Abstract
This study investigated the effects of dietary osteopontin (OPN)-enriched algal protein on growth, immune status, and fecal fermentation profiles of weaned pigs challenged with a live infection of F18-fimbriated enterotoxigenic E. coli (ETEC). At 21 d of age, 54 pigs (5.95 ± 0.28 kg BW; blocked by BW) were allotted to 1 of 3 experimental groups combining dietary and health statuses. A control diet, containing 1% wild-type algal protein, was fed to both sham-inoculated (NC) and ETEC-inoculated (PC) pigs, while the test diet contained 1% OPN-enriched algal protein as fed only to ETEC-inoculated pigs (OA). All pigs received their assigned dietary treatment starting at study initiation to permit a 10-d acclimation period prior to inoculation. Growth performance, fecal dry matter, as well as hematological, histopathological, immune, and microbiota outcomes were analyzed by ANOVA, where treatment and time were considered as fixed effects and pig as a random effect; significance was accepted at P < 0.05. Overall, ETEC-inoculated pigs (PC and OA) exhibited decreased (P < 0.05) ADG and G:F, as well as increased (P < 0.05) peripheral blood helper T-cells and total leukocyte counts, compared with NC pigs during the postinoculation period. The OA treatment also elicited the highest (P < 0.05) concentrations of circulating tumor necrosis factor-α and volatile fatty acid concentrations in luminal contents at various postinoculation time-points, compared with other treatments. A principal coordinate analysis based on Unifrac weighted distances indicated that NC and OA groups had similar overall bacterial community structures, while PC pigs exhibited greater diversity, but infection status had no impact on α-diversity. Osteopontin-specific effects on microbial community structure included enrichment within Streptococcus and Blautia genera and decreased abundance of 12 other genera as compared with PC pigs. Overall, ETEC-infected pigs receiving 1% OPN-enriched algal protein exhibited changes immunity, inflammatory status, and colonic microbial community structure that may benefit weanling pigs experiencing F18 ETEC infection.
Keywords: Escherichia coli, microbiota, osteopontin, tumor necrosis factor-α, volatile fatty acids
Introduction
In commercial swine production systems, newly weaned pigs may experience subclinical or clinical disease challenges that stimulate an inflammatory response and compromise growth performance and animal well-being. Enterotoxigenic Escherichia coli (ETEC) is a common enteric infectious pathogen that causes diarrhea in weaned piglets (Fekete et al., 2002). With shifts in the U.S. swine industry to more judicious use of broad-spectrum antibiotics, there is a need to develop strategies for supporting the health and well-being of pigs when faced with common pathogens, and nutritional solutions are attractive in being both practical and cost-effective (Suchner et al., 2000; Le Floc’h et al., 2006).
The use of algae biomass and algae-derived compounds in human and animal nutrition has shown promise as a source of vitamins, essential amino acids, polyunsaturated fatty acids, minerals, carotenoids, enzymes, and even compounds with fiber-like properties (Wells et al., 2017). Additionally, algae-based ingredients have also been proven to benefit the immune response by altering inflammatory processes during times of challenge (Caporgno and Mathys, 2018). More specifically, the green algae, Chlamydomonas reinhardtii, has served as a model commercial eukaryotic system that can be genetically altered to allow for recombinant expression of proteins, including that of osteopontin (OPN; Specht et al., 2010; Ravi et al., 2018). As secreted from multiple cell types and found in colostrum, OPN is a glycoprotein that serves as a key mediator of recruitment and retention of macrophages and T-cells to sites of inflammation (Mazzali et al., 2002) and is also essential for efficient development of Th1-mediated immune responses (Rollo et al., 2005). In vivo, enterocytes exposed to antigens of microbial origin exhibited increased OPN expression as a response to infection (van der Windt et al., 2011; Chagan-Yasutan et al., 2014). Studies involving fecal microbial profiling of OPN-deficient mice also confirm this expressed protein likely contributes to the maintenance of commensal bacterial communities (Ito et al., 2017).
Considering the dynamic relationship between the microbiota and host, disruption of bacterial community structure via the presence of enteric pathogens has profound influence on the host’s growth performance, immune response, and susceptibility to gastrointestinal disorders such as postweaning diarrhea, which are key points for animal agriculture. Therefore, the objectives of this study were to determine the effects of an OPN-enriched algae protein-containing ingredient on growth performance, immunity, and microbiota outcomes in newly weaned pigs exposed to an F18-fimbriated ETEC challenge.
Materials and Methods
The protocol for this experiment was approved by the Institutional Animal Care and Use Committee and the Institutional Biosafety Committee of the University of Illinois at Urbana-Champaign.
Animal husbandry and experimental design
At weaning (21 d of age), 54 barrows [5.95 ± 0.28 kg body weight (BW)] were obtained from a commercial herd (1050 Cambro genetics; Carthage Veterinary Service, Ltd., Carthage, IL) confirmed to be negative for both porcine reproductive and respiratory syndrome virus and porcine epidemic diarrhea virus, and group-housed at the Innovative Swine Solutions Veterinary Research Facility, a Biosafety Level 2 production containment facility located near Champaign, IL. The containment facility consisted of 4 isolated production rooms (9.1 m × 4.3 m) each containing 4 pens capable of housing 9 pigs per pen. Each room was equipped with a high-efficiency particulate air filtration system, plastic slotted flooring, and provided appropriate floor space for nursery pigs. Lighting and ambient temperature were maintained as appropriate for life stage.
Following transportation to the biocontainment facility, pigs were individually weighed, identified with a unique ear tag, and allotted to 1 of 3 uniform treatment groups based on BW. The 3 experimental treatment groups used in this experiment were comprised of two experimental diets and two states of infection (Table 1). Both diets were formulated to meet all nutrient recommendations for nursery pigs (NRC, 2012), with dietary treatments formulated to be isocaloric, isonitrogenous, and contain equal standardized ileal digestible amino acid concentrations (Table 2). The control diet, containing 1% wild-type algal protein, was fed to both sham-inoculated (NC) and ETEC-challenged (PC) pigs, while the test diet, containing 1% of an OPN-enriched algal protein (OA; Ravi et al., 2018), was only fed to ETEC-challenged pigs.
Table 1.
Experimental treatment groups
| Treatment | Dietary treatment1 | Infection status |
|---|---|---|
| NC | 1.0% wild-type algal protein | Uninfected |
| PC | 1.0% wild-type algal protein | Infected |
| OA | 1.0% OPN-enriched algal protein | Infected |
1Percent inclusion of algal protein ingredient is expressed on a total mixed diet basis.
Table 2.
Ingredient and calculated composition of experimental diets (as-fed basis)1
| Item | Dietary treatment | |
|---|---|---|
| Control | Test | |
| Ingredient, % | ||
| Corn | 48.5 | 48.5 |
| SBM | 20.0 | 20.0 |
| Dried whey | 17.2 | 17.2 |
| Fish meal | 10.0 | 10.0 |
| Choice white grease | 1.50 | 1.50 |
| Ground limestone | 0.50 | 0.50 |
| Sodium chloride | 0.50 | 0.50 |
| Vitamin and mineral premix2 | 0.30 | 0.30 |
| Choline chloride | 0.07 | 0.07 |
| l-Lys HCl | 0.25 | 0.25 |
| dl-Met | 0.05 | 0.05 |
| l-Thr | 0.05 | 0.05 |
| l-Trp | 0.04 | 0.04 |
| Wild-type algal protein | 1.00 | — |
| Osteopontin-enriched algal protein | — | 1.00 |
| Calculated composition 3 | ||
| ME, kcal/kg | 3,389 | 3,389 |
| CP, % | 22.3 | 22.3 |
| SID amino acids, % | ||
| Lys | 1.51 | 1.51 |
| Met + Cys | 0.78 | 0.78 |
| Trp | 0.27 | 0.27 |
| Thr | 0.85 | 0.85 |
| Val | 0.96 | 0.96 |
1All pigs received allotted treatment diet upon starting –10 DPI. Abbreviations: DPI, day postinoculation; WA, wild-type algal protein (i.e., control diet); OA, OPN-type algal protein (i.e., test diet); SID, standardized ileal digestibility.
2Vitamin–mineral premix (JBS United, Sheridan, IN) included the following per kilogram complete diet: Vitamin A (retinyl acetate), 11,128 IU; Vitamin D3 (cholecalciferol), 2,204 IU; vitamin E (dl-α tocopheryl acetate), 66 IU; vitamin K (menadione nicotinamide bisulfite), 1.42 mg; thiamine (thiamine mononitrate), 0.24 mg; riboflavin, 6.58 mg; pyridoxine (pyridoxine hydrochloride), 0.24 mg; vitamin B12, 0.03 mg; d-pantothenic acid (d-calcium pantothenate), 23.5 mg; niacin (nicotinamide and nicotinic acid), 44 mg; folic acid, 1.58 mg; biotin, 0.44 mg; Cu (copper sulfate), 10 mg; Fe (iron sulfate), 125 mg; I (potassium iodate), 1.26 mg; Mn (manganese sulfate), 60 mg; Se (sodium selenite), 0.3 mg; and Zn (zinc oxide), 100 mg.
3Metabolizable energy and standardized ileal digestible (SID) amino acid values were calculated using NRC (2012). Analyzed crude protein determined as total nitrogen × 6.25.
Experimental diets were fed in a single feeding phase to reduce confounds associated with a diet switch around the time of inoculation. Pigs were started on their assigned dietary treatment upon arrival at the animal facility and had 10 d of acclimation prior to inoculation with ETEC as further described below. Overall, 18 individual pigs were assigned to each treatment group as housed in 2 pens per treatment. Each treatment group involving challenged pigs was represented only once per room (1 pen of 9 pigs), while the negative control treatment (sham inoculated) was represented by 2 pens in an isolated room to maintain biosecurity.
Enterotoxigenic E. coli challenge
Following a 10-d acclimation to the diets and facility, pigs were orally inoculated on each of 3 consecutive days [i.e., 0–2 d postinoculation (DPI)] with either 3 mL of sterile phosphate-buffered saline (sham-control) or a live culture of a pathogenic F18-fimbriated enterotoxigenic E. coli strain (1010 CFU ETEC/3-mL dose, isolate number UI-VDL 05-27242, Dr. Carol Maddox, College of Veterinary Medicine, University of Illinois, Urbana, IL). In aligning experimental treatment groups and infection status, only the NC group remained uninfected throughout the study duration.
Growth performance
Growth performance was collected on a pen basis (n = 2 per treatment). Individual pig and feeder weights were captured at –10, 0, and 9 DPI to allow for the calculation of average daily gain (ADG), average daily feed intake (ADFI), and feed efficiency (gain to feed, G:F). Growth performance data are reported in reference to the inoculation schedule (–10 to 9 DPI). It should be noted that growth performance was not a primary outcome and the study was not adequately powered to permit interpretation of growth performance parameters; emphasis is placed on other outcomes that were captured using individual pig as the experimental unit.
Blood collection and analyses
Whole blood (up to 8 mL total) was collected from the jugular vein of up to 4 pigs per pen (n = 8 pigs per treatment with the same pigs collected when possible) into evacuated tubes containing EDTA as an anticoagulant (BD, Franklin Lakes, NJ) at 0, 7, and 10 DPI. Following collection, blood samples were immediately placed on ice and subsequently submitted to the University of Illinois College of Veterinary Medicine Clinical Pathology Lab for quantitation of complete blood cell counts and differential analyses using a multi-parameter, automated hematology analyzer (CELL-DYN 3700, Abbott Laboratories, Abbott Park, IL). Additional whole blood collected at 7 DPI was used to perform a T-cell immunophenotyping procedure via flow cytometry as detailed below.
Serum samples were collected into evacuated serum tubes, allowed to clot at room temperature for 30 to 60 min, and centrifuged at 1,300 × g for 15 min at 20 °C. Serum was removed from centrifuged samples and stored in 0.5 mL aliquots at –80 °C, pending subsequent analyses. A single aliquot was utilized for detection of serum concentrations of cytokines, including tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), interleukin-1β (IL-1β), interleukin-10 (IL-10), interleukin-8 (IL-8), interferon-α (IFN-α), and interleukin-4 (IL-4), measured at 0, 7, and 10 DPI using the Swine Cytokine Magnetic 7-Plex Panel according to manufacturer instructions (Novex by Life Technologies, Frederick, MD).
T-cell immunophenotyping
Whole blood collected on 7 DPI was used for isolation of peripheral blood mononuclear cells for a T-cell immunophenotyping procedure using flow cytometry. In brief, peripheral blood mononuclear cells were isolated by placing individual whole blood samples over a density gradient [SepMate 15 (IVD) and Lymphoprep, StemCell Technologies, Cambridge, MA] and centrifuging at 1,200 × g for 25 min at 20 °C. Isolated cells were then washed with phosphate-buffered saline containing 2% fetal bovine serum before being counted using the Moxi Z Mini Automated Cell Counter (ORFLO Technologies, Ketchum, ID). Individual samples were prepared with 1.0 × 106 cells and labeled with external fluorescent antibodies against cell surface markers CD3 (FITC mouse anti-pig CD3ε, BD Pharmingen, San Jose, CA), CD4 (Alexa Fluor 647 mouse anti-pig CD4a, BD Pharmingen), and CD8 (PE mouse anti-pig CD8b, BD Pharmingen). Following the application of external antibodies for 45 min, cells were fixed using 4% paraformaldehyde solution for 20 min. Prepared, labeled cells were evaluated at the University of Illinois Flow Cytometry Facility using a BD LSR II Flow Cytometry Analyzer (BD Biosciences), and flow cytometer outputs were analyzed using FCS Express 5 Plus (De Novo Software, Glendale, CA).
To summarize our analysis and gating procedures, recorded cellular events were plotted by density using forward and side scatter areas on the y- and x-axes, respectively, to exclude dead cells and cellular debris and allow for the selection of lymphocyte cells based on size. A single gate was applied to the lymphocyte population, the content of which was then plotted by density using forward scatter width and side scatter on the y- and x-axes, respectively, to allow for the gating and analysis of single cell events within our lymphocyte population only. Utilizing single cellular events within our lymphocyte population, single-stain control samples were used to set our detection thresholds for each individual fluorochrome and creation of a CD3+ gate. Gated on CD3+ lymphocytes, a final density plot using our CD8+ and CD4+ fluorochrome channels on the y- and x-axes, respectively, was created and our individual fluorochrome detection levels were used to apply quadrants to the density plot. Statistics from each quadrant were then used for our individual effector T-cell proportions (e.g., helper T-cells and CD3+/CD4+). Once relative T-cell populations had been generated, the ratio of helper-to-cytotoxic T-cells was calculated at each study time-point as a clinical indicator of immune status.
Intestinal tissues and contents
On the terminal day of the study, 10 DPI, 2.5 to 5 cm sections of the ileum, ascending colon, and descending colon were collected from up to 4 pigs per pen (8 pigs per treatment) and preserved in 10% neutral buffered formalin for histological analyses of mucosal surface morphology by a board-certified veterinary histologist (Veterinary Diagnostic Pathology, LLC, Fort Valley, VA). Histologic evaluations utilized were 5-μm hematoxylin and eosin-stained sections prepared from formalized samples. Microscopic examinations included semi-quantitative severity scoring of histopathology lesions and microscopic histologic features. A 5-level severity scoring system was employed in which: 0 = absent, 1 = minimal, 2 = mild, 3 = moderate, 4 = marked, and 5 = severe. When uncertainty existed between scoring groups in assigning a value, half values were given. Morphometric measurements of the ileal villus heights and crypt depths were also made and the villus:crypt ratios were then calculated. A single measurement for each determination was made in micrometer for an estimated average length or height. All evaluations were made in a blinded fashion without the histopathologist having knowledge of study time-point, pig age, treatment assignment, or study management practices that had been employed.
Following collection of intestinal tissue sections, ascending colon contents were collected from the same sections of each pig by expressing them into a clean plastic weigh boat and aliquoted into several separate samplings prior to flash freezing in liquid nitrogen. Samples from the ascending colon were used for analyses of volatile chain fatty acid (VFA) concentrations and bacterial composition and the ascending colon microbiota, for which procedures are described in the following sections.
Fecal outcomes
Fecal samples were collected from up to 6 pigs per pen (n = 12 pigs per treatment, same pigs collected when possible) at both 0 and 7 DPI using either a sterile cotton swab or a fecal loop. Individual sterile swabs were utilized for each animal, but in the event that a fecal loop was required for collection the loop was cleaned with a 70% ethanol solution between pigs. A small amount of feces was separated and submitted to the University of Illinois College of Veterinary Medicine Veterinary Diagnostic lab for total bacteria, total E. coli, and F18-fimbriated E. coli enumeration via quantitative real-time polymerase chain reaction (PCR). Total nucleic acid was extracted from 100 to 120 mg of fecal material using a Biosprint 96 Work Station (Qiagen, Hayward, CA). Fecal samples were resuspended in 500 µL of RNEasy lysis buffer and vortexed for 15 s to produce homogeneous mixtures. Then, 100 µL of this mixture was processed according to manufacturer’s recommendations using the One-For-All-Vet Kit (Qiagen), and nucleic acids were eluted in 75 µL of elution buffer.
Total bacteria, total E. coli, and F18-fimbriated E. coli were quantified by PCR using the rrs, gadAB, and fedA genes, respectively. Quantitative real-time PCR was performed on an Eppendorf Mastercycler ep Realplex System (Eppendorf North America, Westbury Road, NY). The reaction mixture contained 1× Quanta PerfeCTa SYBR Green Supermix (Quanta Biosciences, Gaithersberg, MD 2087); 0.3 µM each of the forward and reverse primers (gadAB and rrs) or 0.625 µM of primers for fedA, and 1 µL of DNA in a total volume of 20 µL. The challenge E. coli strain used for inoculating pigs in the infected treatments served as a positive control. A separate fecal sample was collected at 7 DPI for in-house analyses of fecal DM as a measure of clinical response to ETEC infection.
Volatile fatty acids
Ascending colon contents and fecal samples collected at 10 DPI were thawed, weighed (100 mg each), and acidified with an equal volume of 2 N HCl or 6.25% m-phosphoric acid, respectively, and subsequently sonicated and stored overnight at −20 °C. Samples were then thawed and centrifuged for 10 min at 16,500 × g, and the supernatant was collected for analysis via gas chromatography. All samples were assessed as previously described (Smiricky-Tjardes et al., 2003) for VFA, which were further divided into short chain fatty acids (SCFA; i.e., acetate, propionate, and butyrate) and branched chain fatty acids (BCFA; i.e., valerate, isovalerate, and isobutyrate). Acetic, propionic, n-butyric, valeric, isovaleric, and isobutyric acid solutions were used as standards (Sigma-Aldrich, St. Louis, MO) to quantify individual VFA concentrations.
Microbiota outcomes
DNA was isolated from ascending colon contents collected at 10 DPI using the QIAamp Fast DNA Stool Mini Kit (Qiagen, Valencia, CA) in combination with bead beating on the FastPrep-24 System (MP Biomedicals, Carlsbad, CA) as previously described (Reznikov et al. 2018). PCR amplification and sequencing of the V3-V4 region of 16S rRNA genes were performed at the University of Illinois DNA Services Lab using primers V3f (5′-CCTACGGGAGGCAGCAG-3′) and V4r (5′-GGACTACHVGGGTWTCTAAT-3′) and an Illumina MiSeq flow cell with a MiSeq Reagent Nano Kit version 2 (2 × 250 nt paired end reads) as described previously (Monaco et al., 2018).
Sequences were processed and visualized using QIIME 2 pipeline (Bolyen et al., 2019). Paired-end reads obtained from the sequencing facility were imported into QIIME 2 and demultiplexed using the plugin demux with emp-paired method. Demultiplexed reads were primer removed, chimera checked, and dereplicated using command DADA2 denoise-paired (Callahan et al., 2019). A feature table, representing the counts of exact sequence variants for each sample, was generated, and representative sequences were picked from each feature. The representative sequences were aligned with mafft (Katoh and Standley, 2013), and the alignment was filtered to remove unconserved and gapped columns with the use of mask (Lane, 1991). The phylogenetic tree was constructed from filtered alignment via fasttree (Price et al., 2010). Alpha diversity and UniFrac distance metrics were computed through the plugin diversity with the core metrics-phylogenetic method on the feature table and phylogenetic tree. Samples were rarefied to an equal number of reads (24 630) when α-diversity and UniFrac distance metrics were calculated.
For taxonomic analysis, the feature table was filtered to remove features that presented in <10% of samples and with abundances <10. Taxonomic assignment to features was performed using plugin feature-classifier with classify sklearn method (Pedregosa et al., 2011) in which a pretrained Naïve–Bayes classifier with SILVA 132 99% operational taxonomic units (OTU) from V3–V4 regions of sequences was used (https://www.arb-silva.de/; Quast et al., 2013). Taxonomic bar plots were generated using the plugin taxa with the taxa barplot method. The bar plots were visualized and count table at genus level was downloaded using QIMME 2 View (https://view.qiime2.org/) for differential abundance analysis.
Statistical analyses
For all data excluding microbiome outcomes, statistical analysis was dependent on whether outcomes were measured at a single time-point [1-way analysis of variance (ANOVA)] or at multiple time-points (two-way ANOVA) for the same subject. Individual pig served as the experimental unit for all fecal, blood, histological, and immune outcomes (n = 18 pigs per treatment). For growth performance outcomes, pen served as the experimental unit (n = 2 pens per treatment), but the study design was not powered to consider growth performance as a primary outcome. As such, growth performance data are merely shown for reference as pigs were raised under conditions mimicking group housing in a commercial setting.
For all single time-point outcomes, a 1-way ANOVA was conducted using the MIXED procedure of SAS (v. 6.1; SAS Institute, Inc., Cary, NC). For repeated measures, a 2-way ANOVA including the effect of time sliced by DPI was conducted for all outcomes involving samples collected from the same subject at multiple time-points. When a model was found to be significant, means separation was conducted and least-square means and their associated standard errors of the mean (SEM) were generated. In all cases, outliers were identified as having an absolute Studentized residual value of 3 or greater, and statistical significance was considered at P < 0.05.
For microbiota outcomes, two planned contrasts (i.e., pairwise comparisons) were used: (1) NC vs. PC to assess the effects of F18 ETEC infection and (2) PC vs. OA to assess the effect of algal protein source when consumed by ETEC challenged pigs. β-Diversity (i.e., difference between samples) was evaluated with plugins in QIIME2 (Bolyen et al., 2019). Principal co-ordinate analysis (PCoA) was performed on weighted UniFrac distance matrices using plugin diversity with the diversity PCoA method. The PCoA plot was visualized by plugin emperor. The group significance in β-diversity was statistical confirmed by permutational multivariate analysis of variance (PERMANOVA) as implemented in plugin diversity. α-Diversity was analyzed using the PROC MIXED procedure of SAS (version 9.4, SAS Institute) with infection or algal protein as a fixed effect. For differential abundance analysis, counts of bacterial genera were tested using a negative binomial Wald test implemented in the DESeq2 package of R (Love et al., 2014). Data are reported as means ± SEM or log2 fold change. For α- and β-diversity analysis, statistical significance was defined as P < 0.05. When examining differential abundance of genera, P-values were adjusted (Padj) for multiple testing by the Benjamini–Hochberg procedure and a Padj < 0.1 was considered statistically significant.
Results
Clinical blood indicators
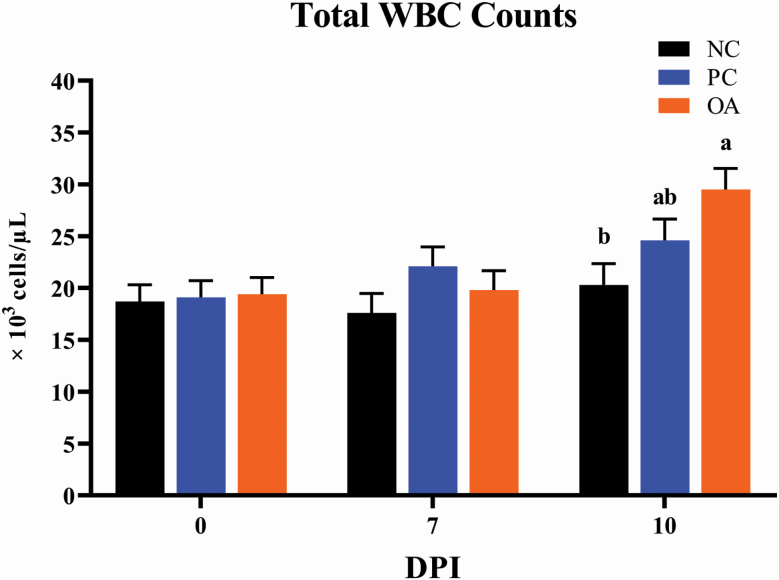
There were no differences between experimental treatments for any erythrogram outcomes at 0 DPI (Table 3). At 7 DPI, PC elicited the greatest (P < 0.05) RBC count and mean corpuscular hemoglobin concentrations (MCHC) compared with the other treatments. Compared with the NC, ETEC infection (both PC and OA) elicited decreased (P < 0.05) erythrocyte cell volume (MCV) and mean corpuscular hemoglobin (MCH). At 10 DPI, PC again had the highest (P < 0.05) MCHC compared with other treatments and ETEC infection decreased (P < 0.05) MCV and MCH when comparing NC with either PC or OA treatments. In terms of leukocyte outcomes, OA pigs exhibited increased (P < 0.05) WBC counts relative to NC pigs at 10 DPI, while PC pigs were intermediate (Figure 1). Only minimal differences were observed for WBC and relative proportions of circulating WBC sub-populations across the other experimental time-points (Supplementary Table 1).
Table 3.
Effects of dietary algal protein supplementation and ETEC infection on erythrogram parameters in weanling pigs1
| Uninfected | ETEC-infected | Model P-value | |||
|---|---|---|---|---|---|
| Item | NC | PC | OA | SEM | DPI × TRT |
| DPI 0 | |||||
| RBC, ×106 cells/µL | 6.41 | 6.65 | 6.56 | 0.124 | 0.375 |
| HGB, g/dL | 12.5 | 12.5 | 12.5 | 0.248 | 0.989 |
| HCT, % | 39.1 | 39.1 | 38.9 | 0.741 | 0.955 |
| MCV, fl | 61.0 | 58.8 | 59.3 | 0.735 | 0.089 |
| MCH, pg | 19.5 | 18.7 | 19.0 | 0.249 | 0.093 |
| MCHC, g/dL | 32.0 | 31.8 | 32.1 | 0.161 | 0.511 |
| DPI 7 | |||||
| RBC, ×106 cells/µL | 5.95b | 6.32a | 5.90b | 0.143 | 0.049 |
| HGB, g/dL | 11.4 | 11.5 | 10.8 | 0.287 | 0.180 |
| HCT, % | 36.2 | 35.3 | 33.8 | 0.855 | 0.125 |
| MCV, fl | 60.9a | 56.0b | 57.4b | 0.848 | <0.001 |
| MCH, pg | 19.1a | 18.2b | 18.3b | 0.288 | 0.034 |
| MCHC, g/dL | 31.4c | 32.5a | 32.0b | 0.186 | <0.001 |
| DPI 10 | |||||
| RBC, ×106 cells/µL | 5.57 | 5.93 | 5.56 | 0.157 | 0.134 |
| HGB, g/dL | 10.6 | 10.3 | 9.99 | 0.314 | 0.390 |
| HCT, % | 34.3 | 32.4 | 32.1 | 0.937 | 0.137 |
| MCV, fl | 59.3a | 55.7b | 57.7b | 0.929 | <0.001 |
| MCH, pg | 18.9a | 17.7b | 18.0b | 0.315 | 0.005 |
| MCHC, g/dL | 30.8b | 31.8a | 31.1b | 0.203 | 0.002 |
1Values represent least square means of 4 to 8 pigs per treatment. All pigs were started on their assigned dietary treatment starting at –10 DPI. Experimental treatments: NC, sham-inoculated pigs receiving 1.0% wild-type algal protein; PC, ETEC-inoculated pigs receiving 1.0% wild-type algal protein; OA, ETEC-inoculated pigs receiving 1.0% OPN-enriched algal protein. Abbreviations: ETEC, enterotoxigenic E. coli; DPI = days postinoculation; TRT, treatment; RBC, red blood cell; HGB, hemoglobin; HCT, hematocrit; MCV, mean cell volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration.
a–cMeans without a common superscript letter within a single time-point differ (P < 0.05).
Figure 1.
Effects of dietary algal protein supplementation and F18-fimbriated ETEC infection on white blood cell counts of whole blood collected from weanling pigs collected at 0, 7, and 10 DPI. Means without a common superscript letter differ (P < 0.05). Values represent least square means of 4 to 8 pigs per treatment. All pigs were started on their assigned dietary treatment starting at –10 DPI. Experimental treatments: NC, sham-inoculated pigs receiving 1.0% wild-type algal protein; PC, ETEC-inoculated pigs receiving 1.0% wild-type algal protein; OA, ETEC-inoculated pigs receiving 1.0% OPN-enriched algal protein. Abbreviations: ETEC, enterotoxigenic E. coli; DPI, days postinoculation; TRT, treatment; WBC, white blood cells.
Serum cytokines
In general, more than 90% of serum samples analyzed at each time-point had IL-1β, IL-10, IFN-γ, and IL-4, concentrations that were below the detectable limit of each assay, and thus, results for these cytokines are not presented. Additionally, TNF-α concentrations were below the detectable limit for more than 90% of serum samples collected at 0 or 7 DPI, so data for these time-points are also not shown. There were no differences (P > 0.05) in the concentrations of IFN-α or IL-8 between experimental treatments at 0 or 7 DPI (Table 4). However, at 10 DPI, OA pigs exhibited the highest (P < 0.05) concentration of TNF-α compared with other treatments, but no differences were observed for IFN-α or IL-8.
Table 4.
Effects of dietary algal protein supplementation and ETEC infection on serum inflammatory cytokine concentrations (pg/mL) in weanling pigs1
| Uninfected | ETEC-infected | Model P-value | |||
|---|---|---|---|---|---|
| Item2 | NC | PC | OA | SEM | DPI × TRT |
| 0 DPI | |||||
| IFN-α | 4.72 | 10.4 | 6.52 | 19.5 | 0.972 |
| TNF-α | — | — | — | — | — |
| IL-8 | 164 | 171 | 206 | 80.9 | 0.905 |
| 7 DPI | |||||
| IFN-α | 28.0 | 29.7 | 18.5 | 19.5 | 0.853 |
| TNF-α | — | — | — | — | — |
| IL-8 | 215 | 387 | 304 | 99.0 | 0.232 |
| 10 DPI | |||||
| IFN-α | 53.9 | 68.5 | 50.4 | 15.9 | 0.628 |
| TNF-α | 107b | 138b | 332a | 65.3 | 0.044 |
| IL-8 | 143 | 144 | 139 | 99.0 | 0.999 |
1Values represent least square means of 2 to 8 pigs per treatment. All pigs were started on their assigned dietary treatment starting at –10 DPI. Experimental treatments: NC, sham-inoculated pigs receiving 1.0% wild-type algal protein; PC, ETEC-inoculated pigs receiving 1.0% wild-type algal protein; OA, ETEC-inoculated pigs receiving 1.0% OPN-enriched algal protein. Abbreviations: ETEC, enterotoxigenic E. coli; DPI, days post-inoculation; TRT, treatment; IFN-α, interferon alpha; TNF-α, tumor necrosis factor alpha; IL-8, interleukin 8, ND, non-detectable.
2More than 90% of serum samples analyzed at 0 and 7 DPI were below the assay detectable limit for TNF-α and were excluded from the analysis (missing data denoted by ‘-‘).
abMeans without a common superscript letter differ (P < 0.05).
T-cell immunophenotyping
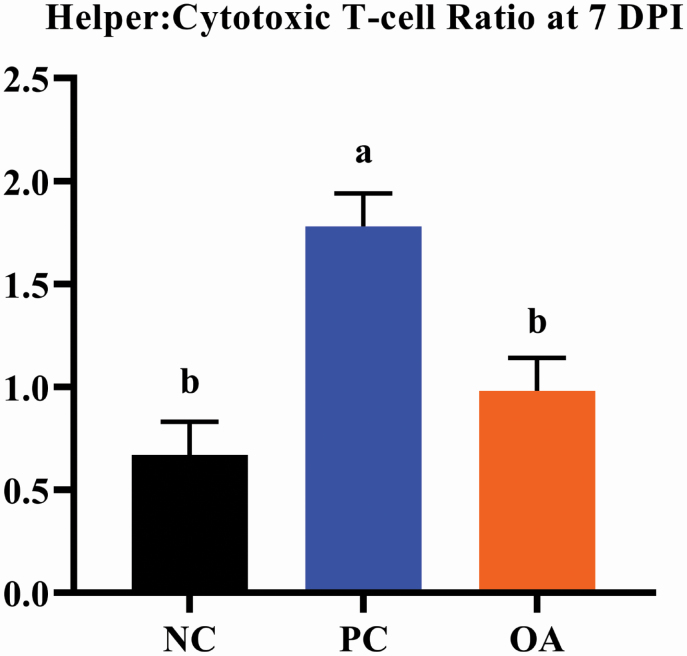
While there were no differences (P > 0.05) in the total proportion of circulating mature T-cells (i.e., CD3+ cells; Figure 2), ETEC infection increased (P < 0.05) the relative proportion of circulating helper T-cells (i.e., CD3+/CD4+ cells) when comparing NC to either PC or OA treatments. Additionally, the PC treatment elicited the lowest (P < 0.05) relative proportion of circulating cytotoxic T-cells (i.e., CD3+/CD8+ cells) compared with other treatments, which resulted in the highest (P < 0.05) helper:cytotoxic T-cell ratio. Additional information regarding T-cell immunophenotyping data can be found in Supplementary Table 2.
Figure 2.
Effects of dietary algal protein supplementation and F18-fimbriated ETEC infection on the ratio of peripheral blood helper-to-cytotoxic T-cells in weanling pigs at 7 DPI. Means without a common superscript letter differ (P < 0.05). Values represent least square means of 6 to 8 pigs per treatment. All pigs were started on their assigned dietary treatment starting at –10 DPI. Experimental treatments: NC, sham-inoculated pigs receiving 1.0% wild-type algal protein; PC, ETEC-inoculated pigs receiving 1.0% wild-type algal protein; OA, ETEC-inoculated pigs receiving 1.0% OPN-enriched algal protein. Helper T-cells are the percent of total CD3-positive lymphocytes that are also positive for cell-surface marker CD4 (CD3+/CD4+). Cytotoxic T-cells are the percent of total CD3-positive lymphocytes that are also positive for cell-surface marker CD8 (CD3+/CD8+). Abbreviations: ETEC, enterotoxigenic E. coli; DPI, days postinoculation; TRT, treatment.
Growth performance
Whereas this study was not designed to evaluate growth performance as a primary outcome, data are presented to provide context for the degree to which dietary and immune challenge treatments affected the growth of weanling pigs (Table 5). During the pre-inoculation period (–10 to 0 DPI; i.e., while pigs were receiving assigned diets but before ETEC challenge), no differences were observed in individual pig BW or pen ADG, ADFI, or G:F outcomes. During the post-inoculation period (0 to 9 DPI) and over the entire study (–10 to 9 DPI), ETEC infection resulted in decreased ADG and G:F when comparing NC pigs with either PC or OA pigs, which is why ETEC-challenged pigs exhibited decreased (P < 0.05) final BW compared with NC pigs. However, there were never differences noted for any growth performance outcomes between PC and OA treatments.
Table 5.
Effects of dietary algal protein supplementation and ETEC infection on BW and growth performance of weanling pigs1
| Uninfected | ETEC-infected | Model P-value | |||
|---|---|---|---|---|---|
| Item | NC | PC | OA | SEM | DPI × TRT |
| BW, kg | |||||
| DPI –10 | 5.98 | 5.97 | 5.90 | 0.281 | 0.977 |
| DPI 0 | 7.14 | 7.16 | 7.37 | 0.281 | 0.814 |
| DPI 9 | 11.8a | 8.71b | 9.32b | 0.359 | <0.001 |
| –10 to 0 DPI | |||||
| ADFI, g/d | 542 | 531 | 525 | 11.2 | 0.593 |
| ADG, g/d | 116 | 118 | 147 | 26.3 | 0.696 |
| G:F, g/kg | 0.21 | 0.22 | 0.28 | 0.06 | 0.681 |
| 0 to 9 DPI | |||||
| ADFI, g/d | 595 | 636 | 733 | 27.7 | 0.128 |
| ADG, g/d | 458a | 202b | 276b | 27.8 | 0.043 |
| G:F, g/kg | 0.77a | 0.32b | 0.38b | 0.04 | 0.022 |
| –10 to 9 DPI | |||||
| ADFI, g/d | 404 | 424 | 469 | 13.3 | 0.130 |
| ADG, g/d | 291a | 160b | 194b | 8.54 | 0.016 |
| G:F, g/kg | 0.72a | 0.38b | 0.41b | 0.01 | 0.005 |
1Values represent least square means of 10 to 18 pigs per treatment. Pigs were removed from analysis for ADG, ADFI, and G:F if their BW measurement was deemed as a statistical outlier. All pigs were started on their assigned dietary treatment starting at –10 DPI. Experimental treatments: NC, sham-inoculated pigs receiving 1.0% wild-type algal protein; PC, ETEC-inoculated pigs receiving 1.0% wild-type algal protein; OA, ETEC-inoculated pigs receiving 1.0% OPN-enriched algal protein. Abbreviations: ETEC, enterotoxigenic E. coli; DPI, days postinoculation; TRT, treatment; ADG, average daily gain; ADFI, average daily feed intake; G:F, feed efficiency.
abMeans without a common superscript letter differ (P < 0.05).
Fecal PCR bacterial profiling
No pigs had detectable expression of the F18 gene fecal samples at 0 DPI (i.e., before inoculation procedures had commenced; Table 6). Pigs assigned to the PC treatment expressed the highest (P < 0.05) fecal total bacterial expression (as demonstrated by a lower Ct value) compared with other treatments at 0 DPI, though this effect did not continue thereafter. At 7 DPI, ETEC infection increased (P < 0.05) fecal total E. coli expression when comparing NC with PC or OA treatments. Moreover, fecal F18 E. coli expression remained undetectable in NC pigs at 7 DPI, while F18 E. coli expression was increased (P < 0.05) in both ETEC-inoculated treatments at this time-point when compared with the NC treatment.
Table 6.
Effects of dietary algal protein supplementation and ETEC infection on prevalence of fecal bacterial gene expression (raw Ct values) in weanling pigs1
| Uninfected | ETEC-infected | Model P-value3 | |||
|---|---|---|---|---|---|
| Item2 | NC | PC | OA | SEM | DPI × TRT |
| DPI 0 | |||||
| Total bacteria (rrs) | 13.75a | 12.21b | 13.64a | 0.37 | 0.001 |
| Total E. coli (gadAB) | 35.64 | 36.68 | 32.96 | 1.34 | 0.151 |
| F18 ETEC (fedA) | ND | ND | ND | — | — |
| DPI 7 | |||||
| Total bacteria (rrs) | 13.64 | 13.53 | 12.98 | 0.43 | 0.468 |
| Total E. coli (gadAB) | 37.23a | 24.49b | 26.19b | 1.50 | <0.001 |
| F18 ETEC (fedA) | ND | 33.35 | 35.20 | 0.90 | 0.673 |
1Values represent least square means of 6 to 8 pigs per treatment. All pigs were started on their assigned dietary treatment starting at –10 DPI. Experimental treatments: NC, sham-inoculated pigs receiving 1.0% wild-type algal protein; PC, ETEC-inoculated pigs receiving 1.0% wild-type algal protein; OA, ETEC-inoculated pigs receiving 1.0% OPN-enriched algal protein. Abbreviations: ETEC, enterotoxigenic E. coli; DPI, days postinoculation; TRT, treatment; Ct, cycle threshold; ND, nondetectable.
2Bacterial gene name shown in parentheses. Data shown as raw Ct values, with higher values denoting lower expression of a particular gene.
3P-values represented were calculated on log2 transformed data.
abMeans without a common superscript letter differ (P < 0.05).
Fecal dry matter
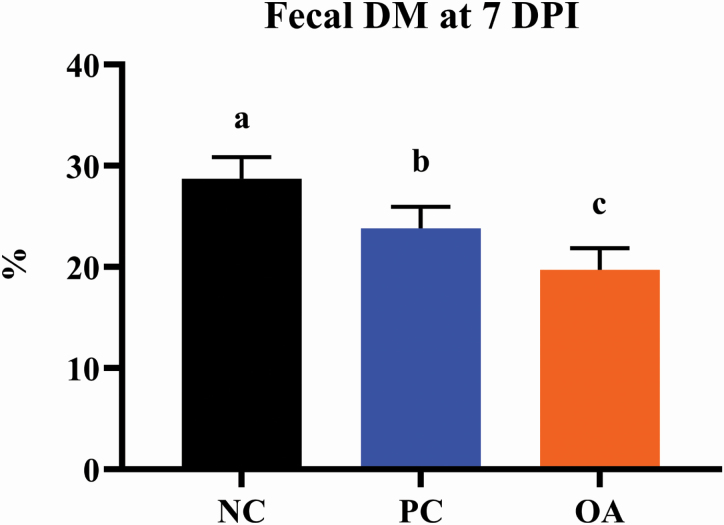
Fecal DM content at 0 and 7 DPI was quantified as an objective measure of fecal consistency in lieu of fecal scoring. Fecal DM content was highest (P < 0.05) in pigs assigned to the NC treatment, with OA pigs exhibiting the lowest DM content and PC pigs being intermediate (Figure 3). Anecdotally, pigs from ETEC-inoculated treatments had obvious signs of diarrhea throughout the post-inoculation period, though this could not be quantified on an individual basis due to group housing of pigs. With that in mind, we speculate that fecal sampling earlier in the postinoculation period may have been needed to elucidate more pronounced differences between noninfected and infected pigs.
Figure 3.
Effects of dietary algal protein supplementation and strain F18 ETEC infection on dry matter content of feces from weanling pigs collected on 7 DPI. Means without a common superscript letter differ (P < 0.05). Values represent least square means of 8 to 12 pigs per treatment. All pigs were started on their assigned dietary treatment starting at –10 DPI. Experimental treatments: NC, sham-inoculated pigs receiving 1.0% wild-type algal protein; PC, ETEC-inoculated pigs receiving 1.0% wild-type algal protein; OA, ETEC-inoculated pigs receiving 1.0% OPN-enriched algal protein. Abbreviations: ETEC, enterotoxigenic E. coli; DPI, days postinoculation; TRT, treatment.
Intestinal tissue histopathology
Across all histomorphology measures, there were no effects of experimental treatment observed (Supplementary Table 3).
Ascending colon luminal contents
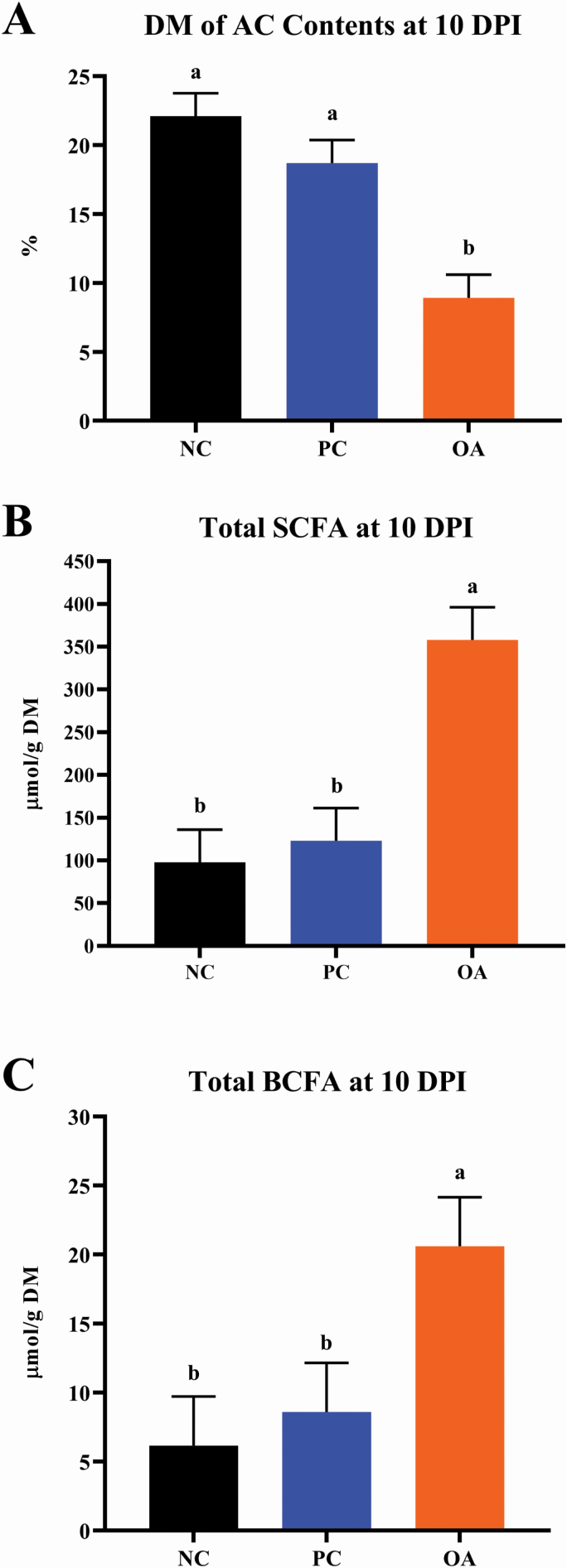
In general, OA pigs exhibited ascending colon DM concentrations that were roughly half (P < 0.05) those observed for NC and PC pigs (Figure 4). Additionally, OA pigs exhibited the highest total concentrations of SCFA and BCFA compared with either the NC or PC treatments, which was a reflection of individual VFA components (Supplementary Table 4).
Figure 4.
Effects of dietary algal protein supplementation and F18-fimbriated ETEC infection on (A) DM, (B) SCFA, and (C) BCFA concentrations of ascending colon contents at 10 DPI in weanling pigs. Means without a common superscript letter differ (P < 0.05). Values represent least square means of 4 to 8 pigs per treatment. All pigs were started on their assigned dietary treatment starting at –10 DPI. Experimental treatments: NC, sham-inoculated pigs receiving 1.0% wild-type algal protein; PC, ETEC-inoculated pigs receiving 1.0% wild-type algal protein; OA, ETEC-inoculated pigs receiving 1.0% OPN-enriched algal protein. Abbreviations: ETEC, enterotoxigenic E. coli; DPI, days postinoculation; TRT, treatment; DM, dry matter; SCFA, short chain fatty acids; BCFA, branched chain fatty acids.
Microbiota analyses of ascending colon contents
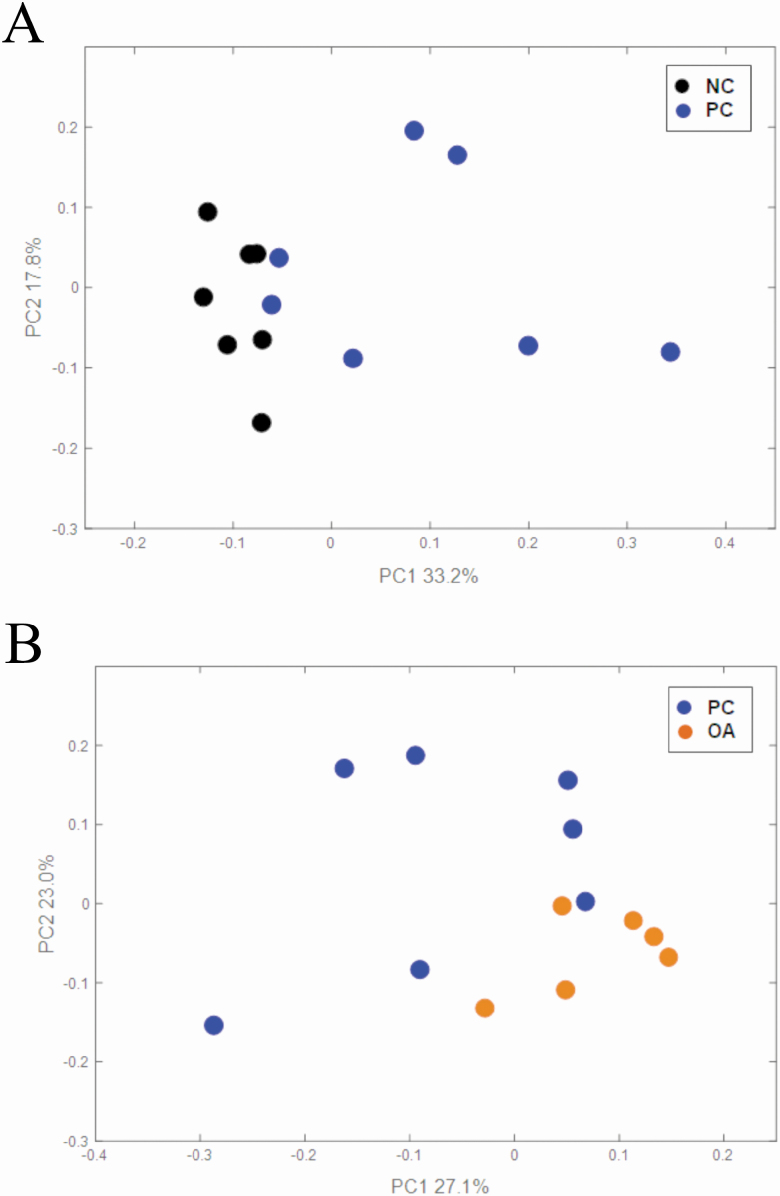
Illumina sequencing of 16S rRNA amplicons of V3–V4 region yielded 1.86 million paired-end reads (92,849 ± 9676 reads per sample) across all samples analyzed. After assembling of paired-end reads, quality filtering, and removing of singletons, 911,939 sequences (49,597 ± 4,526 reads per sample) were utilized for further analysis. PCoA of weighted UniFrac distances generated from ascending colon contents are shown in Figure 5. PERMANOVA analysis revealed both F18 ETEC infection (NC vs. PC; P = 0.003) and dietary algal protein source (PC vs. OA; P = 0.015) influenced overall bacterial community structures. Compared with NC pigs, 6 bacterial genera were increased, and 7 were decreased in PC pigs (Padj < 0.1; Table 7). Abundances of 2 genera were increased and 12 were decreased in OA pigs compared with PC pigs (Padj < 0.1; Table 8). However, neither ETEC infection nor dietary algal protein source influenced α-diversity outcomes (Table 9), though OA pigs exhibited a lower (P = 0.033) Shannon index compared with PC pigs.
Figure 5.
Principal coordinate analysis based on weighted UniFrac distances generated from ascending colon lumenal contents from 6 to 7 pigs per treatment at 10 DPI: (A) effect of F18 ETEC infection, PERMANOVA P = 0.003 and (B) effect of dietary algal protein, PERMANOVA P = 0.015. Experimental treatments: NC, sham-inoculated pigs receiving 1.0% wild-type algal protein; PC, ETEC-inoculated pigs receiving 1.0% wild-type algal protein; OA, ETEC-inoculated pigs receiving 1.0% OPN-enriched algal protein.
Table 7.
Effects of dietary algal protein supplementation and ETEC infection on α-diversity of microbiota profiles in ascending colon contents at 10 DPI in weaning pigs1
| Uninfected | ETEC-infected | P-value | |||
|---|---|---|---|---|---|
| Item | NC | PC | OA | NC vs. PC | PC vs. OA |
| Observed OTU | 361.7 ± 34.1 | 295.7 ± 19.0 | 244.2 ± 31.9 | 0.090 | 0.093 |
| Shannon | 6.17 ± 0.19 | 5.98 ± 0.17 | 5.35 ± 0.26 | 0.443 | 0.033 |
| Evenness | 0.73 ± 0.01 | 0.73 ± 0.02 | 0.68 ± 0.02 | 0.939 | 0.062 |
| Faith’s PD | 28.49 ± 1.63 | 28.12 ± 2.39 | 22.50 ± 2.05 | 0.890 | 0.071 |
1Values are least-square means ± SEM of 6 or 7 pigs per treatment. All pigs were started on their assigned dietary treatment starting at –10 DPI. Experimental treatments: NC, sham-inoculated pigs receiving 1.0% wild-type algal protein; PC, ETEC-inoculated pigs receiving 1.0% wild-type algal protein; OA, ETEC-inoculated pigs receiving 1.0% OPN-enriched algal protein. Abbreviations: ETEC, enterotoxigenic E. coli; OTU, operational taxonomic unit; PD, phylogenetic diversity.
Table 8.
Effects of ETEC infection on bacterial genera of ascending colon contents at 10 DPI in weanling pigs1
| Phylum | Family | Genus | Log2 fold-change PC/NC | P adj | Shift |
|---|---|---|---|---|---|
| Firmicutes | Ruminococcaceae | Oscillospira | 8.05 | <0.001 | Increase |
| Proteobacteria | Burkholderiaceae | Sutterella | 4.73 | 0.010 | Increase |
| Proteobacteria | Succinivibrionaceae | Succinivibrio | 4.52 | <0.001 | Increase |
| Firmicutes | Ruminococcaceae | Ruminiclostridium 9 | 3.84 | 0.001 | Increase |
| Firmicutes | Acidaminococcaceae | Phascolarctobacterium | 2.71 | 0.010 | Increase |
| Bacteroidetes | Muribaculaceae | Uncultured | 1.95 | 0.010 | Increase |
| Firmicutes | Lachnospiraceae | Oribacterium | –2.02 | 0.062 | Decrease |
| Firmicutes | Veillonellaceae | Dialister | –2.64 | 0.010 | Decrease |
| Firmicutes | Streptococcaceae | Streptococcus | –2.79 | 0.016 | Decrease |
| Firmicutes | Erysipelotrichaceae | Catenibacterium | –2.95 | 0.037 | Decrease |
| Firmicutes | Erysipelotrichaceae | Catenisphaera | –5.31 | 0.014 | Decrease |
| Firmicutes | Veillonellaceae | Unclassified | –6.51 | 0.042 | Decrease |
| Firmicutes | Lachnospiraceae | Lachnospiraceae NK3A20 group | –6.81 | 0.009 | Decrease |
1Data derived from 6 to 7 pigs per treatment and analyzed using the DESeq2 package of R. All pigs were started on their assigned dietary treatment starting at –10 DPI. Experimental treatments: NC, sham-inoculated pigs receiving 1.0% wild-type algal protein; PC, ETEC-inoculated pigs receiving 1.0% wild-type algal protein. Abbreviations: ETEC, enterotoxigenic E. coli; Padj, P-value adjusted by the Benjamini–Hochberg method to control for false discovery rate.
Table 9.
Effects of dietary algal protein supplementation during ETEC-infection on bacterial genera of ascending colon contents at 10 DPI in weanling pigs1
| Phylum | Family | Genus | Log2 fold-change OA/PC | P adj | Shift |
|---|---|---|---|---|---|
| Firmicutes | Streptococcaceae | Streptococcus | 3.44 | 0.032 | Increase |
| Firmicutes | Lachnospiraceae | Blautia | 1.36 | 0.081 | Increase |
| Bacteroidetes | Rikenellaceae | Rikenellaceae RC9 gut group | –1.86 | 0.035 | Decrease |
| Bacteroidetes | Muribaculaceae | Uncultured | –2.27 | 0.062 | Decrease |
| Bacteroidetes | Prevotellaceae | Prevotella 2 | –2.72 | 0.041 | Decrease |
| Bacteroidetes | Prevotellaceae | Prevotella 7 | –3.81 | 0.008 | Decrease |
| Bacteroidetes | Prevotellaceae | Prevotella 1 | –2.58 | 0.062 | Decrease |
| Proteobacteria | Burkholderiaceae | Sutterella | –5.10 | 0.035 | Decrease |
| Firmicutes | Ruminococcaceae | Uncultured | –5.59 | 0.014 | Decrease |
| Firmicutes | Ruminococcaceae | Ruminococcaceae UCG-010 | –5.97 | 0.035 | Decrease |
| Firmicutes | Lachnospiraceae | Lachnospiraceae UCG-010 | –6.32 | 0.078 | Decrease |
| Firmicutes | Ruminococcaceae | Candidatus Soleaferrea | –6.76 | 0.020 | Decrease |
| Firmicutes | Lachnospiraceae | Lachnospiraceae UCG-004 | –6.91 | 0.099 | Decrease |
| Bacteroidetes | Rikenellaceae | dgA-11 gut group | –7.60 | 0.008 | Decrease |
1Data derived from 6 to 7 pigs per treatment and analyzed using the DESeq2 package of R. All pigs were started on their assigned dietary treatment starting at –10 DPI. Experimental treatments: OA, ETEC-inoculated pigs receiving 1.0% OPN-enriched algal protein; PC, ETEC-inoculated pigs receiving 1.0% wild-type algal protein. Abbreviations: ETEC, enterotoxigenic E. coli; Padj, P-value adjusted by the Benjamini–Hochberg method to control for false discovery rate.
Discussion
Considering the multitude of stressors faced by weanling pigs, the use of dietary approaches to support health and well-being offers a number of practical advantages. Our research involved use of a live F18-fimbriated ETEC model to mimic clinical pathogenesis commonly experienced in a commercial setting (Fekete et al., 2002; Luise et al., 2019). We hypothesized that consumption of a novel algae-based ingredient enriched in OPN before and after ETEC infection would afford immune support to challenged pigs. Osteopontin is a naturally occurring glycoprotein that serves as a key mediator of recruitment and retention of macrophages and T-cells to sites of inflammation (Mazzali et al., 2002) and is also essential for efficient development of Th1-mediated immune responses (Rollo et al., 2005). Under these conditions, evidence from the current study supports a role for OPN-enriched algal protein to elicit changes in adaptive immunity, inflammatory status, and colonic fermentation patterns and microbiota community structure in ETEC-challenged pigs.
As part of an enteric infection, the host’s innate immune system reacts by increasing production of leukocytes as a primary defense against pathogens (Jiang et al., 2007; Rondina and Garraud, 2014; Hottz et al., 2018). Such a response typically results in both an increase in the total number of WBC in addition to proportional shifts in specific cell sub-types, including basophils and monocytes (Otsuka et al., 2016; Yamanishi and Karasuyama, 2016). Owing to the mild-to-moderate nature of the ETEC challenge that we used, immune system stimulation caused a change in basophil proportions at 7 DPI, though an elevation in WBC counts was not evident until 10 DPI. Whereas induction of an ETEC infection did not alter circulating inflammatory cytokine concentrations to a large extent, growth of infected pigs was reduced during the postinoculation period and the F18-fimbriated ETEC strain was only quantified in pigs assigned to infected treatments. Thus, differential effects between NC and PC treatments prove the effectiveness of inducing an F18 ETEC infection in weanling pigs (Mazzali et al., 2002; Caporgno and Mathys, 2018).
As germane to the study objective, we sought to identify whether ingestion of an OPN-enriched algal protein would alter immune outcomes following an ETEC infection when compared with pigs receiving a wild-type algal protein. Interestingly, OA pigs exhibited an elevation of monocytes even before ETEC inoculation (i.e., 0 DPI), and the innate response to infection continued as WBC counts were numerically higher at 10 DPI in OA pigs compared with PC pigs. The OPN-induced, pre-inoculation increase in monocytes may have indicated a primed innate immune response in pigs because circulating monocytes are precursors of activated macrophages (Ishikawa et al., 2018). The OPN protein is known to be expressed at low concentrations in monocytes, but its expression becomes continuous and elevated in activated macrophages that coordinate a pro-inflammatory response following infection (Gao et al., 2004). In the absence of OPN expression, macrophages exhibit impairments in motility and capacity for cytokine production (Sodek et al., 2006). Moreover, intestinal macrophages underlying the subepithelial lamina propria play a crucial role in the intestinal homeostasis (Toyonaga et al., 2015), suggesting that macrophages were likely important not only in the immune response but also intestinal tissue-level reactions involving the microbiota.
Concomitant with expansion of WBC counts at 7 to 10 DPI, OA pigs also experienced an exaggerated inflammatory response (i.e., increased circulating TNF-α) and a shift in the helper:cytotoxic T-cell ratio, thereby suggesting a differential response of the innate and adaptive arms of the immune system. TNF-α is a pro-inflammatory cytokine secreted by activated innate immune cells, including macrophages, dendritic cells, and basophils (Otsuka et al., 2016; Yamanishi and Karasuyama, 2016). As the largest granulocyte, basophils are able to migrate into affected tissues and produce cytokines (Mukai et al., 2005), but the extent to which basophil infiltration occurs during active bacterial infections remains unknown (Piliponsky et al., 2019). While not quantified in our study, dendritic cells are known to release TNF-α upon activation by OPN (Renkl et al., 2005), and OPN-activated dendritic cells can subsequently induce naïve T-cells to differentiate into Th1 helper cells (Renkl et al., 2005). Endogenously OPN may also modulate the differentiation and proliferation of cytotoxic T-cells (Higuchi et al., 2004).
As partial justification for OPN being used as a dietary strategy to alter the immune response, activated T-cells synthesize OPN that can subsequently modulate cell-mediated immunity by promoting a Th1 response (Nagai et al., 2001). The importance of OPN in such a response was emphasized in knock-out studies where OPN-null mice exhibited compromised host defenses during a rotavirus infection (Rollo et al., 2005). Based on these associations, it is possible that OPN is required for cell-mediated immunity and the development of Th1 cells, as well as having great importance in macrophage activity (Ashkar et al., 2000). In our study, OA pigs exhibited a proportion of helper T-cells that was similar to PC pigs, while OA prevented the reduction in cytotoxic T-cells due to ETEC infection. This ultimately caused a shift in T-cell populations to produce a helper:cytotoxic T-cell ratio that did not differ between NC and OA pigs at 10 DPI, while PC pigs maintained an elevated ratio, thereby indicating an extended active immune response. These OPN-induced responses are congruent with studies in rodents, where OPN has been shown to activate innate immunity, reduce tissue damage, and initiate mucosal repair under acute inflammatory conditions (Chen et al., 2013), while it promotes a Th1 response during chronic inflammatory states (Heilmann et al., 2009). Collectively, evidence from our pig study confirms that dietary OPN-stimulated innate immune cells of primary importance for combatting an enteric ETEC infection.
Whereas our study was not designed to focus on detriments in growth performance due to ETEC infection, there was a clear reduction in individual pig BW at 9 DPI for the PC and OA treatments. This evidence confirms a successful enteric infection even in the absence of a robust cytokine response, which is known to play a major role in regulating feed intake and inducing local tissue (Webel et al., 1997), but is not a hallmark feature of F18 ETEC infection in weanling pigs (Li et al., 2019; Luise et al., 2019). Immune system stimulation can cause repartitioning of nutrients from growth to support immunity due to the acute-phase protein response and subsequent changes in metabolism (Le Floc’h et al., 2004, 2006; Wu et al., 2012; Wellington et al., 2019). Typical alterations of an F18 ETEC infection include the presence of catarrhal enteritis, hyperplasia of the intestinal crypts, and the presence of bacteria adhered to the intestinal mucosa (Fekete et al., 2002). Whereas we observed no discernable changes in intestinal histomorphology at 10 DPI, what was clear was the canonical reduction in fecal consistency in ETEC-infected pigs as evident in fecal DM content at 7 DPI when comparing NC and PC pigs. This effect was exaggerated when considering OA pigs exhibited reduced fecal DM at 7 DPI and reduced ascending colon contents DM at 10 DPI compared with PC pigs, thereby indicating a response specific to OPN consumption. We chose to quantify the DM content as an objective measure because assessment of fecal scores is subject to interobserver variation and is not possible at the individual pig level when raised in a group setting (Pedersen et al., 2011). Moreover, it is recognized that changes in DM content must be considered relative to a proper control, because variability exists between studies depending on the pig’s age, diet, and amount of feed consumed (Pedersen et al., 2011, 2012; Spitzer et al., 2014; Sugiharto et al., 2014).
Diarrhea can be caused by adherence of bacteria to brush border receptors, via its fimbria, with the subsequent release enterotoxins (Fekete et al., 2002; Luise et al., 2019). Along with the aforementioned immune responses at the local and system levels, enteric bacterial infection in pigs is known to compromise digestive efficiency, especially with regard to carbohydrates, which can lead to osmotic diarrhea (Liu et al., 2009; Pi et al., 2014). With reduced digestive and absorptive efficiency during an enteric infection, the profile of nutrients passed to the colonic microbiota will shift, even when considering the overall reduction in nutrient intake. For this reason, we focused on changes occurring within the colonic luminal environment, including the production of microbial end-products (i.e., VFA) and composition of the microbiota. Again, whereas the OPN-induced reduction in fecal DM at 7 DPI was significant relative to the PC treatment, the reduction in DM content of ascending colon lumenal samples from OA pigs by more than 50% relative to both the NC and PC treatments was striking. This decrease in DM content (i.e., increase in water) was likely an osmotic response to the elevated production of SCFA and BCFA as end-products of microbial fermentation. Compared with the PC treatment, OA pigs exhibited a nearly 3-fold increase in total SCFA concentrations of ascending colon contents at 10 DPI, with similar shifts in the concentrations of acetate, propionate, and butyrate due to OPN consumption. The only dietary different between the OA and PC treatments was the enrichment of OPN, while the concentration of dietary algae protein and the other 99% of the diet formulation remain static, suggesting that oral OPN ingestion had a profound influence on composition of the colonic microbial community structure.
The etiology of postweaning diarrhea in pigs is well documented, but the structure and function of the intestinal microbiota and influence of dietary OPN thereon remain unknown. To gain insight into the compositional and functional characteristics of microbiota of postweaning piglets, we comprehensively evaluated the microbiota of ascending colon luminal samples from ETEC-challenged pigs using 16S rRNA gene and metagenomics sequencing. The principal coordinate analysis (based on Unifrac weighted distances) indicated changes in the structure of bacterial composition between samples (i.e., β-diversity), with NC and OA pigs expressing greater similarity within their overall bacterial community structures, and PC pigs exhibiting a more dispersed distribution. As such, this evidence should be interpreted as clustered samples representing high species composition similarity compared with separated samples. Previous evidence suggests that enteric disease challenge in pigs causes a destabilization of microbiome community structures (Leser et al. 2000; Kim et al. 2015). Most of the commensal bacteria present in the healthy gastrointestinal tract decrease when there is an active infection, thus inducing microbiota imbalance (Koh et al., 2015). In our study, pigs assigned to the OA treatment were better able to maintain bacterial community structure relative to PC pigs receiving the wild-type algae protein.
Another approach to understanding alterations of the microbiota is to characterize bacterial diversity within a sample (i.e., α-diversity). Pertinent indices include Shannon (species richness and equity), evenness (similarity of the population size of each of the species present), and Faith’s phylogenetic diversity, where the relation between species is considered. Based on these indexes, our data affirm that ETEC infection did not affect the total number of species present (NC vs. PC), as we conclude that the enteric infection severity was insufficient to cause a change in the total number of species present in ascending colon luminal samples. However, when comparing PC and OA pigs, it became evident that ingestion of OPN-enriched algal protein elicited shifts in α-diversity. The Shannon index of ascending colon luminal samples was lower in OA pigs compared with PC pigs, indicating a lower number of bacterial species, which may correlate with growth performance metrics (Quan et al., 2018). These changes in α-diversity that were specific to ETEC-challenged pigs receiving dietary OPN-enriched algal protein may be further characterized by shifts in bacterial genera.
Overall, OA pigs exhibited a reduction in genera belonging to the phylum Firmicutes and Bacteroidetes as compared with ETEC-challenged PC pigs receiving the wild-type algal protein. Links between the microbiota composition, immune response, and inflammatory status have certainly been explored (Kamada et al., 2013), and additional research is warranted to identify whether the OPN-induced increase in circulating TNF-α at 10 DPI observed in our study may have been related to changes in the microbiota composition. Such findings can be further delineated using metagenomics-based approaches to evaluate changes in bacterial abundance. Here, species within the Sutterella and Succinivibrio genera (both members of the Proteobacteria phylum) exhibited increased abundance in ETEC-infected pigs receiving the wild-type algal protein (PC) when compared with uninfected NC pigs receiving the same diet. The presence of species within the Sutterella genera was also greater in PC pigs compared with OA pigs, which may relate to the incidence of diarrhea in weanling pigs (Yang et al., 2017). Moreover, increased abundance of species within the Sutterella genera have been reported in intestinal disorders (Minamoto et al., 2015; Song et al., 2017). Such changes to enrich species within the Sutterella genera may also cause decreased abundance of Lactobacillus species (Mann et al., 2014), which are known to be involved in supporting a well-balanced colonic microbiota and overall healthy intestinal environment (Konstantinov et al., 2006; Hu et al., 2015). Whereas metagenomics evidence from our pig study did not suggest changes in species within the Lactobacillus genera, the observed increase species within the Sutterella genera may indicate intestinal dysbiosis involved in poor intestinal health of ETEC-infected pigs.
With respect to OPN-induced differences in ETEC-infected pigs, species within the Streptococcus and Blautia genera were specifically enriched in OA pigs. Such shifts have been observed previously in pigs experiencing compromised intestinal health and incidence of diarrhea (Yang et al., 2017), but ingestion of OPN appears to have exacerbated this effect. The dramatic increase in water and VFA concentrations of ascending colon contents at 10 DPI for OA vs. PC pigs directly implicates species within the Streptococcus and Blautia genera, which were the only genera shown to be increased by OPN consumption. The 1.4- and 3.4-fold increases in abundances of species within the Streptococcus and Blautia genera of OA pigs was concomitant with abundance decreases in 12 additional genera. Within the Bacteroidetes phylum, decreased abundance was observed for genera including Rikenellaceae, RC9 gut group, dgA-11 gut group, an uncultured bacterium genus of the Muribaculaceae family, and 3 genera of the Prevotellaceae family (Prevotella 2, Prevotella 7, and Prevotella 1). Moreover, genera within the Firmicutes phylum (Candidatus, Soleaferrea, and Lachnospiraceae) were also decreased in pigs consuming the OPN-enriched algal protein, and this may also relate to the changes in lumenal concentrations of DM and VFA as many of these species are known to ferment various polysaccharides and proteins (Morotomi et al., 2009; Meehan and Beiko, 2014; Su et al., 2014; Haq and Akram, 2017; Israeli-Ruimy et al., 2017). Overall, there appear to be OPN-specific effects on bacterial community structures that relate to measures of intestinal health, including increases in water and VFA concentrations observed in ETEC-infected pigs.
In conclusion, we present evidence that when provided before and throughout an F18 ETEC infection, consumption of an OPN-enriched algal protein elicited changes in immunity, inflammatory status, and the colonic microbial ecology as compared with pigs fed wild-type algal protein. While these changes were insufficient to allow ETEC-infected pigs to grow at the same rate as uninfected pigs, the numerical increase in BW at study conclusion relative to PC pigs, improvement in the helper:cytotoxic T-cell ratio, and elevated TNF-α concentration suggest OPN may be beneficial as a nutritional technology for supporting the health of newly weaned pigs. Identifying the underlying mechanisms by which OPN causes these responses warrants further investigation if this protein is to be used as an alternative strategy to reduce reliance on antibiotic in weanling pigs.
Implications
The ability to perform genetic manipulation of green algae (Chlamydomonas reinhardtii) as a model system affords many opportunities to express proteins with potential to benefit animal health. Osteopontin is an endogenous glycoprotein involved in cell-to-cell communication and immune function that showed potential to alter inflammatory status, immunity, and microbiota outcomes in weanling pigs experiencing an F18 ETEC infection. Therefore, dietary provision of OPN delivered via an algal protein-containing ingredient has potential to serve as a practical approach for managing enteric bacterial infection progression in weanling pigs.
Supplementary Material
Glossary
Abbreviations
- ADFI
average daily feed intake
- ADG
average daily gain
- BAND
immature band neutrophil
- BASO
basophil
- BCFA
branched chain fatty acid
- BW
body weight
- DPI
days post-inoculation
- DM
dry matter
- ETEC
enterotoxigenic E. coli
- G:F
feed efficiency (gain-to-feed ratio)
- HCT
hematocrit
- HGB
hemoglobin
- IFN
interferon (-α or -γ)
- IL
interleukin (1β, -4, -8, or -10)
- LYMPH
lymphocyte
- MCH
mean corpuscular hemoglobin
- MCHC
mean corpuscular hemoglobin concentrations
- MCV
erythrocyte cell volume
- MONO
monocyte
- NEU
neutrophil
- OPN
osteopontin
- OTU
operational taxonomic unit
- PCoA
principal co-ordinate analysis
- PCR
polymerase chain reaction
- PERMANOVA
permutational multivariate analysis of variance
- RBC
red blood cell
- SCFA
short chain fatty acid
- TNF-α
tumor necrosis factor-α
- VFA
volatile fatty acid
- WBC
white blood cell
Funding
This material is based upon work supported by the National Science Foundation under grant no. 1447905, with additional support from Triton Algae Innovations (San Diego, CA).
Conflict of interest statement
The authors declare no real or perceived conflicts of interest.
Literature Cited
- Ashkar, S, G F Weber, V Panoutsakopoulou, M E Sanchirico, M Jansson, S Zawaideh, S R Rittling, D T Denhardt, M J Glimcher, and H Cantor. . 2000. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science. 287:860–864. doi: 10.1126/science.287.5454.860 [DOI] [PubMed] [Google Scholar]
- Bolyen, E, J R Rideout, M R Dillon, N. A. Bokulich, C. C. Abnet, G. A. Al-Ghalith, H. Alexander, E. J. Alm, M. Arumugam, F. Asnicar, . et al. 2019. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 37:852–857. doi: 10.1038/s41587-019-0209-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan, B J, J Wong, C Heiner, S Oh, C M Theriot, A S Gulati, S K McGill, and M K Doughterty. . 2019. High-throughput amplicon sequencing of the full-length 16S rRNA gene with single-nucleotide resolution. Nucleic Acids Res. 47:e103. doi: 10.1093/nar/gkz569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporgno, M P, and A Mathys. . 2018. Trends in microalgae incorporation into innovative food products with potential health benefits. Front. Nutr. 5:58. doi: 10.3389/fnut.2018.00058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chagan-Yasutan, H, T L Lacuesta, L C Ndhlovu, S Oguma, P S Leano, E F Telan, T Kubo, K Morita, T Uede, E M Dimaano, . et al. 2014. Elevated levels of full-length and thrombin-cleaved osteopontin during acute dengue virus infection are associated with coagulation abnormalities. Thromb. Res. 134:449–454. doi: 10.1016/j.thromres.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, F, H Liu, Q Shen, S Yuan, L Xu, X Cai, J Lian, and S Y Chen. . 2013. Osteopontin: participation in inflammation or mucosal protection in inflammatory bowel diseases? Dig. Dis. Sci. 58:1569–1580. doi: 10.1007/s10620-012-2556-y. [DOI] [PubMed] [Google Scholar]
- Fekete, P Z, J Gerardin, E Jacquemin, J G Mainil, and B Nagy. . 2002. Replicon typing of F18 fimbriae encoding plasmids of enterotoxigenic and verotoxigenic Escherichia coli strains from porcine postweaning diarrhea and oedema disease. Vet. Microbiol. 85:275–284. doi: 10.1016/S0378-1135(01)00515-6 [DOI] [PubMed] [Google Scholar]
- Gao, C, H Guo, J Wei, Z Mi, P Wai, and P C Kuo. . 2004. S-nitrosylation of heterogeneous nuclear ribonucleoprotein a/b regulates osteopontin transcription in endotoxin-stimulated murine macrophages. J. Biol. Chem. 279:11236–11243. doi: 10.1074/jbc.M313385200 [DOI] [PubMed] [Google Scholar]
- Haq, I U, and F Akram. . 2017. Enhanced production of a recombinant multidomain thermostable GH9 processive endo-1,4-β-glucanase (CenC) from Ruminiclostridium thermocellum in a mesophilic host through various cultivation and induction strategies. Appl. Biochem. Biotechnol. 183:171–188. doi: 10.1007/s12010-017-2437-0. [DOI] [PubMed] [Google Scholar]
- Heilmann, K, U Hoffmann, E Witte, C Loddenkemper, C Sina, S Schreiber, C Hayford, P Holzlöhner, K Wolk, E Tchatchou, . et al. 2009. Osteopontin as two-sided mediator of intestinal inflammation. J. Cell. Mol. Med. 13:1162–1174. doi: 10.1111/j.1582-4934.2008.00428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi, Y, Y Tamura, T Uchida, K Matsuura, N Hijiya, and S Yamamoto. . 2004. The roles of soluble osteopontin using osteopontin-transgenic mice in vivo: proliferation of CD4+ T lymphocytes and the enhancement of cell-mediated immune responses. Pathobiology. 71:1–11. doi: 10.1159/000072956 [DOI] [PubMed] [Google Scholar]
- Hottz, E D, F A Bozza, and P T Bozza. . 2018. Platelets in immune response to virus and immunopathology of viral infections. Front. Med. 5:121. doi: 10.3389/fmed.2018.00121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, Y, Y Dun, S Li, D Zhang, N Peng, S Zhao, and Y Liang. . 2015. Dietary Enterococcus faecalis LAB31 improves growth performance, reduces diarrhea, and increases fecal Lactobacillus number of weaned piglets e0116635. PLoS ONE. 10:e0116635. doi: 10.1371/journal.pone.0116635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa, M, R Mashiba, K Kawakatsu, N K Tran, and T Nishikata. . 2018. A high-throughput quantitative assay system for macrophage phagocytic activity. Macrophage. 5:e1627. doi: 10.14800/macrophage.1627 [DOI] [Google Scholar]
- Israeli-Ruimy, V, P Bule, S Jindou, B Dassa, S Moraïs, I Borovok, Y Barak, M Slutzki, Y Hamberg, V Cardoso, . et al. 2017. Complexity of the Ruminococcus flavefaciens FD-1 cellulosome reflects an expansion of family-related protein-protein interactions. Sci. Rep. 7:42355. doi: 10.1038/srep42355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito, K, A Nakajima, Y Fukushima, K Suzuki, K Sakamoto, Y Hamazaki, K Ogasawara, N Minato, and M Hattori. . 2017. The potential role of osteopontin in the maintenance of commensal bacteria homeostasis in the intestine. PLoS One. 12:e0173629. doi: 10.1371/journal.pone.0173629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, N, N S Tan, B Ho, and J L Ding. . 2007. Respiratory protein-generated reactive oxygen species as an antimicrobial strategy. Nat. Immunol. 8:1114–1122. doi: 10.1038/ni1501 [DOI] [PubMed] [Google Scholar]
- Kamada, N, S U Seo, G Y Chen, and G Núñez. . 2013. Role of the gut microbiota in immunity and inflammatory disease. Nat. Rev. Immunol. 13:321–335. doi: 10.1038/nri3430 [DOI] [PubMed] [Google Scholar]
- Katoh, K, and D M Standley. . 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J, S G Nguyen, R B Guevarra, I Lee, and T Unno. . 2015. Analysis of swine fecal microbiota at various growth stages. Arch. Microbiol. 197:753–759. doi: 10.1007/s00203-015-1108-1. [DOI] [PubMed] [Google Scholar]
- Koh, H W, M S Kim, J S Lee, H Kim, and S J Park. . 2015. Changes in the swine gut microbiota in response to porcine epidemic diarrhea infection. Microbes Environ. 30:284–287. doi: 10.1264/jsme2.ME15046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantinov, S R, A A Awati, B A Williams, B G Miller, P Jones, C R Stokes, A D L Akkermans, H Smidt, and W M de Vos. . 2006. Post-natal development of the porcine microbiota composition and activities. Environ. Microbiol. 8:1191–1199. doi: 10.1111/j.1462-2920.2006.01009.x [DOI] [PubMed] [Google Scholar]
- Lane, D J. 1991. 16S/23S rRNA sequencing. In: E Stackebrandt and M Goodfellow, editors. Nucleic acid techniques in bacterial systematics. New York: John Wiley & Sons; p. 115–175. [Google Scholar]
- Le Floch, N, C Jondreville, J J Matte, and B Seve. . 2006. Importance of sanitary environment for growth performance and plasma nutrient homeostasis during the post-weaning period in piglets. Arch. Anim. Nutr. 60:23–34. doi: 10.1080/17450390500467810. [DOI] [PubMed] [Google Scholar]
- Le Floc’h, N, D Melchior, and C Obled. . 2004. Modifications of protein and amino acid metabolism during inflammation and immune system activation. Liv. Prod. Sci. 87:37–45. doi: 10.1016/j.livprodsci.2003.09.005 [DOI] [Google Scholar]
- Leser, T D, R H Lindecrona, T K Jensen, B B Jensen, and K Møller. . 2000. Changes in bacterial community structure in the colon of pigs fed different experimental diets and after infection with Brachyspira hyodysenteriae. Appl. Environ. Microbiol. 66:3290–3296. doi: 10.1128/aem.66.8.3290-3296.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Q, E R Burrough, N K Gabler, C L Loving, O Sahin, S A Gould, and J F Patience. . 2019. A soluble and highly fermentable dietary fiber with carbohydrases improved gut barrier integrity markers and growth performance in F18 ETEC challenged pigs. J. Anim. Sci. 97:2139–2153. doi: 10.1093/jas/skz093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y, J Han, J Huang, X Wang, F Wang, and J Wang. . 2009. Dietary L-arginine supplementation improves intestinal function in weaned pigs after an Escherichia coli lipopolysaccharide challenge. Asian-Australasian J. Anim. Sci. 22:1667–1675. doi: 10.5713/ajas.2009.90100 [DOI] [Google Scholar]
- Love, M I, W Huber, and S Anders. . 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15:550. doi: 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luise, D, C Lauridsen, P Bosi, and P Trevisi. . 2019. Methodology and application of Escherichia coli F4 and F18 encoding infection models in post-weaning pigs. J. Anim. Sci. Biotechnol. 10:53. doi: 10.1186/s40104-019-0352-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann, E, S Schmitz-Esser, Q Zebeli, M Wagner, M Ritzmann, and B U Metzler-Zebeli. . 2014. Mucosa-associated bacterial microbiome of the gastrointestinal tract of weaned pigs and dynamics linked to dietary calcium-phosphorus. PLoS One 9:e86950. doi: 10.1371/journal.pone.0086950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzali, M, T Kipari, V Ophascharoensuk, J A Wesson, R Johnson, and J Hughes. . 2002. Osteopontin – a molecule for all seasons. QJM – Int. J. Med. 95:3–13. doi: 10.1093/qjmed/95.1.3 [DOI] [PubMed] [Google Scholar]
- Meehan, C J, and R G Beiko. . 2014. A phylogenomic view of ecological specialization in the Lachnospiraceae, a family of digestive tract-associated bacteria. Genome Biol. Evol. 6:703–713. doi: 10.1093/gbe/evu050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamoto, Y, C C Otoni, S M Steelman, O Büyükleblebici, J M Steiner, A E Jergens, and J S Suchodolski. . 2015. Alteration of the fecal microbiota and serum metabolite profiles in dogs with idiopathic inflammatory bowel disease. Gut Microbes. 6:33–47. doi: 10.1080/19490976.2014.997612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco, M H, M Wang, X Pan, Q Li, J D Richards, M Chichlowski, B M Berg, R N Dilger, and S M Donovan. . 2018. Evaluation of sialyllactose supplementation to a prebiotic-containing formula on growth, intestinal development and bacterial colonization in the neonatal piglet. Curr. Dev. Nutr. 2:nzy067. doi: 10.1093/cdn/nzy067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morotomi, M, F Nagai, H Sakon, and R Tanaka. . 2009. Paraprevotella clara gen. nov., sp. nov. and Paraprevotella xylaniphila sp. nov., members of the family ‘Prevotellaceae’ isolated from human faeces. Int. J. Syst. Evol. Microbiol. 59:1895–1900. doi: 10.1099/ijs.0.008169-0 [DOI] [PubMed] [Google Scholar]
- Mukai, K, K Matsuoka, C Taya, H Suzuki, H Yokozeki, K Nishioka, K Hirokawa, M Etori, M Yamashita, T Kubota, Y Minegishi, H Yonekawa, and H Karasuyama. . 2005. Basophils play a critical role in the development of IgE-mediated chronic allergic inflammation independently of T cells and mast cells. Immunity. 23:191–202. doi: 10.1016/j.immuni.2005.06.011 [DOI] [PubMed] [Google Scholar]
- Nagai, S, S I Hashimoto, T Yamashita, N Toyoda, T Satoh, T Suzuki, and K Matsushima. . 2001. Comprehensive gene expression profile of human activated Th1- and Th2-polarized cells. Int. Immunol. 13:367–376. doi: 10.1093/intimm/13.3.367 [DOI] [PubMed] [Google Scholar]
- National Research Council (NRC) . 2012. Nutrient requirements of swine: eleventh revised edition. Washington, DC: The National Academies Press. doi: 10.17226/13298 [DOI] [Google Scholar]
- Otsuka, A, Y Nonomura, and K Kabashima. . 2016. Roles of basophils and mast cells in cutaneous inflammation. Semin. Immunopathol. 38:563–570. doi: 10.1007/s00281-016-0570-4 [DOI] [PubMed] [Google Scholar]
- Pedersen, K S, R Skrubel, H Stege, O Angen, M Stahl, C Hjulsager, L E Larsen, and J P Nielsen. . 2012. Association between average daily gain, faecal dry matter content and concentrations of Lawsonia intracellularis in faeces. Acta Vet. Scan. 54:58. doi: 10.1186/1751-0147-54-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen, K S, H Stege, and J P Nielsen. . 2011. Evaluation of a microwave method for dry matter determination in faecal samples from weaned pigs with or without clinical diarrhoea. Prev. Vet. Med. 100:163–170. doi: 10.1016/j.prevetmed.2011.04.014 [DOI] [PubMed] [Google Scholar]
- Pedregosa, F, G Varoquaux, A Gramfort, V Michel, B Thirion, O Grisel, M Blondel, P Prettenhofer, R Weiss, V Dubourg, . et al. 2011. Scikit-learn: machine learning in python. J. Mach. Learn. Res. 12:2825–2830. doi: 10.5555/1953048.2078195 [DOI] [Google Scholar]
- Pi, D, Y Liu, H Shi, S Li, J Odle, X Lin, H Zhu, F Chen, Y Hou, and W Leng. . 2014. Dietary supplementation of aspartate enhances intestinal integrity and energy status in weanling piglets after lipopolysaccharide challenge. J. Nutr. Biochem. 25:456–462. doi: 10.1016/j.jnutbio.2013.12.006 [DOI] [PubMed] [Google Scholar]
- Piliponsky, A M, N J Shubin, A K Lahiri, P Truong, M Clauson, K Niino, A L Tsuha, S A Nedospasov, H Karasuyama, L L Reber, . et al. 2019. Basophil-derived tumor necrosis factor can enhance survival in a sepsis model in mice. Nat. Immunol. 20:129–140. doi: 10.1038/s41590-018-0288-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price, M N, P S Dehal, and A P Arkin. . 2010. FastTree 2–approximately maximum-likelihood trees for large alignments. PLoS One 5:e9490. doi: 10.1371/journal.pone.0009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan, J, G Cai, J Ye, M Yang, R Ding, X Wang, E Zheng, D Fu, S Li, S Zhou, . et al. 2018. A global comparison of the microbiome compositions of three gut locations in commercial pigs with extreme feed conversion ratios. Sci. Rep. 8:4536. doi: 10.1038/s41598-018-22692-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quast, C, E Pruesse, P Yilmaz, J Gerken, T Schweer, P Yarza, J Peplies, and F Glöckner. . 2013. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucl. Acids Res. 41:D590–D596. doi: 10.1093/nar/gks1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravi, A, S Guo, B Rasala, M Tran, S Mayfield, and Z L Nikolov. . 2018. Separation options for phosphorylated osteopontin from transgenic microalgae Chlamydomonas reinhardtii. Int. J. Mol. Sci. 19:585. doi: 10.3390/ijms19020585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renkl, A C, J Wussler, T Ahrens, K Thoma, S Kon, T Uede, S F Martin, J C Simon, and J M Weiss. . 2005. Osteopontin functionally activates dendritic cells and induces their differentiation toward a Th1-polarizing phenotype. Blood. 106:946–955. doi: 10.1182/blood-2004-08-3228 [DOI] [PubMed] [Google Scholar]
- Reznikov, E A, S S Comstock, J L Hoeflinger, M Wang, M J Miller, and S M Donovan. . 2018. Dietary bovine lactoferrin reduces Staphylococcus aureus in the tissues and modulates the immune response in piglets systemically infected with S. aureus. Curr. Dev. Nutr. 2:nzy001. doi: 10.1093/cdn/nzy001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollo, E E, S J Hempson, A Bansal, E Tsao, I Habib, S R Rittling, D T Denhardt, E R Mackow, and R D Shaw. . 2005. The cytokine osteopontin modulates the severity of rotavirus diarrhea. J. Virol. 79:3509–3516. doi: 10.1128/jvi.79.6.3509-3516.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondina, M T, and O Garraud. . 2014. Emerging evidence for platelets as immune and inflammatory effector cells. Front. Immunol. 5:653. doi: 10.3389/fimmu.2014.00653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiricky-Tjardes, M R, E A Flickinger, C M Grieshop, L L Bauer, M R Murphy, and G C Fahey. . 2003. In vitro fermentation characteristics of selected oligosaccharides by swine fecal microflora. J. Anim. Sci. 81:2505–2514. doi: 10.2527/2003.81102505x [DOI] [PubMed] [Google Scholar]
- Sodek, J, A P Batista, and R Zohar. . 2006. Osteopontin and mucosal protection. J. Dent. Res. 85:404–415. doi: 10.1177/154405910608500503 [DOI] [PubMed] [Google Scholar]
- Song, D, Q Peng, Y Chen, X Zhou, F Zhang, A Li, D Huang, Q Wu, Y Ye, H He, L Wang, and Y Tang. . 2017. Altered gut microbiota profiles in sows and neonatal piglets associated with porcine epidemic diarrhea virus infection. Sci. Rep. 7:17439. doi: 10.1038/s41598-017-17830-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specht, E, S Miyake-Stoner, and S Mayfield. . 2010. Micro-algae come of age as a platform for recombinant protein production. Biotechnol. Lett. 32:1373–1383. doi: 10.1007/s10529-010-0326-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer, F, W Vahjen, R Pieper, B Martinez-Vallespin, and J Zentek. . 2014. A standardised challenge model with an enterotoxigenic F4+ Escherichia coli strain in piglets assessing clinical traits and faecal shedding of fae and est-II toxin genes. Arch. Anim. Nutr. 68:448–459. doi: 10.1080/1745039X.2014.968701 [DOI] [PubMed] [Google Scholar]
- Su, X L, Q Tian, J Zhang, X Z Yuan, X S Shi, R B Guo, and Y L Qiu. . 2014. Acetobacteroides hydrogenigenes gen. nov., sp. nov., an anaerobic hydrogen-producing bacterium in the family Rikenellaceae isolated from a reed swamp. Int. J. Syst. Evol. Microbiol. 64:2986–2991. doi: 10.1099/ijs.0.063917-0 [DOI] [PubMed] [Google Scholar]
- Suchner, U, K S Kuhn, and P Fürst. . 2000. The scientific basis of immunonutrition. Proc. Nutr. Soc. 59:553–563. doi: 10.1017/S0029665100000793 [DOI] [PubMed] [Google Scholar]
- Sugiharto, S, M S Hedemann, and C Lauridsen. . 2014. Plasma metabolomic profiles and immune responses of piglets after weaning and challenge with E. coli. J. Anim. Sci. Biotech. 5:17. doi: 10.1186/2049-1891-5-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyonaga, T, H Nakase, S Ueno, M Matsuura, T Yoshino, Y Honzawa, A Itou, K Namba, N Minami, S Yamada, . et al. 2015. Osteopontin deficiency accelerates spontaneous colitis in mice with disrupted gut microbiota and macrophage phagocytic activity. PLoS One 10:e0135552. doi: 10.1371/journal.pone.0135552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webel, D M, B N Finck, D H Baker, and R W Johnson. . 1997. Time course of increased plasma cytokines, cortisol, and urea nitrogen in pigs following intraperitoneal injection of lipopolysaccharide. J. Anim. Sci. 75:1514–1520. doi: 10.2527/1997.7561514x. [DOI] [PubMed] [Google Scholar]
- van der Windt, G J, A J Hoogendijk, M Schouten, T J Hommes, A F de Vos, S Florquin, and T van der Poll. . 2011. Osteopontin impairs host defense during pneumococcal pneumonia. J. Infect. Dis. 203:1850–1858. doi: 10.1093/infdis/jir185. [DOI] [PubMed] [Google Scholar]
- Wellington, M O, A K Agyekum, K Hamonic, J K Htoo, A G Van Kessel, and D A Columbus. . 2019. Effect of supplemental threonine above requirement on growth performance of Salmonella typhimurium challenged pigs fed high-fiber diets1. J. Anim. Sci. 97:3636–3647. doi: 10.1093/jas/skz225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells, M L, P Potin, J S Craigie, J A Raven, S S Merchant, K E Helliwell, A G Smith, M E Camire, and S H Brawley. . 2017. Algae as nutritional and functional food sources: revisiting our understanding. J. Appl. Phycol. 29:949–982. doi: 10.1007/s10811-016-0974-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, S, F Zhang, Z Huang, H Liu, C Xie, J Zhang, P A Thacker, and S Qiao. . 2012. Effects of the antimicrobial peptide cecropin AD on performance and intestinal health in weaned piglets challenged with Escherichia coli. Peptides 35:225–230. doi: 10.1016/j.peptides.2012.03.030. [DOI] [PubMed] [Google Scholar]
- Yamanishi, Y, and H Karasuyama. . 2016. Basophils and mast cells in immunity and inflammation. Semin. Immunopathol. 38:535–537. doi: 10.1007/s00281-016-0582-0 [DOI] [PubMed] [Google Scholar]
- Yang, Q, X Huang, S Zhao, W Sun, Z Yan, P Wang, S Li, W Huang, S Zhang, L Liu, and S Gun. . 2017. Structure and function of the fecal microbiota in diarrheic neonatal piglets. Front. Microbiol. 8:502. doi: 10.3389/fmicb.2017.00502 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.