table 1 ).
Table 1.
Clinical trials evaluating the therapeutic efficacy and safety of ebselen (SPI-1005) in COVID-19 patients (www.clinicaltrials.gov).
| NCT No. | Title | Status | Phase | Population | Interventions |
|---|---|---|---|---|---|
| NCT04484025 | SPI-1005 Treatment in Moderate COVID-19 Patients | Not yet recruiting | Phase 2 | 60 participants (18 years and older) | Arm 1–400 mg BID orally for 7 days Arm 2–800 mg BID orally for 7 days |
| NCT04483973 | SPI-1005 Treatment in Severe COVID-19 Patients | Not yet recruiting | Phase 2 | 60 participants (18 years and older) | Arm 1–400 mg BID orally for 7 days Arm 2–800 mg BID orally for 7 days |
Although ebselen exhibits potential antiviral activity against SARS-CoV-2, its effectiveness can be impeded by certain unknown factors and therefore require further studies. For successful therapeutic use, ebselen must attain therapeutic plasma concentration sufficient to exert antiviral action. The inhibition of Mpro can be efficiently increased via the combination of ebselen with N-acetyl cysteine, a drug with cytoprotective effects that can act against SARS-CoV-2 by inhibiting virus replication, attributing to its synergistic action against the virus [24,25]. Since ebselen already possess antiviral activity against several viruses and exhibits potent antiviral activity against SARS-CoV-2 via Mpro inhibition [2], assuming the same is realized in the in vivo and clinical studies, repurposing it for SARS-CoV-2 treatment seems to be a reasonable option [3]. Therefore, ebselen can be considered as a potential therapeutic candidate for COVID-19 patients. However, before including it in the treatment guidelines and widespread use as a potential antiviral drug, further studies should be undertaken to establish its efficacy using in vitro, in vivo, and clinical studies.
Ethical approval
Not applicable.
Source of funding
The authors received no funding in relation to this article.
Author contribution
All authors equally contributed to the analysis and writing of the manuscript.
Trial registry number
1. Name of the registry: Not applicable.
2. Unique Identifying number or registration ID: Not applicable.
3. Hyperlink to your specific registration (must be publicly accessible and will be checked): Not applicable.
Guarantor
Khan Sharun, Division of Surgery, ICAR-Indian Veterinary Research Institute, Izatnagar, Bareilly, Uttar Pradesh, India Email: sharunkhansk@gmail.com.
Provenance and peer review
Not Commissioned, internally reviewed.
Data statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.
Declaration of competing interest
All authors declare that there exist no commercial or financial relationships that could, in any way, lead to a potential conflict of interest.
Acknowledgements
None.
References
- 1.Abbasi-Oshaghi E., Mirzaei F., Farahani F., Khodadadi I., Tayebinia H. Diagnosis and treatment of coronavirus disease 2019 (COVID-19): laboratory, PCR, and chest CT imaging findings. Int. J. Surg. 2020 Jul;79:143–153. doi: 10.1016/j.ijsu.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jin Z., Du X., Xu Y., Deng Y., Liu M., Zhao Y., Zhang B., Li X., Zhang L., Peng C., Duan Y., Yu J., Wang L., Yang K., Liu F., Jiang R., Yang X., You T., Liu X., Yang X., Bai F., Liu H., Liu X., Guddat L.W., Xu W., Xiao G., Qin C., Shi Z., Jiang H., Rao Z., Yang H. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature. 2020 Jun;582(7811):289–293. doi: 10.1038/s41586-020-2223-y. [DOI] [PubMed] [Google Scholar]
- 3.Sies H., Parnham M.J. Potential therapeutic use of ebselen for COVID-19 and other respiratory viral infections. Free Radic. Biol. Med. 2020 Aug 20;156:107–112. doi: 10.1016/j.freeradbiomed.2020.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang D.W., Yan H.L., Xu X.S., Xu L., Yin Z.H., Chang S., Luo H. The selenium-containing drug ebselen potently disrupts LEDGF/p75-HIV-1 integrase interaction by targeting LEDGF/p75. J. Enzym. Inhib. Med. Chem. 2020 Dec;35(1):906–912. doi: 10.1080/14756366.2020.1743282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mukherjee S., Weiner W.S., Schroeder C.E., Simpson D.S., Hanson A.M., Sweeney N.L., Marvin R.K., Ndjomou J., Kolli R., Isailovic D., Schoenen F.J., Frick D.N. Ebselen inhibits hepatitis C virus NS3 helicase binding to nucleic acid and prevents viral replication. ACS Chem. Biol. 2014 Oct 17;9(10):2393–2403. doi: 10.1021/cb500512z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haddad el-B, McCluskie K., Birrell M.A., Dabrowski D., Pecoraro M., Underwood S., Chen B., De Sanctis G.T., Webber S.E., Foster M.L., Belvisi M.G. Differential effects of ebselen on neutrophil recruitment, chemokine, and inflammatory mediator expression in a rat model of lipopolysaccharide-induced pulmonary inflammation. J. Immunol. 2002 Jul 15;169(2):974–982. doi: 10.4049/jimmunol.169.2.974. [DOI] [PubMed] [Google Scholar]
- 7.Simanjuntak Y., Liang J.J., Chen S.Y., Li J.K., Lee Y.L., Wu H.C., Lin Y.L. Ebselen alleviates testicular pathology in mice with Zika virus infection and prevents its sexual transmission. PLoS Pathog. 2018 Feb 15;14(2) doi: 10.1371/journal.ppat.1006854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thenin-Houssier S., de Vera I.M., Pedro-Rosa L., Brady A., Richard A., Konnick B., Opp S., Buffone C., Fuhrmann J., Kota S., Billack B., Pietka-Ottlik M., Tellinghuisen T., Choe H., Spicer T., Scampavia L., Diaz-Griffero F., Kojetin D.J., Valente S.T. Ebselen, a small-molecule capsid inhibitor of HIV-1 replication. Antimicrob. Agents Chemother. 2016 Mar 25;60(4):2195–2208. doi: 10.1128/AAC.02574-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hüther A.M., Zhang Y., Sauer A., Parnham M.J. Antimalarial properties of ebselen. Parasitol. Res. 1989;75(5):353–360. doi: 10.1007/BF00931130. [DOI] [PubMed] [Google Scholar]
- 10.Lu J., Vodnala S.K., Gustavsson A.L., Gustafsson T.N., Sjöberg B., Johansson H.A., Kumar S., Tjernberg A., Engman L., Rottenberg M.E., Holmgren A. Ebsulfur is a benzisothiazolone cytocidal inhibitor targeting the trypanothione reductase of Trypanosoma brucei. J. Biol. Chem. 2013 Sep 20;288(38):27456–27468. doi: 10.1074/jbc.M113.495101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kil J., Lobarinas E., Spankovich C., Griffiths S.K., Antonelli P.J., Lynch E.D., Le Prell C.G. Safety and efficacy of ebselen for the prevention of noise-induced hearing loss: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2017 Sep 2;390(10098):969–979. doi: 10.1016/S0140-6736(17)31791-9. [DOI] [PubMed] [Google Scholar]
- 12.Singh N., Sharpley A.L., Emir U.E., Masaki C., Herzallah M.M., Gluck M.A., Sharp T., Harmer C.J., Vasudevan S.R., Cowen P.J., Churchill G.C. Effect of the putative lithium mimetic ebselen on brain myo-inositol, sleep, and emotional processing in humans. Neuropsychopharmacology. 2016 Jun;41(7):1768–1778. doi: 10.1038/npp.2015.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parnham M.J., Sies H. The early research and development of ebselen. Biochem. Pharmacol. 2013 Nov 1;86(9):1248–1253. doi: 10.1016/j.bcp.2013.08.028. [DOI] [PubMed] [Google Scholar]
- 14.Menendez C.A., Bylehn F., Perez-Lemus G.R., Alvarado W., de Pablo J.J. Molecular characterization of ebselen binding activity to SARS-CoV-2 main protease. Science Advances. 2020 doi: 10.1126/sciadv.abd0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zumla A., Chan J.F., Azhar E.I., Hui D.S., Yuen K.Y. Coronaviruses - drug discovery and therapeutic options. Nat. Rev. Drug Discov. 2016 May;15(5):327–347. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petronilho F., Florentino D., Silvestre F., Danielski L.G., Nascimento D.Z., Vieira A., Kanis L.A., Fortunato J.J., Badawy M., Barichello T., Quevedo J. Ebselen attenuates lung injury in experimental model of carrageenan-induced pleurisy in rats. Inflammation. 2015 Aug;38(4):1394–1400. doi: 10.1007/s10753-015-0113-5. [DOI] [PubMed] [Google Scholar]
- 17.Oostwoud L.C., Gunasinghe P., Seow H.J., Ye J.M., Selemidis S., Bozinovski S., Vlahos R. Apocynin and ebselen reduce influenza A virus-induced lung inflammation in cigarette smoke-exposed mice. Sci. Rep. 2016 Feb 15;6:20983. doi: 10.1038/srep20983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ismail H.T.H. Hematobiochemical disturbances and oxidative stress after subacute manganese chloride exposure and potential protective effects of ebselen in rats. Biol. Trace Elem. Res. 2019 Feb;187(2):452–463. doi: 10.1007/s12011-018-1395-x. [DOI] [PubMed] [Google Scholar]
- 19.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J., HLH Across Speciality Collaboration, Uk COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020 Mar 28;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koyanagi T., Nakamuta M., Enjoji M., Iwamoto H., Motomura K., Sakai H., Nawata H. The selenoorganic compound ebselen suppresses liver injury induced by Propionibacterium acnes and lipopolysaccharide in rats. Int. J. Mol. Med. 2001 Mar;7(3):321–327. doi: 10.3892/ijmm.7.3.321. [DOI] [PubMed] [Google Scholar]
- 21.Feng G., Zheng K.I., Yan Q.Q., Rios R.S., Targher G., Byrne C.D., Poucke S.V., Liu W.Y., Zheng M.H. COVID-19 and liver dysfunction: current insights and emergent therapeutic strategies. J Clin Transl Hepatol. 2020 Mar 28;8(1):18–24. doi: 10.14218/JCTH.2020.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gladilin S., Bidmon H.J., Divanach A., Arteel G.E., Witte O.W., Zilles K., Sies H. Ebselen lowers plasma interleukin-6 levels and glial heme oxygenase-1 expression after focal photothrombotic brain ischemia. Arch. Biochem. Biophys. 2000 Aug 15;380(2):237–242. doi: 10.1006/abbi.2000.1943. [DOI] [PubMed] [Google Scholar]
- 23.Wichmann D., Sperhake J.P., Lütgehetmann M., Steurer S., Edler C., Heinemann A., Heinrich F., Mushumba H., Kniep I., Schröder A.S., Burdelski C., de Heer G., Nierhaus A., Frings D., Pfefferle S., Becker H., Bredereke-Wiedling H., de Weerth A., Paschen H.R., Sheikhzadeh-Eggers S., Stang A., Schmiedel S., Bokemeyer C., Addo M.M., Aepfelbacher M., Püschel K., Kluge S. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann. Intern. Med. 2020 Aug 18;173(4):268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ezeriņa D., Takano Y., Hanaoka K., Urano Y., Dick T.P. N-acetyl cysteine functions as a fast-acting antioxidant by triggering intracellular H2S and sulfane sulfur production. Cell Chem Biol. 2018 Apr 19;25(4):447–459. doi: 10.1016/j.chembiol.2018.01.011. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang G. H2S as a potential defense against COVID-19? Am. J. Physiol. Cell Physiol. 2020 Aug 1;319(2):C244–C249. doi: 10.1152/ajpcell.00187.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]