Abstract
Background
The population of adults with congenital heart disease (CHD) is growing, and increasingly more patients with CHD reach older ages. Patients with CHD are at an increased risk of myocardial infarction (MI) with increased age. Diagnosing MI in patients with CHD can be challenging in clinical practice owing to a high prevalence of aberrant electrocardiograms, ventricular hypertrophy, and heart failure, among other factors. The National Swedish Patient Register (NPR) is widely used in epidemiological studies; however, MI diagnoses specifically in patients with CHD have never been validated in the NPR.
Methods
We contacted hospitals and medical archive services to request medical records for 249 patients, born during 1970–2012, with both CHD and MI diagnoses and who were randomly selected from the NPR by the Swedish National Board of Health and Welfare. Follow-up was until 2015. We performed a medical chart review to validate the MI diagnoses; we also validated CHD diagnoses to ensure that only patients with confirmed CHD diagnoses were included in the MI validation process.
Results
We received medical records for 96.4% (n = 238/249) of patients for validation of CHD diagnoses. In total, 74.8% (n = 178/238) had a confirmed CHD diagnosis; of these, 70.2% (n = 167) had a fully correct CHD diagnosis in the NPR; a further 4.6% (n = 11) had a CHD diagnosis, but it was misclassified. MI diagnoses were validated in 167 (93.8%) patients with confirmed CHD. Of the patients with confirmed CHD, 88.0% (n = 147/167) had correct MI diagnoses. Patients with non-complex CHD diagnoses had more correct MI diagnoses than patients with complex CHD (91.0%, n = 131 compared with 69.6%, n = 16). The main cause for incorrect MI diagnoses was typographical error, contributing to 50.0% of the incorrect diagnoses.
Conclusions
The validity of MI diagnoses in patients with confirmed CHD in the NPR is high, with nearly 9 of 10 MI diagnoses being correct (88.0%). MI in patients with CHD can safely be studied using the NPR.
Keywords: Myocardial infarction, Congenital heart disease, Validation, Swedish patient register
Background
Congenital heart disease (CHD) is the most common congenital anomaly affecting about 1% of all living born children [1, 2]. Today, more than 90% of children born with CHD survive into adulthood [3–5]; the number of geriatric patients with CHD is also increasing [6, 7]. With increasing life expectancy, patients with CHD are also at risk of acquired cardiovascular diseases, such as myocardial infarction (MI) [8–11]. Published data on the prevalence of MI in patients with CHD are still relatively scarce; however, several observational cohort studies and large registry studies have shown an increased risk of MI and coronary artery disease (CAD) in patients with CHD compared with patients who do not have CHD [8, 12–14].
Healthcare data based on large national administrative registers is increasingly used in many observational studies [8, 12, 15–18], making it possible to include large patient populations to study a wide range of outcomes in a time-effective and cost-effective manner. It is therefore important to validate the diagnoses in these registers, to ensure that the studied diagnoses are correct. The Swedish National Patient Register (NPR) is a nationwide register that is widely used for epidemiological studies [19]. A diagnosis of MI has repeatedly been shown to have a high level of validity in the NPR [15, 20]; however, an MI diagnosis specifically in patients with CHD has not yet been validated in the NPR.
Diagnosing MI in patients with CHD can be challenging in clinical practice for several reasons; patients with CHD often show abnormal electrocardiogram (ECG) patterns, either as a consequence of previous surgeries, right or left ventricular hypertrophy, coronary anomalies, arrhythmias, atrioventricular (AV) node displacement such as in AV canal defect, congenitally corrected transposition of the great arteries, and univentricular hearts [21]; in addition, as heart failure is relatively common in patients with CHD [22–24], cardiac troponin (cTN) levels can be chronically increased. Further, patients with CHD often report relatively high levels of pain/discomfort [25]. It is also possible that CHD patients are at an increased risk of type 2 MI due to vulnerability for coronary embolization (e.g. patients with Fontan circulation), and oxygen supply/demand mismatch because of high prevalence of arrhythmia and heart failure [26–30].
Therefore, the aim of this study was to validate the diagnosis of MI in patients with CHD in the NPR. We also validated the diagnosis of CHD for patients with MI, to ensure that only patients with a confirmed CHD diagnosis were included in the MI validation process.
Methods
Swedish national patient register and cause of death register
The NPR is a nationwide register administered by the Swedish National Board of Health and Welfare. The NPR was funded in 1964 and has had nationwide coverage since 1987. Since 2001, the NPR includes all diagnoses from hospital outpatient clinics; however, diagnoses made in primary care are not included in the register [19]. It is compulsory for hospitals to report to the NPR. Hence, for every hospital admission or outpatient visit, information including the main and complementary diagnoses, admission dates, and hospital and department types are reported to the NPR [19].
The Cause of Death register is also administered by the Swedish National Board of Health and Welfare and contains all causes of death as well as contributory causes [31].
Study population
The Swedish National Board of Health and Welfare randomly selected 600 patients, born between 1930 and 2012, from the NPR and/or Cause of Death register who had a diagnosis of congenital heart or vascular conditions, using the following International Classification of Disease (ICD) codes: ICD-8: 746–747, ICD-9: 745–747, ICD-10: Q20–28 and myocardial infarction or angina pectoris (ICD-8: 410; ICD-9: 410–411B; ICD-10: I20–I21). Follow-up of both CHD and MI diagnoses started in 1970 and went on until 2015. As we mainly aimed to validate MI diagnoses in the contemporary ICD era, only 100 of the 600 selected patients had MI/angina diagnoses according to the ICD-8 or ICD-9 versions.
From the data received from the Swedish National Board of Health and Welfare, we identified all patients with a CHD diagnosis (ICD-8: 746–746.99; 747–747.59, ICD-9: 745A–747E and ICD-10: Q20–Q26 except Q26.5 and Q26.6, which are vena portae anomalies). Among patients with a CHD diagnosis, we then identified all patients with an MI diagnosis (ICD-8 and ICD-9: 410; ICD-10: I21).
We validated only MI diagnoses that were primary diagnoses for patients identified in the hospital discharge register. For patients registered with MI in the outpatient register, we included both primary and secondary/complementary diagnoses of MI.
Additional file 1: Figure S1 shows the flow chart of patient selection. In total, 249 patients with the CHD diagnoses above and at least one MI diagnosis were included in the study.
Data collected for validation process
We contacted the individual hospitals and medical archive services in writing, to request the following information regarding the selected MI admissions: the full medical chart and discharge summary, laboratory test reports, first and last electrocardiogram (ECG) during admission, cardiac ultrasound investigation report, coronary angiogram report, and magnetic resonance imaging (MRI) and computed tomography (CT) data, if performed.
When a patient had several admissions for MI in the register, we validated the most recent MI diagnosis. If the patient had been admitted for angina pectoris, we also requested the medical records for the most recent admission owing to angina pectoris. In cases where the medical records from the most recent MI were missing, we retrieved the records for the next most recent admission for MI, or used information about the requested MI episode from other medical records that we received.
To validate the CHD diagnoses for patients with MI, we requested the medical records for the most recent admission or hospital visit with a CHD diagnosis. We also asked for the CHD operative report and the last cardiac ultrasound and cardiac CT or cardiac MRI report. If several CHD diagnoses were found in the register we retrieved medical records for each of them.
Reminder letters were sent out to hospitals and medical archive services that did not respond to the initial letter, and phone calls were also made to non-responding hospitals and medical archive services.
Validation process
Four of the authors reviewed the medical notes. In unclear cases, the notes were reviewed by the senior cardiologist and discussed until consensus was reached.
Validation of MI diagnoses
For validation of MI, we used the Fourth Universal Definition of Myocardial Infarction (2018) [32], which requires elevated cTN over the 99th percentile with a rising/falling pattern, as well as any of the following: symptoms of myocardial ischemia, new ischemic ECG changes, new Q waves, imaging evidence of new loss of viable myocardium or new regional wall motion abnormality in a pattern consistent with an ischemic etiology or evidence of thrombus formation on coronary angiogram or autopsy.
As our study also included MI diagnoses in the era before the use of cTN, a diagnosis was accepted as correct if stated by the physician in charge and supported by information of symptoms and/or ECG pattern and/or increased biomarkers currently used at the time of diagnosis.
We also accepted MI diagnoses as correct even if the criteria of rising/falling pattern in cTN or other biomarkers was not fulfilled, in cases when it was reasonable not to expect a rising/falling pattern in cTN levels (e.g., patient presented late). In a few cases, we also accepted an MI diagnosis as correct when it was stated in the medical records that the patient had experienced an MI and undergone percutaneous coronary intervention (PCI).
Cases were identified as “correct diagnosis”, “incorrect diagnosis” or “insufficient data in the medical records to validate diagnosis”.
Validation of CHD diagnoses
For patients with several diagnoses of CHD in the register, we validated the main diagnosis. If that diagnosis was correct, the patient was classified as having a correct CHD diagnosis, even if there were other diagnoses in the register that were not correct. Confirmed CHD diagnoses but where the CHD diagnosis was not correct were classified as “misclassified CHD diagnosis”.
If we did not receive the requested CHD medical records and did not find any evidence of a CHD diagnosis in the MI admission medical record or other medical records, and assessed that it is unlikely that the patient has a CHD diagnosis, the CHD diagnosis was considered incorrect. Suspected and unconfirmed diagnoses such as “suspected left to right shunt” on echocardiography were considered incorrect diagnoses. Diagnoses of bicuspid aortic valves (BAV) with any degree of aortic stenosis were considered correct when there was a diagnosis of either BAV or aortic stenosis.
We classified the CHD diagnoses into complex and non-complex CHD diagnoses and used a widely used CHD classification system originally published by Botto et al. [33] and further modified by Liu et al. [18, 34]. Complex CHD diagnoses were defined as conotruncal defects and severe non-conotruncal defects (i.e. lesion group 1 and 2). Conotruncal defects included the following diagnoses with corresponding ICD 8, 9 and 10 codes: Common truncus (ICD codes 746.09, 745A, Q200), aortopulmonary septum defect (ICD codes 746.09, 745A, Q214), transposition of great vessels (ICD codes 746.19, 745B, Q203, Q205), double outlet right ventricle (ICD codes 746.19, 745B, Q201), double outlet left ventricle (ICD codes 746.19, 745B, Q202), tetralogy of Fallot (ICD codes 746.29, 745C, Q213). Severe non-conotruncal defects included endocardial cushion defects (ICD codes 746.47, 746.46, 746.43, 745G, Q212), common ventricle (ICD codes 746.37, 745D, Q204), hypoplastic left heart syndrome (ICD codes 746.74, 746H, Q234). In the complex group we also included patients with pulmonary atresia (ICD codes 746.64, 746A, Q220). Non-complex CHD diagnoses were defined as all other CHD diagnoses not included in the complex CHD group (lesion groups 3–6).
Statistical analyses
R version 3.5.2 was used to perform the statistical analyses (R Foundation for Statistical Computing, Vienna, Austria). Microsoft Excel was used to produce the figures. Categorical data are presented as mean and percentage. Continuous data are presented as mean, standard deviation, and percentage of patients or median and interquartile range (IQR).
Results
CHD diagnoses
In total, we requested medical records for 249 patients with a diagnosis of CHD and MI. The CHD diagnosis was validated in 238 patients (for 9 patients we did not receive the medical records and in further 2 patients the medical records were incomplete).
Figure 1 shows the results of validation of the CHD diagnoses. In total, 74.8% (n = 178/238) of patients had a confirmed CHD diagnosis. Of these, 70.2% (n = 167) of patients had a correct CHD diagnosis and further 4.6% (n = 11) had confirmed CHD but the CHD diagnosis was misclassified. Half of the patients with confirmed CHD had a diagnosis of atrial septal defect (ASD) or patent foramen ovale (PFO); (50.6%, n = 90), with one-fourth of these PFO (26.7%, n = 24). A total 3.9% (n = 7) of patients had a bicuspid aortic valve as the main CHD diagnosis.
Fig. 1.
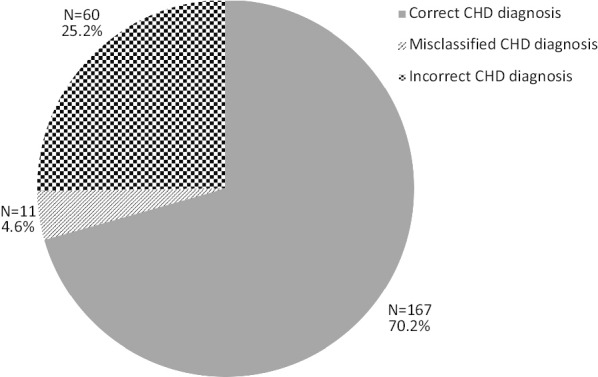
Results of validation of congenital heart disease diagnoses. CHD congenital heart disease
25.2% (n = 60) of patients did not have a CHD diagnosis. The most frequent incorrect CHD diagnosis was VSD (746.39; 745E, Q210), with half of VSD diagnoses being incorrect (50.0%, n = 21). The main reason for this was incorrect assignment of a congenital VSD diagnostic code to patients with post-MI VSD. Also, patients with a valvular disease diagnosis had a high proportion of incorrect diagnoses; of patients with aortic or mitral valvular heart disease diagnoses (including supravalvular aortic stenosis), half (48.5%, n = 16/33) did not have a confirmed congenital lesion.
Slightly more patients with non-complex CHD diagnoses had a correct CHD diagnosis, as compared with patients with complex CHD (71.4%, n = 147 in non-complex CHD compared with 62.5%, n = 20 in complex CHD). Misclassified CHD diagnoses were more common among complex CHD diagnoses. Table 1 shows the distribution of correct, misclassified and incorrect CHD diagnoses grouped according to “non-complex” and “complex” CHD lesion groups.
Table 1.
CHD diagnoses for complex/non-complex CHD diagnoses and number/percentage of correct/incorrect CHD diagnoses per group
| CHD diagnosis | Number of patients | Confirmed CHD | Incorrect CHD diagnosis | |
|---|---|---|---|---|
| Correct CHD diagnosis | Misclassified CHD diagnosis | |||
| All CHD | N = 238 | 167 (70.2%) | 11 (4.6%) | 60 (25.2%) |
| Complex CHD | N = 32 | 20 (62.5%) | 5 (15.6%) | 7 (21.9%) |
| Non-complex CHD | N = 206 | 147 (71.4%) | 6 (2.9%) | 53 (25.2%) |
CHD congenital heart disease
MI diagnoses in patients with confirmed CHD
MI diagnosis was validated in 167 patients with confirmed CHD (median age 58.0 (range 0–85) years, 65.3% male); of the medical records requested for 178 patients with confirmed CHD, we received 169 medical records; however, two of these were incomplete. Most validated MI diagnoses were in the contemporary ICD-10 version (81.4%, n = 136).
Figure 2 shows the results of MI validation in patients with confirmed CHD. Of the 167 patients with confirmed CHD, 88.0% (n = 147/167) had a correct MI diagnosis. Patients with correct MI diagnoses were older than those with incorrect MI diagnoses; median age 59.0 (range 0–85) years in patients with correct MI diagnosis compared with 46.0 (range 0–75) years in patients with incorrect MI diagnosis.
Fig. 2.
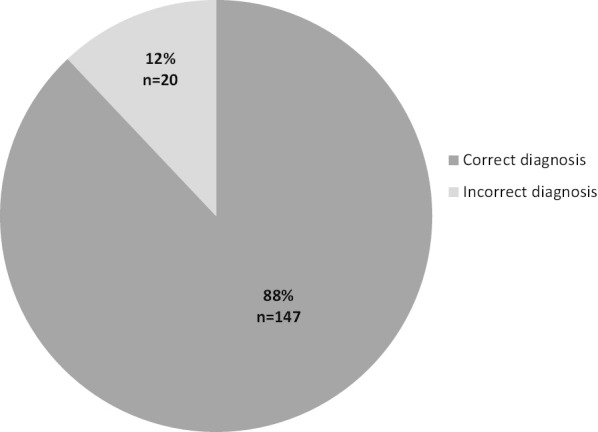
Validation results for myocardial infarction diagnoses in patients with confirmed congenital heart disease
Twenty patients had incorrect MI diagnoses. The main reason for an incorrect MI diagnosis in the register was typographical error (50.0%, n = 10). In a further two cases, the diagnosis in the medical records did not correspond to the diagnosis in the register. Three patients (15.0%) did not fulfill the diagnostic criteria of MI because of either normal cTN levels or cTN without the timely rise and fall required for a correct diagnosis of MI. Other conditions in which an incorrect MI diagnosis was assigned were pericarditis/perimyocarditis (n = 2), Takutsubo cardiomyopathy (n = 1), unstable angina pectoris (n = 1), and hypokinesia on echocardiography related to a previous surgical procedure that included resection of a part of the myocardium (n = 1).
Slightly more patients with incorrect CHD diagnoses had correct MI diagnoses (93.1%, n = 54/58), in comparison with patients with confirmed CHD diagnoses (88.0%, n = 147/167).
Results of MI diagnosis validation in relation to CHD diagnoses
Patients with complex CHD diagnoses had more incorrect MI diagnoses than patients with non-complex CHD. Among patients with complex CHD, only 69.6% (n = 16) had correct MI diagnoses, compared with 91.0% (n = 131) of patients with non-complex CHD. Table 2 shows the distribution of correct and incorrect MI diagnoses in patients with complex and non-complex CHD lesions. Of the 7 patients with complex CHD and incorrect MI diagnosis, 57.1% (n = 4) were due to typographical error.
Table 2.
All confirmed1 CHD diagnoses divided into complex/non-complex CHD lesions, and distribution of correct/incorrect MI diagnoses
| CHD | Number of patients | Correct MI diagnosis | Incorrect MI diagnosis |
|---|---|---|---|
| All CHD | N = 167 | 147 (88.0%) | 20 (12.0%) |
| Complex CHD | N = 23 | 16 (69.6%) | 7 (30.4%) |
| Type 1 MI | 11 (68.8%) | ||
| Type 2 MI | 4 (25.0%) | ||
| Other | 1 (6.2%) | ||
| Non-complex CHD | N = 144 | 131 (91.0%) | 13 (9.0%) |
| Type 1 MI | 96 (73.3%) | ||
| Type 2 MI | 31 (23.7%) | ||
| Other | 4 (3.1%) |
MI myocardial infarction; CHD congenital heart disease
1Confirmed CHD includes correct CHD and misclassified CHD diagnoses
More than half of patients (59.2%, n = 87) had a known CHD diagnosis at the time of MI, and 29 patients (19.7%) were diagnosed with CHD while being investigated for a validated MI episode.
Clinical characteristics of patients with confirmed CHD and correct MI diagnoses
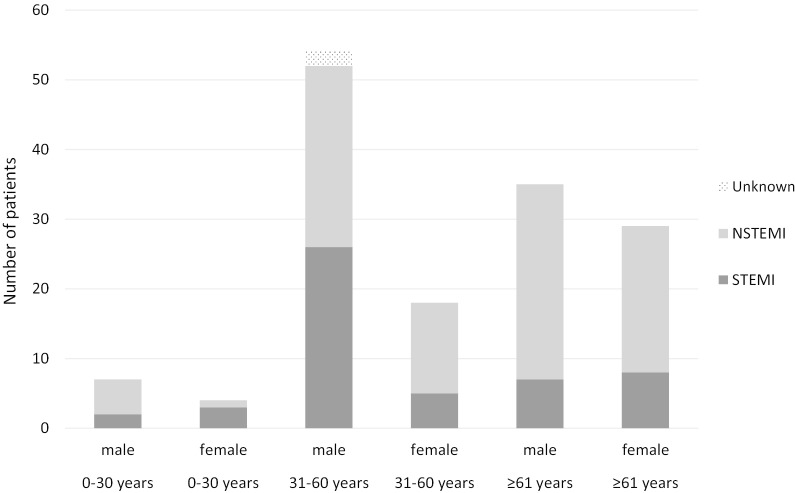
Table 3 describes the characteristics of patients with confirmed CHD and with correct MI and MI-related information. The median age at MI was 59 (range 0–85) years, and 65.3% (n = 96) of patients were male. 34.7% (n = 51 patients) had ST-elevation MI; information of MI type was missing for 2 patients. The proportion of males and females with STEMI/NSTEMI according to age groups is presented in Fig. 3. STEMI occurred in 31.2% (n = 5/16) of the patients with complex CHD diagnoses, compared with 35.1% (n = 46/131) of patients with non-complex CHD. According to information in the medical records, we assessed that 72.8% (n = 107) of patients had MI type 1 and 23.8% (n = 35) had MI type 2.
Table 3.
Baseline data and MI related information in patients with confirmed CHD1 and correct MI diagnoses
| Variable | Total number of patients | Number of patients (%) |
|---|---|---|
| Sex | 147 | |
| Male | 96 (65.3%) | |
| Female | 51 (34.7%) | |
| CHD diagnosis | 147 | |
| ASD secundum/PFO | 73 (49.7%) | |
| VSD | 16 (10.9%) | |
| Other | 58 (39.5%) | |
| Age at MI | 147 | 59 (IQR 50–67) |
| Previous MI or ischemic heart disease | 147 | |
| Yes | 39 (26.5%) | |
| No | 107 (72.8%) | |
| Info missing | 1 (0.7%) | |
| Symptoms | 147 | |
| Typical | 118 (80.3%) | |
| Atypical | 13 (8.8%) | |
| No symptoms | 8 (5.4%) | |
| Info missing | 8 (5.4%) | |
| Cardiac enzymes and biomarkers2 | 147 | |
| Lablist available | 92 (62.6%) | |
| Values only mentioned in text | 40 (27.2%) | |
| Not taken | 4 (2.7%) | |
| Info missing | 11 (7.5%) | |
| Troponin T or I measured | 147 | |
| Yes | 95 (64.6%) | |
| No | 40 (27.2%) | |
| Info missing | 12 (8.2%) | |
| Enzymes/biomarkers elevated | 147 | |
| Yes | 124 (84.4%) | |
| No | 4 (2.7%) | |
| Not taken | 4 (2.7%) | |
| Info missing | 15 (10.2%) | |
| ECG report available | 147 | |
| Yes | 82 (55.8%) | |
| No | 5 (3.4%) | |
| Mentioned in text | 58 (39.5%) | |
| Info missing | 2 (1.4%) | |
| ECG | 140 | |
| ST elevation | 52 (37.1%) | |
| Non-ST elevation (ST-depression, Q-waves, LBBB/RBBB, T-wave inversion) | 71 (50.7%) | |
| Other | 11 (7.9%) | |
| Normal | 6 (4.3%) | |
| CABG | 147 | |
| Yes | 21 (14.3%) | |
| No | 125 (85.0%) | |
| Info missing | 1 (0.7%) | |
| Trombolysis | 147 | |
| Yes | 11 (7.5%) | |
| No | 133 (90.5%) | |
| Info missing | 3 (2.0%) | |
| PCI | 147 | |
| Yes | 46 (31.3%) | |
| No | 99 (67.3%) | |
| Info missing | 2 (1.4%) | |
| Coronary angiogram | 147 | |
| Yes | 95 (64.6%) | |
| No | 49 (33.3%) | |
| Info missing | 3 (2.0%) | |
| Coronary angiogram results3 | 95 | |
| Oclusion in 1 vessel | 35 (36.8%) | |
| Oclusion in 2 vessels | 16 (16.8%) | |
| Oclusion in 3 vessels | 22 (23.2%) | |
| No oclusion | 16 (16.8%) | |
| Other | 5 (5.3%) | |
| Info missing | 1 (1.1%) | |
| MI type as stated in medical records | 147 | |
| Type 1 | 2 (1.4%) | |
| Type 2 | 11 (7.5%) | |
| Other | 2 (1.4%) | |
| Info missing | 132 (89.8%) | |
| Assessment of MI type | 147 | |
| Type 1 | 107 (72.8%) | |
| Type 2 | 35 (23.8%) | |
| Type 3 | 3 (2.0%) | |
| Type 4 | 0 (0.0%) | |
| Type 5 | 2 (1.4%) | |
| Known CHD diagnosis before MI | 147 | |
| Yes | 87 (59.2%) | |
| No | 58 (39.5%) | |
| Info missing | 2 (1.4%) | |
| CHD diagnosed under investigation for MI | 147 | |
| Yes | 29 (19.7%) | |
| No | 116 (78.9%) | |
| Info missing | 2 (1.4%) |
1Confirmed CHD includes correct CHD and misclassified CHD diagnoses
2 TNT/TNI/CK/CK-MB/CK-B/ASAT/ALAT/LD
3defined as > 50% stenosis or mentioning in text “significant stenosis” or “occlusion”
Abbreviations: CHD congenital heart disease, ASD atrial septal defect, PFO patent foramen ovale, VSD ventricular septal defect, MI myocardial infarction, PCI percutaneous coronary intervention, CABG coronary artery bypass grafting, ECG electrocardiogram
Fig. 3.
Number of patients with STEMI and NSTEMI according to age decades groups in patients with confirmed congenital heart disease and correct myocardial infarction diagnoses. STEMI ST-Elevation Myocardial Infarction; NSTEMI Non-ST-Segment Elevation Myocardial Infarction
Cardiovascular risk factors in patients with confirmed CHD and correct MI diagnoses
The most common cardiovascular risk factor in the confirmed CHD population with correct MI was smoking; 25.9% were current smokers (n = 38) and 33.0% (n = 36) smoked previously. Nearly 40% of patients had previously known hypertension (n = 59, 40.1%) and approximately 25% had known hyperlipidemia (n = 36, 24.5%) and diabetes mellitus (n = 35, 23.8%).
Discussion
In the present study, we found that the validity of MI diagnoses in patients with confirmed CHD (median age 58.0 (range 0–85) years, 65.3% male) was high, with nearly 9 of 10 MI diagnoses being correct (88.0%). The main cause for incorrect diagnosis was typographical errors contributing to 50.0% of the incorrect diagnoses; another common reason was not fulfilling the criteria for a rise and fall in cTN /biomarkers.
Hammar et al. found that 86% of MI diagnoses in the NPR between 1987 and 1995 were fully correct [20]. Another validation study of MI diagnosis in the NPR published in 1993 showed that 95.7% of patients had definitive MI [35]. The results of our study are in line with those of previously published studies that have validated MI diagnoses in the NPR. However, comparisons with our study are difficult to make because both of the abovementioned studies were conducted 20–30 years ago, when the diagnostic criteria for MI was different and cTN levels were not widely used. MI diagnosis in the Swedish NPR has not been validated recently; however, two relatively recent validation studies of MI diagnosis in the Danish Patient Register, published in 2009 and 2003, showed similar trends as in our study, with 81.9% [36] and 93.6% [37].
Patients with CHD represent a rapidly growing patient group owing to the recent advancements in both surgical and medical treatment; patients with CHD are also aging. Compared with patients who do not have CHD, the causes of MI in the population with CHD are multifactorial. Apart from true atherosclerotic CAD, MI in patients with CHD can be caused by emboli, reduced blood supply owing to volume/pressure overload, anomalous coronary arteries, scars or manipulation of the coronary arteries during a procedure, such as the arterial switch procedure in neonates [26, 38–40]. Interestingly, in our study we assessed that 23.8% of the patients had MI type 2 which is higher compared with a Swedish cohort, however, lower compared with international cohorts [41, 42].
There are relatively scarce data on MI in patients with CHD; however, two large registry studies have shown that patients with CHD have an increased risk of MI, as compared with patients who did not have CHD [8, 12]. In our study, we found a high validity of MI diagnoses in patients with confirmed CHD in the NPR. It can be implied from our results that the potentially aberrant ECG pattern and chronically increased cTN values in patients with CHD do not significantly decrease the validity of MI diagnosis.
We found that 74.8% of patients with a CHD diagnosis in NPR had confirmed CHD. A significant proportion of the false CHD diagnoses can be attributed to wrongly classifying post-MI VSDs as congenital VSDs. In addition, patients with valvular heart disease of probable degenerative origin often received a diagnosis of CHD. While administrative databases vary in accuracy of CHD diagnoses [43–46] it seems that in our system, the NPR is dependable for diagnosis of MI in patients with CHD.
Our results of MI and CHD validation are in line with studies that have validated other diagnoses in the NPR. Validation of inflammatory bowel disease (IBD) diagnoses showed a 93% positive predictive value (PPV) for any IBD; however, lower PPV was found for specific diagnoses such as Crohn’s disease (72%) and ulcerative colitis (79%) [47]. Validation studies of ankylosing spondylitis showed a PPV of 70%–89% [48]. A validation study of rheumatoid arthritis in the NPR showed a 91% PPV [49], and pancreatitis showed a 83% PPV [50]. Ludvigsson et al. reviewed studies on validation of diagnoses in the NPR and found a PPV of 85%–95% for most diagnoses [15].
Strengths and limitations
One strength of our study is the high generalizability, as we validated MI diagnoses from the entire NPR independently of hospital type or geographic area. Another strength is the low number of missing or unavailable medical records. Further, we validated diagnoses in the ICD-8, ICD-9, and ICD-10 versions, allowing us to validate MI diagnoses in different time periods. This is especially important as the MI diagnostic criteria have changed much during recent decades, with the introduction of highly sensitive cTN.
One limitation of this study was that we did not always have access to all clinical data; for example, some ECGs, laboratory blood test results, and data on onset symptoms were missing. It is therefore possible that a small proportion of patients with unstable angina could have been diagnosed with correct MI. In addition, at times the specifically requested CHD medical record was missing and we relied on information in other medical records that were available to us. Although not likely, it is possible that in those cases, the patient actually could have had a CHD diagnosis, although we did not find any evidence of this in the medical records. Further, we classified uncertain/unconfirmed CHD diagnoses as incorrect, to ensure that we validated MI-only patients with confirmed CHD. It is possible that a few patients who we classified as incorrect CHD might have had a confirmed CHD, if further investigation were undertaken (e.g., patients with a suspected shunt on atrial level that was not further confirmed). Further, as the NPR does not include diagnoses from primary care and outpatient clinics before 2001, it is possible that a few diagnoses of non-complex CHD are not included in the NPR.
Conclusion
Our findings showed that 74.8% of patients with at least one CHD diagnosis had confirmed CHD. Among patients who had a confirmed CHD diagnosis (65.3% male, median age 58.0 (range 0–85)) the validity of an MI diagnosis was high, with nearly 9 of 10 MI diagnoses being correct (88.0%). The main cause for false MI diagnosis was typographical errors, which contributed to half of the false diagnoses. MI in patients with CHD can safely be studied using the NPR.
Supplementary information
Additional file 1. Figure S1: Flow chart of patient selection.
Acknowledgements
We would like to thank all administrative personnel in the hospitals and in the medical archive services around the country for help with retrieving the medical journals. We would also like to thank Kok Wai Giang for help with calculations in R studio. Finally, we thank Analisa Avila, ELS, of Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Abbreviations
- NPR
National Patient Register
- CHD
Congenital heart disease
- MI
Myocardial infarction
- CAD
Coronary artery disease
- ASD
Atrial septal defect
- PFO
Patent foramen ovale
- VSD
Ventricular septal defect
- BAV
Bicuspid aortic valve
- ICD
International Classification of Disease
- IQR
Interquartile range
- ECG
Electrocardiogram
- cTN
Cardiac troponin
- CABG
Coronary artery bypass grafting
- PCI
Percutaneous coronary intervention
- MRI
Magnetic resonance imaging
- CT
Computed tomography
- PPV
Positive predictive value
- IBD
Inflammatory bowel disease
- STEMI
ST-elevation myocardial infarction
- NSTEMI
Non-ST-segment elevation myocardial infarction
Authors' contributions
MF, MD, and ZM planned the study. MF, MD, GHO, and HD performed the medical records review and validation of the diagnoses. MF performed the statistical analyses and wrote the first draft of the manuscript. MD, ZM, PE, GHO, and HD critically reviewed the manuscript. All authors read and approved the final manuscript.
Funding
Open Access funding provided by Gothenburg University Library. This work was funded by the Swedish state under an agreement between the Swedish government and county councils, the ALF Agreement (Grant Number: 236611) and the Swedish Heart‐Lung Foundation (Grant Number: 20090724). The funding sources had no role in the study design, collection, analysis, interpretation of data and in writing the manuscript.
Availability of data and materials
The data generated and/or analyzed in the current study will not be available to the public due to patient confidentiality and risk of patient identification due to small numbers of patients and rare diagnoses. De-identified data can be made available to other researchers from the corresponding author upon reasonable request.
Ethics approval and consent to participate
The study was approved by the Regional Ethical Board in Gothenburg, Sweden (921-16, T302-17, T763-18) and complied with the Declaration of Helsinki. As this study was retrospective in nature, consent to participate was waived by the Regional Ethical Board in Gothenburg.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12872-020-01737-1.
References
- 1.Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39(12):1890–1900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- 2.Khoshnood B, Lelong N, Houyel L, Thieulin AC, Jouannic JM, Magnier S, Delezoide AL, Magny JF, Rambaud C, Bonnet D, et al. Prevalence, timing of diagnosis and mortality of newborns with congenital heart defects: a population-based study. Heart (British Cardiac Society) 2012;98(22):1667–1673. doi: 10.1136/heartjnl-2012-302543. [DOI] [PubMed] [Google Scholar]
- 3.Mandalenakis Z, Rosengren A, Skoglund K, Lappas G, Eriksson P, Dellborg M. Survivorship in children and young adults with congenital heart disease in Sweden. JAMA Intern Med. 2017;177(2):224–230. doi: 10.1001/jamainternmed.2016.7765. [DOI] [PubMed] [Google Scholar]
- 4.Moons P, Bovijn L, Budts W, Belmans A, Gewillig M. Temporal trends in survival to adulthood among patients born with congenital heart disease from 1970 to 1992 in Belgium. Circulation. 2010;122(22):2264–2272. doi: 10.1161/CIRCULATIONAHA.110.946343. [DOI] [PubMed] [Google Scholar]
- 5.Khairy P, Ionescu-Ittu R, Mackie AS, Abrahamowicz M, Pilote L, Marelli AJ. Changing mortality in congenital heart disease. J Am Coll Cardiol. 2010;56(14):1149–1157. doi: 10.1016/j.jacc.2010.03.085. [DOI] [PubMed] [Google Scholar]
- 6.Afilalo J, Therrien J, Pilote L, Ionescu-Ittu R, Martucci G, Marelli AJ. Geriatric congenital heart disease: burden of disease and predictors of mortality. J Am Coll Cardiol. 2011;58(14):1509–1515. doi: 10.1016/j.jacc.2011.06.041. [DOI] [PubMed] [Google Scholar]
- 7.Tutarel O, Kempny A, Alonso-Gonzalez R, Jabbour R, Li W, Uebing A, Dimopoulos K, Swan L, Gatzoulis MA, Diller GP. Congenital heart disease beyond the age of 60: emergence of a new population with high resource utilization, high morbidity, and high mortality. Eur Heart J. 2014;35(11):725–732. doi: 10.1093/eurheartj/eht257. [DOI] [PubMed] [Google Scholar]
- 8.Olsen M, Marino B, Kaltman J, Laursen H, Jakobsen L, Mahle W, Pearson G, Madsen N. Myocardial infarction in adults with congenital heart disease. Am J Cardiol. 2017;120(12):2272–2277. doi: 10.1016/j.amjcard.2017.08.050. [DOI] [PubMed] [Google Scholar]
- 9.Yalonetsky S, Horlick EM, Osten MD, Benson LN, Oechslin EN, Silversides CK. Clinical characteristics of coronary artery disease in adults with congenital heart defects. Int J Cardiol. 2013;164(2):217–220. doi: 10.1016/j.ijcard.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 10.Giannakoulas G, Dimopoulos K, Engel R, Goktekin O, Kucukdurmaz Z, Vatankulu MA, Bedard E, Diller GP, Papaphylactou M, Francis DP, et al. Burden of coronary artery disease in adults with congenital heart disease and its relation to congenital and traditional heart risk factors. Am J Cardiol. 2009;103(10):1445–1450. doi: 10.1016/j.amjcard.2009.01.353. [DOI] [PubMed] [Google Scholar]
- 11.Bokma JP, Zegstroo I, Kuijpers JM, Konings TC, van Kimmenade RRJ, van Melle JP, Kies P, Mulder BJM, Bouma BJ. Factors associated with coronary artery disease and stroke in adults with congenital heart disease. Heart (British Cardiac Society) 2018;104(7):574–580. doi: 10.1136/heartjnl-2017-311620. [DOI] [PubMed] [Google Scholar]
- 12.Fedchenko M, Mandalenakis Z, Rosengren A, Lappas G, Eriksson P, Skoglund K, Dellborg M. Ischemic heart disease in children and young adults with congenital heart disease in Sweden. Int J Cardiol. 2017;248:143–148. doi: 10.1016/j.ijcard.2017.06.120. [DOI] [PubMed] [Google Scholar]
- 13.Lin YS, Liu PH, Wu LS, Chen YM, Chang CJ, Chu PH. Major adverse cardiovascular events in adult congenital heart disease: a population-based follow-up study from Taiwan. BMC Cardiovasc Disord. 2014;14:38. doi: 10.1186/1471-2261-14-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saha P, Potiny P, Rigdon J, Morello M, Tcheandjieu C, Romfh A, Fernandes SM, McElhinney DB, Bernstein D, Lui GK, et al. Substantial cardiovascular morbidity in adults with lower-complexity congenital heart disease. Circulation. 2019;139(16):1889–1899. doi: 10.1161/CIRCULATIONAHA.118.037064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, Heurgren M, Olausson PO. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. doi: 10.1186/1471-2458-11-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sorensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. doi: 10.2147/CLEP.S91125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marelli AJ, Ionescu-Ittu R, Mackie AS, Guo L, Dendukuri N, Kaouache M. Lifetime prevalence of congenital heart disease in the general population from 2000 to 2010. Circulation. 2014;130(9):749–756. doi: 10.1161/CIRCULATIONAHA.113.008396. [DOI] [PubMed] [Google Scholar]
- 18.Liu S, Joseph KS, Lisonkova S, Rouleau J, Van den Hof M, Sauve R, Kramer MS. Canadian Perinatal Surveillance S: association between maternal chronic conditions and congenital heart defects: a population-based cohort study. Circulation. 2013;128(6):583–589. doi: 10.1161/CIRCULATIONAHA.112.001054. [DOI] [PubMed] [Google Scholar]
- 19.National Patient Register [https://www.socialstyrelsen.se/en/statistics-and-data/registers/register-information/the-national-patient-register/]. Retrieved 22 oct 2019
- 20.Hammar N, Alfredsson L, Rosen M, Spetz CL, Kahan T, Ysberg AS. A national record linkage to study acute myocardial infarction incidence and case fatality in Sweden. Int J Epidemiol. 2001;30(Suppl 1):S30–34. doi: 10.1093/ije/30.suppl_1.S30. [DOI] [PubMed] [Google Scholar]
- 21.Khairy P, Marelli AJ. Clinical use of electrocardiography in adults with congenital heart disease. Circulation. 2007;116(23):2734–2746. doi: 10.1161/CIRCULATIONAHA.107.691568. [DOI] [PubMed] [Google Scholar]
- 22.Gilljam T, Mandalenakis Z, Dellborg M, Lappas G, Eriksson P, Skoglund K, Rosengren A. Development of heart failure in young patients with congenital heart disease: a nation-wide cohort study. Open Heart. 2019;6(1):e000858. doi: 10.1136/openhrt-2018-000858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodriguez FH, 3rd, Moodie DS, Parekh DR, Franklin WJ, Morales DL, Zafar F, Adams GJ, Friedman RA, Rossano JW. Outcomes of heart failure-related hospitalization in adults with congenital heart disease in the United States. Congenit Heart Dis. 2013;8(6):513–519. doi: 10.1111/chd.12019. [DOI] [PubMed] [Google Scholar]
- 24.Zomer AC, Vaartjes I, van der Velde ET, de Jong HM, Konings TC, Wagenaar LJ, Heesen WF, Eerens F, Baur LH, Grobbee DE, et al. Heart failure admissions in adults with congenital heart disease; risk factors and prognosis. Int J Cardiol. 2013;168(3):2487–2493. doi: 10.1016/j.ijcard.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Berghammer M, Karlsson J, Ekman I, Eriksson P, Dellborg M. Self-reported health status (EQ-5D) in adults with congenital heart disease. Int J Cardiol. 2013;165(3):537–543. doi: 10.1016/j.ijcard.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Hastings RS, McElhinney DB, Saric M, Ngai C, Skolnick AH. Embolic myocardial infarction in a patient with a Fontan circulation. World J Pediatr Congenit Heart Surg. 2014;5(4):631–634. doi: 10.1177/2150135114540180. [DOI] [PubMed] [Google Scholar]
- 27.Shamoon R, Habib H, Rampal U, Hamdan A, Bikkina M, Shamoon F. A rare case of embolic ST-elevation myocardial infarction in an adult patient with repaired hypoplastic left heart syndrome. World J Pediatr Congenit Heart Surg. 2017;8(4):543–549. doi: 10.1177/2150135116651860. [DOI] [PubMed] [Google Scholar]
- 28.Norozi K, Wessel A, Alpers V, Arnhold JO, Geyer S, Zoege M, Buchhorn R. Incidence and risk distribution of heart failure in adolescents and adults with congenital heart disease after cardiac surgery. Am J Cardiol. 2006;97(8):1238–1243. doi: 10.1016/j.amjcard.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 29.Bouchardy J, Therrien J, Pilote L, Ionescu-Ittu R, Martucci G, Bottega N, Marelli AJ. Atrial arrhythmias in adults with congenital heart disease. Circulation. 2009;120(17):1679–1686. doi: 10.1161/CIRCULATIONAHA.109.866319. [DOI] [PubMed] [Google Scholar]
- 30.Walsh EP, Cecchin F. Arrhythmias in adult patients with congenital heart disease. Circulation. 2007;115(4):534–545. doi: 10.1161/CIRCULATIONAHA.105.592410. [DOI] [PubMed] [Google Scholar]
- 31.Dödsorsaksregistret (Cause of Death Register) [https://www.socialstyrelsen.se/statistik-och-data/register/alla-register/dodsorsaksregistret/] (retrieved 22/10/2019)
- 32.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD. Executive Group on behalf of the Joint European Society of Cardiology /American College of Cardiology /American Heart Association /World Heart Federation Task Force for the Universal Definition of Myocardial I: Fourth Universal Definition of Myocardial Infarction (2018) Circulation. 2018;138(20):e618–e651. doi: 10.1161/CIR.0000000000000617. [DOI] [PubMed] [Google Scholar]
- 33.Botto LD, Lin AE, Riehle-Colarusso T, Malik S, Correa A. National birth defects prevention S: seeking causes: classifying and evaluating congenital heart defects in etiologic studies. Birth Defects Res A Clin Mol Teratol. 2007;79(10):714–727. doi: 10.1002/bdra.20403. [DOI] [PubMed] [Google Scholar]
- 34.Liu S, Joseph KS, Luo W, Leon JA, Lisonkova S, Van den Hof M, Evans J, Lim K, Little J, Sauve R, et al. Effect of folic acid food fortification in canada on congenital heart disease subtypes. Circulation. 2016;134(9):647–655. doi: 10.1161/CIRCULATIONAHA.116.022126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lindblad U, Rastam L, Ranstam J, Peterson M. Validity of register data on acute myocardial infarction and acute stroke: the Skaraborg Hypertension Project. Scand J Soc Med. 1993;21(1):3–9. doi: 10.1177/140349489302100102. [DOI] [PubMed] [Google Scholar]
- 36.Joensen AM, Jensen MK, Overvad K, Dethlefsen C, Schmidt E, Rasmussen L: Predictive values of acute coronary syndrome discharge diagnoses differed in the Danish National Patient Registry. J Clin Epidemiol. 2009, 62. [DOI] [PubMed]
- 37.Madsen M, Davidsen M, Rasmussen S, Abildstrom SZ, Osler M: The validity of the diagnosis of acute myocardial infarction in routine statistics: a comparison of mortality and hospital discharge data with the Danish MONICA registry. J Clin Epidemiol. 2003, 56. [DOI] [PubMed]
- 38.Moll M, Michalak KW, Sobczak-Budlewska K, Moll JA, Kopala M, Szymczyk K, Dryzek P, Moll JJ. Coronary artery anomalies in patients with transposition of the great arteries and their impact on postoperative outcomes. Ann Thorac Surg. 2017;104(5):1620–1628. doi: 10.1016/j.athoracsur.2017.03.078. [DOI] [PubMed] [Google Scholar]
- 39.Ou P, Khraiche D, Celermajer DS, Agnoletti G, Le Quan Sang KH, Thalabard JC, Quintin M, Raisky O, Vouhe P, Sidi D, et al. Mechanisms of coronary complications after the arterial switch for transposition of the great arteries. J Thorac Cardiovasc Surg. 2013;145(5):1263–1269. doi: 10.1016/j.jtcvs.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 40.Lui GK, Fernandes S, McElhinney DB. Management of cardiovascular risk factors in adults with congenital heart disease. J Am Heart Assoc. 2014;3(6):e001076. doi: 10.1161/JAHA.114.001076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raphael CE, Roger VL, Sandoval Y, Singh M, Bell M, Lerman A, Rihal CS, Gersh BJ, Lewis B, Lennon RJ et al: Incidence, Trends and Outcomes of Type 2 Myocardial Infarction in a Community Cohort. Circulation 2020. [DOI] [PMC free article] [PubMed]
- 42.Gard A, Lindahl B, Batra G, Hjort M, Szummer K, Baron T. Diagnosing type 2 myocardial infarction in clinical routine A validation study. Scand Cardiovasc J. 2019;53(5):259–265. doi: 10.1080/14017431.2019.1638961. [DOI] [PubMed] [Google Scholar]
- 43.Khan A, Ramsey K, Ballard C, Armstrong E, Burchill LJ, Menashe V, Pantely G, Broberg CS: Limited accuracy of administrative data for the identification and classification of adult congenital heart disease. J Am Heart Assoc. 2018, 7(2). [DOI] [PMC free article] [PubMed]
- 44.Cohen S, Jannot A-S, Iserin L, Bonnet D, Burgun A, Escudié J-B. Accuracy of claim data in the identification and classification of adults with congenital heart diseases in electronic medical records. Archiv Cardiovasc Dis. 2019;112(1):31–43. doi: 10.1016/j.acvd.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 45.Frohnert BK, Lussky RC, Alms MA, Mendelsohn NJ, Symonik DM, Falken MC. Validity of hospital discharge data for identifying infants with cardiac defects. J Perinatol. 2005;25(11):737–742. doi: 10.1038/sj.jp.7211382. [DOI] [PubMed] [Google Scholar]
- 46.Larsen H, Nielsen GL, Bendsen J, Flint C, Olsen J, Sorensen HT. Predictive value and completeness of the registration of congenital abnormalities in three Danish population-based registries. Scand J Public Health. 2003;31(1):12–16. doi: 10.1080/14034940210134194. [DOI] [PubMed] [Google Scholar]
- 47.Jakobsson GL, Sternegard E, Olen O, Myrelid P, Ljung R, Strid H, Halfvarson J, Ludvigsson JF. Validating inflammatory bowel disease (IBD) in the Swedish National Patient Register and the Swedish Quality Register for IBD (SWIBREG) Scand J Gastroenterol. 2017;52(2):216–221. doi: 10.1080/00365521.2016.1246605. [DOI] [PubMed] [Google Scholar]
- 48.Lindstrom U, Exarchou S, Sigurdardottir V, Sundstrom B, Askling J, Eriksson JK, Forsblad-d'Elia H, Turesson C, Kristensen LE, Jacobsson L. Validity of ankylosing spondylitis and undifferentiated spondyloarthritis diagnoses in the Swedish National Patient Register. Scand J Rheumatol. 2015;44(5):369–376. doi: 10.3109/03009742.2015.1010572. [DOI] [PubMed] [Google Scholar]
- 49.Waldenlind K, Eriksson JK, Grewin B, Askling J. Validation of the rheumatoid arthritis diagnosis in the Swedish National Patient Register: a cohort study from Stockholm County. BMC Musculoskeletal Disorders. 2014;15:432. doi: 10.1186/1471-2474-15-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Razavi D, Ljung R, Lu Y, Andren-Sandberg A, Lindblad M: Reliability of acute pancreatitis diagnosis coding in a National Patient Register: a validation study in Sweden. Pancreatology: Official Journal of the International Association of Pancreatology (IAP) [et al] 2011, 11(5):525–532. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Figure S1: Flow chart of patient selection.
Data Availability Statement
The data generated and/or analyzed in the current study will not be available to the public due to patient confidentiality and risk of patient identification due to small numbers of patients and rare diagnoses. De-identified data can be made available to other researchers from the corresponding author upon reasonable request.