Abstract
Purpose:
To investigate the effectiveness of combination treatment of vascular targeted photodynamic therapy and anti-CTLA-4 immunotherapy in a mouse model of urothelial carcinoma
Materials and Methods:
We used C57BL/6 mice injected with murine bladder 49 cell line. Mice were randomly allocated into four treatment groups: vascular targeted photodynamic therapy only, anti-CTLA-4 only, combination therapy, and control. We conducted three separate experiments that used distinct cohorts of mice: tumor growth and development of lung metastases monitored with bioluminescent imaging (n=91); survival evaluated with Kaplan-Meier curves (n=111); and tumor cell population studied with flow cytometry (n=20). In a fourth experiment, we re-challenged tumors in previously treated mice and compared tumor growth to that of naïve mice.
Results:
Combination therapy provided significant benefits over the other three treatment groups: prolonged survival (p<0.0001), lower tumor signal (p<0.0001), and decreased lung signal uptake (p≤0.002). We also observed that mice previously treated with vascular targeted photodynamic therapy only or combination therapy did not present tumor growth after re-challenged tumors.
Conclusions:
Combination of vascular targeted photodynamic therapy with anti-CTLA-4 is an effective therapy in a urothelial carcinoma syngeneic mouse model. Our results suggest this therapy as a potential treatment option for both bladder and upper tract tumors in future clinical trials.
Keywords: CTLA4 antigen, MB-49 cell line, photochemotherapy, urinary bladder neoplasms, WST11 compound
INTRODUCTION
Urothelial carcinoma (UC) is the most common urinary tract malignancy and the majority of affected patients present with non-muscle-invasive disease at diagnosis. In this disease stage, in addition to tumor resection, the intravesical injection of adjuvant BCG immunotherapy is important in decreasing tumor recurrence and progression.1 However, when this treatment is not tolerated or fails, these patients are left with no other conservative and effective options. In this scenario, the options are radical cystectomy for bladder tumors and nephroureterectomy for upper tract tumors.1,2 There is an unmet need for alternative approaches when radical surgery is undesirable or not recommended.
Classic photodynamic therapy treats tumors by inducing the formation of cytotoxic ROS in tumor tissues. The ROS formation occurs after the light has activated a nontoxic photosensitizer selectively accumulated in the tumor cells. In comparison, VTP targets tumor vasculature via the photosensitizing agent WST11 (Tookad® Soluble, Steba Biotech, France), which stays in the circulation.3 The photo-activation of this circulatory sensitizing WST11 generates a local burst of ROS that leads to complete tumor vascular destruction, blood stasis, and consequently, tumor necrosis.4 Furthermore, WST11-VTP induces long-lasting systemic antitumor immunity, involving both cellular and humoral components.5
The importance of immunotherapy on the treatment of UC was first reported in 1971, when Morales et al. showed the successful treatment of non-muscle-invasive bladder cancer with BCG6; providing a clear rationale for studying the role of other immunotherapy agents on the treatment of UC. Some preclinical studies investigated the role of the immune checkpoint CTLA-4 in UC carcinogenesis and aggressiveness, and its polymorphism has been associated with bladder cancer risk in a case-control study by Wang et al.7 This immune checkpoint is expressed on the surface of T-cells and plays a critical role in T-lymphocyte-mediated tumor response.8 CTLA-4 overexpression by tumor cells affects tumor-specific T-cell immunity, inhibits T-cell proliferation and effector function,9 and reshapes tumor progression and metastasis.10 The concept of CTLA-4 blockage has been used in the clinical setting and showed promising antitumor responses in patients with lymphoma, melanoma, and prostate cancer.11-13
Based on the importance of immunotherapy on the management of UC, the favorable results for WST11-VTP in the treatment of tumor models in previous studies3,14 and favorable results for anti-CTLA-4 drugs as monotherapy,11-13 we assessed the effect of combining WST11-VTP and anti-CTLA-4 immunotherapy in the treatment of UC in a mouse model.
MATERIALS AND METHODS
The animal use protocol for this study was approved by the MSKCC institutional animal care and use committee. A total of 227 seven- to eight-week-old male C57BL/6 mice (Taconic Farms) were used across all four different experiments conducted: tumor and lung metastasis assessment (91), survival (111), flow cytometry (20), and rechallenge (5 naïve and 10 pretreated). Timelines for the four experiments are shown in Figure 1.
Figure 1 –
Timelines for the experiments described in the manuscript. A) Tumor and lung metastasis experiment. B) Survival experiment. C) Flow cytometry experiment. D) Rechallenge experiment.
Cells from the MB-49 mouse bladder cell line which had been treated to express luciferase (MB-49-luc cells; see Supplemental Appendix) were washed and resuspended in PBS pH 7.4; viable cells were counted using Trypan blue exclusion and a hemocytometer prior to injection. For the tumor and lung metastasis assessment, survival, and flow cytometry experiments, mice were injected on Day 1 with 100 μl of the cell-containing solution (5 x 104 MB-49-luc cells diluted in PBS) in the right flank and randomly divided into four groups: WST11-VTP only, anti-CTLA-4 only, combination therapy, and control. For the rechallenge experiment, the same quantity of MB-49-luc cells was injected into the left flank of five naïve mice and the left flank of ten mice from the tumor/lung metastasis experiment that had shown complete tumor response with treatment and was without evidence of disease 111 days after tumor cell injection. These ten mice were from the WST11-VTP only (n=3) and combination therapy (n=7) groups.
WST11-VTP treatment took place on Day 12 for the tumor and lung metastasis assessment and survival experiments, and on Day 16 for the flow cytometry experiment. Details of WST11-VTP treatment are supplied in the Appendix. Figure 1 also shows the timing of antibody administration (anti-mouse CTLA-4 or mouse isotype IgG) for the tumor/lung metastasis assessment, survival, and flow cytometry experiments, with administration details given in the Appendix. The control group and the WST11-VTP-only group received IgG injections as placebo for anti-CTLA-4. We did not inject WST 11 into control group since there is no report about its immune effects without being photo activated. Bioluminescent imaging was used to evaluate mice from the tumor/lung metastasis, survival, and rechallenge experiments (details are given in the Appendix).
Experiments
Tumor and lung metastasis assessment.
For this experiment, mice were kept alive until Day 37. Bioluminescent imaging was performed on days 12, 19, 27, and 32, and on day 37 before mice were sacrificed. To measure the lungs signal we had to hide the primary tumors otherwise the high signal from the primary tumor would interfere in the lung signal uptake measurement. After sacrifice, the lungs were harvested to evaluate for metastasis; details are given in the Appendix.
Survival.
The 111 mice in the survival experiment were kept alive until spontaneous death or until the tumors exceeded the limit established in our protocol (2 cm3). Both situations were considered death from cancer. Animals that achieved the euthanasia endpoint were sacrificed in less than 12 hours. After 112 days (90 days from WST11-VTP treatment), 10/32 (31%) mice from the combination group and 4/32 (12%) from the WST11-VTP group were still alive. All mice from the control and anti-CTLA-4 groups were dead by Days 47 and 72, respectively (35 and 60 days post WST11-VTP). Bioluminescent imaging was performed before the mice were sacrificed.
Rechallenge.
Both the ten rechallenged and the five naïve mice were evaluated with bioluminescent imaging 32 days after the left-flank injection of tumor cells, and then sacrificed.
Flow Cytometry.
On this experiment, WST11-VTP treatment occurred 15 days after tumor implantation (later than in the other experiments to ensure having enough tumor tissue for analysis). Antibodies (anti-CTLA-4 or IgG) were injected on Days 16, 19, and 22 (0, 3, and 6 days after WST11-VTP; slightly different time-points from the other experiments to allow 3 doses of antibodies before sacrificing the mice). Mice were sacrificed on Day 23; tumor tissues, draining lymph nodes, and spleens were collected for flow analysis.
Combinations of surface and intracellular antibodies were used to stain the cells. Events were acquired using a multi-channel flow cytometer (BD LSR, BD Science, San Diego, CA). FlowJo software (FlowJo, Ashland, OR) was then used to analyze data. Forward-scattered light (FSC) and side-scattered light (SSC) were used to identify population and FSC-A/FSC-W gating was used to exclude doublets. Dead cells were excluded using Fixable Viability Dye eFluor506 stain and Fc block was used on all specimens. CD45-APC/Cy7, CD8-PerCp, CD4-v450, FoxP3-APC, and Ki67-FITC antibodies were used for staining of T-cell population. Antibodies were obtained from eBioscience (San Diego, CA) or BD Biosciences (San Diego, CA).
Statistical Analysis
Comparative analysis of primary tumor measurements and signal, and lung signal on the sacrifice day, were performed with the Mann-Whitney test. This method was also used to compare results from the flow cytometry experiment. The comparison between the mean tumor signal and lung signal at different time points was performed with two-way ANOVA test. Survival was plotted using the Kaplan-Meier method, and differences in survival between groups were compared using the log-rank test. The survival curve of each group was compared to the combination group curve separately. All statistical evaluations were performed using the GraphPad Prism® software (GraphPad Software, San Diego, CA).
RESULTS
Primary Treatment Response
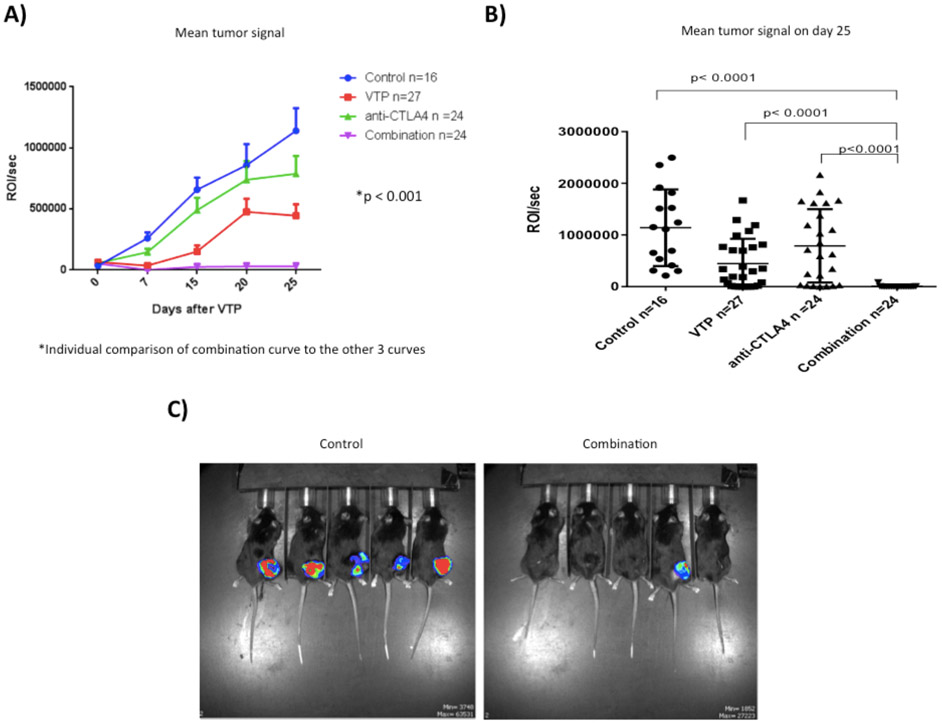
Tumor growth was monitored approximately weekly using luminescence imaging, starting on Day 12, the day of WST11-VTP treatment (Fig. 2A). Before starting the experiments with MB-49-luc cells, the cells had shown stable luciferase expression both in vivo and in vitro (data not shown). The difference between the mean primary tumor signal curves was significant when the combination group curve was compared to the other three groups (Fig. 2A; p<0.001). The analysis of the mean tumor signal 25 days post WST11-VTP (Day 37) significantly favored the combination group in comparison with the other three groups (Fig. 2B -2C). Tumor growth was significantly suppressed by the combination therapy compared to both monotherapy groups and the control group (p<0.05) (Fig. 2B-2C).
Figure 2 –
Effect of different therapies on primary tumor. A) Mean primary tumor growth curves. B) Mean and individual tumor signal on Day 37 (25 days post WST11-VTP). The combination of WST11-VTP + anti-CTLA-4 was significantly associated with a decreased primary tumor signal. C) Pictures of primary tumor imaging with bioluminescent imaging on the 25th day after treatment with WST11-VTP. The picture on the right shows 5 mice from the combination treatment group and the picture on the left is from the control group. Among the 5 mice from each group, tumors were palpable in 1 mouse from the combination group and all 5 mice from the control group.
Prevention of Lung Metastasis
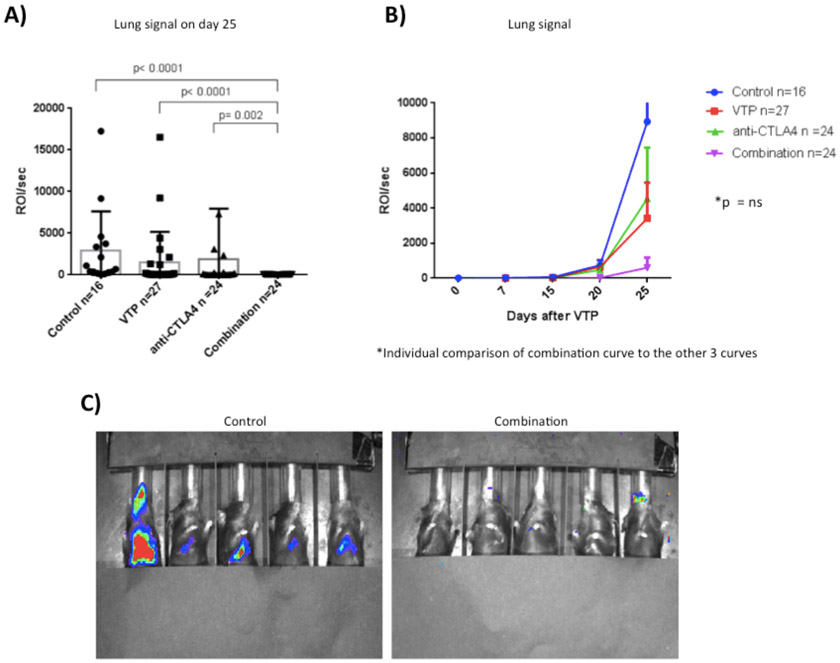
The development of lung metastasis was monitored with bioluminescent imaging on the same schedule as the primary tumor. On day 25 post WST11-VTP, tumor lung signal was decreased in the combination group (Fig. 3A). The mean signal difference was significant compared to the control (p<0.0001), WST11-VTP only (p<0.0001), and anti-CTLA-4 only (p=0.002) groups.
Figure 3 –
Effect of different therapies on prevention of lung metastasis. A) Mean and individual lung signal on the 25th day post WST11-VTP. We observed decreased lung signal in the group receiving combination therapy. B) Mean lung imaging curves. The development of lung metastasis begins between the 15th and 20th day after WST11-VTP treatment in all groups except for the combination group where it began between the 20th and 25th day after WST11-VTP treatment. The development of lung metastasis was postponed and decreased in the combination therapy group. C) Pictures of mice lung imaging on the 25th day after WST11-VTP therapy, illustrating the effect of combination therapy lung metastasis development observed in the graphs.
All four groups presented with negligible lung signal intensity from day 0 to day 15 after WST11-VTP treatment (Fig. 3B). Subsequently, the ROI/sec from the control and monotherapy groups started to increase (Fig. 3B), suggesting the development of lung metastasis (Fig. 3C). When the combination group curve was compared to those of the other three groups, the difference was not significant (Fig. 3B).
Lungs collected from mice were analyzed macroscopically under dissecting microscope (Fig. 4A and 4C) and the presence of metastasis was evaluated histologically (Fig. 4B and 4D).
Figure 4 –
Analysis of mice lungs. A) Macroscopic analysis of lungs from a combination therapy group mouse did not show presence of metastasis. B) No microscopic metastasis was identified with histologic analysis of lungs from this combination therapy group mouse. C) Macroscopic analysis of lungs from a control group mouse showed several metastases. D) Histologic identification of lung metastasis in this control group mouse.
Survival Experiment
In order to assess the effect of the different treatments on overall survival, mice were kept alive until spontaneous death or the primary tumor reached over 2 cm3. Comparing survival curves, the combination therapy provided longer survival than the control and monotherapy groups (Fig. 5; p<0.05). After 112 days (90 days from WST11-VTP treatment), 10/32 (31%) mice from the combination group and 4/32 (12%) from the WST11-VTP group were still alive. All mice from the control and anti-CTLA-4 groups were dead by Days 47 and 72, respectively (35 and 60 days post WST11-VTP) (Fig. 5). Median survival was 40, 43, 44, and 52 days after tumor implantation (29, 32, 33, and 41 days post WST11-VTP) for the control, WST11-VTP only, anti-CTLA-4 only, and combination groups, respectively.
Figure 5 -.
Survival curves for the 111 mice in the survival experiment. Combination treatment with WST11-VTP + anti-CTLA-4 was associated with increased survival compared to the other 3 groups.
Rechallenge Experiment
In order to study if any of the treatment modalities studied could protect from the development of new tumors, we performed tumor rechallenge in the mice from the tumor/lung metastasis assessment experiment that had survived 111 days after tumor implantation and were free of tumors following initial treatment. Tumors did not grow in any of the pretreated mice; however, they did in all five naïve mice injected on the same day. Flank tumors were evaluated with bioluminescent imaging (Fig. 6). Mice were sacrificed 32 days after tumor rechallenging since the animals from the naïve group started to present with ulcerated tumors.
Figure 6 –
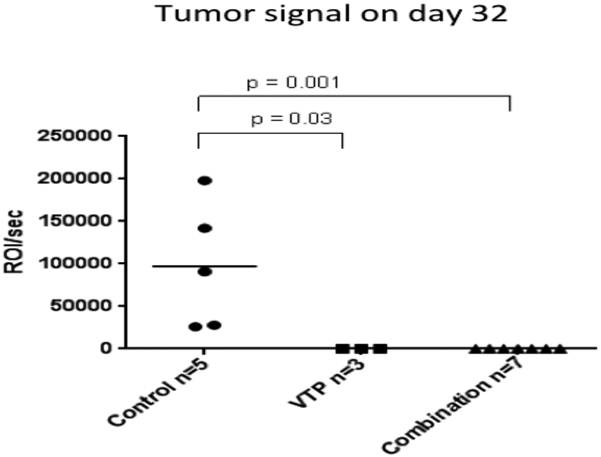
Mean tumor signal 32 days after rechallenge injection. Second tumors were implanted in the left flank of the 10 mice (7 combination therapy, 3 WST11-VTP-only) who had achieved complete primary tumor remission and survived 111 days from the first implantation. Tumors were also implanted in the left flank of 5 naïve mice. After 32 days, none of the 10 previously-treated mice had tumors, but all 5 of the naïve mice did.
Flow Cytometry Experiment
To understand the mechanisms underlying the superior response of the combination group, we studied the T-cell populations in the tumors, spleens, and draining lymph nodes for each treatment group of five mice.
Lymph nodes from the combination group showed a significantly increased rate of CD8 Ki67+ cells compared to the control group (p=0.007). No significant difference was observed regarding the rate of effector lymphocyte T cell (Teff) Ki67+ cells or regulatory T lymphocyte (Treg) Ki67+ cells (Fig. 7A). Spleens from the combination group had an increased rate of CD8 Ki67+ and Teff Ki67+ cells, and no significant difference in Treg Ki67+ cells when compared to the control group (Fig. 7B). Finally, tumor tissues from the control group showed an increased rate of Teff CD45+ cells and Treg CD45+ cells, though only the latter was clearly significant. However, the combination group tumor tissues showed an increased Teff:Treg ratio compared to control group tumor tissues (Fig. 7C).
Figure 7 –

Effect of the combination therapy on T-cell population of different tissues. A) In draining lymph nodes, the combination therapy was associated with a significant increase in CD8 Ki67+ cells. No significant difference was seen in Teff and Treg Ki67+ cells. B) Mice treated with combination therapy presented with an increased rate of CD8 Ki67+ and Teff Ki67+ cells in their spleens, compared to mice from the control group. Rates of Treg Ki67+ cells were not different between the two groups. C) Tumor tissues from the combination group mice had a decreased rate of Treg CD45+ cells and Teff CD45+ cells. However, the Teff:Treg ratio was significantly increased in this group, compared to the tumor tissues from the control group mice.
We also compared the tumor tissue T-cell populations from the combination group with those of the monotherapy groups, but the differences were not significant (data not shown).
DISCUSSION
The lack of alternative options following failure of UC endoscopic resection and BCG treatment offers a compelling rationale for developing different therapeutic strategies to manage UC. In the present study we combined two treatment modalities, WST11-VTP and anti-CTLA-4, previously described 3,15,16 in cancer treatment as monotherapy and assessed their synergistic effect in a mouse UC model. The cell line used, MB-49, shares several similarities with human UC regarding cell surface markers, sensitivity to apoptosis, and immunologic profile.17 We monitored primary tumor growth of MB-49 and metastasis development with in vivo bioluminescent imaging.18 This method reflects the number of metabolically active tumor cells and is a sensitive and efficient way to monitor and track tumor and lung metastasis development.19 Previous studies showed strong correlations between bioluminescent imaging, caliper measurements,20 and MRI-determined tumor volume.21
The WST11-VTP success in treating different tumor tissues has been reported in several animal 22,23 and clinical 3,14,15studies. Madar-Balakirski et al. showed that WST11-VTP was responsible for the permanent occlusion of tumor-feeding arteries resulting in tumor necrosis in a rat mammary carcinoma model.23 In another preclinical study, Chevalier et al. showed the safety and efficacy of prostate tissue ablation with WST11-VTP in a canine model.22 In the clinical scenario, WST11-VTP has been applied in the treatment of prostate cancer in phase I and II clinical trials.3,14,24 Among them, in a Six-month post-treatment analysis of 56 men with low-risk prostate cancer biopsies, WST11-VTP successfully ablated tumors in 38 (68%). Moreover, it effectively destroyed cancer foci in the targeted area and no residual tumor was found in the scar tissue in any case.14 In a recent pooled analysis of three phase II trials, with a total of 117 patients with low- and moderate-risk prostate cancer who were treated with WST11-VTP, Azzouzi et al.15 showed that the tolerability and adverse effects of the treatment were satisfactory. The adverse effects were mild or moderate and 68.4% of the patients presented with negative biopsies after six months.15 To date, there are no published clinical trials addressing the role of WST11-VTP in UC treatment; our study is the first to show its effectiveness on ablate heterotopic UC tumors in a syngeneic model.
Approved by FDA in 2011, anti-CTLA-4 is one of the most important medications in the treatment of metastatic melanoma and is associated with increased survival in patients with this disease.16 It mediates its effects by Treg depletion or via blockage of inhibitory signaling on Teff. Preclinical studies have shown enhanced T-cell activation and an increased ratio of Teff/Treg cells, which correlated with tumor regression in animals treated with anti-CTLA-4.8,25 These findings are in line with that from Schneider et al., who showed that the administration of anti-CTLA-4 increased T-cell motility both in vivo and in vitro.26 In UC treatment, Carthon et al. studied the effects of anti-CTLA-4 in a clinical trial with 12 patients, reporting an increase in the frequency of CD4+ICOSHI cells, a subpopulation of Teff cells, in peripheral blood and tumor tissues of the patients treated with anti-CTLA-4.27 The sustained increase in this cell subpopulation was associated with improved survival in melanoma patients treated with anti-CTLA-4 and represents a potential biomarker in the metastatic disease scenario. Although anti-CTLA-4 by itself was responsible for only small effects on the outcomes assessed in our analyses, its combination with WST11-VTP was important for improving survival and tumor treatment in mice as well as preventing lung metastasis.
Photo-activation of WST11 is known to generate ROS in the tumor vasculature and, consequently, tumor necrosis. Unlike surgery, when the tumor is treated with ablative modalities like WST11-VTP, the necrotic tumor cells remain in the body and tumor antigens from dying cells are released in the treated area. Consequently, these antigens are presented by antigen-presenting cells to T cells and activate a tumor-specific immune response.28
WST11-VTP’s specific antitumor immunity has been reported by Preise et al.,5 showing a substantial recruitment of T cells into the tumor 24 hours after WST11-VTP treatment. These authors found that sensitized splenocytes isolated from WST11-VTP-treated mice adhered better to cultured tumor cells compared to splenocytes isolated from naive mice. Therefore, we hypothesized that combining WST11-VTP with therapies that target antigen-presenting cells or modulate T-cell function could increase this immune response. Indeed, our results confirmed this hypothesis, as the association of anti-CTLA-4 with WST11-VTP led to a significant decrease in primary tumor burden and rendered a higher number of mice tumor-free compared to the WST11-VTP-only and other groups. Furthermore, this combination therapy in mice increased survival and prevented the development of lung metastasis. Preise et al.5 also demonstrated this protection from lung metastasis development; they showed that, after intravenous tumor injection, mice previously treated with WST11-VTP were completely protected from lung metastasis while positive control mice developed multiple lung tumor foci.5
We hypothesized that WST11-VTP and the combination of WST11-VTP and anti-CTLA-4 could have a role in protection from tumor recurrence. Although we did not assess the T cell population of the tumor area, we showed that none of the mice that had a complete treatment response after their previous treatment with WST11-VTP or combination therapy developed tumors after rechallenge. Waitz et al. observed similar results showing that the combination of cryoablation with anti-CTLA-4 in a mouse prostate cancer model led to a decreased growth or rejection of the second tumor. These authors also found that CD8+ and CD4+FoxP3− T cells were increased in these secondary tumors in the combination group, suggesting that these cell populations mediated tumor rejection.28
We addressed the cellular mechanisms involved in the described treatment modality and showed a significantly increased Teff:Treg ratio in tumor tissues from mice treated with the combination therapy, compared to non-treated (control) mice. Previous clinical studies showed that the intratumor Teff:Treg ratio was associated with tumor necrosis in anti-CTLA-4 treated patients 29 and was prognostic of improved survival in patients with ovarian cancer.30 We also observed that combination therapy-treated mice had significant increases in Teff Ki67+ in spleens and in CD8 Ki67+ in spleens and draining lymph nodes, suggesting proliferation and immune effect. However, we did not observe significant differences in tumor tissue between mice treated with combination therapy and mice treated with anti-CTLA-4 or WST11-VTP monotherapy. The precise day when T cells start to respond to the different treatment modalities is still unknown and may be different for each type of therapy. This might explain the non-significant differences observed when we compared the combination therapy to the monotherapy groups. Moreover, it should also explain the higher Teff concentration, in percentage of CD45 cells, in the control compared to the combination group. Future research approaching the mechanistic cellular effects of WST11-VTP with anti-CTLA-4 on this UC cell line will hopefully shed light on the cellular components induced by this combined therapy.
The combination of WST11-VTP with anti-CTLA-4 proved to be an effective therapy in the treatment of an UC syngeneic mouse model. It was associated with increased survival, prevention of lung metastasis, and prevention of tumor recurrence in mice. Our results present this therapy as a potential treatment option for both bladder and upper tract tumors in future clinical trials. It may be an alternative to patients who failed to respond to BCG and are unfit to undergo radical surgery or chemotherapy.
Supplementary Material
1 – Matherial and Methods - Cell Culture
2 - Matherial and Methods - Generation of MB-49 Line Expressing Luciferase
3 - Matherial and Methods - WST11-VTP
4 - Matherial and Methods - Antibody Administration
5 - Matherial and Methods - Luminescent Imaging
6 - Matherial and Methods - Tumor and Lung Metastasis Assessment Experiment
ACKNOWLEDGEMENTS
We thank: Dr. Emily Cheng (Human Oncology and Pathogenesis Program, MSKCC) for providing MSCV-puro-Luciferase-GFP construct; Dr. Jedd Wolchok’s lab (MSKCC) for providing MB-49 tumor cells; Dr. Sebastien Monette for help interpreting the results of our histology experiment; Dr. Patricia Corradi for help with graphics and figures editing; and the MSKCC editorial group, especially Amy Plofker, for editorial assistance.
Grant Support: This study received support from the following grants or institutions: NIH grant P30-CA008748 (cancer center support grant); Sidney Kimmel Center for Prostate and Urologic Cancers for all authors except A. Scherz; Wade Thompson Family Foundation.
ABBREVIATIONS AND ACRONYMS
- BCG
bacillus Calmette-Guerin
- MSKCC
Memorial Sloan Kettering Cancer Center
- ROS
reactive oxygen species
- UC
urothelial carcinoma
- VTP
vascular targeted photodynamic therapy
Footnotes
DISCLOSURE OF POTENTIAL CONFLICT OF INTERESTS
The authors declare that they have no conflicts of interest.
RESEARCH INVOLVING ANIMALS - ETHICAL STATEMENTS
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted. The project has been approved by the Institutional Review Board and Institutional animal care use committee.
REFERENCES
- 1.Babjuk M, Burger M, Zigeuner R et al. : EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur Urol 2013; 64: 639. [DOI] [PubMed] [Google Scholar]
- 2.Rouprêt M, Babjuk M, Compérat E et al. : European guidelines on upper tract urothelial carcinomas: 2013 update. Eur Urol 2013; 63: 1059. [DOI] [PubMed] [Google Scholar]
- 3.Azzouzi AR, Barret E, Moore CM et al. : TOOKAD(®) Soluble vascular-targeted photodynamic (VTP) therapy: determination of optimal treatment conditions and assessment of effects in patients with localised prostate cancer. BJU Int 2013; 112: 766. [DOI] [PubMed] [Google Scholar]
- 4.Tempel-Brami C, Pinkas I, Scherz A et al. : Detection of light images by simple tissues as visualized by photosensitized magnetic resonance imaging. PLoS One 2007; 2(11): e1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Preise D, Oren R, Glinert I et al. : Systemic antitumor protection by vascular-targeted photodynamic therapy involves cellular and humoral immunity. Cancer Immunol Immunother 2009; 58: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J Urol 2002; 167(2 Pt 2): 891. [DOI] [PubMed] [Google Scholar]
- 7.Wang L, Su G, Zhao X et al. : Association between the cytotoxic T-lymphocyte antigen 4 +49A/G polymorphism and bladder cancer risk. Tumor Biol 2014; 35: 1139. [DOI] [PubMed] [Google Scholar]
- 8.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science 1996; 271 (5256): 1734. [DOI] [PubMed] [Google Scholar]
- 9.Pentcheva-Hoang T, Simpson TR, Montalvo-Ortiz W et al. : Cytotoxic T lymphocyte antigen-4 blockade enhances antitumor immunity by stimulating melanoma-specific T-cell motility. Cancer Immunol Res 2014; 2: 970. [DOI] [PubMed] [Google Scholar]
- 10.Carosella ED, Ploussard G, LeMaoult J et al. : A systematic review of immunotherapy in urologic cancer: evolving roles for targeting of CTLA-4, PD-1/PD-L1, and HLA-G. Eur Urol 2015; 68: 267. [DOI] [PubMed] [Google Scholar]
- 11.Phan GQ, Yang JC, Sherry RM et al. : Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A 2003; 100: 8372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Small EJ, Tchekmedyian NS, Rini BI et al. : A pilot trial of CTLA-4 blockade with human anti-CTLA-4 in patients with hormone-refractory prostate cancer. Clin Cancer Res 2007; 13: 1810. [DOI] [PubMed] [Google Scholar]
- 13.Ansell SM, Hurvitz SA, Koenig PA et al. : Phase I study of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with relapsed and refractory B-cell non-Hodgkin lymphoma. Clin Cancer Res 2009; 15: 6446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eymerit-Morin C, Zidane M, Lebdai S et al. : Histopathology of prostate tissue after vascular-targeted photodynamic therapy for localized prostate cancer. Virchows Arch 2013; 463: 547. [DOI] [PubMed] [Google Scholar]
- 15.Azzouzi AR, Barret E, Bennet J et al. : TOOKAD® Soluble focal therapy: pooled analysis of three phase II studies assessing the minimally invasive ablation of localized prostate cancer. World J Urol 2015; 33: 945. doi: 10.1007/s00345-015-1505-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hodi FS, O’Day SJ, McDermott DF et al. : Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363: 711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loskog A, Dzojic H, Vikman S et al. : Adenovirus CD40 ligand gene therapy counteracts immune escape mechanisms in the tumor microenvironment. J Immunol 2004; 172: 7200. doi: 10.4049/jimmunol.172.11.7200. [DOI] [PubMed] [Google Scholar]
- 18.Nogawa M, Yuasa T, Kimura S et al. : Intravesical administration of small interfering RNA targeting PLK-1 successfully prevents the growth of bladder cancer. J Clin Invest 2005; 115: 978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jenkins DE, Oei Y, Hornig YS et al. : Bioluminescent imaging (BLI) to improve and refine traditional murine models of tumor growth and metastasis. Clin Exp Metastasis 2003; 20: 733. doi: 10.1023/B:CLIN.0000006815.49932.98. [DOI] [PubMed] [Google Scholar]
- 20.Jenkins DE, Yu SF, Hornig YS et al. : In vivo monitoring of tumor relapse and metastasis using bioluminescent PC-3M-luc-C6 cells in murine models of human prostate cancer. Clin Exp Metastasis 2003; 20: 745. [DOI] [PubMed] [Google Scholar]
- 21.Rehemtulla A, Stegman LD, Cardozo SJ et al. : Rapid and quantitative assessment of cancer treatment response using in vivo bioluminescence imaging. Neoplasia 2000; 2: 491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chevalier S, Anidjar M, Scarlata E et al. : Preclinical study of the novel vascular occluding agent, WST11, for photodynamic therapy of the canine prostate. J Urol 2011; 186: 302. [DOI] [PubMed] [Google Scholar]
- 23.Madar-Balakirski N, Tempel-Brami C, Kalchenko V et al. : Permanent occlusion of feeding arteries and draining veins in solid mouse tumors by vascular targeted photodynamic therapy (VTP) with Tookad. PLoS One 2010; 5: e10282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore CM, Azzouzi AR, Barret E et al. : Determination of optimal drug dose and light dose index to achieve minimally invasive focal ablation of localized prostate cancer using WST11-vascular-targeted photodynamic (VTP) therapy. BJU Int 2015; 116: 888. [DOI] [PubMed] [Google Scholar]
- 25.Quezada SA, Peggs KS, Curran MA et al. : CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest 2006; 116: 1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schneider H, Downey J, Smith A et al. : Reversal of the TCR stop signal by CTLA-4. Science 2006; 313(5795): 1972. [DOI] [PubMed] [Google Scholar]
- 27.Carthon BC, Wolchok JD, Yuan J et al. : Preoperative CTLA-4 blockade: tolerability and immune monitoring in the setting of a presurgical clinical trial. Clin Cancer Res 2010; 16: 2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waitz R, Solomon SB, Petre EN et al. : Potent induction of tumor immunity by combining tumor cryoablation with anti-CTLA-4 therapy. Cancer Res 2012; 72: 430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hodi FS, Butler M, Oble DA et al. : Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci U S A 2008; 105: 3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sato E, Olson SH, Ahn J et al. : Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A 2005; 102: 18538. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1 – Matherial and Methods - Cell Culture
2 - Matherial and Methods - Generation of MB-49 Line Expressing Luciferase
3 - Matherial and Methods - WST11-VTP
4 - Matherial and Methods - Antibody Administration
5 - Matherial and Methods - Luminescent Imaging
6 - Matherial and Methods - Tumor and Lung Metastasis Assessment Experiment