Abstract
Cells are surrounded by a protective lipid bilayer membrane, and membrane proteins in the bilayer control the flow of chemicals, information, and energy across this barrier. Many therapeutics target membrane proteins, and some directly target the lipid membrane itself. However, interactions within biological membranes are challenging to study due to their heterogeneity and insolubility. Mass spectrometry (MS) has become a powerful technique for studying membrane proteins, especially how membrane proteins interact with their surrounding lipid environment. Although detergent micelles are the most common membrane mimetic, nanodiscs are emerging as a promising platform for MS. Nanodiscs, nanoscale lipid bilayers encircled by two scaffold proteins, provide a controllable lipid bilayer for solubilizing membrane proteins. This Young Scientist Perspective focuses on native MS of intact nanodiscs and highlights the unique experiments enabled by making membranes fly, including studying membrane protein-lipid interactions and exploring the specificity of fragile transmembrane peptide complexes. It will also explore current challenges and future perspectives for interfacing nanodiscs with MS.
Keywords: Native mass spectrometry, nanodiscs, membrane proteins, antimicrobial peptides, lipids, protein-lipid interactions
Graphical Abstract

1. Introduction: Membrane Proteins
1.1. The Importance and Challenges of Membrane Proteins.
Each cell is surrounded by a diverse lipid bilayer membrane, which forms a chemical barrier separating the cell from the external environment. Membrane proteins embedded in lipid membranes serve as transporters that move chemicals across the barrier, receptors that transmit information in and out of the cell, and enzymes that play important roles in energy conversion, signaling, and metabolism. Membrane proteins thus play critical roles in nearly all biochemical processes. Due to these important biological roles and relative accessibility on the outside of the cell, membrane proteins make up a major fraction of drug targets.1,2
Despite the pharmaceutical importance of membrane proteins, there is a significant gap in our understanding of their structure and interactions. For example, membrane proteins make up around 30% of the proteome3 but only around 2–3% of high-resolution structures. One challenge in studying membrane proteins that contributes to this gap is their unique amphipathic nature. Natural membranes are highly heterogeneous and insoluble, so membrane proteins generally need to be solubilized and isolated prior to analysis using an artificial membrane mimetic that replaces this natural lipid environment. However, because lipids can be critical in membrane protein structure and function,4,5 it can be challenging to extract membrane proteins from natural lipid bilayers while still preserving their natural behavior.
Although lipids can clearly be important for membrane protein activity, the molecular mechanisms of membrane protein-lipid interactions are often unclear because it is challenging to study these polydisperse interactions. Thus, there is a gap between measuring the global lipid composition and understanding local membrane protein-lipid interactions. For example, the global membrane lipid composition changes as a function of age, diet, and disease,6–8 but it is largely unclear how these changes modulate membrane protein activity. Understanding how lipids modulate membrane proteins at the molecular level will help trace the mechanisms between global lipidomic information and biological outcomes. Moreover, understanding membrane protein-lipid interactions will help optimize membrane mimetics for drug discovery and structural biology that better capture the natural lipid environment of membrane proteins.
1.2. Key Questions about Membrane Protein-Lipid Interactions.
Diving further, there are many unanswered questions about how membrane proteins and lipids interact. First, how do the lipids that surround a given membrane protein differ from the bulk lipid bilayer? Localization and/or binding of specific lipids to the membrane protein may mean that the local lipids that touch the membrane protein, referred to as annular lipids (Figure 1), are different than the bulk lipids. Second, how and where do lipids bind on the membrane protein surface? How specific are lipid binding sites, and what drives this specificity? Do specific lipids modulate protein interactions or dynamics? Finally, are functional effects driven more by specific binding or by general bulk membrane properties? Because mass spectrometry (MS) can identify lipids and quantify both the number of bound lipids as well as relative lipid ratios, MS has a significant role to play in answering these mechanistic questions about membrane protein-lipid interactions.
Figure 1:

(A) Model of a membrane protein nanodisc with a cutout side view. (B) Top view of membrane protein nanodiscs with bulk lipids (light grey) and annular lipids (black) shown.
1.3. Membrane Mimetics.
One challenge with MS of membrane proteins is addressing the unique solubility challenges of membrane proteins in an MS-compatible format. Detergent micelles, which consist of loose micellar clusters of amphipathic small molecules, are the most commonly used membrane mimetic.9–11 Detergents are highly effective at solubilizing membrane proteins but may fail to suitably reproduce the lipid bilayer environment found in natural membranes.12–14 Often a range of different detergents need to be screened to optimize detergent conditions for MS.15 Thus, there is a need for membrane mimetics that better replicate the natural lipid environment of membrane proteins.
To address this challenge, alternative membrane mimetics have emerged for solubilizing membrane proteins,16 including liposomes, bicelles, amphipols, and lipid nanoparticles surrounded by protein, peptide, or polymer belts. The most commonly used alternative membrane mimetics for MS are nanodiscs (Figure 1), which were developed by Stephen Sligar and coworkers in 2002.17 Nanodiscs contain a roughly 10 nm wide lipid bilayer encircled by two membrane scaffold protein (MSP) belts. Building on this initial design, larger and smaller scaffolds are now also available by using longer or shorter MSP sequences.18,19
This Young Scientist Perspective will cover the growing use of nanodiscs in mass spectrometry. Unlike a traditional review, I will focus primarily on my research developing native MS of intact nanodiscs as a tool to study membrane proteins and transmembrane peptides. Along the way, I will also highlight the exciting MS applications of nanodiscs by others, starting with a brief survey of other uses of nanodiscs in MS. Finally, I will offer some commentary on current challenges and future directions for research. The title pays subtle homage to John Fenn, who pioneered the electrospray ionization (ESI) that made these studies possible,20,21 and this Perspective will broadly explore how making membranes “fly” inside the mass spectrometer can lead to unique new insights and experiments.
2. Why use Nanodiscs for MS?
Nanodiscs complement the strengths and weaknesses of other membrane mimetics. The main strengths of nanodiscs for MS are: 1) They solubilize membrane proteins without detergents, which avoids introducing large amounts of detergent into the mass spectrometer. 2) Nanodiscs provide a lipid bilayer, which is more physiologically relevant than detergents. 3) Although the artificial lipid bilayer may not perfectly model natural membranes, the lipid content can be controlled to create membranes with specific lipid compositions. 4) Both sides of the protein are accessible for binding studies, which is not true of liposomes. 5) The MSP belt is homogeneous. 6) Finally, nanodiscs are the most monodisperse membrane mimetic.
The primary disadvantages of nanodiscs are: 1) They require initial detergent solubilization of the membrane, which may disrupt natural interactions. 2) Not all lipid combinations form sufficiently flat bilayers that will assemble into nanodiscs. 3) Because both sides of the nanodisc are accessible, transport assays are essentially impossible. 4) Nanodisc assembly inevitably leads to loss of material. 5) Finally, because nanodiscs create complex native MS spectra, pure and monodisperse membrane proteins are often required.
Overall, provided the membrane protein can be isolated with sufficient yield and purity prior to assembly, nanodiscs provide a unique combination of relative monodispersity and a controllable lipid bilayer that makes them well suited for MS. To date, nanodiscs are the only membrane mimetic with sufficient homogeneity to resolve an intact lipid bilayer with a membrane protein embedded by native MS.
3. A Brief Survey of Nanodiscs in Mass Spectrometry
The earliest MS application of nanodiscs from the labs of Stephen Sligar and Milan Mrksich was in using matrix-assisted laser desorption/ionization (MALDI) to monitor transducin binding to rhodopsin in nanodiscs that were tethered on a surface.22 Conventional ESI and MALDI continue to be used for detecting the types of lipids and membrane proteins embedded in nanodiscs23–25 as well as for measuring enzymatic activity and ligand binding for membrane proteins inside the nanodisc.26,27
Nanodiscs are especially powerful in interactomics experiments to capture and identify soluble binding partners to membrane proteins in nanodiscs.28–30 Here, nanodiscs with a target membrane protein or lipid are the “bait” and soluble proteins are the pool of unknown binders. Alternatively, nanodiscs can be formed from an unpurified mixture of membrane proteins that capture the membrane proteome, a solubilized membrane protein library (SMPL).31–34 Here, a soluble protein target serves as the “bait”, and the nanodisc library provides a pool of unknown binders.
Finally, nanodiscs are becoming more widely used in structural proteomics. John Engen and coworkers were the first to interface nanodiscs with HDX to measure the dynamics of membrane proteins in lipid bilayers,35–38 and this combination has since been used broadly.39,40 Michael Gross and Robert Blankenship used fast photooxidation of proteins (FPOP) to probe solvent accessibility and membrane topology of light-harvesting complex 2 embedded in nanodiscs.41 Nanodiscs have also been used for limited proteolysis.42,43 These applications demonstrate the broad utility of nanodiscs for studying membrane proteins with a diverse array of MS methods.
4. Interfacing Nanodiscs and Native MS
4.1. Native MS of Empty Nanodiscs.
As a graduate student with Stephen Sligar, I collaborated with Michael Gross to examine nanodiscs by native MS. We started with the simplest systems: “empty” nanodiscs with dimyristoyl-phosphatidylcholine (DMPC) or palmitoyloleoyl-phosphatidylcholine (POPC) lipids without embedded membrane proteins.44 Nanodiscs were buffer exchanged into ammonium acetate and ionized by nano-ESI. The first FTICR spectra showed a complex series of peaks that contained broad distributions with sharp peaks on top. The sharp peaks corresponded to intact nanodiscs with different numbers of lipids. For example, some nanodiscs had 155 DMPC molecules while others had 156 or 154. This intrinsic polydispersity manifested as a series of sharp peaks separated by the mass of the lipid (Figure 2). A subsequent study showed that the broader distributions were caused by constructive overlap between adjacent charge states.45 Algorithms initially designed to analyze these complex nanodisc spectra45 later evolved into UniDec, which has become widely used in native MS.46 Overall, these first studies demonstrated that intact nanodiscs could be preserved inside the mass spectrometer and that individual lipids could be resolved.
Figure 2:

Native mass spectrum of DMPC nanodiscs with deconvolved charge states separated below in various colors. The inset shows the deconvolved mass distribution. Each peak represents nanodiscs with a specific number of lipids.
Since these initial studies, empty nanodiscs have continued to be a proving ground for native MS. Ion mobility of empty nanodiscs suggested that they likely retain a disc shape at lower collision energies that collapses into a spherical shape at higher energies.46 Iain Campuzano, Joe Loo, and coworkers have used empty nanodiscs to compare native MS methods on a range of different instrument platforms.47,48 Finally, empty nanodiscs provide a predictable complexity that makes them ideal for testing new data analysis approaches.49–51 Methods for studying polydisperse spectra have been integrated into the PMI Intact algorithm from Protein Metrics and tested on nanodisc spectra.52 Jim Prell and coworkers have developed a range of Fourier-based data analysis approaches that were used to study nanodiscs53–55 but also are useful for studying polymers, proteins with bound salt, and membrane proteins in polydisperse detergent micelles.56 Prell and coworkers have also provided critical computational insights into the mechanisms of lipid ejections from nanodiscs that reveal how gas phase protonation of lipid head group effects dissociation pathways.57
4.2. Native MS of Membrane Proteins Ejected from Nanodiscs.
The first uses of nanodisc native MS were from John Klassen’s lab in a “catch-and-release” assay to study soluble proteins binding to glycolipids embedded in nanodiscs.58–62 Although the intact nanodisc complex was not resolved, collision induced dissociation (CID) released the protein with any bound glycolipids. Similar experiments with glycolipid micelles showed lower and likely not physiological affinities.63 Thus, this research first demonstrated the unique potential of nanodiscs to study protein-lipid interactions and showed an application where nanodiscs were more suitable than micelles.
The first report of integral membrane proteins in nanodiscs came from Carol Robinson’s lab.64 Here, native mass spectra were compared from membrane proteins in micelles, amphipols, bicelles, and nanodiscs. High levels of collision energy were used to eject membrane proteins from the different membrane mimetics. Interestingly, the relative amounts of monomer, dimer, and trimer for DgkA (14 kDa monomer mass) were different among the four membrane mimetics, with bicelles and nanodiscs (the two lipid bilayer systems) favoring higher oligomers. These results revealed that lipids can stabilize membrane protein oligomers.65 They also showed that membrane mimetics can be hard to break apart by CID. A modified instrument with high collision voltage was needed to release membrane proteins from the mimetic with few lipids bound. In the process, this high energy may disrupt the true oligomeric states present in the original membrane.
As a postdoc with Carol Robinson, my goal was to examine membrane protein nanodiscs at lower collision energies. This was enabled by development of high mass Orbitrap instrumentation,66 which improved the effective resolution of the data, and UniDec software, which allowed unbiased analysis of complex spectra.46 Using nanodiscs with an embedded trimeric ammonium transporter (AmtB, 126 kDa trimer mass) or tetrameric aquaporin (AqpZ, 99 kDa tetramer mass) membrane protein complexes with DMPC and POPC lipids, we discovered that the first resolved species at low collision voltages were the membrane proteins bound to dozens of lipids, the full lipid annulus.67 The two MSP belts were lost at low energies prior to the complex being resolved. Under increasing collisional activation, the annular lipids could be further stripped off before the membrane protein oligomers broke apart. These results demonstrated the unique potential of native MS of nanodiscs to study weakly bound annular lipids that could not be captured in detergent micelles.
Since these initial studies, ejection of membrane proteins from nanodiscs has since been used in several applications. A recent report from Neil Kelleher, Amy Rosenzweig, and coworkers demonstrated that nanodiscs could be used as a launchpad for top-down proteomics.68 Here, a copper-dependent membrane metalloenzyme in nanodiscs (pMMO, 99 kDa timer mass) was subjected to multiple levels of activation to first release the complex from the nanodisc, then dissociate the complex into isolated proteins, and finally break the peptide backbone to locate the copper binding sites. Nanodiscs were complementary to detergents, opening dissociation pathways for top-down fragmentation that were not possible in micelles.
Another approach to nanodisc native MS is with laser-induced-liquid-bead-ion-desorption (LILBID). Increasing the laser power during LILBID ionization can cause dissociation through unique pathways that are not generally seen through CID. Nina Morgner and coworkers showed that LILBID favors ejecting membrane protein complexes that retain the MSP belts but few lipids.69–71 Because LILBID generally produces singly charged fragments, nanodiscs with complex lipids and polydisperse membrane proteins can be analyzed. The primary challenges are that the spectra have generally lower resolution, which may limit studying lipid-protein interactions with small mass differences between the lipids. Moreover, increasing laser intensities dissociate complexes as they are ejected from the nanodisc, so it may be hard to rule out some disruption of oligomeric states present in the intact membrane.
4.3. Rejecting Ejecting: Chasing Intact Membrane Protein Nanodiscs.
As I started my research group at the University of Arizona, my goal was to use native MS to study membrane protein-lipid interactions in mixed lipid nanodiscs. I was also interested in measuring the oligomeric state of a membrane protein in an intact nanodisc, avoiding all potential artifacts of CID. There were two primary challenges. First, the combination of different lipids, MSP belts, and membrane protein oligomers meant that spectra could be impossible to assign unambiguously. To address this challenge, we engineered MSP belts with added amino acids that would shift the mass in predictable ways.72 By mixing two belts prior to assembly, we could use the peak shape patterns to encode the oligomeric states of the MSP belts and disambiguate the assignment.
The second challenge was that we lacked good tools for controlling the dissociation of nanodiscs. To address this challenge, we began exploring charge manipulation reagents. Charge reducing reagents, such as imidazole or triethylamine, were known to stabilize complexes for native MS by reducing the overall charge and lowering the Coulombic repulsion that drives dissociation.73–75 Conversely, supercharging reagents increased the charge acquired during ESI and generally destabilized complexes for native MS.76–78 Thus, our hypothesis was that supercharging reagents would destabilize nanodiscs whereas charge reducing reagents would stabilize nanodiscs.
Empty nanodiscs and nanodiscs with small peptide complexes behaved predicted; they were stabilized by charge reducing reagents and destabilized supercharging reagents.79–81 Charge reducing has since become indispensable strategy for retaining labile lipids and antimicrobial peptides nanodiscs during native MS.80–83 However, membrane protein nanodiscs behaved unexpectedly.79 Nanodiscs with either AmtB or AqpZ were slightly destabilized by the charge reducing reagent, imidazole. Supercharging reagents showed a range of behaviors depending on the reagent and instrument polarity. In positive ion mode, propylene carbonate was partially stabilizing, capturing nanodiscs that had lost around 20–40 lipids but retained the membrane protein complex and both MSP belts. contrast, glycerol carbonate was strongly destabilizing in positive mode, ejecting membrane protein complexes with only 0–20 bound lipids retained. In negative ionization mode, these supercharging reagents were uniformly stabilizing, capturing the membrane protein complex in an almost fully intact nanodisc (Figure 3). Using macromolecular mass defect analysis67 and mixed MSP belts72 to unambiguously assign the spectra, we demonstrated that AmtB was a trimer and AqpZ was a tetramer in the intact lipid bilayers.
Figure 3:
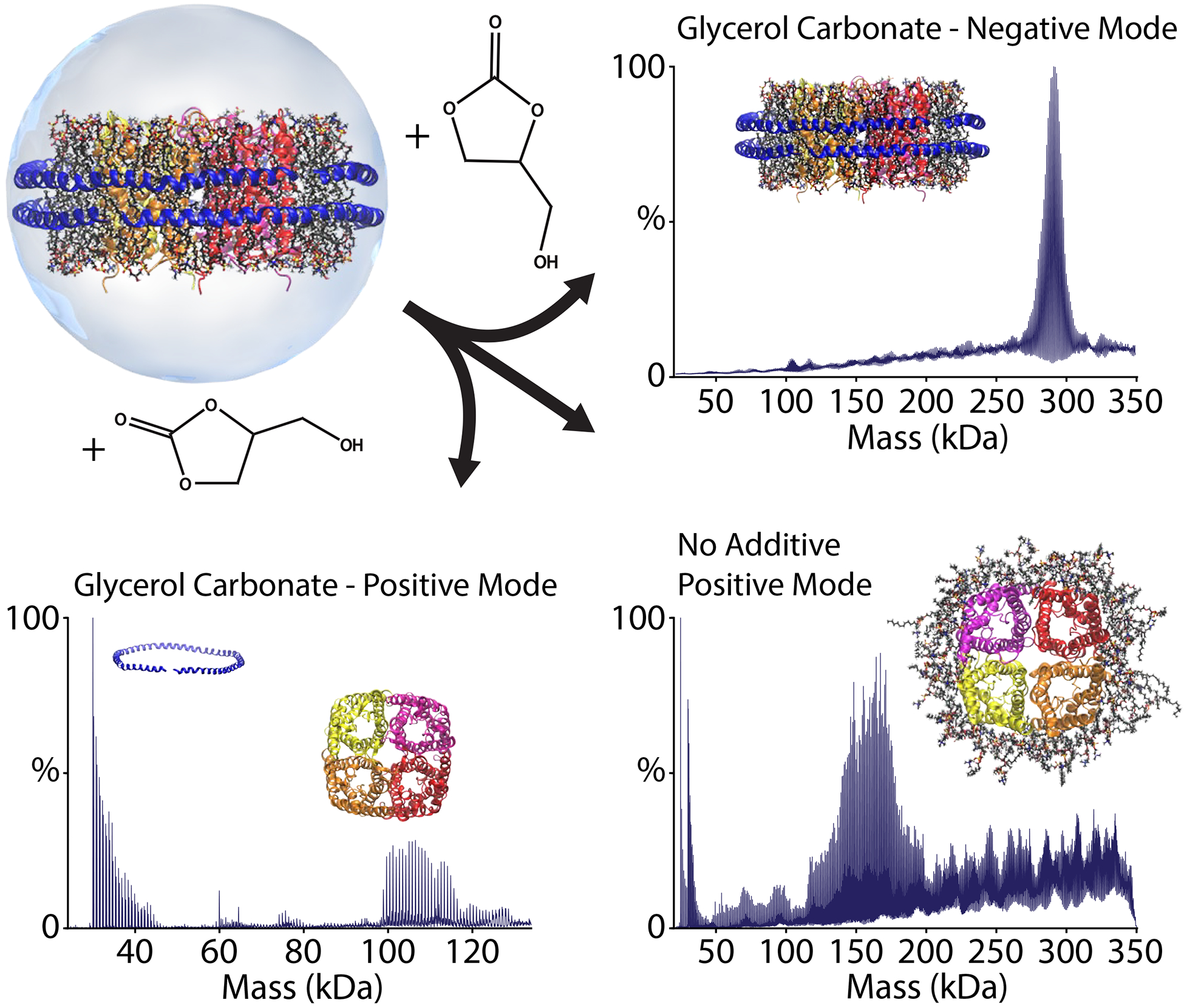
POPC nanodiscs with embedded AqpZ eject AqpZ with many bound lipids under conventional conditions (bottom right). Adding glycerol carbonate causes ejection with few bound lipids in positive mode (bottom left) or preserves the intact nanodisc in negative mode (top right). Figure taken from ref. 71.
This study provided a toolbox of reagents to modulate nanodisc stability and access new states in the gas phase, which we later used to characterize membrane protein-lipid interactions in mixed lipid nanodiscs (Section 4.4). It also showed the first resolved mass spectra of membrane proteins in intact lipid bilayers. Preserving the intact bilayer captures membrane proteins in their most natural environment possible for native MS and avoids disrupting fragile complexes, which we later employed to study antimicrobial peptide complexes (Section 4.5).
4.4. Capturing Membrane Protein-Lipid Interactions in Flight.
Building on this initial study79 with a single type of lipid in the nanodisc, our hypothesis was that mixed lipid nanodiscs could be used to measure the ratio of different lipids bound to the membrane protein in varying states of activation. We assembled AmtB into nanodiscs with a 50/50 mixture of POPC and palmitoyl-oleoyl-phosphatidylglycerol (POPG).84 Using propylene carbonate, we detected the nearly intact nanodisc complex with both MSP belts and around 120–175 lipids (Figure 4). The average mass of lipids in the intact nanodiscs was 745.5 Da, which is the average mass of a 50/50 mixture of POPC (760 Da) and POPG (749 Da). Thus, the intact nanodiscs had the expected 50/50 POPC/POPG distribution. Next, the spectra without charge manipulation reagents showed ejected AmtB with 40–130 bound lipids. The average mass of bound lipids in this complex was slightly below 754.5 Da, indicating an enrichment in POPG as we strip away the bulk lipids and retain only the bound annular lipids. Finally, we used glycerol carbonate to eject AmtB from the nanodisc with 0–40 bound lipids. Lipids 7–40 also showed lower average lipid masses, indicating POPG enrichment for the 20 most tightly bound lipids. However, lipids 1–6 showed higher average lipid masses, indicating an enrichment in POPC for the 6 most tightly bound lipids. Combining these data with results from novel lipid exchange-mass spectrometry experiments revealed that AmtB has a few specific POPC binding sites but overall remodels its local lipid environment to become enriched in POPG. Recently, a similar approach was used to detect PG binding to LmrP, a bacterial multidrug exporter (46 kDa monomer mass), ejected from nanodiscs.85
Figure 4:

(A) Deconvolved mass spectra of AmtB in 50/50 POPC/POPG nanodiscs with propylene carbonate (red), no additive (blue), or glycerol carbonate (black) show progressively less lipid bound. Masses are shown as the number of lipids bound to the AmtB trimer. (B) The average lipid mass for different numbers of bound lipids. Average masses higher than 754.5 Da (dashed line) are enriched in POPC (black) whereas lower masses are enriched in POPG (green). Figure adapted from ref. 76.
Together, these data demonstrate that nanodisc native MS can provide unique details into the specificities of a wide range of protein-lipid interactions. Using a toolbox of supercharging reagents, we can measure lipid enrichment in the full nanodisc complex, in the annulus of lipids surrounding the protein, and in the tightly bound lipids that are retained upon ejection. With AmtB, we quantified the ratios of lipids bound in every state from the first to the 175th bound lipid. We discovered that there are multiple types of lipid binding sites with different specificities and strengths. Future work will explore additional membrane protein targets and lipid types to help understand broadly how membrane proteins remodel their surrounding lipid environment.
4.5. Diving into Antimicrobial Peptides.
Enabled by the ability to measure the oligomeric state of membrane proteins in intact nanodiscs, we next explored small transmembrane peptides, focusing on antimicrobial peptides (AMPs). AMPs are generally cationic and amphipathic. The cationic charge drives selectivity towards bacterial membranes, which are generally rich in anionic lipids, and the amphipathicity drives insertion into lipid bilayers to disrupt the bacterial membrane, potentially by forming a toxic pore complex.86,87 We had two questions: 1) what stoichiometries of AMPs are present in membranes; and 2) how do the lipids affect these complexes? Micelles would not be suitable because the lipid bilayer is critical to the interactions.
Klassen and coworkers were the first to investigate an AMP, gramicidin A (GA), in nanodiscs using native MS.88 Due to the instrumentation, intact GA-nanodisc complexes were not resolved, and GA had to be ejected from the nanodiscs. The advantage of this approach is that clean signals for ejected GA could be studied by ion mobility. However, a significant fraction of the ejected GA was monomeric rather than the expected dimer. Ejection from the nanodisc partially disrupted these small, fragile AMP complexes.
To avoid disruption by CID, our goal was to study AMPs in intact nanodiscs with native MS. Adding AMPs directly to nanodiscs allowed AMPs to dive into nanodisc membrane as they would naturally. We titrated AMPs at increasing ratios of peptide/nanodisc and used native MS to measure the stoichiometries of AMPs associated with the nanodisc (Figure 5).81 Because these small peptides did not significantly shift the overall mass of the nanodiscs, subtle shifts in the mass defect were used to quantify relative amounts of different AMP stoichiometries. Formation of specific oligomeric complexes was inferred from the stoichiometry distribution. For example, GA had a distribution of even stoichiometries, indicating that GA forms dimers in the membrane. In contrast, melittin showed a random distribution of stoichiometries, indicating that no specific oligomers were formed, either because melittin incorporated as monomers or formed complexes without specificity for oligomeric state. LL37, a human AMP, showed non-random stoichiometries that suggested partial specificity.
Figure 5:

Schematic of (A) adding AMPs to nanodiscs for (B) native MS analysis. (C) Deconvolved mass spectra were analyzed using (D) macromolecular mass defect analysis to determine the stoichiometries of AMPs associated with the nanodisc as (E) increasing amounts of peptides were added. Figure taken from ref. 73.
To explore how lipids influenced membrane interactions, we tested nanodiscs with DMPC or dimyristoylphosphatidylglycerol (DMPG), modelling neutral mammalian membranes and anionic bacterial membranes respectively. GA had almost no specificity, showing the same stoichiometries in both types of nanodiscs. Melittin associated with both lipids but incorporated to a greater degree in DMPG, demonstrating selectivity for anionic lipids. As we continued to examine a wider range of peptides,82 we discovered that most behaved similarly to melittin, showing little if any preference for formation of specific oligomers but strong preferences for anionic DMPG. Many AMPs that we tested had no interactions with DMPC nanodiscs and only associated with DMPG nanodiscs, showing that the charge of the lipid head groups is critical in driving AMP specificity.
Overall, these studies demonstrate the unique power of nanodiscs and native MS to capture interactions of fragile and polydisperse complexes within lipid membranes. Conventional structural biology techniques struggle with the polydispersity and small size of AMPs. Other biophysical methods usually require labels that may distort their interactions and provide ensemble measurements rather than the stoichiometry distribution. Native MS uniquely captures the oligomeric state distribution to reveal the specificity of complex formation. Only nanodiscs provide the right type of membrane mimetic for native MS of AMPs, providing a controllable lipid bilayer that is homogeneous enough to capture and interpret spectra of the intact membrane with embedded AMPs.
5. Current Challenges and Future Perspectives
Looking forward, the primary challenge for native MS of nanodiscs is complexity. Unlike detergent micelles, where naked protein complexes can often be easily ejected, membrane proteins are difficult to eject from nanodiscs. Supercharging is highly enabling for destabilizing nanodiscs,79,84 but usually many lipids still remain bound to the ejected membrane protein, forming complex mass distributions. New data analysis approaches are critical to addressing this polydispersity. Furthermore, new charge manipulation reagents may open new dissociation pathways for studying oligomerization and lipid binding. Modifications to the MSP belt such as covalent circularization89 may also help to stabilize the nanodiscs and alter dissociation pathways. For all of these cases, molecular dynamics may prove useful to understand both the effects of charge manipulation regents90 and broader mechanisms of how nanodiscs behave in the mass spectrometer. Finally, surface induced dissociation (SID)91 may be a promising path for ejecting membrane protein and peptide complexes without the dissociation observed by CID. It is important to note that only a handful of proteins have been studied in nanodiscs by native MS, and new behaviors may be discovered as more complexes are explored and new methods are employed.
Future research using nanodiscs native MS will also explore a wider range of lipids. However, as different lipids are added to nanodiscs, complexity again becomes a significant challenge. The combination of lipids with different masses can easily overlap and become unresolvable. We have been working to address this complexity by using lipids with similar92 or resonant masses.83 By choosing lipids with masses that overlap, we can resolve intact nanodiscs with cholesterol, cardiolipin, and glycolipids in addition to standard phospholipids. We are working on developing MS-compatible mixtures that model the head group composition of natural membranes using this approach, but not all combinations of lipids form stable bilayers amenable for nanodisc formation. Ultimately, our goal is to provide resolvable nanodiscs with a rich lipid composition that models natural biological membranes. As methods develop, it may eventually be possible to work with nanodiscs made from natural lipids, potentially using polymer belts to extract these directly from the membrane.93,94 However, these approaches are currently limited by the mass heterogeneity (except with LILBID95) and will likely require extensive dissociation of the complex.
Finally, another challenge for native MS broadly is the need for new ionization methods that are higher throughput and that can be coupled with online separations. Currently, static nano-ESI needles are the gold standard for nanodisc native MS. We have tested several online ESI sources that work well for more stable systems, but nanodiscs have proven too fragile, showing partial dissociation when higher flow rates and larger tips diameters are used. Further research is needed for nondenaturing ionization techniques that can be coupled with online flow injection. High-throughput injection techniques, such as RapidFire or Echo-MS,96,97 could have enormous impact in native MS if the ionization conditions can be optimized for preserving fragile noncovalent complexes like nanodiscs.
In summary, nanodisc native MS has grown into a unique platform for studying the interactions of membrane proteins. Although detergent micelles provide the cleanest system for measuring membrane protein oligomeric states or doing lipid titrations, nanodiscs can reveal how membrane proteins broadly remodel the surrounding lipid bilayer. In other words, detergents are best for studying tight, specific lipid interactions whereas nanodiscs are better for analyzing weaker, less specific interactions. Furthermore, nanodiscs are essential for measuring interactions of AMPs and other fragile transmembrane complexes that are highly lipid dependent and/or too fragile to survive CID. By making intact membranes fly, nanodiscs provide a unique and exciting platform for native MS to study interactions with and within membranes.
Highlights.
This feature explores interfacing native MS and nanodiscs to study lipid membranes.
An overview highlights the broad uses of nanodiscs and MS.
Applications discuss protein-lipid interactions and antimicrobial peptides.
Future challenges and opportunities of complexity in nanodisc native MS.
Acknowledgements
The author thanks many friends, collaborators, and mentors for outstanding support, especially Carol Robinson, Stephen Sligar, and Michael Gross. Recent work was done by a talented team of high school students, undergraduates, graduates, and staff scientists, and the author thanks them all for making the lab such a fun and exciting place to work. Current research interfacing nanodiscs and native MS is funded by the National Science Foundation (CHE-1845230) and the National Institute of General Medical Sciences and National Institutes of Health (R35 GM128624). The content is solely the responsibility of the author and does not necessarily represent the official views of the NIH.
Biography

Michael Marty, Ph.D., is an Assistant Professor in the Department of Chemistry & Biochemistry and Bio5 Institute at The University of Arizona. Prof. Marty earned his B.A. in chemistry and mathematics at St. Olaf College in 2010, and he completed his Ph.D. in chemistry as a Springborn Fellow at the University of Illinois Urbana-Champaign in 2013 under the direction of Prof. Stephen Sligar. He then performed postdoctoral research at the University of Oxford with Prof. Dame Carol Robinson before joining the faculty at The University of Arizona in 2016. Prof. Marty has been awarded the ASMS Research Award, the Bisgrove Scholar Award, an NSF CAREER award, and an NIH R35 Maximizing Investigators’ Research Award (MIRA). His research applies lipoprotein nanodiscs with mass spectrometry to study membrane proteins, antimicrobial peptides, and their interactions with lipid bilayers. As the developer of UniDec, he is also interested in mass spectrometry data analysis and deconvolution.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- (1).Rask-Andersen M; Masuram S; Schiöth HB The druggable genome: Evaluation of drug targets in clinical trials suggests major shifts in molecular class and indication. Annu. Rev. Pharmacol. Toxicol 2014, 54, 9–26. [DOI] [PubMed] [Google Scholar]
- (2).Bull SC; Doig AJ Properties of Protein Drug Target Classes. PloS one 2015, 10, e0117955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Almen MS; Nordstrom KJ; Fredriksson R; Schioth HB Mapping the human membrane proteome: a majority of the human membrane proteins can be classified according to function and evolutionary origin. BMC Biol 2009, 7, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Corradi V; Sejdiu BI; Mesa-Galloso H; Abdizadeh H; Noskov SY; Marrink SJ; Tieleman DP Emerging Diversity in Lipid-Protein Interactions. Chem. Rev 2019, 119, 5775–5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Overduin M; Esmaili M Memtein: The fundamental unit of membrane-protein structure and function. Chem. Phys. Lipids 2019, 218, 73–84. [DOI] [PubMed] [Google Scholar]
- (6).Nicolson GL; Ash ME Membrane Lipid Replacement for chronic illnesses, aging and cancer using oral glycerolphospholipid formulations with fructooligosaccharides to restore phospholipid function in cellular membranes, organelles, cells and tissues. Biochim. Biophys. Acta 2017, 1859, 1704–1724. [DOI] [PubMed] [Google Scholar]
- (7).Escribá PV Membrane-lipid therapy: A historical perspective of membrane-targeted therapies — From lipid bilayer structure to the pathophysiological regulation of cells. Biochim. Biophys. Acta 2017, 1859, 1493–1506. [DOI] [PubMed] [Google Scholar]
- (8).Dumas F; Haanappel E Lipids in infectious diseases – The case of AIDS and tuberculosis. Biochim. Biophys. Acta 2017, 1859, 1636–1647. [DOI] [PubMed] [Google Scholar]
- (9).Barrera NP; Di Bartolo N; Booth PJ; Robinson CV Micelles protect membrane complexes from solution to vacuum. Science 2008, 321, 243–246. [DOI] [PubMed] [Google Scholar]
- (10).Bolla JR; Agasid MT; Mehmood S; Robinson CV Membrane Protein-Lipid Interactions Probed Using Mass Spectrometry. Annu. Rev. Biochem 2019, 88, 85–111. [DOI] [PubMed] [Google Scholar]
- (11).Frick M; Schmidt C Mass spectrometry-A versatile tool for characterising the lipid environment of membrane protein assemblies. Chem. Phys. Lipids 2019, 221, 145–157. [DOI] [PubMed] [Google Scholar]
- (12).Arana MR; Fiori MC; Altenberg GA Functional and structural comparison of the ABC exporter MsbA studied in detergent and reconstituted in nanodiscs. Biochem. Biophys. Res. Commun 2019, 512, 448–452. [DOI] [PubMed] [Google Scholar]
- (13).Ganapathy S; Opdam L; Hontani Y; Frehan S; Chen Q; Hellingwerf KJ; de Groot HJM; Kennis JTM; de Grip WJ Membrane matters: The impact of a nanodisc-bilayer or a detergent microenvironment on the properties of two eubacterial rhodopsins. Biochim. Biophys. Acta 2020, 1862, 183113. [DOI] [PubMed] [Google Scholar]
- (14).Guo Y Be Cautious with Crystal Structures of Membrane Proteins or Complexes Prepared in Detergents. Crystals 2020, 10, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Laganowsky A; Reading E; Hopper JTS; Robinson CV Mass spectrometry of intact membrane protein complexes. Nat. Protoc 2013, 8, 639–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Marty MT; Hoi KK; Robinson CV Interfacing Membrane Mimetics with Mass Spectrometry. Acc. Chem. Res 2016, 49, 2459–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Bayburt TH; Grinkova YV; Sligar SG Self-assembly of discoidal phospholipid bilayer nanoparticles with membrane scaffold proteins. Nano Lett. 2002, 2, 853–856. [Google Scholar]
- (18).Denisov IG; Grinkova YV; Lazarides AA; Sligar SG Directed self-assembly of monodisperse phospholipid bilayer nanodiscs with controlled size. J. Am. Chem. Soc 2004, 126, 3477–3487. [DOI] [PubMed] [Google Scholar]
- (19).Hagn F; Etzkorn M; Raschle T; Wagner G Optimized phospholipid bilayer nanodiscs facilitate high-resolution structure determination of membrane proteins. J. Am. Chem. Soc 2013, 135, 1919–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Dellisanti CD Electrospray makes molecular elephants fly. Nat. Methods 2015, 12, 15–15. [Google Scholar]
- (21).Fenn J Nobel lecture: electrospray wings for molecular elephants. http://www.nobelprize.org/nobel_prizes/chemistry/laureates/2002/fenn-lecture.html 2002. [DOI] [PubMed]
- (22).Marin VL; Bayburt TH; Sligar SG; Mrksich M Functional assays of membrane-bound proteins with SAMDI-TOF mass spectrometry. Angew. Chem. Int. Ed 2007, 46, 8796–8798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Marty MT; Das A; Sligar SG Ultra-thin layer MALDI mass spectrometry of membrane proteins in nanodiscs. Anal. Bioanal. Chem 2012, 402, 721–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Maric S; Skar-Gislinge N; Midtgaard S; Thygesen MB; Schiller J; Frielinghaus H; Moulin M; Haertlein M; Forsyth VT; Pomorski TG; Arleth L Stealth carriers for low-resolution structure determination of membrane proteins in solution. Acta Crystallogr. D Biol. Crystallogr 2014, 70, 317–328. [DOI] [PubMed] [Google Scholar]
- (25).Häusler E; Fredriksson K; Goba I; Peters C; Raltchev K; Sperl L; Steiner A; Weinkauf S; Hagn F Quantifying the insertion of membrane proteins into lipid bilayer nanodiscs using a fusion protein strategy. Biochim. Biophys. Acta 2020, 1862, 183190. [DOI] [PubMed] [Google Scholar]
- (26).Ma J; Lu Y; Wu D; Peng Y; Loa-Kum-Cheung W; Peng C; Quinn RJ; Shui W; Liu Z-J Ligand identification of the adenosine A2A receptor in self-assembled nanodiscs by affinity mass spectrometry. Anal. Methods 2017, 9, 5851–5858. [Google Scholar]
- (27).McDougle DR; Palaria A; Magnetta E; Meling DD; Das A Functional studies of N-terminally modified CYP2J2 epoxygenase in model lipid bilayers. Protein Sci. 2013, 22, 964–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Borch J; Roepstorff P; Moeller-Jensen J Nanodisc-based coimmunoprecipitation for mass spectrometric identification of membrane-interacting proteins. Mol. Cell. Proteomics 2011, 10, O110 006775, 006779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Zhang XX; Chan CS; Bao H; Fang Y; Foster LJ; Duong F Nanodiscs and SILAC-based mass spectrometry to identify a membrane protein interactome. J. Proteome Res 2012, 11, 1454–1459. [DOI] [PubMed] [Google Scholar]
- (30).Chung KY; Day PW; Velez-Ruiz G; Sunahara RK; Kobilka BK Identification of GPCR-interacting cytosolic proteins using HDL particles and mass spectrometry-based proteomic approach. PloS one 2013, 8, e54942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Marty MT; Wilcox KC; Klein WL; Sligar SG Nanodisc-solubilized membrane protein library reflects the membrane proteome. Anal. Bioanal. Chem 2013, 405, 4009–4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Moutal A; Wang Y; Yang X; Ji Y; Luo S; Dorame A; Bellampalli SS; Chew LA; Cai S; Dustrude ET; Keener JE; Marty MT; Vanderah TW; Khanna R Dissecting the role of the CRMP2-neurofibromin complex on pain behaviors. Pain 2017, 158, 2203–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Roy J; Pondenis H; Fan TM; Das A Direct Capture of Functional Proteins from Mammalian Plasma Membranes into Nanodiscs. Biochemistry 2015, 54, 6299–6302. [DOI] [PubMed] [Google Scholar]
- (34).Wilcox KC; Marunde MR; Das A; Velasco PT; Kuhns BD; Marty MT; Jiang H; Luan C-H; Sligar SG; Klein WL Nanoscale Synaptic Membrane Mimetic Allows Unbiased High Throughput Screen That Targets Binding Sites for Alzheimer’s-Associated Aβ Oligomers. PloS one 2015, 10, e0125263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Hebling CM; Morgan CR; Stafford DW; Jorgenson JW; Rand KD; Engen JR Conformational analysis of membrane proteins in phospholipid bilayer nanodiscs by hydrogen exchange mass spectrometry. Anal. Chem 2010, 82, 5415–5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Morgan CR; Hebling CM; Rand KD; Stafford DW; Jorgenson JW; Engen JR Conformational transitions in the membrane scaffold protein of phospholipid bilayer nanodiscs. Mol. Cell. Proteomics 2011, 10, M111 010876, 010811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Parker CH; Morgan CR; Rand KD; Engen JR; Jorgenson JW; Stafford DW A Conformational Investigation of Propeptide Binding to the Integral Membrane Protein γ-Glutamyl Carboxylase Using Nanodisc Hydrogen Exchange Mass Spectrometry. Biochemistry 2014, 53, 1511–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Nasr ML; Shi XM; Bowman AL; Johnson M; Zvonok N; Janero DR; Vemuri VK; Wales TE; Engen JR; Makriyannis A Membrane phospholipid bilayer as a determinant of monoacylglycerol lipase kinetic profile and conformational repertoire. Protein Sci. 2013, 22, 774–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Redhair M; Clouser AF; Atkins WM Hydrogen-deuterium exchange mass spectrometry of membrane proteins in lipid nanodiscs. Chem. Phys. Lipids 2019, 220, 14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Martens C; Shekhar M; Lau AM; Tajkhorshid E; Politis A Integrating hydrogen-deuterium exchange mass spectrometry with molecular dynamics simulations to probe lipid-modulated conformational changes in membrane proteins. Nat. Protoc 2019, 14, 3183–3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Lu Y; Zhang H; Niedzwiedzki DM; Jiang J; Blankenship RE; Gross ML Fast Photochemical Oxidation of Proteins Maps the Topology of Intrinsic Membrane Proteins: Light-Harvesting Complex 2 in a Nanodisc. Anal. Chem 2016, 88, 8827–8834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Heuveling J; Frochaux V; Ziomkowska J; Wawrzinek R; Wessig P; Herrmann A; Schneider E Conformational changes of the bacterial type I ATP-binding cassette importer HisQMP2 at distinct steps of the catalytic cycle. Biochim. Biophys. Acta 2014, 1838, 106–116. [DOI] [PubMed] [Google Scholar]
- (43).Periasamy A; Shadiac N; Amalraj A; Garajova S; Nagarajan Y; Waters S; Mertens HD; Hrmova M Cell-free protein synthesis of membrane (1,3)-beta-d-glucan (curdlan) synthase: co-translational insertion in liposomes and reconstitution in nanodiscs. Biochim. Biophys. Acta 2013, 1828, 743–757. [DOI] [PubMed] [Google Scholar]
- (44).Marty MT; Zhang H; Cui W; Blankenship RE; Gross ML; Sligar SG Native mass spectrometry characterization of intact nanodisc lipoprotein complexes. Anal. Chem 2012, 84 8957–8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Marty MT; Zhang H; Cui W; Gross ML; Sligar SG Interpretation and deconvolution of nanodisc native mass spectra. J. Am. Soc. Mass Spectrom 2014, 25, 269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Marty MT; Baldwin AJ; Marklund EG; Hochberg GK; Benesch JL; Robinson CV Bayesian deconvolution of mass and ion mobility spectra: from binary interactions to polydisperse ensembles. Anal. Chem 2015, 87, 4370–4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Campuzano IDG; Li HL; Bagal D; Lippens JL; Svitel J; Kurzeja RJM; Xu H; Schnier PD; Loo JA Native MS Analysis of Bacteriorhodopsin and an Empty Nanodisc by Orthogonal Acceleration Time-of-Flight, Orbitrap and Ion Cyclotron Resonance. Anal. Chem 2016, 88, 12427–12436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Campuzano IDG; Nshanian M; Spahr C; Lantz C; Netirojjanakul C; Li H; Wongkongkathep P; Wolff JJ; Loo JA High Mass Analysis with a Fourier Transform Ion Cyclotron Resonance Mass Spectrometer: From Inorganic Salt Clusters to Antibody Conjugates and Beyond. J. Am. Soc. Mass Spectrom 2020, 31, 1155–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Reid DJ; Diesing JM; Miller MA; Perry SM; Wales JA; Montfort WR; Marty MT MetaUniDec: High-Throughput Deconvolution of Native Mass Spectra. J. Am. Soc. Mass Spectrom 2019, 30, 118–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Marty MT A Universal Score for Deconvolution of Intact Protein and Native Electrospray Mass Spectra. Anal. Chem 2020, 92, 4395–4401. [DOI] [PubMed] [Google Scholar]
- (51).Marty MT Eliminating Artifacts in Electrospray Deconvolution with a SoftMax Function. J. Am. Soc. Mass Spectrom 2019, 30, 2174–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Campuzano IDG; Robinson JH; Hui JO; Shi SD; Netirojjanakul C; Nshanian M; Egea PF; Lippens JL; Bagal D; Loo JA; Bern M Native and Denaturing MS Protein Deconvolution for Biopharma: Monoclonal Antibodies and Antibody-Drug Conjugates to Polydisperse Membrane Proteins and Beyond. Anal. Chem 2019, 91, 9472–9480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Cleary SP; Li H; Bagal D; Loo JA; Campuzano IDG; Prell JS Extracting Charge and Mass Information from Highly Congested Mass Spectra Using Fourier-Domain Harmonics. J. Am. Soc. Mass Spectrom 2018, 29, 2067–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Cleary SP; Prell JS Distinct Classes of Multi-Subunit Heterogeneity: Analysis using Fourier Transform Methods and Native Mass Spectrometry. Analyst 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Cleary SP; Thompson AM; Prell JS Fourier Analysis Method for Analyzing Highly Congested Mass Spectra of Ion Populations with Repeated Subunits. Anal. Chem 2016, 88, 6205–6213. [DOI] [PubMed] [Google Scholar]
- (56).Wilson JW; Rolland AD; Klausen GM; Prell JS Ion mobility-mass spectrometry reveals that α-hemolysin from Staphylococcus aureus simultaneously forms hexameric and heptameric complexes in detergent micelle solutions. Anal. Chem 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Miller ZM; Zhang JD; Donald WA; Prell JS Gas-Phase Protonation Thermodynamics of Biological Lipids: Experiment, Theory, and Implications. Anal. Chem 2020, 92, 10365–10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Zhang Y; Liu L; Daneshfar R; Kitova EN; Li C; Jia F; Cairo CW; Klassen JS Protein-glycosphingolipid interactions revealed using catch-and-release mass spectrometry. Anal. Chem 2012, 84, 7618–7621. [DOI] [PubMed] [Google Scholar]
- (59).Lin H; Kitova EN; Klassen JS Quantifying Protein-Ligand Interactions by Direct Electrospray Ionization-MS Analysis: Evidence of Nonuniform Response Factors Induced by High Molecular Weight Molecules and Complexes. Anal. Chem 2013, 85, 8919–8922. [DOI] [PubMed] [Google Scholar]
- (60).Leney AC; Fan X; Kitova EN; Klassen JS Nanodiscs and Electrospray Ionization Mass Spectrometry: A Tool for Screening Glycolipids Against Proteins. Anal. Chem 2014, 86, 5271–5277. [DOI] [PubMed] [Google Scholar]
- (61).Han L; Kitova EN; Li J; Nikjah S; Lin H; Pluvinage B; Boraston AB; Klassen JS Protein–Glycolipid Interactions Studied in Vitro Using ESI-MS and Nanodiscs: Insights into the Mechanisms and Energetics of Binding. Anal. Chem 2015, 87, 4888–4896. [DOI] [PubMed] [Google Scholar]
- (62).Leney AC; Rezaei Darestani R; Li J; Nikjah S; Kitova EN; Zou C; Cairo CW; Xiong ZJ; Prive GG; Klassen JS Picodiscs for Facile Protein-Glycolipid Interaction Analysis. Anal. Chem 2015, 87, 4402–4408. [DOI] [PubMed] [Google Scholar]
- (63).Han L; Kitova EN; Klassen JS Detecting Protein-Glycolipid Interactions Using Glycomicelles and CaR-ESI-MS. J. Am. Soc. Mass Spectrom 2016, 27, 1878–1886. [DOI] [PubMed] [Google Scholar]
- (64).Hopper JT; Yu YT; Li D; Raymond A; Bostock M; Liko I; Mikhailov V; Laganowsky A; Benesch JL; Caffrey M; Nietlispach D; Robinson CV Detergent-free mass spectrometry of membrane protein complexes. Nat. Methods 2013, 10, 1206–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Gupta K; Donlan JAC; Hopper JTS; Uzdavinys P; Landreh M; Struwe WB; Drew D; Baldwin AJ; Stansfeld PJ; Robinson CV The role of interfacial lipids in stabilizing membrane protein oligomers. Nature 2017, 541, 421–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Gault J; Donlan JAC; Liko I; Hopper JTS; Gupta K; Housden NG; Struwe WB; Marty MT; Mize T; Bechara C; Zhu Y; Wu B; Kleanthous C; Belov M; Damoc E; Makarov A; Robinson CV High-resolution mass spectrometry of small molecules bound to membrane proteins. Nat. Meth 2016, 13, 333–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Marty MT; Hoi KK; Gault J; Robinson CV Probing the Lipid Annular Belt by Gas-Phase Dissociation of Membrane Proteins in Nanodiscs. Angew. Chem. Int. Ed. Engl 2016, 55, 550–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Ro SY; Schachner LF; Koo CW; Purohit R; Remis JP; Kenney GE; Liauw BW; Thomas PM; Patrie SM; Kelleher NL; Rosenzweig AC Native top-down mass spectrometry provides insights into the copper centers of membrane-bound methane monooxygenase. Nat. Commun 2019, 10, 2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Peetz O; Henrich E; Laguerre A; Lohr F; Hein C; Dotsch V; Bernhard F; Morgner N Insights into Cotranslational Membrane Protein Insertion by Combined LILBID-Mass Spectrometry and NMR Spectroscopy. Anal. Chem 2017, 89, 12314–12318. [DOI] [PubMed] [Google Scholar]
- (70).Henrich E; Lohr F; Pawlik G; Peetz O; Dotsch V; Morgner N; de Kroon AI; Bernhard F Lipid Conversion by Cell-Free Synthesized Phospholipid Methyltransferase Opi3 in Defined Nanodisc Membranes Supports an in Trans Mechanism. Biochemistry 2018, 57, 5780–5784. [DOI] [PubMed] [Google Scholar]
- (71).Henrich E; Peetz O; Hein C; Laguerre A; Hoffmann B; Hoffmann J; Dötsch V; Bernhard F; Morgner N Analyzing native membrane protein assembly in nanodiscs by combined non-covalent mass spectrometry and synthetic biology. eLife 2017, 6, e20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Reid DJ; Keener JE; Wheeler AP; Zambrano DE; Diesing JM; Reinhardt-Szyba M; Makarov A; Marty MT Engineering Nanodisc Scaffold Proteins for Native Mass Spectrometry. Anal. Chem 2017, 89, 11189–11192. [DOI] [PubMed] [Google Scholar]
- (73).Sun J; Kitova EN; Klassen JS Method for Stabilizing Protein–Ligand Complexes in Nanoelectrospray Ionization Mass Spectrometry. Anal. Chem 2007, 79, 416–425. [DOI] [PubMed] [Google Scholar]
- (74).Lemaire D; Marie G; Serani L; Laprévote O Stabilization of Gas-Phase Noncovalent Macromolecular Complexes in Electrospray Mass Spectrometry Using Aqueous Triethylammonium Bicarbonate Buffer. Anal. Chem 2001, 73, 1699–1706. [DOI] [PubMed] [Google Scholar]
- (75).Mehmood S; Marcoux J; Hopper JTS; Allison TM; Liko I; Borysik AJ; Robinson CV Charge Reduction Stabilizes Intact Membrane Protein Complexes for Mass Spectrometry. J. Am. Chem. Soc 2014, 136, 17010–17012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Going CC; Xia Z; Williams ER New supercharging reagents produce highly charged protein ions in native mass spectrometry. Analyst 2015, 140, 7184–7194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Sterling HJ; Williams ER Origin of Supercharging in Electrospray Ionization of Noncovalent Complexes from Aqueous Solution. J. Am. Soc. Mass Spectrom 2009, 20, 1933–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Zenaidee MA; Donald WA Extremely supercharged proteins in mass spectrometry: profiling the pH of electrospray generated droplets, narrowing charge state distributions, and increasing ion fragmentation. Analyst 2015, 140, 1894–1905. [DOI] [PubMed] [Google Scholar]
- (79).Keener JE; Zambrano DE; Zhang G; Zak CK; Reid DJ; Deodhar BS; Pemberton JE; Prell JS; Marty MT Chemical additives enable native mass spectrometry measurement of membrane protein oligomeric state within intact nanodiscs. J. Am. Chem. Soc 2019, 141, 1054–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).Townsend JA; Keener JE; Miller ZM; Prell JS; Marty MT Imidazole Derivatives Improve Charge Reduction and Stabilization for Native Mass Spectrometry. Anal. Chem 2019, 91, 14765–14772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Walker LR; Marzluff EM; Townsend JA; Resager WC; Marty MT Native Mass Spectrometry of Antimicrobial Peptides in Lipid Nanodiscs Elucidates Complex Assembly. Anal. Chem 2019, 91, 9284–9291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Walker LR; Marty MT Revealing the Specificity of a Range of Antimicrobial Peptides in Lipid Nanodiscs by Native Mass Spectrometry. Biochemistry 2020, 59, 2135–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Kostelic MM; Ryan AM; Reid DJ; Noun JM; Marty MT Expanding the Types of Lipids Amenable to Native Mass Spectrometry of Lipoprotein Complexes. J. Am. Soc. Mass Spectrom 2019, 30, 1416–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Zhang G; Keener JE; Marty MT Measuring Remodeling of the Lipid Environment Surrounding Membrane Proteins with Lipid Exchange and Native Mass Spectrometry. Anal. Chem 2020, 92, 5666–5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).Debruycker V; Hutchin A; Masureel M; Ficici E; Martens C; Legrand P; Stein RA; McHaourab HS; Faraldo-Gómez JD; Remaut H; Govaerts C An embedded lipid in the multidrug transporter LmrP suggests a mechanism for polyspecificity. Nat. Struct. Mol. Biol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Sani M-A; Separovic F How Membrane-Active Peptides Get into Lipid Membranes. Acc. Chem. Res 2016, 49, 1130–1138. [DOI] [PubMed] [Google Scholar]
- (87).Guha S; Ghimire J; Wu E; Wimley WC Mechanistic Landscape of Membrane-Permeabilizing Peptides. Chem. Rev 2019, 119, 6040–6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (88).Li J; Richards MR; Kitova EN; Klassen JS Delivering Transmembrane Peptide Complexes to the Gas Phase Using Nanodiscs and Electrospray Ionization. J. Am. Soc. Mass Spectrom 2017, 28, 2054–2065. [DOI] [PubMed] [Google Scholar]
- (89).Nasr ML; Baptista D; Strauss M; Sun Z-YJ; Grigoriu S; Huser S; Pluckthun A; Hagn F; Walz T; Hogle JM; Wagner G Covalently circularized nanodiscs for studying membrane proteins and viral entry. Nat. Meth 2017, 14, 49–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (90).Konermann L; Metwally H; Duez Q; Peters I Charging and supercharging of proteins for mass spectrometry: recent insights into the mechanisms of electrospray ionization. Analyst 2019. [DOI] [PubMed] [Google Scholar]
- (91).Harvey SR; Liu Y; Liu W; Wysocki VH; Laganowsky A Surface induced dissociation as a tool to study membrane protein complexes. Chem. Commun 2017, 53, 3106–3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (92).Hoi KK; Robinson CV; Marty MT Unraveling the Composition and Behavior of Heterogeneous Lipid Nanodiscs by Mass Spectrometry. Anal. Chem 2016, 88, 6199–6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (93).Ravula T; Hardin NZ; Ramamoorthy A Polymer nanodiscs: Advantages and limitations. Chem. Phys. Lipids 2019, 219, 45–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (94).Overduin M; Esmaili M Native Nanodiscs and the Convergence of Lipidomics, Metabolomics, Interactomics and Proteomics. Applied Sciences-Basel 2019, 9. [Google Scholar]
- (95).Hellwig N; Peetz O; Ahdash Z; Tascon I; Booth PJ; Mikusevic V; Diskowski M; Politis A; Hellmich Y; Hanelt I; Reading E; Morgner N Native mass spectrometry goes more native: investigation of membrane protein complexes directly from SMALPs. Chem. Commun 2018, 54, 13702–13705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (96).Sawyer WS; Srikumar N; Carver J; Chu PY; Shen A; Xu A; Williams AJ; Spiess C; Wu C; Liu Y; Tran JC High-throughput antibody screening from complex matrices using intact protein electrospray mass spectrometry. Proc. Natl. Acad. Sci 2020, 117, 9851–9856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (97).Sinclair I; Stearns R; Pringle S; Wingfield J; Datwani S; Hall E; Ghislain L; Majlof L; Bachman M Novel Acoustic Loading of a Mass Spectrometer:Toward Next-Generation High-Throughput MS Screening. J. Lab. Autom 2016, 21, 19–26. [DOI] [PubMed] [Google Scholar]


