ABSTRACT
The aim of this study was to investigate the role of tumor-associated neutrophils (TANs) in the metastasis of pancreatic ductal adenocarcinoma (PDAC), to explore the regulation of TANs, and to determine the mechanisms governing the metastasis of PDAC. The correlation between neutrophils and the patient’s clinical pathological data was first evaluated. Then, the effects of neutrophils on the invasion of PDAC were analyzed using a combination of conditioned media, direct and indirect coculture of human peripheral blood neutrophils, and PDAC cell lines (Panc-1, MiaPaCa-2 and AsPC-1). The cytokines secreted by neutrophils were detected through ELISA. TAN density was significantly correlated with poor metastasis-free survival (P < .05). Through coculture, it was found that the effect of neutrophils on pancreatic cancer cells was dependent on concentration, and a high concentration of neutrophils showed a lethal effect, while a low concentration of neutrophils primarily promoted the migration ability of cancer cells. The results of the wound-healing assay, the Transwell invasion assay, and laser confocal microscopy confirmed the promoting effect and indicated that the effect of neutrophils toward cancer cells may function indirectly by releasing a series of cytokines. The results of ELISA show that this effect may be achieved through the secretion of a large amount of TNF-α and TGF-β1 by neutrophils. Our study indicated that neutrophils may increase the metastasis of PDAC by releasing a series of cell cytokines, such as TNF-α and TGF-β1.
KEYWORDS: PDAC, neutrophils, metastasis, TNF-ɑ, TGF-Β1
Introduction
It is expected that pancreatic ductal adenocarcinoma (PDAC) will become the second leading cause of cancer-associated death in the next decade. With a 5-year overall survival (OS) rate of 7%, PDAC is the cancer of the gastrointestinal tract with the poorest prognosis.1,2 One of the main reasons for the poor prognosis of PDAC is its strong metastatic tendency. Our previous study suggested that even in patients with resectable PDAC, the majority of those patients (56%) were found to have metastases to the local lymph nodes during surgery.3 Metastasis is the most common cause of death after surgery, especially liver metastasis.4–6 Our previous study showed that the postoperative PDAC metastasis rate was up to 70% (liver metastasis accounted for 84.3%).7 Therefore, it is particularly important to explore the mechanism of metastasis of PDAC.
Neutrophils are one of the primary components of the human immune system and represent an important weapon against tumors.8,9 However, neutrophils may not be effective against tumors in patients with depressed immunity. Yan et al. have shown that the anticancer activity of neutrophil granulocytes in cancer patients is poor compared to healthy people.9 Evidence-based medicine has shown that tumor-associated neutrophils (TANs) have a significant correlation with the prognosis of cancer patients.10 A number of studies have shown that neutrophils can promote the epithelial-mesenchymal transition (EMT) ability of cancer cells to promote tumor invasion and metastasis.11–13 However, related evidence is still lacking in PDAC. Therefore, the present study investigates the role of TANs in PDAC patients to detect its correlation with the clinical features, metastasis and prognosis of PDAC patients, as well as the related possible mechanism in PDAC.
Materials and methods
Patients
All patients were admitted to the General Surgery Department of Peking University Third Hospital (China, Beijing) between October 2015 and October 2017, were diagnosed with PDAC, underwent radical surgery and were confirmed by pathological examination. Patients who received other oncotherapy before surgery were excluded. The degree of tumor differentiation and lymph node metastasis were determined by pathological examination. Pathological staging was assessed according to the American Joint Commission on Cancer (AJCC) criteria, version 7.14 After discharge from the hospital, all patients were followed at 3-month intervals for the first year and at 4- to 6-month intervals thereafter, and the end point was December 2017. The collected clinical information included serum CA19-9 levels, chest X-rays, abdominal ultrasound, abdominal magnetic resonance imaging (MRI) or computed tomography (CT). Metastasis was diagnosed when a new lesion was detected by CT scan or ultrasound, and other metastases or recurrences were excluded. Some of the metastases were detected by positron emission tomography (PET-CT). Biopsy was not routinely performed due to the risk of needle tract metastasis.
Detection and evaluation of TANs
Immunohistochemistry was used to identify tumor-associated neutrophils (TANs) in primary tumor tissue with the specific anti-CD15 antibodies (ab212396, Abcam), a specific marker of neutrophils.15 Round cells with a diameter of 10–15 µm, abundant cytoplasm, rod-shaped and lobulated nuclei, and rough and uneven chromatin arranged in small clumps, as well as CD15 expression, were recognized as neutrophils. The area for TAN assessment was defined as within the borders of the invasive tumors, which did not include immune infiltrates in adjacent normal tissue or ductal carcinoma in situ. TANs in the tumor area with crush artifacts, necrosis, or regressive hyalinization were also excluded. For patients whose TAN density was heterogeneous in a single tumor section, we evaluated different regions and reported the average level. A consensus evaluation of all surgical specimens was reached through reexamination. Two pathologists were blinded to clinical outcomes and evaluated TANs independently. The Spearman correlation coefficient between the two pathologists was 0.85 (P < .001).
Cell culture
Peripheral blood neutrophils from pancreatic cancer patients were isolated and purified by density gradient centrifugation on PolymorphPrep (Axis-Shield PoC AS, Oslo, Norway) according to the protocol supplied by the manufacturer. Three human pancreatic cancer cell lines, Panc-1, MiaPaCa-2 and AsPC-1 (American Type Culture Collection, ATCC, Manassas, VA), were cultured in Dulbecco’s Modified Eagle Medium (DMEM) medium containing 10% fetal bovine serum at 24-well culture plates (Costar, Corning, NY, USA) for 24 h. Neutrophil viability, which was tested by Trypan blue exclusion after 24 h of incubation with cancer cells, was 62.2% (±0.04%). The following day, isolated neutrophils (3 × 106/L in DMEM medium) were added. Each cell line was divided into three groups (monoculture, direct coculture and indirect coculture) (Supplementary Figure 1). For direct coculture of cancer cells with neutrophils, neutrophils were resuspended in DMEM and added to each well. For the indirect coculture group, 12-well Transwell inserts (membrane pore size: 0.4 mm, Costar, Corning, NY, USA) were used to separate 400 cancer cells (at lower chambers) from DMEM (at upper chambers). For the control group (defined as monoculture), cancer cells were cultured in the culture plate alone in equal amounts of DMEM. Cells (1 × 105) were grown at 37°C in a humidified incubator in the presence of 5% CO2.
Cell proliferation assay
Proliferation assays were performed using CCK8 (Dojindo). Cells were plated in 96-well plates in triplicate at approximately 1000 cells per well and cultured in growth medium. Cells were then cocultured with neutrophils, lymphocytes or no other cells, and the number of cells per well was measured by the absorbance (450 nm) of reduced water-soluble tetrazolium salt (WST) at the indicated time points. Lymphocytes were isolated via lymphocyte separation media (HLSM1077, Multi Sciences) from fresh blood from healthy donors according to the manufacturer’s instructions. According to OD, a cell growth curve was produced, with cell number serving as the X-axis and OD value serving as the Y-axis.
In vitro wound-healing assay of the effect of neutrophils
A wound-healing assay was performed to assess the effects of neutrophils on the migration of PDAC cells. Cancer cell line cells (8 × 105/mL) were plated into 6-well plates and grown overnight to near confluence. Cells in the experimental group were either treated with neutrophils of different concentrations, and the control group was left untreated. Using a pipette tip, a cell-free area was scraped in each well, and culture was continued. Images were taken at different time points, and cells migrating into the cell free space were quantified.
Observation under a laser confocal microscope
After culture for 24 h, the cell slides were fixed in 3.7% PFA and penetrated by 0.2% Triton x-100 for 10 minutes. Two secondary antibodies, goat anti-mouse IgG1 heavy chain (FITC) (ab97239) and DyLight649 goat anti-rabbit IgG (H + L) (GAR6492, MultiSciences) were added to the media 30 minutes after being treated with 5 mg/mL of anti-E-cadherin or 10 mg/mL of anti-vimentin antibodies overnight and visualized by epifluorescence microscopy after staining with Hoechst solution (Sigma) for 30 minutes.
Transwell invasion assay
Transwell assays were performed as described previously.16 Each cell line was divided into three groups (monoculture, direct coculture and indirect coculture) (Figure 2b). Briefly, 8.0-mm Matrigel-coated Transwell supports from Becton Dickinson Canada (BD) were used to evaluate cell invasion. Fifty thousand cancer cells were suspended in 500 mL of 10% FBS/DMEM and seeded in the upper chamber. The bottom chamber was filled with 1 mL of 10% FBS/DMEM. In experiments with indirect coculture, 50,000 (1:1) or 200,000 (4:1) human peripheral blood neutrophils were added to the bottom chamber. In direct coculture experiments, neutrophils were added to the upper chamber mixed with the cancer cells. Membranes were equilibrated at room temperature for 10 minutes before cells were added, and the cells were allowed to invade for 24 h followed by fixation in 3.7% PFA. Cell invasion was calculated as the average cell coverage area in µm2 on the underside of the membrane compared with the cancer cell control. The results were calculated based on the analysis of 20 fields (×20) in five independent experiments.
Figure 2.
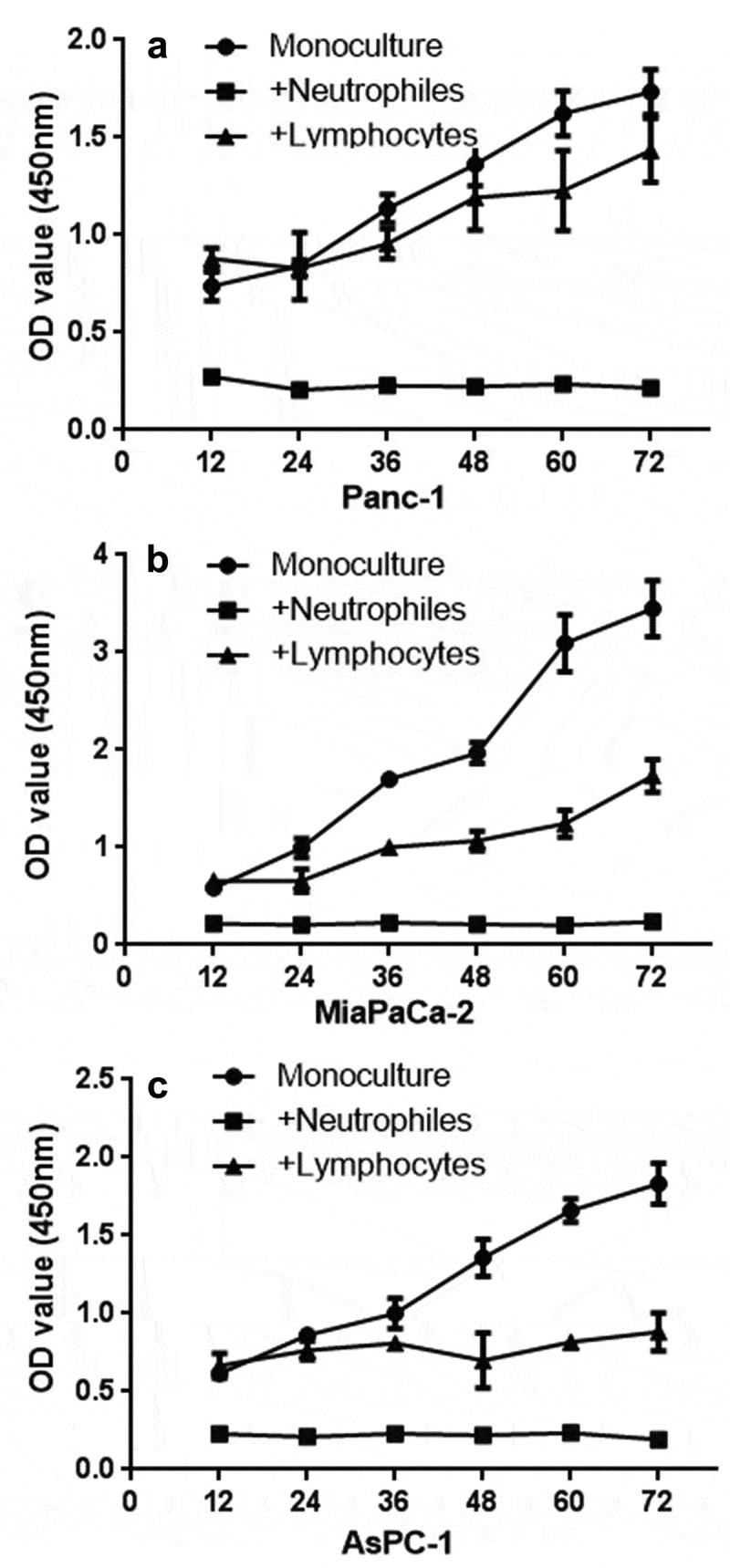
CCK8 test. PDAC cells (panc-1, miapaca-2, aspc-1 cells, 4 × 106) were cocultured with neutrophils (1 × 109) after 12 h of seeding (equal PDAC cells seeded first, neutrophils and lymphocytes were added after 12 h; therefore, the OD value showed a difference at 12 h). Compared with lymphocytes (1 × 109) and the monoculture group, neutrophils show a lethal effect on PDAC cells
ELISA
For the ELISA experiments, 5,000 cancer cells (Panc-1, MiaPaCa-2 and AsPC-1) were cultured in 1 mL of culture media in a 24-well plate. Where indicated, neutrophils were added at a 1:1 ratio or 4:1 ratio. For all experiments, the media was centrifuged at 400 × g for 5 minutes to remove cells and stored at −80°C until use. ELISA kits for IL-8, TNF-ɑ, TGF-β1, MMP9, EGF, IL-6, IL-12, IL-1β, GM-CSF and G-CSF were purchased from Multi Sciences, China. The ELISA experiments followed the manufacturer’s protocol for each cytokine tested.
Statistical analysis
The Mann–Whitney U-test was used to describe the normality of each continuous parameter’s distribution. The χ2 test was used for the analysis of categorical variables in the TAN-negative and TAN-positive groups. Quantitative results are reported as the means ± standard deviations. Associations between clinical and histopathological parameters with overall survival (OS) were analyzed using Kaplan-Meier curves and compared with the log-rank test. All analyzes were performed using SPSS 22.0 statistical software (SPSS, IL, USA). Significance levels were determined at p < .05.
Results
Clinical parameters of PDAC are associated with the expression of TANs
Due to the time required for follow-up and the completeness of the patients’ information, 57 patients with ductal adenocarcinoma were included in this study, and the relevant data of the patients are shown in Table 1. Representative images of different TAN levels are shown in Figure 1 A–H. The evaluation criteria of the TANs with PDAC were evaluated as A and B (0 points), C and D (1 points), E and F (2 points), and G and H (3 points) according to the occurrence rate of the TANs. Both 0 points and 1 point were rated as negative TANs, and 2 points and 3 points were rated as positive TANs (Figure 1). Through immunohistochemical staining score, 24 patients were recognized as TAN-positive, and 33 were TAN-negative. In December 2017, thirty-nine patients developed metastases after surgery. Among the patients, thirty-four have already died. By comparing the clinical parameters of the two groups, the results showed that the number of neutrophils in the blood of the TAN-positive group was significantly higher than that of the TAN-negative group (P = .039, Table 1). Moreover, the traditional markers of CA199 in the TAN-positive group were significantly higher than those in the negative group. Although there was no significant difference between the TAN-positive and TAN-negative groups in overall survival (P = .059, Figure 1j), further analysis indicated that patients with a higher level of TANs tended to have a shorter metastasis-free survival (P = .004, Figure 1i).
Table 1.
Comparison and evaluation of the clinical parameters of TANs classification in PDAC patients
| Classification |
TANs negative |
TANs positive |
|||
|---|---|---|---|---|---|
| Variable | Mean | SD | Mean | SD | P Value |
| Age(years) | 61.14 | 6.98 | 62.53 | 3.87 | 0.381 |
| Gender(male/female) | 18/15 | 11/13 | 0.703 | ||
| Platelet(×103/mL) | 210 | 70.2 | 227.5 | 75.51 | 0.673 |
| Lymphocytes(×103/mL) | 1.46 | 0.57 | 1.55 | 0.49 | 0.923 |
| Neutrophil (×103/mL) | 3.44 | 0. 597 | 5.25 | 2.09 | 0.039 |
| AFP(U/mL) | 3.544 | 0. 921 | 4.35 | 1.74 | 0.674 |
| CEA(ng/mL) | 23.9 | 31. 321 | 14.29 | 5. 35 | 0.087 |
| CA125(U/mL) | 21.42 | 16.22 | 23.23 | 9.35 | 0.645 |
| CA199(U/mL) | 379.66 | 622.85 | 512.31 | 821.01 | 0.026 |
| CA242(U/mL) | 71.78 | 112.12 | 141.66 | 155.12 | 0.051 |
| Diameter(cm) | 3.14 | 1.21 | 3.32 | 1.58 | 0.418 |
| PLR | 151.12 | 45.32 | 168.12 | 48.34 | 0.336 |
| NLR | 2.47 | 2.85 | 4.61 | 3.24 | 0.321 |
| AJCC-stage(<ⅡB/≥ⅡB) | 19/15 | 11/13 | 0.595 | ||
| Location (Head/Others) | 30/3 | 23/1 | 0.643 | ||
| Differentiation (well to moderate/others) | 11/22 | 10/14 | 0.585 | ||
Abbreviations: PLR: platelet/lymphocyte ratio; NLR: neutrophil/lymphocyte ratio
Figure 1.
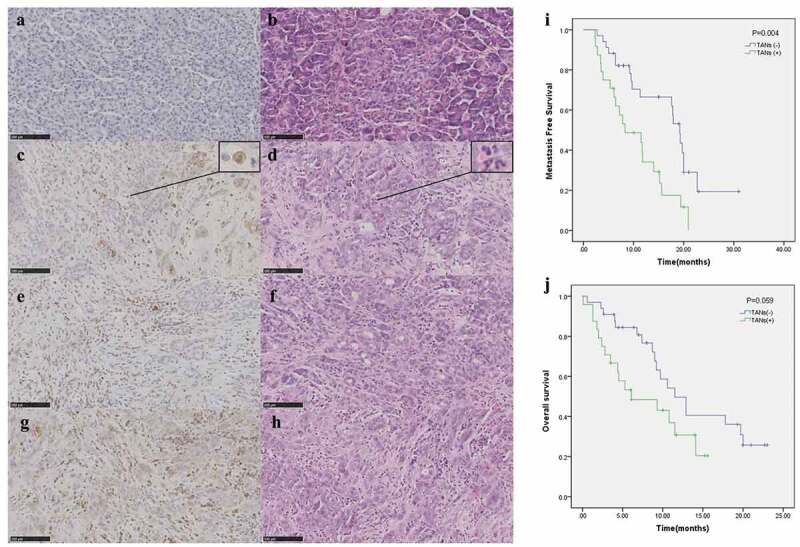
Evaluation criteria of TANs for PDAC: figure A, C, E and G were immunohistochemistry (CD15) (20 × 100) of TANs, respectively. The same rows of B, D, F and H were the results of HE in the PDAC tissue of the same patient. Figures C and D were enlarged as the typical TANs. Plot of metastasis-free survival (i) and overall survival (j) for all patients stratified based upon TAN groups. Abbreviations: TAN tumor-associated neutrophils
Effects of neutrophils on proliferation and migration of PDAC cells
By comparing with the untreated group, three PDAC cell lines (Panc-1, AsPC-1, MiaPaCa-2, 1 × 109) showed significant differences in shape after treatment with neutrophils for three days. The deformed cells resembled boats, which is a classic transformation of epithelial-mesenchymal transition (EMT) (Supplementary Figure 2). Moreover, some of the cancer cells also show signs of death. Therefore, whether neutrophils have a lethal effect on cancer cells was further evaluated. CCK8 was used to detect the effect of neutrophils on three PDAC cell lines (Panc-1, AsPC-1, MiaPaCa-2), and it was found that neutrophils had a lethal effect on tumor cells. This killing effect of neutrophils is significantly stronger than that of lymphocytes (Figure 2).
To test whether the effect of neutrophils on cancer cells depends on concentration, cell lines of PDAC (Miapaca-2) were cocultured with neutrophils in different concentration gradients. High concentrations of neutrophils (1 × 109) show obvious promotion of the migration of cancer cells in the early stage but show a lethal effect later, while low concentrations of neutrophils (1 × 108) mainly promote the migration of cancer cells (Supplementary Figure 3).
An in vitro wound-healing assay indicated that a low concentration of neutrophils mainly promoted the migration of all three PDAC cell lines (Panc-1, Miapaca-2 and Aspc-1) (Figure 3). By comparing the separately cultured cancer cell groups, Transwell invasion experiments indicated that indirect and direct co-culture with neutrophils significantly increased the transmigration of all three PDAC cell lines (Panc-1, Miapaca-2 and Aspc-1) (Figure 4). The effect of neutrophils on cancer cells may be indirect.
Figure 3.
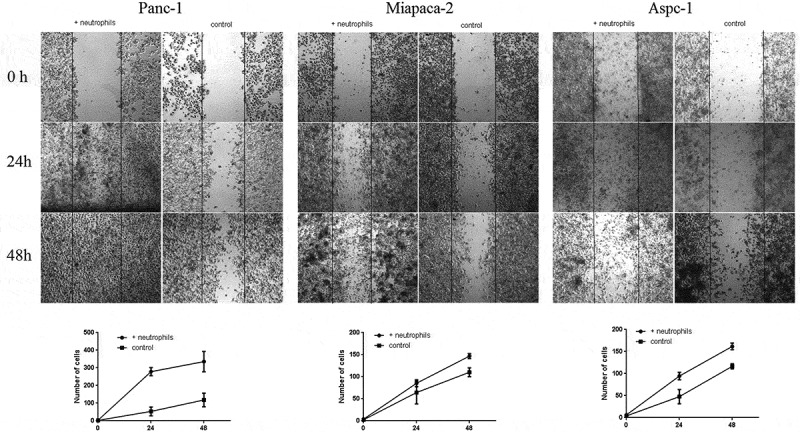
In vitro wound-healing assay of three PDAC cells (Panc-1, MiaPaCa-2, AsPC-1) cocultured with neutrophils and without (controls) at 0 h (Panc-1: photo after neutrophils added; MiaPaCa-2 and AsPC-1 photo before neutrophils added), 24 h and 48 h, respectively. As shown in the figure, neutrophils can promote the migration of all three PDAC cells (Panc-1, MiaPaCa-2, AsPC-1)
Figure 4.
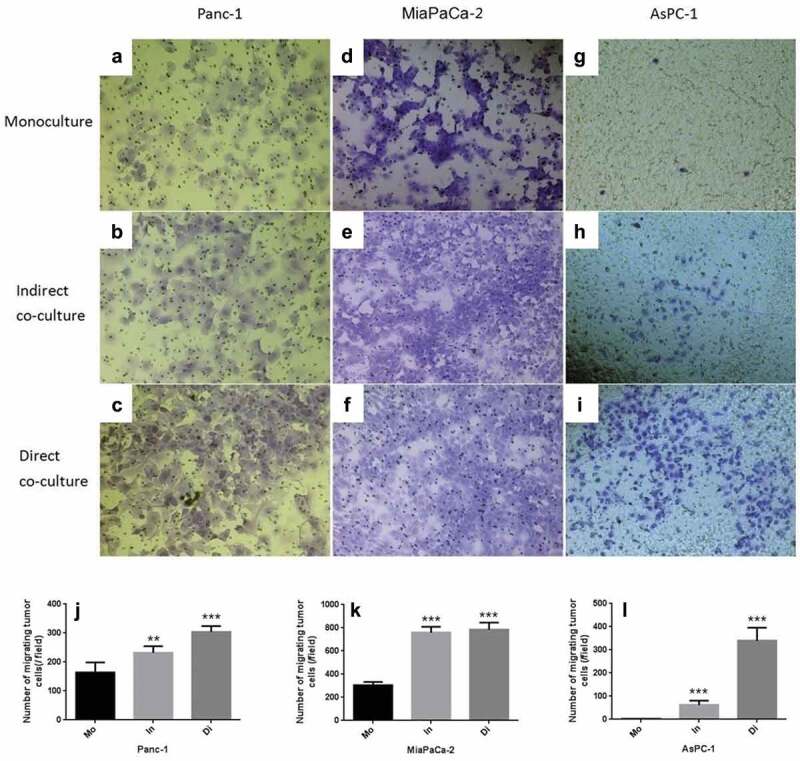
Transwell invasion experiments of Panc-1, Miapaca-2 and Aspc-1 cells separately cultured (Mo, Monoculture, FIG. ADG), indirectly cocultured with neutrophils (In, FIG. BEH) and directly cocultured (Di, Direct coculture, FIG. CFI). Compared with the monoculture, both indirect and direct co-culture with neutrophils increased the transmigration of all three PDAC cell lines significantly (JKL) (* P < .05; ** P < .01; ***P < .001)
To further detect the effect of neutrophils on the EMT of cancer cells and the mode of action, we used laser confocal microscopy to detect the EMT proteins (E-cadherin and vimentin) of tumor cells in different coculture methods (direct coculture and indirect coculture). PDAC cell lines (Panc-1, MiaPaCa-2, and AsPC-1) cultured in three groups: trained directly with neutrophils (direct coculture), trained indirectly (indirect coculture), and untrained (monoculture). The results showed that through training with neutrophils, E-cadherin expression was significantly decreased, and vimentin expression was significantly increased. Such a change is a significant trend of EMT, which is found in both direct and indirect coculture. Thus, the effect of neutrophils toward cancer cells may function indirectly through such means as releasing cytokines (Figure 5).
Figure 5.
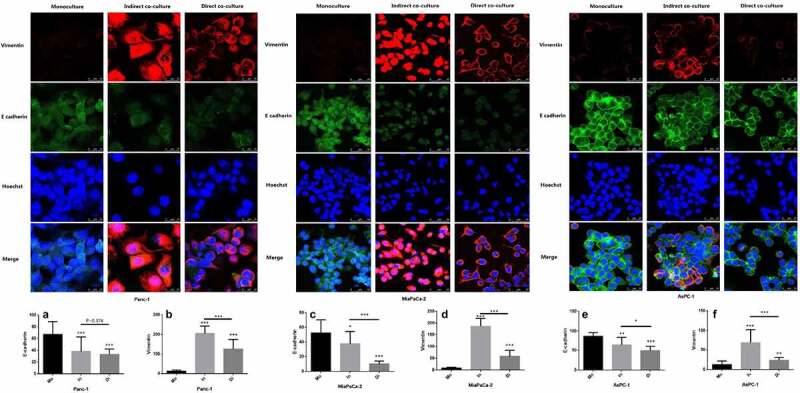
Expression of E-cadherin in three PDAC cells (Panc-1, MiaPaCa-2 and AsPC-1) was significantly reduced when direct cocultured (Di) or indirect cocultured (In) with neutrophils compared with that monocultured (Mo), while vimentin was significantly increased. The expression of E-cadherin and vimentin in Panc-1 at A and B, MiaPaCa-2 at C and D for AsPC-1 at E and F. To make each group comparable, the fluorescence brightness of the nucleus was set at the same level in the laser confocal imaging of the three groups. Vimentin (expressed in the cell membrane) was highly expressed in the indirect culture group in MiaPaCa-2, resulting in a higher brightness, so that it could not be distinguished in the image whether the expression site was the cell membrane or the nucleus. (* P < .05; ** P < .01; *** P < .001)
Detection of cytokines in the coculture system of neutrophils and PDAC by ELISA
Neutrophils may stimulate PDAC cells by releasing a series of cytokines. Through searching relevant literature, we found that IL-8, TNF-ɑ, TGF-β1, MMP9, EGF, IL-6, IL-12, IL-1β, GM-CSF, and G-CSF were reported. To test whether these cytokines participated in the effect of neutrophils on PDAC cells, the concentration of cytokines was detected in the culture medium, where the neutrophil and PDAC cell lines were cocultured in the 6-well plates at different ratios (direct coculture and indirect coculture) for 24 h. The results indicated that the neutrophils secreted a large amount of TNF-ɑ and TGF-β1 after coculturing with PDAC cells (Figure 6).
Figure 6.
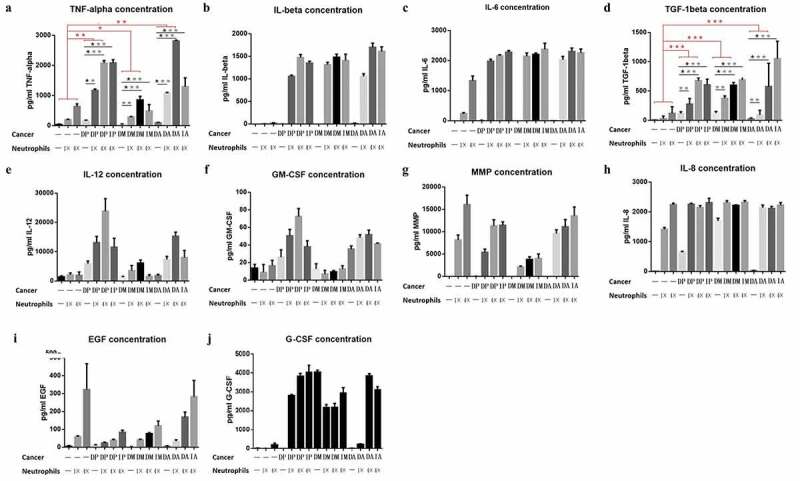
Detection of cytokines in three groups of cell lines (P: Panc-1, M:Miapaca-2, A:Aspc-1) in culture medium. (D: directly cocultured with neutrophils) and (In: indirectly cocultured with neutrophils). Detection of the concentrations of TNF-alpha, IL-6, TGF-beta, IL-12, GM-CSF, MMP, IL-8, EGF and G-CSF in cell culture medium (Fig A, B, C, D, E, F, G, H, I and J, respectively). Among all the cytokines, only TNF-α and TGF-β concurred with the experimental hypothesis: secreted by neutrophils, especially when stimulated by pancreatic cancer cells (1. the secretion of the cytokines was significantly increased in the coculture system; 2. their secretion was positively proportional to the concentration of neutrophils). (* P < .05; ** P < .01; ***P < .001)
Discussion
Our results showed that the number of neutrophils in the blood of the TAN-positive patients was significantly higher than that of the TAN-negative patients. Our previous study suggested that the neutrophil evaluation parameter NLR in the blood system could predict postoperative metastasis.7 Moreover, our study found that CA199, a widely used clinical indicator for the prognosis of PDAC, was significantly higher in the TAN-positive group than in the TAN-negative group. Multiple studies have shown that these parameters are significantly correlated with postoperative metastasis of PDAC.17,18 Both of the above results indirectly proved the correlation between TANs and PDAC metastasis. Although this study fails to develop an association between overall survival, it found that patients with a higher level of TANs tend to have a shorter metastasis-free survival. Similar results are also found in other tumors, such as lung cancer, breast cancer, colon cancer, liver cancer, oral cancer and esophageal cancer.19–25 It has been shown that TANs not only promote tumor growth of PDAC but also regulate their tolerance to treatment and induce immune suppression.26 Reid et al. showed that TANs are more frequently found in the micropapillae and undifferentiated types of pancreatic carcinoma of pancreatic ducts.27
Using a coculture technique, our study found a two-sided effect of neutrophils on PDAC cells. On the one hand, when cocultured with neutrophils at a low concentration, pancreatic cancer cells show a classic transformation of epithelial-mesenchymal transition (EMT). EMT refers to the phenomenon of epithelial cells transforming to mesenchymal cells under normal physiological or specific pathological conditions, which is thought to be a morphological change before tumor infiltration and metastasis. This transformation is demonstrated by the detection of EMT proteins through western blotting and laser confocal microscopy. Moreover, the promotion of metastasis by low concentrations of neutrophils was also demonstrated in wound-healing assays and Transwell invasion assays. These results indicated that a low concentration of neutrophils could significantly promote the metastasis of PDAC cells.
However, neutrophils are the most important weapon for the body’s immune system to resist tumors, which is also indicated in our study. However, this lethal effect on tumor cells was only observed when neutrophils were present at a high concentration. PDAC is mostly rich in fibrous tissue; therefore, the infiltrated neutrophils in the tumor often cannot reach a high concentration. Although some pancreatic cancer tissues are rated as TAN-positive, the neutrophils are still detected at a relatively low level. Therefore, such a killing effect on cancer cells may not be observed in PDAC patients.28 Although neutrophils in a very low concentration often fail to constitute an effective killing ability, they inevitably stimulate cancer cells, which may lead to the invasion and metastasis of pancreatic cancer. Therefore, neutrophils in PDAC tissue may be an important factor to promote the invasion and metastasis of tumor cells.
Furthermore, the ability of neutrophils to promote cell metastasis in PDAC is observed in both direct and indirect coculture. Therefore, we speculate that the interaction between the two types of cells is mainly regulated by the secretion of cytokines. A large number of studies have suggested that neutrophils can promote the infiltration and metastasis of tumor cells by releasing cytokines to alter the microenvironment.11,13,29–31 In this study, cytokines in the culture medium of the coculture system of neutrophils and PDAC were detected by ELISA, and the results typically indicated that neutrophils secreted a large amount of TNF-ɑ and TGF-β1 after coculture. High concentrations of TNF-α kill cancer cells. The role of TNF-α in the development of cancer has been reported previously, and this cytokine has been revealed as an important participant in facilitating the development of lung cancer cells induced by BaP.32 This cytokine has also been suggested as a useful tumor marker for the poor prognosis of breast cancer.33 TGF-β signaling events are well-known to control diverse processes and numerous responses, such as cell proliferation, differentiation, apoptosis, and migration, as well as metastasis.34–36 It has been proven that TGF-β1 expression is an independent prognostic indicator for intrahepatic cholangiocarcinoma patients.37 TGF-β1 is an upstream effector of MMPs and VEGF, which could promote invasion, angiogenesis, and proliferation in many cancers.34–37
Conclusions
Neutrophils are an important weapon of the human immune system against tumor development. However, tumor cells cannot be completely killed or suppressed by neutrophils due to their low concentrations. Although neutrophils cannot effectively kill tumor cells, they inevitably stimulate tumor cells, enhancing their EMT function and thereby inducing the invasion and metastasis of cancer cells. The abovementioned modes of action can be achieved by secreting a series of cytokines, such as TNF-ɑ and TGF-β1.
Supplementary Material
Funding Statement
This study was supported by a grant from the National Natural Science Foundation of China [No. 81672862], the Capital Characteristic Clinical Application Research and Achievement Promotion Project [No. Z171100001017121], the Doctoral Venture Capital fund of Henan Provincial People’s Hospital [No. ZC20180077], and the Special Project of Henan Provincial Key Research, Development and Promotion (Science and Technology) [No. 192102310119],Joint project of Medical science and Technology Research Program of Henan Province [No. LHGJ20190577]. Medical Science and Technology Research Plan of Henan Province, project co-built by provincial department [No. SB20190319].
Abbreviation
| AJCC | American Joint Committee on Cancer |
| EGF | Epidermal growth factor |
| EGFR | Epidemic growth factor receptor |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| IL | Interleukin |
| MMP9 | Matrix metallopeptidase 9 |
| PDAC | Pancreatic ductal adenocarcinoma |
| PMNs | polymorphonuclear leukocytes |
| TANs | Tumor-associated neutrophils |
| TGF-β1 | Transforming growth factor-beta 1 |
| TNF-ɑ | Tumor necrosis factor-alpha |
Authors’ contributions
Dr. Xiu Dianrong and Tao Lianyuan conceived and designed this study; Tao Lianyuan performed the HE, IHC, confocal laser scanning and cell culture; Tao Lianyuan,Li Gang, Tao Ming, Xiu Dianrong, Yuan Chunhui, Ma Zhaolai and Jian Bing performed the statistical analysis and interpreted the data; Xiu Dianrong and Tao Lianyuan wrote the manuscript. All authors reviewed and accepted the final manuscript for submission.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Barhli A, Cros J, Bartholin L, Neuzillet C.. Prognostic stratification of resected pancreatic ductal adenocarcinoma: past, present, and future. Dig Liver Dis. 2018;50(10):979–990. doi: 10.1016/j.dld.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 2.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM.. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 3.Tao L, Zhang L, Peng Y, Tao M, Li G, Xiu D, Yuan C, Ma C, Jiang B. Preoperative neutrophil-to-lymphocyte ratio and tumor-related factors to predict lymph node metastasis in patients with pancreatic ductal adenocarcinoma (PDAC). Oncotarget. 2016;7(45):74314–74324. doi: 10.18632/oncotarget.11031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zewu M, Yanling C, Shenghua H, Jinhai Z, Liangyi Z. Risk factors of liver metastasis in patients after radical resection of pancreatic cancer. Zhonghua Zhong Liu Za Zhi [Chin J Oncol]. 2015;37(4):312–316. [PubMed] [Google Scholar]
- 5.Shi H, Li J, Fu D. Process of hepatic metastasis from pancreatic cancer: biology with clinical significance. J Cancer Res Clin Oncol. 2016;142:1137–1161. [DOI] [PubMed] [Google Scholar]
- 6.Swaid F, Downs D, Rosemurgy AS. A practical approach to liver metastasis from unknown primary cancer: what surgeons need to know. Cancer Genet. 2016;209(12):559–566. doi: 10.1016/j.cancergen.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Tao L, Zhang L, Peng Y, Tao M, Li L, Xiu D, Yuan C, Ma Z, Jiang B. Neutrophils assist the metastasis of circulating tumor cells in pancreatic ductal adenocarcinoma: A new hypothesis and a new predictor for distant metastasis. Medicine (Baltimore). 2016;95(39):e4932. doi: 10.1097/MD.0000000000004932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singel KL, Segal BH. Neutrophils in the tumor microenvironment: trying to heal the wound that cannot heal. Immunol Rev. 2016;273(1):329–343. doi: 10.1111/imr.12459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan J, Kloecker G, Fleming C, Bousamra M 2nd, Hansen R, Hu X, Ding C, Cai Y, Xiang D, Donninger H, et al. Human polymorphonuclear neutrophils specifically recognize and kill cancerous cells. Oncoimmunology. 2014;3(7):e950163. doi: 10.4161/15384101.2014.950163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen M, Hu P, Donskov F, Wang G, Liu Q, Du J. Tumor-associated neutrophils as a new prognostic factor in cancer: a systematic review and meta-analysis. PloS One. 2014;9:e98259. doi: 10.1371/journal.pone.0098259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grosse-Steffen T, Giese T, Giese N, Longerich T, Schirmacher P, Hansch GM, Gaida MM. Epithelial-to-mesenchymal transition in pancreatic ductal adenocarcinoma and pancreatic tumor cell lines: the role of neutrophils and neutrophil-derived elastase. Clin Dev Immunol. 2012;2012:720768. doi: 10.1155/2012/720768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lopez-Lago MA, Posner S, Thodima VJ, Molina AM, Motzer RJ, Chaganti RS. Neutrophil chemokines secreted by tumor cells mount a lung antimetastatic response during renal cell carcinoma progression. Oncogene. 2013;32(14):1752–1760. doi: 10.1038/onc.2012.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mayer C, Darb-Esfahani S, Meyer AS, Hubner K, Rom J, Sohn C, Braicu I, Sehouli J, Hänsch GM, Gaida MM, et al. Neutrophil Granulocytes in Ovarian Cancer - Induction of Epithelial-To-Mesenchymal-Transition and Tumor Cell Migration. J Cancer. 2016;7(5):546–554. doi: 10.7150/jca.14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 15.Shen S, Ke Y, Dang E, Fang H, Chang Y, Zhang J, Zhu Z, Shao S, Qiao P, Zhang T, et al. Semaphorin 4D from CD15+ granulocytes via ADAM10-induced cleavage contributes to antibody production in bullous pemphigoid. J Invest Dermatol. 2018;138(3):588–597. doi: 10.1016/j.jid.2017.09.037. [DOI] [PubMed] [Google Scholar]
- 16.Magalhaes MA, Larson DR, Mader CC, Bravo-Cordero JJ, Gil-Henn H, Oser M, Chen X, Koleske AJ, Condeelis J. Cortactin phosphorylation regulates cell invasion through a pH-dependent pathway. J Cell Biol. 2011;195(5):903–920. doi: 10.1083/jcb.201103045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ni XG, Bai XF, Mao YL, Shao YF, Wu JX, Shan Y, Wang CF, Wang J, Tian YT, Liu Q, et al. The clinical value of serum CEA, CA19-9, and CA242 in the diagnosis and prognosis of pancreatic cancer. Eur J Surg Oncolo. 2005;31(2):164–169. doi: 10.1016/j.ejso.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Tao LY, Cai L, He XD, Liu W, Qu Q. Comparison of serum tumor markers for intrahepatic cholangiocarcinoma and hepatocellular carcinoma. Am Surg. 2010;76(11):1210–1213. doi: 10.1177/000313481007601119. [DOI] [PubMed] [Google Scholar]
- 19.Berry RS, Xiong MJ, Greenbaum A, Mortaji P, Nofchissey RA, Schultz F, Martinez C, Luo L, Morris KT, Hanson JA, et al. High levels of tumor-associated neutrophils are associated with improved overall survival in patients with stage II colorectal cancer. PLoS One. 2017;12(12):e0188799. doi: 10.1371/journal.pone.0188799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eruslanov EB. Phenotype and function of tumor-associated neutrophils and their subsets in early-stage human lung cancer. Cancer Immunol Immunother. 2017;66(8):997–1006. doi: 10.1007/s00262-017-1976-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu H, Wu B, Ma G, Zheng D, Song R, Huang E, Mao M, Lu B. Melatonin represses oral squamous cell carcinoma metastasis by inhibiting tumor-associated neutrophils. Am J Transl Res. 2017;9(12):5361–5374. [PMC free article] [PubMed] [Google Scholar]
- 22.Soto-Perez-de-Celis E, Chavarri-Guerra Y, Leon-Rodriguez E, Gamboa-Dominguez A. Tumor-associated neutrophils in breast cancer subtypes. Asian Pac J Cancer Prev. 2017;18(10):2689–2693. doi: 10.22034/APJCP.2017.18.10.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagasaka M, Kukreja G, Abdulfatah E, Vaishampayan U, Sukari A. Role of molecular profiling in diagnosis of papillary renal-cell cancer presenting as cancer of unknown primary site. Clin Genitourin Cancer. 2017;15(4):e713–e7. doi: 10.1016/j.clgc.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J, Zhang G, Hu P, Deng G, Liu Q, Qiao L, Luo H, Zhang J. Vasculogenic mimicry is associated with increased tumor-infiltrating neutrophil and poor outcome in esophageal squamous cell carcinoma. Onco Targets Ther. 2017;10:2923–2930. doi: 10.2147/OTT.S135477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamamoto T, Kawada K, Itatani Y, Inamoto S, Okamura R, Iwamoto M, Miyamoto E, Chen-Yoshikawa TF, Hirai H, Hasegawa S, et al. Loss of SMAD4 promotes lung metastasis of colorectal cancer by accumulation of CCR1+Tumor-associated neutrophils through CCL15-CCR1 axis. Clin Cancer Res. 2017;23(3):833–844. doi: 10.1158/1078-0432.CCR-16-0520. [DOI] [PubMed] [Google Scholar]
- 26.Chao T, Furth EE, Vonderheide RH. CXCR2-dependent accumulation of tumor-associated neutrophils regulates T-cell immunity in pancreatic ductal adenocarcinoma. Cancer Immunol Res. 2016;4(11):968–982. doi: 10.1158/2326-6066.CIR-16-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reid MD, Basturk O, Thirabanjasak D, Hruban RH, Klimstra DS, Bagci P, Altinel D, Adsay V. Tumor-infiltrating neutrophils in pancreatic neoplasia. Mod Pathol. 2011;24(12):1612–1619. doi: 10.1038/modpathol.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang D, DuBois RN. Immunosuppression associated with chronic inflammation in the tumor microenvironment. Carcinogenesis. 2015;36(10):1085–1093. doi: 10.1093/carcin/bgv123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huh SJ, Liang S, Sharma A, Dong C, Robertson GP. Transiently entrapped circulating tumor cells interact with neutrophils to facilitate lung metastasis development. Cancer Res. 2010;70(14):6071–6082. doi: 10.1158/0008-5472.CAN-09-4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu P, Shen M, Zhang P, Zheng C, Pang Z, Zhu L, Du J. Intratumoral neutrophil granulocytes contribute to epithelial-mesenchymal transition in lung adenocarcinoma cells. Tumour Biol. 2015;36(10):7789–7796. doi: 10.1007/s13277-015-3484-1. [DOI] [PubMed] [Google Scholar]
- 31.Yu PF, Huang Y, Han YY, Lin LY, Sun WH, Rabson AB, Wang Y, Shi YF. TNFalpha-activated mesenchymal stromal cells promote breast cancer metastasis by recruiting CXCR2+ neutrophils. Oncogene. 2017;36(4):482–490. doi: 10.1038/onc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao G, Wang Z, Huang Y, Ye L, Yang K, Huang Q, Chen X, Li G, Chen Y, Wang J, et al. Effects of Benzoapyrene on migration and invasion of lung cancer cells functioning by TNF-α. J Cell Biochem. 2018;119(8):6492–6500. doi: 10.1002/jcb.26683. [DOI] [PubMed] [Google Scholar]
- 33.Tripsianis G, Papadopoulou E, Anagnostopoulos K, Botaitis S, Katotomichelakis M, Romanidis K, KONTOMANOLIS E, TENTES I, KORTSARIS A. Coexpression of IL-6 and TNF-alpha: prognostic significance on breast cancer outcome. Neoplasma. 2014;61(2):205–212. doi: 10.4149/neo_2014_026. [DOI] [PubMed] [Google Scholar]
- 34.Calon A, Tauriello DV, Batlle E. TGF-beta in CAF-mediated tumor growth and metastasis. Semin Cancer Biol. 2014;25:15–22. doi: 10.1016/j.semcancer.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 35.Drabsch Y, Ten Dijke P. TGF-beta signalling and its role in cancer progression and metastasis. Cancer Metastasis Rev. 2012;31(3–4):553–568. doi: 10.1007/s10555-012-9375-7. [DOI] [PubMed] [Google Scholar]
- 36.Xie F, Ling L, van Dam H, Zhou F, Zhang L. TGF-beta signaling in cancer metastasis. Acta Biochim Biophys Sin (Shanghai). 2018;50(1):121–132. doi: 10.1093/abbs/gmx123. [DOI] [PubMed] [Google Scholar]
- 37.Chen Y, Ma L, He Q, Zhang S, Zhang C, Jia W. TGF-beta1 expression is associated with invasion and metastasis of intrahepatic cholangiocarcinoma. Biol Res. 2015;48(1):26. doi: 10.1186/s40659-015-0016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


