Abstract
Non-alcoholic fatty liver disease (NAFLD) is among the most frequent etiologies of cirrhosis worldwide, and it is associated with features of metabolic syndrome; the key factor influencing its prognosis is the progression of liver fibrosis. This review aimed to propose a practical and stepwise approach to the evaluation and management of liver fibrosis in patients with NAFLD, analyzing the currently available literature. In the assessment of NAFLD patients, it is important to identify clinical, genetic, and environmental determinants of fibrosis development and its progression. To properly detect fibrosis, it is important to take into account the available methods and their supporting scientific evidence to guide the approach and the sequential selection of the best available biochemical scores, followed by a complementary imaging study (transient elastography, magnetic resonance elastography or acoustic radiation force impulse) and finally a liver biopsy, when needed. To help with the selection of the most appropriate method a Fagan′s nomogram analysis is provided in this review, describing the diagnostic yield of each method and their post-test probability of detecting liver fibrosis. Finally, treatment should always include diet and exercise, as well as controlling the components of the metabolic syndrome, +/- vitamin E, considering the presence of sleep apnea, and when available, allocate those patients with advanced fibrosis or high risk of progression into clinical trials. The final end of this approach should be to establish an opportune diagnosis and treatment of liver fibrosis in patients with NAFLD, aiming to decrease/stop its progression and improve their prognosis.
Keywords: Non-alcoholic fatty liver disease, Liver fibrosis, Clinical assessment, Diagnosis, Treatment, Test accuracy
Core Tip: The most important liver-related factor associated with adverse clinical outcomes and mortality in patients with non-alcoholic fatty liver disease (NAFLD) is the presence and progression of fibrosis; its progression depends upon genetic, clinical, and biochemical risk factors, that must be assessed in order to identify patients at risk. To be able to accurately identify fibrosis we must take into account the diagnostic ability of each method and its possible variations according to the local prevalence and the selected cutoffs. This review summarizes the available data on assessment and management of NAFLD with a comprehensive analysis of the current diagnostic methods.
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is one of the main etiologies of cirrhosis worldwide[1], given its close relationship with features of the metabolic syndrome, including obesity and insulin resistance, NAFLD is becoming one of the most frequent and the fastest-growing cause of chronic liver disease in the world, and it is expected to grow exponentially in the following years, thus increasing the health system and economic burden[2,3].
NAFLD is defined as the accumulation of fat in the liver (> 5%), after the exclusion of other potential causes such as alcohol, viral infections, and drugs, among others. The next entity in the spectrum of the disease is non-alcoholic steatohepatitis (NASH) which is defined as the presence of hepatocellular injury and cell death, with lobular and portal inflammation. The final stages, fibrosis and cirrhosis arise as a consequence of the deposition of collagen and subsequent vascular remodeling[4,5]. Finally, within the spectrum of the disease, hepatocellular carcinoma should be included as a complication after this series of pathophysiological events.
The frequency of hepatic steatosis varies significantly according to ethnicity, being more frequent in Hispanics (45%) than in Caucasians (10%-33%) and African Americans (24%), which is probably related to the higher prevalence of obesity and insulin resistance in this ethnic group, as well as the influence of genetic factors[3,6,7]. Genetic polymorphisms in NAFLD have been identified as associated with the presence of features of the metabolic syndrome (glucose and lipid metabolism, as well as hypertension) and inflammation[8]. The prevalence of NAFLD in high-risk groups, like type 2 diabetes mellitus (T2DM) is even higher, being present in almost 70% of this group[9]. Other high-risk populations include those with hypertension, obesity, and dyslipidemia[2,10]. In terms of fibrosis, according to the National Health and Nutrition Examination Survey up to 10.3% of the patients with NAFLD have advanced fibrosis[11].
The presence of fibrosis rather than the diagnosis of steatohepatitis is the most relevant feature associated with liver-related events and overall mortality. This effect is seen even in the early stages of fibrosis, showing a stepwise increase in adverse outcomes as the stage of fibrosis progresses[11-14]. Fibrosis parallels the development of the two major components of chronic liver diseases: Portal hypertension and functional hepatocyte insufficiency. However, the importance of liver fibrosis is beyond “liver prognosis” itself, as it is associated with other adverse clinical outcomes, including cardiovascular events[15], ischemic stroke[16], metabolic complications[17], and all-cause and cardiovascular mortality[18,19]. This could be explained by a more pronounced systemic inflammation profile influencing different organs and systems, and the interaction between them leading to further inflammation and activation of the immune response.
Therefore, early recognition and proper management of liver fibrosis in NAFLD are of major importance. In this review, we will address the risk factors for NAFLD and the risk of progression, as well as the currently available methods used to assess liver fibrosis and the new treatments available. This will help the clinicians to early recognize liver fibrosis in patients with NAFLD and use the best available methods for its evaluation and management.
DETERMINANTS OF FIBROSIS PROGRESSION
It is important to note that both biopsy-proven NASH and simple steatosis can progress to liver fibrosis, which contradicts the classic theory in which NAFLD has a benign curse while NASH has a more aggressive one[20]. Significant fibrosis can be observed in approximately one-third of patients with NAFLD in the absence of NASH[21]. The etiology of fibrosis in non-NASH patients is not entirely clear, although there are several theories; it has been hypothesized that these cases represent a form of NASH in remission as aminotransferase levels improve regardless of whether or not fibrosis progress or that T2DM by itself could be fibrogenic[22-24].
The progression of fibrosis in patients with NAFLD and NASH is variable, on average 40% have progression of fibrosis over a mean period of 3-6 years. Despite the incidence of fibrosis, the change is slow, being about 0.02 stages overall per year. There is considerable variability, with one out of six patients having a relatively rapid progression of more than 0.5 stages per year, and some patients progressing from no fibrosis to advanced fibrosis (F3-F4) on average in 12 years[23,24]. The determinants of fibrosis progression can be divided into genetic and clinical determinants.
Genetic determinants
Genetic factors are of major importance in the development[25] and the risk of fibrosis progression in NAFLD[26]. At least four genetic variants have been associated with fibrosis progression. The variant rs738409 in the human patatin-like phospholipase domain-containing 3 (PNPLA3) gene located in chromosome 22[25], is the best described and major genetic determinant of liver fibrosis development and progression in NAFLD[21,26-28]. The variant rs58542926, of the transmembrane 6 superfamily member 2 (TM6SF2) gene has also been implicated in de progression of fibrosis in NAFLD, however, the data is conflicting[28-30], probably due to the heterogeneity of the populations evaluated[27]. It is possible, however, that there is an additive effect of TM6SF2 and PNAPLA3 variants on the histological severity of NAFLD[27]. Finally, the rs641738 C>T genetic variant in Membrane-bound O-acyltransferase domain containing 7 (MBOAT7) and the variation in the glucokinase regulator have also been associated with higher severity of necroinflammation and fibrosis[31,32].
In a cohort of 515 patients with NAFLD, PCR-based assays were used to genotype the PNPLA3, TM6SF2, and MBOAT7 variants. The three variants were associated with increased liver injury. The TM6SF2 variant was associated predominantly with hepatic fat accumulation, whereas the MBOAT7 polymorphism was linked to fibrosis. The PNPLA3 polymorphism conferred a higher risk for both steatosis and fibrosis[33].
Clinical and environmental determinants
There are several clinical determinants of fibrosis progression in NAFLD and NASH, however, by far the presence of insulin resistance and T2DM are the major predictors of fibrosis progression[23]. Other clinical determinants of fibrosis progression rate are body mass index (BMI), sarcopenia, absence of treatment with renin-angiotensin system (RAS) inhibitors, non-obese NASH patients, alanine aminotransferase (ALT) levels above the upper limit of normal (ULN), and the severity of hepatic fat accumulation[22-24,34-36].
EVALUATION OF LIVER FIBROSIS IN NAFLD
Clinical evaluation
Most patients diagnosed with NAFLD are asymptomatic, with only a few of them complaining of mild upper quadrant pain related to fatty infiltration of the liver. Three general scenarios could arise the suspicion of NAFLD, including abnormalities on imaging performed for other reasons, abnormal liver enzymes, or based on high-risk features of NAFLD such as metabolic syndrome[37]. There are no specific signs or symptoms related to the early stages of NAFLD fibrosis, once the patient presents with advanced fibrosis, portal hypertension or/and liver dysfunction they will develop specific symptoms of hepatic decompensation (ascites, splenomegaly, spider angiomas, palmar erythema, caput medusae, hepatic encephalopathy, and jaundice). Since most of the cases will present as NAFLD alone or mild fibrosis the physician should have a high suspicion index in patients with high-risk factors such as insulin resistance, T2DM, hypertension, obesity, and dyslipidemia. Some of the clinical hallmarks the clinician should pay attention to include acanthosis nigricans and skin tags, usually located in the lateral area around the neck and axillae, as well as hirsutism, polycystic ovary syndrome, and a high waist/hip ratio[38,39]. In the case of obstructive sleep apnea (OSA), clinical evaluation includes the presence of fatigue, sleepiness and snoring, and the use of the Epworth Sleepiness scale, measurement of neck circumference, and Mallampati scale evaluation[40]. Although these features are not specific of fibrosis, their presence should raise suspicion of a high-risk phenotype, rendering further assessment mandatory.
Blood tests and scores
Serologic tests for fibrosis detection can be divided into direct and indirect markers. Indirect markers of fibrosis aim to obtain information from the overall liver function, whereas direct serologic markers are molecules that are obtained directly from byproducts or products related to collagen deposition[37].
Indirect markers, such as routine laboratory tests are unreliable to accurately and promptly detect liver fibrosis in NAFLD unless advanced fibrosis and subsequent portal hypertension exist when thrombocytopenia and high levels of aspartate aminotransferase (AST) are observed. However, these variables provide a rather overt diagnosis, relying on the personal experience of their interpretation.
Several biomarkers have been proposed as direct markers of liver fibrosis, one of the most described is cytokeratin 18 (CK-18). Cytokeratins are proteins of keratin-containing intermediate filaments located in the intracytoplasmic cytoskeleton of epithelial tissue, being CK-18 the predominant in the liver and released as a consequence of increased apoptosis and associated with fibrosis in NASH[41]. CK-18 has been investigated as a potential biomarker of severity and liver fibrosis in different etiologies[42-44]. In NAFLD, CK-18 increased significantly with steatosis and fibrosis stages, however, it has a low sensitivity and specificity, ranging from 54% to 62% and 69% to 85%, respectively, limiting its use as a reliable diagnostic tool[43,45].
Other direct markers of liver fibrosis are collagens and their fragments since they represent the principal component of the fibrotic scars. The most validated biomarkers for the measurement of type III collagen formation are the amino-terminal propeptide of procollagen type III (PIIINP) and N-terminal pro-collagen III peptide (PRO-C3) biomarkers[46]. PRO-C3 which is a collagen fragment is significantly higher in NASH patients with advanced fibrosis than those without advanced fibrosis. PRO-C3 levels have a direct correlation with worsening of liver fibrosis, in the same manner, PRO-C3 levels decrease with fibrosis improvement, identifying patients with active fibrogenesis. It is noteworthy that patients with advanced fibrosis can have an inactive disease, implying lower production of collagen and therefore having normal levels of PRO-C3[47]. The European Liver Fibrosis project developed the enhanced liver fibrosis (ELF™) test, a blood-based score that was comprised of ELISA measurements of hyaluronic acid, PIIINP, and tissue inhibitor of matrix metalloproteinases; this score has a sensitivity and specificity for severe liver fibrosis of 78% and 76%, respectively[48].
None of the currently available biomarkers by itself has sufficient accuracy for diagnosing fibrosis which is why predictive scores play an important role in providing a cutoff able to discern between no fibrosis or the presence of advanced fibrosis. Among the predictive scores fibrosis-4 index (FIB-4) index, NAFLD fibrosis score (NFS), the BARD score, FibroTest, HepatoScore, hepamet fibrosis score (HFS), and AST to platelets ratio index (APRI) score, are the most widely used (Table 1)[49,50].
Table 1.
Diagnostic performance of blood tests and scores for fibrosis assessment methods from studies made in patients with non-alcoholic fatty liver disease
| Population (n) | Ref. | Cutoffs | Se | Sp | PPV | NPV | AUROC | |
| FIB-4 (age, platelet count, AST and ALT) | 541 | [52] | ≤ 1.3 NF | 74% | 71% | 43% | 90% | 0.802 |
| ≥ 2.67 AF | 33% | 98% | 80% | 83% | ||||
| 153 | [53] | ≥ 1.3 AF | 87% | 60% | NA | NA | 0.895 | |
| 452 | [56] | ≥ 1.5 AF | 75% | 67% | 58% | 82% | 0.780 | |
| 328 | [57] | ≤ 1.3 NF | 56% | 56% | 22% | 85% | 0.540 | |
| ≥ 2.67 AF | 22% | 87% | 27% | 84% | ||||
| APRI (AST and platelet count) | 153 | [53] | > 1 AF | 78% | 82% | NA | NA | 0.830 |
| > 2 AF | 28% | 92% | NA | NA | ||||
| 452 | [56] | > 0.559 AF | 62% | 76% | 61% | 76% | 0.754 | |
| NFS (age, glycaemia, BMI, platelet count, albumin, AST and ALT) | 480 | [55] | ≤ -1.455 NAF | 82% | 77% | 56% | 93% | 0.820 |
| ≥ 0.676 AF | 51% | 98% | 90% | 85% | ||||
| 126 | [58] | ≤ -1.455 NAF; ≥ 0.676 AF | 96% | 84% | 70% | 98% | 0.919 | |
| 138 | [59] | ≤ -1.455 NAF | 22% | 100% | 100% | 81% | 0.680 | |
| ≥ 0.676 AF | ||||||||
| 452 | [56] | > -1.036 AF | 77% | 60% | 54% | 81% | 0.732 | |
| 328 | [57] | ≤ -1.455 NAF | 53% | 67% | 26% | 87% | 0.640 | |
| 122 | [61] | ≥ 0.676 AF | 9% | 98% | 50% | 83% | 0.840 | |
| NA | NA | 59% | 89% | |||||
| BARD (BMI > 28 kg/m2, AST/ALT ratio > 0.8 and diabetes) | 126 | [58] | 0-1 NAF | 89% | 89% | 69% | 97% | 0.919 |
| 2-4 AF | ||||||||
| 138 | [59] | 0-1 NAF | 51% | 77% | 45% | 81% | 0.670 | |
| 2-4 AF | ||||||||
| 160 | [60] | 0-1 NAF | NA | NA | 27% | 97% | 0.780 | |
| 2-4 AF | ||||||||
| 452 | [56] | 2-4 AF | 79% | 51% | 50% | 80% | 0.695 | |
| 122 | [61] | 2-4 AF | NA | NA | 59% | 77% | 0.730 | |
| 328 | [57] | 2-4 AF | 83% | 37% | 22% | 91% | 0.594 | |
| Fibrometer NAFLD | 452 | [56] | ≥ 0.311 AF | 80% | 62% | 56% | 83% | 0.817 |
| Hepascore (age, sex, bilirubin, GGT, hyaluronic acid, and a2-macroglobulin) | 452 | [56] | ≥ 0.322 AF | 67% | 76% | 63% | 79% | 0.778 |
| Fibrotest (α-2-macroglobulin, haptoglobin, apolipoprotein A1, GGT, and TB) | 452 | [56] | ≥ 0.316 AF | 81% | 57% | 54% | 83% | 0.736 |
| HFS (sex, age, HOMA score, diabetes, AST, albumin, and platelets) | 2452 | [69] | < 0.12 NAF | 73.9% | 77.4% | 46% | 91.9% | 0.848 |
| ≥ 0.47 AF | 35.2% | 97.2% | 76.3% | 85.2% | ||||
| 49 | [70] | ≥ 0.47 AF | 11% | 100% | 100% | 83% | NA |
Se: Sensitivity; Sp: Specificity; PPV: Positive predictive value; NPV: Negative predictive value; AUROC: Area under the curve; FIB-4: Fibrosis-4 index; APRI: Aspartate aminotransferase to platelet ratio index; NFS: Non-alcoholic fatty liver disease fibrosis score; HFS: Hepamet fibrosis score; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; BMI: Body mass index; GGT: Gamma-Glutamyltransferase; TB: Total bilirubin; HOMA: Homeostatic model assessment; NF: No fibrosis; AF: Advanced fibrosis; NAF: Non-advanced fibrosis; NA: Not available.
FIB-4 index: FIB-4 index is a complex marker, based on age, platelet count, AST, and ALT, it was developed in 2006 as a non-invasive panel to stage liver disease in subjects with human immunodeficiency virus and hepatitis C virus co-infection[51]. In NAFLD, a cutoff value < 1.45 has a negative predictive value (NPV) of 90% and a sensitivity of 84% to exclude advanced fibrosis. A cutoff > 3.25, had a positive predictive value (PPV) of 65% and a specificity of 68%[50,52]. This indicates that 84% of patients with suspected NAFLD-related advanced fibrosis would be identified by the FIB-4 index and avoid a liver biopsy. However, more than 30% of NAFLD patients diagnosed as non-advanced fibrosis by the FIB-4 index may have advanced fibrosis in liver biopsy. Given that about a third of patients could be misdiagnosed as non-advanced fibrosis, FIB-4 cannot replace liver biopsy[53,54].
NFS score: NFS score includes age, glycemia, BMI, platelet count, albumin, AST, and ALT; unlike other prognostic scores in NAFLD which were created for other etiologies, NFS was developed in 733 patients with biopsy-proven NAFLD[55]. NFS uses two diagnostic cutoffs, the low cutoff score (-1.455) to exclude advanced fibrosis (NPV 88%-93%), and the high cutoff score (0.676) to diagnose advanced fibrosis (PPV 82%-90%)[55], leaving one-third of patients in a “grey zone” where liver biopsy is still required[56]. In further validations, NFS has remained with good NPV (81%-98%) for advanced fibrosis (F3-F4), however, PPV has had more fluctuation (50%-100%)[54,57-59].
The BARD score: BARD score is a simple index defined by the presence of three clinical and laboratory parameters, BMI (> 28 kg/m2, 1 point), AST/ALT ratio (> 0.8, 2 points), and diabetes (1 point), ranging from 0 to 4. The BARD score was developed in 2008 in 827 patients with NAFLD. The result of the score is dichotomized as 0-1 and 2-4, for low and high risk of advanced fibrosis, respectively[60]. The PPV and NPV range from 26% to 68% and 81% to 96%, respectively[58-60]; while the sensitivity and specificity ranged from 51% to 88% and 66% to 88%, respectively[54,58,59]. The score can be easily derived from clinical data, however, as the BARD score takes into account the BMI, it may be less reliable for excluding the presence of advanced fibrosis in countries where subjects with NAFLD are not overweight or obese[61].
APRI score: This score is a simple ratio that takes into account the value of AST and platelets; it was developed in 2003 to predict liver fibrosis in patients with hepatitis C[62]. With an APRI threshold of 1.5, the sensitivity and specificity are 84.0% and 96.1%, respectively, for advanced fibrosis in NAFLD[63]. The score is a reliable tool to differentiate between patients with no fibrosis and patients with advanced fibrosis/cirrhosis, but it cannot reliably discriminate between intermediate stages of fibrosis. The area under the curve (AUROC) for the APRI score in patients with NADLF ranges from 0.8307 to 0.95[64,65].
FibroTest: FibroTest is a commercial algorithm that has shown good predictive values for diagnosing advanced fibrosis (AUROC = 0.81-0.88) in patients with NAFLD, however, its diagnostic accuracy may be affected by acute inflammation, sepsis or extrahepatic cholestasis[66,67]. This score integrates the value of five serum biomarkers (α-2-macroglobulin, haptoglobin, apolipoprotein A1, γ-glutamyltranspeptidase (GGT), and total bilirubin, adjusted for sex and age) for liver fibrosis (FibroTest), plus ALT for the necroinflammatory activity (ActiTest), into an equation-based algorithm, obtaining finally a result between 0 and 1, where higher values indicate a greater probability of liver fibrosis[68]. The AUROC to differentiate between fibrosis stages is lower, for intermediate stages, F1 vs F2 is 0.66 and for advanced fibrosis, F3 vs F4 is 0.69[67].
Hepascore: Hepascore is an algorithm to detect fibrosis in many chronic liver diseases, it combines clinical variables including age and sex with blood-based parameters such as bilirubin, gamma-glutamyl transferase, hyaluronic acid, and a-2-macroglobulin. In patients with NAFLD, a threshold of 0.37 helps to identify individuals with advanced fibrosis, with an AUROC of 0.778, NPV, and PPV of 79% and 63%, respectively[56].
Hepamet fibrosis scoring system: The HFS is the most recently developed score system, created from data of 2452 patients with biopsy proven-NAFLD at medical centers in Spain, Italy, France, Cuba, and China from the Hepamet registry. The HSF is calculated by a complex formula, using the following items: Patient sex, age, homeostatic model assessment score, presence of diabetes, AST levels, albumin, and platelet count; it is available online for public usage. HSF had an AUROC of 0.85 to discriminate between advanced fibrosis and no advanced fibrosis. In the validation cohort, a cut-off of 0.12 for low risk and 0.47 for high risk, identified patients with and without advanced fibrosis with 97.2% specificity, 74% sensitivity, a 92% NPV, and a 76.3% PPV. HFS is not affected by patient age, BMI, hypertransaminasemia, or the presence of T2DM. HFS was developed and validated in a large and heterogeneous population, giving it an advantage above other scores[69]. Recently, this score was evaluated in a cohort of 49 patients with morbid obesity undergoing bariatric surgery, a cut-off 0.47 for advanced fibrosis had a sensitivity of 11%, a specificity of 100%, a PPV of 100%, and an NPV of 83%[70].
After analyzing the data related to prediction scores, it is clear that overall, predictive scores for fibrosis have a good NPV for excluding advanced fibrosis with low PPV. Therefore, these scores may be confidently used for baseline risk stratification to exclude advanced fibrosis; however, due to their low specificity, a result of advanced fibrosis based in these scores should be further confirmed by other methods, such as imaging studies or liver biopsy depending upon availability[4].
Imaging studies
Conventional imaging studies such as ultrasonography (US), computed tomography (CT), and magnetic resonance imaging (MRI) are available techniques for the detection of fatty liver[71,72]. However, they have low to moderate accuracy to identify liver fibrosis[73,74].
Currently, elastography has become the non-invasive method of choice to quantify liver stiffness (elasticity). The basic principle is that fibrotic tissue is stiffer than normal tissue, therefore the waves spread faster in fibrotic tissue than in the normal liver. This technique uses a force to move the hepatic tissue, measuring this movement by US or MRI[75], giving the value of liver stiffness measurement (LSM) in kilopascal (kPa)[76]. The LSM obtained depends on the frequency of waves applied, therefore, it cannot be compared between different methods.
Transient elastography: One dimensional transient elastography (TE) is a non-invasive ultrasound-based method that uses shear wave velocity, providing LSM and controlled attenuation parameter (CAP) for the evaluation of steatosis. The benefit of TE compared with liver biopsy is that it measures a larger region, 100 times larger than the volume of the tissue obtained by biopsy[64]. The limitation is that occasionally measurements cannot be obtained in obese patients[49,77], reliable LSM measurements are obtained in about 80% to 90% of NASH/NAFLD patients[78,79]. It is noteworthy that at least three hours of fasting are necessary before evaluation with TE since a significant increase in LSM has been observed in patients with less than two hours of fasting[80]. In addition to this, there are quality standards that should be met to consider the result as adequate: At least 10 valid measurements (60% of success) and an IQR < 30%[81].
The cutoff value of CAP for differentiating hepatic steatosis is highly variable due to the heterogeneity of the populations evaluated, ranging from 214 to 289 dB/m, with moderate to high sensitivity and specificity (78%-91.9% and 79%-85.7%), as well as moderate to good predictive values (PPV of 77%-85.0% and NPV of 76%-92.3%)[78,82,83]. In one meta-analysis, in which 2735 patients were included, the optimal CAP cutoff was 248 dB/m, however, only 7% of the population had NAFLD diagnosis. Siddiqui et al[84], reported a prospective study of 393 patients with biopsy-proven NAFLD, in whom TE was performed, the median CAP scores for steatosis grades were 306 for S1 and, 340 for S2 and S3, the best cutoff, balancing sensitivity and specificity, to differentiate steatosis from no-steatosis was CAP of 285 dB/m, with a sensitivity of 80% and a specificity of 77%. It is noteworthy that the prevalence in the population, the etiology of liver disease, diabetes, and BMI deserve consideration when interpreting CAP, and perhaps lower thresholds should be applied to patients with high pretest probability, and higher thresholds in groups with low pretest probability[78].
For the detection of advanced fibrosis, TE has excellent accuracy (F3: Sensitivity 85%, specificity 82%, and F4: Sensitivity 92%, specificity 92%), whereas it has moderate accuracy for significant fibrosis (F2: Sensitivity 79%, specificity 75%)[61]. The diagnostic performance according to the AUROC values for the diagnosis of severe fibrosis and cirrhosis is good, ranging from 0.86 to 0.87; for the diagnosis of intermediate fibrosis (≥ F2), the AUROC is 0.82[85]. In general, the performance of TE is superior to prediction scores[63]. According to Siddiqui et al[84], a cutoff value of < 6.5 kPa excludes fibrosis with high accuracy (NPV 0.91), as well as a cutoff < 12.1 kPa excludes cirrhosis in NAFLD patients. Therefore we suggest that the cutoffs for the evaluation of steatosis and fibrosis should be the ones given by this work since the study evaluated only NAFLD patients with confirmed histology.
It is not clear if TE should be performed at defined intervals; recently a cohort of 611 patients with T2DM was followed with serial TE for 3.5 years, the majority of patients had NAFLD at baseline, and another 50% developed NAFLD during the period evaluated. Around 20% had advanced liver fibrosis at baseline, but only 4% developed advanced fibrosis in 3 years. Baseline BMI, ALT, and ∆ALT independently predicted LSM increase[80]. Based on this data, we could advise performing TE every three years only in patients with risk factors such as T2DM, elevated BMI, and ALT above the UNL, otherwise, TE could be performed every 5 years.
Acoustic radiation force impulse: Acoustic radiation force impulse (ARFI) is an elastography technique that uses modified commercially available ultrasound machines, combining both elastography and conventional B-mode US. The liver is mechanically excited using short-duration acoustic pulses with a frequency of 2.67 MHz to generate localized tissue displacements in tissue[86]. Mean normal values range from 0.8 to 1.7 m/s and mean values indicating advanced fibrosis range from 1 to 3.4 m/s, with a clear overlap of values[87]. According to Fierbinteanu-Braticevici et al[88], the optimal cutoff value to identify advanced fibrosis is 1.54 m/s, with sensitivity and specificity of 97% and 100% respectively. In a meta-analysis of 13 studies, including 1163 patients, for detection of intermediate fibrosis (≥ F2), the summary sensitivity was 74% and the specificity was 83%, for the diagnosis of cirrhosis, the sensitivity was 87% and specificity was 87%. The diagnostic odds ratio of ARFI and TE did not differ significantly in the detection of significant fibrosis[89]. In NAFLD patients, several studies have investigated the best cutoff for the detection of advanced fibrosis, ranging from 1.15 m/s to 1.77 m/s with varying sensitivities (59%-90%) and specificities (63%-91%)[85,90-93].
Magnetic resonance elastography: Magnetic resonance elastography (MRE) is a phase contrast-based MRI technique[94] in which a passive drive generates vibrations at 60 Hz by varying acoustic pressure waves transmitted from an active driver device[95]. LSM is performed by drawing region of interest (ROI) on the elastograms, which cover regions of the liver with sufficient wave amplitude. The mean ROIs from 4 slices are averaged and reported as the mean LSM of the liver[96]. An advantage of MRE is that the area measured in the liver is larger than in TE or liver biopsy, which can avoid the sampling variability caused by the heterogeneity of advanced fibrosis[77]. The cutoff LSM for the detection of advanced fibrosis in NAFLD patients varies according to the population evaluated, ranging from 3.6 to 4.8 kPa with high sensitivity and specificity[63,77,97-99]. The diagnostic accuracy of MRE for liver fibrosis in NAFLD is higher than that of clinical scoring systems and TE[77]. A 15% increase in liver stiffness on repeated MRE may be associated with histologic fibrosis progression (≥ 1 stage) and progression from early fibrosis to advanced fibrosis[100]. Magnetic resonance also quantifies hepatic steatosis with high accuracy by measuring the proton density fat fraction which is the fraction of MRI-visible protons bound to fat divided by all protons in the liver[101].
Combined methods
Recently there have been reports of test combinations for better detection of liver fibrosis in NAFLD. The serial combination of NFS or FIB-4 with TE improves the performance of the tests when applying the second test only in patients in the uncertainty area of the first test. This algorithm is a low-cost alternative that can be applied in daily practice allowing the correct classification of a high proportion of NAFLD patients[102].
The FibroScan-AST (FAST) score, was recently proposed as a reliable algorithm to identify among patients with NAFLD, those with NASH, intermediate liver fibrosis (≥ F2), and elevated NAFLD activity score (NAS ≥ 4) (i.e., those at high risk of progression of the disease). The results of the FAST score range from 0 to 1, and derive from a logarithm-based equation, considering 3 values, AST, CAP, and LSM, and are further divided into three zones, according to the value: Rule-out (≤ 0.35), gray (0.35-0.67) and rule-in (≥ 0.67). The performance of the test is good, with an AUROC of 0.85 (0.83-0.87), and a sensitivity, NPV, specificity, and PPV of 0.89, 0.94, 0.92, and 0.69, respectively[103].
Determining which method is best for the patient relies on several factors: The availability of the methods in a daily clinical care setting, the performance of the operator, the prevalence of the disease in the specific population, and the preference of the patient. As shown in some studies, a combination of non-invasive methods could help to improve the accuracy of the diagnosis.
Liver biopsy
Liver biopsy is the gold standard for the diagnosis and assessment of the severity of liver fibrosis in NAFLD[4]. In the context of NAFLD, liver biopsy is usually obtained via a percutaneous approach using ultrasound guidance. The use of a 16 gauge or wider needle is recommended for the biopsy. An adequate histology specimen should have at least 2 cm long and comprising 10 or more portal tracts, and the review of specimens should be carried out by two pathologists[104,105].
Specimens should be processed with hematoxylin and eosin staining and specifically with Masson’s trichrome or Sirius red staining to assess fibrosis. Liver fibrosis has a singular pattern in NAFLD, frequently beginning in the pericentral zone 3 and eventually progressing to bridging fibrosis and cirrhosis[106]. There are several systems to evaluate NAFLD biopsies. The NAFLD activity score was developed as a tool to measure changes in NAFLD during therapeutic trials, the maximum score is 8, comprises steatosis (0-3), ballooning (0-2) and lobular inflammation (0-3), with a major drawback as it does not take into account the amount of fibrosis[104]. For the evaluation of fibrosis in NAFLD, there are three scoring assessment systems, the Brunt system, the NASH Clinical Research Network (CRN) system, and the Steatosis, Activity, Fibrosis (SAF) system[107,108]. In the Brunt system, fibrosis stages are divided into four, stage 1, zone 3 perisinusoidal fibrosis; stage 2, portal fibrosis; stage 3, bridging fibrosis; and stage 4, liver cirrhosis[109]. The NASH CRN system is a modification of the Brunt system in which stage 1 is subdivided into three stages, to include a distinction between delicate (1a) and dense (1b) perisinusoidal fibrosis, and to detect portal-only fibrosis, without perisinusoidal fibrosis (stage 1c), showing reasonable interrater agreement among experienced pathologists[110]. The SAF system which includes the NASH CRN system was built from a cohort of morbidly obese patients undergoing bariatric surgery. This system separately assesses the grade of steatosis (S0 to S3), the grade of activity (A0 to A4) and the stage of fibrosis (F0 to F4), the latter according to the NASH-CRN staging system, with the single modification of pooling the three substages (1a, 1b, and 1c) into a single F1 score[111]. Some computerized techniques also have been developed for the quantification of fibrosis in NAFLD/NASH histology, representing promising and accurate methods for the evaluation of liver fibrosis in this group of patients[112,113].
Liver biopsy has some relevant inconveniences, including the fact that only 1/50000 of the whole liver tissue is sampled, therefore sampling error is a major concern. To prevent sampling error, it is important to collect a sufficient amount of tissue; the use of a thick needle and a collection of at least 2 samples are recommended[114]. Inter-observer variability of the pathologist is another important concern, for the diagnosis of liver fibrosis in NAFLD, pathologists have a moderate inter-observer agreement (κ scores of 0.52-0.56) for fibrosis stage[115], therefore, at least two pathologists should review the specimens. Finally, complications associated with percutaneous liver biopsy are rare, only 1% to 3% of patients require hospitalization and the mortality rate is extremely low, 1 in 10000 to 1 in 12000 liver biopsies[114].
Due to the high prevalence of steatosis, it is not practical to perform a liver biopsy in every patient with NAFLD. The European Association for the Study of the Liver (EASL) guidelines recommend that for the identification of advanced fibrosis or cirrhosis, serum biomarkers/scores and/or TE are less accurate, and it is important to confirm these advanced stages by liver biopsy. According to the clinical context and in selected patients at high risk of liver disease progression, monitoring should include a repeat liver biopsy after at least a 5-year follow-up[4]. The American Association for the Study of Liver Disease (AASLD) guidelines recommend performing a liver biopsy when the diagnosis is not clear (i.e., there is a suspicion of another liver disease), or in those with a high probability of NASH/advanced fibrosis, especially in those considered for treatment with vitamin E or pioglitazone[116].
With the evidence above mentioned, it seems more practical to perform a liver biopsy in NAFLD only in patients with high suspicion of advanced fibrosis and/or rapid progression, when other causes of liver damage cannot be ruled out or for clinical trials exploring new-generation drugs.
Tables 1 and 2 summarize the diagnostic performance of the previously mentioned methods. It is of great importance to consider that some methods have different cutoff values, as can be seen in the table, and each cutoff holds different predictive values. This must be taken into account at the time of the patient's evaluation in the clinical practice and it is especially important to use the same cutoff if follow-up is to be done.
Table 2.
Diagnostic performance of imaging studies for fibrosis assessment methods from studies made in patients with non-alcoholic fatty liver disease
| Population | Ref. | Cutoffs | Se | Sp | PPV | NPV | AUROC | |
| TE [LSM (kPA)] | n = 291 | [85] | ≥ 8.2 kPa AF | 90% | 61% | NA | NA | 0.870 |
| ≥ 12.5 kPa AF | 57% | 90% | NA | NA | NA | |||
| 452 | [56] | ≥ 8.7 kPa AF | 88% | 63% | 59% | 90% | 0.831 | |
| 142 | [77] | ≥ 11.7 kPa AF | 86% | 84% | 75% | 92% | 0.880 | |
| 79 | [83] | ≥ 9.5 kPa AF | 92% | 63% | 54% | 94% | NA | |
| MRE [LSM (kPA)] | 142 | [77] | ≥ 4.8 kPa AF | 74% | 87% | 74% | 81% | 0.890 |
| 628 | [63] | ≥ 3.6 kPa AF | 86% | 91% | 71% | 93% | NA | |
| 117 | [97] | ≥ 3.63 kPa AF | 81% | 89% | 68% | 97% | NA | |
| 142 | [98] | ≥ 4.15 kPa AF | 85% | 92% | NA | NA | 0.954 | |
| 102 | [99] | > 3.64 kPa AF | 92% | 90% | NA | NA | 0.957 | |
| ARFI [SWV (m/s)] | 291 | [85] | ≥ 1.15 AF | 90% | 63% | NA | NA | 0.840 |
| ≥ 1.53 AF | 59% | 90% | NA | NA | NA | |||
| 57 | [90] | ≥ 1.45 AF | 76% | 68% | NA | NA | 0.910 | |
| 32 | [91] | ≥ 1.3 AF | 85% | 83% | NA | NA | NA | |
| 23 | ≥ 1.47 AF | 100% | 75% | NA | NA | 0.942 | ||
| NASH | [92] | |||||||
| 54 | [93] | ≥ 1.77 AF | 100% | 91% | NA | NA | 0.930 |
Se: Sensitivity; Sp: Specificity; PPV: Positive predictive value; NPV: Negative predictive value; AUROC: Area under the curve; TE: Transient elastography; MRE: Magnetic resonance elastography; ARFI: Acoustic radiation force impulse; LSM: Liver stiffness measurement; kPa: Kilopascal; SWV: Shear wave velocity; NASH: Non-alcoholic steatohepatitis; NF: No fibrosis; AF: Advanced fibrosis; NAF: Non-advanced fibrosis; NA: Not available.
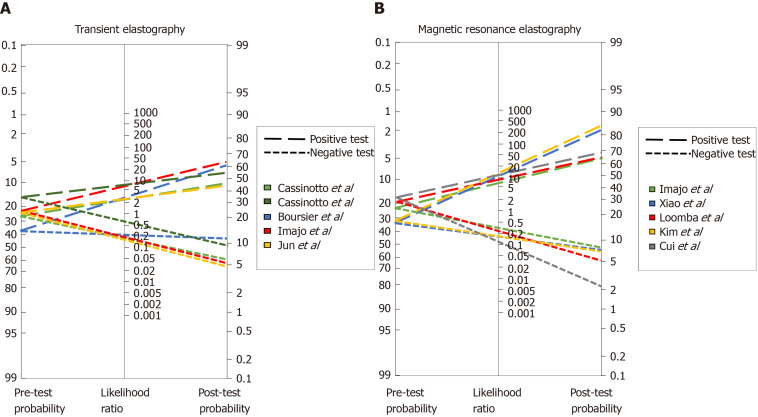
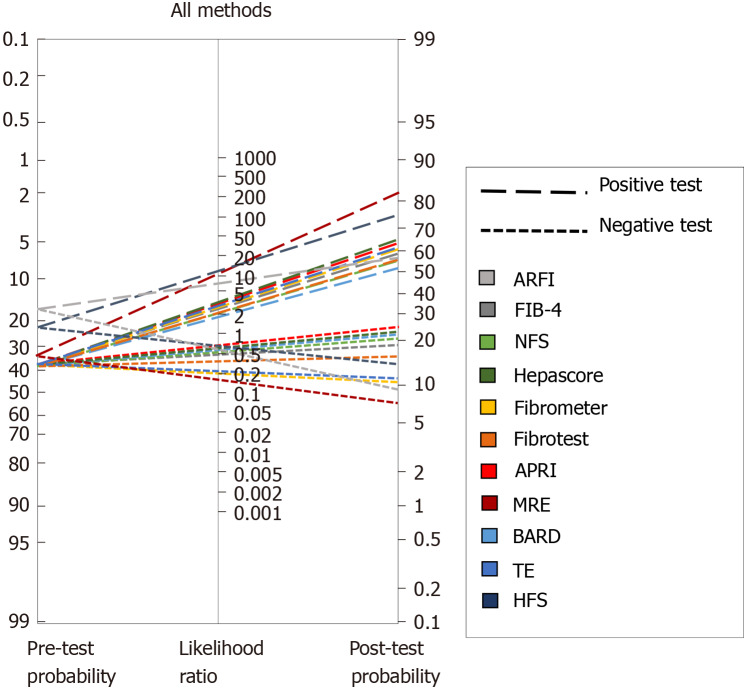
To further address this issue, we analyzed the diagnostic capabilities of several methods based on Fagan′s nomogram, which considers the pre-test probability, or prevalence, of the disease we are looking to diagnose and, uses the likelihood ratio that derives from the sensitivity and specificity reported in published studies, to estimate a final post-test probability, which simply represents the probability of a patient truly having the condition of interest if the test is positive or truly not having the disease if the test is negative.
Figure 1A depicts the analysis of all TE studies presented in Table 2, and Figure 1B shows the MRE studies. As mentioned before, each study proposes different cutoffs, therefore it is of great importance to evaluate the behavior of each one by its post-test probability and not only by their sensitivity and specificity. In Figure 1A one can conclude that overall, the best cutoff to use is the one derived from the study by Imajo et al[77] where > 11.7 kPa has the highest probability to detect advance fibrosis if the patient actually has it, and the lowest probability of diagnosing it if the patient does not have advanced fibrosis. With respect to MRE studies in Figure 1B, two of the cutoffs have a great performance with a post-test probability > 80%; overall the best cutoff derives from the study of Kim et al[98] with a post-test probability of diagnosing advanced fibrosis of 84% in patients with the disease when the employed cutoff is > 4.15 kPa. Interestingly once we compare both methods in Figure 1 it is apparent that MRE has better diagnostic capabilities, hence we created a Fagan′s nomogram using all the methods presented in Tables 1 and 2, to evaluate which one could diagnose or exclude AF better (Figure 2). Since the study by Boursier et al[56] evaluated most of the methods in their study we used those results to create the graph, for the remaining methods: MRE, HFS and AFRI, we selected the study by Xiao et al[63], Ampuero et al[69], and Cassinotto et al[85] respectively. The analysis of this graph shows that the best methods with higher post-test probability to detect advanced fibrosis in patients with the disease in order of detection are MRE, Hepamet fibrosis scoring system, NFS score, APRI, TE, fibrometer and FIB-4, the best methods to exclude advanced fibrosis in patients without the disease are, MRE, AFRI, Fibrometer and TE.
Figure 1.
Fagan′s nomogram for (A) transient elastography and (B) magnetic resonance elastography studies.
Figure 2.
Fagan′s nomogram for all diagnostic methods. ARFI: Acoustic radiation force impulse; FIB-4: Fibrosis-4 index; NFS: Non-alcoholic fatty liver disease fibrosis score; APRI: Aspartate aminotransferase to platelet ratio index; MRE: Magnetic resonance elastography; TE: Transient elastography; HFS: Hepamet fibrosis score.
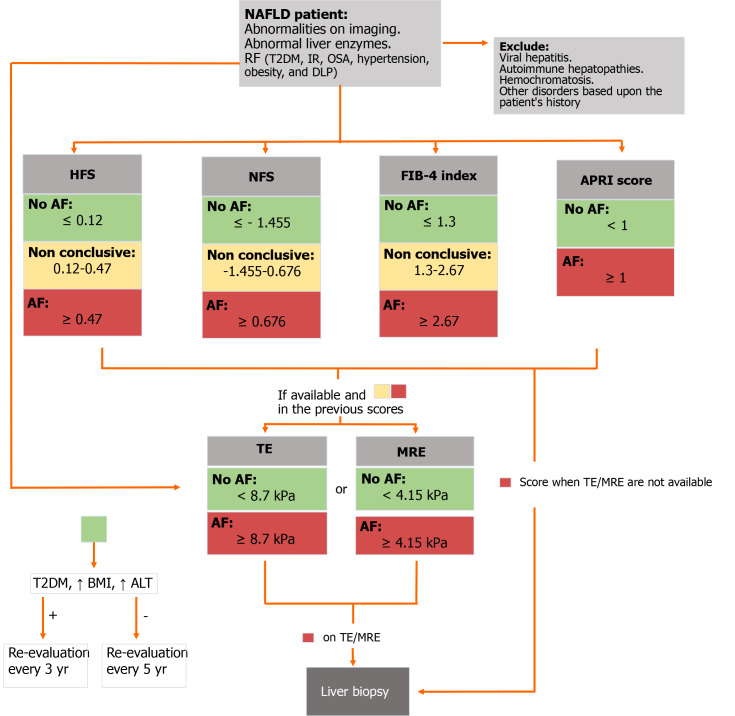
Finally, after a comprehensive evaluation we propose a stepwise assessment of fibrosis in NAFLD patients, according to the available data, which can be found in Figure 3.
Figure 3.
Diagnostic flow-chart to assess liver fibrosis in patients with non-alcoholic fatty liver disease. NAFLD: Non-alcoholic fatty liver disease; T2DM: Type 2 diabetes mellitus; IR: Insulin resistance; OSA: Obstructive sleep apnoea; DLP: Dyslipidemia; HFS: Hepamet fibrosis score; NFS: Non-alcoholic fatty liver disease fibrosis score; FIB-4: Fibrosis-4 index; APRI: Aspartate aminotransferase to platelet ratio index; AF: Advanced fibrosis; TE: Transient elastography; MRE: Magnetic resonance elastography; BMI: Body mass index; ALT: Alanine aminotransferase.
MANAGEMENT OF LIVER FIBROSIS IN NAFLD
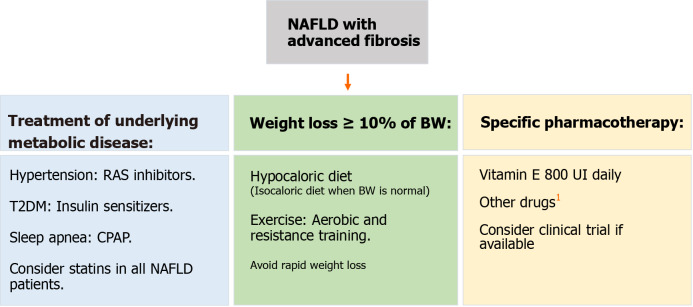
The appropriate management of patients with NAFLD and fibrosis should be a comprehensive treatment that takes into account three major components: The treatment of underlying metabolic diseases, weight loss (WL), and pharmacological therapy (Figure 4).
Figure 4.
Management flow-chart for patients with non-alcoholic fatty liver disease advanced fibrosis. We suggest vitamin E as it has demonstrated higher transplant-free survival and lower rates of hepatic decompensation. 1Other drugs such as cenicriviroc, obethicholic acid, dapagliflozin, and selonsertib have shown benefit in clinical trials and must be considered as well, especially as results continue to show beneficial results. NAFLD: Non-alcoholic fatty liver disease; RAS: Renin-angiotensin system; T2DM: Type 2 diabetes mellitus; CPAP: Continuous positive airway pressure; BW: Body weight.
Treatment of underlying metabolic diseases
As referred before, the importance of liver fibrosis is beyond the liver prognosis[18,19]. Therefore, to improve the prognosis of patients with NAFLD and fibrosis the treatment of concomitant diseases must be a priority.
Nearly half of the patients with hypertension have concomitant NAFLD[117]. RAS seems to contribute to the development of liver fibrosis, interestingly, the administration of RAS inhibitors showed an improvement in liver histology and decrease in protein expression of alpha-smooth muscle actin and hepatic content of hydroxyproline in a murine model of NAFLD[118]. In a cross-sectional study in hypertensive patients with biopsy-proven NAFLD, the use of RAS blockers was associated with less advanced hepatic fibrosis suggesting a beneficial effect of RAS blockers in NAFLD[119]. A similar effect was found in a retrospective cohort of 118 NAFLD patients with paired liver biopsies, where the use of RAS inhibitors was associated with decreased fibrosis progression rate only in patients with T2DM[22]. Given these potential benefits, the high safety profile of the drugs, and the fact that RAS blockers are one of the main classes of drugs recommended as initial therapy by hypertension guidelines[120,121], we suggest that RAS blockers should be considered as first-line therapy in patients with NAFLD and hypertension, after assessing the potential contraindications.
More than three-quarters of T2DM patients have coexistent NAFLD[9]; insulin resistance and hyperinsulinemia are the hallmarks of NAFLD and have an important role in stellate cell activation[122]. Therefore, treatment of patients with T2DM and NAFLD should be focused on improving insulin resistance. Even though metformin has not shown benefit in the histology of NAFLD, it remains the first-line drug for T2DM for its effects in the reduction of body weight, serum levels of lipids, and glucose[123,124]. Other benefits of metformin have been reported, such as the reduction in the risk of liver cancer[125,126]. Other insulin sensitizers such as dipeptidyl peptidase 4 inhibitors and glucagon-like peptide-1 analogs could also be good choices for the treatment of T2DM in patients with NAFLD[127]. Thiazolidinediones and Sodium-glucose co-transporter-2 (SGLT2) inhibitors are reviewed in the pharmacological management section[127]. Hence, insulin sensitizers should be the gold standard therapy for patients with T2DM and NAFLD.
OSA induces insulin resistance and systemic inflammation which as mentioned before are major features in NAFLD pathogenesis[128]. OSA patients should be screened for NAFLD and vice versa those with NAFLD for OSA[129]. The first-line treatment for OSA is continuous positive airway pressure (CPAP), which has several benefits, among which stand out the improvement of blood pressure and glucose resistance, and the reduction of overall and cardiovascular mortality[130-132]. The evidence of the benefit of CPAP on liver fibrosis in patients with NAFLD is scarce, at least three studies have studied this, one showed improvement while the other two did not show this effect[132-134]. Longer trials may be needed to demonstrate a clear benefit. Although there is no clear evidence of a benefit to the liver in patients with OSA and NAFDL, CPAP should be encouraged in all patients with OSA.
Cardiovascular disease is one of the main causes of NAFLD-related deaths. Statins reduce NAFLD/NASH cardiovascular events, moreover, statins could reduce the risk of hepatocellular carcinoma related to NAFLD/NASH. Given these benefits and the fact that these medications have a high-security profile, they should be considered even in NAFLD patients without dyslipidemia[135,136].
Weight loss
To date, lifestyle modifications including diet and exercise are the first line and cornerstone of NAFLD/NASH treatment. Most studies have demonstrated a reduction of steatosis or/and steatohepatitis without improvement in liver fibrosis[137-143], nevertheless some have shown fibrosis regression, especially when a WL of 10% or more is achieved or when exercise therapy is included[144,145].
The Mediterranean diet (MD) with components such as fish, nuts, fruits, olive oil, whole grains, and vegetables is proposed by EASL guidelines for the treatment of NAFLD[4]. The MD has shown an inverse relationship with NAFLD prevalence and a reduction in liver steatosis[146-148]. In a non-randomized, open-label, 24-wk prospective study, 44 untreated NAFLD patients with non-significant fibrosis received nutritional counsel to increase adherence to MD with significant improvements in liver fibrosis at the end of the follow-up. It is noteworthy that the patients did not have significant fibrosis and that the technique for measurement was elastography ultrasound which could lead to unprecise data[149]. At the moment there is no evidence that diet per se could improve liver fibrosis, however, we must take into account that the diet that is going to have the maximum benefit is the one that will be followed by the patient in the long-term, therefore highly restrictive diets that induce a rapid WL should not be considered.
Regarding exercise, there is evidence that in selected patients with cirrhosis, moderate-intensity aerobic or resistance exercise, 4 d per week, 20 min, for at least 8 wk can have a positive physiological impact[150]. Moreover, it has been demonstrated that supervised exercise in cirrhotic patients can significantly lower the hepatic venous pressure gradient[151]. Exercise intervention studies in NAFLD are limited by low statistical power, and most have shown a reduction in intrahepatic triglyceride content without an improvement in liver fibrosis[152,153]. Few studies have shown a reduction in fibrosis, in particular, high-intensity interval training has recently been recognized as a novel exercise modality that demonstrated an improvement in liver stiffness (-16.8%), these benefits appeared to be independent of WL[154,155]. More evidence regarding the effect of exercise independently of the WL in fibrosis is required. As before concerning the diet, we consider that the exercise that is going to have the maximum impact is the one that can be maintained long-term, therefore the prescription of exercise should take into account the patients′ preferences and capabilities.
Bariatric surgery could be another approach in the management of NAFLD fibrosis, possibly through improvement of insulin resistance and hyperinsulinemia, which are related to the development of fibrosis in NAFLD[122]. In this context, bariatric surgery has proven to be an effective method for sustained WL in properly selected obese individuals[156-158], and importantly, in selected patients with T2DM, bariatric surgery is an effective therapy for glycemic control[159,160]. These changes have an additive effect considering that if 10% or more of WL is achieved with bariatric surgery, an improvement in fibrosis may be seen. There is some evidence of this benefit in retrospective and prospective cohorts with follow-up to 5 years[161-163]. Nevertheless, bariatric surgery should not be considered alone for the treatment of NAFLD fibrosis and the other indications of the procedure must be considered. The recommendation concerning this surgery in patients with advanced fibrosis must be individualized and evaluated by an experienced and interdisciplinary group[116].
Specific pharmacological management
Pharmacological management should be restricted to patients with NASH and/or advanced fibrosis. The current recommended drugs thus far in the guidelines are pioglitazone and vitamin E[4]. Although there are multiple treatments with different mechanisms of action under development for the treatment of NASH, and specifically aimed to reduce liver fibrosis, these drugs remain experimental and are considered for their use only in clinical trials.
Vitamin E is an antioxidant of polyunsaturated lipids and has a role in the treatment of NASH through antioxidant dependent and independent mechanisms[164]. In a NASH animal model, vitamin E supplementation decreased baseline levels of transforming growth factor beta 1 mRNA, suggesting a potential interference with both the initiation and progression of fibrosis[165]. Vitamin E improves transaminase activity, steatosis, inflammation, and ballooning in NASH patients[166-169]. Nevertheless, the data about its impact on liver fibrosis is controversial, several studies have shown an improvement in liver fibrosis[168-170], while others have not[124,169-172]. In the classical PIVENS study, 247 adults with NASH without T2DM were randomized to receive pioglitazone (30 mg/d), vitamin E (800 IU/d), or placebo, for 96 wk. Vitamin E therapy was associated with a significantly higher rate of improvement in NASH, with no reductions in fibrosis scores[167]. However, in a post hoc analysis, WL (≥ 2 kg) was associated with improvement on liver fibrosis scores, while weight gain (≥ 2 kg) was associated with worsening of fibrosis scores. These data reinforce the evidence-based recommendation for lifestyle modifications and WL as the basis of NASH/NAFLD treatment[116]. It is important to notice that since the PIVENS study did not include patients with T2DM[167], the evidence of Vitamin E in this group is not strong, and the few studies including T2DM have not shown benefit in liver fibrosis[173]. In a retrospective study, 90 patients with NASH and advanced fibrosis or cirrhosis consumed vitamin E (800 IU/d) for ≥ 2 years and were propensity-matched to 90 adults who did not take vitamin E, with a median follow-up was 5.6 years; vitamin E users had higher adjusted transplant-free survival, lower rates of hepatic decompensation than controls, and these benefits were evident in both, with and without T2DM patients[174]. There are some concerns with regard to the safety of vitamin E supplementation. A large long-term randomized trial (SELECT trial) showed a slight increase risk of prostate cancer in patients with vitamin E supplementation (Hazard ratio: 1.17; CI: 1.004-1.36)[175]. Furthermore, another large study associated vitamin E with an increased risk of hemorrhagic stroke, also with a marginal increment (Relative risk 1.22, 1.00-1.48)[176]. The significance of these side effects is not entirely clear since large-scale population studies tend to find statistical differences without clinical relevance, nevertheless, vitamin E must be prescribed with caution in high-risk groups.
Pioglitazone has a role in the treatment of NASH through modulation by an adiponectin-mediated effect on insulin sensitivity and hepatic fatty acid metabolism, as well as acting as a PPAR (gamma with Greek letter)[177]. Like vitamin E, pioglitazone has shown conflicting evidence regarding the reduction of fibrosis. The PIVENS study did not show an improvement[167], while two other randomized studies in diabetic and non-diabetic patients showed a reduction in liver fibrosis[171,172]. Cusi et al[172], studied 101 patients with prediabetes or T2DM and NASH, all patients were prescribed a hypocaloric diet and then randomly assigned to pioglitazone (45 mg/d), or placebo for 18 mo, followed by an 18-mo open-label phase with pioglitazone treatment. The pioglitazone group had improvement in the mean fibrosis score, however, this treatment was associated with significant weight gain, suggesting that pioglitazone could alter the natural history of the disease.
Cenicriviroc (CVC) is a dual antagonist of CCR2 and CCR5. CVC significantly reduced monocyte/macrophage recruitment and collagen deposition in animal models of fibrosis[178]. The CENTAUR study, a multicenter phase 2b clinical trial, randomized 289 patients to receive CVC (150 mg/d) or placebo for 12 mo, showed no differences in NAS score between the CVC and placebo group, however, twice as many subjects on CVC achieved a reduction in the fibrosis stage (1 stage) with no worsening of steatohepatitis compared to those on placebo; this benefit was seen across all stages of fibrosis, particularly for stage 2 and 3[179]. Based on the prior results, a large phase 3 trial, the AURORA study, is currently recruiting patients with NASH and histopathological evidence of stage 2 or 3 liver fibrosis to evaluate the benefit of CVC on the improvement in fibrosis and determine long-term clinical outcomes[180].
Obeticholic acid (OCA), is a farnesoid-X receptor (FXR) agonist[181]. FXR is a nuclear hormone receptor that regulates glucose and lipid metabolism. In a double-blind, placebo-controlled, proof-of-concept study in patients with NAFLD and T2DM, OCA increased insulin sensitivity and decreased significantly markers of liver fibrosis, nevertheless, no histopathology was obtained[182]. The FLINT study, a multicenter, randomized, placebo-controlled trial in non-cirrhotic NASH patients to assess treatment with OCA (25 mg/d) or placebo for 72 wk. OCA improved fibrosis, hepatocellular ballooning, steatosis, and lobular inflammation when compared with placebo[183]. Finally, in a recent multicenter double-blind, placebo-controlled study, 1968 patients with stage F1-F3 fibrosis (931 with F2-F3) were randomized to receive oral placebo, OCA 10 mg, or OCA 25 mg daily for 18 mo; an improvement of at least 1 stage of liver fibrosis was observed in 18% of the 10 mg group, and 23% in the 25 mg group, however, mild to moderate pruritus was reported in half of the patients with 25 mg of OCA[184]. A baseline NAS > 5, baseline triglyceride level 154 mg/dL, baseline INR 1, baseline AST level 49 U/L, and decrease in ALT level at week 24, were significant predictors of histologic response in NASH patients treated with OCA[185]. Tropifexor is another FXR agonist[186]. In a murine model, tropifexor reversed established fibrosis and reduced the NAFLD activity score, hepatic triglycerides, and profibrogenic gene expression[187]. Currently, at least three clinical trials are evaluating the safety, tolerability, and efficacy of different doses of tropifexor in NASH patients[188-190].
Elafibranor is an agonist of the peroxisome proliferator-activated receptor-α and peroxisome proliferator-activated receptor-δ. In a randomized, double-blind placebo-controlled trial, including 276 patients for 52 wk, NASH resolved without fibrosis worsening in a higher proportion of patients in the Elafibranor group (120 mg/d), and NASH resolution was associated with a reduction in liver fibrosis stage[191].
SGLT2 inhibitors, a relatively novel class of oral antidiabetic drugs that reduce hyperglycemia by promoting the urinary excretion of glucose[192]. Dapagliflozin, an SGLT2 inhibitor, has proven beneficial effects other than lower glucose levels in T2DM, such as a significant reduction in total body weight, predominantly by reducing total body fat mass and visceral fat[193]. In an open-label trial, 57 patients with T2DM and NAFLD were randomized to dapagliflozin (5 mg/d) (n = 33) or standard treatment (n = 24) for 24 wk. In 14 patients from the dapagliflozin group who had significant fibrosis (≥ 8.0 kPa), LSM decreased significantly from 14.7 ± 5.7 to 11.0 ± 7.3 kPa[194]. Licoglicoflzin, a dual sodium-glucose co-transporter 1/2 inhibitor which has proven positive effects on body weight in obese patients[195,196], in combination with tropifexor is currently under recruitment to evaluate the efficacy, safety, and tolerability in patients with NASH and significant fibrosis (F2 and F3)[189].
Finally, selonsertib, an inhibitor of apoptosis signal-regulating kinase 1, a serine/threonine signaling kinase, that can lead to fibrosis was evaluated alone or in combination with simtuzumab, in NAFLD patients with stage 2 or 3 liver fibrosis for 24 wk. Reductions in LSM on MRE and collagen content and lobular inflammation on liver biopsy were observed[197].
It is expected that shortly evidence from these and new drugs will increase exponentially, however, given the complex physiopathology of NAFLD fibrosis, it is unlikely that one drug by itself will deliver significant clinical outcomes, and perhaps the combinations of drugs with different targets will be necessary to obtain better results. Additionally, it is important to mention that until now, there is no evidence showing a clear effect of the “hepatoprotectors” on liver fibrosis (ursodeoxycholic acid, pentoxifylline, antioxidants among others), and therefore should not be used indiscriminately.
CONCLUSION
The main concern in NAFLD/NASH patients is the presence and progression of liver fibrosis, therefore all the efforts should center on it. There are several available scores to predict and stratify the risk of advanced fibrosis although the accuracy is limited in intermediate stages. To improve the accuracy of the diagnosis a combination of methods such as TE or US may be used, while MRE or liver biopsy alone are considered as the best options to accurately diagnose fibrosis. To select a diagnostic test or tests the prevalence of the disease in the specific center should be considered, as well as the experience of the center and the observers. The treatment should always take into account the presence of comorbidities such as the features of metabolic syndrome and should always include lifestyle modifications considering the preferences of the patient to ensure long-term adherence. The only approved pharmacological treatments so far are Vitamin E and pioglitazone, however, they have shown conflicting results on liver fibrosis improvement. Therefore, several new drugs and trials are being created and conducted aiming to improve both steatosis and liver fibrosis with very promising results thus far.
Footnotes
Conflict-of-interest statement: All authors declare no conflicts of interest related to this article.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: American Association for the Study of Liver Diseases; and European Association for the Study of Liver Disease.
Peer-review started: May 15, 2020
First decision: May 21, 2020
Article in press: September 22, 2020
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Mexico
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bodenheimer HC, Chen GX S-Editor: Huang P L-Editor: A P-Editor: Wang LL
Contributor Information
Alejandro Campos-Murguía, Department of Gastroenterology, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City 14080, Mexico.
Astrid Ruiz-Margáin, Department of Gastroenterology, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City 14080, Mexico.
José A González-Regueiro, Department of Gastroenterology, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City 14080, Mexico.
Ricardo U Macías-Rodríguez, Department of Gastroenterology, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City 14080, Mexico.
References
- 1.GBD 2017 Cirrhosis Collaborators. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:245–266. doi: 10.1016/S2468-1253(19)30349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 3.Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 4.European Association for the Study of the Liver (EASL) ; European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–1402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Brunt EM, Wong VW, Nobili V, Day CP, Sookoian S, Maher JJ, Bugianesi E, Sirlin CB, Neuschwander-Tetri BA, Rinella ME. Nonalcoholic fatty liver disease. Nat Rev Dis Primers. 2015;1:15080. doi: 10.1038/nrdp.2015.80. [DOI] [PubMed] [Google Scholar]
- 6.Zelber-Sagi S, Nitzan-Kaluski D, Halpern Z, Oren R. Prevalence of primary non-alcoholic fatty liver disease in a population-based study and its association with biochemical and anthropometric measures. Liver Int. 2006;26:856–863. doi: 10.1111/j.1478-3231.2006.01311.x. [DOI] [PubMed] [Google Scholar]
- 7.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 8.Miyaaki H, Nakao K. Significance of genetic polymorphisms in patients with nonalcoholic fatty liver disease. Clin J Gastroenterol. 2017;10:201–207. doi: 10.1007/s12328-017-0732-5. [DOI] [PubMed] [Google Scholar]
- 9.Targher G, Bertolini L, Padovani R, Rodella S, Tessari R, Zenari L, Day C, Arcaro G. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2007;30:1212–1218. doi: 10.2337/dc06-2247. [DOI] [PubMed] [Google Scholar]
- 10.Oikonomou D, Georgiopoulos G, Katsi V, Kourek C, Tsioufis C, Alexopoulou A, Koutli E, Tousoulis D. Non-alcoholic fatty liver disease and hypertension: coprevalent or correlated? Eur J Gastroenterol Hepatol. 2018;30:979–985. doi: 10.1097/MEG.0000000000001191. [DOI] [PubMed] [Google Scholar]
- 11.Le MH, Devaki P, Ha NB, Jun DW, Te HS, Cheung RC, Nguyen MH. Prevalence of non-alcoholic fatty liver disease and risk factors for advanced fibrosis and mortality in the United States. PLoS One. 2017;12:e0173499. doi: 10.1371/journal.pone.0173499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Keach JC, Lafferty HD, Stahler A, Haflidadottir S, Bendtsen F. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2015;149:389–397.e10. doi: 10.1053/j.gastro.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dulai PS, Singh S, Patel J, Soni M, Prokop LJ, Younossi Z, Sebastiani G, Ekstedt M, Hagstrom H, Nasr P, Stal P, Wong VW, Kechagias S, Hultcrantz R, Loomba R. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology. 2017;65:1557–1565. doi: 10.1002/hep.29085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hagström H, Nasr P, Ekstedt M, Hammar U, Stål P, Hultcrantz R, Kechagias S. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol. 2017;67:1265–1273. doi: 10.1016/j.jhep.2017.07.027. [DOI] [PubMed] [Google Scholar]
- 15.Shili-Masmoudi S, Wong GL, Hiriart JB, Liu K, Chermak F, Shu SS, Foucher J, Tse YK, Bernard PH, Yip TC, Merrouche W, Chan HL, Wong VW, de Lédinghen V. Liver stiffness measurement predicts long-term survival and complications in non-alcoholic fatty liver disease. Liver Int. 2020;40:581–589. doi: 10.1111/liv.14301. [DOI] [PubMed] [Google Scholar]
- 16.Kim SU, Song D, Heo JH, Yoo J, Kim BK, Park JY, Kim DY, Ahn SH, Kim KJ, Han KH, Kim YD. Liver fibrosis assessed with transient elastography is an independent risk factor for ischemic stroke. Atherosclerosis. 2017;260:156–162. doi: 10.1016/j.atherosclerosis.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Önnerhag K, Hartman H, Nilsson PM, Lindgren S. Non-invasive fibrosis scoring systems can predict future metabolic complications and overall mortality in non-alcoholic fatty liver disease (NAFLD) Scand J Gastroenterol. 2019;54:328–334. doi: 10.1080/00365521.2019.1583366. [DOI] [PubMed] [Google Scholar]
- 18.Baik M, Kim SU, Kang S, Park HJ, Nam HS, Heo JH, Kim BK, Park JY, Kim DY, Ahn SH, Han KH, Lee HS, Kim YD. Liver Fibrosis, Not Steatosis, Associates with Long-Term Outcomes in Ischaemic Stroke Patients. Cerebrovasc Dis. 2019;47:32–39. doi: 10.1159/000497069. [DOI] [PubMed] [Google Scholar]
- 19.Mangla N, Ajmera VH, Caussy C, Sirlin C, Brouha S, Bajwa-Dulai S, Madamba E, Bettencourt R, Richards L, Loomba R. Liver Stiffness Severity is Associated With Increased Cardiovascular Risk in Patients With Type 2 Diabetes. Clin Gastroenterol Hepatol. 2020;18:744–746.e1. doi: 10.1016/j.cgh.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 21.Pelusi S, Cespiati A, Rametta R, Pennisi G, Mannisto V, Rosso C, Baselli G, Dongiovanni P, Fracanzani AL, Badiali S, Maggioni M, Craxi A, Fargion S, Prati D, Nobili V, Bugianesi E, Romeo S, Pihlajamaki J, Petta S, Valenti L. Prevalence and Risk Factors of Significant Fibrosis in Patients With Nonalcoholic Fatty Liver Without Steatohepatitis. Clin Gastroenterol Hepatol. 2019;17:2310–2319.e6. doi: 10.1016/j.cgh.2019.01.027. [DOI] [PubMed] [Google Scholar]
- 22.Pelusi S, Petta S, Rosso C, Borroni V, Fracanzani AL, Dongiovanni P, Craxi A, Bugianesi E, Fargion S, Valenti L. Renin-Angiotensin System Inhibitors, Type 2 Diabetes and Fibrosis Progression: An Observational Study in Patients with Nonalcoholic Fatty Liver Disease. PLoS One. 2016;11:e0163069. doi: 10.1371/journal.pone.0163069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McPherson S, Hardy T, Henderson E, Burt AD, Day CP, Anstee QM. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol. 2015;62:1148–1155. doi: 10.1016/j.jhep.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 24.Adams LA, Sanderson S, Lindor KD, Angulo P. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol. 2005;42:132–138. doi: 10.1016/j.jhep.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 25.Schwimmer JB, Celedon MA, Lavine JE, Salem R, Campbell N, Schork NJ, Shiehmorteza M, Yokoo T, Chavez A, Middleton MS, Sirlin CB. Heritability of nonalcoholic fatty liver disease. Gastroenterology. 2009;136:1585–1592. doi: 10.1053/j.gastro.2009.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eslam M, Valenti L, Romeo S. Genetics and epigenetics of NAFLD and NASH: Clinical impact. J Hepatol. 2018;68:268–279. doi: 10.1016/j.jhep.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Koo BK, Joo SK, Kim D, Bae JM, Park JH, Kim JH, Kim W. Additive effects of PNPLA3 and TM6SF2 on the histological severity of non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2018;33:1277–1285. doi: 10.1111/jgh.14056. [DOI] [PubMed] [Google Scholar]
- 28.Akuta N, Kawamura Y, Arase Y, Suzuki F, Sezaki H, Hosaka T, Kobayashi M, Kobayashi M, Saitoh S, Suzuki Y, Ikeda K, Kumada H. Relationships between Genetic Variations of PNPLA3, TM6SF2 and Histological Features of Nonalcoholic Fatty Liver Disease in Japan. Gut Liver. 2016;10:437–445. doi: 10.5009/gnl15163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sookoian S, Castaño GO, Scian R, Mallardi P, Fernández Gianotti T, Burgueño AL, San Martino J, Pirola CJ. Genetic variation in transmembrane 6 superfamily member 2 and the risk of nonalcoholic fatty liver disease and histological disease severity. Hepatology. 2015;61:515–525. doi: 10.1002/hep.27556. [DOI] [PubMed] [Google Scholar]
- 30.Liu YL, Reeves HL, Burt AD, Tiniakos D, McPherson S, Leathart JB, Allison ME, Alexander GJ, Piguet AC, Anty R, Donaldson P, Aithal GP, Francque S, Van Gaal L, Clement K, Ratziu V, Dufour JF, Day CP, Daly AK, Anstee QM. TM6SF2 rs58542926 influences hepatic fibrosis progression in patients with non-alcoholic fatty liver disease. Nat Commun. 2014;5:4309. doi: 10.1038/ncomms5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mancina RM, Dongiovanni P, Petta S, Pingitore P, Meroni M, Rametta R, Borén J, Montalcini T, Pujia A, Wiklund O, Hindy G, Spagnuolo R, Motta BM, Pipitone RM, Craxì A, Fargion S, Nobili V, Käkelä P, Kärjä V, Männistö V, Pihlajamäki J, Reilly DF, Castro-Perez J, Kozlitina J, Valenti L, Romeo S. The MBOAT7-TMC4 Variant rs641738 Increases Risk of Nonalcoholic Fatty Liver Disease in Individuals of European Descent. Gastroenterology. 2016;150:1219–1230.e6. doi: 10.1053/j.gastro.2016.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petta S, Miele L, Bugianesi E, Cammà C, Rosso C, Boccia S, Cabibi D, Di Marco V, Grimaudo S, Grieco A, Pipitone RM, Marchesini G, Craxì A. Glucokinase regulatory protein gene polymorphism affects liver fibrosis in non-alcoholic fatty liver disease. PLoS One. 2014;9:e87523. doi: 10.1371/journal.pone.0087523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krawczyk M, Rau M, Schattenberg JM, Bantel H, Pathil A, Demir M, Kluwe J, Boettler T, Lammert F, Geier A NAFLD Clinical Study Group. Combined effects of the PNPLA3 rs738409, TM6SF2 rs58542926, and MBOAT7 rs641738 variants on NAFLD severity: a multicenter biopsy-based study. J Lipid Res. 2017;58:247–255. doi: 10.1194/jlr.P067454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Q, You H, Ou X, Zhao X, Sun Y, Wang M, Wang P, Wang Y, Duan W, Wang X, Wu S, Kong Y, Saxena R, Gouw ASH, Jia J. Non-obese histologically confirmed NASH patients with abnormal liver biochemistry have more advanced fibrosis. Hepatol Int. 2019;13:766–776. doi: 10.1007/s12072-019-09982-z. [DOI] [PubMed] [Google Scholar]
- 35.Petta S, Ciminnisi S, Di Marco V, Cabibi D, Cammà C, Licata A, Marchesini G, Craxì A. Sarcopenia is associated with severe liver fibrosis in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2017;45:510–518. doi: 10.1111/apt.13889. [DOI] [PubMed] [Google Scholar]
- 36.Koo BK, Kim D, Joo SK, Kim JH, Chang MS, Kim BG, Lee KL, Kim W. Sarcopenia is an independent risk factor for non-alcoholic steatohepatitis and significant fibrosis. J Hepatol. 2017;66:123–131. doi: 10.1016/j.jhep.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 37.Patel V, Sanyal AJ, Sterling R. Clinical Presentation and Patient Evaluation in Nonalcoholic Fatty Liver Disease. Clin Liver Dis. 2016;20:277–292. doi: 10.1016/j.cld.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 38.Pandey A, Sonthalia S. Skin Tags. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Aug 8. [Google Scholar]
- 39.Wu J, Yao XY, Shi RX, Liu SF, Wang XY. A potential link between polycystic ovary syndrome and non-alcoholic fatty liver disease: an update meta-analysis. Reprod Health. 2018;15:77. doi: 10.1186/s12978-018-0519-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benotti P, Wood GC, Argyropoulos G, Pack A, Keenan BT, Gao X, Gerhard G, Still C. The impact of obstructive sleep apnea on nonalcoholic fatty liver disease in patients with severe obesity. Obesity (Silver Spring) 2016;24:871–877. doi: 10.1002/oby.21409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feldstein AE, Wieckowska A, Lopez AR, Liu YC, Zein NN, McCullough AJ. Cytokeratin-18 fragment levels as noninvasive biomarkers for nonalcoholic steatohepatitis: a multicenter validation study. Hepatology. 2009;50:1072–1078. doi: 10.1002/hep.23050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li J, Verhaar AP, Pan Q, de Knegt RJ, Peppelenbosch MP. Serum levels of caspase-cleaved cytokeratin 18 (CK18-Asp396) predict severity of liver disease in chronic hepatitis B. Clin Exp Gastroenterol. 2017;10:203–209. doi: 10.2147/CEG.S135526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Darweesh SK, AbdElAziz RA, Abd-ElFatah DS, AbdElazim NA, Fathi SA, Attia D, AbdAllah M. Serum cytokeratin-18 and its relation to liver fibrosis and steatosis diagnosed by FibroScan and controlled attenuation parameter in nonalcoholic fatty liver disease and hepatitis C virus patients. Eur J Gastroenterol Hepatol. 2019;31:633–641. doi: 10.1097/MEG.0000000000001385. [DOI] [PubMed] [Google Scholar]
- 44.Alt Y, Grimm A, Schlegel L, Grambihler A, Kittner JM, Wiltink J, Galle PR, Wörns MA, Schattenberg JM. The Impact of Liver Cell Injury on Health-Related Quality of Life in Patients with Chronic Liver Disease. PLoS One. 2016;11:e0151200. doi: 10.1371/journal.pone.0151200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cusi K, Chang Z, Harrison S, Lomonaco R, Bril F, Orsak B, Ortiz-Lopez C, Hecht J, Feldstein AE, Webb A, Louden C, Goros M, Tio F. Limited value of plasma cytokeratin-18 as a biomarker for NASH and fibrosis in patients with non-alcoholic fatty liver disease. J Hepatol. 2014;60:167–174. doi: 10.1016/j.jhep.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 46.Karsdal MA, Daniels SJ, Holm Nielsen S, Bager C, Rasmussen DGK, Loomba R, Surabattula R, Villesen IF, Luo Y, Shevell D, Gudmann NS, Nielsen MJ, George J, Christian R, Leeming DJ, Schuppan D. Collagen biology and non-invasive biomarkers of liver fibrosis. Liver Int. 2020;40:736–750. doi: 10.1111/liv.14390. [DOI] [PubMed] [Google Scholar]
- 47.Luo Y, Oseini A, Gagnon R, Charles ED, Sidik K, Vincent R, Collen R, Idowu M, Contos MJ, Mirshahi F, Daita K, Asgharpour A, Siddiqui MS, Jarai G, Rosen G, Christian R, Sanyal AJ. An Evaluation of the Collagen Fragments Related to Fibrogenesis and Fibrolysis in Nonalcoholic Steatohepatitis. Sci Rep. 2018;8:12414. doi: 10.1038/s41598-018-30457-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xie Q, Zhou X, Huang P, Wei J, Wang W, Zheng S. The performance of enhanced liver fibrosis (ELF) test for the staging of liver fibrosis: a meta-analysis. PLoS One. 2014;9:e92772. doi: 10.1371/journal.pone.0092772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kwok R, Tse YK, Wong GL, Ha Y, Lee AU, Ngu MC, Chan HL, Wong VW. Systematic review with meta-analysis: non-invasive assessment of non-alcoholic fatty liver disease -- the role of transient elastography and plasma cytokeratin-18 fragments. Aliment Pharmacol Ther. 2014;39:254–269. doi: 10.1111/apt.12569. [DOI] [PubMed] [Google Scholar]
- 50.Kobayashi N, Kumada T, Toyoda H, Tada T, Ito T, Kage M, Okanoue T, Kudo M. Ability of Cytokeratin-18 Fragments and FIB-4 Index to Diagnose Overall and Mild Fibrosis Nonalcoholic Steatohepatitis in Japanese Nonalcoholic Fatty Liver Disease Patients. Dig Dis. 2017;35:521–530. doi: 10.1159/000480142. [DOI] [PubMed] [Google Scholar]
- 51.Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT, Thomas DL, Messinger D, Nelson M APRICOT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 52.Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ Nash Clinical Research Network. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7:1104–1112. doi: 10.1016/j.cgh.2009.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peleg N, Issachar A, Sneh-Arbib O, Shlomai A. AST to Platelet Ratio Index and fibrosis 4 calculator scores for non-invasive assessment of hepatic fibrosis in patients with non-alcoholic fatty liver disease. Dig Liver Dis. 2017;49:1133–1138. doi: 10.1016/j.dld.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 54.Sun W, Cui H, Li N, Wei Y, Lai S, Yang Y, Yin X, Chen DF. Comparison of FIB-4 index, NAFLD fibrosis score and BARD score for prediction of advanced fibrosis in adult patients with non-alcoholic fatty liver disease: A meta-analysis study. Hepatol Res. 2016;46:862–870. doi: 10.1111/hepr.12647. [DOI] [PubMed] [Google Scholar]
- 55.Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, Enders F, Saksena S, Burt AD, Bida JP, Lindor K, Sanderson SO, Lenzi M, Adams LA, Kench J, Therneau TM, Day CP. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846–854. doi: 10.1002/hep.21496. [DOI] [PubMed] [Google Scholar]
- 56.Boursier J, Vergniol J, Guillet A, Hiriart JB, Lannes A, Le Bail B, Michalak S, Chermak F, Bertrais S, Foucher J, Oberti F, Charbonnier M, Fouchard-Hubert I, Rousselet MC, Calès P, de Lédinghen V. Diagnostic accuracy and prognostic significance of blood fibrosis tests and liver stiffness measurement by FibroScan in non-alcoholic fatty liver disease. J Hepatol. 2016;65:570–578. doi: 10.1016/j.jhep.2016.04.023. [DOI] [PubMed] [Google Scholar]
- 57.Jun DW, Kim SG, Park SH, Jin SY, Lee JS, Lee JW, Kim MY, Choi DH, Cho YK, Yeon JE, Sohn JH Korean NAFLD study group (KNSG) External validation of the non-alcoholic fatty liver disease fibrosis score for assessing advanced fibrosis in Korean patients. J Gastroenterol Hepatol. 2017;32:1094–1099. doi: 10.1111/jgh.13648. [DOI] [PubMed] [Google Scholar]
- 58.Cichoż-Lach H, Celiński K, Prozorow-Król B, Swatek J, Słomka M, Lach T. The BARD score and the NAFLD fibrosis score in the assessment of advanced liver fibrosis in nonalcoholic fatty liver disease. Med Sci Monit. 2012;18:CR735–CR740. doi: 10.12659/MSM.883601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ruffillo G, Fassio E, Alvarez E, Landeira G, Longo C, Domínguez N, Gualano G. Comparison of NAFLD fibrosis score and BARD score in predicting fibrosis in nonalcoholic fatty liver disease. J Hepatol. 2011;54:160–163. doi: 10.1016/j.jhep.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 60.Harrison SA, Oliver D, Arnold HL, Gogia S, Neuschwander-Tetri BA. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut. 2008;57:1441–1447. doi: 10.1136/gut.2007.146019. [DOI] [PubMed] [Google Scholar]
- 61.Fujii H, Enomoto M, Fukushima W, Tamori A, Sakaguchi H, Kawada N. Applicability of BARD score to Japanese patients with NAFLD. Gut. 2009;58:1566–1567 author reply 1567. doi: 10.1136/gut.2009.182758. [DOI] [PubMed] [Google Scholar]
- 62.Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 63.Xiao G, Zhu S, Xiao X, Yan L, Yang J, Wu G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: A meta-analysis. Hepatology. 2017;66:1486–1501. doi: 10.1002/hep.29302. [DOI] [PubMed] [Google Scholar]
- 64.Mikolasevic I, Orlic L, Franjic N, Hauser G, Stimac D, Milic S. Transient elastography (FibroScan(®)) with controlled attenuation parameter in the assessment of liver steatosis and fibrosis in patients with nonalcoholic fatty liver disease - Where do we stand? World J Gastroenterol. 2016;22:7236–7251. doi: 10.3748/wjg.v22.i32.7236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kolhe KM, Amarapurkar A, Parikh P, Chaubal A, Chauhan S, Khairnar H, Walke S, Ingle M, Pandey V, Shukla A. Aspartate transaminase to platelet ratio index (APRI) but not FIB-5 or FIB-4 is accurate in ruling out significant fibrosis in patients with non-alcoholic fatty liver disease (NAFLD) in an urban slum-dwelling population. BMJ Open Gastroenterol. 2019;6:e000288. doi: 10.1136/bmjgast-2019-000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vilar-Gomez E, Chalasani N. Non-invasive assessment of non-alcoholic fatty liver disease: Clinical prediction rules and blood-based biomarkers. J Hepatol. 2018;68:305–315. doi: 10.1016/j.jhep.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 67.Poynard T, Morra R, Halfon P, Castera L, Ratziu V, Imbert-Bismut F, Naveau S, Thabut D, Lebrec D, Zoulim F, Bourliere M, Cacoub P, Messous D, Munteanu M, de Ledinghen V. Meta-analyses of FibroTest diagnostic value in chronic liver disease. BMC Gastroenterol. 2007;7:40. doi: 10.1186/1471-230X-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ratziu V, Massard J, Charlotte F, Messous D, Imbert-Bismut F, Bonyhay L, Tahiri M, Munteanu M, Thabut D, Cadranel JF, Le Bail B, de Ledinghen V, Poynard T LIDO Study Group; CYTOL study group. Diagnostic value of biochemical markers (FibroTest-FibroSURE) for the prediction of liver fibrosis in patients with non-alcoholic fatty liver disease. BMC Gastroenterol. 2006;6:6. doi: 10.1186/1471-230X-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ampuero J, Pais R, Aller R, Gallego-Durán R, Crespo J, García-Monzón C, Boursier J, Vilar E, Petta S, Zheng MH, Escudero D, Calleja JL, Aspichueta P, Diago M, Rosales JM, Caballería J, Gómez-Camarero J, Lo Iacono O, Benlloch S, Albillos A, Turnes J, Banales JM, Ratziu V, Romero-Gómez M HEPAmet Registry. Development and Validation of Hepamet Fibrosis Scoring System-A Simple, Noninvasive Test to Identify Patients With Nonalcoholic Fatty Liver Disease With Advanced Fibrosis. Clin Gastroenterol Hepatol. 2020;18:216–225.e5. doi: 10.1016/j.cgh.2019.05.051. [DOI] [PubMed] [Google Scholar]
- 70.Meneses D, Olveira A, Corripio R, Del Carmen Méndez M, Romero M, Calvo-Viñuelas I, Herranz L, Vicent D, de-Cos-Blanco AI. Performance of Noninvasive Liver Fibrosis Scores in the Morbid Obese Patient, Same Scores but Different Thresholds. Obes Surg. 2020;30:2538–2546. doi: 10.1007/s11695-020-04509-0. [DOI] [PubMed] [Google Scholar]
- 71.Gu J, Liu S, Du S, Zhang Q, Xiao J, Dong Q, Xin Y. Diagnostic value of MRI-PDFF for hepatic steatosis in patients with non-alcoholic fatty liver disease: a meta-analysis. Eur Radiol. 2019;29:3564–3573. doi: 10.1007/s00330-019-06072-4. [DOI] [PubMed] [Google Scholar]
- 72.Kim M, Kang BK, Jun DW. Comparison of conventional sonographic signs and magnetic resonance imaging proton density fat fraction for assessment of hepatic steatosis. Sci Rep. 2018;8:7759. doi: 10.1038/s41598-018-26019-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Allan R, Thoirs K, Phillips M. Accuracy of ultrasound to identify chronic liver disease. World J Gastroenterol. 2010;16:3510–3520. doi: 10.3748/wjg.v16.i28.3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lo GC, Besa C, King MJ, Kang M, Stueck A, Thung S, Wagner M, Smith AD, Taouli B. Feasibility and reproducibility of liver surface nodularity quantification for the assessment of liver cirrhosis using CT and MRI. Eur J Radiol Open. 2017;4:95–100. doi: 10.1016/j.ejro.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li Q, Dhyani M, Grajo JR, Sirlin C, Samir AE. Current status of imaging in nonalcoholic fatty liver disease. World J Hepatol. 2018;10:530–542. doi: 10.4254/wjh.v10.i8.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ozturk A, Grajo JR, Dhyani M, Anthony BW, Samir AE. Principles of ultrasound elastography. Abdom Radiol (NY) 2018;43:773–785. doi: 10.1007/s00261-018-1475-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Imajo K, Kessoku T, Honda Y, Tomeno W, Ogawa Y, Mawatari H, Fujita K, Yoneda M, Taguri M, Hyogo H, Sumida Y, Ono M, Eguchi Y, Inoue T, Yamanaka T, Wada K, Saito S, Nakajima A. Magnetic Resonance Imaging More Accurately Classifies Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease Than Transient Elastography. Gastroenterology. 2016;150:626–637.e7. doi: 10.1053/j.gastro.2015.11.048. [DOI] [PubMed] [Google Scholar]
- 78.Karlas T, Petroff D, Sasso M, Fan JG, Mi YQ, de Lédinghen V, Kumar M, Lupsor-Platon M, Han KH, Cardoso AC, Ferraioli G, Chan WK, Wong VW, Myers RP, Chayama K, Friedrich-Rust M, Beaugrand M, Shen F, Hiriart JB, Sarin SK, Badea R, Jung KS, Marcellin P, Filice C, Mahadeva S, Wong GL, Crotty P, Masaki K, Bojunga J, Bedossa P, Keim V, Wiegand J. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2017;66:1022–1030. doi: 10.1016/j.jhep.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 79.Oeda S, Takahashi H, Imajo K, Seko Y, Ogawa Y, Moriguchi M, Yoneda M, Anzai K, Aishima S, Kage M, Itoh Y, Nakajima A, Eguchi Y. Accuracy of liver stiffness measurement and controlled attenuation parameter using FibroScan® M/XL probes to diagnose liver fibrosis and steatosis in patients with nonalcoholic fatty liver disease: a multicenter prospective study. J Gastroenterol. 2020;55:428–440. doi: 10.1007/s00535-019-01635-0. [DOI] [PubMed] [Google Scholar]
- 80.Lee HW, Wong GL, Kwok R, Choi KC, Chan CK, Shu SS, Leung JK, Chim AM, Luk AO, Ma RC, Chan HL, Chan JC, Kong AP, Wong VW. Serial transient elastography examinations to monitor patients with type 2 diabetes: A prospective cohort study. Hepatology. 2020 doi: 10.1002/hep.31142. [DOI] [PubMed] [Google Scholar]
- 81.Boursier J, Zarski JP, de Ledinghen V, Rousselet MC, Sturm N, Lebail B, Fouchard-Hubert I, Gallois Y, Oberti F, Bertrais S, Calès P Multicentric Group from ANRS/HC/EP23 FIBROSTAR Studies. Determination of reliability criteria for liver stiffness evaluation by transient elastography. Hepatology. 2013;57:1182–1191. doi: 10.1002/hep.25993. [DOI] [PubMed] [Google Scholar]
- 82.Shi KQ, Tang JZ, Zhu XL, Ying L, Li DW, Gao J, Fang YX, Li GL, Song YJ, Deng ZJ, Wu JM, Tang KF. Controlled attenuation parameter for the detection of steatosis severity in chronic liver disease: a meta-analysis of diagnostic accuracy. J Gastroenterol Hepatol. 2014;29:1149–1158. doi: 10.1111/jgh.12519. [DOI] [PubMed] [Google Scholar]
- 83.Jun BG, Park WY, Park EJ, Jang JY, Jeong SW, Lee SH, Kim SG, Cha SW, Kim YS, Cho YD, Kim HS, Kim BS, Jin SY, Park S. A prospective comparative assessment of the accuracy of the FibroScan in evaluating liver steatosis. PLoS One. 2017;12:e0182784. doi: 10.1371/journal.pone.0182784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Siddiqui MS, Vuppalanchi R, Van Natta ML, Hallinan E, Kowdley KV, Abdelmalek M, Neuschwander-Tetri BA, Loomba R, Dasarathy S, Brandman D, Doo E, Tonascia JA, Kleiner DE, Chalasani N, Sanyal AJ NASH Clinical Research Network. Vibration-Controlled Transient Elastography to Assess Fibrosis and Steatosis in Patients With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2019;17:156–163.e2. doi: 10.1016/j.cgh.2018.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cassinotto C, Boursier J, de Lédinghen V, Lebigot J, Lapuyade B, Cales P, Hiriart JB, Michalak S, Bail BL, Cartier V, Mouries A, Oberti F, Fouchard-Hubert I, Vergniol J, Aubé C. Liver stiffness in nonalcoholic fatty liver disease: A comparison of supersonic shear imaging, FibroScan, and ARFI with liver biopsy. Hepatology. 2016;63:1817–1827. doi: 10.1002/hep.28394. [DOI] [PubMed] [Google Scholar]
- 86.Nightingale K, Soo MS, Nightingale R, Trahey G. Acoustic radiation force impulse imaging: in vivo demonstration of clinical feasibility. Ultrasound Med Biol. 2002;28:227–235. doi: 10.1016/s0301-5629(01)00499-9. [DOI] [PubMed] [Google Scholar]
- 87.D'Onofrio M, Crosara S, De Robertis R, Canestrini S, Demozzi E, Gallotti A, Pozzi Mucelli R. Acoustic radiation force impulse of the liver. World J Gastroenterol. 2013;19:4841–4849. doi: 10.3748/wjg.v19.i30.4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fierbinteanu-Braticevici C, Andronescu D, Usvat R, Cretoiu D, Baicus C, Marinoschi G. Acoustic radiation force imaging sonoelastography for noninvasive staging of liver fibrosis. World J Gastroenterol. 2009;15:5525–5532. doi: 10.3748/wjg.15.5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bota S, Herkner H, Sporea I, Salzl P, Sirli R, Neghina AM, Peck-Radosavljevic M. Meta-analysis: ARFI elastography versus transient elastography for the evaluation of liver fibrosis. Liver Int. 2013;33:1138–1147. doi: 10.1111/liv.12240. [DOI] [PubMed] [Google Scholar]
- 90.Friedrich-Rust M, Romen D, Vermehren J, Kriener S, Sadet D, Herrmann E, Zeuzem S, Bojunga J. Acoustic radiation force impulse-imaging and transient elastography for non-invasive assessment of liver fibrosis and steatosis in NAFLD. Eur J Radiol. 2012;81:e325–e331. doi: 10.1016/j.ejrad.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 91.Guzmán-Aroca F, Frutos-Bernal MD, Bas A, Luján-Mompeán JA, Reus M, Berná-Serna Jde D, Parrilla P. Detection of non-alcoholic steatohepatitis in patients with morbid obesity before bariatric surgery: preliminary evaluation with acoustic radiation force impulse imaging. Eur Radiol. 2012;22:2525–2532. doi: 10.1007/s00330-012-2505-3. [DOI] [PubMed] [Google Scholar]
- 92.Osaki A, Kubota T, Suda T, Igarashi M, Nagasaki K, Tsuchiya A, Yano M, Tamura Y, Takamura M, Kawai H, Yamagiwa S, Kikuchi T, Nomoto M, Aoyagi Y. Shear wave velocity is a useful marker for managing nonalcoholic steatohepatitis. World J Gastroenterol. 2010;16:2918–2925. doi: 10.3748/wjg.v16.i23.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yoneda M, Suzuki K, Kato S, Fujita K, Nozaki Y, Hosono K, Saito S, Nakajima A. Nonalcoholic fatty liver disease: US-based acoustic radiation force impulse elastography. Radiology. 2010;256:640–647. doi: 10.1148/radiol.10091662. [DOI] [PubMed] [Google Scholar]
- 94.Venkatesh SK, Ehman RL. Magnetic resonance elastography of liver. Magn Reson Imaging Clin N Am. 2014;22:433–446. doi: 10.1016/j.mric.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Muthupillai R, Lomas DJ, Rossman PJ, Greenleaf JF, Manduca A, Ehman RL. Magnetic resonance elastography by direct visualization of propagating acoustic strain waves. Science. 1995;269:1854–1857. doi: 10.1126/science.7569924. [DOI] [PubMed] [Google Scholar]
- 96.Hoodeshenas S, Yin M, Venkatesh SK. Magnetic Resonance Elastography of Liver: Current Update. Top Magn Reson Imaging. 2018;27:319–333. doi: 10.1097/RMR.0000000000000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Loomba R, Wolfson T, Ang B, Hooker J, Behling C, Peterson M, Valasek M, Lin G, Brenner D, Gamst A, Ehman R, Sirlin C. Magnetic resonance elastography predicts advanced fibrosis in patients with nonalcoholic fatty liver disease: a prospective study. Hepatology. 2014;60:1920–1928. doi: 10.1002/hep.27362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim D, Kim WR, Talwalkar JA, Kim HJ, Ehman RL. Advanced fibrosis in nonalcoholic fatty liver disease: noninvasive assessment with MR elastography. Radiology. 2013;268:411–419. doi: 10.1148/radiol.13121193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cui J, Ang B, Haufe W, Hernandez C, Verna EC, Sirlin CB, Loomba R. Comparative diagnostic accuracy of magnetic resonance elastography vs. eight clinical prediction rules for non-invasive diagnosis of advanced fibrosis in biopsy-proven non-alcoholic fatty liver disease: a prospective study. Aliment Pharmacol Ther. 2015;41:1271–1280. doi: 10.1111/apt.13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ajmera VH, Liu A, Singh S, Yachoa G, Ramey M, Bhargava M, Zamani A, Lopez S, Mangla N, Bettencourt R, Rizo E, Valasek M, Behling C, Richards L, Sirlin C, Loomba R. Clinical Utility of an Increase in Magnetic Resonance Elastography in Predicting Fibrosis Progression in Nonalcoholic Fatty Liver Disease. Hepatology. 2020;71:849–860. doi: 10.1002/hep.30974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dulai PS, Sirlin CB, Loomba R. MRI and MRE for non-invasive quantitative assessment of hepatic steatosis and fibrosis in NAFLD and NASH: Clinical trials to clinical practice. J Hepatol. 2016;65:1006–1016. doi: 10.1016/j.jhep.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Petta S, Wong VW, Cammà C, Hiriart JB, Wong GL, Vergniol J, Chan AW, Di Marco V, Merrouche W, Chan HL, Marra F, Le-Bail B, Arena U, Craxì A, de Ledinghen V. Serial combination of non-invasive tools improves the diagnostic accuracy of severe liver fibrosis in patients with NAFLD. Aliment Pharmacol Ther. 2017;46:617–627. doi: 10.1111/apt.14219. [DOI] [PubMed] [Google Scholar]
- 103.Newsome PN, Sasso M, Deeks JJ, Paredes A, Boursier J, Chan WK, Yilmaz Y, Czernichow S, Zheng MH, Wong VW, Allison M, Tsochatzis E, Anstee QM, Sheridan DA, Eddowes PJ, Guha IN, Cobbold JF, Paradis V, Bedossa P, Miette V, Fournier-Poizat C, Sandrin L, Harrison SA. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol. 2020;5:362–373. doi: 10.1016/S2468-1253(19)30383-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Boyd A, Cain O, Chauhan A, Webb GJ. Medical liver biopsy: background, indications, procedure and histopathology. Frontline Gastroenterol. 2020;11:40–47. doi: 10.1136/flgastro-2018-101139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tublin ME, Blair R, Martin J, Malik S, Ruppert K, Demetris A. Prospective Study of the Impact of Liver Biopsy Core Size on Specimen Adequacy and Procedural Complications. AJR Am J Roentgenol. 2018;210:183–188. doi: 10.2214/AJR.17.17792. [DOI] [PubMed] [Google Scholar]
- 106.Gramlich T, Kleiner DE, McCullough AJ, Matteoni CA, Boparai N, Younossi ZM. Pathologic features associated with fibrosis in nonalcoholic fatty liver disease. Hum Pathol. 2004;35:196–199. doi: 10.1016/j.humpath.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 107.Bedossa P, Patel K, Castera L. Histologic and noninvasive estimates of liver fibrosis. Clin Liver Dis (Hoboken) 2015;6:5–8. doi: 10.1002/cld.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cheah MC, McCullough AJ, Goh GB. Current Modalities of Fibrosis Assessment in Non-alcoholic Fatty Liver Disease. J Clin Transl Hepatol. 2017;5:261–271. doi: 10.14218/JCTH.2017.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 110.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 111.Bedossa P, Poitou C, Veyrie N, Bouillot JL, Basdevant A, Paradis V, Tordjman J, Clement K. Histopathological algorithm and scoring system for evaluation of liver lesions in morbidly obese patients. Hepatology. 2012;56:1751–1759. doi: 10.1002/hep.25889. [DOI] [PubMed] [Google Scholar]
- 112.De Rudder M, Bouzin C, Nachit M, Louvegny H, Vande Velde G, Julé Y, Leclercq IA. Automated computerized image analysis for the user-independent evaluation of disease severity in preclinical models of NAFLD/NASH. Lab Invest. 2020;100:147–160. doi: 10.1038/s41374-019-0315-9. [DOI] [PubMed] [Google Scholar]
- 113.Masseroli M, Caballero T, O'Valle F, Del Moral RM, Pérez-Milena A, Del Moral RG. Automatic quantification of liver fibrosis: design and validation of a new image analysis method: comparison with semi-quantitative indexes of fibrosis. J Hepatol. 2000;32:453–464. doi: 10.1016/s0168-8278(00)80397-9. [DOI] [PubMed] [Google Scholar]
- 114.Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344:495–500. doi: 10.1056/NEJM200102153440706. [DOI] [PubMed] [Google Scholar]
- 115.Gawrieh S, Knoedler DM, Saeian K, Wallace JR, Komorowski RA. Effects of interventions on intra- and interobserver agreement on interpretation of nonalcoholic fatty liver disease histology. Ann Diagn Pathol. 2011;15:19–24. doi: 10.1016/j.anndiagpath.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 116.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 117.López-Suárez A, Guerrero JM, Elvira-González J, Beltrán-Robles M, Cañas-Hormigo F, Bascuñana-Quirell A. Nonalcoholic fatty liver disease is associated with blood pressure in hypertensive and nonhypertensive individuals from the general population with normal levels of alanine aminotransferase. Eur J Gastroenterol Hepatol. 2011;23:1011–1017. doi: 10.1097/MEG.0b013e32834b8d52. [DOI] [PubMed] [Google Scholar]
- 118.Saber S, Mahmoud AAA, Helal NS, El-Ahwany E, Abdelghany RH. Renin-angiotensin system inhibition ameliorates CCl4-induced liver fibrosis in mice through the inactivation of nuclear transcription factor kappa B. Can J Physiol Pharmacol. 2018;96:569–576. doi: 10.1139/cjpp-2017-0728. [DOI] [PubMed] [Google Scholar]
- 119.Goh GB, Pagadala MR, Dasarathy J, Unalp-Arida A, Sargent R, Hawkins C, Sourianarayanane A, Khiyami A, Yerian L, Pai R, McCullough AJ, Dasarathy S. Renin-angiotensin system and fibrosis in non-alcoholic fatty liver disease. Liver Int. 2015;35:979–985. doi: 10.1111/liv.12611. [DOI] [PubMed] [Google Scholar]
- 120.Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, Ramirez A, Schlaich M, Stergiou GS, Tomaszewski M, Wainford RD, Williams B, Schutte AE. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension. 2020;75:1334–1357. doi: 10.1161/HYPERTENSIONAHA.120.15026. [DOI] [PubMed] [Google Scholar]
- 121.Abraham HM, White CM, White WB. The comparative efficacy and safety of the angiotensin receptor blockers in the management of hypertension and other cardiovascular diseases. Drug Saf. 2015;38:33–54. doi: 10.1007/s40264-014-0239-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Svegliati-Baroni G, Ridolfi F, Di Sario A, Casini A, Marucci L, Gaggiotti G, Orlandoni P, Macarri G, Perego L, Benedetti A, Folli F. Insulin and insulin-like growth factor-1 stimulate proliferation and type I collagen accumulation by human hepatic stellate cells: differential effects on signal transduction pathways. Hepatology. 1999;29:1743–1751. doi: 10.1002/hep.510290632. [DOI] [PubMed] [Google Scholar]
- 123.Haukeland JW, Konopski Z, Eggesbø HB, von Volkmann HL, Raschpichler G, Bjøro K, Haaland T, Løberg EM, Birkeland K. Metformin in patients with non-alcoholic fatty liver disease: a randomized, controlled trial. Scand J Gastroenterol. 2009;44:853–860. doi: 10.1080/00365520902845268. [DOI] [PubMed] [Google Scholar]
- 124.Lavine JE, Schwimmer JB, Van Natta ML, Molleston JP, Murray KF, Rosenthal P, Abrams SH, Scheimann AO, Sanyal AJ, Chalasani N, Tonascia J, Ünalp A, Clark JM, Brunt EM, Kleiner DE, Hoofnagle JH, Robuck PR Nonalcoholic Steatohepatitis Clinical Research Network. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA. 2011;305:1659–1668. doi: 10.1001/jama.2011.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cunha V, Cotrim HP, Rocha R, Carvalho K, Lins-Kusterer L. Metformin in the prevention of hepatocellular carcinoma in diabetic patients: A systematic review. Ann Hepatol. 2020;19:232–237. doi: 10.1016/j.aohep.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 126.Zhang ZJ, Zheng ZJ, Shi R, Su Q, Jiang Q, Kip KE. Metformin for liver cancer prevention in patients with type 2 diabetes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2012;97:2347–2353. doi: 10.1210/jc.2012-1267. [DOI] [PubMed] [Google Scholar]
- 127.Ozturk ZA, Kadayifci A. Insulin sensitizers for the treatment of non-alcoholic fatty liver disease. World J Hepatol. 2014;6:199–206. doi: 10.4254/wjh.v6.i4.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang L, Zhang X, Meng H, Li Y, Han T, Wang C. Obstructive sleep apnea and liver injury in severely obese patients with nonalcoholic fatty liver disease. Sleep Breath. 2020 doi: 10.1007/s11325-020-02018-z. [DOI] [PubMed] [Google Scholar]
- 129.Aron-Wisnewsky J, Clement K, Pépin JL. Nonalcoholic fatty liver disease and obstructive sleep apnea. Metabolism. 2016;65:1124–1135. doi: 10.1016/j.metabol.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 130.Salord N, Fortuna AM, Monasterio C, Gasa M, Pérez A, Bonsignore MR, Vilarrasa N, Montserrat JM, Mayos M. A Randomized Controlled Trial of Continuous Positive Airway Pressure on Glucose Tolerance in Obese Patients with Obstructive Sleep Apnea. Sleep. 2016;39:35–41. doi: 10.5665/sleep.5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fu Y, Xia Y, Yi H, Xu H, Guan J, Yin S. Meta-analysis of all-cause and cardiovascular mortality in obstructive sleep apnea with or without continuous positive airway pressure treatment. Sleep Breath. 2017;21:181–189. doi: 10.1007/s11325-016-1393-1. [DOI] [PubMed] [Google Scholar]
- 132.Jullian-Desayes I, Tamisier R, Zarski JP, Aron-Wisnewsky J, Launois-Rollinat SH, Trocme C, Levy P, Joyeux-Faure M, Pepin JL. Impact of effective versus sham continuous positive airway pressure on liver injury in obstructive sleep apnoea: Data from randomized trials. Respirology. 2016;21:378–385. doi: 10.1111/resp.12672. [DOI] [PubMed] [Google Scholar]
- 133.Kim D, Ahmed A, Kushida C. Continuous Positive Airway Pressure Therapy on Nonalcoholic Fatty Liver Disease in Patients With Obstructive Sleep Apnea. J Clin Sleep Med. 2018;14:1315–1322. doi: 10.5664/jcsm.7262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Buttacavoli M, Gruttad'Auria CI, Olivo M, Virdone R, Castrogiovanni A, Mazzuca E, Marotta AM, Marrone O, Madonia S, Bonsignore MR. Liver Steatosis and Fibrosis in OSA patients After Long-term CPAP Treatment: A Preliminary Ultrasound Study. Ultrasound Med Biol. 2016;42:104–109. doi: 10.1016/j.ultrasmedbio.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 135.Athyros VG, Boutari C, Stavropoulos K, Anagnostis P, Imprialos KP, Doumas M, Karagiannis A. Statins: An Under-Appreciated Asset for the Prevention and the Treatment of NAFLD or NASH and the Related Cardiovascular Risk. Curr Vasc Pharmacol. 2018;16:246–253. doi: 10.2174/1570161115666170621082910. [DOI] [PubMed] [Google Scholar]
- 136.Doumas M, Imprialos K, Dimakopoulou A, Stavropoulos K, Binas A, Athyros VG. The Role of Statins in the Management of Nonalcoholic Fatty Liver Disease. Curr Pharm Des. 2018;24:4587–4592. doi: 10.2174/1381612825666190117114305. [DOI] [PubMed] [Google Scholar]
- 137.Sekiya M, Yahagi N, Matsuzaka T, Najima Y, Nakakuki M, Nagai R, Ishibashi S, Osuga J, Yamada N, Shimano H. Polyunsaturated fatty acids ameliorate hepatic steatosis in obese mice by SREBP-1 suppression. Hepatology. 2003;38:1529–1539. doi: 10.1016/j.hep.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 138.Levy JR, Clore JN, Stevens W. Dietary n-3 polyunsaturated fatty acids decrease hepatic triglycerides in Fischer 344 rats. Hepatology. 2004;39:608–616. doi: 10.1002/hep.20093. [DOI] [PubMed] [Google Scholar]
- 139.Huang MA, Greenson JK, Chao C, Anderson L, Peterman D, Jacobson J, Emick D, Lok AS, Conjeevaram HS. One-year intense nutritional counseling results in histological improvement in patients with non-alcoholic steatohepatitis: a pilot study. Am J Gastroenterol. 2005;100:1072–1081. doi: 10.1111/j.1572-0241.2005.41334.x. [DOI] [PubMed] [Google Scholar]
- 140.Eckard C, Cole R, Lockwood J, Torres DM, Williams CD, Shaw JC, Harrison SA. Prospective histopathologic evaluation of lifestyle modification in nonalcoholic fatty liver disease: a randomized trial. Therap Adv Gastroenterol. 2013;6:249–259. doi: 10.1177/1756283X13484078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ueno T, Sugawara H, Sujaku K, Hashimoto O, Tsuji R, Tamaki S, Torimura T, Inuzuka S, Sata M, Tanikawa K. Therapeutic effects of restricted diet and exercise in obese patients with fatty liver. J Hepatol. 1997;27:103–107. doi: 10.1016/s0168-8278(97)80287-5. [DOI] [PubMed] [Google Scholar]
- 142.Tendler D, Lin S, Yancy WS, Jr, Mavropoulos J, Sylvestre P, Rockey DC, Westman EC. The effect of a low-carbohydrate, ketogenic diet on nonalcoholic fatty liver disease: a pilot study. Dig Dis Sci. 2007;52:589–593. doi: 10.1007/s10620-006-9433-5. [DOI] [PubMed] [Google Scholar]
- 143.Promrat K, Kleiner DE, Niemeier HM, Jackvony E, Kearns M, Wands JR, Fava JL, Wing RR. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51:121–129. doi: 10.1002/hep.23276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, Friedman SL, Diago M, Romero-Gomez M. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology. 2015;149:367–78.e5; quiz e14-5. doi: 10.1053/j.gastro.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 145.Glass LM, Dickson RC, Anderson JC, Suriawinata AA, Putra J, Berk BS, Toor A. Total body weight loss of ≥ 10 % is associated with improved hepatic fibrosis in patients with nonalcoholic steatohepatitis. Dig Dis Sci. 2015;60:1024–1030. doi: 10.1007/s10620-014-3380-3. [DOI] [PubMed] [Google Scholar]
- 146.Zelber-Sagi S, Salomone F, Mlynarsky L. The Mediterranean dietary pattern as the diet of choice for non-alcoholic fatty liver disease: Evidence and plausible mechanisms. Liver Int. 2017;37:936–949. doi: 10.1111/liv.13435. [DOI] [PubMed] [Google Scholar]
- 147.Baratta F, Pastori D, Polimeni L, Bucci T, Ceci F, Calabrese C, Ernesti I, Pannitteri G, Violi F, Angelico F, Del Ben M. Adherence to Mediterranean Diet and Non-Alcoholic Fatty Liver Disease: Effect on Insulin Resistance. Am J Gastroenterol. 2017;112:1832–1839. doi: 10.1038/ajg.2017.371. [DOI] [PubMed] [Google Scholar]
- 148.Ryan MC, Itsiopoulos C, Thodis T, Ward G, Trost N, Hofferberth S, O'Dea K, Desmond PV, Johnson NA, Wilson AM. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J Hepatol. 2013;59:138–143. doi: 10.1016/j.jhep.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 149.Kaliora AC, Gioxari A, Kalafati IP, Diolintzi A, Kokkinos A, Dedoussis GV. The Effectiveness of Mediterranean Diet in Nonalcoholic Fatty Liver Disease Clinical Course: An Intervention Study. J Med Food. 2019;22:729–740. doi: 10.1089/jmf.2018.0020. [DOI] [PubMed] [Google Scholar]
- 150.Locklear CT, Golabi P, Gerber L, Younossi ZM. Exercise as an intervention for patients with end-stage liver disease: Systematic review. Medicine (Baltimore) 2018;97:e12774. doi: 10.1097/MD.0000000000012774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Macías-Rodríguez RU, Ilarraza-Lomelí H, Ruiz-Margáin A, Ponce-de-León-Rosales S, Vargas-Vorácková F, García-Flores O, Torre A, Duarte-Rojo A. Changes in Hepatic Venous Pressure Gradient Induced by Physical Exercise in Cirrhosis: Results of a Pilot Randomized Open Clinical Trial. Clin Transl Gastroenterol. 2016;7:e180. doi: 10.1038/ctg.2016.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhang HJ, He J, Pan LL, Ma ZM, Han CK, Chen CS, Chen Z, Han HW, Chen S, Sun Q, Zhang JF, Li ZB, Yang SY, Li XJ, Li XY. Effects of Moderate and Vigorous Exercise on Nonalcoholic Fatty Liver Disease: A Randomized Clinical Trial. JAMA Intern Med. 2016;176:1074–1082. doi: 10.1001/jamainternmed.2016.3202. [DOI] [PubMed] [Google Scholar]
- 153.Keating SE, Hackett DA, George J, Johnson NA. Exercise and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. 2012;57:157–166. doi: 10.1016/j.jhep.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 154.Hamasaki H. Perspectives on Interval Exercise Interventions for Non-Alcoholic Fatty Liver Disease. Medicines (Basel) 2019;6 doi: 10.3390/medicines6030083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Oh S, So R, Shida T, Matsuo T, Kim B, Akiyama K, Isobe T, Okamoto Y, Tanaka K, Shoda J. High-Intensity Aerobic Exercise Improves Both Hepatic Fat Content and Stiffness in Sedentary Obese Men with Nonalcoholic Fatty Liver Disease. Sci Rep. 2017;7:43029. doi: 10.1038/srep43029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pedroso FE, Angriman F, Endo A, Dasenbrock H, Storino A, Castillo R, Watkins AA, Castillo-Angeles M, Goodman JE, Zitsman JL. Weight loss after bariatric surgery in obese adolescents: a systematic review and meta-analysis. Surg Obes Relat Dis. 2018;14:413–422. doi: 10.1016/j.soard.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 157.O'Brien PE, Hindle A, Brennan L, Skinner S, Burton P, Smith A, Crosthwaite G, Brown W. Long-Term Outcomes After Bariatric Surgery: a Systematic Review and Meta-analysis of Weight Loss at 10 or More Years for All Bariatric Procedures and a Single-Centre Review of 20-Year Outcomes After Adjustable Gastric Banding. Obes Surg. 2019;29:3–14. doi: 10.1007/s11695-018-3525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sjöström L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, Dahlgren S, Larsson B, Narbro K, Sjöström CD, Sullivan M, Wedel H Swedish Obese Subjects Study Scientific Group. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 159.Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, Navaneethan SD, Singh RP, Pothier CE, Nissen SE, Kashyap SR STAMPEDE Investigators. Bariatric Surgery versus Intensive Medical Therapy for Diabetes - 5-Year Outcomes. N Engl J Med. 2017;376:641–651. doi: 10.1056/NEJMoa1600869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Nanni G, Castagneto M, Bornstein S, Rubino F. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet. 2015;386:964–973. doi: 10.1016/S0140-6736(15)00075-6. [DOI] [PubMed] [Google Scholar]
- 161.Lassailly G, Caiazzo R, Buob D, Pigeyre M, Verkindt H, Labreuche J, Raverdy V, Leteurtre E, Dharancy S, Louvet A, Romon M, Duhamel A, Pattou F, Mathurin P. Bariatric Surgery Reduces Features of Nonalcoholic Steatohepatitis in Morbidly Obese Patients. Gastroenterology. 2015;149:379–388; quiz e15-e16. doi: 10.1053/j.gastro.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 162.Caiazzo R, Lassailly G, Leteurtre E, Baud G, Verkindt H, Raverdy V, Buob D, Pigeyre M, Mathurin P, Pattou F. Roux-en-Y gastric bypass versus adjustable gastric banding to reduce nonalcoholic fatty liver disease: a 5-year controlled longitudinal study. Ann Surg. 2014;260:893–899; discussion 898-899. doi: 10.1097/SLA.0000000000000945. [DOI] [PubMed] [Google Scholar]
- 163.Moretto M, Kupski C, da Silva VD, Padoin AV, Mottin CC. Effect of bariatric surgery on liver fibrosis. Obes Surg. 2012;22:1044–1049. doi: 10.1007/s11695-011-0559-y. [DOI] [PubMed] [Google Scholar]
- 164.Galli F, Azzi A, Birringer M, Cook-Mills JM, Eggersdorfer M, Frank J, Cruciani G, Lorkowski S, Özer NK. Vitamin E: Emerging aspects and new directions. Free Radic Biol Med. 2017;102:16–36. doi: 10.1016/j.freeradbiomed.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 165.Parola M, Muraca R, Dianzani I, Barrera G, Leonarduzzi G, Bendinelli P, Piccoletti R, Poli G. Vitamin E dietary supplementation inhibits transforming growth factor beta 1 gene expression in the rat liver. FEBS Lett. 1992;308:267–270. doi: 10.1016/0014-5793(92)81290-3. [DOI] [PubMed] [Google Scholar]
- 166.Sanyal AJ, Mofrad PS, Contos MJ, Sargeant C, Luketic VA, Sterling RK, Stravitz RT, Shiffman ML, Clore J, Mills AS. A pilot study of vitamin E versus vitamin E and pioglitazone for the treatment of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2004;2:1107–1115. doi: 10.1016/s1542-3565(04)00457-4. [DOI] [PubMed] [Google Scholar]
- 167.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, Van Natta M, Clark J, Brunt EM, Kleiner DE, Hoofnagle JH, Robuck PR, NASH CRN. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sumida Y, Naito Y, Tanaka S, Sakai K, Inada Y, Taketani H, Kanemasa K, Yasui K, Itoh Y, Okanoue T, Yoshikawa T. Long-term (>=2 yr) efficacy of vitamin E for non-alcoholic steatohepatitis. Hepatogastroenterology. 2013;60:1445–1450. doi: 10.5754/hge11421. [DOI] [PubMed] [Google Scholar]
- 169.Xu R, Tao A, Zhang S, Deng Y, Chen G. Association between vitamin E and non-alcoholic steatohepatitis: a meta-analysis. Int J Clin Exp Med. 2015;8:3924–3934. [PMC free article] [PubMed] [Google Scholar]
- 170.Harrison SA, Torgerson S, Hayashi P, Ward J, Schenker S. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2003;98:2485–2490. doi: 10.1111/j.1572-0241.2003.08699.x. [DOI] [PubMed] [Google Scholar]
- 171.Aithal GP, Thomas JA, Kaye PV, Lawson A, Ryder SD, Spendlove I, Austin AS, Freeman JG, Morgan L, Webber J. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology. 2008;135:1176–1184. doi: 10.1053/j.gastro.2008.06.047. [DOI] [PubMed] [Google Scholar]
- 172.Cusi K, Orsak B, Bril F, Lomonaco R, Hecht J, Ortiz-Lopez C, Tio F, Hardies J, Darland C, Musi N, Webb A, Portillo-Sanchez P. Long-Term Pioglitazone Treatment for Patients With Nonalcoholic Steatohepatitis and Prediabetes or Type 2 Diabetes Mellitus: A Randomized Trial. Ann Intern Med. 2016;165:305–315. doi: 10.7326/M15-1774. [DOI] [PubMed] [Google Scholar]
- 173.Bril F, Biernacki DM, Kalavalapalli S, Lomonaco R, Subbarayan SK, Lai J, Tio F, Suman A, Orsak BK, Hecht J, Cusi K. Role of Vitamin E for Nonalcoholic Steatohepatitis in Patients With Type 2 Diabetes: A Randomized Controlled Trial. Diabetes Care. 2019;42:1481–1488. doi: 10.2337/dc19-0167. [DOI] [PubMed] [Google Scholar]
- 174.Vilar-Gomez E, Vuppalanchi R, Gawrieh S, Ghabril M, Saxena R, Cummings OW, Chalasani N. Vitamin E Improves Transplant-Free Survival and Hepatic Decompensation Among Patients With Nonalcoholic Steatohepatitis and Advanced Fibrosis. Hepatology. 2020;71:495–509. doi: 10.1002/hep.30368. [DOI] [PubMed] [Google Scholar]
- 175.Klein EA, Thompson IM, Jr, Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, Minasian LM, Ford LG, Parnes HL, Gaziano JM, Karp DD, Lieber MM, Walther PJ, Klotz L, Parsons JK, Chin JL, Darke AK, Lippman SM, Goodman GE, Meyskens FL, Jr, Baker LH. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2011;306:1549–1556. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Schürks M, Glynn RJ, Rist PM, Tzourio C, Kurth T. Effects of vitamin E on stroke subtypes: meta-analysis of randomised controlled trials. BMJ. 2010;341:c5702. doi: 10.1136/bmj.c5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lutchman G, Promrat K, Kleiner DE, Heller T, Ghany MG, Yanovski JA, Liang TJ, Hoofnagle JH. Changes in serum adipokine levels during pioglitazone treatment for nonalcoholic steatohepatitis: relationship to histological improvement. Clin Gastroenterol Hepatol. 2006;4:1048–1052. doi: 10.1016/j.cgh.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 178.Lefebvre E, Moyle G, Reshef R, Richman LP, Thompson M, Hong F, Chou HL, Hashiguchi T, Plato C, Poulin D, Richards T, Yoneyama H, Jenkins H, Wolfgang G, Friedman SL. Antifibrotic Effects of the Dual CCR2/CCR5 Antagonist Cenicriviroc in Animal Models of Liver and Kidney Fibrosis. PLoS One. 2016;11:e0158156. doi: 10.1371/journal.pone.0158156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Friedman SL, Ratziu V, Harrison SA, Abdelmalek MF, Aithal GP, Caballeria J, Francque S, Farrell G, Kowdley KV, Craxi A, Simon K, Fischer L, Melchor-Khan L, Vest J, Wiens BL, Vig P, Seyedkazemi S, Goodman Z, Wong VW, Loomba R, Tacke F, Sanyal A, Lefebvre E. A randomized, placebo-controlled trial of cenicriviroc for treatment of nonalcoholic steatohepatitis with fibrosis. Hepatology. 2018;67:1754–1767. doi: 10.1002/hep.29477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Mayo Clinic. AURORA: Phase 3 Study for the Efficacy and Safety of CVC for the Treatment of Liver Fibrosis in Adults With NASH. Available from: www.mayo.edu/research/clinical-trials/cls-20425267.
- 181.Markham A, Keam SJ. Obeticholic Acid: First Global Approval. Drugs. 2016;76:1221–1226. doi: 10.1007/s40265-016-0616-x. [DOI] [PubMed] [Google Scholar]
- 182.Mudaliar S, Henry RR, Sanyal AJ, Morrow L, Marschall HU, Kipnes M, Adorini L, Sciacca CI, Clopton P, Castelloe E, Dillon P, Pruzanski M, Shapiro D. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology. 2013;145:574–582.e1. doi: 10.1053/j.gastro.2013.05.042. [DOI] [PubMed] [Google Scholar]
- 183.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, Chalasani N, Dasarathy S, Diehl AM, Hameed B, Kowdley KV, McCullough A, Terrault N, Clark JM, Tonascia J, Brunt EM, Kleiner DE, Doo E NASH Clinical Research Network. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956–965. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Younossi ZM, Ratziu V, Loomba R, Rinella M, Anstee QM, Goodman Z, Bedossa P, Geier A, Beckebaum S, Newsome PN, Sheridan D, Sheikh MY, Trotter J, Knapple W, Lawitz E, Abdelmalek MF, Kowdley KV, Montano-Loza AJ, Boursier J, Mathurin P, Bugianesi E, Mazzella G, Olveira A, Cortez-Pinto H, Graupera I, Orr D, Gluud LL, Dufour JF, Shapiro D, Campagna J, Zaru L, MacConell L, Shringarpure R, Harrison S, Sanyal AJ REGENERATE Study Investigators. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2019;394:2184–2196. doi: 10.1016/S0140-6736(19)33041-7. [DOI] [PubMed] [Google Scholar]
- 185.Loomba R, Sanyal AJ, Kowdley KV, Terrault N, Chalasani NP, Abdelmalek MF, McCullough AJ, Shringarpure R, Ferguson B, Lee L, Chen J, Liberman A, Shapiro D, Neuschwander-Tetri BA. Factors Associated With Histologic Response in Adult Patients With Nonalcoholic Steatohepatitis. Gastroenterology. 2019;156:88–95.e5. doi: 10.1053/j.gastro.2018.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Tully DC, Rucker PV, Chianelli D, Williams J, Vidal A, Alper PB, Mutnick D, Bursulaya B, Schmeits J, Wu X, Bao D, Zoll J, Kim Y, Groessl T, McNamara P, Seidel HM, Molteni V, Liu B, Phimister A, Joseph SB, Laffitte B. Discovery of Tropifexor (LJN452), a Highly Potent Non-bile Acid FXR Agonist for the Treatment of Cholestatic Liver Diseases and Nonalcoholic Steatohepatitis (NASH) J Med Chem. 2017;60:9960–9973. doi: 10.1021/acs.jmedchem.7b00907. [DOI] [PubMed] [Google Scholar]
- 187.Hernandez ED, Zheng L, Kim Y, Fang B, Liu B, Valdez RA, Dietrich WF, Rucker PV, Chianelli D, Schmeits J, Bao D, Zoll J, Dubois C, Federe GC, Chen L, Joseph SB, Klickstein LB, Walker J, Molteni V, McNamara P, Meeusen S, Tully DC, Badman MK, Xu J, Laffitte B. Tropifexor-Mediated Abrogation of Steatohepatitis and Fibrosis Is Associated With the Antioxidative Gene Expression Profile in Rodents. Hepatol Commun. 2019;3:1085–1097. doi: 10.1002/hep4.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Pedrosa M, Seyedkazemi S, Francque S, Sanyal A, Rinella M, Charlton M, Loomba R, Ratziu V, Kochuparampil J, Fischer L, Vaidyanathan S, Anstee QM. A randomized, double-blind, multicenter, phase 2b study to evaluate the safety and efficacy of a combination of tropifexor and cenicriviroc in patients with nonalcoholic steatohepatitis and liver fibrosis: Study design of the TANDEM trial. Contemp Clin Trials. 2020;88:105889. doi: 10.1016/j.cct.2019.105889. [DOI] [PubMed] [Google Scholar]
- 189.Efficacy, Safety and Tolerability of the Combination of Tropifexor & Licogliflozin Compared to Each Monotherapy, in Adult Patients With NASH and Liver Fibrosis. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT04065841 ClinicalTrials.gov Identifier: NCT04065841.
- 190.Study of Safety and Efficacy of Tropifexor (LJN452) in Patients With Non-alcoholic Steatohepatitis (NASH). In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT02855164 ClinicalTrials.gov Identifier: NCT02855164.
- 191.Ratziu V, Harrison SA, Francque S, Bedossa P, Lehert P, Serfaty L, Romero-Gomez M, Boursier J, Abdelmalek M, Caldwell S, Drenth J, Anstee QM, Hum D, Hanf R, Roudot A, Megnien S, Staels B, Sanyal A GOLDEN-505 Investigator Study Group. Elafibranor, an Agonist of the Peroxisome Proliferator-Activated Receptor-α and -δ, Induces Resolution of Nonalcoholic Steatohepatitis Without Fibrosis Worsening. Gastroenterology. 2016;150:1147–1159.e5. doi: 10.1053/j.gastro.2016.01.038. [DOI] [PubMed] [Google Scholar]
- 192.Idris I, Donnelly R. Sodium-glucose co-transporter-2 inhibitors: an emerging new class of oral antidiabetic drug. Diabetes Obes Metab. 2009;11:79–88. doi: 10.1111/j.1463-1326.2008.00982.x. [DOI] [PubMed] [Google Scholar]
- 193.Bolinder J, Ljunggren Ö, Kullberg J, Johansson L, Wilding J, Langkilde AM, Sugg J, Parikh S. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab. 2012;97:1020–1031. doi: 10.1210/jc.2011-2260. [DOI] [PubMed] [Google Scholar]
- 194.Shimizu M, Suzuki K, Kato K, Jojima T, Iijima T, Murohisa T, Iijima M, Takekawa H, Usui I, Hiraishi H, Aso Y. Evaluation of the effects of dapagliflozin, a sodium-glucose co-transporter-2 inhibitor, on hepatic steatosis and fibrosis using transient elastography in patients with type 2 diabetes and non-alcoholic fatty liver disease. Diabetes Obes Metab. 2019;21:285–292. doi: 10.1111/dom.13520. [DOI] [PubMed] [Google Scholar]
- 195.Yokote K, Sano M, Tsumiyama I, Keefe D. Dose-dependent reduction in body weight with LIK066 (licogliflozin) treatment in Japanese patients with obesity. Diabetes Obes Metab. 2020;22:1102–1110. doi: 10.1111/dom.14006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Bays HE, Kozlovski P, Shao Q, Proot P, Keefe D. Licogliflozin, a Novel SGLT1 and 2 Inhibitor: Body Weight Effects in a Randomized Trial in Adults with Overweight or Obesity. Obesity (Silver Spring) 2020;28:870–881. doi: 10.1002/oby.22764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Loomba R, Lawitz E, Mantry PS, Jayakumar S, Caldwell SH, Arnold H, Diehl AM, Djedjos CS, Han L, Myers RP, Subramanian GM, McHutchison JG, Goodman ZD, Afdhal NH, Charlton MR GS-US-384-1497 Investigators. The ASK1 inhibitor selonsertib in patients with nonalcoholic steatohepatitis: A randomized, phase 2 trial. Hepatology. 2018;67:549–559. doi: 10.1002/hep.29514. [DOI] [PMC free article] [PubMed] [Google Scholar]