Figure 2.
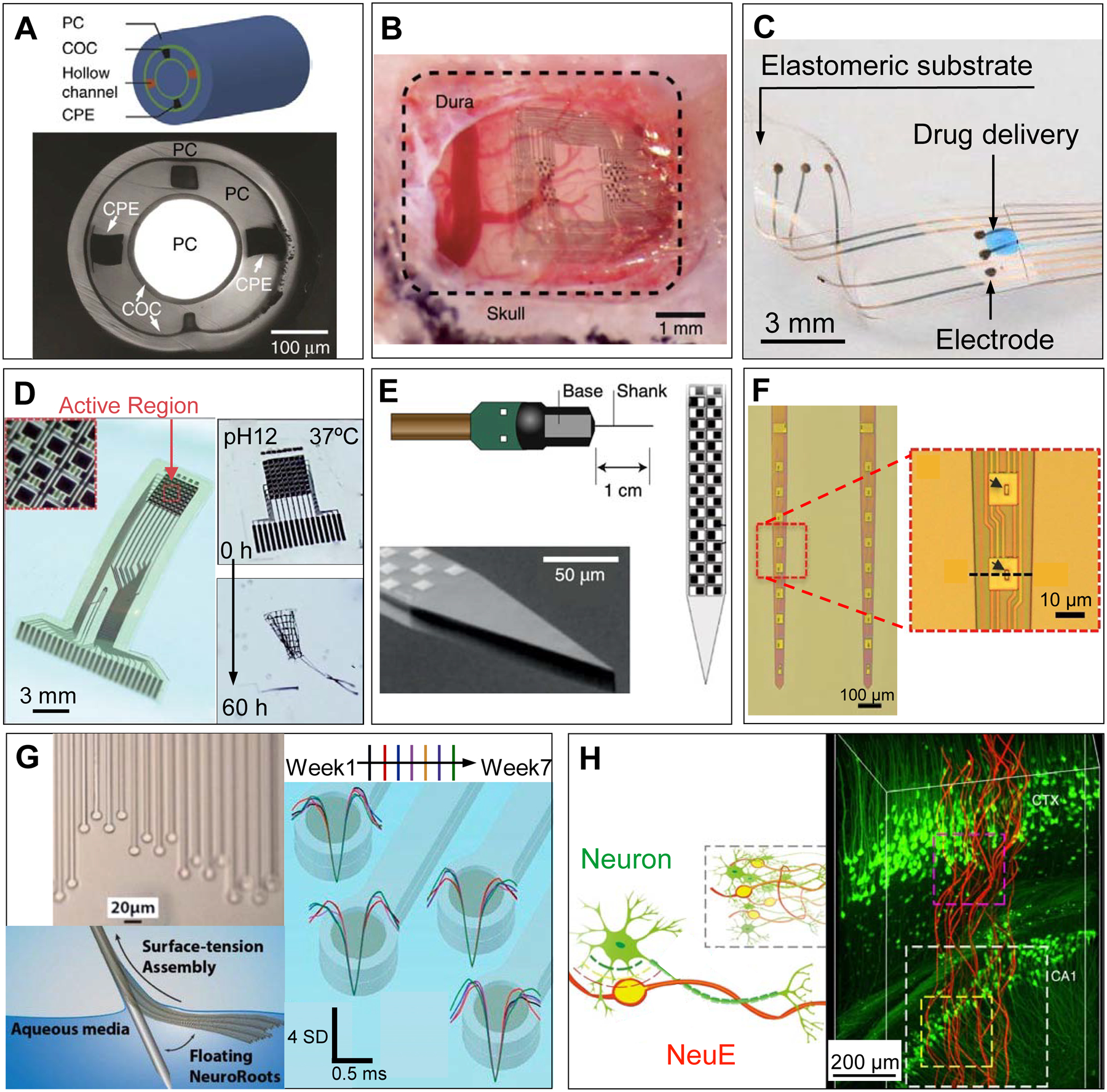
Examples of novel in vivo bioelectronic neural interfaces. (A) Multifunctional fibers, constructed via thermal drawing processes, offer a multimodal means of interacting with neurons. As demonstrated in the schematic drawing (top) and a cross-sectional image (bottom) of the multifunctional fiber, this design incorporates one cylindrical optical waveguide comprising a polycarbonate (PC) core and a cyclic olefin copolymer (COC) shell, two microfluidic channels (‘hollow channels’) for local drug delivery, and two conductive polyethylene electrodes (CPE) for recording extracellular action potentials. Adapted with permission from Ref. 50. (B) NeuroGrid features poly(3,4-ethylenedioxythiophene) polystyrene sulfonate (PEDOT:PSS) coated electrodes with sizes and interelectrode spacing inspired by the average size of neuron somata and neuronal density within the neocortex, and is capable of resolving both local field potentials (LFPs) and single unit activity when conformally interfacing the curvilinear cortical surface. Adapted with permission from Ref. 52. (C) e-dura is designed to mimic the mechanical properties of the dura mater of the brain and spinal cord. By integrating flexible silicone with thin films of conductive platinum-silicone composites and a microfluidic channel, the e-dura affords seamless integration with the spinal cord and potentially superficial regions of the brain, enabling electrical recording and stimulation in addition to local drug delivery. Adapted with permission from Ref. 51. (D) Bioresorbable silicon electronics are designed to seamlessly interface with cortical tissue for transient monitoring and modulation of brain activity (left). By integrating functional materials that can dissolve under physiological conditions, bioresorbable electronics show rapid dissolution upon immersion in an aqueous buffer solution with pH = 12 at 37 °C (right). Adapted with permission from Ref. 55. (E) Neuropixels facilitates electrophysiological recordings that demand high channel counts (left). Neuropixels incorporates 960 electrodes (layout shown in the right), with 384 active processing units at any one time. Adapted with permission from Ref. 57. (F) Ultraflexible nanoelectronic probes (shown: NET-50, left) combine reduced feature sizes of electrodes (right) and flexible materials to produce a device capable of chronically stable single unit recording. Adapted with permission from Ref. 56. (G) NeuroRoots takes inspiration from axon sizes and distribution within the brain (top left) and can be inserted into the brain through self-assembly, mediated by capillary forces, along the direction of the microwire shuttle (bottom left). Owing to NeuroRoots’ flexibility and small footprint, stable single unit recording is demonstrated for up to 7 weeks (right). Adapted with permission from Ref. 61. (H) Neuron-like electronics (NeuE) combine bioinspired feature sizes, mechanical compliance and topological properties (left) to optimize device-brain interfacing (right) and affords chronic recording stability. Adapted with permission from Ref. 63.
