Mutations in Filamin C (FLNC) are associated with different forms of cardiomyopathies 1, 2. However, the role of FLNC in mammalian cardiomyocytes (CMs) remains largely unexplored. FLNC is predominantly expressed in striated muscle, localizing to Z-discs, intercalated discs (ICDs), and costameres 3. Mice expressing a C-terminal truncated FLNC mutant protein display perinatal lethality with severe defects in skeletal myogenesis but no cardiac defects 4. The latter is puzzling, given that multiple distinct FLNC mutations lead to cardiomyopathy. As this mutant Flnc allele might be hypomorphic, a true null Flnc and a Flnc CM-specific knockout model are essential to further probe requirements for Flnc in CMs.
We generated Flnc global (gKO) and cardiac specific knockout (cKO) mice by crossing Sox2-Cre or cardiac troponin T-Cre to FLNC-floxed mice (Flnctm1a(EUCOMM)Hmgu, MGI:4847813), in which exons 9 to 13 of Flnc are flanked by two LoxP sites. Cre-mediated deletion results in loss of the Flnc region between exons 9–13, frameshift of Flnc, and subsequent loss of the protein. All mouse protocols were approved by the Institutional Animal Care and Use Committee. Both gKO and cKO mice were embryonic lethal, demonstrating that Flnc is critical in developing CMs (data not shown).
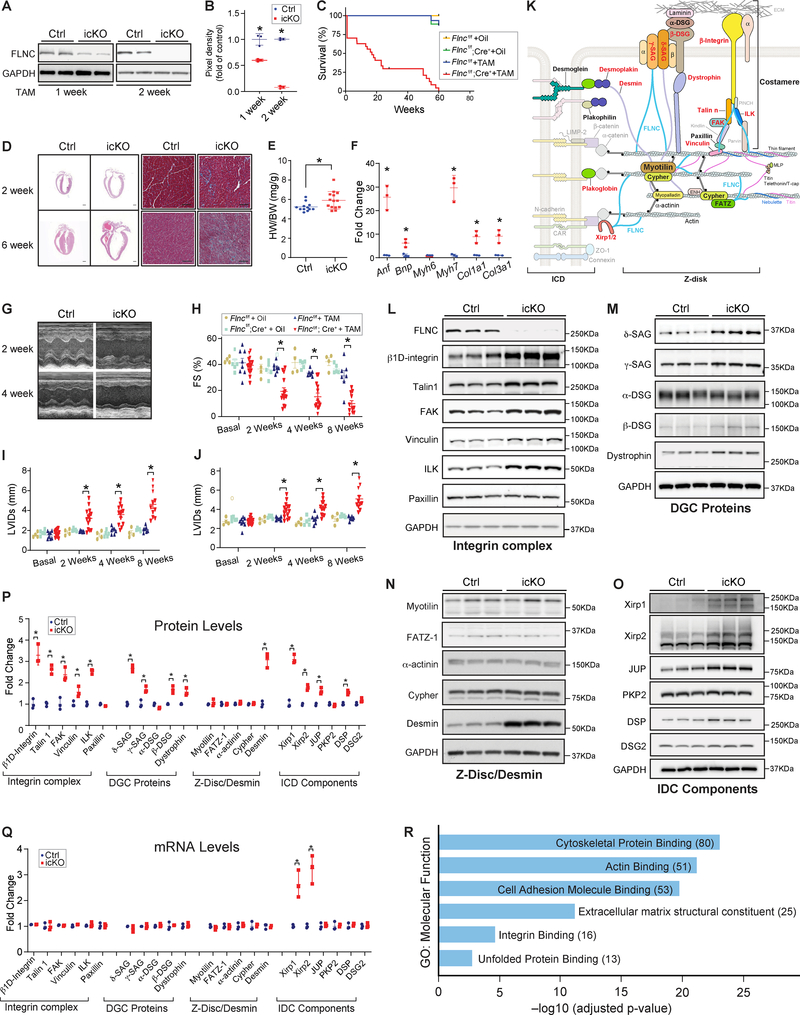
To study Flnc in adult CMs, we generated inducible CM specific knockouts (icKOs) by crossing Flnc-floxed mice to αMHC-MerCreMer mice, and subjected them to tamoxifen treatment (TAM) at two months of age. FLNC protein was efficiently ablated in icKO mouse hearts two weeks post TAM (Figs. A–B). Flnc-icKO mice began to die 1-week post TAM, with 73% dying by 25 weeks (Fig. C). No icKO mice survived past 60 weeks of age (Fig. C). IcKO hearts at 2 weeks and 6 weeks-post TAM displayed marked cardiac dilation and extensive fibrosis 6 weeks-post TAM (Fig. D). Analysis of heart weight to body weight (HW/BW) ratios revealed significantly increased heart mass in icKO at 2 weeks-post TAM, compared to controls, while no differences were observed in body weight. Cardiac stress markers, Anf, Bnp, and Myh7, were significantly increased in icKO hearts at 2-weeks post TAM, as were profibrotic genes Col1a1 and Col3a1 (Fig. F).
Figure. Loss of Filamin C is catastrophic for heart function.
(A-B) Representative western blot (A) and quantitation (B) showing efficient loss of Flnc in icKO mouse hearts 2 weeks post tamoxifen treatment (TAM) (40 mg/kg/day for 3 days). Flncf/f, Cre− littermates post TAM served as controls (Ctrl). n= 3 per group. (C) Kaplan-Meier survival curves of Flncf/f and Flncf/f; Cre+ mice injected with TAM or vehicle control. n= 16, 8, 26, and 9 respectively. (D) Microscopic cross-sectional views of Hematoxylin & Eosin (Left, scale bar, 1 mm) and Masson Trichrome (Right, Scale bar: 50 μm) stained hearts isolated from Flnc icKO or Ctrl mice, 2 weeks or 6 weeks post TAM. n=3. (E) HW to BW ratio for icKO (n = 14) and Ctrl (n = 12) mice 2 weeks post TAM. (F) qRT-PCR analysis in Ctrl and icKO mouse hearts at 2 weeks post TAM. (G) Representative echocardiographic images of icKO and Ctrl mice at 2 and 4 weeks post TAM. (H-J) Echocardiographic measurements for Flncf/f and Flncf/f;Cre+ mice at baseline or 2, 4, 8 weeks post TAM or vehicle control. n = 8, 4, 15, and 5 respectively. (K) Diagram of intercalated disc (ICD), Z-disc, and costamere components. Proteins noted in red were upregulated, while those in black were unchanged, in Flnc icKO hearts. (L-P) Representative western blots and quantification of Integrin complex, DGC proteins, Z-disk protein and Desmin and ICD components in icKO and Ctrl hearts at 2 weeks post TAM (n=3). Other than FAK, δ-SAG, β-DSG, and Dystrophin which were not detected in our mass spectrometry studies, all other proteins tested in the western blot analysis have similar changes in mass spectrometry studies. (Q) qRT-PCR analysis for mRNAs of proteins examined in Ctrl and icKO mouse hearts at 2 weeks post TAM injection. (R) Functional enrichment analysis of proteins upregulated in icKO. For western blot analysis, GAPDH served as a loading control. The pixel density of each protein was normalized to the level of GAPDH. In qRT-PCR assays, mRNA levels of each gene were normalized to corresponding 18S levels, with icKO values being expressed as fold-change versus control. Data are represented as the mean ± SEM. *P < 0.05, by 2-tailed Student’s t test.
Echocardiographic studies revealed a rapid and progressive decrease in LV systolic function (fractional shortening) in icKOs relative to controls (Figs. G–H). LV chamber dilation was evidenced by a significant increase in end-diastolic LV internal diameter (LVIDd) and end-systolic LV internal diameter (LVIDs) (Figs. I–J).
FLNC interacts with multiple cytoskeletal proteins, including integrins and the dystrophin-associated glycoprotein complex (DGC) at the costamere, Myotilin and FATZ-1 at the Z-disk, and Xin proteins at the ICD 3. We investigated whether loss of Flnc in CMs affected levels of major cytoskeletal proteins comprising these complexes (Figs. K–P) 5. Loss of Flnc resulted in significant increases in β1D-integrin, as well as Talin1, Kindlin2, FAK, Vinculin, and ILK, while Paxillin was not changed (Fig. L). δ− and γ−SAG, Dystrophin, and β-DSG, but not α-DSG, were increased in icKO hearts (Fig. M). Desmin, an intermediate filament protein was also increased. Z-disc proteins, Myotilin, FATZ-1, α-actinin and Cypher, were not changed (Fig. N). ICD proteins Xirp1 and Xirp2, JUP, and DSP, were significantly increased, while PKP2 and DSG2 were not changed (Fig. O). These results demonstrated that loss of Flnc resulted in increased levels of a subset of costameric and ICD proteins (Fig. P). qRT-PCR data indicated that mRNA levels of increased proteins were not changed, with the exception of Xirp1 and Xirp2 mRNAs that were significantly increased (Fig. Q). Immunostaining for upregulated proteins showed no protein aggregation or mislocalization in icKO hearts (data not shown).
Mass spectrometry (MS) analysis identified 380 proteins that were upregulated more than 2-fold, and 27 proteins that were downregulated more than 2-fold, in icKO hearts relative to controls. These data confirmed increased levels of specific proteins found by initial Western blot analyses. Functional clustering of upregulated proteins revealed most significant pathway enrichment in “cytoskeletal protein binding”, “actin binding”, and “extracellular matrix structural constituent” (Fig. R).
Previous mutation of Flnc in mice resulted in defects in skeletal muscle development, with no evident cardiac phenotype 4, despite the observation that recessive mutations in FLNC lead to human congenital dilated cardiomyopathy 1, 2. Our results have addressed this apparent contradiction, by demonstrating a key and critical role for FLNC in both developing and adult CMs. In embryonic CMs, loss of FLNC resulted in fetal demise. In adult CMs, upon loss of FLNC, mice developed rapid and fulminant DCM within 2 weeks. Intriguingly, loss of FLNC was accompanied by upregulation of multiple proteins, including those that directly interact with FLNC, representing components of the costamere and ICD, and the intermediate filament protein, Desmin (Fig. K). The dire effects of loss of FLNC are likely to reflect its significant impact on all three of these major constituents of the CM cytoskeleton, essential for normal contraction.
Acknowledgments
Sources of Funding
JC, XF and SME are funded by grants from the National Institutes of Health, the National Heart, Lung, and Blood Institute, and JC holds an American Heart Association Endowed Chair in Cardiovascular Research. MZ was supported by an American Heart Association Summer Undergraduate fellowship.
Data Sharing: The data, analytical methods, and study materials that support the findings of this study will be available to other researchers from the corresponding authors on reasonable request.
Footnotes
Conflict of Interest Disclosures
None
Reference
- 1.Ader F, De Groote P, Reant P, Rooryck-Thambo C, Dupin-Deguine D, Rambaud C, Khraiche D, Perret C, Pruny JF, Mathieu-Dramard M, Gerard M, Troadec Y, Gouya L, Jeunemaitre X, Van Maldergem L, Hagege A, Villard E, Charron P and Richard P. FLNC pathogenic variants in patients with cardiomyopathies: Prevalence and genotype-phenotype correlations. Clin Genet. 2019;96:317–329. [DOI] [PubMed] [Google Scholar]
- 2.Reinstein E, Gutierrez-Fernandez A, Tzur S, Bormans C, Marcu S, Tayeb-Fligelman E, Vinkler C, Raas-Rothschild A, Irge D, Landau M, Shohat M, Puente XS, Behar DM and Lopez-Otin C. Congenital dilated cardiomyopathy caused by biallelic mutations in Filamin C. Eur J Hum Genet. 2016;24:1792–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Ven PF, Ehler E, Vakeel P, Eulitz S, Schenk JA, Milting H, Micheel B and Furst DO. Unusual splicing events result in distinct Xin isoforms that associate differentially with filamin c and Mena/VASP. Exp Cell Res. 2006;312:2154–67. [DOI] [PubMed] [Google Scholar]
- 4.Dalkilic I, Schienda J, Thompson TG and Kunkel LM. Loss of FilaminC (FLNc) results in severe defects in myogenesis and myotube structure. Mol Cell Biol. 2006;26:6522–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Z, Mu Y, Zhang J, Zhou Y, Cattaneo P, Veevers J, Peter AK, Manso AM, Knowlton KU, Zhou X, Evans SM, Ross RS and Chen J. Kindlin-2 Is Essential for Preserving Integrity of the Developing Heart and Preventing Ventricular Rupture. Circulation. 2019;139:1554–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]