Abstract
Background.
Little is known about antibiotic prescribing practices of dentists. The objective of this study was to gain a better understanding of dentists’ beliefs and behaviors regarding the use of antibiotic prophylaxis (AP) before invasive dental procedures.
Methods.
A multidisciplinary team developed and disseminated a questionnaire to 3,584 dentist members of the National Dental Practice-Based Research Network.
Results.
Overall, 2,169 network dentists (61%) responded. Respondents saw patients at risk of developing infective endocarditis (IE) and prosthetic joint infection (PJI) at least once per week (35% and 65%, respectively). Although 78% of dentists agreed that the 2007 American Heart Association guidelines for the prevention of IE are well-defined and clear, only 49% agreed concerning PJI guidelines. Differences for the IE and PJI patient populations also existed for questions regarding dentists’ understanding of the specific patient groups at risk, the recommended antibiotic regimens, and the need to consult with a patient’s cardiologist or orthopedic surgeon.
Conclusions.
The survey results indicate that decision making regarding the use of AP occurs frequently among dentists. Moreover, dentists reported uncertainty about the appropriate use of AP as defined by both IE and PJI guidelines, which may have resulted in a lack of concordance between dentists’ beliefs and their practice behaviors.
Practical Implications.
The results reported by the authors highlight the need to develop better educational programs that address antimicrobial stewardship in AP for patients at risk of developing IE and PJI and target the dental profession.
Keywords: Endocarditis, surveys, antibiotic prophylaxis, antibiotics, practice guidelines, cardiovascular diseases, infection
The use of antibiotics to prevent infection at an anatomic location distant to the site of an invasive procedure is referred to as secondary prophylaxis. Support for antibiotic prophylaxis (AP) is based on several factors, the most important of which is a concern for preventing rare but devastating complications such as infective endocarditis (IE), with its high morbidity and mortality rates.1–4
A systematic review of studies conducted over the past 40 years found that the incidence, duration, nature, and magnitude of bacteremia from a variety of invasive dental procedures and from activities of daily living (for example, toothbrushing).5 The studies in the systematic review have contributed to an emphasis on dental procedures as a primary source of transient bacteremia and the potential for distant-site infections, including IE and prosthetic joint infections (PJIs). There is, however, an increasing worldwide concern about the unnecessary and unsupported use of antibiotics for prophylactic as well as therapeutic purposes, given marked risks of experiencing adverse drug effects for people and society.6–8 Although there have been no randomized trials, results of some large observational studies suggest a potential benefit from AP in certain “at-risk” cardiac patient populations, furthering confusion regarding the use of AP.9–12
Since the initial formal recommendations for AP were issued by the American Heart Association (AHA) in 1955, there has been a major increase in the use of secondary AP for a wide variety of patient populations, most notably for a variety of cardiac conditions, as well as for prosthetic joints.2,4,13 However, the nature of the patient populations, the number of patients at risk, and the frequency of AP use are unclear. Studies suggest that there are wide variations in dentist and physician opinions regarding the use of AP for various patient populations, clinical settings, and dental procedures, as well as on compliance with AP guidelines both for patients with cardiac conditions and prosthetic joints.4,14–17 It is not clear what factors dental practitioners use when making decisions about secondary AP for these patient populations.
The primary objective of our study was to quantify the beliefs and behaviors related to dentists’ use of secondary AP, with a focus on patients at risk of developing IE or PJI. Secondary objectives were to explore factors related to dentists’ adherence to AP guidelines, the influence of these guidelines on AP prescribing practices, and their knowledge about risks for bloodstream infections and the utility of AP in preventing distant-site infections.
METHODS
A multidisciplinary study team of clinicians and research experts in oral medicine, psychology, informatics, statistics, and survey methodology developed a questionnaire with 15 multiple-response questions on AP prescribing practices. We used an extensive process for the development of this survey to ensure that the data derived from these dental practices would best reflect the beliefs and behaviors of dentists in the United States. The complexity and length of the methodology was such that it necessitated publication of a separate article.18 In brief, the development of the survey involved 3 stages. In stage 1, we determined the time line, collected supporting documentation, and established ad hoc a preliminary survey draft of 90 questions, which was refined by team members. We then implemented a think-aloud test with a group of 11 dental practitioners to identify and reduce cognitive demand and fatigue, thereby optimizing the response rate. In stage 2, we organized the survey into themes, and the National Institute of Dental and Craniofacial Research carried out an informal review. A final survey of 15 multiple-response questions was reviewed by the National Dental Practice-Based Research Network Central Institutional Review Board (IRB), University of Alabama at Birmingham, and the IRB at Atrium Healths Carolinas Medical Center. In stage 3, we configured the final survey using Research Electronic Data Capture (REDCap) software. We distributed e-mail invitations, which included a link to the questionnaire, through REDCap to 3,584 actively practicing dentists, including general dentists and specialists in endodontics, periodontics, prosthodontics, orthodontics, pediatric dentistry, dental public health, and oral and maxillofacial surgery. We considered dentists who did not complete the questionnaire within 2 weeks of a third e-mailed invitation as nonresponders. All dentists were members of The National Dental Practice-Based Research Network (“network”), a consortium of dental practices and dental organizations focused on improving the scientific basis for clinical decision making.19,20 All activities for these investigations were approved by the IRBs governing each of the 6 regions encompassing the network. We also collected data about each practitioner using the network’s Enrollment Questionnaire of reported information about themselves, their practices, and their patient population.20 The typical enrollee completed the questionnaire online, although a paper option was also available.
Dental practitioners eligible for this study were all US-licensed, clinically active general and specialty dentists, and current members of the network.
We assessed the dentists’ prescribing practice behaviors with questions that covered the following:
how often they see specific patient populations;
their sense of clarity of AP guidelines;
the extent to which they consult with the patient’s physician and who has the responsibility to make decisions concerning the need for AP;
their adherence to AP guidelines;
their opinions on efficacy of AP and its use for different patient populations and dental procedures.
Statistical analyses
We conducted power analysis on the basis of an anticipated sample size of 2,400 completed questionnaires. This sample size would yield sufficient precision to estimate response percentages with a mean (standard deviation) margin of error of 3.15% (0.34) per region, with a 95% confidence level. We present descriptive statistics as counts and percentages for categorical variables and as means (standard deviations) for continuous measures. We conducted the analysis using SAS Enterprise Guide Version 7.1 on platform of SAS Version 9.4 (SAS Institute).
RESULTS
Overall, 2,169 eligible dentists (61%) responded to the questionnaire, which included 1,706 (79%) general practitioners and 458 (21%) specialists (information on practice type was missing for 5 respondents). Five respondents did not provide practice types. The remaining demographics are shown in the table.
Table 1.
Demographic characteristics of all practitioners who completed the antibiotic prophylaxis questionnaire, based on responses from the The Practice-Based Research Network enrollment questionnaire.
| CHARACTERISTIC | NO. (%) |
|---|---|
| Total | 2,169 (100.0) |
| Age Groups, y | |
| 25–35 | 94 (4.3) |
| 35–45 | 501 (23.1) |
| 45–55 | 444 (20.5) |
| 55–65 | 686 (31.6) |
| ≥ 65 | 421 (19.4) |
| Missing | 23 (1.1) |
| Network Region | |
| Western | 313(14.4) |
| Midwest | 247 (11.4) |
| Southwest | 437 (20.1) |
| South Central | 418(19.3) |
| South Atlantic | 293 (13.5) |
| Northeast | 459 (21.2) |
| Missing | 2(0.1) |
| Sex | |
| Male | 1,507 (69.5) |
| Female | 649 (29.9) |
| Missing | 13 (0.6) |
| Race | |
| White | 1,751 (80.7) |
| African American | 87 (4.0) |
| American Indian or Alaska Native | 5 (0.2) |
| Asian | 216 (10.0) |
| Native Hawaiian or Other Pacific Islander | 4 (0.2) |
| Other and missing | 106 (4.9) |
| Hispanic or Latino Origin | |
| Yes | 115 (5.3) |
| No | 2,021 (93.2) |
| Missing | 33(1.5) |
| Primary Practice Location | |
| Inner city of urban area | 272 (12.6) |
| Urban | 612 (28.2) |
| Suburban | 968 (44.6) |
| Rural | 298 (13.7) |
| Missing | 19 (0.9) |
| Practice Time Type | |
| Full time | 1,798 (82.9) |
| Part time | 344 (15.9) |
| Missing | 27 (1.2) |
| Practice Type | |
| General dentist | 1,706 (78.7) |
| Specialist | 458 (21.1) |
| Missing | 5 (0.2) |
| Practice Locations, No. | |
| 1 | 1,701 (78.4) |
| 2 | 347 (16.0) |
| 3 | 67 (3.1) |
| ≥ 3 | 52 (2.4) |
| Missing | 2 (0.1) |
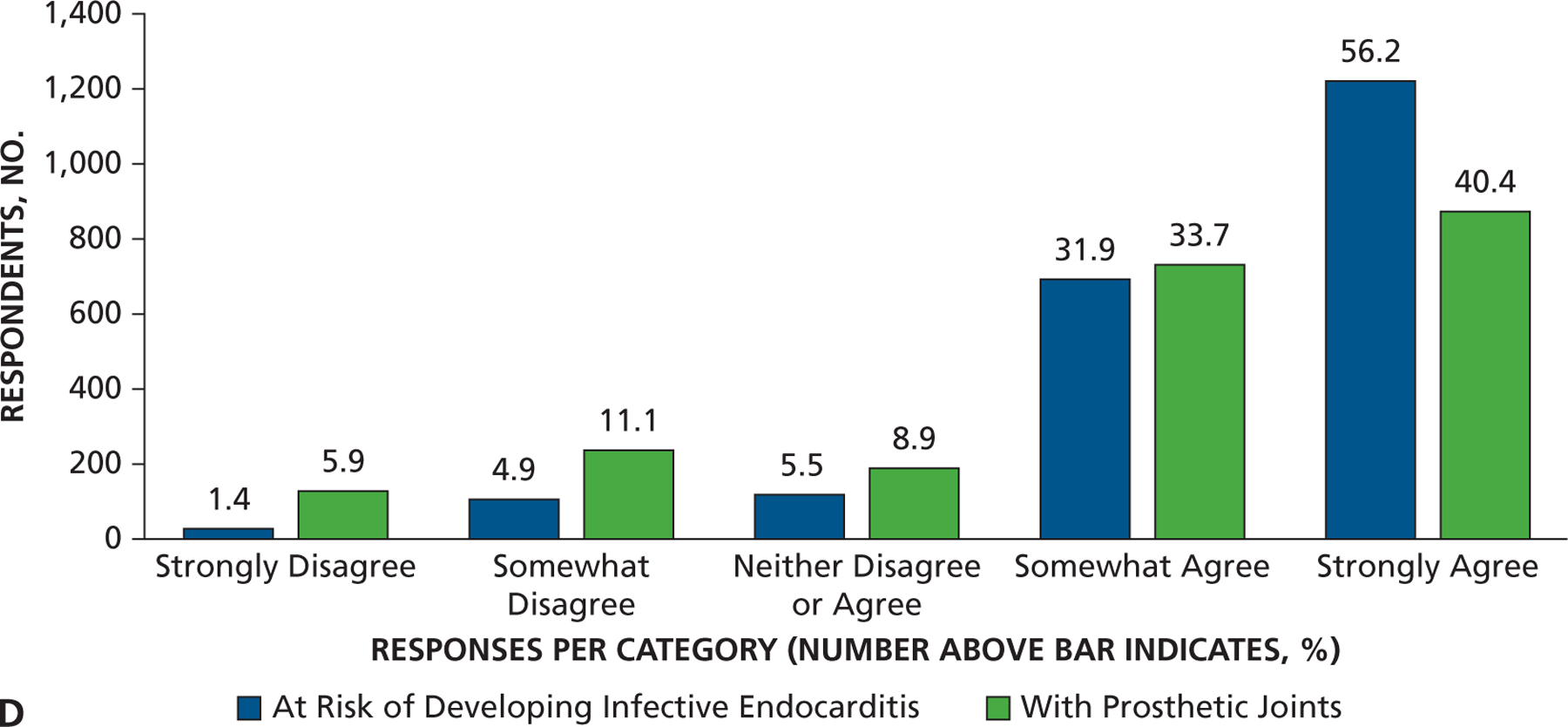
Most dentist respondents saw patients at risk of developing PJI once per week or more often (65%) and patients at risk of developing IE once per month or more often (73%), with 35% seeing them at least once per week (Figure 1 and eTable, available online at the end of this article). Seventy-eight percent of dentists responded that they either “strongly agree” or “somewhat agree” that the AHA IE prevention guidelines were well defined and clear, but only 49% felt that way concerning patients at risk of developing PJI (Figure 2A). Similarly, 75% of dentists agreed that IE-risk patient groups were “well defined and clear” versus 47% for PJI-risk patients (Figure 2B). Seventy-two percent of respondents acknowledged that dental procedures of concern were well defined and clear for patients at risk of developing IE versus 55% for PJI (Figure 2C). Differences in clarity also exist for questions regarding appropriate antibiotic regimens (88% for IE and 74% for PJI) (Figure 2D). Similarly, substantial percentages of respondents felt a need to consult with a patient’s cardiologist or orthopedic surgeon (48% for IE and 59% for PJI) about the need for AP, and many preferred that a patient’s physician make the decision regarding the need for AP (63% for IE and 71% for PJI) (Figures 3A and 3B). When asked about the antibiotic they preferred, dentists rarely prescribed an alternative to those recommended by the AHA or American Dental Association (ADA) (Figure 4). When asked what they would do if a patient’s physician advised AP that was not consistent with standard guidelines, the most common response (45%) was to ask the physician or surgeon to provide the prescription to the patient, although other common responses included following the physician’s or surgeon’s instructions (25%) or calling the physician or surgeon to discuss the issue (21%) (Figure 5). Dentists were asked about the AHA-recommended dose and timing for AP and how often they gave prophylaxis for more than the 1 recommended dose. Most (86%) replied “never” or “rarely” (Figure 6).
Figure 1.
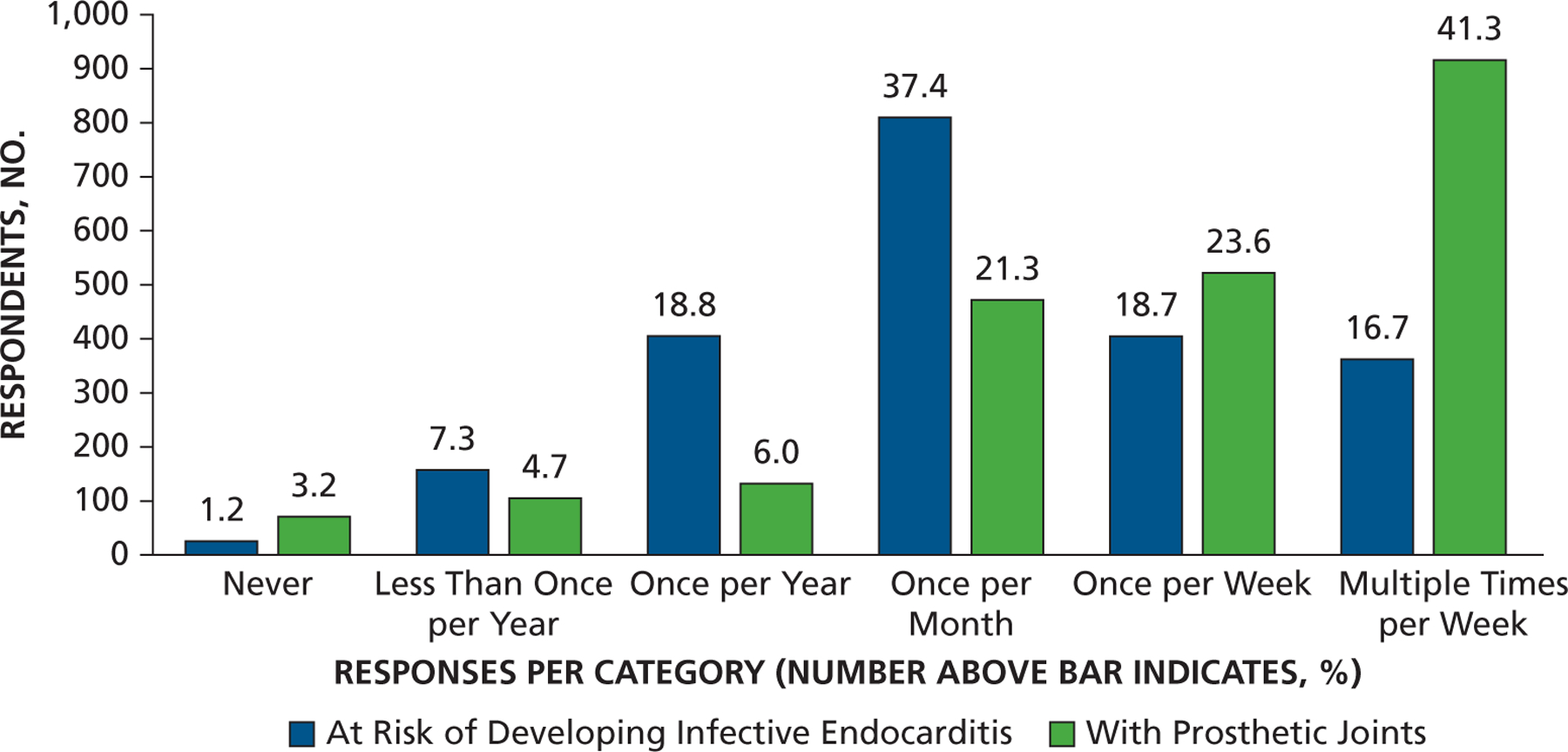
Responses to the question “Approximately how often do you see the following patient populations in your practice?”
Figure 2.
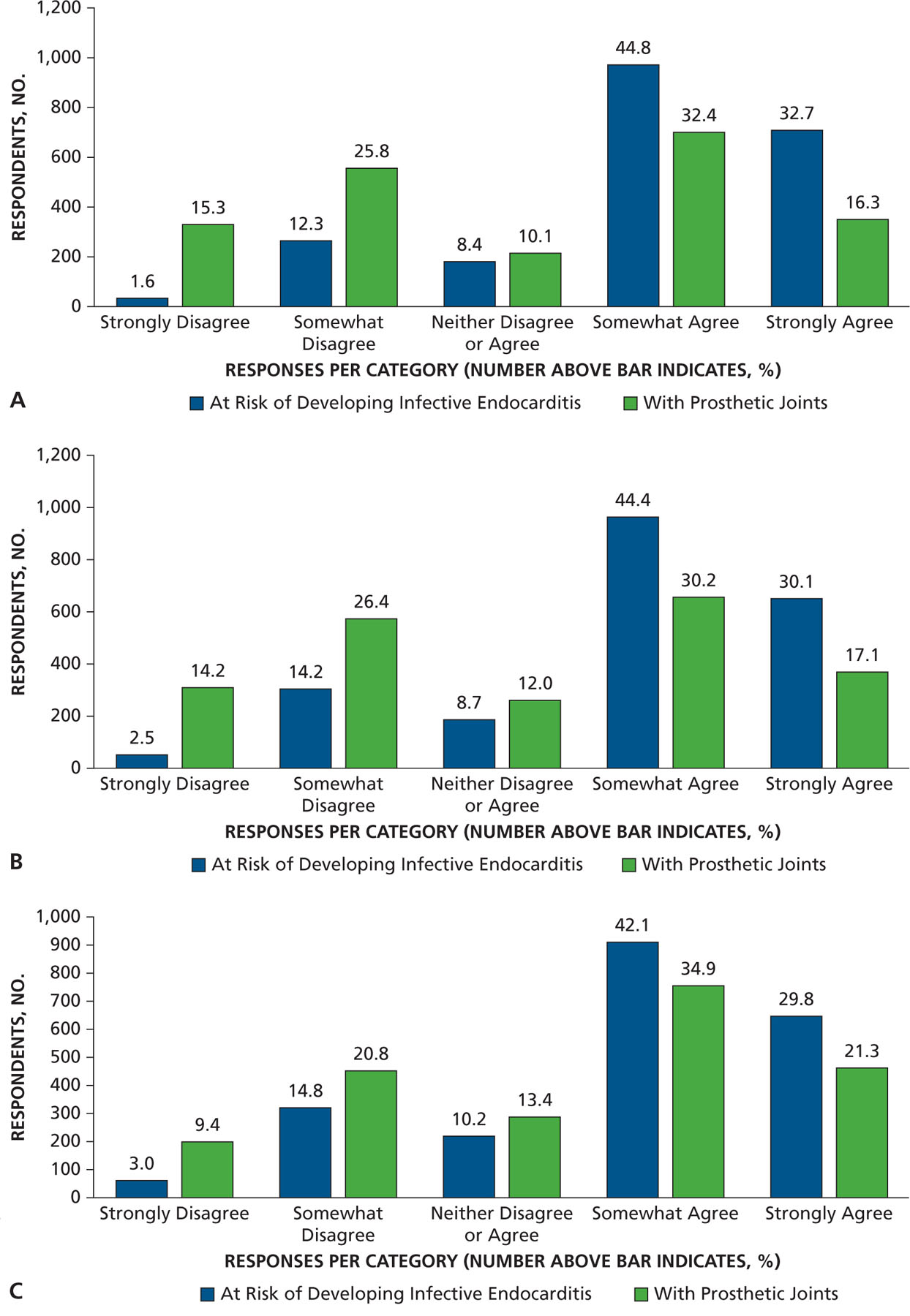
A. Responses to the question “To what extent do you agree with the following statement? Guidelines concerning the use of antibiotic prophylaxis are well defined and clear.” B. Responses to the question “To what extent do you agree with the following statement? The patient groups who should receive antibiotic prophylaxis are well defined and clear.” C. Responses to the question “To what extent do you agree with the following statement? The dental procedures that require antibiotic prophylaxis are well defined and clear.” D. Responses to the question “To what extent do you agree with the following statement? The antibiotic prophylactic regimens (drugs and dosages) are well defined and clear.”
Figure 3.
A. Responses to the question “To what extent do you agree with the following statement? I feel the need to consult with the patient’s cardiologist, orthopedic surgeon, or physician about whether or not antibiotic prophylaxis is needed.” B. Responses to the question “To what extent do you agree with the following statement? I think the patient’s cardiologist, orthopedic surgeon, or physician should decide if a patient needs antibiotic prophylaxis when undergoing invasive dental procedures.”
Figure 4.
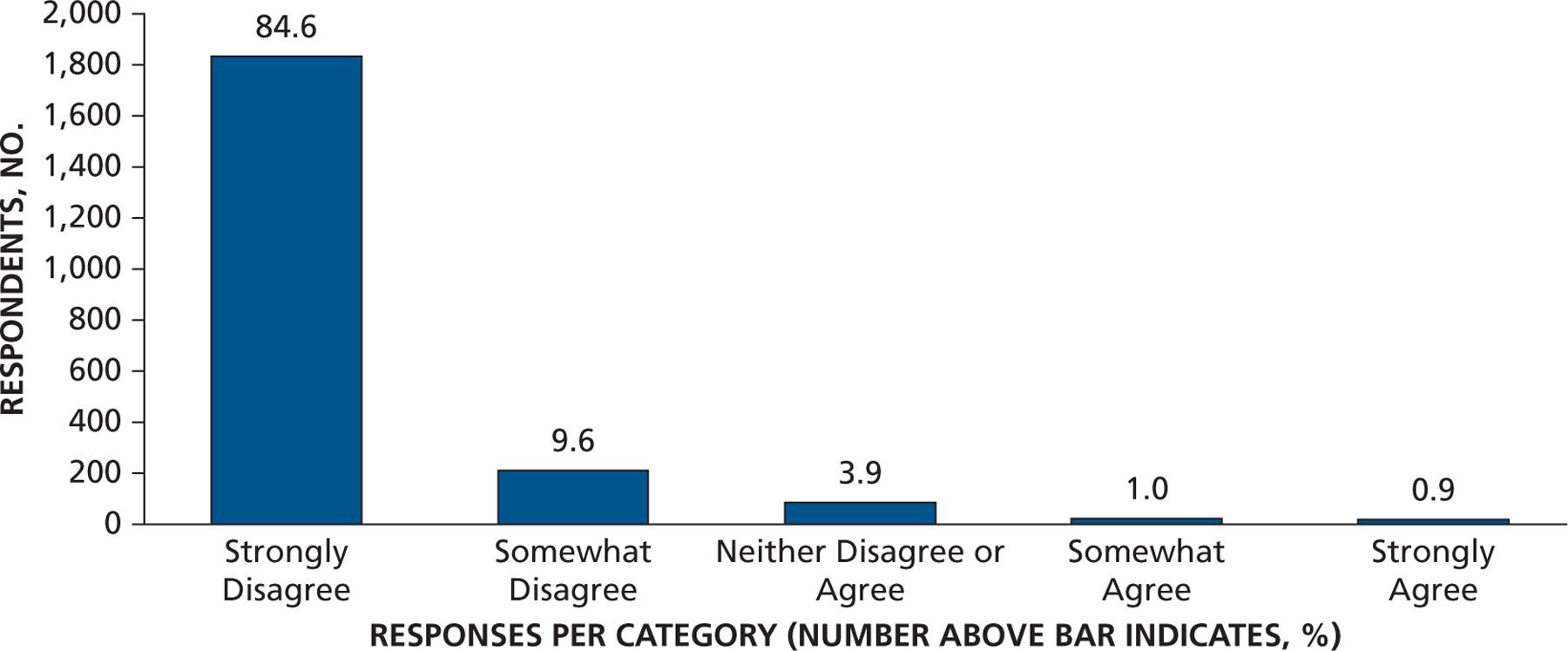
Responses to the statement “I prescribe alternative antibiotics rather than those recommended by the AHA or ADA for my patients who require antibiotic prophylaxis prior to dental procedures.” ADA: American Dental Association. AHA: American Heart Association.
Figure 5.
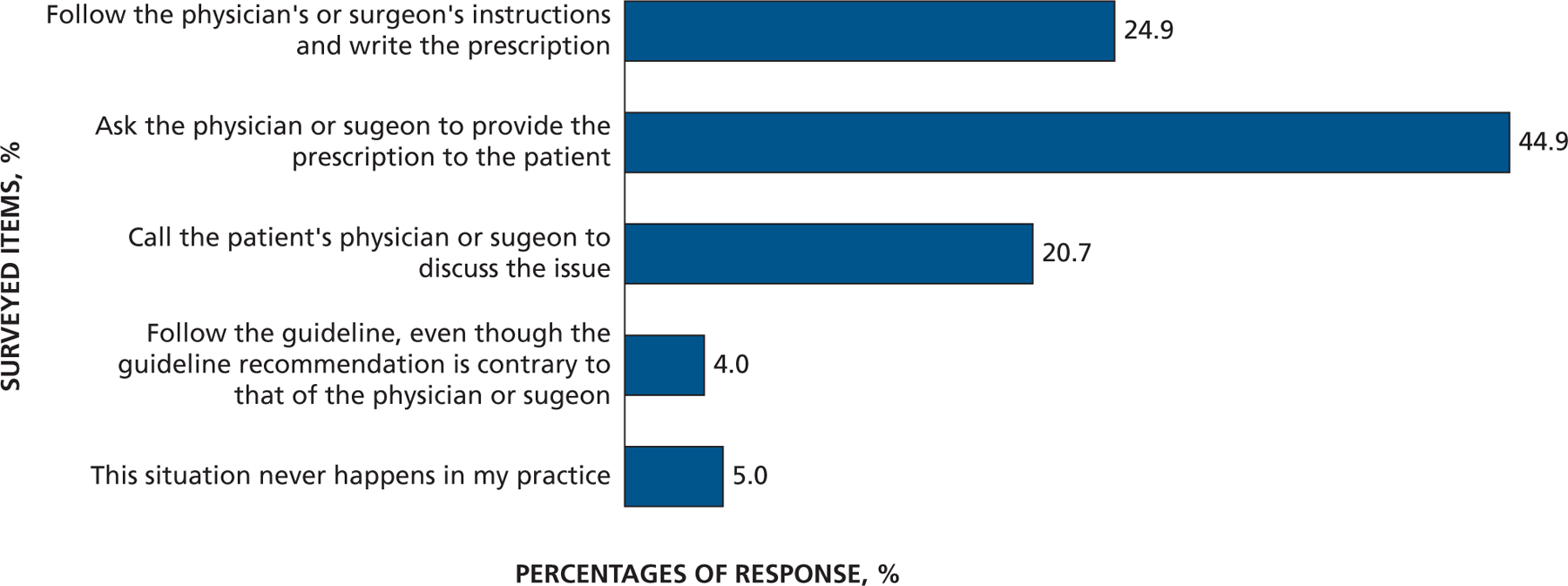
Responses to the question “Thinking about the antibiotic prophylaxis regimens, if a patient’s physician or surgeon advises prescribing antibiotic prophylaxis that is not consistent with standard guidelines, would you most likely …”
Figure 6.
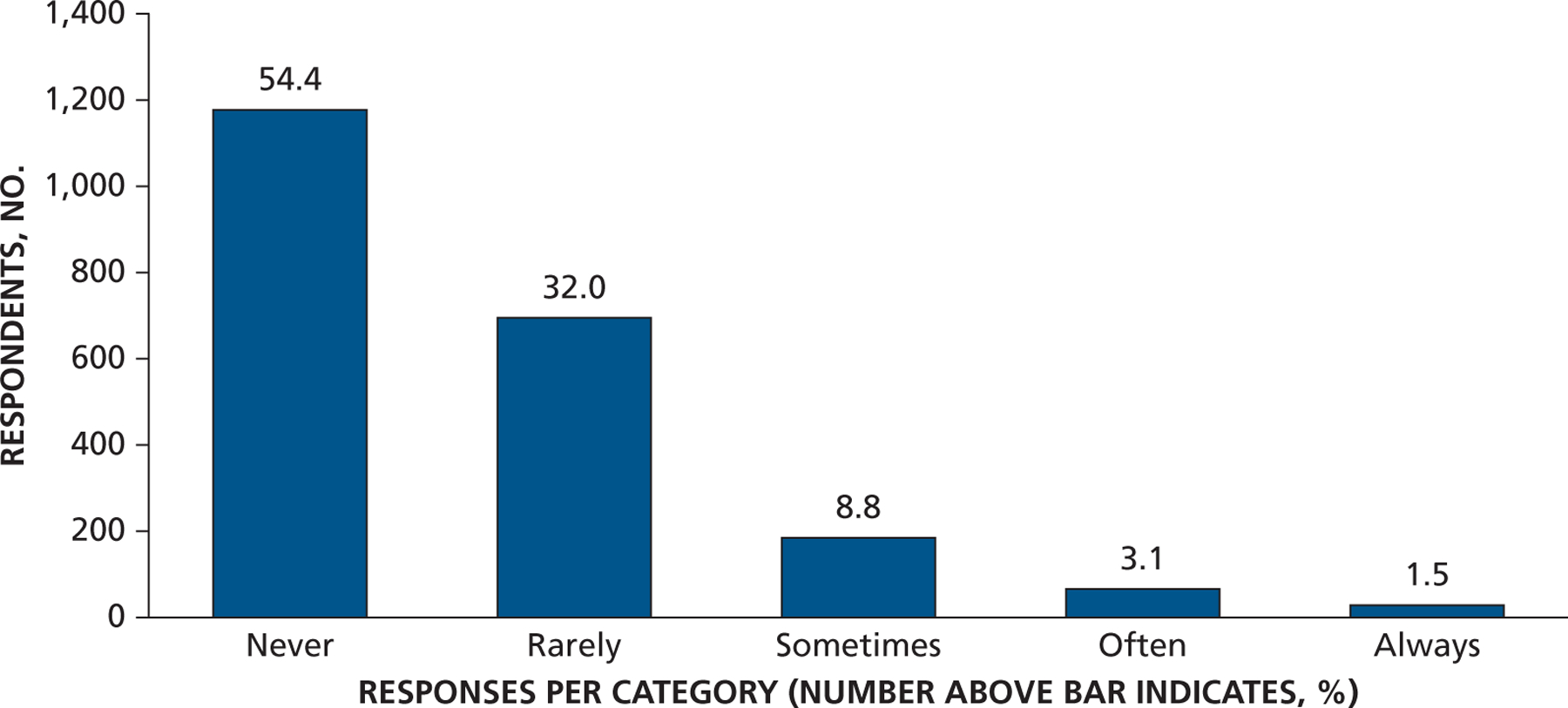
Responses to the question “AHA guidelines recommend a specific dose of antibiotic given 30–60 minutes before the procedure. How often do you give prophylactic antibiotics for longer than the 1 recommended dose?” AHA: American Heart Association.
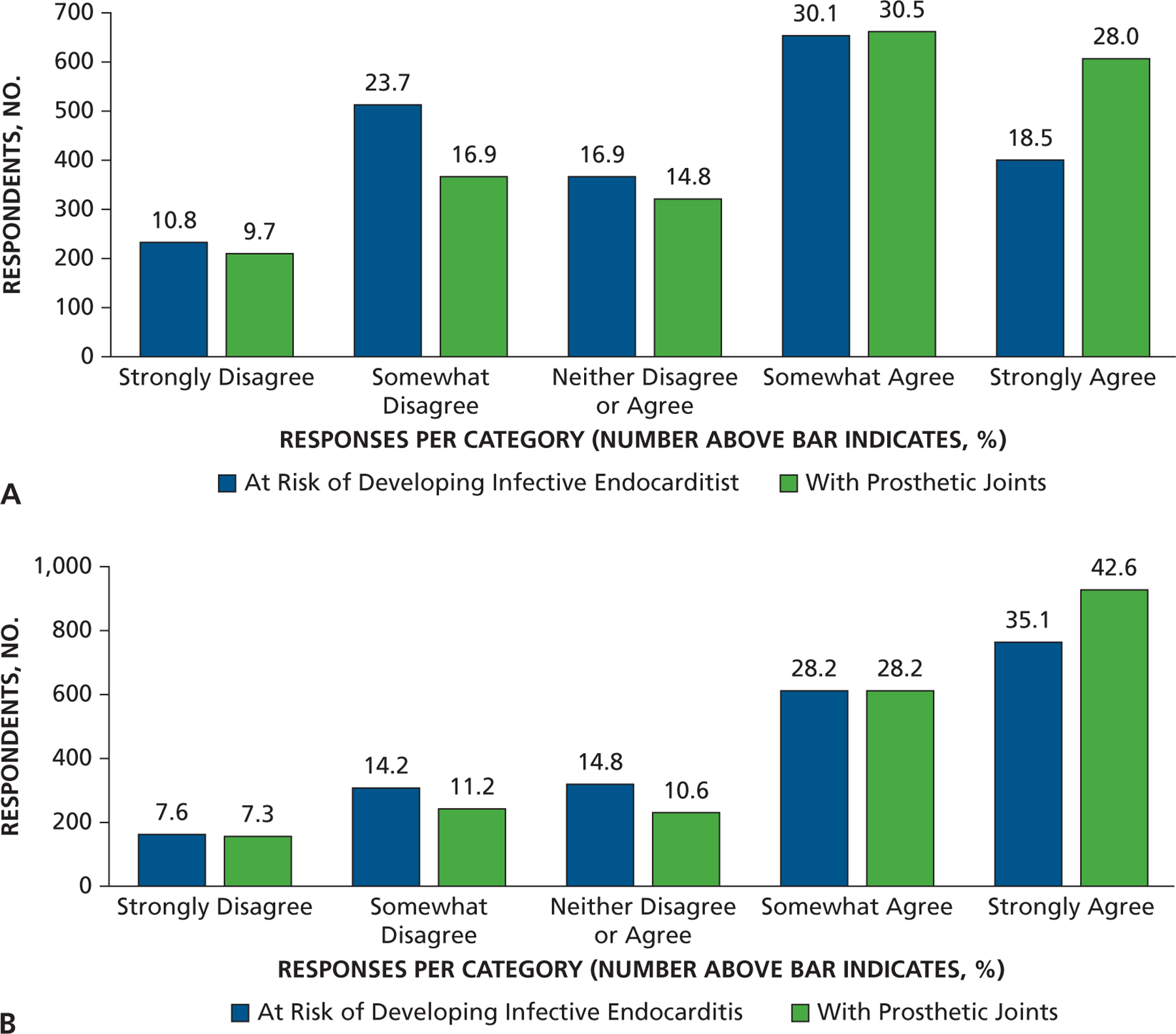
Another series of questions addressed other patient populations that might be at risk of developing distant-site infections. When asked about the extent to which AP prevents infection, more dentists somewhat or strongly agreed that AP prevented infection in those with a prosthetic heart valve (recommended for AP by the AHA) (80%) compared with patients with a prosthetic joint (43%). Far fewer dentists somewhat or strongly agreed that AP prevented infection in those with a heart murmur (not recommended for AP by the AHA) (17%). However, when asked about patients with a coronary artery bypass graft (not recommended for AP by the AHA), 42% of dentists somewhat or strongly agreed that AP prevented infection, close to the response concerning patients with a prosthetic joint (Figure 7). When asked if they ever prescribe AP before invasive dental procedures for other patient populations, most dentists said they would defer to the patient’s physician about the need for AP in patients who are immunosuppressed owing to corticosteroids (59%), cancer chemotherapeutic drugs (65%), organ transplant immunosuppression (66%), or disease (for example, HIV and AIDS) (61%). Far fewer (34%) felt the need to defer to a patient’s physician about need for AP in people with diabetes who are insulin dependent, and most (50%) would not give AP, although 15% would (Figure 8). Finally, with regard to risk of developing IE, only 19% of dentists strongly or somewhat agreed that local anesthetic injection posed a risk, whereas 31% strongly or somewhat agreed that home care posed a risk. More dentists strongly or somewhat agreed that extractions (77%), scaling (68%), and, to a lesser extent, restorations that involve the gingival margin (46%) pose a risk (Figure 9).
Figure 7.
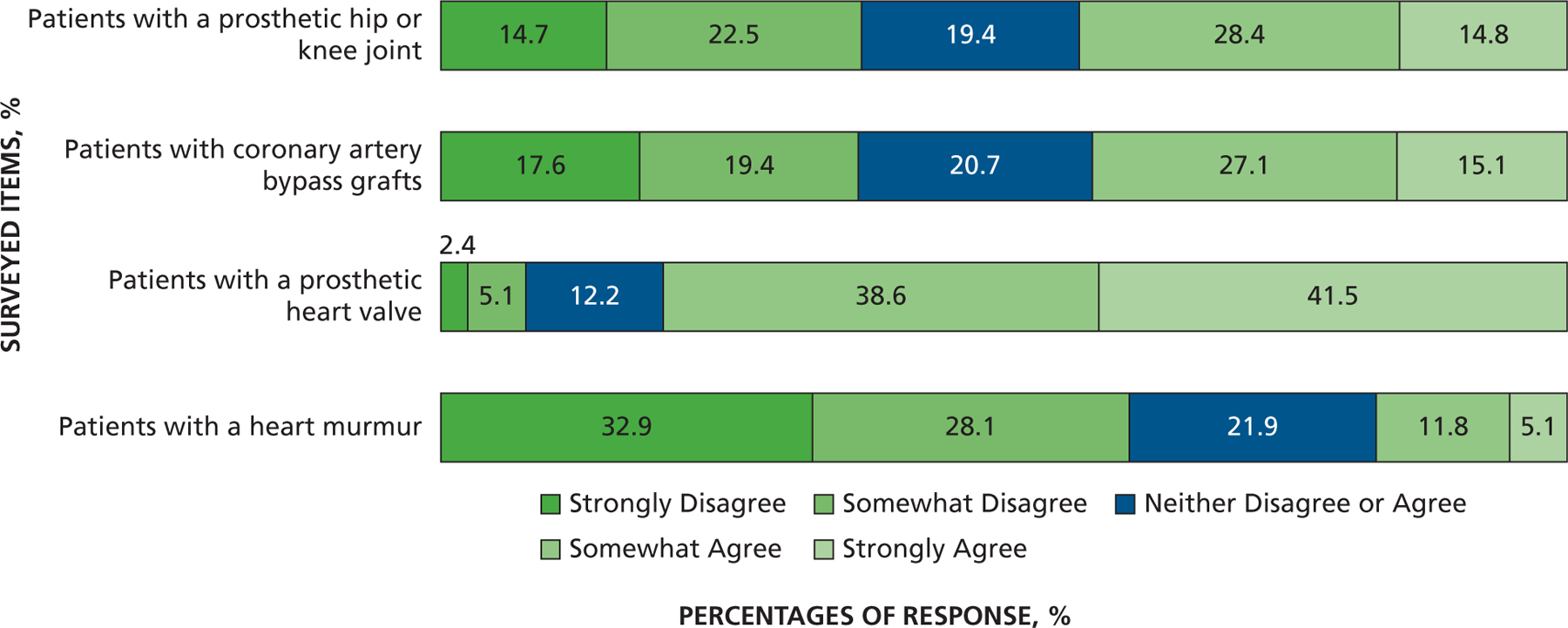
Responses to the question “To what extent do you agree that antibiotic prophylaxis prevents infection in the following patient populations?”
Figure 8.
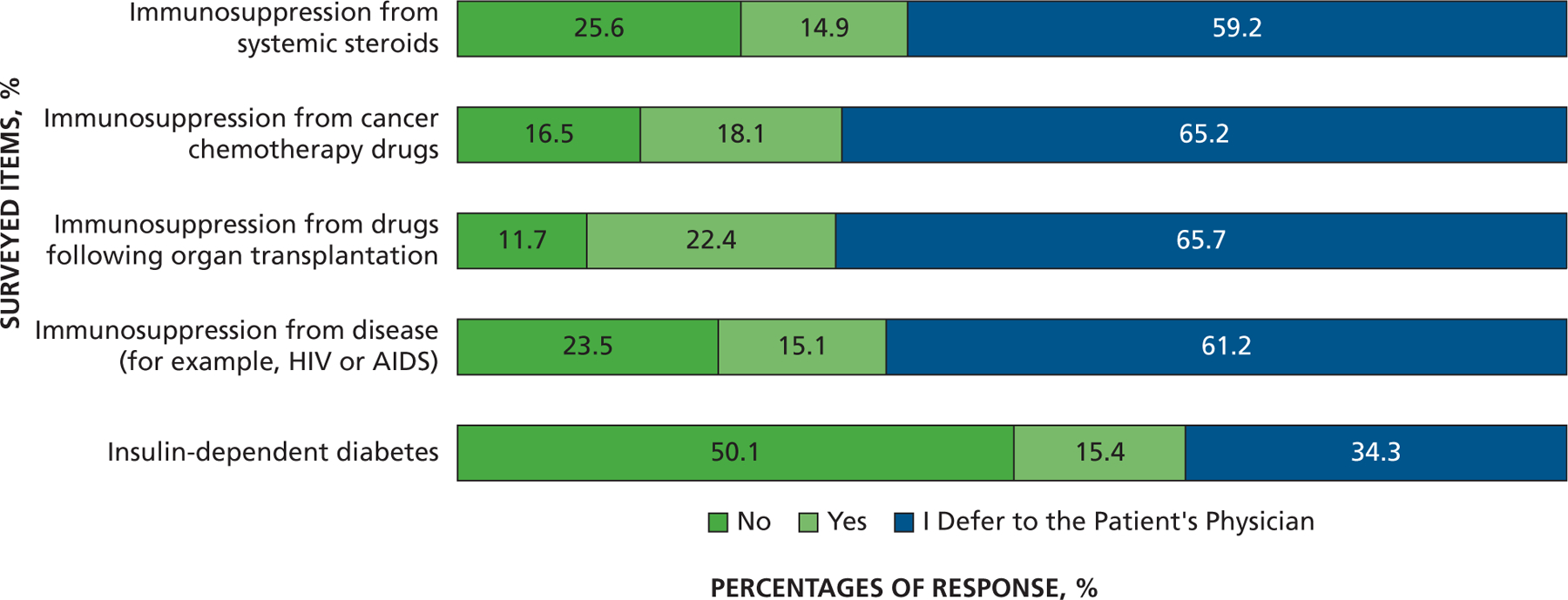
Responses to the question “Do you ever prescribe antibiotic prophylaxis prior to invasive dental procedures in your office for patients with: …”
Figure 9.
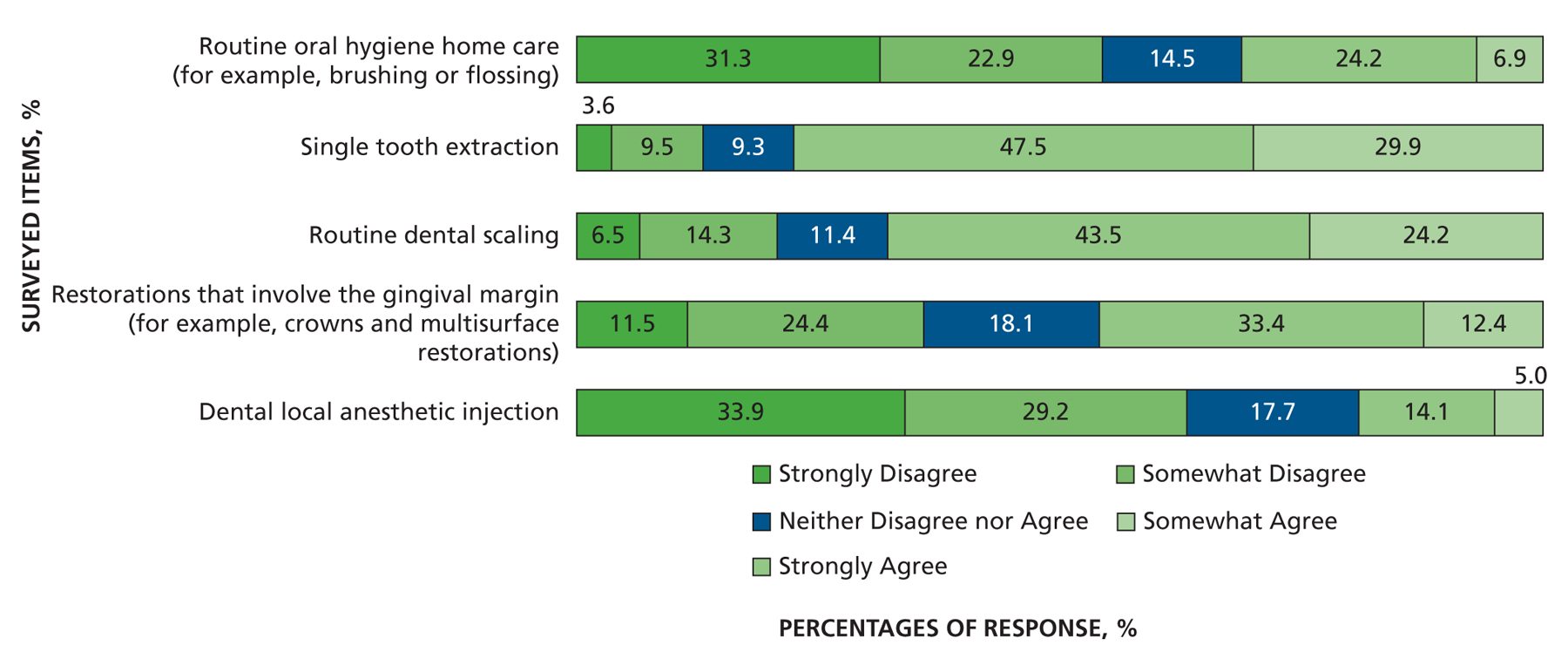
Responses to the question “To what extent do you agree that each of the following put patients at some risk for infective endocarditis?”
DISCUSSION
The results of this survey are important and show a paradox in practice. They indicate that despite the commonality of AP use in dental practices, the understanding of recommended guidelines for its use in patient groups at risk of developing both IE and PJI is not optimal. Educational efforts, therefore, are warranted because the indications for AP use are a frequent clinical issue encountered by dentists.
We found a high frequency of patients at risk of developing IE and PJI seen in network practices and this likely contributed to our high response rate of 61% and suggests a strong interest in AP. A survey of 530 French dentists found that 94% treat patients at risk of developing IE at least once per month.21 The response rate to our questionnaire exceeds those for practitioner surveys in general,22 and this may reflect network practitioners’ desire to contribute to the scientific base for clinical practice.
Consistent differences between dentists’ beliefs and behaviors regarding AP use in patients at risk of developing IE versus PJI may reflect, in part, the history of professional guidelines addressing these 2 patient populations. AHA guidelines restrict AP to patients at highest risk from (not of developing) IE and are not intended for those at moderate risk,13 who represent nearly 90% of patients previously recommended for AP. This moderate-risk group deletion in 2007 therefore resulted in a 90% reduction in the number of patients recommended for AP.2,23 The use of AP for patients with prosthetic joints, however, is a long-standing and controversial issue. The role of oral bacterial species in IE is well established, and it may have prompted orthopedic surgeons to support AP to prevent the devastating consequences of late PJI.24 However, despite their role in IE, oral bacterial species are a rare cause of PJI.
There is ongoing controversy regarding which, if any, patients with prosthetic joints are sufficiently at risk to warrant regular exposure to antibiotics for invasive dental procedures.2,25 The long-standing practice of using AP before a dental procedure for all patients with a prosthetic joint changed in 1997 when a joint committee representing the ADA and the American Association of Orthopaedic Surgeons recommended that AP only be used for 2 years after placement of a prosthetic joint and beyond 2 years for only a select group of medically complex patients.26,27 This well-accepted standard, however, was reversed in 2009 when the AAOS reverted back to the informal pre-1997 standard that essentially called for AP for all patients with prosthetic joints and for life. Since then, there have been 3 formal attempts to resolve this controversy,25,28,29 but the outcome has been ongoing confusion.30,31 This likely explains why, in our study, only 16% of dentists strongly agreed with the statement that guidelines concerning the use of AP are well defined and clear for patients with prosthetic joints.
The long history of the AHA guidelines, along with consistent involvement of the dental profession in producing them, probably explains why more dentists somewhat or strongly agreed that the AHA guidelines for the patient groups who should receive AP are more “well defined and clear” for IE than for PJI (Figure 2B). However, even for AHA guidelines, only 32.7% of dentists strongly agreed that they were well defined and clear, and only 30.1% felt that the patient groups recommended for AP in the AHA guidelines were well defined and clear. This may explain recent findings that suggest many US dentists are continuing to prescribe AP for patients in the AHA moderate-risk group.12,23 These observations are not unique to the US. A French survey found that 88% of dentists still prescribe AP to patients at moderate risk of developing IE despite French guidance recommending that they not do so.21
In addition, since the 2007 AHA guidelines were issued, efforts to reduce AP in those at moderate risk of developing IE inadvertently may have resulted in a substantial fall in AP prescribing for those at high risk.23 This may reflect the difficulties dentists experience in distinguishing between the different cardiac conditions that constitute high and moderate risk. It also may reflect the pressures of the antibiotic stewardship message to reduce antibiotic prescribing wherever possible.
When asked about dental procedures that put some patients at risk of developing IE, respondents gave opinions that covered the spectrum from strongly disagree to strongly agree (Figure 9). The level of confusion appears to be high, exceeding 90% in 1 survey.17 Of interest, the dental procedures that may put patients at risk are stated in the AHA guidelines with a simple sentence that was intended to result in a common understanding of risk of undergoing the many dental procedures with widely varying invasiveness (Figure 9).
Our results also show that most dentists somewhat or strongly agree that a patient’s cardiologist, orthopedic surgeon, or physician should decide if a patient needs AP. This likely reflects concerns about the lack of clarity of the IE and PJI guidelines and the feeling that cardiologists and orthopedic surgeons are better able to make this decision. It may also reflect medicolegal concerns about who should take responsibility for these decisions. In the past, the AHA produced a wallet card for cardiologists to give to patients to indicate if AP were recommended.
It also appears that 15% through 22% of dentists use AP for patients who may be immunosuppressed owing to drug use or disease and for those who have insulin-dependent diabetes. There are no published guidelines on AP for these patient populations and no scientific studies to suggest a risk
Our data have certain limitations, and conclusions should take into account that we measured beliefs about treatment recommendations in hypothetical clinical scenarios, which may not reflect clinical treatment behavior. In addition, although the response rate was good, it is possible that nonrespondents would have reported different beliefs and behavior. Although network practitioners have much in common with dentists at large,32 they are not recruited randomly, and their responses may not be representative of all dentists in the United States. However, a case can be made that network dentists are representative of US dentists. This conclusion is warranted because
substantial percentages of network general dentists were represented in the different response categories of the enrollment questionnaire;
findings from several network studies document that network dentists report patterns of diagnosis and treatment that are similar to patterns determined from nonnetwork dentists;33,34
an ADA Survey of Dental Practice showed the similarity of network and nonnetwork dentists.35
These results reinforce the need for continuing education and antibiotic stewardship programs specifically designed for the dental practice setting. Dentists are high prescribers of antibiotics in general,36–40 and they write more than 2.9 million prescriptions per year.16 Because of increasing concerns about antibiotic resistance, dentists can be an important part of the solution.16,41–44 Similar to the actions taken in response to an increased awareness of dentists’ roles in opioid prescribing (for example, mandatory prescription drug monitoring programs and mandatory continuing education),45 it is possible that similar actions will be seen as partial solutions to foster improved antibiotic stewardship.
The volume of information, figures, and tables derived from this survey of over 2,000 dentists was such that it could not be covered in 1 article. For this reason, we pooled the data for specialists and general dentists together and plan a separate article that will allow for a discussion of these data broken down by generalist and dental specialty, and other demographics.
CONCLUSIONS
The findings of our study clearly suggest the need to explore in more detail the opinions of dentists concerning their prescribing of AP to all patient populations, not just those at risk of developing IE and PJI, and what they do in practice for all patients who may benefit from primary as well as secondary prophylaxis. Data that support better targeting of antibiotics to patients and situations in which they are justified and a reduction in antibiotic prescribing overall are in the interests of all patients and society in general.
Supplementary Material
Acknowledgments
The National Dental Practice-Based Research Network Collaborative Group comprises practitioner, faculty, and staff investigators who contributed to this network activity. A list of those who contributed is at http://www.nationaldentalpbrn.org/collaborative-group.php.
The authors extend their gratitude to Thomas Paumier, DDS, and John Robinson, DDS, for the expert advice given during the planning phase for the questionnaire instrument; Kathleen Sullivan, MA, for her expertise in medical editing; and Casey Stephens, MPH, for her implementation of automatic survey and management of the data base.
This study was funded by grants U19-DE-22516 and U19-DE-28717 from the National Institutes of Health. Opinions and assertions contained herein are those of the authors and are not to be construed as necessarily representing the views of the respective organizations or the National Institutes of Health. A Web site devoted to details about The National Dental Practice-Based Research Network is available at http://NationalDentalPBRN.org.
The authors are grateful to The National Dental Practice-Based Research Network practitioners who participated in the study and to Dena Fischer, DDS, MSD, MS, NIDCR program official. They are also grateful to Stephanie Reyes, BA, from the network’s Southwest region, who served the networkwide role of principal regional coordinator for this study. This role is responsible for contributions focused on designing protocol procedures so that they are feasible and practical in the dental setting and entails responsibilities in both the study development and implementation phases. The authors are also grateful to the network’s regional coordinators who followed up with network practitioners to improve the response rate. Midwest region: Tracy Shea, RDH, BSDH, Chris Enstad, BS, RDH; Western region: Natalia Tommasi, MA, Celeste Machen, BA, Sacha Reich, BA, PMP, Stephanie Hodge, MA; Northeast region: Vi Luong, BS, MS, Christine O’Brien, RDH, Pat Regusa, BA; South Atlantic region: Danny Johnson, Deborah McEdward, RDH, BS, CCRP; South Central region: Claudia Carcelén, MPH, Shermetria Massengale, MPH, CHES, Ellen Sowell, BA; Southwest region: Meredith Buchberg, MP).
ABBREVIATION KEY
- ADA
American Dental Association
- AHA
American Heart Association
- AP
Antibiotic prophylaxis
- IE
Infective endocarditis
- IRB
Institutional review board
- PJI
Prosthetic joint infection
Footnotes
SUPPLEMENTAL DATA
Supplemental data related to this article can be found at https://doi.org/10.1016/j.adaj.2020.04.027.
Disclosure. None of the authors reported any disclosures.
Contributor Information
Peter B. Lockhart, Department of Oral Medicine, Carolinas Medical Center, 1000 Blythe Blvd, Charlotte, NC 28203.
Martin H. Thornhill, Department of Oral Medicine, Atrium Healths Carolina Medical Center, Charlotte, NC;; Department of Oral and Maxillofacial Medicine, Surgery and Pathology, University of Sheffield School of Clinical Dentistry, Sheffield, UK.
Jing Zhao, Center for Outcomes Research and Evaluation (CORE), Atrium Healths Carolinas Medical Center, Charlotte, NC.
Larry M. Baddour, Departments of Medicine and Cardiovascular Diseases, Division of Infectious Diseases, Mayo Clinic, Rochester, MN.
James Davis, Department of Oral Medicine, Atrium Healths Carolinas Medical Center, Charlotte, NC..
Patrick E. McKnight, Department of Psychology, George Mason University, Fairfax, VA..
Gregg H. Gilbert, Department of Clinical and Community Sciences, University of Alabama at Birmingham, Birmingham, AL..
Rahma Mungia, Department of Periodontics, University of Texas Health San Antonio, San Antonio, TX..
Jean-Luc Mougeot, Department of Oral Medicine, Atrium Healths Carolinas Medical Center, Charlotte, NC..
References
- 1.Newman HN. Focal infection. J Dent Res. 1996; 75(12):1912–1919. [DOI] [PubMed] [Google Scholar]
- 2.Lockhart PB, Loven B, Brennan MT, Fox PC. The evidence base for the efficacy of antibiotic prophylaxis in dental practice. JADA. 2007;138(4):458–474. [DOI] [PubMed] [Google Scholar]
- 3.Cahill T, Harrison J, Jewell P, et al. Antibiotic prophylaxis for infective endocarditis: a systematic review and meta-analysis. Heart. 2017;103(12):937–944. [DOI] [PubMed] [Google Scholar]
- 4.Lockhart PB, Brennan MT, Fox PC, Norton HJ, Jernigan DB, Strausbaugh LJ. Decision-making on the use of antimicrobial prophylaxis for dental procedures: a survey of infectious disease consultants and review. Clin Infect Dis. 2002;34(12):1621–1626. [DOI] [PubMed] [Google Scholar]
- 5.Lafaurie GI, Noriega LA, Torres CC, et al. Impact of antibiotic prophylaxis on the incidence, nature, magnitude, and duration of bacteremia associated with dental procedures: a systematic review. JADA. 2019;150(11):948–959. e944. [DOI] [PubMed] [Google Scholar]
- 6.Thornhill MH, Dayer MJ, Prendergast B, Baddour LM, Jones S, Lockhart PB. Incidence and nature of adverse reactions to antibiotics used as endocarditis prophylaxis. J Antimicrob Chemother. 2015;70(8):2382–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thornhill MH, Dayer MJ, Durkin MJ, Lockhart PB, Baddour LM. Risk of adverse reactions to oral antibiotics prescribed by dentists. J Dent Res. 2019;98(10):1081–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States. Atlanta, GA: U.S. Department of Health and Human Services; 2019. [Google Scholar]
- 9.Thornhill MH, Dayer M, Lockhart PB, Prendergast B. Antibiotic prophylaxis of infective endocarditis. Curr Infect Dis Rep. 2017;19(2):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dayer MJ, Jones S, Prendergast B, Baddour LM, Lockhart PB, Thornhill MH. Incidence of infective endocarditis in England, 2000–13: a secular trend, interrupted time-series analysis. Lancet. 2015;385(9974):1219–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dayer M, Thornhill M. Is antibiotic prophylaxis to prevent infective endocarditis worthwhile? J Infect Chemother. 2018;24(1):18–24. [DOI] [PubMed] [Google Scholar]
- 12.Suda KJ, Calip GS, Zhou J, et al. Assessment of the appropriateness of antibiotic prescriptions for infection prophylaxis before dental procedures, 2011 to 2015. JAMA Netw Open. 2019;2(5):e193909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Associationda guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2007;116(15):1736–1754. [DOI] [PubMed] [Google Scholar]
- 14.Lockhart PB, Hanson NB, Ristic H, Menezes AR, Baddour L. Acceptance among and impact on dental practitioners and patients of American Heart Association recommendations for antibiotic prophylaxis. JADA. 2013; 144(9):1030–1035. [DOI] [PubMed] [Google Scholar]
- 15.Dayer MJ, Chambers JB, Prendergast B, Sandoe JA, Thornhill MH. NICE guidance on antibiotic prophylaxis to prevent infective endocarditis: a survey of clinicians’ attitudes. QJM. 2013;106(3):237–243. [DOI] [PubMed] [Google Scholar]
- 16.Durkin MJ, Hsueh K, Sallah YH, et al. ; Centers for Disease Control and Prevention Epicenters. An evaluation of dental antibiotic prescribing practices in the United States. JADA. 2017;148(12):878–886. e871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spittle LS, Muzzin KB, Campbell PR, DeWald JP, Rivera-Hidalgo F. Current prescribing practices for antibiotic prophylaxis: a survey of dental practitioners. J Contemp Dent Pract. 2017;18(7):559–566. [DOI] [PubMed] [Google Scholar]
- 18.Mougeot JL, Davis JM, Zhao J, et al. Methodology for the development of a national Dental Practice-Based Research Network survey on dentist’s beliefs and behaviors concerning antibiotic prophylaxis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2020. June 12:4332 10.1016/j.oooo.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilbert GH, Williams OD, Korelitz JJ, et al. ; National Dental PBRN Collaborative Group. Purpose, structure, and function of the United States National Dental Practice-Based Research Network. J Dent. 2013;41(11): 1051–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The National Dental Practice-Based Research Network. The nation’s network. Available at: http://nationaldentalpbrn.org/. Accessed October 11, 2019. [Google Scholar]
- 21.Cloitre A, Duval X, Hoen B, Alla F, Lesclous P. A nationwide survey of French dentists’ knowledge and implementation of current guidelines for antibiotic prophylaxis of infective endocarditis in patients with predis-posing cardiac conditions. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;125(4):295–303. [DOI] [PubMed] [Google Scholar]
- 22.Cunningham CT, Quan H, Hemmelgarn B, et al. Exploring physician specialist response rates to web-based surveys. BMC Med Res Methodol. 2015;15:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thornhill M, Gibson TB, Cutler E, et al. Antibiotic prophylaxis and incidence of endocarditis before and after the 2007 AHA recommendations. J Am Col Cardiol. 2018; 72(20):2443–2454. [DOI] [PubMed] [Google Scholar]
- 24.Lattimer GL, Keblish PA, Dickson TB Jr., Vernick CG, Finnegan WJ. Hematogenous infection in total joint replacement: recommendations for prophylactic antibiotics. JAMA. 1979;242(20):2213–2214. [PubMed] [Google Scholar]
- 25.Sollecito TP, Abt E, Lockhart PB, et al. The use of prophylactic antibiotics prior to dental procedures in patients with prosthetic joints: evidence-based clinical practice guideline for dental practitionersda report of the American Dental Association Council on Scientific Affairs. JADA. 2015;146(1):11–16.e8. [DOI] [PubMed] [Google Scholar]
- 26.American Dental Association, American Academy of Orthopedic Surgeons. Antibiotic prophylaxis for dental patients with total joint replacements. JADA. 1997; 128(7):1004–1008. [DOI] [PubMed] [Google Scholar]
- 27.American Dental Association, American Academy of Orthopedic Surgeons. Antibiotic prophylaxis for dental patients with total joint replacements Advisory Statement. JADA. 2003;134(7):895–899. [DOI] [PubMed] [Google Scholar]
- 28.Watters W III, Rethman MP, Hanson NB, et al. Prevention of orthopaedic implant infection in patients undergoing dental procedures. J Am Acad Orthop Surg. 2013;21(3):180–189. [DOI] [PubMed] [Google Scholar]
- 29.Quinn RH, Murray JN, Pezold R, Sevarino KS. Members of the Voting and Writing Panels of the AUC for Management of Patients with Orthopedic Implants Undergoing Dental Procedures. The American Academy of Orthopaedic Surgeons appropriate use criteria for the management of patients with orthopaedic implants undergoing dental procedures. J Bone Joint Surg Am. 2017; 99(2):161–163. [DOI] [PubMed] [Google Scholar]
- 30.Lockhart PB. Antibiotic prophylaxis guidelines for prosthetic joints: much ado about nothing? [editorial]. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116(1): 1–3. [DOI] [PubMed] [Google Scholar]
- 31.Lockhart PB, Garvin KL, Osmon DR, et al. The antibiotic prophylaxis guideline for prosthetic joints: trying to do the right thing. J Am Acad Orthop Surg. 2013; 21(3):193–194. [DOI] [PubMed] [Google Scholar]
- 32.Makhija SK, Gilbert GH, Rindal DB, et al. ; DPBRN Collaborative Group. Practices participating in a dental PBRN have substantial and advantageous diversity even though as a group they have much in common with dentists at large. BMC Oral Health. 2009;9:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Norton WE, Funkhouser E, Makhija SK, et al. ; National Dental Practice-Based Research Network Collaborative Group. Concordance between clinical practice and published evidence: findings from The National Dental Practice-Based Research Network. JADA. 2014;145(1): 22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gilbert GH, Riley JL, Eleazer PD, Benjamin PL, Funkhouser E; National Dental PBRN Collaborative Group. Discordance between presumed standard of care and actual clinical practice: the example of rubber dam use during root canal treatment in the National Dental Practice-Based Research Network. BMJ Open. 2015;5(12): e009779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.American Dental Association Survey Center. The 2010 Survey of Dental Practice. Chicago, IL: American Dental Association; 2012. [Google Scholar]
- 36.Suda KJ, Henschel H, Patel U, Fitzpatrick MA, Evans CT. Use of antibiotic prophylaxis for tooth extractions, dental implants, and periodontal surgical procedures. Open Forum Infect Dis. 2018;5(1):ofx250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Durkin MJ, Feng Q, Warren K, et al. ; Centers for Disease Control and Prevention Epicenters. Assessment of inappropriate antibiotic prescribing among a large cohort of general dentists in the United States. JADA. 2018; 149(5):372–381. e371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suda KJ, Roberts RM, Hunkler RJ, Taylor TH. Antibiotic prescriptions in the community by type of provider in the United States, 2005–2010. J Am Pharm Assoc (2003). 2016;56(6):621–626. e621. [DOI] [PubMed] [Google Scholar]
- 39.Stein K, Farmer J, Singhal S, Marra F, Sutherland S, Quinonez C. The use and misuse of antibiotics in dentistry: a scoping review. JADA. 2018;149(10):869–884. e865. [DOI] [PubMed] [Google Scholar]
- 40.Koppen L, Suda KJ, Rowan S, McGregor J, Evans CT. Dentists’ prescribing of antibiotics and opioids to Medicare Part D beneficiaries: medications of high impact to public health. JADA. 2018;149(8):721–730. [DOI] [PubMed] [Google Scholar]
- 41.Goff DA, Mangino JE, Glassman AH, Goff D, Larsen P, Scheetz R. Review of guidelines for dental antibiotic prophylaxis for prevention of endocarditis and prosthetic joint infections and need for dental stewardship. Clin Infect Dis. 2019. 10.1093/cid/ciz1118. [DOI] [PubMed] [Google Scholar]
- 42.Gross AE, Hanna D, Rowan SA, Bleasdale SC, Suda KJ. Successful implementation of an antibiotic stewardship program in an academic dental practice. Open Forum Infect Dis. 2019;6(3):ofz067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sanchez GV, Fleming-Dutra KE, Roberts RM, Hicks LA. Core elements of outpatient antibiotic stewardship. MMWR Recomm Rep. 2016;65(6):1–12. [DOI] [PubMed] [Google Scholar]
- 44.Fluent MT, Jacobsen PL, Hicks LA; for OSAP, the Safest Dental Visit. Considerations for responsible antibiotic use in dentistry. JADA. 2016;147(8):683–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCauley JL, Gilbert GH, Cochran DL, et al. ; National Dental PBRN Collaborative Group. Prescription drug monitoring program use: National Dental PBRN Results. JDR Clin Trans Res. 2019;4(2): 178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


