Abstract
Red Seaweed “Rhodophyta” are an important group of macroalgae that include approximately 7000 species. They are a rich source of structurally diverse bioactive constituents, including protein, sulfated polysaccharides, pigments, polyunsaturated fatty acids, vitamins, minerals, and phenolic compounds with nutritional, medical, and industrial importance. Polysaccharides are the main components in the cell wall of red algae and represent about 40–50% of the dry weight, which are extensively utilized in industry and pharmaceutical compounds, due to their thickening and gelling properties. The hydrocolloids galactans carrageenans and agars are the main red seaweed cell wall polysaccharides, which had broad-spectrum therapeutic characters. Generally, the chemical contents of seaweed are different according to the algal species, growth stage, environment, and external conditions, e.g., the temperature of the water, light intensity, nutrient concentrations in the ecosystem. Economically, they can be recommended as a substitute source for natural ingredients that contribute to a broad range of bioactivities like cancer therapy, anti-inflammatory agents, and acetylcholinesterase inhibitory. This review touches on the main points of the pharmaceutical applications of red seaweed, as well as the exploitation of their specific compounds and secondary metabolites with vital roles.
Keywords: bioactive compounds, drugs, seaweed, Rhodophyta
1. Introduction
Macroalgae are macroscopic benthic marine algae (seaweed) living in the intertidal zone. They are characterized by autotrophic nutrition and fast-growth; they do not need land for cultivation and their growth rate is faster than terrestrial plants [1]. They are categorized into three different groups Chlorophyta; Phaeophyta, and Rhodophyta, based on their pigment composition and storage food Figure 1.
Figure 1.
Photo of common red seaweed species with potential from Egypt coasts.
Red seaweed are the critical source of numerous bioactive compounds, in contrast with the other two groups of green and brown seaweed like polysaccharides “floridean starch and sulfated galactans, such as carrageenans or agars”, minerals, unsaturated fatty acids, amino acids, vitamins, phycobiliproteins, other pigments, phycolectins and mycosporine-like amino acids, which have many biological and industrial applications [2,3].
The protein content in red seaweed varied between 10–50% of the dry weight and being higher than macroalgal groups and some foods [4]. In addition, they contain the essential amino acid, about 25–50% of the total amino acids, is like other protein sources like leguminous plants [5].
Red and green seaweed contain the largest amount of phenolic compounds like flavonoids, phenolics acids, and bromophenols, which had different medical applications, due to the reaction of these components with proteins, e.g., enzymes or cellular receptors. While, phlorotannins, are the major polyphenolic secondary compounds synthesized only in marine brown seaweed [6].
For decades and at present, seaweed is used in food in many countries, as well as in traditional drugs and cosmetics, due to their richness in natural metabolites. The therapeutic trend has begun searching forward for new medications from natural products like algae. Since ancient times, macroalgae are used for treating different diseases. The approximate numbers of biochemicals are more than up 700 from red species. The majority of these contents have shown promising biological abilities, including antimicrobial, antiviral, antitumor, antioxidant, anticoagulant, anti-inflammatory, antidiabetic, antiallergic, and analgesics efficiencies [6,7].
2. Antimicrobial Activity
The antimicrobial mechanism of macroalgae depended on the modification of the cell porousness of pathogen species that led to loss of macromolecules and disrupted the function of the membrane and finally destroy the pathogen cells [7,8].
The variation in the biological ability of red seaweed may be related to seasonal variations in algal biological compounds, algal growth phases, and the efficiency of the extraction methods. Carbohydrates, glycosides and phenolic compounds from Kappaphycus alvarezii can act as considered as antibacterial agents toward different human pathogenic [9]. Jania rubens had antimicrobial activity toward thirty-two multidrug-resistant bacterial isolates which related to its long-chain saturated fatty acids, for example n-hexadecanoic acid or found in ester form such as docosanoic acid 1,2,3-propanetriyl ester and hexanedioic acid, dioctyl ester were responsible for antibacterial ability [8]. Agar and carrageenan oligosaccharides from red seaweed had antimicrobial ability against different human pathogenic microbe [10]. Gloiopeltis tenax extracts using supercritical CO2 modified with ethanol, primarily composed of fatty acids, phenols sesquiterpenes, ketones and sterols exhibited remarkable antimicrobial activity [11]. Brominated cyclic diterpenes from red alga Sphaerococcus coronopifolius has antibacterial activity toward methicillin-resistant bacteria Staphylococcus aureus strains [12]. Laurencia spp. synthesized halogenated biomolecules with antimicrobial, and cytotoxic potential [13]. Red alga Plocamium and Chondrococcus secreted polyhalogenated monoterpenes which exhibit antimicrobial and antitumor [14].
The red seaweed from India Southern Coasts showed higher antifungal ratio “37%” than brown “33.3%” and green seaweed “8.3%” [15]. The Methanolic extract of Corallina mediterranea, Hypnea musciformis and Laurencia papillosa had antifungal activity toward human pathogenic fungi Aspergillus and Candida [16]. The new diterpene, sphaerodactylomelol fraction 2 extracted from Sphaerococcus coronopifolius exhibited a highest antifungal activity toward Candida albicans [17]. The antifungal efficacy of methanolic extracts of Acanthaphora spicifra had a similar activity of commercial drugs like ciprofloxacin and amphotericin [18].
3. Antiviral Activity
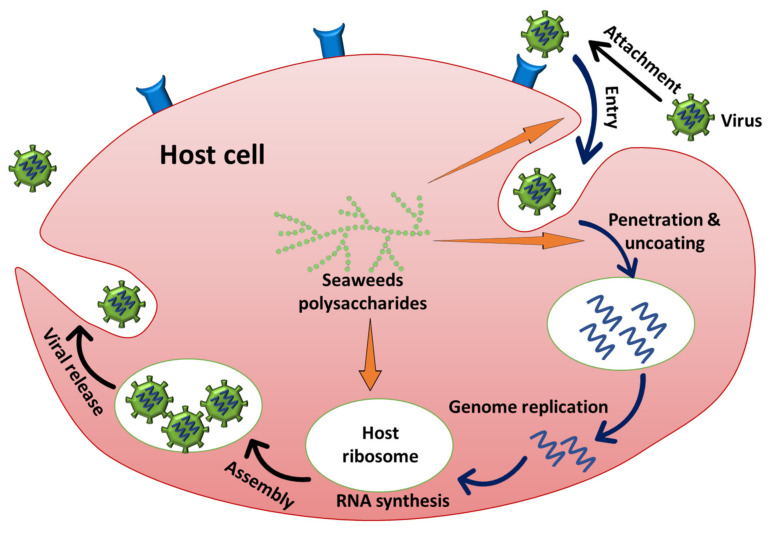
Macroalgae have antiviral properties that provide a protective effect against several virus species by obstructing the spread of the human immune deficiency virus (HIV) and other sexually transmitted viruses, such as herpes simplex virus (HSV) and genital warts [19]. Sulfated polysaccharides “SPs” and phenolic compounds from seaweed have better antiviral activity than other bioactive substances via preventing viral adsorption (simultaneous-treatment assay) and replication (post-treatment assay) [20]. The mechanism of macroalgal polysaccharides against viral diseases is focusing on the viral attachment phase via 1) attaching immediately with virions and/or 2) connecting to the respective protein receptors as explained in Figure 2 and Figure 3) immunomodulators that activate Natural killer cells (NK) or prompt immune reactions [21].
Figure 2.
The viral infection stages and the antiviral potency of macroalgal polysaccharides, modified after [21].
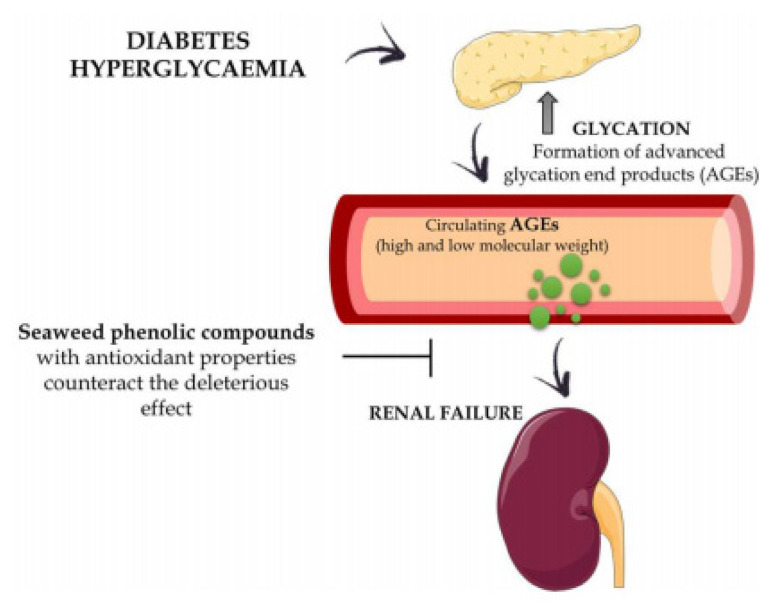
Figure 3.
Graphical illustrating the antioxidant activities of macroalgal phenolic compounds toward the harmful impacts related to the hyperglycemia [6].
Oligopolysaccharides agars and carrageenans from different red seaweed species showed antiviral capacities [3]. The crude polysaccharides from red algae (Pterocladia capillacea and Laurencia obtusa) from Abo Qir, Alexandria showed antiviral efficiency against hepatitis C (HCV) in vitro [22]. Carrageenans from Gigartina skottsbergii may act as models for structuring novel anti- HSV 1 and 2 agents through the mechanism of blocking viral attachment. Also, it could raise the efficiency of NK cells and increase the lymphocytes generation amount [23]. Iota carrageenan from Solieria chordalis had antiviral efficiency toward HSV1 [24].
Polyphenol-rich extracts synthesized by Mexican red seaweed Solieria filiformis have major virucidal abilities toward the Measles virus and avoid virus dissemination in vitro [25].
4. Antioxidant Activity
Antioxidants substances from seaweed have attracted the principal interest in pharmaceutical manufacturing, since these compounds effectively prevent or retard the adverse impacts caused by free radicals. There are two groups of antioxidants (1) the reaction breaking antioxidants and (2) preventive antioxidants [26]. They demonstrate the main roles in the prohibition of many maladies such as a tumor, inflammatory disorders, coronary heart, neurological degeneration, and aging [27].
There are many specialists occupied with discovering natural antioxidants posing security and adequacy, which can be replaced the manufactured ones butylated hydroxyl anisole (BHA), butylated hydroxyl toluene (BHT), α-tocopherol and propyl gallate which led to destroying the liver and causing cancer [28]. Ina and Kamei [29] classified the algal antioxidants into two groups; (1) water-soluble antioxidants vitamins, phycobiliproteins and polyphenols, and (2) fat-soluble antioxidants carotenoids and vitamin E (α-tocopherol).
Carrageenans, porphyrans, and agars have strong antioxidant and immunostimulatory [30]. The antioxidant activity of polysaccharides relies upon the level of sulfate, molecular weight, sort, and branching of the main sugar. Hence, the antioxidant efficacy of low molecular weight SPs is better than those of high molecular weight SPs whereas, the smallest polysaccharides may integrate into the cells more effortlessly and give protons higher adequately contrasted with larger polysaccharides [31]. Benattouche et al. [32] recommended the SPs from Corallina officinalis for use in natural antioxidants in food industry applications.
Phycobiliproteins are hydrophilic groups of pigment-proteins from red macroalgae, have antioxidant ability in vitro and in vivo toward free radicals and selenium [33]. R-phycoerythrin from Mastocarpus stellatus has antioxidant ability [34]. R-phycoerythrin from Palmaria palmata and Polysiphonia urceolata identified as antioxidant agents [35]. Mycosporine from red seaweed had antioxidant activities [3].
Phenol compounds play a vital role against external stress. These compounds vary from simple acids to more complex polyphenolic compounds. These compounds are great antioxidants and act as chelators of metals and free radicals, inhibiting lipid, and scavengers of Reactive Oxygen Species (ROS) like H2O2; OH•; O2•− & 1O2 [6]. The phenolic and flavonoid contents of red seaweed from the Alexandria coast are significantly correlated to their DPPH antioxidant ability [2,36]. The antioxidant potency of macroalgal phenolic compounds counteracts the deleterious impacts of the advanced glycation end products (AGEs), which are the toxic effects associated with hyperglycemia (Figure 3) [6]. The red alga Symphyocladia latiuscula bromophenols had antioxidant efficiency [37]. The antioxidant capacity of Polysiphonia stricta relates to brominated units and its degrees [38].
Despite the low macroalgal lipid content but it is characterized by a high content of polyunsaturated fatty acid PUFA, especially the arachidonic, eicosapentaenoic, α-linoleic, and octadecatetraenoic acids which had the prohibition of cardiovascular infections, diabetes, antimicrobial antiviral and antioxidant abilities [39]. The antioxidant activities of the extracted fatty acids from Pterocladia capillacea and Osmundaria obtusiloba were more than 50% Ascorbic Acid Activity (AsA) [40]. Bioactive peptides from red seaweed Polysiphonia urceolata and Palmaria palmata are known as antioxidant agents [35]. The protective role of lipophilic constituents of macroalgae toward lipid peroxidation is related to low polarity and high solubility [26].
Also, enzymes from red algae Palmaria palmata, such as proteases and carbohydrases showed antioxidant efficiency [41].
5. Anticancer and Antiproliferative Activities
Cancer is a noteworthy medical issue worldwide and, until now, lacks fully effective medicines. Macroalgae have cancer-fighting agents that may demonstrate valuable in relieving tumors and other cancer conditions like breast, colon cancer, and leukemia [19,42]. In the 1960s, the first anticancer medicine from marine algae (algasol T331) has been detected in Italy. Recently, new anticancer medicine was be produced from red seaweed Gracilaria foliifera and green seaweed Cladophoropsis sp. [43].
The antioxidant efficiency of the polysaccharide fractions itself might play a role in their antitumor action. Polysaccharides from J. rubens were a potential candidate for anticancer treatment [42]. Carrageenan oligosaccharides from different red species showed anticarcinogenic action with less cytotoxicity and synergistic impacts with usual medicines, as well as enhancing the immunocompetence destroyed cells by these mediciness [44]. Luo et al. [45] recommended that the λ-carrageenan injection that could repress malignant development in B16-F10 and 4T1 bearing mice and upgrade tumor resistant reactions by rising the tumor-infiltrating M1 macrophages number dendritic cell antigen “DCs” and extra stimulated CD4 + CD8 + T lymphocytes in the spleen. Agar extracted from cold-water extraction of red algae Gracilaria species exhibits antitumor efficiency with antioxidant activity [37]. Porphyrans are an anionic polysaccharide, isolated from Porphyra sp. shows anticancer activity [46]. Coura et al. [47] reported the biological activities of agars oligosaccharides anti-tumoral and immunomodulatory.
Phycobiliproteins from red macroalgal species have an important role as anticancer agents, due to their high efficiency and low toxicity. They can enhance the activity of conventional anticancer medicines, decreasing their side impacts, and act as photosensitizers for the infected cells treatment [48]. Especially, phycocyanin (PC) has the antitumor impact that can obstruct the cancer cells multiplication and kill the infected cell, thereby PC can serve as a promising anticancer agent [49]. PC exhibited many anticancer mechanisms, such adjusting the mitochondrial membrane potential (MMP), which invigorates the produce of cytochrome c and elevate the ROS formation, at last lead to cancer cell apoptosis. Also, it can repress the Cyclooxygenase-2 (COX-2) and prostaglandin E2 (PGE2) definition and down-regulate the MMP-9 explanation via mitogen-activated protein kinase (MAPK) signaling pathway. Moreover, they are a toxin on tumor cells and are non-toxic to ordinary cells [50]. The anti-proliferation activity of PC is mediated by BCR-ABL signaling and suppression of the downstream PI3K/Akt “Phosphatidylinositol-4,5-bisphosphate 3-kinase/ protein kinase B” pathway. Also, (MAPK), (PI3K/Akt/mTOR the mammalia target of rapamycin), and Nuclear Factor (NF-κB) pathways involved phycocyanin-induced cell death [49].
Macroalgal phenolic compounds play an important role as tumor fighter through inhibition of the tumor cells via a xenobiotic processing, which changes the carcinogen activation, or through disturbing the cellular division during mitosis at the telophase stage. They also decrease the amount of cellular protein and mitotic index, while some flavonoids can alter hormone production and inhibit aromatase to restrain the malignancy cells progress [51]. Terpernoids, isolated from red seaweed, have demonstrated a high antitumor ability [37]. The water extracts of Laurencia obusta indicated significant antiproliferative ability toward both HCT15, A549 and MCF7 due to their polyphenols contents [52]. The bis (2,3-dibromo-4,5-dihydroxybenzyl) ether from most red seaweed had apoptotic ability toward K562 human myelogenous leukemia cells [53]. The organic extract from Rhodomela confervoides contain 3-bromo-4,5-dihydroxy-benzaldehyde bromophenols and 3-bromo-4,5-dihydroxy benzoic acid methyl ester with a maximum activity toward KB, A549 cancer cell lines and Bel-7402 (Human papillomavirus-related endocervical adenocarcinoma) [38]. The diterpene, sphaerodactylomelol fraction 1 from Sphaerococcus coronopifolius inhibited the cell proliferation [15].
6. Anti-Inflammatory Activity
Inflammation is defined as a defense reaction in a broad assortment of physiological and pathological procedures, caused by injury, harm, and contagion, or it may be due to the release of chemical substances from migrating cells [54].
Macroalgal polysaccharides exhibited anti-inflammatory potency with no toxic effects on human health. Orally administration of polysaccharide fractions from Gracilaria verrucosa to mice and intraperitoneally increased the anti-inflammatory and antioxidant capacities and stimulating phagocytosis [55]. A sulfated polysaccharide fraction from Gracilaria cornea shows anti-inflammatory activity via inhibition of histamine, neutrophil migration, and vascular permeability [47]. Porphyrans extracted from Porphyra sp. shows anti-inflammatory activity in humans by the suppression of nitric oxide (NO) production and hindering NF-B activation in the mouse macrophages of RAW264.7 cells [46]. Carrageenans from red species act as anti-inflammatory inducing agents in experimental animals [56].
Phycobiliproteins are commercially used for their therapeutic value as anti-inflammatory agents [57]. Especially R-phycoerythrin from most red seaweed such as Gelidium pusillum, Chondrus crispus, and Gracilaria verrucosa exhibited anti-inflammatory capacity [58]. Phycocyanin exhibited an anti-inflammatory activity with antioxidative ability [37].
PUFA from red seaweed had anti-thrombotic and anti-inflammatory properties [59]. Van Ginneken et al. [60] recommend that the n-6/n-3 ratio between fatty acids should < 10 in the diet, which preventing cardiovascular, inflammatory, and nervous system disorders.
7. Analgesic and Antipyretic Activities
Algal terpenes, peptides, and sulfated polysaccharides act as painkiller agent [61]. The red alga Dichotomaria obtusata aqueous extract inhibited the production of endogenous mediators in response to acetic acid, due to its metabolites, such as polysaccharides and phenols [62]. The analgesic activity of red algae Vidalia obtusaloba and Ceratodictyon spongiosum was related to bromophenolic and peptide metabolites [63].
Antipyretics reduce the elevated body temperature. Macroalgal bioactive compounds act as novel and safe for antipyretic agents, e.g., flavonoids like baicalin, which indicated antipyretic impact by suppressing Tumor Necrosis Factor (TNF-α) and hindrance of arachidonic acid peroxidation that decrease the prostaglandin ratio and reduce fever and pains [64]. The possible antipyretic mechanism of macroalgae may be due to the inhibition of prostaglandin, such as paracetamol by blocking the cyclooxygenase enzyme activity and/or inhibition of any of mediators of pyrexia [65]. The antipyretic capacity of Hypnea musciformis and Gracilaria dura methanolic extract was dose-dependent on albino mice and caused a decrease in body temperature up to 4 h following its administration, due to the inhibition of prostaglandin synthesis, compared with standard paracetamol [66].
8. Anticoagulant and Antithrombotic Activities
Anticoagulants are defined as a substance that treats or stops blood clots and minimizes the risk of stroke, cardiac disappointment, and obstruction within blood vessels. The anticoagulant capacity of seaweed may be attributed to its polysaccharides composition, molecular weight, sulfate content, and position, e.g., uronic acids, carrying a negative charge, which gives it the capacity to bind calcium ions, and therefore, prevents the formation of a clot [67]. The algal anticoagulation mechanism may be due to their direct impact on thrombin and enhancing of antithrombin III.; Moreover, algal polysaccharides delayed activated partial thromboplastin time (APTT), proposing the obstacle of intrinsic factors, extended essential pathway-dependent coagulating times, and decreased platelet aggregation.
Commonly, galactans from red seaweed could be alternative sources of new anticoagulant agents [68]. Sulfated galactans from Grateloupia indica had anticoagulant efficiency as heparin [69]. Carrageenans from red species contains –O-SO3H group, which plays a vital role in blood clotting inhibition by inhibiting platelet aggregation [70]. Carrageenans had about one-fifteenth of the heparin action. The λ-carrageenan exhibited superior anticoagulant ability than κ-carrageenan relating to its higher sulfate content [71]. Depolymerization of agars extracted from Porphyra yezoensis and Gracilaria birdiae by ultrasound assisted increased the anticoagulant activity [47].
9. Antidiabetic Activity
Diabetes mellitus is a metabolic disorder resulting from an imperfection in insulin secretion and/or insulin activity. It is an almost coarse serious metabolic disease in the world.
Seaweed species have a unique antidiabetic way in search of natural alpha-glucosidase inhibitors that reduce the absorption of glucose from the gut itself [6].
However, seaweed species have α-amylase and α-glucosidase inhibitory actions [72]. The sulfated galactans extracted from Gracilaria opuntia was responsible for the antidiabetic properties through the deactivation of α-glucosidase, a-amylase, and dipeptidyl peptidase-4 [73]. Laurencia dendroidea could be a natural source for the production of antidiabetic agents [74]. Chen et al. [75] demonstrated glucosidase inhibitory action of agar polysaccharides, which increased the acid hydrolysis process.
Mittal et al. [57] demonstrated the antidiabetic efficiency of R-PE and R-PC from Chondrus crispus, Gelidium pusillum, Heterosiphonia japonica, and Palmaria palmata. The organic extracts of red algae Gracilaria changii, G.; lemaneiformis, Gelidium amansii, Osmundea pinnatifida, containing phenolic compounds, showed glucose uptake regulation [76]. There are other active compounds extracted from Rhodophyta spp. (Polysiphonia urceolata, Rhodomela confervoides and Symphyocladia latiuscula) exhibited antidiabetic properties like bromophenols, benzene acetamide, 2-piperidione, and n-hexadecanoic acid [77].
10. Anti-Obesity Activity
Obesity is excessive fat accumulation, and is known to increase the risk of many dangerous diseases like type II diabetes, hypertension, hyperlipidemia, and cardiovascular diseases. Obesity is gaining increased attention because of the high expense and dangerous symptoms of anti-obesity drugs. Rhodophyta species were shown to have anti-obesity properties [78].
The ethanolic extract of Grateloupia elliptica (60%) reduced the accumulation of the lipid in 3T3-L1 cells and inhibited the adipogenic proteins expression. In addition to a significant decreasing in body weight of C57 BL/6J male mice, as well as reducing white adipose tissue (WAT) weight, e.g., fatty liver, leptin, total cholesterol, and serum triglycerides contents in vivo without cytotoxic effect [79]. Forty % of Plocamium telfairiae ethanolic extract showed anti-obesity ability via reducing the fat accumulation and suppressed the expression of major adipogenesis factors, like peroxisome proliferator-activated receptor-γ (PPAR-γ),CCAAT/enhancer-binding protein (C/EBP)-α, sterol regulatory element-binding protein 1 (SREBP-1), and phosphorylated ACC (pACC) in 3T3-L1 cells [80]. Seo et al. [81] demonstrated the antidiabetic activity of extract from Gelidium amansii via reduction the accumulation of lipid in 3T3-L1 adipocyte cell line.
Generally, marine algal polysaccharides are considered to be dietary fibers so they are not digested by humans [82]; hence SPs can hinder adipogenesis through the mitogen activated protein kinase (MAPK) in 3T3-L1 pre-adipocytes [83].
11. Antihypertensive Activity
Seaweed exhibits significant anti-hypertensive activities [84]. Macroalgae consumption led to decreased blood pressure, which might be linked to the hypotensive effects of the dietary fiber and their rich nitrate content [19]. The antihypertensive effects of macroalgal peptides maintained a healthy heart by stimulating circulation in the blood vessels, and avoiding deadly conditions, such as heart breakdown, atherosclerosis, and peripheral vascular disease [85].
The secondary metabolites of seaweed act as hypoglycemic agents, reduce blood pressure and regulate cholesterol levels, inhibition of hepatic cholesterol biosynthesis, also for hyperplasia prevention, gastrointestinal, regenerative Nori-peptides from Porphyra yezoensis have the important antihypertensive ability in hypertensive patients, as well as spontaneously hypertensive rats [86].
12. Acetylcholinesterase Inhibitory ‘’Alzheimer’s Disease”
Alzheimer’s disease (AD) is a progressive and degenerative problem in brain regions, chiefly campus, and neocortex responsible for mental functions that reduced neurotransmitter acetylcholine (ACh). AD can prompt amnesia, abnormalities, and cognitive disturbances [87]. In the cholinergic theory, serious damage of cholinergic neurotransmitter AChE in the central nervous system (CNS) gives AD indications [87]. The principle therapeutic strategy against AD is acetylcholinesterase hindrance.
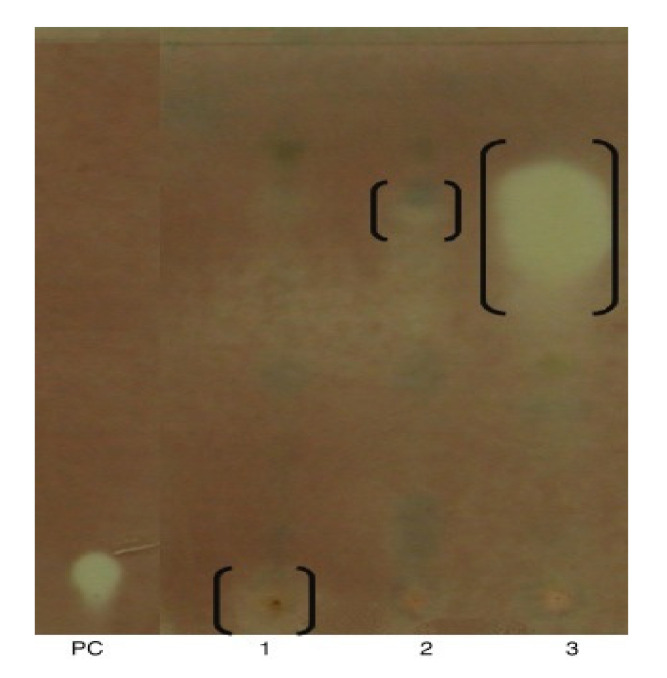
There is very few research reporting on the AChE inhibitory (AChEI) impact of seaweed. The AChEI ability of plant extracts is classified into potent inhibitors (> 50% inhibition), moderate inhibitors (30–50% inhibition), and weak inhibitors (<30% inhibition) [88]. As clarified in Figure 4, Ochtodes secundiramea extracts exhibited moderate potency (48.59 ± 0.8%), while the red algae Hypnea musciformis (7.21%) and Pterocladia capillacea (5.38%) extracts had a weak action. The AChEI abilities of these algae are related to solely compose of halogenated monoterpenes [89].
Figure 4.
TLC qualitative AChEI assay. PC: +ve control, physostigmine (0.03 μg). DCM/MeOH extracts (100 μg) of (1) Hypnea musciformis, (2) Pterocladia capillacea, and (3) Ochtodes secundiramea. TLC elution system: hexane:ethyl acetate:methanol (2:7:1 v/v/v) [89].
The alcoholic extract of Gracilaria corticata (IC50 9.5) and G.; salicornia (IC50 8.7 mg/mL) extracts showed moderate AChEI efficiency [90]. The sulfated polysaccharides from Gelidium pristoides exhibited inhibitory potency on acetylcholinesterase that related to their antioxidant and neuroprotective potentials [91].
Some red seaweed species synthesize homotaurine, aminosulfonate compounds, which could be a promising medicine for Alzheimer’s disease prevention [92].
13. Macroalgae for Skincare
Macroalgae metabolites reduced the appearance of redness and blemishes, the appearance of sun damage, brightening, re-mineralizing, hydrating and firming skin [93]. They have a reaction mechanism toward the hazardous ultraviolet ‘UV-A and -B’ impacts via delivering phenolic compounds, mycosporine, amino acid, and carotenoids, which act as UV-absorbing [94]. The extracts of red seaweed Asparagopsis armata, Gelidium corneum, Corallina officinalis had skin softness, whitening/lightening, and elasticity restoring anti-aging properties so they can be used as skincare products including creams, oil, soap, mask, or lotion [95]. Agarose from Gracilaria cornea and G.; lemaneiformis are used for skin whitening, due to its anti-melanogenic activity by inhibiting melanin synthesis [96,97]. Fatty acid-like palmitic acid and its derivated ascorbyl palmitate from seaweed are used in cosmetics as emulsifiers and antioxidant agents for anti-wrinkle and anti-aging characteristics [98]. Amino acid extracted from Asparagopsis armata is inserted in some anti-aging lotions [37]. Mycosporine from different Rhodophyta species act as photoprotective substances for skin care products with antioxidant properties [3].
14. Conclusions
This review aims to provide an overview of some medical and cosmetic importance of red macroalgae. They represent renewable biomass and are a potentially fruitful source of vital bioactive compounds that ecofriendly and safe. These bioactive compounds are already used mainly in food and medicine. For example, sulfated polysaccharides from red algae are commercially applied as a useful ingredient as a thickening agent, stabilizer, emulsifier, texture modifier, and dietary fiber in food and pharmaceutical industries. There are some species from Rhodophyta whose feasibility medical potential is higher, like Gracilaria spp., Pterocladia spp. Jania spp. and Corallina spp. Due to the economic importance of seaweed, more studies should be undertaken, focusing on improving seaweed production on a large scale by adjusting the culture conditions. Cultivation of economic species in seaweed aquaculture may be the future road for the sustainability of seaweed and controlling the production of active compounds. Optimization extraction methods, purification, and fractionation of bioactive compounds led to the production of more active and safe compounds. Therefore, more clinical studies should be carried out on a large scale for economic production.
Author Contributions
Conceptualization, M.M.I.; and M.M.E.-S.; software, B.S.A.; validation, M.M.E.-S.; and M.M.I.; resources, M.M.I.; data curation, B.S.A.; writing—original draft preparation, M.M.I.; writing—review and editing, M.M.E.-S.; visualization, B.S.A.; supervision, M.M.E.-S.; project administration, M.M.E.-S.; funding acquisition, B.S.A.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Wan A.H., Davies S.J., Soler-Vila A., Fitzgerald R., Johnson M.P. Macroalgae as a sustainable aquafeed ingredient. Rev. Aquac. 2019;11:458–492. doi: 10.1111/raq.12241. [DOI] [Google Scholar]
- 2.Ismail M.M., Gheda S.F., Pereira L. Variation in bioactive compounds in some seaweed from Abo Qir bay, Alexandria, Egypt. Rend Lincei-Sci. Fis. 2016;27:269–279. [Google Scholar]
- 3.Torres M.D., Flórez-Fernández N., Domínguez H. Integral utilization of red seaweed for bioactive production. Mar. Drugs. 2019;17:314. doi: 10.3390/md17060314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vieira E.F., Soares C., Machado S., Correia M., Ramalhosa M.J., Oliva-Teles M.T., Carvalho A.P., Domingues V.F., Antunes F., Oliveira T.A.C., et al. Seaweeds from the Portuguese coast as a source of proteinaceous material, total and free amino acid composition profile. Food Chem. 2018;269:264–275. doi: 10.1016/j.foodchem.2018.06.145. [DOI] [PubMed] [Google Scholar]
- 5.Paiva L., Lima E., Patarra R.F., Neto A.I., Baptista J. Edible Azorean macroalgae as source of rich nutrients with impact on human health. Food Chem. 2014;164:128–135. doi: 10.1016/j.foodchem.2014.04.119. [DOI] [PubMed] [Google Scholar]
- 6.Gómez-Guzmán M., Rodríguez-Nogales A., Algieri F., Gálvez J. Potential role of seaweed polyphenols in cardiovascular-associated disorders. Mar. Drugs. 2018;16:250. doi: 10.3390/md16080250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abu-Khudir R., Ismail G.A., Diab T. Antimicrobial, antioxidant, and anti-Tumor activities of Sargassum linearifolium and Cystoseira crinita from Egyptian Mediterranean Coast. Nutr. Cancer. 2020 doi: 10.1080/01635581.2020.1764069. [DOI] [PubMed] [Google Scholar]
- 8.El-Sheekh M., El Sabbagh S., Abd El Samea B. Control of some microbial skin diseases by some marine algal extract. J. Agric. Chem. Biotechnol. 2016;7:67–74. doi: 10.21608/jacb.2016.40743. [DOI] [Google Scholar]
- 9.Bhuyar P., Rahim M.H., Sundararaju S., Maniam G.P., Govindan N. Antioxidant and antibacterial activity of red seaweed; Kappaphycus alvarezii against pathogenic bacteria. Global J. Environ. Sci. Manag. 2020;6:47–58. [Google Scholar]
- 10.Ratnawati R., Prasetyaningrum A., Wardhani D.H. Kinetics and thermodynamics of ultrasound-assisted depolymerization of ҡ-carrageenan. Bull. Chem. React. Eng. Catal. 2016;11:48–58. doi: 10.9767/bcrec.11.1.415.48-58. [DOI] [Google Scholar]
- 11.Zheng J., Chen Y., Yao F., Chen W., Shi G. Chemical composition and antioxidant/antimicrobial activities in supercritical carbon dioxide fluid extract of Gloiopeltis tenax. Mar. Drugs. 2012;10:2634–2647. doi: 10.3390/md10122634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smyrniotopoulos V., Vagias C., Rahman M.M., Gibbons S., Roussis V. Ioniols I and II, tetracyclic diterpenes with antibacterial activity, from Sphaerococcus coronopifolius. Chem. Biodivers. 2010;7:666–676. doi: 10.1002/cbdv.200900026. [DOI] [PubMed] [Google Scholar]
- 13.Brito I., Cueto M., Díaz-Marrero A.R., Darias J., San Martín A. Oxachamigrenes, New Halogenated Sesquiterpenes from Laurencia obtusa. J. Nat. Prod. 2002;65:946–948. doi: 10.1021/np010580t. [DOI] [PubMed] [Google Scholar]
- 14.Blunt J.W., Copp B.R., Munro M.H.G., Northcote P.T., Prinsep M.R. Marine natural products. Nat. Prod. Rep. 2003;20:1–48. doi: 10.1039/b207130b. [DOI] [PubMed] [Google Scholar]
- 15.Padmakumar K., Ayyakkannu K. Seasonal variation of antibacterial and antifungal activities of the extract of marine algae from southern coasts of India. Bot. Mar. 1997;40:507–515. doi: 10.1515/botm.1997.40.1-6.507. [DOI] [Google Scholar]
- 16.Saleh B., Al-Mariri A. Antifungal activity of crude seaweed extracts collected from Lattakia Coast, Syria. J. Fish. Aquat. Sci. 2018;13:49–55. doi: 10.3923/jfas.2018.49.55. [DOI] [Google Scholar]
- 17.Rodrigues D., Alves C., Horta A., Pinteus S., Silva G., Culioli G., Thomas O.P., Pedrosa R. Antitumor and antimicrobial potential of bromoditerpenes isolated from the Red Alga, Sphaerococcus coronopifolius. Mar. Drugs. 2015;13:713–726. doi: 10.3390/md13020713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pandian P., Selvamuthukumar S., Manavalan R., Parthasarathy V. Screening of antibacterial and antifungal activities of red marine algae Acanthaphora spicifera (Rhodophyceae) J. Biomed. Sci. Res. 2011;3:444–448. [Google Scholar]
- 19.Mendis E., Kim S.K. Present and future prospects of seaweed in developing functional foods. Adv. Food Nutr. Res. 2011;64:1–15. doi: 10.1016/B978-0-12-387669-0.00001-6. [DOI] [PubMed] [Google Scholar]
- 20.Kwon H.J., Ryu Y.B., Kim Y.M., Song N., Kim C.Y., Rho M.-C., Jeong J.-H., Cho K.-O., Lee W.S., Park S.J. In vitro antiviral activity of phlorotannins isolated from Ecklonia cava against porcine epidemic diarrhea coronavirus infection and hemagglutination. Bioorg. Med. Chem. 2013;21:4706–4713. doi: 10.1016/j.bmc.2013.04.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi Q., Wang A., Lu Z., Qin C., Hu J., Yin J. Overview on the antiviral activities and mechanisms of marine polysaccharides from seaweed. Carbohydr. Res. 2017:453–454. doi: 10.1016/j.carres.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 22.Gheda S.F., El-Adawib H.L., EL-Deebc N.M. Antiviral profile of brown and red seaweed polysaccharides against hepatitis C virus. Iran. J. Pharmaceut. Res. 2016;15:483–491. [PMC free article] [PubMed] [Google Scholar]
- 23.Diogo J.V., Novo S.G., Gonzalez M.J., Ciancia M., Bratanich A.C. Antiviral activity of lambda-carrageenan prepared from red seaweed (Gigartina skottsbergii) against BoHV-1 and SuHV-1. Res. Veter. Sci. 2015;98:142–144. doi: 10.1016/j.rvsc.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 24.Boulho R., Marty C., Freile-Pelegrín Y., Robledo D., Bourgougnon N., Bedoux G. Antiherpetic (HSV-1) activity of carrageenans from the red seaweed Solieria chordalis (Rhodophyta, Gigartinales) extracted by microwave-assisted extraction (MAE) J. Appl. Phycol. 2017;29:2219–2228. [Google Scholar]
- 25.Morán-Santibañez K., Peña-Hernández M.A., Cruz-Suárez L.E., Ricque-Marie D., Skouta R., Vasquez A.H., Rodríguez-Padilla C., Trejo-Avila L. Virucidal and synergistic activity of polyphenol-rich extracts of seaweeds against measles virus. Viruses. 2018;10:465. doi: 10.3390/v10090465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang H.L., Wang B.G. Antioxidant capacity and lipophilic content of seaweed collected from the Qingdao coastline. J. Agric. Food Chem. 2004;52:4993–4997. doi: 10.1021/jf049575w. [DOI] [PubMed] [Google Scholar]
- 27.Collins K.G., Fitzgerald G.F., Stanton C., Ross R.P. Looking beyond the terrestrial, the potential of seaweed derived bioactives to treat non-communicable diseases. Mar. Drugs. 2016;14:60. doi: 10.3390/md14030060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vizetto-Duarte C., Cust-odio L., Acosta G., Lago J.H.G., Morais T.R., de Sousa C.B., Gangadhar K.N., Rodrigues M.J., Pereira H., Lima R.T., et al. Can macroalgae provide promising anti-tumoral compounds? A closer look at Cystoseira tamariscifolia as a source for antioxidant and anti-hepatocarcinoma compounds. Peer J. 2016;4:1704. doi: 10.7717/peerj.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ina A., Kamei Y. Vitamin B (12), a chlorophyll-related analog to pheophytin a from marine brown algae, promotes neurite outgrowth and stimulates differentiation in PC12 cells. Cytotechnol. 2006;52:181–187. doi: 10.1007/s10616-006-9038-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manlusoc J.K.T., Hsieh C.-L., Hsieh C.-Y., Salac E.S., Lee Y.-T., Tsai P.-W. Pharmacologic application potentials of sulfated polysaccharide from marine algae. Polymers. 2019;11:1163. doi: 10.3390/polym11071163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wijesekara I., Pangestuti R., Kim S. Biological activities and potential health benefits of sulphate polysaccharides derived from marine algae. Carbohydr. Polym. 2011;84:14–21. doi: 10.1016/j.carbpol.2010.10.062. [DOI] [Google Scholar]
- 32.Benattouche Z., Raho G.B., Sahnouni F., Hariri A., Bouhadi G., Benchohra M. Anioxidant activities of sulfated polysaccharide obtained from red algae Corallina officinalis. IJP. 2017;4:88–91. [Google Scholar]
- 33.Punampalam R., Khoo K.S., Sit N.M. Evaluation of antioxidant properties of phycobiliproteins and phenolic compounds extracted from Bangia atropurpurea. Malays. J. Fundam. Appl Sci. 2018;14:289–297. doi: 10.11113/mjfas.v14n2.1096. [DOI] [Google Scholar]
- 34.Nguyen H.P.T., Morançais M., Fleurence J., Dumay J. Mastocarpus stellatus as a source of R-phycoerythrin, Optimization of enzyme assisted extraction using response surface methodology. J. Appl. Phycol. 2017;29:1563–1570. doi: 10.1007/s10811-016-1024-z. [DOI] [Google Scholar]
- 35.Fitzgerald C., Gallagher E., O’Connor P., Prieto J., Mora-Soler L., Grealy M., Hayes M. Development of a seaweed derived platelet activating factor acetylhydrolase (PAF-AH) inhibitory hydrolysate, synthesis of inhibitory peptides and assessment of their toxicity using the Zebrafish larvae assay. Peptides. 2013;50:119–124. doi: 10.1016/j.peptides.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 36.Ismail M.M., El Zokm G.M., El-Sayed A.M. Variation in biochemical constituents and master elements in common seaweed from Alexandria Coast, Egypt, with special reference to their antioxidant activity and potential food uses, prospective equations. Environ. Monit Assessm. 2017;189:648. doi: 10.1007/s10661-017-6366-8. [DOI] [PubMed] [Google Scholar]
- 37.Cotas J., Leandro A., Monteiro P., Pacheco D., Figueirinha A., Gonçalves A.M.M., da Silva G.J., Pereira L. Seaweed Phenolics, From Extraction to Applications. Mar. Drugs. 2020;18:384. doi: 10.3390/md18080384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu M., Hansen P.E., Lin X. Bromophenols in marine algae and their bioactivities. Mar. Drugs. 2011;9:1273–1292. doi: 10.3390/md9071273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kendel M., Wielgosz-Collin G., Bertrand S., Roussakis C., Bourgougnon N., Bedoux G. Lipid composition, fatty acids and sterols in the seaweed Ulva armoricana, and Solieria chordalis from Brittany (France), An analysis from nutritional, chemotaxonomic, and antiproliferative activity perspectives. Mar. Drugs. 2015;13:5606–5628. doi: 10.3390/md13095606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De alencar D., Diniz J., Rocha S., Pires-cavalcante K., De Lima R.L., De Sousa K.C., Freitas J.O., Bezerra R.M., Baracho B.M., Sampaio A.H., et al. Fatty acid composition from the marine red algae Pterocladiella capillacea (S. G. Gmelin) Santelices & Hommersand 1997 and Osmundaria obtusiloba (C.; Agardh) R. E. Norris 1991 and its antioxidant activity. An. Acad Bras. Cienc. 2018;90:449–459. doi: 10.1590/0001-3765201820160315. [DOI] [PubMed] [Google Scholar]
- 41.Wang T., Jónsdóttir R., Kristinsson H.G., Hreggvidsson G.O., Jonsson J.O., Thorkelsson G., Olafasdottir G. Enzyme-enhanced extraction of antioxidant ingredients from red algae Palmaria palmata. LWT Food Sci. Tech. 2010;43:1387–1393. doi: 10.1016/j.lwt.2010.05.010. [DOI] [Google Scholar]
- 42.Gheda S., El-Sheekh M., Abou-Zeid A. In vitro anticancer activity of polysaccharide extracted from red alga Jania rubens against breast and colon cancer cell lines. Asian Pacif. J. Trop. Med. 2018;11:583–589. [Google Scholar]
- 43.Erfani N., Nazemosadat Z., Moein M. Cytotoxic activity of ten algae from the Persian Gulf and Oman Sea on human breast cancer cell lines; MDA-MB-231, MCF-7, and T-47D. Pharm. Res. 2015;7:133–137. doi: 10.4103/0974-8490.150539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raman M., Doble M. ҡ-Carrageenan from marine red algae, Kappaphycus alvarezii—A functional food to prevent colon carcinogenesis. J. Funct. Foods. 2015;15:354–364. doi: 10.1016/j.jff.2015.03.037. [DOI] [Google Scholar]
- 45.Luo M., Shao B., Nie W.X., Wei X., Li Y.-L., Wang B.-L., He Z.-Y., Liang Z., Ye T.-H., Wei Y.-Q. Antitumor and adjuvant activity of lamb-dacarrageenan by stimulating immune response in cancer immunotherapy. Sci. Rep. 2015;5:12. doi: 10.1038/srep11062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang Z., Hama Y., Yamaguchi K., Oda T. Inhibitory effect of sulphated polysaccharide porphyran on nitric oxide production in lipopolysaccharide-stimulated RAW264 7 mcrophages. J. Biochem. 2012;151:65–74. doi: 10.1093/jb/mvr115. [DOI] [PubMed] [Google Scholar]
- 47.Coura C.O., Souza R.B., Rodrigues J.A.G., Vanderlei E.D.S.O., De Araújo I.W.F., Ribeiro N.A., Frota A.F., Ribeiro K.A., Chaves H.V., Pereira K.M.A., et al. Mechanisms involved in the anti-inflammatory action of a polysulfated fraction from Gracilaria cornea in rats. PLoS ONE. 2015;10:e0119319. doi: 10.1371/journal.pone.0119319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gantar M., Dhandayuthapani S., Rathinavelu A. Phycocyanin induces apoptosis and enhances the effect of topotecan on prostate cell line LNCaP. J. Med. Food. 2012;15:1091–1095. doi: 10.1089/jmf.2012.0123. [DOI] [PubMed] [Google Scholar]
- 49.Jiang L., Wang Y., Yin Q., Liu G., Liu H., Huang Y., Li B. Phycocyanin, A potential drug for cancer treatment. J. Cancer. 2017;8:3416–3429. doi: 10.7150/jca.21058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ravi M., Tentu S., Baskar G., Prasad S.R., Raghavan S., Jayaprakash P., Jeyakanthan J., Rayala S.K., Venkatraman G. Molecular mechanism of anti-cancer activity of phycocyanin in triple-negative breast cancer cells. BMC Cancer. 2015;15:768. doi: 10.1186/s12885-015-1784-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dellai A., Laajili S., Le Morvanb V., Robert J., Bouraoui A. Antiproliferative activity and phenolics of the Mediterranean seaweed Laurencia obusta. Ind. Crop. Prod. 2013;47:252–255. doi: 10.1016/j.indcrop.2013.03.014. [DOI] [Google Scholar]
- 52.Mhadhebi L., Mhadhebi A., Robert J., Bouraoui A. Antioxidant, anti-inflammatory and antiproliferative effects of aqueous extracts of three Mediterranean brown seaweeds of the genus Cystoseira. Iran. J. Pharm Res. 2014;13:207–220. [PMC free article] [PubMed] [Google Scholar]
- 53.Liu M., Zhang W., Wei J., Qiu L., Lin X. Marine bromophenol bis(2,3-dibromo-4,5-dihydroxybenzyl) ether, induces mitochondrial apoptosis in K562 cells and inhibits topoisomerase I in vitro. Toxicol. Lett. 2012;211:126–134. doi: 10.1016/j.toxlet.2012.03.771. [DOI] [PubMed] [Google Scholar]
- 54.Vaughan V.C., Hassing M.R., Lewandowski P.A. Marine polyunsaturated fatty acids and cancer therapy. Br. J. Cancer. 2013;108:486–492. doi: 10.1038/bjc.2012.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gunerken E., D’Hondt E., Eppink M.H., Garcia-Gonzalez L., Elst K., Wijffels R.H. Cell disruption for microalgae biorefneries. Biotechnol Adv. 2015;33:243–260. doi: 10.1016/j.biotechadv.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 56.Morris C.E. How does fertility of the substrate affect intra-specific competition? Evidence and synthesis from self-thinning. Ecol. Res. 2003;18:287–305. doi: 10.1046/j.1440-1703.2003.00555.x. [DOI] [Google Scholar]
- 57.Mittal R., Raghavarao K.S.M.S. Extraction of R-Phycoerythrin from marine macro-algae, Gelidium pusillum, employing consortia of enzymes. Algal Res. 2018;34:1–11. [Google Scholar]
- 58.Lee D., Nishizawa M., Shimizu Y., Saeki H. Anti-inflammatory effects of dulse Palmaria palmata resulting from the simultaneous water-extraction of phycobiliproteins and chlorophyll a. Food Res. Int. 2017;100:514–521. doi: 10.1016/j.foodres.2017.06.040. [DOI] [PubMed] [Google Scholar]
- 59.Kumari P., Kumar M., Gupta V., Reddy C.R.K., Jha B. Tropical marine macroalgae as potential sources of nutritionally important PUFAs. Food Chem. 2010;120:749–757. [Google Scholar]
- 60.Van Ginneken C., Schafer K.H., Van Dam D., Huygelen V., De Deyn P.P. Morphological changes in the enteric nervous system of aging and APP23 transgenic mice. Brain Res. 2011;1378:43–53. doi: 10.1016/j.brainres.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 61.Hong D.D., Hien H.M., Anh H.T. Studies on the analgesic and anti-inflammatory activities of Sargassum swartzii (Turner) C. Agardh (Phaeophyta) and Ulva reticulata Forsskal (Chlorophyta) in experiment animal models. Afr. J. Biotechnol. 2011;10:2308–2314. [Google Scholar]
- 62.Vázquez A.I., Sánchez C.M.D., Delgado N.G., Alfonso A.M.S., Ortega Y.S., Sánchez H.C. Anti-inflammatory and analgesic activities of red seaweed Dichotomaria obtusata. Braz J. Pharmaceut Scis. 2011;47:111–118. [Google Scholar]
- 63.Tan L.T., Williamson R.T., Gerwick W.H., Watts K.H., Mcgough K., Jacobs R. Cis and trans, transceratospongamide, new bioactive cyclic heptapeptides from the Indonesian red alga Ceratodictyon spongiosum and symbiotic sponge Sigmadocia symbiotica. J. Org. Chem. 2000;65:419–425. doi: 10.1021/jo991165x. [DOI] [PubMed] [Google Scholar]
- 64.Germain S., Bum E.N., Talla E., Dimo T., Weiss N., Sidiki N., Dawe A., Moto F.C.O., Dzefiet P.D., Waard M. Antipyretic and antinociceptive effects of Nauclealatifolia roots decoction and possible mechanisms of action. Pharm. Biol. 2011;49:15–25. doi: 10.3109/13880209.2010.492479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chandrasekharan N.V., Simmons D.L. The cycloox-ygenases. Genome Biol. 2004;5:241. doi: 10.1186/gb-2004-5-9-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Paul J.P., Devi S.D.K. Evaluation of antipyretic activity of methanol extract of Hypnea musciformis (wulf) Lamouroux (red seaweed) in Manapad coast, Tamil Nadu. Ind. Int. J.; Med. Chem. Anal. 2015;5:74–78. [Google Scholar]
- 67.Carvalhal F., Cristelo R.R., Resende D.I., Pinto M.M., Sousa A., Correia-da-Silva M. Antithrombotics from the Sea, Polysaccharides and Beyond. Mar. Drugs. 2019;16:17. doi: 10.3390/md17030170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Adrien A., Bonnet A., Dufour D., Baudouin S., Maugard T., Bridiau N. Anticoagulant activity of sulfated ulvan isolated from the green macroalga Ulva rigida. Mar. Drugs. 2019;14:17. doi: 10.3390/md17050291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Holtkamp A.D., Kelly S., Ulber R., Lang S. Fucoidans and fucoidanas esfocus on techniques for molecular structure elucidation and modification of marine polysaccharides. Appl. Microbiol. Biotechnol. 2009;82:1–11. doi: 10.1007/s00253-008-1790-x. [DOI] [PubMed] [Google Scholar]
- 70.Güven K.C., Coban B., Sezik E. Anticoagulant and antilipaemic activities of polysaccharides from marine algae. J. Black Sea Med. Env. 2019;25:22–257. [Google Scholar]
- 71.Necas J., Bartosikova L. Carrageenan, A Review. Vet. Med. 2013;58:187–205. doi: 10.17221/6758-VETMED. [DOI] [Google Scholar]
- 72.Venkatraman A., Yahoob S.A.M., Nagarajan Y., Harikrishnan S., Vasudevan S., Murugasamy T. Pharmacological activity of biosynthesized gold nanoparticles from brown algae seaweed Turbinaria conoide. Nanoworld J. 2018;4:17–22. doi: 10.17756/nwj.2018-055. [DOI] [Google Scholar]
- 73.Makkar F., Chakraborty K. Antidiabetic and anti-inflammatory potential of Sulphated polygalactans from red seaweed Kappaphycus alvarezii and Gracilaria opuntia. Int. J. Food Prop. 2017;20:1326–1337. doi: 10.1080/10942912.2016.1209216. [DOI] [Google Scholar]
- 74.Nguyen T.H., Nguyen T.H., Nguyen V.M., Nguyen T.L.P., Tran T.V.A., Do A.D., Kim S.M. Antidiabetic and antioxidant activities of red seaweed Laurencia dendroidea. Asian Pac. J. Trop. Biomed. 2019;9:501–509. doi: 10.4103/2221-1691.271723. [DOI] [Google Scholar]
- 75.Chen H.M., Zheng L., Yan Agaro X.J. Bioactivity research of oligosaccharides. Food Technol. Biotechnol. 2005;43:29–36. [Google Scholar]
- 76.Chan P.T., Matanjun P., Yasir S.M., Tan T.S. Histopathological studies on liver, kidney and heart of normal and dietary induced hyperlipidaemic rats fed with tropical red seaweed Gracilaria changii. J. Funct. Foods. 2015;17:202–213. doi: 10.1016/j.jff.2015.05.019. [DOI] [Google Scholar]
- 77.Seung-Hong L., You-Jin J. Anti-diabetic effects of brown algae derived phlorotannins, marine polyphenols through diverse mechanisms. Fitoterapia. 2013;86:129–136. doi: 10.1016/j.fitote.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 78.Kang M.-C., Ko N.K., Kim Y.-B., Jeon Y.J. Anti-obesity effects of seaweeds of Jeju Island on the differentiation of 3T3-L1 preadipocytes and obese mice fed a high-fat diet. Food Chem. Toxicol. 2016;90:36–44. doi: 10.1016/j.fct.2016.01.023. [DOI] [PubMed] [Google Scholar]
- 79.Lee H.-G., Lu Y.A., Li X., Hyun J.-M., Kim H.-S., Lee J.J., Kim T.H., Kim H.M., Kang M.-C. Anti-Obesity effects of Grateloupia elliptica, a red seaweed, in mice with high-fat diet-induced obesity via suppression of adipogenic factors in white adipose tissue and increased thermogenic factors in brown adipose tissue. Nutrients. 2020;12:308. doi: 10.3390/nu12020308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lu Y.A., Lee H.G., Li X., Hyun J.-M., Kim H.-S., Kim T.H., Kim H.-M., Lee J.J., Kang M.-C., Jeon Y.J. Anti-obesity effects of red seaweed, Plocamium telfairiae, in C57BL/6 mice fed a high-fat diet. 2020 doi: 10.1039/C9FO02924A. [DOI] [PubMed] [Google Scholar]
- 81.Seo M.-J., Lee O.-H., Choi H.-S., Lee B.-Y. Extract from edible red seaweed (Gelidium amansii) inhibits lipid accumulation and ros production during differentiation in 3T3-L1 cells. Prev. Nutr. Food Sci. 2012;17:129–135. doi: 10.3746/pnf.2012.17.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mabeau S., Fleurence J. Seaweed in Food Products, Biochemical and Nutritional Aspects. Trends Food Sci. Technol. 1993;4:103–107. [Google Scholar]
- 83.Kim K.-J., Lee O.-H., Le B.-Y. Fucoidan, a sulfated polysaccharide, inhibits adipogenesis through the mitogen-activated protein kinase pathway in 3T3-L1 preadipocytes. Life Sci. 2010;86:791–797. doi: 10.1016/j.lfs.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 84.Seca A.M.L., Diana C.G.A., Pinto D.C.G.A. Overview on the Antihypertensive and Anti-Obesity Effects of Secondary Metabolites from Seaweeds. Mar. Drugs. 2018;16:237. doi: 10.3390/md16070237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fitzgerald C., Gallagher E., Tasdemir D., Hayes M. Heart health peptides from macroalgae and their potential use in functional foods. J. Agricul. Food Chem. 2011;59:6829–6836. doi: 10.1021/jf201114d. [DOI] [PubMed] [Google Scholar]
- 86.Saito M., Kawai M., Hagino H., Okada J., Yamamoto K., Hayashida M., Ikeda T.P. Antihypertensive effect of Nori-peptides derived from red alga Porphyra yezoensis in hypertensive patients. Amer. J. Hypertension. 2002;15:210A. [Google Scholar]
- 87.McNamara Y. Alzheimer’s association international conference (AAIC) copenhagen, denmark-July 12–17, 2014. Drugs Future. 2014;39:651–656. [Google Scholar]
- 88.Vinutha B., Prashanth D., Salma K., Sreeja S.L., Pratiti D., Padmaja R., Radhika S., Amit A., Venkateshwarlu K., Deepak M. Screening of selected Indian medicinal plants for acetylcholinesterase inhibitory activity. J. Ethnopharmacol. 2007;109:359–363. doi: 10.1016/j.jep.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 89.Machado L.P., Carvalho L.R., Young M.C.M., Cardoso-Lopes E.M., Centeno D.C., Zambotti-Villela L., Colepicolo P., Yokoya N.S. Evaluation of acetylcholinesterase inhibitory activity of Brazilian red macroalgae organic extracts. Rev. Bras. Farmacogn. 2015;25:657–662. [Google Scholar]
- 90.Ghannadi A., Plubrukarn A., Zandi K., Sartavi K., Yegdaneh A. Screening for antimalarial and acetylcholinesterase inhibitory activities of some Iranian seaweeds. Res. Pharm. Sci. 2013;8:113–118. [PMC free article] [PubMed] [Google Scholar]
- 91.Olasehindea T.A., Mabinyaa L.V., Olaniranc A.O., Okoha A.I. Chemical characterization, antioxidant properties, cholinesterase inhibitory and anti-amyloidogenic activities of sulfated polysaccharides from some seaweeds. Bioact. Carbohydr Diet. Fibre. 2019;18:1–10. doi: 10.1016/j.bcdf.2019.100182. [DOI] [Google Scholar]
- 92.Caltagirone C., Ferrannini L., Marchionni N., Nappi G., Scapagnini G., Trabucchi M. The potential protective effect of Tramiprosate (homotaurine) against Alzheimer’s disease, A review. Aging Clin. Exp. Res. 2012;24:580–587. doi: 10.3275/8585. [DOI] [PubMed] [Google Scholar]
- 93.Pereira L. Seaweeds as source of bioactive substances and skin care therapy-cosmeceuticals, algotheraphy and thalassotherapy. Cosmetics. 2018;5:68. doi: 10.3390/cosmetics5040068. [DOI] [Google Scholar]
- 94.Chan J.N., Poon B.P., Salvi J., Olsen J.B., Emili A., Mikhail A. Perinuclear cohibin complexes maintain replicative life span via roles at distinct silent chromatin domains. Dev. Cell. 2011;20:867–879. doi: 10.1016/j.devcel.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 95.Leandro A., Leonel Pereira L., Gonçalves A.M.M. Diverse applications of marine macroalgae. Mar. Drugs. 2020;18:17. doi: 10.3390/md18010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jin M., Liu H., Hou Y., Chan Z., Di W., Li L., Zeng R. Preparation, characterization and alcoholic liver injury protective effects of algal oligosaccharides from Gracilaria lemaneiformis. Food Res. Int. 2017;100:186–195. doi: 10.1016/j.foodres.2017.08.032. [DOI] [PubMed] [Google Scholar]
- 97.Kim J.H., Yun E.J., Yu S., Kim K.H., Kang N.J. Different levels of skin whitening activity among 3,6-Anhydro-L-galactose, agarooligosaccharides, and neoagarooligosaccharides. Mar. Drugs. 2017;15:321. doi: 10.3390/md15100321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yarnpakdee S., Benjakul S., Senphan T. Antioxidant activity of the extracts from freshwater macroalgae (Cladophora glomerata) grown in northern Thailand and its preventive effect against lipid oxidation of refrigerated eastern little tuna slice. Turk. J. Fish. Aquat Sci. 2018;19:209–219. [Google Scholar]