Abstract
The value and suitability of cytology specimens for molecular diagnosis has been demonstrated by numerous studies. In practice, however, the success rates vary widely across institutions depending on the disease setting, institutional practices of acquisition, handling/processing, and testing methodologies. As the number of clinically relevant biomarkers continues to increase, more laboratories are turning to next-generation sequencing platforms for testing. Although amplicon-based next-generation sequencing assays, interrogating a limited genomic territory, can be performed with minimal input material, broader-based next-generation sequencing assays have higher DNA input requirements that may not be met if the small tissue samples are not acquired and handled appropriately. We briefly describe some of the process changes we have instituted in our laboratories when handling cytologic material to maximize the tissue available for broad hybrid-capture–based next-generation sequencing assays. Among the key changes established were the consolidation and preservation of previously discarded supernatant material in cytologic samples, the introduction of mineral oil for deparaffinization of cell blocks, and adjustments in the molecular laboratory process and bioinformatics pipelines. We emphasize that even minimal changes can have broad implications for test performance, highlighting the importance of a cohesive group-based approach among clinical, cytopathology, surgical pathology, molecular, and bioinformatics teams.
Molecular diagnostics is a rapidly evolving field with broad applications in modern clinical practice. Increasing roles in tumor classification, risk assessment, prognosis, disease burden monitoring, treatment optimization, and personalized targeted therapies are drastically redefining daily pathology practice.1,2 The traditional surgical pathology approach, historically defined by tumor morphology, is rapidly transforming to encompass a comprehensive array of testing modalities, all hinging upon the availability of suitable tissue. In parallel, as the number of clinically relevant molecular markers has continued to increase, so has the trend toward minimally invasive procedures.2 The use of cytopathology samples as the sole tissue source for comprehensive assessment is markedly increasing and becoming routine practice. Consequently, these changes are driving the need to adapt and optimize procedures for such samples.3–6
Currently in the presurgical setting, small biopsy specimens (core biopsies or mucosal forceps biopsies) are typically considered the preferred source of material for molecular testing, even if concurrent cytologic samples are available. However, in some instances, cytologic samples may be the only diagnostic material available for a patient. Furthermore, it is becoming recognized that cytology samples may offer several advantages over small biopsies, particularly if the processes in cytology and molecular laboratories are optimized accordingly. The major advantage of cytology samples is that, because they are often collected in non–formalin-based fixatives, they offer the possibility of testing of higher-quality nucleic acids.7 In addition, fine-needle aspirates (FNAs) frequently have higher tumor to normal cell ratio compared with core needle biopsies,8 which is a critical consideration in genomic analyses. Thus, even though the overall tissue volume is typically, although not always, lower in FNA samples than in needle biopsies, the higher quality of nucleic acid and the higher tumor DNA fraction represent critical advantages of cytology samples.
Although the suitability of cytology specimens for molecular diagnosis has been demonstrated in numerous studies,3–5,9 testing represents a significant challenge in most clinical laboratories given the limited room for error that exists in handling extremely limited samples. Success rates vary widely across institutions depending on the disease setting, institutional practices of acquisition, handling/processing, and testing methodologies.10–12
A prototype of a cancer type with challenging testing needs is lung adenocarcinoma.13–15 Because most patients present at an advanced stage, cytology and small biopsies constitute the majority of diagnostic samples in this setting. At the same time, the landscape of targetable biomarkers for this disease is rapidly evolving to encompass a wide array of genetic alterations in multiple genes, including structural chromosomal rearrangements, point mutations, insertions, deletions, and copy number changes. Although amplicon capture–based next generation sequencing (NGS) assays with low DNA requirements are being increasingly adopted in many laboratories for testing samples with limited material, this approach is suboptimal for lung samples given the need to detect amplifications and fusions in addition to mutations. Hybrid capture–based NGS assays are a more suitable approach in this setting. In the last 2 years, our institution has adopted a hybrid capture–based, targeted NGS assay, Memorial Sloan Kettering–Integrated Mutation Profiling of Actionable Cancer Targets (MSK–IMPACT), which detects point mutations, small insertions/deletions, copy number alterations, and selected fusions in 410 cancer-associated genes.16,17 Although providing comprehensive genomic profiling, hybrid capture–based assays generally have higher nucleic assay requirements that may not be met in a large proportion of small biopsies and cytology samples if not acquired and handled appropriately.12,18 This new testing reality sharply contrasts with the early days of molecular testing of lung carcinoma samples, which generally required primarily EGFR analysis, and which we reported could be achieved with high success rates in cytology samples without major adjustments of standard processes in cytology and molecular laboratories.19,20
Using lung carcinoma as a disease model, we describe some of the process changes we have instituted in handling cytology samples to maximize the tissue available for broad hybrid capture–based testing (Figure 1).
Figure 1.
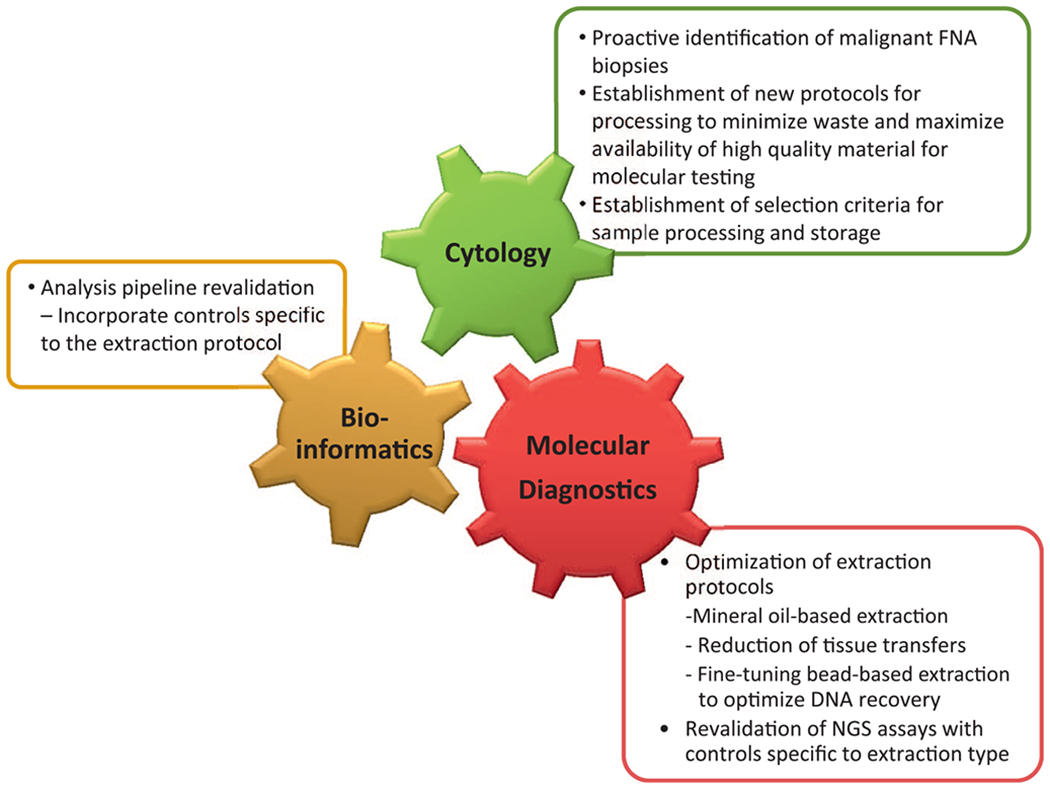
Integrated process optimization to maximize success of next-generation sequencing (NGS) testing on fine-needle aspirate (FNA) samples. Optimizing workflows and processing protocols requires an integrated effort where minor changes in one process may have major effects on the other and highlights the need for communication and cohesive work among all teams.
PROCESSING OF CELL BLOCKS TO MAXIMIZE YIELD
Formalin-fixed, paraffin-embedded (FFPE) cell blocks represent the most common source of material for ancillary studies in cytopathology. Currently, standard guidelines for lung carcinoma molecular testing recommend cell blocks as the preferred specimen for molecular studies.21 As an adaptation to classical surgical pathology tissue processing, this approach is preferred in most laboratories because it provides similar morphology to traditional tissue sections and the same workflows and storage capabilities, as well as similar capabilities for downstream ancillary studies such as immunohistochemistry, fluorescent in situ hybridization, and molecular testing. Even if a cytology sample is collected in non–formalin-based solution (such as alcohol-based CytoLyt [Cytyc Corporation, Marlborough, Massachusetts] or cell culture media), the process of paraffin embedding typically includes formalin fixation steps using the standard histologic processors. This results in a marked reduction in the quality of already limited material that very often compromises the performance of more comprehensive molecular assays. Given the broad use of cell blocks, instituting solutions to maximize the yield from this specific preparation is critical. Currently, cell block preparation methods vary widely across institutions.12 The difficulty of capturing cells in a cell block from hypocellular suspensions is a well-known limitation of molecular testing on cytology samples, particularly in the currently common scenario where testing is limited entirely to cell block material.22 At our institution, we have developed and implemented an improved HistoGel (Thermo Scientific, Waltham, Massachusetts)–based cell block preparation method, which resulted in a substantially improved cell block sufficiency for molecular studies.23 Other key steps in this process have been to reduce noncritical ancillary tests and consolidate workflows to reduce excessive recutting of tissue blocks, as discussed in detail in previous literature.24
REDEFINING CYTOLOGY SAMPLE PROCESSING FOR MOLECULAR TESTING
Recognizing that optimized cell block processing was not enough to meet molecular testing demands in many cytopathology cases, we then focused our efforts on the tissue preparation process. Important measures were to identify and eliminate any step that would lead to tissue wasting and to move away from the practice of preparing FFPE tissue blocks as the only source of tissue available for molecular studies. Our current approach is to encourage concurrent FNA and core needle biopsies, whenever clinically feasible, to ensure a dual source of available material for testing. When an FNA is performed, the laboratory receives the needle rinse material collected in CytoLyt in addition to smears. The rinse material is centrifuged to create a cell pellet, which is used to make both the cell block and a ThinPrep (Hologic, Bedford, Massachusetts) slide. Specifically for the ThinPrep slide, a small aliquot of pelleted material is transferred into the standard PreservCyt fixative jar (Hologic) per manufacturer’s protocol and processed accordingly. Based on a recent study showing a substantial amount of nucleic acid in the supernatants of FNA needle rinses after cell pelleting,25 we have modified our process to preserve the CytoLyt supernatants, material that has traditionally been discarded in the majority of cytopathology laboratories, for molecular studies. In addition, any unused cell suspension from the PreservCyt container is also saved.26 The 2 saved cytology materials are later combined and ultimately concentrated into a single pellet in a microcentrifuge tube that is directly compatible with our automated DNA extraction processes, minimizing any losses from unnecessary material transfer steps (Figure 2). The microcentrifuge tubes are prepared for all cases positive for lung non–small cell carcinoma, as well as other select tumors, and are stored at −20°C until a molecular order is received. In the molecular laboratory, the pelleted material is directly homogenized into lysis buffer with proteinase K in the same tube in which the specimen is received. The emulsion then joins the workflow of our standard FFPE processing at the tissue lysis step and follows an identical downstream assessment and processing protocols.
Figure 2.
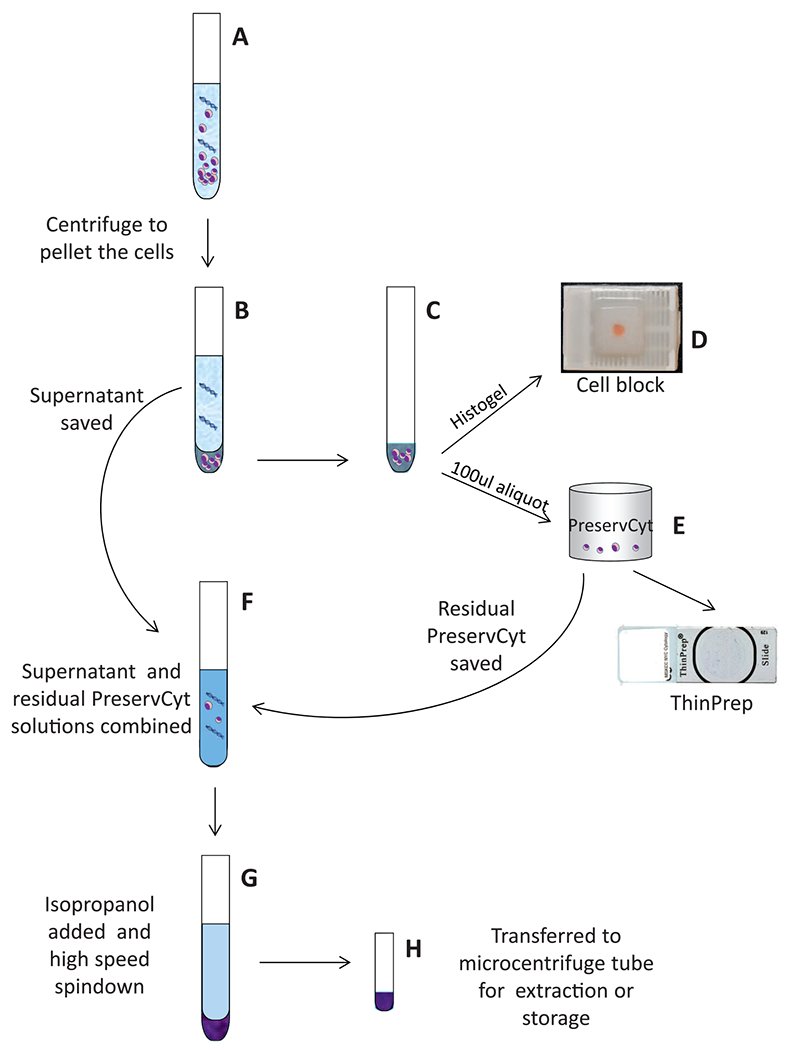
Repurposing of the unused cytology material for molecular testing. Key steps in the process are illustrated. A, Cytology suspension in CytoLyt (Cytyc Corporation, Marlborough, Massachusetts) is pelleted by gentle centrifugation (600g) to preserve cell morphology. B through D, The pellet is processed into a cell block using a modified HistoGel (Thermo Scientific, Waltham, Massachusetts) method. E, An aliquot of the pellet is transferred into a PreservCyt container (Hologic, Bedford, Massachusetts) and used to prepare a ThinPrep slide (Hologic). F through H, The CytoLyt supernatant and residual PreservCyt are combined. Isopropanol is added to precipitate cell-free DNA, followed by high-speed (17 000–18 000g) centrifugation. The pelleted material is transferred into a microcentrifuge tube for extraction and storage.
The use of this repurposed cytologic material has greatly improved our ability to offer comprehensive and clinically relevant molecular genetic testing in the setting of very limited tissue samples, without affecting cell block preparations. Currently, provided that the corresponding ThinPrep slide shows sufficient tumor cell content (>10% tumor cells from all nucleated cells to meet the sensitivity limits of our standard assays), the DNA extracted from this previously discarded material is quickly becoming our primary choice over the cell block or the core biopsy for NGS assays. The advantages include (1) excellent DNA quality given the lack of formalin fixation, (2) reduced histology and molecular pathology processing costs associated with handling the FFPE (ie, no recut preparation and processing required), and (3) reduced processing turnaround time because of elimination of the preparation and processing of the recuts. Importantly, this process avoids the dependence of molecular testing on the quality of the cell block preparations which, in our experience, even after improved methodology has been instituted, may be inconsistent and operator dependent. The detailed description of this process and validation will be published separately. Molecular analysis using direct aliquots from cytologic samples in suspension is a well-established process in human papillomavirus testing of cervical cytology, and more recently has been used for NGS on thyroid FNAs.27 In addition, NGS on DNA extracted directly from FNA needle rinse and effusion fluids remaining in PreservCyt has been recently described.28 Our process is similar, taking advantage of the residual cells in PreservCyt as well as CytoLyt supernatant remaining after cell block processing.
We acknowledge that cytologic smears also represent an excellent alternative to FFPE cell blocks, and we are aware that smears have been adopted as a primary or major source of material for molecular studies in several institutions.6,29,30 In our practice, we currently sacrifice smears for molecular testing only for cases in which no other material is available.
PROCESS CHANGES IN THE MOLECULAR LABORATORY
To maximize the yield of DNA from cell blocks, extraction protocols were further adapted. An important change was to transition to mineral oil for deparaffinization, thereby improving the quality of extracted DNA and increasing the DNA yield. Mineral oil extractions are associated with significant reduction of hands-on labor and reduction in transfers, centrifugation, and decanting steps that lead to tissue waste.31,32
The deparaffinization process constitutes a key vulnerability to loss of FFPE pathology material, particularly for small biopsies and especially for cytology cell blocks. This is because the disaggregated cells present in FNA and core biopsies are potentially washed away during all dewaxing steps, including pipetting off of the wax-solvent (whether xylene or xylene substitute) mixture and subsequent ethanol washes, of these minute specimens that are already starting out near the limits of suitability for testing. We have validated and adopted a method that maximizes preservation of these specimens by eliminating dedicated dewaxing and sample washing steps prior to specimen lysis. As proteinase K–based sample lysis is critical for maximal nucleic acid recovery, and this is an aqueous reaction requiring paraffin exclusion, tissue lysis is achieved by direct simultaneous incubation of FFPE specimen with mineral oil and aqueous lysis solution. Only after complete tissue lysis is attained are any sample purification steps performed. As mineral oil is an inert reagent with a long history of utility in molecular biology and chemistry as a reaction overlay to prevent evaporation, it eliminates carryover of assay-inhibitory xylene, limonene, and/or alcohols from dedicated dewaxing. Comparison of DNA yields from FFPE cytology specimens and very small biopsies before and after switching to mineral oil is shown in Figure 3. Overall, with the new process we were able to double the total DNA yield per sample in side-by-side comparisons.
Figure 3.
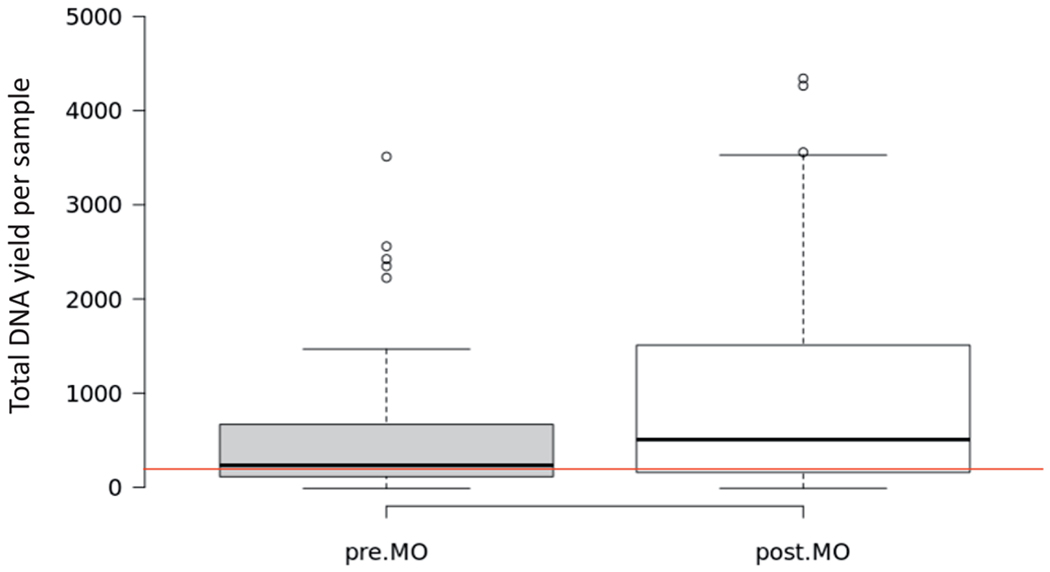
Box plot summarizing the data for extraction of formalin-fixed, paraffin-embedded cytologic samples and very small biopsies in a 2-month period (321 samples). Gray box shows the distribution of samples in 1 month prior to institution of mineral oil (MO) extractions. White box on the right is the distribution for samples in a 1-month period extracted with the mineral oil protocol. The red line marks the 200-ng line, which is the optimal quantity required for Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets. More than 90% of samples in the post-MO extraction period met criteria for testing based on concentration, compared with 83% in the pre-MO category.
BIOINFORMATICS
Evaluation of copy number alterations from NGS data is complicated by variable sequencing coverage of GC- and AT-rich genomic regions. When analyzing the data for clinical use, a comparative normal sample is generally used to cancel out such sequencing coverage artifacts and report high-confidence copy number alterations. Because of the specific steps involved with cytology sample extraction and preparation for NGS sequencing, distinct variations in GC-coverage profiles can be observed when evaluating the sequencing data generated from the FFPE samples extracted through standard and mineral oil processes and from the non–formalin-fixed cytology samples. Regardless of the method, the GC-rich regions of the sequenced areas get a much higher proportion of sequencing reads coverage compared with AT-rich regions of the genome in the MSK-IMPACT assay. However, the specific proportions can be highly variable depending on the extraction process involved. Although the standard mutation analysis is not generally affected if sufficient overall coverage is obtained, distinct challenges arise when normalizing read coverage for identification of clinically relevant copy number alterations if an adequate control is not used. Any changes in the extraction process for the clinical samples therefore require revalidation and normalization with samples processed using a similar protocol. The use of similarly extracted and processed normal samples ensures equivalent sequencing coverage biases of GC- or AT-rich genomic regions, resulting in efficient copy number evaluation. This is demonstrated in Figure 4, where the use of a control sample extracted with a different protocol leads to excess noise, which can often interfere with the assessment of copy number calls. When a similarly prepared cytology sample is used for normalization of copy number, results show a cleaner profile and more accurate P values for significance of results.
Figure 4.
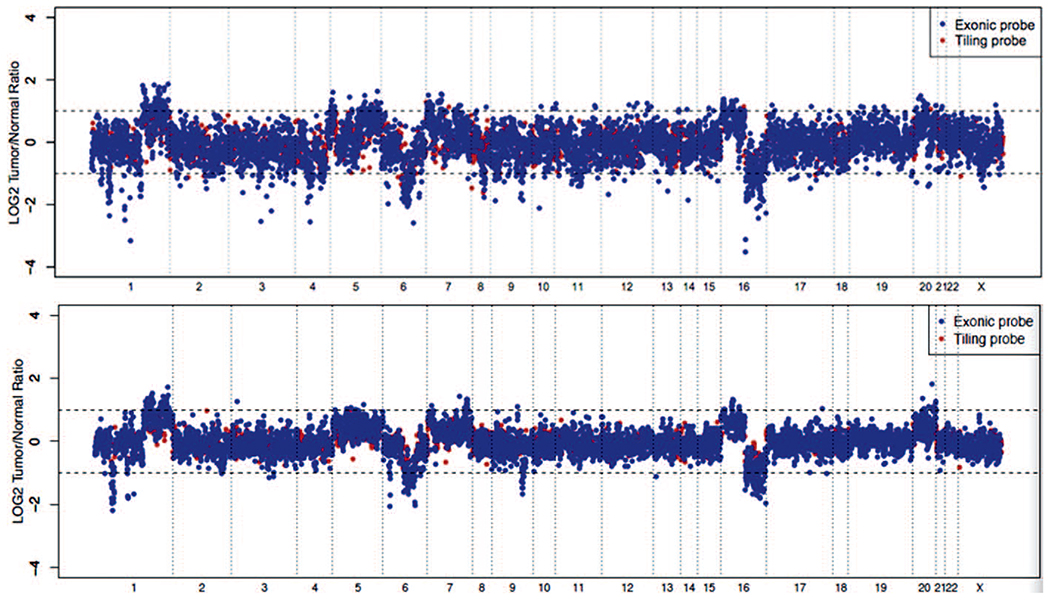
Representative copy number plots of a cell pellet sample. Log ratios comparing tumor versus normal sequencing coverage values are calculated across all targeted regions on different chromosomes. The top panel shows the results when a standard formalin-fixed, paraffin-embedded normal is used as a control; the bottom is normalized using a cytology normal that was processed and extracted using the same protocol as the clinical sample. The bottom panel shows a sharper copy number profile, allowing a more accurate assessment of copy number changes across the genome.
CONCLUSIONS
At our institution, with simple workflow changes and through process optimization, we have been able to provide overall success rates for comprehensive molecular testing in approximately 90% of our lung cytology cases (compared with our prior success rate of ~75%–80%), which is at the same level of what is seen with our needle biopsies. We anticipate that after full integration of the repurposed non-FFPE cytology material into our workflow, the sufficiency rates for cytology samples should increase even further. Furthermore, although both FNA and needle biopsies show similar technical testing success, in many instances the cytologic material is preferred to the needle biopsies given the superior quality of nucleic acid and general enrichment for tumor without the need for manual macrodissection, which proves to be a challenge given the small nature of the samples.
A variety of other cytologic preparations and processes are suitable for testing under any methodology, provided that they are validated and adjusted to the individual needs of the institution. Given the wide variability in testing methods, understanding the specific testing requirements and the methodology for molecular assessment is critical. Establishing specific criteria of suitability and defining and fine-tuning specific tissue handling and processing protocols is an institutional effort. Importantly, all processes are interconnected and even minor changes may have wide ramifications on the performance of the assays and may require additional and often comprehensive validation steps, highlighting communication and cohesive work among clinical, cytopathology, molecular, and bioinformatics services when establishing any new protocol.
Footnotes
The authors have no relevant financial interest in the products or companies described in this article.
References
- 1.Watson IR, Takahashi K, Futreal PA, Chin L. Emerging patterns of somatic mutations in cancer. Nat Rev Genet. 2013;14(10):703–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biankin AV, Piantadosi S, Hollingsworth SJ. Patient-centric trials for therapeutic development in precision oncology. Nature. 2015;526(7573):361–370. [DOI] [PubMed] [Google Scholar]
- 3.Mojica WD, Oh KW, Lee H, Furlani EP, Sands AM. Maximizing derivable information from cytologic specimens for pathologic and molecular diagnostics. J Am Soc Cytopathol. 2015;4(3):141–147. [DOI] [PubMed] [Google Scholar]
- 4.Aisner DL, Sams SB. The role of cytology specimens in molecular testing of solid tumors: techniques, limitations, and opportunities. Diagn Cytopathol. 2012; 40(6):511–524. [DOI] [PubMed] [Google Scholar]
- 5.Gailey MP, Stence AA, Jensen CS, Ma D. Multiplatform comparison of molecular oncology tests performed on cytology specimens and formalin-fixed, paraffin-embedded tissue. Cancer Cytopathol. 2015;123(1):30–39. [DOI] [PubMed] [Google Scholar]
- 6.Roy-Chowdhuri S, Goswami RS, Chen H, et al. Factors affecting the success of next-generation sequencing in cytology specimens. Cancer Cytopathol. 2015;123(11):659–668. [DOI] [PubMed] [Google Scholar]
- 7.Williams C, Ponten F, Moberg C, et al. A high frequency of sequence alterations is due to formalin fixation of archival specimens. Am J Pathol. 1999; 155(5):1467–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chowdhuri SR, Chen H, Staerkel G, Stewart JM. Morphologic assessment of cellularity and tumor fraction in concurrently acquired cytology fine needle aspiration and surgical core needle biopsy for molecular analysis. Poster presented at: 105th Annual Meeting of the United States & Canadian Academy of Pathology; March 16, 2016; Seattle, WA. [Google Scholar]
- 9.Killian JK, Walker RL, Suuriniemi M, et al. Archival fine-needle aspiration cytopathology (FNAC) samples: untapped resource for clinical molecular profiling. J Mol Diagn. 2010;12(6):739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harada S, Agosto-Arroyo E, Levesque JA, et al. Poor cell block adequacy rate for molecular testing improved with the addition of Diff-Quik-stained smears: need for better cell block processing. Cancer Cytopathol. 2015;123(8):480–87. [DOI] [PubMed] [Google Scholar]
- 11.Coley SM, Crapanzano JP, Saqi A. FNA, core biopsy, or both for the diagnosis of lung carcinoma: obtaining sufficient tissue for a specific diagnosis and molecular testing. Cancer Cytopathol. 2015;123(5):318–326. [DOI] [PubMed] [Google Scholar]
- 12.Crapanzano JP, Heymann JJ, Monaco S, Nassar A, Saqi A. The state of cell block variation and satisfaction in the era of molecular diagnostics and personalized medicine. Cytojournal. 2014;11(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Son C, Kang EJ, Roh MS. Strategic management of transthoracic needle aspirates for histological subtyping and EGFR testing in patients with peripheral lung cancer: an institutional experience. Diagn Cytopathol. 2015;43(7):532–538. [DOI] [PubMed] [Google Scholar]
- 14.Song DH, Lee B, Shin Y, et al. Cytomorphological identification of advanced pulmonary adenocarcinoma harboring KRAS mutation in lymph node fine-needle aspiration specimens: comparative investigation of adenocarcinoma with KRAS and EGFR mutations. Diagn Cytopathol. 2015;43(7):539–544. [DOI] [PubMed] [Google Scholar]
- 15.Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014;311(19): 1998–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hyman DM, Solit DB, Arcila ME, et al. Precision medicine at Memorial Sloan Kettering Cancer Center: clinical next-generation sequencing enabling next-generation targeted therapy trials. Drug Discov Today. 2015;20(12):1422–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng DT, Mitchell TN, Zehir A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn. 2015;17(3):251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakajima T, Yasufuku K. How I do it—optimal methodology for multidirectional analysis of endobronchial ultrasound-guided transbronchial needle aspiration samples. J Thorac Oncol. 2011;6(1):203–206. [DOI] [PubMed] [Google Scholar]
- 19.Rekhtman N, Brandt SM, Sigel CS, et al. Suitability of thoracic cytology for new therapeutic paradigms in non-small cell lung carcinoma: high accuracy of tumor subtyping and feasibility of EGFR and KRAS molecular testing. J Thorac Oncol. 2011;6(3):451–458. [DOI] [PubMed] [Google Scholar]
- 20.Arcila ME, Oxnard GR, Nafa K, et al. Rebiopsy of lung cancer patients with acquired resistance to EGFR inhibitors and enhanced detection of the T790M mutation using a locked nucleic acid-based assay. Clin Cancer Res. 2011;17(5): 1169–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Thorac Oncol. 2013;8(7):823–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saqi A The state of cell blocks and ancillary testing: past, present, and future [published online ahead of print August 24, 2016]. Arch Pathol Lab Med. doi: 10.5858/arpa.2016-0125-RA. [DOI] [PubMed] [Google Scholar]
- 23.Rudomina D, Rekhtman N, Friedlander MA, Dsouza C, Aggarwal G, Lin O. Improved cell block preparation method: a protocol for increased cellularity which is suitable for immunocytochemical and molecular assays. J Am Soc Cytopathol. 2013;2(1):S81–S82. [Google Scholar]
- 24.Travis WD, Rekhtman N. Pathological diagnosis and classification of lung cancer in small biopsies and cytology: strategic management of tissue for molecular testing. Semin Respir Crit Care Med. 2011;32(1):22–31. [DOI] [PubMed] [Google Scholar]
- 25.Deftereos G, Finkelstein SD, Jackson SA, et al. The value of mutational profiling of the cytocentrifugation supernatant fluid from fine-needle aspiration of pancreatic solid mass lesions. Mod Pathol. 2014;27(4):594–601. [DOI] [PubMed] [Google Scholar]
- 26.Biscotti CV, Shorie JH, Gramlich TL, Easley KA. ThinPrep vs. conventional smear cytologic preparations in analyzing fine-needle aspiration specimens from palpable breast masses. Diagn Cytopathol. 1999;21(2):137–141. [DOI] [PubMed] [Google Scholar]
- 27.Nikiforova MN, Wald AI, Roy S, Durso MB, Nikiforov YE. Targeted next-generation sequencing panel (ThyroSeq) for detection of mutations in thyroid cancer. J Clin Endocrinol Metab. 2013;98(11):E1852–E1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei S, Lieberman D, Morrissette JJ, Baloch ZW, Roth DB, McGrath C. Using “residual” FNA rinse and body fluid specimens for next-generation sequencing: an institutional experience. Cancer Cytopathol. 2016;124(5):324–329. [DOI] [PubMed] [Google Scholar]
- 29.Knoepp SM, Roh MH. Ancillary techniques on direct-smear aspirate slides: a significant evolution for cytopathology techniques. Cancer Cytopathol. 2013; 121(3):120–128. [DOI] [PubMed] [Google Scholar]
- 30.Shivnarain D, Ladanyi M, Zakowski MF. Detection of BCL2 rearrangement in archival cytological smears of B-cell lymphomas. Mod Pathol. 1994;7(9):915–919. [PubMed] [Google Scholar]
- 31.Heikal N, Nussenzveig RH, Agarwal AM. Deparaffinization with mineral oil: a simple procedure for extraction of high-quality DNA from archival formalin-fixed paraffin-embedded samples. Appl Immunohistochem Mol Morphol. 2014;22(8):623–626. [DOI] [PubMed] [Google Scholar]
- 32.Lin J, Kennedy SH, Svarovsky T, et al. High-quality genomic DNA extraction from formalin-fixed and paraffin-embedded samples deparaffinized using mineral oil. Anal Biochem. 2009;395(2):265–267. [DOI] [PMC free article] [PubMed] [Google Scholar]


