Abstract
Background: Microglia are well known key regulators of neuroinflammation which feature in multiple neurodegenerative disorders. These cells survey the CNS and, under inflammatory conditions, become “activated” through stimulation of toll-like receptors (TLRs), resulting in changes in morphology and production and release of cytokines. In the present study, we examined the roles of the related TAM receptors, Mer and Axl, and of their ligand, Gas6, in the regulation of microglial pro-inflammatory TNF-α production and microglial morphology.
Methods: Primary cultures of murine microglia of wild-type (WT), Mer−/− and Axl−/− backgrounds were stimulated by the TLR4 agonist, lipopolysaccharide (LPS) with or without pre-treatment with Gas6. Gene expression of TNF-α, Mer, and Axl was examined using reverse transcription-quantitative polymerase chain reaction (RT-qPCR), and enzyme-linked immunosorbent assay (ELISA) was used to measure TNF-α release from microglia. Immunofluorescence staining of β-actin and the microglial marker Iba1 was performed to reveal microglial morphological changes, with cellular characteristics (area, perimeter, Feret’s diameter, minimum Feret, roundness, and aspect ratio) being quantified using ImageJ software.
Results: Under basal conditions, TNF-α gene expression was significantly lower in Axl−/− microglia compared to WT cells. However, all microglial cultures robustly responded to LPS stimulation with the upregulation of TNF-α expression to similar degrees. Furthermore, Mer receptor expression was less responsive to LPS stimulation when in Axl knockout cells. The presence of Gas6 consistently inhibited the LPS-induced upregulation of TNF-α in WT, Mer−/− and Axl−/− microglia. Moreover, Gas6 also inhibited LPS-induced changes in the microglial area, perimeter length, and cell roundness in wild-type cells.
Conclusion: Gas6 can negatively regulate the microglial pro-inflammatory response to LPS as well as via stimulation of other TLRs, acting through either of the TAM receptors, Axl and Mer. This finding indicates an interaction between TLR and TAM receptor signaling pathways and reveals an anti-inflammatory role for the TAM ligand, Gas6, which could have therapeutic potential.
Keywords: microglia, Gas6, TAM receptors, toll-like receptors, LPS, inflammation, TNF-α, primary cell culture
Background
Neuroinflammation is a common feature of disorders such as multiple sclerosis or Alzheimer’s disease, among others, occurring at various stages of disease progression (Weggen et al., 2001; Frischer et al., 2009; Heneka et al., 2014; Dendrou et al., 2015). Microglia, the principal immune cells of the CNS, are responsible for surveying the CNS environment in search of damage (Nimmerjahn et al., 2005; Salter and Stevens, 2017). Upon activation, microglia can detect a variety of stimuli through detecting damage- or pathogen-associated molecular patterns (DAMPs or PAMPs, respectively) and respond by creating an inflammatory milieu through the release of cytokines such as TNF-α and IL-1β (Lively and Schlichter, 2018; Subedi et al., 2019). In inflamed tissue, microglia can control the release of pro-inflammatory cytokines, switching to an anti-inflammatory response to promote healing (Heneka et al., 2014). Moreover, microglia undergo morphological changes upon inflammatory stimulation. The transition from their surveillant state to a more “classically” activated phenotype has been well characterized with cells switching from a ramified, extended morphology to a more rounded, amoeboid structure (Djukic et al., 2006; Tam and Ma, 2014; Michell-Robinson et al., 2015; Arcuri et al., 2017). It is this molecular and phenotypic regulation of microglia that may become inefficient during neurodegeneration as chronic inflammation takes hold (Song and Colonna, 2018).
Toll-like receptors (TLRs) are a family of pattern recognition receptors (PRRs), present on the surface of microglia (Bsibsi et al., 2002; Olson and Miller, 2004; Frederiksen et al., 2019). TLR4, for which the gram-negative bacterial cell wall component lipopolysaccharide (LPS) is a strong agonist, is well known for its role in inflammation, providing many opportunities for therapeutics (Jack et al., 2005; Lu et al., 2008; Roy et al., 2016). The TAM (Tyro3, Axl, Mer) subfamily of receptor tyrosine kinases (RTKs) are known to play an important role in the resolution of inflammation, both within and outside the CNS (Lemke and Rothlin, 2008; Shafit-Zagardo et al., 2018; Lee and Chun, 2019). For example, Mer−/− mice treated with LPS show an exacerbated sickness behavior (Camenisch et al., 1999). Furthermore, TAM receptors, along with their ligand Gas6, have been implicated in the negative regulation of pro-inflammatory cytokine release from LPS-stimulated cells (Binder et al., 2008; Alciato et al., 2010).
Within the CNS, TAM receptor expression is highly variable concerning both age and cell type. Microglia have a strong expression of both Axl and Mer receptors but a negligible expression of Tyro3, especially neonatally (Prieto et al., 2000; Shafit-Zagardo et al., 2018; Goudarzi et al., 2020). Although Axl and Mer, along with Gas6, are known to play important roles in the macrophage/microglial inflammatory response, little has been confirmed regarding the specific roles that each receptor plays in the resolution of the response, nor their actions on microglial morphological responses. Therefore, this study aimed to investigate the specific roles of the TAM receptors, Mer and Axl, in regulating TNF-α expression and release in primary mouse microglia as well as to explore the impact of Gas6 on microglial morphological changes under pro-inflammatory conditions. Here, we have used single receptor knockout microglia to study both cytokine release in response to pro-inflammatory stimulation and morphological effects, with and without the influence of ligand stimulation by Gas6. We discovered that both Mer and Axl play important roles in inflammatory resolution and that Gas6 can inhibit a broad range of TLR-mediated inflammatory induction of TNF-α as well as associated morphological changes.
Materials and Methods
Primary Mouse Microglial Cell Cultures
All experimental procedures were performed following the Animals (Scientific Procedures) Act, 1986 under a UK Home Office project license (license number PC2238199) with approval from the institutional ethics committee (AWERB).
Primary microglial cells were derived according to Mecha et al. (2011). In brief, cells were isolated from neonatal (P1–3) wild-type (WT; C57/BL6), Mer mutant (Mer−/−; Jackson Laboratories, Bar Harbor, ME, USA), and Axl mutant (Axl−/−; Jackson Laboratories) mouse brains and cultured in Dulbecco’s Modified Eagle Medium (DMEM; Thermo Fisher Scientific, Loughborough, UK) supplemented with 10% fetal bovine serum (FBS; Lonza, Slough, UK), 10% horse serum (HS; Gibco Invitrogen, Paisley, UK) and 1% penicillin/streptomycin (P/S; Thermo Fisher Scientific) at 37°C for 10–14 days. Cultures were then shaken orbitally at 260 rpm for 3 h to detach the microglia, which were seeded onto poly-D-lysine coated 24-well plates at a cell density of 3 × 105 cells/well for protein/RNA extraction protocols or onto coverslips (circular, 16 mm diameter; Thermo Fisher Scientific) at 4 × 104 cell density for immunofluorescence staining.
Cells were allowed to adhere for 1–2 days before the medium was changed to DMEM containing 1% FBS and 1% P/S into which treatments were added. Pro-inflammatory stimulation was induced by adding LPS (10 ng/ml; Sigma–Aldrich, Gillingham, UK) to cells. Other cell treatments included the TAM receptor ligand, Gas6 (1.6 μg/ml; produced in house as detailed in Stenhoff et al., 2004).
RNA Extraction, Reverse Transcription, and qPCR
After experimental treatments, media was removed from wells, and cells were lysed for total RNA extraction and purification using the RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. RNA concentration and purity were measured using a spectrophotometer (ND-1000; NanoDrop Technologies, Wilmington, DE, USA). Equal amounts of total RNA were reverse transcribed (RT) into cDNA (High Capacity cDNA Reverse Transcription Kit, Applied Biosystems, Foster City, CA, USA) which was used to perform quantitative polymerase chain reaction (qPCR) using gene-specific primer/fluorescent hydrolysis probe sets (Integrated DNA Technologies, IDT, Leuven, Belgium). Gene expression was normalized to Gapdh (Thermo Fisher Scientific) as a reference gene in each sample; relative gene expression was calculated using 2−ΔCt and fold change in expression was calculated using 2−ΔΔ for different experiments, as previously described (Schmittgen and Livak, 2008).
Enzyme-Linked Immunosorbent Assay (ELISA)
Legend Max™ Mouse TNF-α Enzyme-Linked Immunosorbent Assay (ELISA) Kit (BioLegend, San Diego, CA, USA) was used following the manufacturer’s instructions to measure protein levels in cell-conditioned media. In brief, the medium was added to a 96-well plate pre-coated with hamster monoclonal anti-mouse TNF-α capture antibody. After incubation at room temperature for 2 h, samples were washed and the detection antibody was added for a further hour. After further washing, avidin-horseradish peroxidase was used for detection and optical density was measured at 450 nm and 570 nm (Multiskan GO; Thermo Fisher Scientific).
Immunofluorescence Staining
After treatments, media was removed from coverslips by aspiration, and cells were washed twice with PBS, then fixed by incubation with 4% paraformaldehyde (PFA; Sigma–Aldrich) in PBS for 10 min at room temperature. Cells were then washed in PBS (3 × 5 min) before permeabilization with 0.3% Triton X-100 at room temperature for 5 min. Coverslips were washed again (3 × 5 min) before further permeabilization using 100% methanol at −20°C for 10 min. After a final wash, cells were blocked in 5% HS for 1 h.
For staining, coverslips were incubated in primary antibodies made in 1% bovine serum albumin (BSA) solution to appropriate dilutions at 4°C overnight. Primary antibodies were removed, coverslips were washed and secondary antibodies in 1% BSA were added for 1 h at room temperature. Coverslips underwent a final wash in PBS before being mounted onto clear glass microscopy slides using PermaFluor Aqueous Mounting Medium (Thermo Fisher Scientific). Primary antibodies used were rabbit anti-Iba1 (1:300; Fujifilm Wako Pure Chemical Corporation, Japan) and mouse anti-β-actin [1:2,500; Cell Signaling Technologies (CST), London, UK]. Secondary antibodies used were anti-rabbit AlexaFluor 488 (1:1,000; Invitrogen, UK), anti-mouse Cy3 (1:1,000; Invitrogen), and the stain DAPI was used at 1:400 (Invitrogen Molecular Probes). Images were taken on a laser scanning confocal microscope (Zeiss LSM 710, Cambridge, UK).
Image Processing
The program RBS ImageJ was used for all image processing. Firstly, all images were scaled to micrometers and converted to black and white (B&W) images using “Image… Colour Threshold.” Cellular characteristics were then measured using “Analyze…Analyze Particles” where numbered outlines were created for each cell over 100 μm2 that did not cross the image threshold (Supplementary Figure 1) and parameters were automatically measured. Parameters used were area, perimeter, Feret’s diameter, minimum Feret, roundness, and aspect ratio as described in Zanier et al. (2015). A subtype of giant multinucleated phagocytic cells (Fendrick et al., 2007; Hornik et al., 2014) were excluded from data analysis due to the limited number observed in images. Furthermore, extreme data outliers and any cells in contact with others were removed from the analysis. In total, over 300 cells were analyzed for each treatment group.
Statistical Analysis
Statistical analysis was performed using Prism 8 (GraphPad Software, San Diego, CA, USA). The normal distribution of experimental data was determined using the Shapiro–Wilk test. Statistical tests used on normally distributed data were one-way analysis of variance (ANOVA) or paired Student’s t-tests, whereas non-parametric tests used were Kruskal–Wallis, Friedman’s, or Wilcoxon signed-ranked tests. A p ≤ 0.05 was considered as statistically significant. Data analyzed were from a minimum of four independent experiments on separate primary cultures.
Results
Axl Knockout Microglia Show Reduced Baseline TNF-α Expression, but Wild-Type and TAM Knockout Cells Respond Equally to LPS
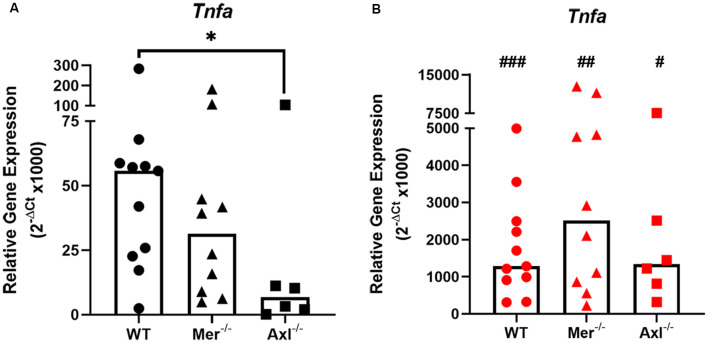
To discover the role of Mer and Axl receptors in intrinsic TNF-α expression in primary microglia, reverse transcription-quantitative polymerase chain reaction (RT-qPCR) was used to measure TNF-α gene expression in non-stimulated microglia of WT, Mer−/− and Axl−/− backgrounds. Under basal conditions, Axl−/− microglia displayed significantly lower TNF-α gene expression when compared with WT microglia, whereas Mer−/− cells displayed no such difference (Figure 1A). LPS (10 ng/ml) addition to WT microglia for 8 h caused a massive upregulation of TNF-α gene expression through TLR4 activation (Roy et al., 2016), which also occurred to the same degree in both Mer−/− and Axl−/− microglia, all differing significantly from basal values (Figure 1B). We also observed this effect with a separate commercial LPS preparation (Supplementary Figure 2), which furthermore showed the TNF-α-inducing effect occurred mainly via distinct TLRs and not all equally.
Figure 1.
Reduced baseline but not an inflammatory-stimulated expression of TNF-α in Mer and Axl deficient microglia. Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) was used to determine the relative gene expression (2−ΔCt) of TNF-α in wild-type (WT), Mer−/− or Axl−/− microglia when (A) unstimulated or (B) stimulated with lipopolysaccharide (LPS; 10 ng/ml) for 8 h. Gapdh was used as a reference gene. Data shown are individual data points with a median bar for n ≥ 6 independent experimental repeats. Statistical significance was determined using Mann-Whitney test comparing Mer−/− or Axl−/− microglia against WT (*) or by using Wilcoxon signed-rank tests to compare pairwise differences between basal and lipopolysaccharide (LPS) values (#); *p < 0.05; #p < 0.05; ##p < 0.01; ###p < 0.005.
Pro-inflammatory Stimulation by LPS Downregulates Mer but Upregulates Axl Expression in Microglia, With Axl Proving Necessary for This Response
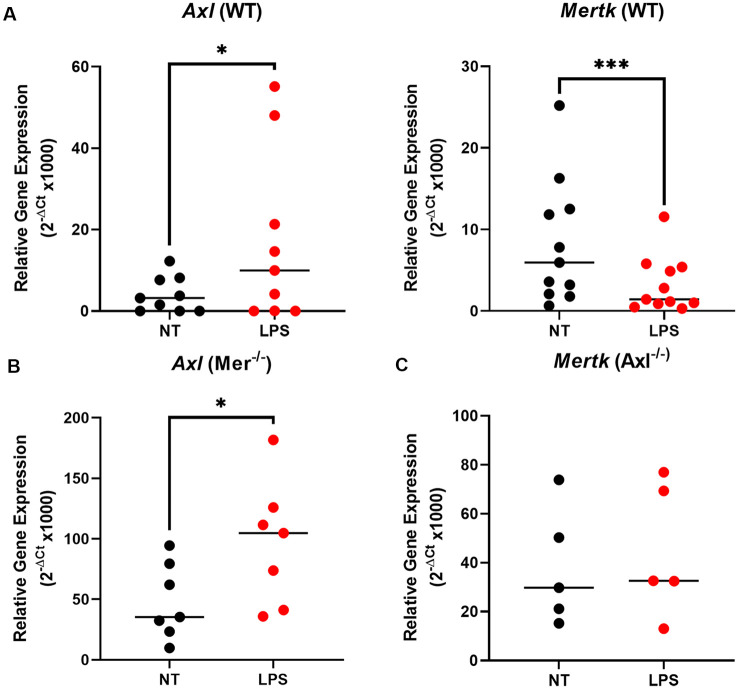
To understand the underlying alterations in TAM receptors in response to LPS, we measured gene expression of Mer and Axl receptors first in WT and then Mer−/− and Axl−/− microglia. In WT microglia, stimulation with LPS (10 ng/ml) for 8 h caused a significant upregulation of Axl expression concomitant with a significant downregulation of Mer (Figure 2A). Interestingly, the LPS-induced upregulation of Axl was also observed in Mer−/− microglia (Figure 2B) whilst, in contrast, LPS did not alter Mer expression in Axl −/− cells (Figure 2C). This response indicates a potential regulatory role of Axl for the expression of Mer in microglia. Furthermore, under baseline, non-LPS-stimulated conditions, single knockout of either Mer or Axl receptor caused a significant increase in the other remaining TAM receptor (Supplementary Figure 3; p = 0.0032, Mer expression for WT vs. Axl−/− baseline; p = 0.0003, Axl expression for WT vs. Mer−/− baseline). This suggests a compensation between the TAM receptors when one is non-functional.
Figure 2.
LPS stimulation alters TAM receptor expression in opposite directions, with Axl regulating the Mer response. After 8 h LPS (10 ng/ml) stimulation or not, RT-qPCR was used to determine the relative gene expression (2−ΔCt) of (A) Axl and Mertk in WT microglia, (B) Axl in Mer−/− microglia, and (C) Mertk in Axl−/− microglia. Gene expression was normalized to Gapdh. Data shown are individual data points with the median line for n ≥ 5 independent experimental repeats. Statistical significance was determined using Wilcoxon signed-rank test; *p < 0.05; ***p < 0.001.
Gas6 Inhibits LPS-Induced TNF-α Upregulation in Both Wild-Type and Knockout Microglia
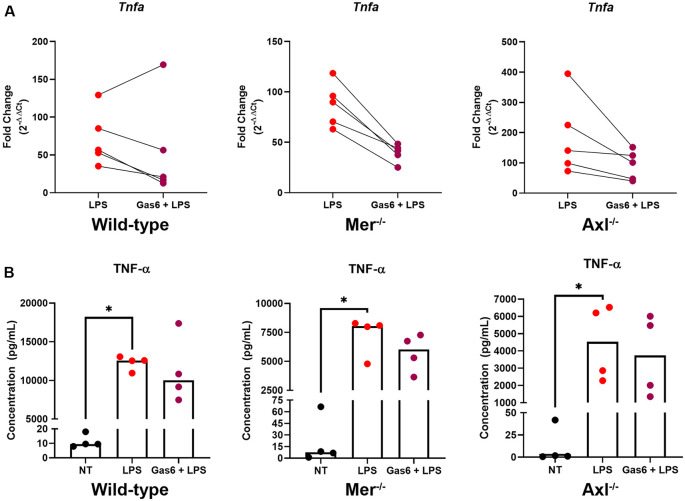
To further distinguish the roles of Mer and Axl receptors, we primed cells with their ligand, Gas6, before stimulating cells with LPS and measuring TNF-α expression. Initially, we confirmed the ability for LPS to almost completely suppress endogenous Gas6 expression in WT microglia (Supplementary Figure 4). This would mean that all experiments under LPS inflammatory conditions using exogenous Gas6 to activate Mer and Axl would be exclusively through exogenous and not endogenous Gas6. Under conditions of LPS stimulation, the presence of Gas6 throughout the 8-h incubation period (including 1 h preceding it) attenuated the upregulation of TNF-α gene expression in both WT and single TAM receptor knockout microglia (Figure 3A). This response occurred in a pairwise trend with the majority of samples showing a substantial down-regulation of gene expression (p = 0.4375, WT LPS vs. LPS + Gas6 vs; p = 0.0625, Mer−/− LPS vs. LPS + Gas6; p = 0.0625, Axl−/− LPS vs. LPS + Gas6). LPS also strongly induced the upregulation of TNF-α at the protein level as measured by ELISA analysis of TNF-α released from cells into the medium (Figure 3B). However, the Gas6 inhibitory effects observed by qPCR were not significant at the protein level. Also, both Axl−/− and Mer−/− cells showed significantly lower levels of LPS-induced TNF-α protein release than WT cells (p = 0.0286, LPS WT vs. LPS Axl−/−; p = 0.0286, LPS WT vs. LPS Mer−/−). The interference of Gas6 in signaling pathways regulating TNF-α expression was not unique to TLR4 activation, as we also observed the same inhibitory effect of Gas6 downstream of other TLR agonists (Supplementary Figure 2).
Figure 3.
Gas6 inhibits TNF-α induction by LPS in WT and single TAM receptor knockout microglia. (A) RT-qPCR was used to determine the fold change (2−ΔΔCt) in TNF-α gene expression in WT, Mer−/− or Axl−/− microglia following 8 h of LPS (10 ng/ml) stimulation with or without 1 h pre-treatment with Gas6 (1.6 μg/ml); fold-change in expression for each experimental repeat (n = 5) is displayed. (B) Enzyme-Linked Immunosorbent Assay(ELISA) measurement of TNF-α protein in culture media under the same experimental conditions as in panel (A), with individual data points from each separate culture and median bar displayed for n = 4 independent experimental repeats. Statistical tests used were (A) Wilcoxon signed-rank tests or (B) Friedman’s tests; *p < 0.05.
Gas6 Limits Changes in Morphological Characteristics in Microglia in Response to LPS Stimulation
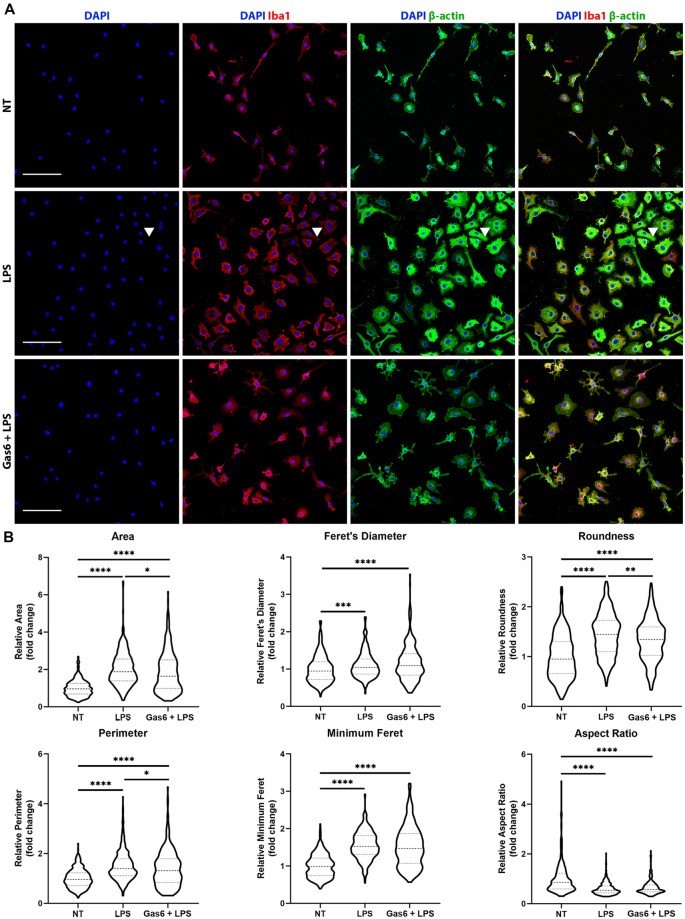
LPS is known to activate microglia to an M1 phenotype, resulting in them becoming more amoeboid in morphology (Tam and Ma, 2014). Here, we confirmed the LPS-induced transition to a more rounded morphology and investigated whether the changes in TNF-α expression observed with Gas6 pre-treatment also translated into morphological changes. WT microglia were seeded onto glass coverslips and treated with LPS (10 ng/ml), with or without 1 h Gas6 (1.6 μg/ml) pre-treatment, for 19 h (Figure 4). DAPI was used for nuclear staining, Iba1 to confirm the purity of microglial cultures, and β-actin to visualize the cytoskeleton and morphology of cells. Data were normalized for each experimental repeat to account for variation among cultures (original data and all images in Supplementary Figure 5). LPS stimulation of microglia had a profound effect on morphology, measured through several parameters (Figure 4B). Moreover, Gas6 pre-treatment of LPS-stimulated microglia significantly reverted the altered morphological parameters area, perimeter, and roundness towards the non-stimulated state, with cells displaying a less pronounced amoeboid shape.
Figure 4.
Gas6 counteracts LPS-induced changes in microglial morphology. (A) Representative images of primary microglial cultures treated with LPS (10 ng/ml for 19 h) with or without 1 h pre-treatment with Gas6 (1.6 μg/ml). Cells were stained with DAPI, Iba1, and β-actin and imaged under fluorescence confocal microscopy. Scale bar −100 μm. Arrowheads indicate a multinucleated cell. (B) Quantification of morphological characteristics (area, Feret’s diameter, roundness, perimeter, minimum Feret, and aspect ratio) for n = 4 individual experimental repeats (n > 300 cells per treatment group). Data normalized to the control no treatment (NT) group is displayed as fold change on violin plots showing median and upper/lower quartiles. Statistical tests used were one-way analysis of variance (ANOVA) with Tukey post hoc analysis; *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
Discussion
In the present study, we have shown that the potent TLR4 agonist, LPS, strongly induced expression of the pro-inflammatory cytokine TNF-α in mouse microglia, in both WT cells and those deficient in either the Mer or Axl receptor. The absence of a single TAM receptor did not influence the ability of microglia to respond to this major pro-inflammatory stimulus, although basal TNF-α expression was reduced in Axl−/− microglia. Interestingly, baseline expression of Mer or Axl was significantly higher in cells in which the other receptor was absent as compared to WT cells, suggesting a compensatory effect. Additionally, we observed that the TAM receptor ligand, Gas6, had an inhibitory effect on LPS-induced microglial TNF-α expression and release, independently of Mer or Axl receptor knockout. Finally, we observed significant alteration of microglial morphology in WT microglia in response to LPS which was also counteracted by Gas6.
The TAM receptor system is well established in immune processes, evident from the increased susceptibility of Mer−/− mice to LPS-induced inflammation (Camenisch et al., 1999) and TAM receptor triple knockout mice displaying autoimmune characteristics (Lu and Lemke, 2001). The effects of TAM receptors on pro-inflammatory responses are often identified through alterations in cytokine expression or changes to intracellular signaling pathways (Rothlin et al., 2007; Ji et al., 2013; Zheng et al., 2015). In keeping with our observations, other studies have also reported the ability of individual TAM receptors to work in concert when regulating the cell’s response to stimuli. For example, Axl and Mer have been shown to work together for optimal clearance of apoptotic cell bodies by microglia (Fourgeaud et al., 2016), and phagocytosis by bone marrow-derived macrophages is dependent on both Mer and Axl kinase activity (Zagórska et al., 2014). Also, recent evidence supports the independent roles of Mer and Axl, whilst working together to suppress inflammatory responses (Zagórska et al., 2020). Furthermore, opposite responses of Mer and Axl to inflammatory conditions have been reported in human myeloid cells (Healy et al., 2016), with the upregulation of Axl being associated with a pro-inflammatory response (Gao et al., 2019).
The present study in microglia also concurs with previous reports of the negative regulatory role of Gas6 in inflammatory signaling in other cells. For example, Gas6 has previously been shown to have a suppressive effect on TLR-mediated pro-inflammatory cytokine production in cardiomyocytes (Grommes et al., 2008), microglial cell lines (Li et al., 2019), mouse macrophages (Deng et al., 2011) and primary murine microglia (Binder et al., 2008). There is growing evidence linking alleviation of inflammation to morphological alterations in microglia which are associated with pro-inflammatory signaling responses and cytokine expression (Zhang et al., 2014, 2019; Kalakh and Mouihate, 2017; Honjoh et al., 2019). This study used WT microglia to observe the potential modulatory effects of Gas6 on microglial morphological characteristics. We did not analyze morphology in TAM single knockout cells as their TNF-α release profiles under LPS conditions were similar to wild-type responses, as were the Gas6 effects on these; therefore, the morphological responses would be expected to be the same.
When measuring TNF-α protein release, the data concurred with the gene expression analysis in some areas but not others. For example, there were no significant differences between the LPS-induced TNF-α gene expression between the three groups of cell cultures, whereas under LPS stimulation, Mer−/− and Axl−/− cells showed lower TNF-α protein release than WT cells. This could be due to a maximal activation by LPS of the Tnfa gene promoter that is too strong to be affected by the lack of a single TAM receptor. In contrast, TNF-α protein release occurs at the end of a multi-level pathway to expression and therefore could be more sensitive to the absence of a TAM receptor through post-transcriptional mechanisms such as protein modification, stability, or trafficking (Vogel and Marcotte, 2012). Also, the lack of difference between baseline TNF-α protein release by WT and Mer−/−/Axl−/− cells could be due to the poorer sensitivity of ELISAs for the detection of low levels of cytokine present in the supernatant, not taking into account cytokines that had been consumed by cells (Amsen et al., 2009). Also, we observed that active stimulation of either the TAM receptor by Gas6 is capable of suppressing the LPS effect at the gene level but not significantly at the protein level. In this case, constant ligand stimulation of TAM signaling pathways appear to have sufficient inhibitory efficacy to interfere with TNF-α gene promoter activation, whereas not sufficiently to affect levels of cytokine accumulated in the medium after a time period. In addition, it was noteworthy that under LPS stimulation conditions, and contrary to resting cells, knockout of a single TAM receptor did not decrease expression of the other. Therefore, single TAM levels were maintained at least at the unstimulated levels and these cells would be responsive to Gas6 stimulation, which can explain why Gas6 was also able to inhibit TNF-α expression in LPS-stimulated Axl−/− cells, as their Mer expression remains robust.
There are other aspects of the TAM system, receptor interactions, and their involvement in inflammation touched on in our work. For example, the distinct responses of Mer and Axl to inflammatory stimuli suggest potential heterodimerization, previously proposed when protein S, a Mer and Tyro3 ligand, was able to stimulate an Axl response (Ray et al., 2017). It is important to know how the different components interact before a full understanding of the TAM receptor system is possible. It is also worth noting that this study focussed solely on Gas6, a ligand for all three TAM receptors, and not protein S (Tsou et al., 2014; Al Kafri and Hafizi, 2019) which has also been purported to have anti-inflammatory functions (Carrera-Silva et al., 2013; Barth et al., 2018). Furthermore, we have shown here that Gas6, via the TAM receptors Axl and Mer, negatively regulates the inflammatory response downstream of multiple TLRs, and other studies are continuing to elucidate these interactions (Wu et al., 2018; Herrera-Rivero et al., 2019; Zahoor et al., 2020). It is vital to probe further to fully clarify how the TAM system is involved in pro-inflammatory cytokine mediation and the underlying signaling pathways involved. This understanding could provide a fresh avenue for the treatment of many debilitating neuroinflammatory disorders.
In this study, we have used the pro-inflammatory cytokine TNF-α as a prominent biomarker to investigate proinflammatory responses (Muhammad, 2019). It is the case however that microglial responses to inflammation involve the complex release and functional interplay of many different cytokines (Lively and Schlichter, 2018). However, our aim here was to gain a clear view of the role of Gas6 in regulating a major pro-inflammatory mechanism, and with future work to be directed at investigating the key signaling pathways and inflammatory markers involved in the TLR response. Also in this study, analysis of TAM receptor protein expression may have supplemented the more sensitive gene expression data; however, the lack of highly sensitive anti-mouse TAM receptor antibodies coupled with the low levels of protein present in the samples precluded a reliable analysis to be done on all the cultures as they were using qPCR. Furthermore, blockade of Gas6 may have been a useful tool to allow us to confirm any ligand-dependent roles of the TAM receptors that occur under basal conditions in microglial cells. However, currently there are no specific Gas6 ligand traps available, and therefore it was not possible to completely negate endogenous Gas6 effects during our experiments. Nevertheless, our data show the clear modulatory role of Gas6 under pro-inflammatory conditions, which are the main findings of this study.
To conclude, we have shown that Gas6, via both TAM receptors Mer and Axl, has a significant negative regulatory influence on the pro-inflammatory response in primary mouse microglia, as observed through TNF-α expression and microglial morphology, and downstream of multiple TLRs. Furthermore, pro-inflammatory conditions alter TAM receptor expression in opposite directions and Axl appears to play a role in the regulation of Mer expression. These findings have expanded the current knowledge on the anti-inflammatory capabilities of Gas6 and TAM receptors in microglia. This new knowledge is of relevance to a greater understanding of the pathophysiology of several neurodegenerative diseases as well as potential novel therapeutic avenues.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Statement
The animal study was reviewed and approved by UK Home Office project licence (number PC2238199); and University of Portsmouth ethics committee (AWERB).
Author Contributions
SH, SG, and SEG conceived and designed the experiments. SEG conducted the experiments, analyzed the results, and drafted the manuscript. SH contributed to data interpretation and manuscript writing. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Glossary
Abbreviations
- ANOVA
analysis of variance
- B&W
black and white
- BSA
bovine serum albumin
- CST
Cell Signaling Technologies
- DAMPs
damage-associated molecular patterns
- DMEM
Dulbecco’s modified eagle medium
- ELISA
enzyme-linked immunosorbent assay
- FBS
fetal bovine serum
- HS
horse serum
- LPS
lipopolysaccharide
- NT
no treatment
- P/S
penicillin/streptomycin
- PAMPs
pathogen-associated molecular patterns
- PFA
paraformaldehyde
- PPRs
pattern recognition receptors
- qPCR
quantitative polymerase chain reaction
- RT
reverse transcription
- RTKs
receptor tyrosine kinases
- TLRs
Toll-like receptors
- WT
wild-type.
Footnotes
Funding. This research was funded by a project grant from the UK Multiple Sclerosis (MS) Society (Award no. 60) and through a University of Portsmouth PhD bursary to SEG.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fncel.2020.576650/full#supplementary-material.
References
- Alciato F., Sainaghi P. P., Sola D., Castello L., Avanzi G. C. (2010). TNF-α, IL-6 and IL-1 expression is inhibited by GAS6 in monocytes/macrophages. J. Leukoc. Biol. 87, 869–875. 10.1189/jlb.0909610 [DOI] [PubMed] [Google Scholar]
- Al Kafri N., Hafizi S. (2019). Tumour-secreted protein S (ProS1) activates a Tyro3-Erk signalling axis and protects cancer cells from apoptosis. Cancers 11:1843. 10.3390/cancers11121843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsen D., de Visser K. E., Town T. (2009). Approaches to determine expression of inflammatory cytokines. Methods Mol. Biol. 511, 107–142. 10.1007/978-1-59745-447-6_5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcuri C., Mecca C., Bianchi R., Giambanco I., Donato R. (2017). The pathophysiological role of microglia in dynamic surveillance, phagocytosis and structural remodeling of the developing CNS. Front. Mol. Neurosci. 10:191. 10.3389/fnmol.2017.00191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth N. D., Marwick J. A., Heeb M. H., Gale A. J., Rossi A. G., Dransfield I. (2018). Augmentation of human monocyte responses to lipopolysaccharide by the protein S and Mer/TYRO3 receptor tyrosine kinase axis. J. Immunol. 201, 2602–2611. 10.4049/jimmunol.1800249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder M. D., Cate H. S., Prieto A. L., Kemper D., Butzkueven H., Gresle M. M., et al. (2008). Gas6 deficiency increases oligodendrocyte loss and microglial activation in response to cuprizone-induced demyelination. J. Neurosci. 28, 5195–5206. 10.1523/JNEUROSCI.1180-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bsibsi M., Ravid R., Gveric D., Van Noort J. M. (2002). Broad expression of toll-like receptors in the human central nervous system. J. Neuropathol. Exp. Neurol. 61, 1013–1021. 10.1093/jnen/61.11.1013 [DOI] [PubMed] [Google Scholar]
- Camenisch T. D., Koller B. H., Earp H. S., Matsushima G. K. (1999). A novel receptor tyrosine kinase, Mer, inhibits TNF-α production and lipopolysaccharide-induced endotoxic shock. J. Immunol. 162, 3498–3503. [PubMed] [Google Scholar]
- Carrera-Silva E. A., Chan P. Y., Joannas L., Errasti A. E., Gagliani N., Bosurgi L., et al. (2013). T cell-derived protein S engages TAM receptor signaling in dendritic cells to control the magnitude of the immune response. Immunity 39, 160–170. 10.1016/j.immuni.2013.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dendrou C. A., Fugger L., Friese M. A. (2015). Immunopathology of multiple sclerosis. Nat. Rev. Immunol. 15, 545–558. 10.1038/nri3871 [DOI] [PubMed] [Google Scholar]
- Deng T., Zhang Y., Chen Q., Yan K., Han D. (2011). Toll-like receptor-mediated inhibition of Gas6 and ProS expression facilitates inflammatory cytokine production in mouse macrophages. Immunology 135, 40–50. 10.1111/j.1365-2567.2011.03511.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djukic M., Mildner A., Schmidt H., Czesnik D., Brück W., Priller J., et al. (2006). Circulating monocytes engraft in the brain, differentiate into microglia and contribute to the pathology following meningitis in mice. Brain 129, 2394–2403. 10.1093/brain/awl206 [DOI] [PubMed] [Google Scholar]
- Fendrick S. E., Xue Q.-S., Streit W. J. (2007). Formation of multinucleated giant cells and microglial degeneration in rats expressing a mutant Cu/Zn superoxide dismutase gene. J. Neuroinflammation 4:9. 10.1186/1742-2094-4-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourgeaud L., Través P. G., Tufail Y., Leal-Bailey H., Lew E. D., Burrola P. G., et al. (2016). TAM receptors regulate multiple features of microglial physiology. Nature 532, 240–244. 10.1038/nature17630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiksen H. R., Haukedal H., Freude K. (2019). Cell type specific expression of toll-like receptors in human brains and implications in Alzheimer’s disease. Biomed Res. Int. 2019:7420189. 10.1155/2019/7420189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischer J. M., Bramow S., Dal-Bianco A., Lucchinetti C. F., Rauschka H., Schmidbauer M., et al. (2009). The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain 132, 1175–1189. 10.1093/brain/awp070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao T., Jernigan J., Raza S. A., Dammer E. B., Xiao H., Seyfried N. T., et al. (2019). Transcriptional regulation of homeostatic and disease-associated-microglial genes by IRF1, LXRβ and CEBPα. Glia 67, 1958–1975. 10.1002/glia.23678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudarzi S., Gilchrist S. E., Hafizi S. (2020). Gas-6 induces myelination through anti-inflammatory IL-10 and TGF-β upregulation in white matter and glia. Cells 9:1779. 10.3390/cells9081779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grommes C., Lee C. Y. D., Wilkinson B. L., Jiang Q., Koenigsknecht-Talboo J. L., Varnum B., et al. (2008). Regulation of microglial phagocytosis and inflammatory gene expression by Gas6 acting on the Axl/Mer family of tyrosine kinases. J. Neuroimmune Pharmacol. 3, 130–140. 10.1007/s11481-007-9090-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy L. M., Perron G., Won S.-Y., Michell-Robinson M. A., Rezk A., Ludwin S. K., et al. (2016). MerTK is a functional regulator of myelin phagocytosis by human myeloid cells. J. Immunol. 196, 3375–3384. 10.4049/jimmunol.1502562 [DOI] [PubMed] [Google Scholar]
- Heneka M. T., Kummer M. P., Latz E. (2014). Innate immune activation in neurodegenerative disease. Nat. Rev. Immunol. 14, 463–477. 10.1038/nri3705 [DOI] [PubMed] [Google Scholar]
- Herrera-Rivero M., Santarelli F., Brosseron F., Kummer M. P., Heneka M. T. (2019). Dysregulation of TLR5 and TAM ligands in the Alzheimer’s brain as contributors to disease progression. Mol. Neurobiol. 56, 6539–6550. 10.1007/s12035-019-1540-3 [DOI] [PubMed] [Google Scholar]
- Honjoh K., Nakajima H., Hirai T., Watanabe S., Matsumine A. (2019). Relationship of inflammatory cytokines from M1-type microglia/macrophages at the injured site and lumbar enlargement with neuropathic pain after spinal cord injury in the CCL21 knockout (plt) mouse. Front. Cell. Neurosci. 13:525. 10.3389/fncel.2019.00525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornik T. C., Neniskyte U., Brown G. C. (2014). Inflammation induces multinucleation of microglia via PKC inhibition of cytokinesis, generating highly phagocytic multinucleated giant cells. J. Neurochem. 128, 650–661. 10.1111/jnc.12477 [DOI] [PubMed] [Google Scholar]
- Jack C. S., Arbour N., Manusow J., Montgrain V., Blain M., McCrea E., et al. (2005). TLR signaling tailors innate immune responses in human microglia and astrocytes. J. Immunol. 175, 4320–4330. 10.4049/jimmunol.175.7.4320 [DOI] [PubMed] [Google Scholar]
- Ji R., Tian S., Lu H. J., Lu Q., Zheng Y., Wang X., et al. (2013). TAM receptors affect adult brain neurogenesis by negative regulation of microglial cell activation. J. Immunol. 191, 6165–6177. 10.4049/jimmunol.1302229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalakh S., Mouihate A. (2017). Androstenediol reduces demyelination-induced axonopathy in the rat corpus callosum: impact on microglial polarization. Front. Cell. Neurosci. 11:49. 10.3389/fncel.2017.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.-H., Chun T. (2019). Anti-Inflammatory role of TAM family of receptor tyrosine kinases via modulating macrophage function. Mol. Cells 42, 1–7. 10.14348/molcells.2018.0419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke G., Rothlin C. V. (2008). Immunobiology of the TAM receptors. Nat. Rev. Immunol. 8, 327–336. 10.1038/nri2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Ye J., Zhao G., Hong G., Hu X., Cao K., et al. (2019). Gas6 attenuates lipopolysaccharide-induced TNF-α expression and apoptosis in H9C2 cells through NF-κB and MAPK inhibition via the Axl/PI3K/Akt pathway. Int. J. Mol. Med. 44, 982–994. 10.3892/ijmm.2019.4275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lively S., Schlichter L. C. (2018). Microglia responses to pro-inflammatory stimuli (LPS, IFNγ+TNFα) and reprogramming by resolving cytokines (IL-4, IL-10). Front. Cell. Neurosci. 12:215. 10.3389/fncel.2018.00215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q., Lemke G. (2001). Homeostatic regulation of the immune system by receptor tyrosine kinases of the Tyro3 family. Science 293, 306–311. 10.1126/science.1061663 [DOI] [PubMed] [Google Scholar]
- Lu Y.-C., Yeh W.-C., Ohashi P. S. (2008). LPS/TLR4 signal transduction pathway. Cytokine 42, 145–151. 10.1016/j.cyto.2008.01.006 [DOI] [PubMed] [Google Scholar]
- Mecha M., Iñigo P. M., Mestre L., Hernangomez M., Borrell J., Guaza C. (2011). An easy and fast way to obtain a high number of glial cells from rat cerebral tissue: a beginners approach. Protoc. Exch. 218:1038 10.1038/protex.2011.218 [DOI] [Google Scholar]
- Michell-Robinson M. A., Touil H., Healy L. M., Owen D. R., Durafourt B. A., Bar-Or A., et al. (2015). Roles of microglia in brain development, tissue maintenance and repair. Brain 138, 1138–1159. 10.1093/brain/awv066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammad M. (2019). “Tumour necrosis factor alpha: a major cytokine of brain neuroinflammation,” in Cytokines, ed Benzadi Payam. (Rijeka, Croatia: IntechOpen; ), 1–14. 10.5772/intechopen.85476 [DOI] [Google Scholar]
- Nimmerjahn A., Kirchhoff F., Helmchen F. (2005). Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308, 1314–1319. 10.1126/science.1110647 [DOI] [PubMed] [Google Scholar]
- Olson J. K., Miller S. D. (2004). Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J. Immunol. 173, 3916–3924. 10.4049/jimmunol.173.6.3916 [DOI] [PubMed] [Google Scholar]
- Prieto A. L., Weber J. L., Lai C. (2000). Expression of the receptor protein-tyrosine kinases Tyro-3, Axl and Mer in the developing rat central nervous system. J. Comp. Neurol. 425, 295–314. [DOI] [PubMed] [Google Scholar]
- Ray A. K., DuBois J. C., Gruber R. C., Guzik H. M., Gulinello M. E., Perumal G., et al. (2017). Loss of Gas6 and Axl signaling results in extensive axonal damage, motor deficits, prolonged neuroinflammation and less remyelination following cuprizone exposure. Glia 65, 2051–2069. 10.1002/glia.23214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothlin C. V., Ghosh S., Zuniga E. I., Oldstone M. B. A., Lemke G. (2007). TAM receptors are pleiotropic inhibitors of the innate immune response. Cell 131, 1124–1136. 10.1016/j.cell.2007.10.034 [DOI] [PubMed] [Google Scholar]
- Roy A., Srivastava M., Saqib U., Liu D., Faisal S. M., Sugathan S., et al. (2016). Potential therapeutic targets for inflammation in toll-like receptor 4 (TLR4)-mediated signaling pathways. Int. Immunopharmacol. 40, 79–89. 10.1016/j.intimp.2016.08.026 [DOI] [PubMed] [Google Scholar]
- Salter M. W., Stevens B. (2017). Microglia emerge as central players in brain disease. Nat. Med. 23, 1018–1027. 10.1038/nm.4397 [DOI] [PubMed] [Google Scholar]
- Schmittgen T. D., Livak K. J. (2008). Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 3, 1101–1108. 10.1038/nprot.2008.73 [DOI] [PubMed] [Google Scholar]
- Shafit-Zagardo B., Gruber R. C., DuBois J. C. (2018). The role of TAM family receptors and ligands in the nervous system: from development to pathobiology. Pharmacol. Ther. 188, 97–117. 10.1016/j.pharmthera.2018.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W. M., Colonna M. (2018). The identity and function of microglia in neurodegeneration. Nat. Immunol. 19, 1048–1058. 10.1038/s41590-018-0212-1 [DOI] [PubMed] [Google Scholar]
- Stenhoff J., Dahlbäck B., Hafizi S. (2004). Vitamin K-dependent Gas6 activates ERK kinase and stimulates growth of cardiac fibroblasts. Biochem. Biophys. Res. Commun. 319, 871–878. 10.1016/j.bbrc.2004.05.070 [DOI] [PubMed] [Google Scholar]
- Subedi L., Lee J. H., Yumnam S., Ji E., Kim S. Y. (2019). Anti-inflammatory effect of sulforaphane on lps-activated microglia potentially through JNK/AP-1/NF-κB inhibition and Nrf2/HO-1 activation. Cells 8:194. 10.3390/cells8020194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam W. Y., Ma C. H. E. (2014). Bipolar/rod-shaped microglia are proliferating microglia with distinct M1/M2 phenotypes. Sci. Rep. 4:7279. 10.1038/srep07279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou W.-I., Nguyen K.-Q. N., Calarese D. A., Garforth S. J., Antes A. L., Smirnov S. V., et al. (2014). Receptor tyrosine kinases, TYRO3, AXL and MER, demonstrate distinct patterns and complex regulation of ligand-induced activation. J. Biol. Chem. 289, 25750–25763. 10.1074/jbc.M114.569020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel C., Marcotte E. M. (2012). Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 13, 227–232. 10.1038/nrg3185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weggen S., Eriksen J. L., Das P., Sagi S. A., Wang R., Pietrzik C. U., et al. (2001). A subset of NSAIDs lower amyloidogenic Aβ42 independently of cyclooxygenase activity. Nature 414, 212–216. 10.1038/35102591 [DOI] [PubMed] [Google Scholar]
- Wu G., McBride D. W., Zhang J. H. (2018). Axl activation attenuates neuroinflammation by inhibiting the TLR/TRAF/NF-κB pathway after MCAO in rats. Neurobiol. Dis. 110, 59–67. 10.1016/j.nbd.2017.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagórska A., Través P. G., Jiménez-García L., Strickland J. D., Tapia F. J., Mayoral R., et al. (2020). Differential TAM receptor regulation of hepatic physiology and injury. Life Science Alliance 3:e202000694 10.26508/lsa.202000694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagórska A., Través P. G., Lew E. D., Dransfield I., Lemke G. (2014). Diversification of TAM receptor tyrosine kinase function. Nat. Immunol. 15, 920–928. 10.1038/ni.2986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahoor A., Yang Y., Yang C., Akhtar M., Guo Y., Shukat A., et al. (2020). Gas6 negatively regulates the staphylococcus aureus-induced inflammatory response via TLR signaling in the mouse mammary gland. J. Cell. Physiol. 235, 7081–7093. 10.1002/jcp.29604 [DOI] [PubMed] [Google Scholar]
- Zanier E. R., Fumagalli S., Perego C., Pischiutta F., De Simoni M.-G. (2015). Shape descriptors of the “never resting” microglia in three different acute brain injury models in mice. Intensive Care Med. Exp. 3:7. 10.1186/s40635-015-0039-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Wei Y.-Z., Wang G.-Q., Li D.-D., Shi J.-S., Zhang F. (2019). Targeting MAPK pathways by naringenin modulates microglia M1/M2 polarization in lipopolysaccharide-stimulated cultures. Front. Cell. Neurosci. 12:531. 10.3389/fncel.2018.00531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.-M., Lund H., Mia S., Parsa R., Harris R. A. (2014). Adoptive transfer of cytokine-induced immunomodulatory adult microglia attenuates experimental autoimmune encephalomyelitis in DBA/1 mice. Glia 62, 804–817. 10.1002/glia.22643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S., Hedl M., Abraham C. (2015). TAM receptor-dependent regulation of SOCS3 and MAPKs contributes to proinflammatory cytokine downregulation following chronic NOD2 stimulation of human macrophages. J. Immunol. 194, 1928–1937. 10.4049/jimmunol.1401933 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.