Abstract
Uvulifer Yamaguti, 1934, is a genus of diplostomoidean digeneans that parasitizes kingfishers worldwide. Species have a Neascus-type metacercaria that encysts in or on fish intermediate hosts, often causing black spot disease. Only 3 prior studies published DNA sequence data for Uvulifer species with only 1 including a single named species (Uvulifer spinatus López-Jiménez, Pérez-Ponce de León, & García-Varela, 2018). Herein we describe 2 new species of Uvulifer from the green-and-rufous kingfisher, Chloroceryle inda (Linnaeus), collected in Peru (Uvulifer batesi n. sp. and Uvulifer pequenae n. sp.). Both new species are readily differentiated from their New World congeners by a combination of morphological characters including distribution of vitelline follicles and prosoma:opisthosoma length ratios. In addition, we used newly generated nuclear 28S rRNA and mitochondrial COI gene sequence data to differentiate among species and examine phylogenetic affinities of Uvulifer. This includes the 2 new species and Uvulifer ambloplitis (Hughes, 1927), as well as Uvulifer elongatus Dubois, 1988, Uvulifer prosocotyle (Lutz, 1928), and Uvulifer weberi Dubois, 1985, none of which have been part of prior molecular phylogenetic studies. Our data on Uvulifer revealed 0.1–2.2% interspecific divergence in 28S sequences and 9.3–15.3% in COI sequences. Our 28S phylogeny revealed at least 6 well-supported clades within the genus. In contrast, the branch topology in the COI phylogenetic tree was overall less supported, indicating that although COI sequences are a great tool for species differentiation, they should be used with caution for phylogenetic inference at higher taxonomic levels. Our 28S phylogeny did not reveal any clear patterns of host association between Uvulifer and particular species of kingfishers; however, it identified 2 well-supported clades uniting Uvulifer species from distant geographical locations and more than 1 biogeographic realm, indicating at least 2 independent dispersal events in the evolutionary history of the New World Uvulifer. Our results clearly demonstrate that the diversity of Uvulifer in the New World has been underestimated.
KEY WORDS: Diplostomidae, Molecular Phylogeny, Uvulifer batesi n. sp, Uvulifer pequenae n. sp, Uvulifer ambloplitis, Chloroceryle inda, Kingfishers, Amazon, Peru, Brazil
The digenean genus Uvulifer Yamaguti, 1934 (Diplostomidae: Crassiphialinae), contains between 16 and 19 species worldwide, with the majority of the species parasitic in kingfishers (see Dubois, 1964; Yamaguti, 1971; Subair et al., 2013). The known life cycles for species of Uvulifer have a Neascus-type metacercaria that encysts on an aquatic vertebrate intermediate host, normally a fish (Hunter, 1933; Niewiadomska, 2002). Often the metacercariae become melanized by the fish host, which manifests as black spot disease (Niewiadomska, 2002; McAllister et al., 2013). Prior to this study, 6 valid species of Uvulifer were recognized from the Americas. Two of these species are distributed only in the Nearctic, 3 are distributed only in the Neotropics, and 1 species is distributed in both the Nearctic and Neotropics (Dubois, 1938, 1985, 1988; Muzzall et al., 2011; López-Jiménez et al., 2018). Uvulifer ambloplitis (Hughes, 1927) and Uvulifer semicircumcisus Dubois and Rausch, 1950, infect the belted kingfisher, Megaceryle alcyon (Linnaeus), in North America (Hunter, 1933; Dubois and Rausch, 1950). Uvulifer prosocotyle (Lutz, 1928) was reported from the ringed kingfisher, Megaceryle torquata Linnaeus, in Brazil and the Amazon kingfisher, Chloroceryle amazona (Latham), in Venezuela (Dubois, 1938; Caballero and Diaz-Ungria, 1958). Uvulifer weberi Dubois, 1985, is known from C. amazona, the green kingfisher, Chloroceryle americana (Gmelin), and the green-and-rufous kingfisher, Chloroceryle inda (Linnaeus), in Paraguay (Dubois, 1985, 1988). Uvulifer elongatus Dubois, 1988, was described from M. torquata in Paraguay (Dubois, 1988), and Uvulifer spinatus López-Jiménez, Pérez-Ponce de León, and García-Varela, 2018, was recently described from C. americana in Mexico and is also found in Guatemala, Honduras, and Nicaragua (López-Jiménez et al., 2018).
In the present study, we describe 2 previously unknown species of Uvulifer from C. inda in the Cordillera Azul National Park, Peruvian Amazon. We generated partial sequences of the nuclear large subunit ribosomal RNA gene (28S) and the mitochondrial cytochrome oxidase 1 gene (COI) from both new species and 5 additional species of Uvulifer collected from various kingfishers from South and North America and a fish from North America. Newly generated sequences were aligned and compared, and observed differences were used for augmenting morphological comparisons among species. Phylogenetic analyses were conducted independently for both gene fragments using new sequence data plus available congeneric sequence data from GenBank.
MATERIALS AND METHODS
Adult specimens belonging to the genus Uvulifer were obtained from C. inda collected in the Cordillera Azul National Park, Peru, C. americana and M. torquata from Pantanal, Fazenda Retiro Novo, Municipality of Poconé, Mato Grosso State, Brazil, M. torquata from the vicinities of Lábrea, state of Amazonas, Brazil, and M. alcyon from Minnesota. In addition, a metacercaria of Uvulifer sp. was collected from a yellow perch, Perca flavescens Mitchill, from Minnesota. Live digeneans removed from the hosts were briefly rinsed in saline, killed with hot water, and preserved in 80% ethanol. Specimens for light microscopy were stained with aqueous alum carmine or Mayer's hematoxylin following Lutz et al. (2017), dehydrated in an ethanol series of ascending concentration, cleared in clove oil, and mounted permanently in Damar gum. Specimens were identified and measured using an Olympus© BX53 microscope (Olympus America, Center Valley, Pennsylvania) equipped with a drawing tube and a digital imaging system operated through iSolution Lite software (Image & Microscope Technology Inc., Vancouver, British Columbia, Canada). All measurements given in the text are in micrometers unless otherwise stated. Type specimens of the new species and adult Uvulifer spp. are deposited in the collection of the Harold W. Manter Laboratory (HWML), University of Nebraska State Museum, Lincoln, Nebraska. We use the terms prosoma and opisthosoma instead of the often used anterior and posterior segments to reflect the fact that these parts of the body in diplostomoideans are not segments (e.g., unlike segments or proglottides in cestodes).
Genomic DNA was extracted from 1 whole individual of each of the new species using the methods described by Tkach and Pawlowski (1999). An approximate 1,300-bp-long fragment at the 5′ end of the 28S rDNA gene (including variable domains D1–D3) was amplified from genomic DNA using the polymerase chain reaction (PCR) protocols in Tkach et al. (2003) and Tkach and Curran (2015), with the same primers used by Tkach and Curran (2015). A fragment of the mitochondrial cytochrome c oxidase subunit 1 (COI) gene was amplified using the previously published forward primer Cox1_Schist_5′ (5′−TCT TTR GAT CAT AAG CG−3′) and reverse primers acox650R (5′−CCA AAA AAC CAA AAC ATA TGC TG−3′) or JB5 (5′−AGC ACC TAA ACT TAA AAC ATA ATG AAA ATG−3′) (Lockyer et al., 2003; Derycke et al. 2005; Kudlai et al., 2015). In some cases, COI was amplified in 2 overlapping fragments using a combination of published primers and new internal primers designed for this study by TJA. The forward primer Cox1_Schist_5′ was used with the new reverse primer BS_CO1_IntR (5′−TAA TAC GAC TCA CTA TAA AAA AAA MAM AGA AGA RAA MAC MGT AGT AAT−3′); the new forward primer BS_CO1_IntF (5′−ATT AAC CCT CAC TAA ATG ATT TTT TTY TTT YTR ATG CC−3′) was used with the reverse primer acox650R. The underlined portions indicate a shortened T3 and T7 tail sequence.
PCR products were purified using the ExoSap PCR clean-up enzymatic kit from Affymetrix (Santa Clara, California) following the manufacturer's protocol. PCR products were cycle-sequenced directly using BrightDye® Terminator Cycle Sequencing Kit (MCLAB, San Francisco, California) chemistry, alcohol precipitated, and run on an ABI 3130 automated capillary sequencer (Life Technologies, Grand Island, New York).
PCR primers and the additional internal forward primer DPL600F (5′−CGG AGT GGT CAC CAC GAC CG−3′) and reverse primer DPL700R (5′−CAG CTG ATT ACA CCC AAA G−3′) were used for sequencing of 28S PCR reactions (Achatz et al., 2019). The PCR primers were used for sequencing of COI PCR reactions. In addition, the shortened T3 tail (5′−ATT AAC CCT CAC TAA A−3′) and shortened T7 tail (5′−TAA TAC GAC TCA CTA TA−3′) primers from Van Steenkiste et al. (2015) were used for sequencing of the PCR reactions prepared with BS_COI_IntF and BS_COI_IntR primers. Contiguous sequences were assembled using Sequencher version 4.2 software (GeneCodes Corp., Ann Arbor, Michigan). Newly generated sequences are deposited in GenBank (Table I).
Table I.
List of diplostomid species used in our phylogenetic analyses of 28S rDNA and COI mtDNA including their host species, geographical origin of material, morphological voucher numbers, and GenBank accession numbers. CNHE: Colección Nacional de Helmintos, Instituto de Biología, Universidad Nacional Autónoma de México, Mexico City. Specimen 98.01_BLV is deposited in the collection of the Biodiversity Institute of Ontario.
| Digenean taxa |
Host species |
Geographic origin |
Museum no. |
Accession no. |
Reference |
|
|
28S |
COI |
|||||
| Ornithodiplostomum scardinii | Scardinius erythrophthalmus | Czech Republic | − | KX931427 | KX931425 | Stoyanov et al., 2017 |
| Posthodiplostomum centrarchi | Ardea herodias | Canada | 98.01_BLV | − | MH581291 | Locke et al., 2018 |
| Uvulifer ambloplitis | Megaceryle alcyon | U.S.A. | HWML-139982 | MK874320 | MK871329 | Present study |
| Uvulifer batesi n. sp. | Chloroceryle inda | Peru | HWML-139983, HWML-139984 | MK874321 | MK871330 | Present study |
| Uvulifer elongatus | Megaceryle torquata | Lábrea, Brazil | − | MK874322 | MK871331 | Present study |
| U. elongatus | M. torquata | Pantanal, Brazil | HWML-139985 | MK874323 | MK871332 | Present study |
| Uvulifer pequenae n. sp. | C. inda | Peru | HWML-139986, HWML-139987 | MK874324 | MK871333 | Present study |
| Uvulifer prosocotyle | M. torquata | Pantanal, Brazil | HWML-139988 | MK874325 | MK871334 | Present study |
| Uvulifer spinatus | Poecilia mexicana | Mexico | CNHE: 10322−10324 | MF568582 | − | López-Jiménez et al., 2018 |
| Uvulifer weberi | Chloroceryle americana | Pantanal, Brazil | HWML-139989 | MK874326 | MK871335 | Present study |
| Uvulifer sp. | Lepomis gibbosus | Canada | − | − | MF124281 | Blasco-Costa and Locke, 2017 |
| Uvulifer sp. | M. alcyon | Mexico | − | MF398332 | − | Hernández-Mena et al., 2017 |
| Uvulifer sp. | M. alcyon | Mexico | − | MF568569 | − | López-Jiménez et al., 2018 |
| Uvulifer sp. | Poecilia sp. | Mexico | − | MF568674 | − | López-Jiménez et al., 2018 |
| Uvulifer sp. | Amatitlania nigrofasciata | Mexico | − | MF568575 | − | López-Jiménez et al., 2018 |
| Uvulifer sp. | Tilapia sparrmanii | South Africa | − | MK604825 | − | Hoogendoorn et al., 2019 |
| Uvulifer sp. | Perca flavescens | U.S.A. | − | MK874327 | MK871336 | Present study |
Phylogenetic interrelationships among members of Uvulifer were analyzed using 28S and COI datasets as separate alignments. Newly obtained and previously published sequences were aligned with Clustal W (Larkin et al., 2007) as implemented in BioEdit version 7.0.5.3 software (Hall, 1999); both alignments were trimmed to the length of the shortest respective sequence. Ornithodiplostomum scardinii (Shulman, 1952) was used as an outgroup in the 28S analysis, and O. scardinii and Posthodiplostomum centrarchi Hoffman, 1958, were used in CO1 analysis based on the topologies presented in the phylogenetic study by López-Jiménez et al. (2018).
The 28S alignment included newly generated sequences of 7 species of Uvulifer and previously published sequences of 6 species-level lineages of Uvulifer, only 1 of them (U. spinatus) representing an identified species. The COI alignment included newly generated sequences of 7 species of Uvulifer and a single previously published compatible sequence of Uvulifer sp. Additional COI sequences of Uvulifer available in GenBank were non-compatible with our sequences or were much shorter in length.
Independent phylogenetic analyses (separate 28S rRNA and COI gene alignments) were conducted using Bayesian Inference (BI) as implemented in MrBayes Ver. 3.2.6 software (Ronquist and Huelsenbeck, 2003). The general time-reversible model with estimates of invariant sites and gamma-distributed among-site variation (GTR + I + G) was identified as the best-fitting nucleotide substitution model for the 28S dataset using Mega7 (Kumar et al., 2016). The Hasegawa-Kishino-Yano and gamma-distributed among-site variation (HKY + G) model was identified as the best-fitting nucleotide substitution model for each of the partitioned nucleotide codon position. BI analyses were performed using MrBayes software as follows: Markov chain Monte Carlo (MCMC) chains were run for 3,000,000 generations with a sample frequency of 1,000, log-likelihood scores were plotted, and only the final 75% of trees were used to produce the consensus trees by setting the “burn-in” parameter at 750. This number of generations was considered sufficient because the SD dropped below 0.01. The trees were visualized in FigTree ver. 1.4 software (Rambaut, 2016) and annotated in Adobe Illustrator®.
DESCRIPTION
Uvulifer pequenae n. sp.
Figures 1, 2.
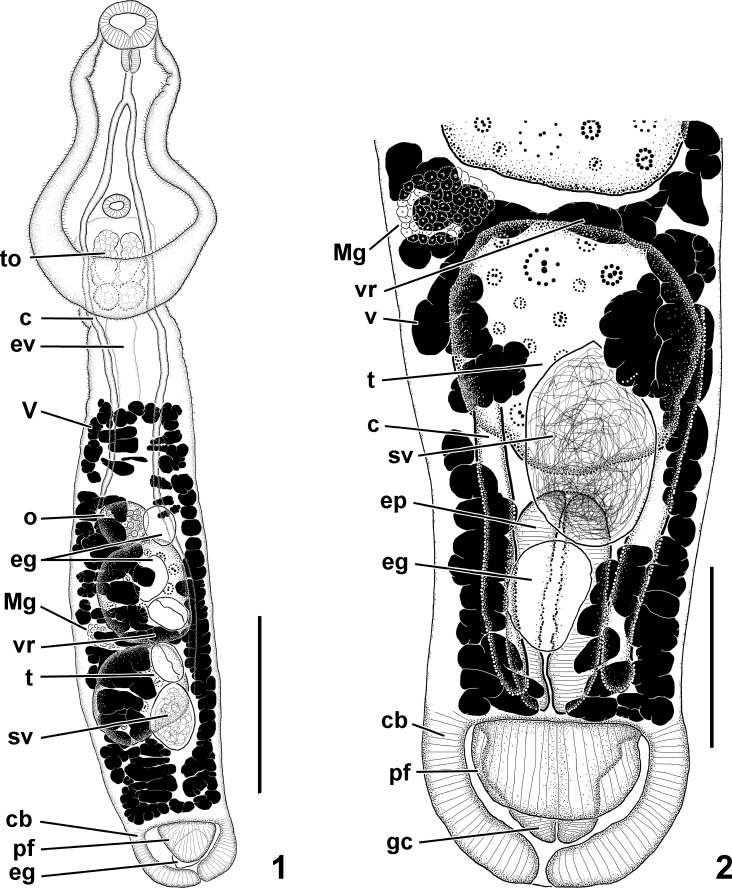
Uvulifer pequenae n. sp. (1) Ventral view of whole mount. Scale bar = 300 μm. (2) Ventral view of posterior body end. Scale bar = 100 μm. Abbreviations: c, ceca; cb, copulatory bursa; eg, egg; ep, ejaculatory pouch; ev, excretory vesicle; gc, genital cone; Mg, Mehlis' gland; o, ovary; pf, preputial fold; sv, seminal vesicle; t, testis; to, tribocytic organ; v, vitelline follicle; vr, vitelline reservoir.
Description (based on 2 fully mature specimens):
Body 1,403–1,432 long, comprising a prosoma and opisthosoma; prosoma pyriform, ventrally concave, 480–517 long, with maximum width in the posterior half (304–318); opisthosoma elongated, 922–932 long and claviform with maximum width near midpoint (202–236). Prosoma: opisthosoma length ratio 0.54–0.57. Tegumental spines covering prosoma but limited to anterior 25% of opisthosoma. Oral sucker nearly terminal, 68–77 × 88–99. Prepharynx absent or not apparent. Pharynx oval, 45–56 × 34–37. Esophagus slightly longer than pharynx. Cecal bifurcation in anterior third of prosoma. Ceca slender, blind, extending to near posterior end of opisthosoma. Ventral sucker delicate, much smaller than oral sucker, 39–40 × 45–48, located at 60–62% of the prosoma length from the anterior end. Tribocytic organ immediately posterior to ventral sucker (72% of the prosoma length from the anterior end); oval with ventral muscular portion having a longitudinal slit-like opening and basal glandular portion embedded in the prosoma, 133–136 × 99–114. Testes tandem, with smooth or slightly irregular margins, anterior testis 167–173 × 142–156, posterior testis 97–153 × 77–82. Seminal vesicle subglobular, ventral to posterior testis, connected to ejaculatory duct; proximal ejaculatory duct tubular and running antero-dorsally, then bending and running posteriorly; distal portion opening into a muscular ejaculatory pouch. Ejaculatory pouch 142–156 × 71–85, draining posteriorly through narrow short male duct posteriorly; duct uniting with female system. Ovary submedian, (slightly dextral), immediately pretesticular (32% of the opisthosoma length from the anterior end), subspherical, 79–85 × 82–91. Ootype surrounded by Mehlis' gland, submedian, (slightly dextral), intertesticular. Seminal receptacle subspherical, immediately dorsal to ootype, smaller than ovary. Uterus ventral in opisthosoma, extending from ovarian level to posterior margin of posterior testis, containing from 2 to 5 eggs (71–81 × 46–57); distal uterus uniting with male duct and forming hermaphroditic canal; hermaphroditic canal descending into genital cone. Genital cone 60–65 × 94–97, extends into a bulbous copulatory bursa; copulatory bursa with muscular ventral preputial fold. Ventrolateral preputial lobe 45–65 × 82–94. Vitelline follicles located in opisthosoma, ventral and lateral to gonads, absent in the anterior 13–16% of the opisthosoma and posterior 11–12% of opisthosoma. Vitelline reservoir intertesticular, sinistral to ootype. Excretory vesicle I-shaped, with main stem dorsal in opisthosoma; stem ascending into prosoma and surrounding tribocytic organ and giving rise to 6 longitudinal branches that extend toward oral sucker; branches interconnected by network of anastomosing channels throughout prosoma. Excretory pore not observed.
Taxonomic summary
Type host:
Chloroceryle inda (Linnaeus) (Coraciiformes: Alcedinidae).
Site of infection:
Small intestine.
Type locality:
San Martín, Tocache Province, Cordillera Azul National Park, Río Pescadero, NE of Shapaja (8°10.694′S, 76°13.422′W), Peru, elev. 953 m above sea level.
Type specimens deposited:
The type series consists of 2 fully mature specimens deposited in the Harold W. Manter Laboratory. Holotype: HWML 139986, labeled ex. C. inda, small intestine, Cordillera Azul National Park, Peru, 13 Nov 2013, coll. K. Patitucci; paratype: HWML 139987, label identical to the holotype. Symbiotype deposited in the Field Museum, Chicago (FMNH 3859910).
Representative DNA sequences:
ZooBank registration:
urn:lsid:zoobank.org:act:ED554410-BFDC-4FBD-AC4B-38A4BAF9213D
Etymology:
The species is named after Tatiana Z. Pequeño Saco who provided invaluable assistance in organizing the field collecting in the Cordillera Azul.
Remarks
The new species clearly belongs to Uvulifer based on the combination of characteristic features that include the vitelline follicles confined to the opisthosoma, the presence of a muscular ejaculatory pouch, and a muscular copulatory bursa containing a retractile or protrusible genital cone partially surrounded by a ventrolateral preputial muscular fold (Niewiadomska, 2002).
We believe only mature specimens of Uvulifer should be used for reliable morphological identification. Uvulifer pequenae is distinguishable from U. elongatus, U. semicircumcisus, U. spinatus, and U. weberi by relatively shorter vitellarium. The vitellarium of all these 4 species occupies almost the whole length of the opisthosoma, whereas in U. pequenae it is absent in the first 13–16% of the opisthosoma. The new species also differs from these 4 species by a greater prosoma:opisthosoma length ratio (see below).
Uvulifer pequenae can be further distinguished from U. elongatus by a much shorter body length (1,403–1,432 in the new species vs. 2,200–3,300 in U. elongatus), a much smaller ventral sucker (39–40 × 45–48 in the new species vs. 85–100 × 100–120 in U. elongatus), and slightly smaller eggs (71–81 in the new species vs. 80–90 in U. elongatus). The most dramatic difference between U. pequenae and U. elongatus is seen in the prosoma:opisthosoma length ratio. It equals 0.54–0.57 in the new species vs. only 0.17–0.19 in our well-fixed specimens of U. elongatus and 0.21 based on our measurements of the original line drawing of the type-specimen. Furthermore, 28S sequences are 0.9% different and COI sequences are 13.3% different between the 2 species.
Uvulifer pequenae can be further distinguished from U. semicircumcisus by somewhat smaller eggs (71–81 in the new species vs. 80–102 in U. semicircumcisus). The prosoma:opisthosoma length ratio in U. pequenae is also larger compared to U. semicircumcisus (0.54–0.57 in the new species vs. 0.28–0.41 in U. semicircumcisus). Additionally, U. semicircumcisus has been reported only from North America, whereas this new species is from the Peruvian Amazon.
Uvulifer pequenae can be further distinguished from U. spinatus by a larger ventral sucker (39–40 × 45–48 in the new species vs. 21–28 × 28–35 in U. spinatus). The prosoma:opisthosoma length ratio in U. pequenae is also larger compared to U. spinatus (0.54–0.57 in the new species vs. 0.28–0.41 in U. spinatus). Our sequence of U. pequenae 28S was similar to U. spinatus; the 2 species differ by 0.4%, which is similar or greater than the differences recorded between other congeneric species within the Diplostomoidea Poirier, 1886 (Locke et al., 2018; Achatz et al., 2019). For example, 28S sequences of 3 species of Parastrigea Szidat, 1928 published by Hernández-Mena et al. (2017) differ by only 0.09–0.71% (1 to 8 bases different out of 1,132). The previously published COI sequences of U. spinatus were not homologous with the sequence obtained in our study.
Uvulifer pequenae can be further distinguished from U. weberi by a larger oral sucker (68–77 × 88–99 in the new species vs. 45–57 × 48–57 in U. weberi) and larger tribocytic organ (133–136 × 99–114 in the new species vs. 60–95 × 60–80 in U. weberi). The prosoma:opisthosoma length ratio in U. pequenae is larger compared to U. weberi (0.54–0.57 in the new species vs. 0.41–0.44 in U. weberi based on our specimens, and 0.35 based on the original line drawing of the type-specimen). The 28S sequence of U. weberi differs by 1.3% from that of U. pequenae, while COI sequences differ by 12.9%.
Uvulifer pequenae can be distinguished from U. ambloplitis as originally described by Hunter (1933) by having smaller eggs (71–81 long in the new species vs. 90–99 long in U. ambloplitis). The vitelline follicles do not reach the anterior margin of testes in U. ambloplitis, but extend anteriorly well beyond this level in the new species. Our sequences of U. ambloplitis and U. pequenae differ from each other by 1.4% in 28S and 12.9% in COI. Additionally, adult U. ambloplitis have not been reported outside the Nearctic.
Uvulifer pequenae is morphologically closest to U. prosocotyle, especially in the prosoma:opisthosoma length ratio (0.54–0.57 in the new species vs. 0.46–0.77 in our specimens of U. prosocotyle and 0.75 based on the original line drawing of the type-specimen). The 2 species differ in the egg size (71–81 long in the new species vs. 83–90 long in U. prosocotyle), and the relative extent of vitelline fields. The vitellarium-free zone occupies the first 13–16% of the opisthosoma in the new species compared to approximately 22–33% in our specimens of U. prosocotyle. The vitellarium of U. pequenae extends to approximately halfway between the anterior margin of the ovary and the anterior margin of the opisthosoma. In contrast, the vitellarium of U. prosocotyle extends to approximately the anterior margin of the ovary. Uvulifer prosocotyle also has a very distinctive ‘neck' region that is much narrower than the rest of the opisthosoma, whereas U. pequenae does not have this narrow part of the opisthosoma. Specimens of both U. pequenae and U. prosocotyle used in our study were heat-killed and fixed in the same manner. While the morphology of both species is very similar, the sequence divergence is very substantial at 1.4% in the 28S sequence and 12.9% in COI. Complete comparison of metric characters for U. pequenae and U. prosocotyle is provided in Table II.
Table II.
Metric characters of new Uvulifer spp. from Peru and the most morphologically similar congeners from the New World. Measurements of Uvulifer spinatus taken from López-Jiménez et al. (2018). Range values are followed by mean after semicolon.
| Character |
Species |
|||
|
Uvulifer pequenae n. sp. (n = 2) |
Uvulifer batesi n. sp. (n = 2) |
Uvulifer prosocotyle (n = 4) |
Uvulifer spinatus (n = 13) |
|
| Geographic origin of material | Peru | Peru | Brazil | Mexico |
| Overall body length | 1,403–1,432; 1,418 | 1,291–1,319; 1,305 | 1,060–1,439; 1,285 | 1,161–1,782; 1,499 |
| Prosoma length | 480–517; 499 | 307–335; 321 | 436–496; 460 | 276–439 |
| Prosoma width | 304–318; 311 | 251–285; 268 | 221–257; 235 | 204–227 |
| Opisthosoma length | 922–932; 927 | 1,032–1,034; 1,033 | 644–983; 846 | 800–1,327 |
| Opisthosoma width | 202–236; 219 | 170–195; 183 | 168–203; 183 | 110–195 |
| Oral sucker length | 68–77; 73 | 43–44; 44 | 55–73; 63 | 57–71; 61 |
| Oral sucker width | 88–99; 94 | 48–51; 50 | 106–113; 109 | 53–74; 62 |
| Pharynx length | 45–56; 51 | 23–25; 24 | 48–58; 54 | 34–46; 37 |
| Pharynx width | 34–37; 36 | 20 | 38–51; 43 | 29–35; 32 |
| Ventral sucker length | 39–40; 40 | 25–26; 26 | 35–38; 37 | 21–28; 24 |
| Ventral sucker width | 45–48; 47 | 29–31; 30 | 42–47; 45 | 28–35; 31 |
| Tribocytic organ length | 133–136; 135 | 105 | 73–106; 88 | 88–121; 97 |
| Tribocytic organ width | 99–114; 107 | 85 | 68–80; 75 | 97–125; 108 |
| Ovary length | 79–85; 82 | Obscured by uterus | 56–70; 62 | 49–72; 59 |
| Ovary width | 82–91; 87 | Obscured by uterus | 60–74; 65 | 56–64; 60 |
| Anterior testis length | 167–173; 170 | 91–94; 93 | 118–150; 136 | 80–144; 113 |
| Anterior testis width | 142–156; 149 | 85–97; 91 | 122–146; 131 | 91–125; 108 |
| Posterior testis length | 97–153; 125 | 97–107; 102 | 119–171; 138 | 78–139; 104 |
| Posterior testis width | 77–82; 80 | 94–97; 96 | 116–137; 124 | 89–124; 107 |
| Genital cone length | 60–65; 63 | 74–80; 77 | 61–94; 78 | 71–117; 89 |
| Genital cone width | 94–97; 96 | 80–86; 83 | 55–88; 67 | – |
| Ejaculatory pouch length | 142–156; 149 | 111 | Not well observed | 110–217; 172 |
| Ejaculatory pouch width | 71–85; 78 | 60–63; 62 | Not well observed | 64–109; 80 |
| Egg number | 2–5; 4 | 4–6; 5 | 0–3 | – |
| Egg length | 71–81; 76 | 76–87; 82 | 83–90; 88 | 65–81; 73 |
| Egg width | 46–57; 53 | 41–52; 47 | 43–44; 44 | 42–48; 44 |
| Ventrolateral preputial lobe length | 45–65; 55 | 68–99; 84 | 42–59; 50 | – |
| Ventrolateral preputial lobe width | 82–94; 88 | 130–142; 136 | 76–103; 89 | – |
| Prosoma:opisthosoma length ratio | 0.54–0.57; 0.56 | 0.31–0.33; 0.32 | 0.46–0.77; 0.56 | 0.28–0.41* |
| Oral sucker:ventral sucker width ratio | 1.76–2.31; 2.04 | 1.39–1.52; 1.46 | 2.28–2.52; 2.44 | 1.67–2.33; 1.99 |
| Anterior vitellarium-free zone: opisthosoma length | 0.13–0.16; 0.15 | 0.25–0.28; 0.27 | 0.22–0.33; 0.25 | – |
| Posterior vitellarium-free zone: opisthosoma length | 0.11–0.12; 0.12 | 0.15–0.16; 0.16 | 0.12–0.14; 0.14 | – |
| Anterior margin of ventral sucker positioned at | 60–62% of prosoma length; 61% | 37–39% of prosoma length; 38% | 57–62% of prosoma length; 59% | – |
| Anterior margin of holdfast positioned at | 72% of prosoma length | 46–47% of prosoma length; 46.5% | 66–72% of prosoma length; 69% | – |
| Anterior margin of ovary positioned at | 32% of opisthosoma length | 50–53% of opisthosoma length; 51.5% | 27–45% of opisthosoma length; 36% | – |
Originally given as opisthosoma: prosoma length ratio by López-Jiménez et al. (2018).
Uvulifer batesi n. sp.
Figures 3, 4.
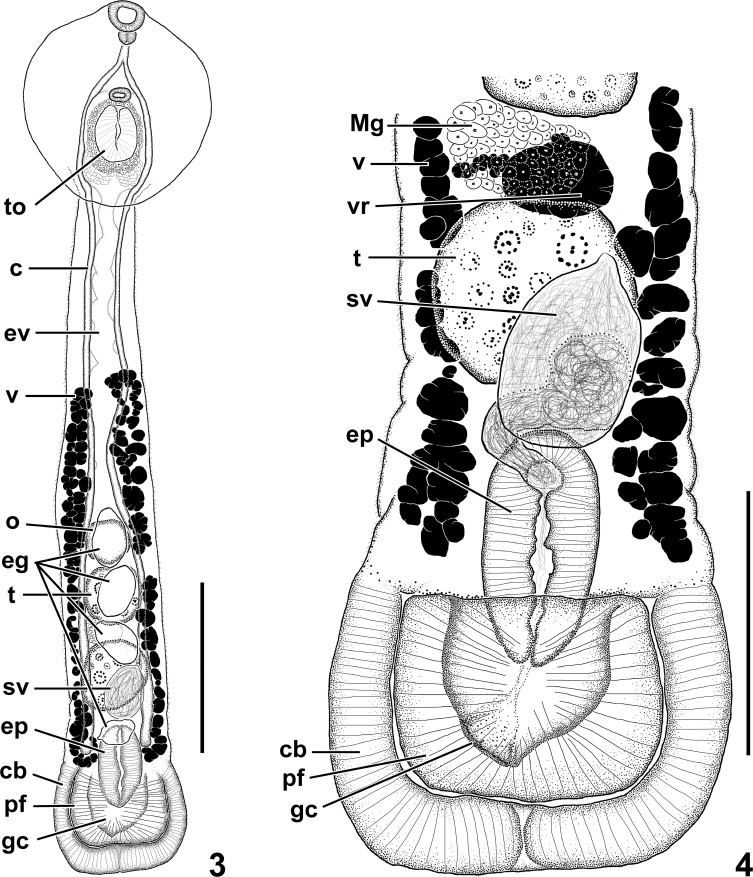
Uvulifer batesi n. sp. (3) Ventral view of holotype. Scale bar 250 μm. (4) Ventral view of posterior body end of holotype with uterus omitted. Scale bar = 150 μm. Abbreviations: c, ceca; cb, copulatory bursa; eg, egg; ep, ejaculatory pouch; ev, excretory vesicle; gc, genital cone; Mg, Mehlis' gland; o, ovary; pf, preputial fold; sv, seminal vesicle; t, testis; to, tribocytic organ; v, vitelline follicle; vr, vitelline reservoir.
Description (based on 2 fully mature specimens):
Body 1,291–1,319 long, comprising prosoma and opisthosoma; prosoma oval, ventrally concave, 307–335 long, with maximum width at midway (251–285); opisthosoma elongated, 1,032–1,034, gradually widening toward bell-shaped posterior end (170–195). Prosoma:opisthosoma length ratio 0.31–0.33. Prosoma devoid of tegumental spines, opisthosoma (excluding bell-shaped posterior end) covered by tegumental spines. Oral sucker nearly terminal, 43–44 × 48–51. Prepharynx absent. Pharynx oval, overlapping with oral sucker, 23–25 × 20. Esophagus about equal in length with pharynx. Cecal bifurcation in anterior third of prosoma. Ceca slender, blind, extending to near posterior end of opisthosoma. Ventral sucker delicate, much smaller than oral sucker, 25–26 × 29–31, located 37–39% of the prosoma length from the anterior end. Tribocytic organ 105 × 85, located immediately posterior to ventral sucker (46–47% of the prosoma length from the anterior end), oval with ventral muscular portion having a deep, longitudinal slit-like opening and basal glandular portion embedded in the prosoma. Testes tandem, with smooth margins, anterior testis 91–94 × 85–97, posterior testis 97–107 × 94–97. Seminal vesicle subglobular, ventral to posterior testis, connected to ejaculatory duct; proximal ejaculatory duct funnel-like with proximal end wide and distal end narrowing and running antero-dorsally, then bending and running posteriorly; distal portion opening into a muscular ejaculatory pouch; ejaculatory pouch 111 × 60–63, draining posteriorly through narrow short male duct. Ovary appearing subspherical with smooth margin (but largely obscured by uterus in both specimens), immediately pretesticular (50–53% of the opisthosoma length from the anterior end). Ootype surrounded by Mehlis' gland, submedian (slightly dextral), intertesticular. Seminal receptacle not observed. Uterus ventral in opisthosoma, extending from a level slightly pre-ovarian to posterior margin of posterior testis, containing 4–6 eggs (76–87 × 41–52); distal uterus uniting with male duct and forming hermaphroditic canal; hermaphroditic canal descending into genital cone. Genital cone 74–80 × 80–86, extending into a highly bulbous copulatory bursa; copulatory bursa with prominent muscular ventrolateral preputial fold. Ventrolateral preputial fold 68–99 × 130–142. Vitelline follicles in opisthosoma, ventral, absent in the anterior 25–28% of the opisthosoma and posterior 15–16% of opisthosoma. Vitelline reservoir intertesticular, sinistral to ootype. Excretory vesicle I-shaped, with main stem dorsal in opisthosoma; main stem appearing wavy, ascending into prosoma and surrounding tribocytic organ and giving rise to 6 secondary longitudinal branches that extend toward oral sucker; branches surrounding suckers and interconnected by network of anastomosing channels throughout prosoma. Excretory pore not observed.
Taxonomic summary
Type host:
Chloroceryle inda (Linnaeus) (Coraciiformes: Alcedinidae).
Site of infection:
Small intestine.
Type locality:
San Martín, Tocache Province, Cordillera Azul National Park, Río Pescadero, NE of Shapaja (8°10.694′S, 76°13.422′W), Peru, elev. 953 m above sea level.
Type specimens deposited:
The type series consists of 2 fully mature specimens deposited in the Harold W. Manter Laboratory. Holotype: HWML 139983, labeled ex. C. inda, small intestine, Cordillera Azul National Park, Peru, 13 Nov 2013, coll. K. Patitucci; paratype: HWML-139984, labeled identical to the holotype. Symbiotype deposited in the Field Museum, Chicago (FMNH 3859910).
Representative DNA sequences:
ZooBank registration:
urn:lsid:zoobank.org:act:F23BE7CF-0942-404F-AD5F-E2E2D373A4AE
Etymology:
The new species is named after Dr. John Bates in recognition of his contributions to the knowledge of South American birds and as the leader of the field crew that collected the new species.
Remarks
The new species clearly belongs to Uvulifer based on the combination of characteristic features such as the presence of a muscular ejaculatory pouch and a muscular copulatory bursa containing a retractile or protrusible genital cone partially surrounded by a ventrolateral preputial muscular fold.
Uvulifer batesi is easily distinguished from the New World congeners by the wide, bell-shaped copulatory bursa region at the posterior body end. This is the widest portion of the opisthosoma in U. batesi, whereas the widest part of the opisthosoma in other New World congeners is at the testicular level.
Uvulifer batesi can also be distinguished from U. elongatus, U. semicircumcisus, U. spinatus, and U. weberi by relatively shorter vitellarium. The vitellarium in all these 4 species occupies almost the whole length of the opisthosoma, whereas in U. batesi it is absent in the first 25–28% of the opisthosoma.
Uvulifer batesi can be further differentiated from U. elongatus by shorter body length (1,291–1,319 in the new species vs. 2,200–3,300 in U. elongatus), a much smaller ventral sucker (25–26 × 29–31 in the new species vs. 85–100 × 100–120 in U. elongatus), and pharynx (23–25 × 20 in the new species vs. 45–55 × 30–37 in U. elongatus). In addition, U. batesi and U. elongatus differ by 0.9% in 28S sequences and 12.9% in COI sequences.
Uvulifer batesi can be further distinguished from U. semicircumcisus by a thinner opisthosoma (170–195 in the new species vs. 270–400 in U. semicircumcisus) and smaller ventral sucker (25–26 × 29–31 in the new species vs. 40–49 in diameter in U. semicircumcisus). Additionally, U. semicircumcisus has been reported only in North America, whereas U. batesi was found in the Peruvian Amazon.
Uvulifer batesi can be further differentiated from the morphologically similar U. spinatus by the distribution of tegumental spines. In U. batesi the tegumental spines cover the majority of the opisthosoma, whereas in U. spinatus they extend only from the anterior margin of the opisthosoma to the anterior testis. Additionally, the 2 species can be differentiated by the more posteriorly positioned gonads in U. batesi, a smaller pharynx (23–25 × 20 in this new species vs. 34–46 × 29–35 in U. spinatus), and a smaller oral sucker:ventral sucker width ratio (1.39–1.52 in this new species vs. 1.67–2.33 in U. spinatus). The 28S sequence of U. batesi was similar to that of U. spinatus; the 2 species differ by only 0.3%. The available COI sequences of U. spinatus were not homologous with our sequences. Complete comparison of metric characters for U. pequenae and U. prosocotyle is provided in Table II.
Uvulifer batesi can be further distinguished from U. weberi by the somewhat, relatively more posterior gonads in U. batesi. In addition, both 28S (1.3%) and COI (13.7%) sequences are quite different between the 2 species.
Uvulifer batesi can be further distinguished from U. ambloplitis, as originally described by Hunter (1933), by having a smaller oral sucker (43–44 × 48–51 in the new species vs. 94–120 diameter in U. ambloplitis), smaller pharynx (23–25 × 20 in our new species vs. 52–63 × 40–45 in U. ambloplitis), smaller ventral sucker (25–26 × 29–31 in our new species vs. 44–52 × 45–56 in U ambloplitis), smaller eggs (76–87 in our new species vs. 90–99 in U. ambloplitis), and relatively longer fields of vitelline follicles that do not reach the anterior margin of testes in U. ambloplitis but extend well beyond this level anteriorly in U. batesi. Our sequences of U. ambloplitis and U. batesi are 1.4% different in 28S and 15.1% different in COI. As stated above, adult specimens of U. ambloplitis have been reported only in the Nearctic, whereas U. batesi is from the Peruvian Amazon.
Uvulifer batesi can be further differentiated from U. prosocotyle by the lower prosoma:opisthosoma length ratio (0.31–0.33 in the new species vs. 0.46–0.77 in our specimens of U. prosocotyle and 0.75 based off the original line drawing of the type-specimen). In addition, U. prosocotyle also has a very distinctive ‘neck' region that is much narrower than the rest of the opisthosoma, while U. batesi does not have this narrowed part of the opisthosoma. In addition, the 2 species differ by 1.4% in 28S sequences and by 13.1% in COI sequences.
Uvulifer batesi can be further distinguished from U. pequenae by the lower prosoma: opisthosoma length ratio (0.31–0.33 in the new species vs. 0.54–0.57 in U. pequenae) and the distribution of tegumental spines. The tegumental spines of U. batesi cover most of the opisthosoma but are completely absent on the prosoma. In contrast, the anterior 25% of the opisthosoma and entire prosoma have tegumental spines in U. pequenae. The 28S sequences were very close with only 0.2% difference; however, the COI sequences showed a much greater difference of 10%.
Molecular phylogenies
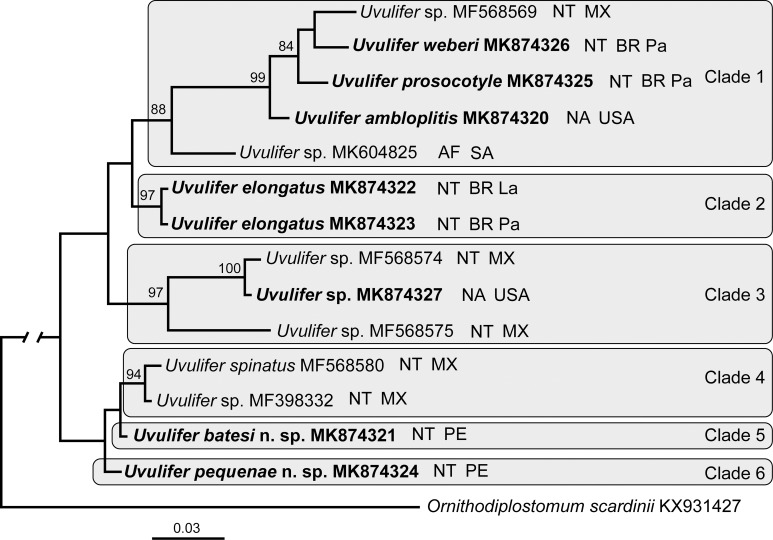
Upon trimming to the length of the shortest sequence the 28S alignment was 1,133 bp long. The phylogenetic tree resulting from the BI analysis contained 6 Uvulifer clades (Fig. 5). The clade 1 (88%) included recently published Uvulifer sp. (MK604825) from South Africa and a well-supported clade (99%) of U. ambloplitis + U. prosocotyle + U. weberi + Uvulifer sp. (GenBank accession MF568569). Notably, this clade included species from the Afrotropics, Nearctic, and Neotropics. The clade 2 (97%) included both of our isolates of U. elongatus collected from Amazonas (Lábrea) and Mato Grosso (Pantanal) states in Brazil. The clade 3 (97% support) was composed of Uvulifer sp. (MF568575) + a well-supported clade (100%) of Uvulifer sp. (MF568574) + Uvulifer sp. (MK874327). This clade was composed of only metacercariae from species from the Nearctic and Neotropics. The clade 4 (94% support) included U. spinatus + Uvulifer sp. (GenBank MF398332). Clades 5 and 6 included a single species each, U. pequenae and U. batesi.
Figure 5.
Phylogenetic interrelationships among 14 Uvulifer taxa based on Bayesian Inference (BI) analysis of partial 28S rRNA gene sequences. Bayesian Inference posterior probability values lower than 70% (BI) are not shown. New sequences obtained in this study are in bold. Branch length scale bar indicates number of substitutions per site. GenBank accession numbers and the biogeographical realm, and geographic origin are provided after the names of species. Abbreviations for biogeographical realms: AF = Afrotropical realm, NA = Nearctic realm, NT = Neotropical realm. Abbreviations for geographic origin: BR La = Lábrea site in Brazil, BR Pa = Pantanal site in Brazil, MX = Mexico, PE = Peru, SA = South Africa, USA = United States of America.
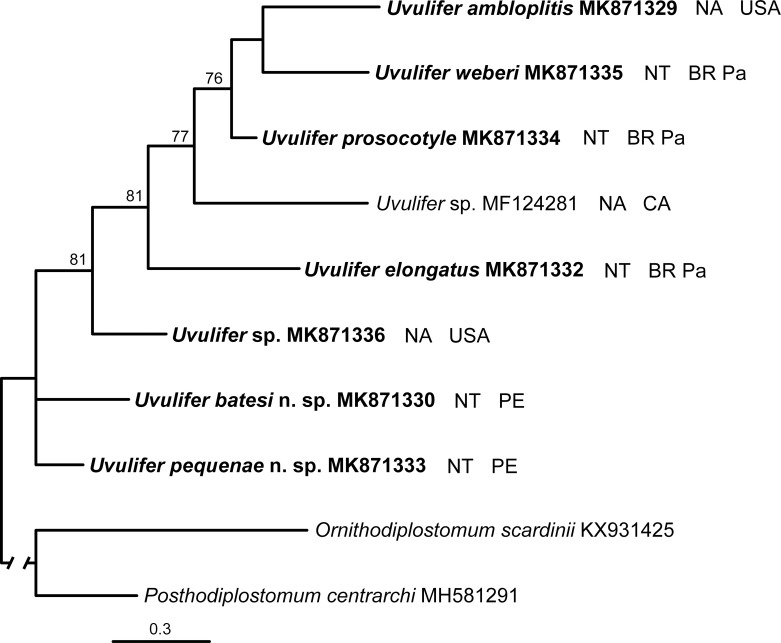
Upon trimming to the length of the shortest sequence the COI alignment was 451 bp long. While the branch topology of the Uvulifer tree was reasonably resolved, the support of the majority of nodes was rather weak (Fig. 6). The 2 new species from Peru appeared on the tree as sister taxa to the rest of the species in the genus. Despite some difference in the composition of the included species the 28S and CO1 phylogenies had an overall very similar branch topology.
Figure 6.
Phylogenetic interrelationships among 8 Uvulifer taxa based on Bayesian Inference (BI) analysis of partial COI mtDNA sequences. Bayesian Inference posterior probability values lower than 70% (BI) are not shown. New sequences obtained in this study are in bold. Branch length scale bar indicates number of substitutions per site. GenBank accession numbers, the biogeographical realm, and the geographic origin are provided after the names of species. Abbreviations for biogeographical realms: NA = Nearctic realm, NT = Neotropical realm. Abbreviations for geographic origin: BR Pa = Pantanal site in Brazil, CA = Canada, PE = Peru, USA = United States of America.
Genetic variation
The interspecific divergence in 28S sequences of Uvulifer spp. was generally low (0.1–2.2% or 1–25 bases out of 1,132). In contrast, COI sequences had much greater interspecific variation (9.3–15.3% or 42–69 bases out of 451). Although the 2 new Uvulifer species from the Peruvian Amazon were very similar in 28S sequences (0.2% or 2 bases out of 1,132), they were 10% different (45 bases out of 451 bases) in COI. Uvulifer pequenae and its morphologically closest congener U. prosocotyle differ by 1.4% (16 bases out of 1,132 bases) in 28S sequences and 12.9% (58 bases out of 451 bases) in COI. Uvulifer batesi and its morphologically closest congener U. spinatus differ by 0.3% (3 bases out of 1,132 bases) in 28S sequences (compatible COI sequences of U. spinatus are not available). Pairwise nucleotide comparisons among all Uvulifer spp. are provided in Tables III and IV. It is noteworthy that our isolate of U. elongatus from Pantanal, Brazil, had a single mixed base (a double peak) in its 28S sequence, whereas our isolate of U. elongatus from Lábrea, Brazil, did not have any mixed bases in 28S. There was only 0.5% difference (2 out of 426 bases) in their COI sequences.
Table III.
Pairwise comparisons of partial sequences of the 28S rRNA gene between Uvulifer species included in this study. Percentage differences are given above diagonal and the number of variable nucleotide positions is given below the diagonal. The 28S results are based on a 1,132-bp-long alignment.
| 1. MK874320 |
2. MK874321 |
3. MK874323 |
4. MK874324 |
5. MK874325 |
6. MK874326 |
7. MF568582 |
8. MK874327 |
9. MF398332 |
10. MF568569 |
11. MF568674 |
12. MF568575 |
13. MK604825 |
|
| 1. Uvulifer ambloplitis MK874320 | — | 1.4% | 1.2% | 1.4% | 0.5% | 0.5% | 1.5% | 1.6% | 1.4% | 0.7% | 1.7% | 2% | 1.2% |
| 2. Uvulifer batesi n. sp. MK874321 | 16 | — | 0.9% | 0.2% | 1.4% | 1.3% | 0.3% | 1.4% | 0.2% | 1.5% | 1.3% | 1.7% | 1.2% |
| 3. Uvulifer elongatus MK874323 | 13 | 10 | — | 0.9% | 1.5% | 1.4% | 1.2% | 1.2% | 1.1% | 1.6% | 1.3% | 1.5% | 0.8% |
| 4. Uvulifer pequenae n. sp. MK874324 | 16 | 2 | 10 | — | 1.4% | 1.3% | 0.4% | 1.4% | 0.4% | 1.5% | 1.3% | 1.7% | 1.2% |
| 5. Uvulifer prosocotyle MK874325 | 6 | 16 | 17 | 16 | — | 0.5% | 1.5% | 1.6% | 1.4% | 0.5% | 1.7% | 2% | 1.2% |
| 6. Uvulifer weberi MK874326 | 6 | 15 | 16 | 15 | 6 | — | 1.6% | 1.7% | 1.5% | 0.5% | 1.8% | 2% | 1.6% |
| 7. Uvulifer spinatus MF568582 | 17 | 3 | 13 | 5 | 17 | 18 | — | 1.7% | 0.1% | 1.6% | 1.6% | 2% | 1.3% |
| 8. Uvulifer sp. MK874327 | 18 | 16 | 14 | 16 | 18 | 19 | 19 | — | 1.6% | 1.7% | 0.1% | 1.3% | 1.4% |
| 9. Uvulifer sp. MF398332 | 16 | 2 | 12 | 4 | 16 | 17 | 1 | 18 | — | 1.5% | 1.5% | 1.9% | 1.2% |
| 10. Uvulifer sp. MF568569 | 8 | 17 | 18 | 17 | 6 | 6 | 18 | 19 | 17 | — | 1.8% | 2.2% | 1.6% |
| 11. Uvulifer sp. MF568674 | 19 | 15 | 15 | 15 | 19 | 20 | 18 | 1 | 17 | 20 | — | 1.4% | 1.5% |
| 12. Uvulifer sp. MF568575 | 22 | 19 | 17 | 19 | 22 | 23 | 22 | 15 | 21 | 25 | 16 | — | 1.6% |
| 13. Uvulifer sp. MK604825 | 14 | 14 | 9 | 14 | 14 | 18 | 15 | 16 | 14 | 18 | 17 | 18 | — |
Table IV.
Pairwise comparisons of partial sequences of the COI mtDNA gene between Uvulifer species included in this study. Percentage differences are given above diagonal and the number of variable nucleotide positions is given below the diagonal. Results are based on a 451-bp-long alignment.
| 1. MK871329 |
2. MK871330 |
3. MK871332 |
4. MK871333 |
5. MK871334 |
6. MK871335 |
7. MK871336 |
8. MF124281 |
|
| 1. Uvulifer ambloplitis MK871329 | — | 15.1% | 14.6% | 12.9% | 10.4% | 11.5% | 13.5% | 13.7% |
| 2. Uvulifer batesi n. sp. MK871330 | 68 | — | 12.9% | 10% | 13.1% | 13.7% | 11.3% | 15.3% |
| 3. Uvulifer elongatus MK871332 | 66 | 58 | — | 13.3% | 11.3% | 13.5% | 14.2% | 14.4% |
| 4. Uvulifer pequenae n. sp. MK871333 | 58 | 45 | 60 | — | 12.9% | 12.9% | 10.4% | 14.2% |
| 5. Uvulifer prosocotyle MK871334 | 47 | 59 | 51 | 58 | — | 9.3% | 10.4% | 11.3% |
| 6. Uvulifer weberi MK871335 | 52 | 62 | 61 | 58 | 42 | — | 13.3% | 13.3% |
| 7. Uvulifer sp. MK871336 | 61 | 51 | 64 | 47 | 47 | 60 | — | 12% |
| 8. Uvulifer sp. MF124281 | 62 | 69 | 65 | 64 | 51 | 60 | 54 | — |
DISCUSSION
The 2 new species of Uvulifer described herein represent the first species of Uvulifer described from Peru, and the seventh and eighth species of Uvulifer species in the New World. Our study is the first to provide DNA sequence data from U. ambloplitis, U. elongatus, U. prosocotyle, and U. weberi. Although a number of studies have involved Uvulifer (e.g., Boyd and Fry, 1971; Muzzall et al., 2011; Flores-Lopes, 2014), our study is only the fourth molecular phylogenetic study to produce DNA sequence data sourced from adult Uvulifer spp. (Hernández-Mena et al., 2017; López-Jiménez et al. 2018; Hoogendoorn et al., 2019) and only the second study to produce DNA sequence data from named adult material (López-Jiménez et al., 2018).
The interspecific genetic variation among partial 28S sequences was lower than demonstrated by López-Jiménez et al. (2018) for U. spinatus and other unnamed lineages of Uvulifer. Our 28S sequences of Uvulifer from South and North America demonstrated 0.2–1.6% interspecific divergence levels (Table III), which is lower than the range of 1.3–1.6% for interspecific differences reported by López-Jiménez et al. (2018). Interspecific divergence in our partial COI sequences showed levels of differences similar to those reported by López-Jiménez et al. (2018). Newly generated COI sequences showed 9.3–15.1% difference among species (Table IV), whereas López-Jiménez et al. (2018) reported 9.3–12.5% differences. The 2 genetically closest named species of Uvulifer in our dataset (U. batesi and U. pequenae) had only a 2 nucleotide difference in 28S while demonstrating a much greater 10% difference in COI sequences. This suggests that as few as a 2 bases difference (assuming high sequence quality) in 28S may be sufficient to differentiate between species in this genus, although it cannot be excluded that some species may have identical 28S sequences.
Our newly generated COI sequences cover the same region of COI as the vast majority of published COI sequences of diplostomoideans (e.g., sequences originating from Blasco-Costa and Locke, 2017; Hernández-Mena et al., 2017; Hoogendoorn et al., 2019). López-Jiménez et al. (2018) opted to amplify and sequence a different region of COI for their Uvulifer spp. We attempted amplification of the region sequenced by López-Jiménez et al. (2018) from our 2 new species. The PCRs were unsuccessful, although we did not experience problems amplifying and sequencing the 28S fragment and the standard “barcoding” region of the COI gene. Only 2 of the newly generated COI sequences (from metacercaria MK871336 and U. prosocotyle MK871334) overlapped with the region of the COI gene sequenced by López-Jiménez et al. (2018). Their sequence MF568574 and our metacercaria from Minnesota differ in 28S only by a single nucleotide; however, in COI they differ by 4.9% (14 bases out of 283). This level of divergence is much lower than differences seen between other named Uvulifer species in the same region of COI (usually ∼10% difference or more). It should be noted that according to López-Jiménez et al. (2018) the COI intraspecific variation in their material did not exceed 1.8%. Sequencing and morphological examination of a greater diversity of adult specimens from broader geographic area is necessary to determine if the metacercaria from our material is an independent species or represents a genetically divergent population of a known species.
Six species of kingfishers occur in the Americas. Megaceryle alcyon inhabits widespread areas of North America north of Mexico and may also winter in Central and South America. Megaceryle torquata inhabits ranges from the Rio Grande valley of North America south throughout Central America and South America. Chloroceryle americana is distributed throughout the southwestern United States south to central Argentina. Chloroceryle amazona ranges from Central America south to northern Argentina; the American pygmy kingfisher, Chloroceryle aenea (Pallas), ranges from southern Mexico south throughout central South America. The range of Chloroceryle inda extends from Nicaragua to Paraguay (Remsen, 1991). Our phylogenetic analyses included Uvulifer spp. from 4 New World kingfisher species: M. alcyon, M. torquata, C. americana, and C. inda. In the phylogeny resulting from our analysis of 28S (Fig. 5), neither of the well-supported clades that included more than 1 species of Uvulifer was limited to a single kingfisher species. In part, this may be the result of the strong overlap of distributions of the South American kingfisher species. It is known that a species of kingfisher can be host to multiple species of Uvulifer; for instance, U. pequenae and U. batesi both parasitize C. inda, and at least 3 species of Uvulifer parasitize M. alcyon (Hernández-Mena et al., 2017; López-Jiménez et al., 2018; present data). However, the potential for a single Uvulifer species to infect multiple species of kingfisher has not been previously tested using molecular tools.
The phylogenetic tree based on the 28S alignment (Fig. 5) revealed 2 strongly supported clades of Uvulifer containing specimens from distant geographical locations. Clade 1 included Uvulifer sp. from the Afrotropical realm, U. ambloplitis from the Nearctic, and Uvulifer sp., U. weberi, and U. prosocotyle from the Neotropics. The clade 3 included 2 unidentified species-level lineages distributed in Mexico and Central America (López-Jiménez et al., 2018) and a form from the northern United States. This likely indicates at least 2 independent dispersal events in the evolutionary history of the New World Uvulifer. The interrelationships and phylogeographic history of Uvulifer will likely be better resolved once DNA sequence data are available from a greater diversity of Uvulifer species including those from the Eastern Hemisphere.
The branch topology in the COI phylogenetic tree was not fully resolved and had overall lowed support values likely due to the mutation saturation effect. Somewhat higher branch support values in the CO1 tree within Uvulifer reported by López-Jiménez et al. (2018) are likely explained by the fact that these authors sequenced a different, somewhat shorter and less variable region of CO1 gene. Our results indicate that while COI sequences are a great tool for species differentiation, they should be used with caution for phylogenetic inference at higher taxonomic levels.
The result of our COI phylogeny (Fig. 6) confirmed the low utility of COI sequence data for phylogenetic inference in this digenean group that was suggested in the recent major publications on this group and digeneans overall (Locke et al., 2018; Pérez-Ponce de León and Hernández-Mena, 2019). Regardless, utilization of ribosomal as well as mitochondrial sequence data as tools for assisting with differentiating among species greatly enhances the power of taxonomic investigations within the Diplostomidae.
Our specimens of U. ambloplitis closely conform morphologically to the form originally described as Uvulifer claviformis Dubois & Rausch, 1948. Boyd and Fry (1971) later noted that Dubois viewed U. claviformis as a synonym of U. ambloplitis based on materials from Boyd and Fry (1971) and other materials in a personal communication. We believe the differences between the 2 forms can be possibly explained by the varying levels of contraction after fixation and/or levels of maturity as noted by Boyd and Fry (1971). Specimens morphologically identical to U. ambloplitis as described by Hunter (1933) should be sequenced for an adequate molecular and morphological comparison and a taxonomic conclusion regarding the form described by Dubois and Rausch (1948) and other previously synonymized species.
The overwhelming majority of ecological studies that report Uvulifer spp. did not include DNA sequence data (e. g., Boyd and Fry, 1971; Pérez-Ponce de León et al., 2010; Muzzall et al., 2011; McAllister et al., 2013; Flores-Lopes, 2014, Zimmermann et al., 2016; Hollander et al., 2019). Based on our results, it is clear that the diversity of Uvulifer in the New World is greater than previously recognized. At present, only 2 named species are currently known in North America north of Mexico (Boyd and Fry, 1971; López-Jiménez et al., 2018). Likely, many of the previous ecological studies dealing with larval stages of Uvulifer included more than a single Uvulifer species. Detailed molecular and morphological comparisons should provide a solution for this problem.
ACKNOWLEDGMENTS
We are grateful to Dr. John M. Bates (Field Museum, Chicago, Illinois), Tatiana Z. Pequeño Saco (Centro de Conservación, Investigación y Manejo de Áreas Naturales–Cordillera Azul), Dr. Eric Pulis (Northern State University, Aberdeen, South Dakota), Dr. Jason D. Weckstein (Drexel University, Philadelphia, Pennsylvania), Dr. Francisco Tiago de Melo (Federal University of Pará, Belém, Pará, Brazil), and Dr. João B. Pinho (Universidade Federal de Mato Grosso, Cuiabá, Mato Grosso, Brazil) for their invaluable help with field collecting. Collecting and processing of the specimens were supported by grant DEB-1120734 from the National Science Foundation and grant R15AI092622 from the National Institutes of Health, U.S.A., to V.V.T., and the Joe K. Neel Memorial Award from the University of North Dakota and Willis A. Reid, Jr. Student Research Grant from the American Society of Parasitologists to T.J.A.
LITERATURE CITED
- Achatz T. J, Pulis E. E, Junker K, Binh T. T, Snyder S. D, Tkach V. V. Molecular phylogeny of the Cyathocotylidae (Digenea, Diplostomoidea) necessitates systematic changes and reveals a history of host and environment switches. Zoologica Scripta. 2019;48:545–556. doi: 10.1111/zsc.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasco-Costa I, Locke S. A. Life history, systematics and evolution of the Diplostomoidea Poirier, 1886: Progress, promises and challenges emerging from molecular studies. Advances in Parasitology. 2017;98:167–225. doi: 10.1016/bs.apar.2017.05.001. [DOI] [PubMed] [Google Scholar]
- Boyd E. M, Fry A. E. Metazoan parasites of the eastern belted kingfisher, Megaceryle alcyon alcyon. Journal of Parasitology. 1971;57:150–156. [Google Scholar]
- Caballero E, Diaz-Ungria C. Intento de un catálogo de los tremátodos digéneos registrados en territorio Venezolano. Memoria de la Sociedad de Ciencias Naturales La Salle. 1958;18:19–36. [Google Scholar]
- Derycke S, Remerie T, Vierstraete A, Backeljau T, Vanfleteren J, Vincx M, Moens T. Mitochondrial DNA variation and cryptic speciation within the free-living marine nematode Pellioditis marina. Marine Ecology Progress Series. 2005;300:91–103. [Google Scholar]
- Dubois G. Monographie des Strigeida (Trematoda) Mémoires de la Société Neuchâteloise des Sciences Naturelles. 1938;6:1–535. [Google Scholar]
- Dubois G. Du statut de quelques Strigeata La Rue, 1926 (Trematoda). I. Bulletin de la Société Neuchâteloise des Sciences Naturelles. 1964;87:27–71. [Google Scholar]
- Dubois G. Quelques Strigeoidea (Trematoda) récoltés chez des oiseaux du Paraguay par la Mission Claude Weber, automne 1983, du Muséum d'Histoire Naturelle de Genève. Revue Suisse de Zoologie. 1985;92:641–648. [Google Scholar]
- Dubois G. Quelques Strigeoidea (Trematoda) récoltés au Paraguay par les expéditions du Muséum d'Histoire naturelle de Genève, au cours des années 1979, 1982 et 1985. Revue Suisse de Zoologie. 1988;95:521–532. [Google Scholar]
- Dubois G, Rausch R. Seconde contribution a l'etude des strigeides (Trematoda) Nord-Américains. Bulletin de la Société Neuchâteloise des Sciences Naturelles. 1948;71:29–61. [Google Scholar]
- Dubois G, Rausch R. A contribution to the study of North American strigeids (Trematoda) American Midland Naturalist. 1950;43:1–31. [Google Scholar]
- Flores-Lopes F. The occurrence of black spot disease in Astyanax aff. fasciatus (Characiformes: Characidae) in the Guaíba Lake basin, RS, Brazil. Brazilian Journal of Biology. 2014;74:127–134. doi: 10.1590/1519-6984.08312. [DOI] [PubMed] [Google Scholar]
- Hall T. A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series. 1999;41:95–98. [Google Scholar]
- Hernández-Mena D. I, García-Varela M, Pérez-Ponce de León G. Filling the gaps in the classification of the Digenea Carus, 1863: Systematic position of the Proterodiplostomidae Dubois, 1936 within the superfamily Diplostomoidea Poirier, 1886, inferred from nuclear and mitochondrial DNA sequences. Systematic Parasitology. 2017;94:833–848. doi: 10.1007/s11230-017-9745-1. [DOI] [PubMed] [Google Scholar]
- Hollander C. A, Griffith B. N, Zimmermann M. R. Differences in endohelminth parasite infection between male morphotypes of bluegill sunfish (Lepomis macrochirus) Journal of Parasitology. 2019;105:135–142. [PubMed] [Google Scholar]
- Hoogendoorn C, Smit N. J, Kudlai O. Molecular and morphological characterization of four diplostomid metacercariae infecting Tilapia sparrmanii (Perciformes: Cichlidae) in the North West Province, South Africa. Parasitology Research. 2019;118:1–14. doi: 10.1007/s00436-019-06285-y. [DOI] [PubMed] [Google Scholar]
- Hunter G. W., III The strigeid trematode, Crassiphiala ambloplitis (Hughes, 1927) Parasitology. 1933;25:510–517. [Google Scholar]
- Kudlai O, Kostadinova A, Pulis E. E, Tkach V. V. A new species of Drepanocephalus Dietz, 1909 (Digenea: Echinostomatidae) from the double-crested cormorant Phalacrocorax auritus (Lesson) (Aves: Phalacrocoracidae) in North America. Systematic Parasitology. 2015;90:221–230. doi: 10.1007/s11230-015-9550-7. [DOI] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Molecular Biology and Evolution. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin M. A, Blackshields G, Brown N. P, Chenna R, McGettigan P. A, McWilliam H, Valentin F, Wallace I. M, Wilm A, Lopez R, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Locke S. A, Van Dam A. R, Caffara M, Pinto H. A, López-Hernández D, Blanar C. A. Validity of the Diplostomoidea and Diplostomida (Digenea, Platyhelminthes) upheld in phylogenomic analysis. International Journal for Parasitology. 2018;48:1043–1059. doi: 10.1016/j.ijpara.2018.07.001. [DOI] [PubMed] [Google Scholar]
- Lockyer A. E, Olson P. D, Østergaard P, Rollinson D, Johnston D. A, Attwood S. W, Southgate V. R, Horak P, Snyder S. D, Le T. H, et al. The phylogeny of the Schistosomatidae based on three genes with emphasis on the interrelationships of Schistosoma Weinland, 1858. Parasitology. 2003;126:203–224. doi: 10.1017/s0031182002002792. [DOI] [PubMed] [Google Scholar]
- López-Jiménez A, Pérez-Ponce de León G, García-Varela M. Molecular data reveal high diversity of Uvulifer (Trematoda: Diplostomidae) in Middle America, with the description of a new species. Journal of Helminthology. 2018;92:725–739. doi: 10.1017/S0022149X17000888. [DOI] [PubMed] [Google Scholar]
- Lutz H. L, Tkach V. V, Weckstein J. D. Methods for specimen-based studies of avian symbionts. In: Webster M, editor. The role of collections in ornithology: The extended specimen. Studies in avian biology. CRC Press; Boca Raton, Florida: 2017. pp. 127–183. (ed.) p. [Google Scholar]
- McAllister C. T, Tumlison R, Robison H. W, Trauth S. E. Initial survey on black-spot disease (Digenea: Strigeoidea: Diplostomidae) in select Arkansas fishes. Journal of the Arkansas Academy of Science. 2013;67:200–203. [Google Scholar]
- Muzzall P. M, Cook V, Sweet D. J. Helminths of belted kingfishers, Megaceryle alcyon Linnaeus, 1758, from a fish hatchery in Ohio, U.S.A. Comparative Parasitology. 2011;78:367–372. [Google Scholar]
- Niewiadomska K. Family Diplostomidae Poirier, 1886. In: Gibson D. I, Jones A, Bray R. A, editors. Keys to the Trematoda, vol. 1. CAB International and Natural History Museum; London, U.K: 2002. pp. 167–196. (eds) p. [Google Scholar]
- Pérez-Ponce de León G, Hernández-Mena D. Testing the higher-level phylogenetic classification of Digenea (Platyhelminthes, Trematoda) based on nuclear rDNA sequences before entering the age of the ‘next-generation' Tree of Life. Journal of Helminthology. 2019;93:260–276. doi: 10.1017/S0022149X19000191. [DOI] [PubMed] [Google Scholar]
- Pérez-Ponce de León G, Rosas-Valdez R, Aguilar-Aguilar R, Mendoza-Garfias B, Mendoza-Palmero C, García-Prieto L, Rojas-Sánchez A, Briosio-Aguilar R, Pérez-Rodríguez R, Domínguez-Domínguez O. Helminth parasites of freshwater fishes, Nazas River basin, northern Mexico. Check List. 2010;6:26–35. [Google Scholar]
- Rambaut A. Figtree (version 1.4.3) 2016 Available at: http://tree.bio.ed.ac.uk/software/figtree/ Accessed 14 March 2019.
- Remsen J. V., Jr Community ecology of Neotropical kingfishers. University of California Publications in Zoology. 1991;124:1–128. [Google Scholar]
- Ronquist F, Huelsenbeck J. P. MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Stoyanov B, Georgieva S, Pankov P, Kudlai O, Kostadinova A, Georgiev B. B. Morphology and molecules reveal the alien Posthodiplostomum centrarchi Hoffman, 1958 as the third species of Posthodiplostomum Dubois, 1936 (Digenea: Diplostomidae) in Europe. Systematic Parasitology. 2017;94:1–20. doi: 10.1007/s11230-016-9680-6. [DOI] [PubMed] [Google Scholar]
- Subair K. T, Brinesh R, Janardanan K. P. Studies on the life-cycle of Uvulifer iruvettiensis sp. nov. (Digenea: Diplostomidae) Acta Parasitologica. 2013;58:91–97. doi: 10.2478/s11686-013-0118-x. [DOI] [PubMed] [Google Scholar]
- Tkach V. V, Curran S. S. Prosthenystera oonastica n. sp. (Digenea: Callodistomidae) from ictalurid catfishes in southeastern United States and molecular evidence differentiating species in the genus across Americas. Systematic Parasitology. 2015;90:39–51. doi: 10.1007/s11230-014-9531-2. [DOI] [PubMed] [Google Scholar]
- Tkach V. V, Littlewood D. T. J, Olson P. D, Kinsella J. M, Swiderski Z. Molecular phylogenetic analysis of the Microphalloidea Ward, 1901 (Trematoda: Digenea) Systematic Parasitology. 2003;56:1–15. doi: 10.1023/a:1025546001611. [DOI] [PubMed] [Google Scholar]
- Tkach V. V, Pawlowski J. A new method of DNA extraction from the ethanol-fixed parasitic worms. Acta Parasitologica. 1999;44:147–148. [Google Scholar]
- Van Steenkiste N, Locke S. A, Castelin M, Marcogliese D. J, Abbott C. New primers for DNA barcoding of digeneans and cestodes (Platyhelminthes) Molecular Ecology Resources. 2015;15:945–952. doi: 10.1111/1755-0998.12358. [DOI] [PubMed] [Google Scholar]
- Yamaguti S. Synopsis of the digenetic trematodes of vertebrates. Vols. 1 and II. Keigaku Publishing; Tokyo, Japan: 1971. 1,074 p. [Google Scholar]
- Zimmermann M. R, Luth K. E, Esch G. W. Transmission pattern differences of miracidia and cercariae larval stages of digenetic trematode parasites. Acta Parasitologica. 2016;61:680–688. doi: 10.1515/ap-2016-0095. [DOI] [PubMed] [Google Scholar]