Abstract
The application of thioallenoates to catalytic enantioselective [2+2]-cycloadditions with unactivated alkenes is reported.In many cases, the thioallenoates examined exhibit superior reactivity and selectivity compared to the alkoxy analogs generally used in these cycloadditions.
Keywords: Cycloaddition, Enantioselective, Lewis-Acid, Allenes, Cyclobutane
Graphical abstract
To create your abstract, type over the instructions in the template box below. Fonts or abstract dimensions should not be changed or altered.

1. Introduction
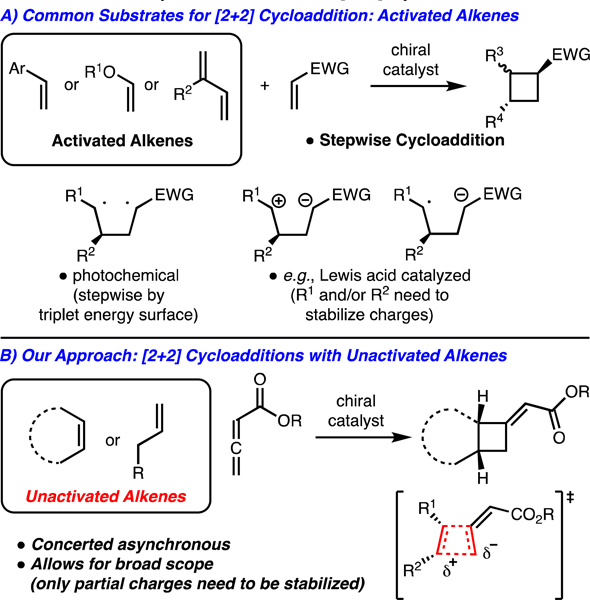
The synthesis of cyclobutane containing molecules through [2+2]-cycloadditions has seen wide application in chemical synthesis.1 This can be attributed to the presence of the cyclobutane ring structure in a large number of biologically relevant molecules, the availability of alkene precursors for [2+2]-cycloadditions, and the rapid molecular complexity built in a single step. Thus, the development of catalytic enantioselective [2+2]-cycloaddition to access cyclobutanes has seen significant developments in recent years.2 Impressive advances have been made in intermolecular enantioselective [2+2] cycloadditions by photochemical activation,3 reactions of highly polarized π-components,2a and radical anion/cation processes initiated by photocatalysts;4 however, all involve stepwise mechanisms and thus activated alkenes are required in nearly every case to stabilize charged/radical intermediates (Scheme 1A).3c Enantioselective [2+2] cycloadditions with unactivated alkenes are significantly more rare. In one instance, Ishihara reported the enantioselective [2+2] cycloaddition with 1,1’- and trisubstituted unactiavted alkenes.5 In a more recent study, Bach and coworkers reported photochemical [2+2] cycloaddition between cyclohexenone derivatives and unactivated alkenes.3c Despite these advances, generally effective approaches for enantioselective [2+2] cycloadditions with unactivated alkenes are lacking. Therefore, our lab initiated a program towards addressing this challenge through the use of allenoates (Scheme 1B).6,7,8 Our mechanistic rationale for development of these reactions is that the cycloadditions are likely concerted, asynchronous processes and thus highly polar or radical intermediates do not need to be supported. Thus, a wide range of unactivated alkenes can engage in these reactions. 8a-c
Scheme 1:
Catalytic Enantioselective [2+2] Cycloadditions.
2. Background
Driven by the motivations described above, in 2015 we reported the first catalytic enantioselective [2+2] cycloaddition of allenoates and unactivated alkenes promoted by N-protonated oxazaborolidene catalysts (generated in situ by protonation of 3 with HNTf2) (Scheme 2A).6a To achieve sufficient reactivity across a range of alkenes, the use of trifluoroethyl allenoate was required (Scheme 2A).6a However, the synthesis of this allene is challenging and thus represents a significant limitation in the utility of this method. The optimized route to 1 starts from propargyl bromide, which upon generation of the Grignard reagent and quench with carbon dioxide provided carboxylic acid 6 in 51% yield. Esterification with trifluoroethanol assisted by DCC and DMAP furnishes the allenoate in 20% yield after in situ isomerization to the allene. The low yield in this reaction is due to the poor nucleophilicity of 2,2,2-trifluoroethanol, the high volatility of 1, and the requirement of sequential purification by column chromatography and distillation. Due to the difficulties with the synthesis, preparation on gram scale was never achieved. In light of this limitation, we set out to identify an allenoate that offers similar reactivity to trifluoroethyl allenoate 1, yet is easier to prepare. Herein, we report a solution to this problem through the synthesis and use of thioallenoates (Scheme 2B).
Scheme 2:
Overview of work
3. Results and Discussion
Using cyclopentene as a test system, initial investigations of different allenic esters were performed (Table 1).9 Whereas improved reaction enantioselectivity was obtained with benzyl allenoate 7, a dramatic decrease in overall reaction yield was observed (Table 1, compare entries 1 and 2). The use of the more reactive phenyl allenoate 8 showed similar reactivity to trifluoroethyl allenoate 1; however, decreased diastereo- and enantioselectivities were obtained (Table 1, compare entries 1 and 3). We next examined thiobenzyl allenoate 9 in the reaction as it proved to be uniquely effective in obtaining high enantioselectivities with aryl alkenes in a recent report from this lab.6d To our delight, a similar yield and high enantioselectivity was obtained from the cycloaddition with 9 compared to trifluoroethyl allenoate 1 (Table 1, compare entries 1 and 4). Furthermore, the desired cycloadduct was obtained as a single alkene isomer marking a drastic improvement in reaction diastereoselectivity. As demonstrated in our previous report, the alkene isomers of products are of opposite absolute configuration.6a Therefore, formation of the product as a single alkene isomer is particularly important.
Table 1:
Evaluation of the Allene
 | |||||
| entry | allene-XR | yield (%)a | E:Zb | erc | product |
| 1 | OCH2CF3(1) | 74 | 10:01 | 94:06:00 | 4 |
| 2 | Obn(7) | 48 | 10:01 | 96:04:00 | 10 |
| 3 | Oph(8) | 76 | 8:01 | 70:30:00 | 11 |
| 4 | SBn(9) | 64 | >20:1 | 94:6 | 12 |
determined by 1H analysis of the unpurified reaction mixture with an internal standard.
Determined by 1H NMR analysis of the unpurified reaction mixture.
Determined by HPLC analysis with a chiral column.
Thioallenoate 9 can be prepared by a straightforward sequence involving Wittig olefination of in situ generated ketene (Scheme 3).9 Importantly, the reaction can be performed on multigram scale and the product is easily purified and isolated. The thioallenoate was also found to be stable for months when stored at −20 °C.
Scheme 3:
Synthesis of Thioallenoate 9
With optimized conditions in hand, we investigated the scope of the reaction with a range of unactivated alkenes.Cyclic unactivated alkenes underwent cycloaddition in high enantioselectivity, with more strained alkenes (e.g., cyclooctene vs. cyclohexene) proceeding in higher overall yield. (Scheme 4, products 12, 16, 18). Cycloaddition of cis-4-octene provided cycloadduct 20 in 67% yield, >20:1 E/Z, and 93:7 er. Notably, the alkene geometry is conserved to provide only the syn-substituted cyclobutane suggesting the reaction is concerted in accordance with our previous reports.6 In addition, reaction with an asymmetric alkene resulted in formation of 22 with improved regioselectivity as compared to reaction with the trifluoroethyl allenoate 1 (compare with product 21).
Scheme 4:
Substrate Scope with Alkenesa
a With 5 equiv alkene. See the Supporting Information for experimental details. Yields reported are the average of two experiments. Enantiomeric ratios (er) determined by HPLC analysis with a chirai column. b Previously reported in ref 3a c Previously reproted in ref 3c. d With 53 mol % 3 and 50 mol % HNTf2 e With 53 mol % 3, 50 mol % HNTf2 and 10 equiv alkene in the absence of CH2CI2. f Reaction run with oxazaborolidine 34 instead of 3 and at −17 °C.
While the thioallenoate was superior in many instances when compared to allenic esters, there are several cases in which yield and/or selectivity suffered (Scheme 4). For example, reaction with terminal alkenes resulted in poor yields (compare products 23 and 24).In addition, reaction of trisubstituted alkenes resulted in lower selectivity and yield compared to the benzyl allenoate (compare products 25 and 26).6c At this time, justification for the divergence in selectivity and yield with these two examples is unclear.
Desymmetrization of TIPS protected alcohol 27 with thioallenoate 9 by [2+2]-cycloaddition was also investigated for the potential to set multiple stereocenters (eq (1)). [3.2.0] bicycloheptane 28 was obtained as a single observable stereoisomer in 51% yield and 94:6 er. The cycloaddition likely proceeds through a transition state in which the sterically demanding protected alcohol is oriented away from the bond formation site thus setting the remote stereocenter.
 |
(1) |
The utility of the thioallenoates was extended to reaction of the γ, γ-dimethyl derivative 30 (Scheme 5).The requisite thioallenoate was prepared by Wittig olefination of in situ generated dimethylketene in 60% yield on multigram scale. Enantioselective cycloaddition with the catalyst derived from oxazaborolidine 3 resulted in formation of product 32 as a 1:1 mixture of alkene isomers; albeit, the E-isomer was formed in high enantioselectivity. Interestingly, use of the oxazaborolidinium catalyst derived from 33, which bears 3,5-(CF3)2C6H3 groups instead of Ph-groups on the catalyst backbone, resulted in an improved selectivity for formation of the E-isomer. The reaction could be extended to other alkenes with mixed results. While cyclopentene and cis-4-octene worked to provide products 34 and 35 in good enantioselectivity, control of alkene geometry was poor. In addition, reaction of 1-hexene resulted in poor enantioselectivity (product 36). Finally, it should also be noted that use of the corresponding benzylester allenoate resulted in low yields, thus further highlighting the increased reactivity with the thioallenoate.
Scheme 5:
Synthesis and use of Thioallenoate 32
4. Summary
In summary, the utility of thioallenoate in [2+2] cycloadditions is presented. In many cases, reactions with the thioallenoate offers superior selectivity compared to reactions with alkoxy analogs. In addition, the synthesis of the thioallenoate is straightforward and can be performed on gram scale, which significantly increases the synthetic utility of these reactions.
5. Experimental section
1H NMR and 13C NMR spectra were recorded at room temperature using a Varian I400 (1H NMR at 400 MHz, 13C NMR at 100 MHz, 19F at 375 MHz), Varian VXR400 (1H NMR at 400 MHz, 13C NMR at 100 MHz), Varian I500 (1H NMR at 500 MHz and 13C NMR at 125 MHz) and Varian I600 (1H NMR at 600 MHz and 13C NMR at 150MHz). Chemical shifts are reported in ppm from tetramethylsilane with the respective solvent resonance as the internal standard (1H NMR CHCl3: δ 7.26 ppm 13C NMR CDCl3: δ 77.2 ppm). Data is reported as follows: chemical shift, multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, p = pentet, br = broad, m = multiplet), coupling constants (Hz) and integration. Infrared spectra (IR) were obtained on a Bruker Tensor II FTIR Spectrometer (ATR sampling technique) and recorded in wavenumbers (cm−1). Bands are characterized as broad (br), strong (s), medium (m), and weak (w). Melting points were obtained on a Thomas Hoover capillary melting point apparatus without correction. High Resolution Mass Spectrometry (HRMS) analysis was obtained using Electron Impact Ionization (EI), Chemical Ionization (CI) or Electrospray Ionization (ESI) and reported as m/z (relative intensity). GC-MS data was acquired using an Agilent 6890N Gas Chromatograph and 5973 Inert Mass Selective Detector. ESI was acquired using a Waters/Micromass LCT Classic (ESI-TOF). Optical rotations were measured on a Perkin-Elmer 241 polarimeter at 589 nm wavelength (sodium D-line) using a standard 10 cm cell (1 mL). Specific rotations, [α]D20, are reported in degree mL/(g·dm) at the specific temperature. Concentrations (c) are given in grams per 100 mL of the specific solvent. Unless otherwise noted, all reactions have been carried out with distilled and degassed solvents under an atmosphere of dry N2 in oven- (135 °C) and flame-dried glassware with standard vacuum-line techniques. Dichloromethane, diethyl ether and tetrahydrofuran were purified under a positive pressure of dry argon by passage through two columns of activated alumina. Toluene was purified under a positive pressure of dry argon by passage through columns of activated alumina and Q5 (Grubbs apparatus). All work-up and purification procedures were carried out with reagent grade solvents (purchased from Sigma-Aldrich) in air. Standard column chromatography techniques using ZEOprep 60/40–63 μm silica gel were used for purification.
General Procedure for [2+2] cycloaddition (unless otherwise stated).
HNTf2 (14.0 mg, 0.05 mmol, 0.20 equiv) was added to a flame dried screw cap test tube in an argon filled glovebox, capped with a septum and removed from the glovebox. CH2Cl2 (0.50 mL) was added and the reaction mixture cooled to −78 °C. Oxazaborolidine 3 or 34 (0.25 M in PhMe, 0.25 mL, 0.06 mmol, 0.25 equiv) was added and allowed to stir for 30 minutes. The alkene (1.25 mmol, 5.00 equiv) was added, followed quickly by the allenoate (38.0 μL, 0.25 mmol, 1.00 equiv) and the reaction was allowed to warm to room temperature. After 16 hours, the reaction was quenched with 100 μL Et3N and the solution was concentrated. The resulting residue was purified directly by silica gel chromatography.
S-benzyl (E)-2-((1S,5S)-bicyclo[3.2.0]heptan-6-ylidene)ethanethioate [12]
Prepared according to the general procedure utilizing cyclopentene as the alkene component. Purification by silica gel chromatography (2% EtOAc:hexanes) to yield 40.7 mg (63% yield, >20:1 E/Z) of the title compound as a bright yellow oil. IR (film): 3029 (s), 2854 (w), 1672 (s), 1638 (s), 1495 (m), 1454 (m), 1403 (m), 1337 (m), 1161 (m), 1035 (s), 752 (s), 701 (s) cm-1. 1H NMR (500 MHz, CDCl3) δ 7.38 – 7.21 (m, 5H), 5.96 – 5.93 (m, 1H), 4.17 (s, 2H), 3.42 – 3.35 (m, 1H), 3.26 (app ddt, J = 19.4, 9.0, 2.5 Hz, 1H), 2.96 – 2.89 (m, 1H), 2.65 – 2.58 (m, 1H), 1.86 – 1.52 (m, 6H).13C NMR (100 MHz, CDCl3) δ 188.1, 169.5, 138.1, 129.0, 128.7, 127.2, 120.0, 49.0, 38.2, 36.2, 33.3, 32.9, 32.7, 24.7. HRMS (CI): Calcd for C16H18O1S1 [M]+: 258.1073, Found: 258.1084. Optical Rotation: [α]D20 +67.0 (c = 1.00, CHCl3) for an enantiomerically enriched sample of 94:6 er. The enantiomeric purity was established by HPLC analysis using a chiral column (Lux 3u Cellulose-4 column, 22 °C, 0.25 mL/min, 99:1 hexanes:isopropanol, 254 nm, tminor = 29.496 min, tmajor = 30.606 min). See supporting information part B for HPLC chromatograms.
S-benzyl (E)-2-((1S,6S)-bicyclo[4.2.0]octan-7-ylidene)ethanethioate [16]:
HNTf2 (35.1 mg, 0.125 mmol, 0.50 equiv) was added to a flame dried screw cap test tube in an argon filled glovebox, capped with a septum and removed from the glovebox. CH2Cl2 (1.25 mL) was added and the reaction mixture cooled to −25 °C. Oxazaborolidine 1 (0.25 M in PhMe, 0.53 mL, 0.13 mmol, 0.53 equiv,) was added and allowed to stir for 30 minutes. Cyclohexene (130 μL, 1.25 mmol, 5.00 equiv) was added followed quickly by the allenoate (38.0 μL, 0.25 mmol, 1.00 equiv) and the reaction was allowed to warm to room temperature. After 16 hours, the reaction was quenched with 100 μL Et3N and the solution was concentrated. The resulting brown residue was purified directly by silica gel chromatography (1 – 2% EtOAc:hexanes) to yield 40.5 mg (59% yield, >20:1 E/Z) of the title compound as a yellow oil. IR (film): 3029 (w), 2926 (m), 2851 (m), 1672 (s), 1638 (s), 1567 (m), 1495 (m), 1453 (m), 1413 (m), 1332 (m), 1276 (m), 1156 (m), 1035 (s), 825 (s), 744 (s), 699 (s) cm-1. 1H NMR (600 MHz, CDCl3) δ 7.33 – 7.27 (m, 4H), 7.25 – 7.21 (m, 1H), 5.99 (app q, J = 2.1 Hz, 1H), 4.20 – 4.13 (m, 2H), 3.12 – 3.04 (m, 2H), 2.82 – 2.77 (m, 1H), 2.44 (app ddq, J = 13.1, 8.4, 4.9, 4.2 Hz, 1H), 1.96 – 1.89 (m, 1H), 1.71 (app dq, J = 13.4, 4.3 Hz, 1H), 1.58 – 1.50 (m, 2H), 1.48 – 1.40 (m, 1H), 1.38 – 1.29 (m, 1H), 1.28 – 1.19 (m, 1H), 1.19 – 1.09 (m, 1H).13C NMR (125 MHz, CDCl3) δ 188.2, 167.7, 138.2, 129.0, 128.7, 127.2, 118.1, 43.0, 40.3, 33.0, 29.9, 28.9, 24.3, 22.5, 21.8. HRMS (EI): Calcd for C17H20O1S1 [M]+: 272.1229, Found: 272.1234. Optical Rotation: [α]D20 −33.4 (c = 1.00, CHCl3) for an enantiomerically enriched sample of 94:6 er. The enantiomeric purity was established by HPLC analysis using a chiral column (Lux 3u Cellulose-4 column, 22 °C, 0.25 mL/min, 99:1 hexanes:isopropanol, 254 nm, tmajor = 30.955 min, tminor = 34.093 min). See supporting information part B for HPLC chromatograms.
S-benzyl (E)-2-((1S,8S)-bicyclo[6.2.0]decan-9-ylidene)ethanethioate [18]:
Prepared according to the general procedure utilizing cyclooctene as the alkene component. Purification by silica gel chromatography (hexanes – 2% EtOAc:hexanes gradient) provided 67.6 mg (90% yield, 20:1 E/Z) of the title compound as a pale yellow oil. IR (film): 3029 (w), 2918 (s), 2850 (m), 1672 (s), 1638 (s), 1495 (w), 1461 (m), 1454 (w), 1407 (w), 1334 (w), 1160 (w), 1136 (w), 1038 (s), 916 (w), 825 (m), 762 (w), 741 (w), 700 (m), 565 (w), 470 (w) cm-1. H NMR (600 MHz, CDCl3) δ 7.33 – 7.27 (m, 4H), 7.25 – 7.21 (m, 1H), 5.98 (q, J = 2.3 Hz, 1H), 4.16 (s, 2H), 3.33 – 3.27 (m, 1H), 3.02 – 2.95 (m, 1H), 2.66 (dddd, J = 18.4, 5.9, 3.7, 2.5 Hz, 1H), 2.48 (app qdd, J = 9.0, 5.6, 2.0 Hz, 1H), 1.79 – 1.43 (m, 8H), 1.38 – 1.23 (m, 4H).13C NMR (100 MHz, CDCl3) δ 188.2, 169.5, 138.2, 129.0, 128.7, 127.2, 118.8, 48.3, 40.0, 36.9, 32.9, 30.3, 29.9, 29.3, 26.3, 25.9, 25.8. HRMS (EI): Calcd for C19H24O1S1 [M]+: 300.1542, Found: 300.1535. Optical Rotation: [α]D20 −33.5 (c = 1.00, CHCl3) for an enantiomerically enriched sample of 98:2 er. The enantiomeric purity was established by HPLC analysis using a chiral column (Lux 3u Cellulose-4 column, 22 °C, 0.25 mL/min, 99:1 hexanes:isopropanol, 254 nm, tmajor = 34.240 min, tminor = 37.805 min). See supporting information part B for HPLC chromatograms.
S-benzyl (E)-2-((2S,3S)-2,3-dipropylcyclobutylidene)ethanethioate [20]:
Prepared according to the general procedure utilizing cis-4-octene as the alkene component. Purification by silica gel chromatography (hexanes – 2% EtOAc:hexanes gradient) provided 50.7 mg (67% yield, >20:1 dr, >20:1 E/Z) of the title compound as a pale yellow liquid. IR (film): 3086 (w), 3062 (w), 3029 (w), 2955(s), 2926 (s), 2870 (s), 1674 (s), 1639 (s), 1495 (m), 1454 (m), 1410 (w), 1378 (w), 1334 (w), 1162 (w), 1118 (w), 1071 (w), 1036 (s), 919 (w), 828 (m), 786 (m), 736 (m), 699 (s), 565 (w), 471 (w) cm-1. H NMR (500 MHz, CDCl3) δ 7.35 – 7.27 (m, 4H), 7.25 – 7.21 (m, 1H), 5.98 (q, J = 2.2 Hz, 1H), 4.16 (s, 2H), 3.16 (app ddt, J = 17.5, 8.6, 1.6 Hz, 1H), 3.05 (app dtdd, J = 9.2, 7.5, 3.4, 1.7 Hz, 1H), 2.73 (app ddt, J = 17.6, 5.6, 2.9 Hz, 1H), 2.50 – 2.40 (m, 1H), 1.54 – 1.40 (m, 3H), 1.40 – 1.16 (m, 5H), 0.92 (td, J = 7.2, 3.4 Hz, 6H).13C NMR (125 MHz, CDCl3) δ 188.2, 168.8, 138.2, 129.0, 128.7, 127.2, 118.7, 47.6, 38.7, 34.6, 32.9, 32.2, 30.1, 21.4, 20.8, 14.3, 14.3.HRMS (EI): Calcd for C19H26O1S1 [M]+: 302.1699, Found: 302.1696. Optical Rotation: [α]D20 −7.7 (c = 1.00, CHCl3) for an enantiomerically enriched sample of 93:7 er. The enantiomeric purity was established by HPLC analysis using a chiral column (Lux 3u Cellulose-1 column, 22 °C, 0.25 mL/min, 99:1 hexanes:isopropanol, 254 nm, tminor = 24.454 min, tmajor = 31.649 min). See supporting information part B for HPLC chromatograms.
S-benzyl (E)-2-((2S,3R)-3-isopropyl-2-methylcyclobutylidene)ethanethioate [22]:
Prepared according to the general procedure utilizing cis-4-methyl-2-pentene as the alkene component. Purification by silica gel chromatography (hexanes – 2% EtOAc:hexanes gradient) provided 40.1 mg (58% yield, 5:1 E/Z, 10:1 rr) of the title compound as an inseparable mixture of isomers. IR (film): 3062 (w), 3029 (w), 2957 (m), 2929 (m), 2870 (w), 1674 (s), 1634 (s), 1454 (m), 1416 (w), 1335 (w), 1160 (w), 1038 (s), 827 (m), 751 (w), 700 (m) cm-1. 1H NMR (500 MHz, CDCl3) δ 7.40 – 7.18 (m, 5H), 6.18 – 6.08 (m, 1H), 4.18 (s, 2H), 3.16 (ddd, J = 17.4, 8.3, 2.4 Hz, 1H), 2.77 – 2.70 (m, 1H), 2.69 – 2.54 (m, 2H), 1.95 – 1.82 (m, 1H), 1.12 (d, J = 6.8 Hz, 3H), 1.05 – 0.98 (m, 3H), 0.91 (d, J = 6.4 Hz, 3H).13C NMR (125 MHz, CDCl3) δ 188.2, 167.5, 138.2, 129.0, 129.0, 129.0, 128.7, 127.2, 119.4, 55.5, 40.3, 33.0, 29.3, 27.3, 21.8, 21.6, 16.0.HRMS (EI): Calcd for C17H22O1S1 [M]+: 274.1386, Found: 274.1384. Optical Rotation: [α]D20 −26.7 (c = 1.00, CHCl3) for an enantiomerically enriched sample of 94:6 er. The enantiomeric purity was established by HPLC analysis using a chiral column (Lux 3u Cellulose-1 column, 22 °C, 0.25 mL/min, 400:1 hexanes:isopropanol, 254 nm, tminor = 38.095 min, tmajor = 56.180 min). See supporting information part B for HPLC chromatograms.
S-Benzyl (S,Z)-2-(3,3-dimethyl-2-(2-((triisopropylsilyl)oxy)ethyl)cyclobutylidene)ethanethioate [26]:
Prepared according to the general procedure utilizing triisopropyl((4-methylpent-3-en-1-yl)oxy)silane as the alkene component. Purification by silica gel chromatography (hexanes – 2% Et2O:hexanes gradient) provided 78.0 mg (70% yield, 1:1 Z/E, mixture of alkene isomers) of the title compound as a colorless oil. For characterization, small amounts of the respective isomers were separated. Z-isomer:IR (film): 2942 (s), 2865 (s), 1738 (m) 1678 (s), 1647 (m), 1071 (m), 1033 (m), 883 (m), 682 (m) cm−1 1H NMR (600 MHz, CDCl3) δ 7.36 – 7.19 (m, 5H), 5.98 (q, J = 2.1 Hz, 1H), 4.14 (s, 2H), 3.84 – 3.72 (m, 1H), 3.72 – 3.64 (m, 1H), 3.01 (ddt, J = 8.5, 5.6, 2.8 Hz, 1H), 2.57 – 2.51 (m, 1H), 2.35 (ddd, J = 16.3, 3.0, 1.7 Hz, 1H), 2.21 – 2.06 (m, 1H), 1.88 – 1.78 (m, 1H), 1.19 (s, 3H), 1.13 (s, 3H), 1.08 (d, J = 5.3 Hz, 21H).13C NMR (125 MHz, CDCl3) δ 187.0, 164.7, 138.2, 129.0, 128.7, 127.2, 120.7, 62.8, 52.4, 44.6, 35.3, 33.1, 32.5, 29.9, 23.9, 18.2, 12.2. HRMS (APCI): Calcd for C26H43O2S1Si1 [M]+: 447.2748, Found: 447.2747. Optical Rotation: [α]D20 +1.6 (c = 0.64, CHCl3) for an enantiomerically enriched sample of 87:13 er. The enantiomeric purity was established by HPLC analysis of the respective deprotected bicyclic thioester, see the supporting information part A for details.
S-benzyl (E)-2-((1S,3R,5S)-3-(((triisopropylsilyl)oxy)methyl)bicyclo[3.2.0]heptan-6-ylidene)ethanethioate [28]:
Prepared according to the general procedure utilizing (cyclopent-3-en-1ylmethoxy)triisopropylsilane as the alkene component. Purification by silica gel chromatography (2% EtOAc:hexanes) provided 58.0 mg (51% yield, >20:1 dr, >20:1 E/Z) of the title compound as a yellow oil. IR (film): 2942 (m), 2864 (m), 1674 (m), 1641 (m), 1495 (w), 1463 (w), 1389 (w), 1276 (m), 1261 (m), 1113 (m), 1035 (m), 882 (w), 764 (s), 750 (s), 700 (w) cm-1. 1H NMR (500 MHz, CDCl3) δ 7.35 – 7.27 (m, 4H), 7.25 – 7.21 (m, 1H), 5.98 – 5.92 (m, 1H), 4.16 (s, 2H), 3.74 – 3.66 (m, 2H), 3.44 – 3.33 (m, 1H), 3.26 (dddd, J = 19.4, 9.0, 2.4 Hz, 1H), 2.99 – 2.86 (m, 1H), 2.68 (dddd, J = 19.4, 4.3, 2.5 Hz, 1H), 2.48 – 2.33 (m, 1H), 1.76 (ddd, J = 25.0, 12.7, 5.9 Hz, 2H), 1.56 – 1.39 (m, 2H), 1.10 – 1.00 (m, 21H). 13C NMR (125 MHz, CDCl3) δ 188.1, 169.4, 138.2, 129.0, 128.7, 127.2, 120.2, 66.4, 49.1, 41.4, 38.7, 36.4, 36.3, 35.8, 32.9, 18.2, 12.1. HRMS (EI): Calcd for C26H40O2S1Si1 [M]+: 444.2513, Found: 444.2522. Optical Rotation: [α]D20 +15.9 (c = 1.00, CHCl3) for an enantiomerically enriched sample of 94:6 er. The enantiomeric purity was established by HPLC analysis using a chiral column (Lux 3u Cellulose-1 column, 22 °C, 0.5 mL/min, 99:1 hexanes:isopropanol, 254 nm, tminor = 10.348 min, tmajor = 10.822 min). See supporting information part B for HPLC chromatograms.
S-(4-chlorobenzyl) 2-((1R,8S)-10,10-dimethylbicyclo[6.2.0]decan-9-ylidene)ethanethioate [35]:
HNTf2 (14.0 mg, 0.05 mmol, 0.20 equiv) was added to a flame dried round bottom flask in an argon filled glovebox, capped with a septum, and removed from the glovebox. CH2Cl2 (0.50 mL, 0.50 M) was added and the reaction mixture was cooled to - 78 °C. Oxazaborolidine 3 (0.25 M in PhMe, 250 μL, 0.06 mmol, 0.25 equiv) was added and allowed to stir for 30 minutes. Cyclooctene (160 μL, 1.25 mmol, 5.00 equiv) was added, followed quickly by S-(4-chlorobenzyl) 4-methylpenta-2,3-dienethioate 32 (63.0 mg, 0.25 mmol, 1.00 equiv) and the reaction was allowed to warm to room temperature. After 16 hours, the reaction was quenched with 100 μL Et3N and the solution was concentrated. Purification by silica gel chromatography (hexanes – 2% Et2O:hexanes gradient) provided 82.4 mg (93% yield, ~1:1 E/Z mixture of alkene isomers) of the title compound as a pale yellow oil. For characterization, small amounts of the respective E-isomer were separated. E-isomer: IR (film): 2918 (br, m), 2850 (m), 1672 (s), 1636 (s), 1490 (m), 1460 (w), 1443 (w), 1339 (w), 1261 (w), 1242 (w), 1092 (m), 1066 (s), 1066 (s), 980 (w), 813 (s), 738 (m), 678 (w), 641 (w), 552 (w), 509 (w cm-1. 1H NMR (500 MHz, CDCl3) δ 7.26 – 7.11 (m, 4H), 5.87 – 5.77 (m, 1H), 4.07 (d, J = 14.5 Hz, 1H), 4.01 (d, J = 13.4 Hz, 1H), 3.27 (t, J = 10.0 Hz, 1H), 2.07 – 1.92 (m, 2H), 1.76 – 1.41 (m, 6H), 1.42 – 1.33 (m, 1H), 1.31 – 1.08 (m, 4H), 1.04 (s, 3H), 1.00 (s, 3H).13C NMR (125 MHz, CDCl3) δ 187.3, 179.8, 137.0, 133.0, 130.3, 128.8, 116.6, 48.4, 46.13, 45.4, 32.3, 31.21, 29.2, 28.1, 27.9, 26.1, 25.7, 22.5, 22.1.HRMS (ESI): Calcd for C21H27O1Cl1S1Na1 [M+Na]+: 385.1369, Found: 385.1369. The enantiomeric purity was established by HPLC analysis using a chiral column. E-isomer: (Lux 3u Cellulose-2 column, 22 °C, 0.25 mL/min, 400:1 hexanes:isopropanol, 254 nm, tminor = 30.711 min, tmajor = 34.576 min): 96:4 er. Z-isomer: (Lux 3u Cellulose-2 0.25mL/min, 400:1 hexanes:isopropanol, 254 nm, tmajor = 36.894 min, tminor = 41.498 min): 86:14 er. Absolute stereochemistry only tentatively assigned. See supporting information part B for HPLC chromatograms. The reaction was repeated using oxazaborolidine 34 under otherwise unchanged conditions. The isolated yield was 84.2 mg (95% yield, ~4:1 E/Z) and the corresponding er was 98:2 for the major E-isomer and 81:19 for the minor Z-isomer. Absolute stereochemistry only tentatively assigned. See supporting information part B for HPLC chromatograms.
S-(4-chlorobenzyl) 2-((1R,5S)-7,7-dimethylbicyclo[3.2.0]heptan-6-ylidene)ethanethioate[36]:
HNTf2 (14.0 mg, 0.05 mmol, 0.20 equiv) was added to a flame dried round bottom flask in an argon filled glovebox, capped with a septum, and removed from the glovebox. CH2Cl2 (0.50 mL, 0.50 M) was added and the reaction mixture cooled to −78 °C. Oxazaborolidine 3 (0.25 M in PhMe, 250 μL, 0.06 mmol, 0.25 equiv) was added and allowed to stir for 30 minutes. Cyclopentene (114 μL, 1.25 mmol, 5.00 equiv) was added followed quickly by a solution of S-(4-chlorobenzyl) 4-methylpenta-2,3-dienethioate (32)(63.0 mg, 0.25 mmol, 1.00 equiv) in PhMe (0.20 mL) and the reaction was allowed to warm to room temperature. After 16 hours, the reaction was quenched with 100 μL Et3N and the solution was concentrated. Purification by silica gel chromatography (1% Et2O:hexanes) provided 59.4 mg (74% yield, 1:1 E/Z, mixture of alkene isomers) of the title compound as a colorless oil. Small amounts of the isomers were separable for analytical characterization. Z-Isomer:IR (film): 2944 (m), 1672 (s), 1635 (m), 1490 (m), 1085 (m), 1042 (m), 1027 (s), 1004 (m), 789 (m), 743 (m) cm-1. 1H NMR (600 MHz, CDCl3) 7.32 – 7.16 (m, 4H), 5.81 (d, J = 1.9 Hz, 1H), 4.08 (s, 2H), 3.29 (app. td, J≈ 7.2, 1.7 Hz, 1H), 2.41 (app. t, J≈ 7.7 Hz, 1H), 1.86 – 1.64 (m, 3H), 1.59 – 1.39 (m, 3H), 1.34 (s, 3H), 1.13 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 187.2, 179.7, 137.0, 133.0, 130.3, 128.8, 117.1, 47.6, 47.5, 43.3, 32.3, 30.9, 30.9, 28.2, 27.0, 19.1. HRMS (EI): Calcd for C18H21Cl1O1S1 [M]+: 320.0996, Found: 320.0999. Optical Rotation: [α]D20 +36.8 (c = 2.30, CHCl3) for an enantiomerically enriched sample of 66:34 er. The enantiomeric purity was established by HPLC analysis using a chiral column (Lux 3u Cellulose-1 column, 22 °C, 0.5 mL/min, 99:1 hexanes:isopropanol, 254 nm, tminor = 8.477 min, tmajor = 9.571 min). E-Isomer:IR (film): 2951 (m), 1673 (s), 1637 (m), 1491 (m), 1491 (w), 1039 (s), 798 (w) cm-1. 1H NMR (600 MHz, CDCl3) δ 7.29 – 7.19 (m, 4H), 5.86 (d, J = 2.4 Hz, 1H), 4.13 (d, J = 14.0 Hz, 1H), 4.07 (d, J = 14.0 Hz, 1H), 3.75 (ddd, J = 8.7, 5.2, 2.0 Hz, 1H), 2.44 (app. t, J≈ 7.2 Hz, 1H), 2.11 – 2.00 (m, 1H), 1.85 – 1.69 (m, 2H), 1.59 – 1.43 (m, 3H), 1.21 (s, 3H), 0.96 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 186.0, 178.4, 136.9, 133.0, 130.3, 128.8, 118.3, 47.4, 45.5, 44.8, 32.5, 32.0, 28.1, 27.8, 26.6, 18.1. HRMS (EI): Calcd for C18H21Cl1O1S1 [M]+: 320.0996, Found: 320.0997. Optical Rotation: [α]D20 −190.4 (c = 2.18, CHCl3) for an enantiomerically enriched sample of 89:11 er. The enantiomeric purity was established by HPLC analysis using a chiral column (Lux 3u Cellulose-4 column, 22 °C, 0.5 mL/min, 99:1 hexanes:isopropanol, 254 nm, tmajor = 8.913 min, tminor = 9.535 min). Absolute stereochemistry only tentatively assigned. See supporting information part B for HPLC chromatograms. The reaction was repeated using oxazaborolidine 34 under otherwise unchanged conditions. The isolated yield was 55.4 mg (69% yield, ~2:1 E/Z) and the corresponding er was 94:6 for the major E-isomer and 77:23 for the minor Z-isomer. Absolute stereochemistry only tentatively assigned. See supporting information part B for HPLC chromatograms.
S-(4-chlorobenzyl) 2-((3R,4S)-2,2-dimethyl-3,4-dipropylcyclobutylidene)ethanethioate[37]:
HNTf2 (14.0 mg, 0.05 mmol, 0.20 equiv) was added to a flame dried round bottom flask in an argon filled glovebox, capped with a septum, and removed from the glovebox. CH2Cl2 (0.50 mL, 0.50 M) was added and the reaction mixture cooled to −78 °C. Oxazaborolidine 3 (0.25 M in PhMe, 250 μL, 0.06 mmol, 0.25 equiv) was added and allowed to stir for 30 minutes. Cis-4-octene (195 μL, 1.25 mmol, 5.00 equiv) was added followed quickly by a solution of S-(4-chlorobenzyl) 4-methylpenta-2,3-dienethioate (32)(63.0 mg, 0.25 mmol, 1.00 equiv) in PhMe (0.20 mL) and the reaction was allowed to warm to room temperature. After 16 hours, the reaction was quenched with 100 μL Et3N and the solution was concentrated. Purification by silica gel chromatography (1% Et2O:hexanes) provided 73.9 mg (81% yield, ~1:3 E/Z, inseparable mixture of alkene isomers) of the title compound as a colorless oil. IR (film): 2955 (m), 2928 (m), 1674 (m), 1635 (m), 1490 (m), 1092 (m), 1030 (s), 1015 (m), 831 (m), 793 (m), 507 (m) cm-1. 1H NMR (600 MHz, CDCl3) Z-Isomer: δ 7.40 – 7.00 (m, 4H), 5.89 (d, J = 2.1 Hz, 1H), 4.11 (d, J = 14.0 Hz, 1H), 4.07 (d, J = 14.0 Hz, 1H), 3.00 – 2.67 (m, 1H), 2.16 (ddd, J = 9.7, 8.2, 6.8 Hz, 1H), 1.60 – 1.22 (m, 8H), 1.30 (s, 3H), 1.26 (s, 3H), 0.96 – 0.86 (m, 6H). 13C NMR (125 MHz, CDCl3)Z-Isomer: δ 186.3, 177.2, 137.0, 133.0, 130.3, 128.8, 118.7, 47.5, 44.6, 42.6, 32.6, 32.1, 28.2, 27.3, 22.4, 21.7, 20.5, 14.6, 14.3. HRMS (EI): Calcd for C21H29Cl1O1S1 [M]+: 364.1622, Found: 364.1624. The enantiomeric purity was established by HPLC analysis using a chiral column. E-isomer: (Lux 3u Cellulose-1 column followed by Lux 3u Amylose 2 column, 22 °C, 0.5 mL/min, 99:1 hexane:isopropanol, 254 nm, tmajor = 25.089 min, tminor = 27.090 min): 91:9 er. Z-isomer: (Lux 3u Cellulose-1 column followed by Lux 3u Amylose 2 column, 22 °C, 0.5 mL/min, 99:1 hexane:isopropanol, 254 nm, tmajor = 23.950 min, tminor = 24.571 min): 74:26 er. Absolute stereochemistry only tentatively assigned. See supporting information part B for HPLC chromatograms. The reaction was repeated using oxazaborolidine 34 under otherwise unchanged conditions. The isolated yield was 79.4 mg (87% yield, ~1:1 E/Z) and the corresponding er was 95:5 for the E-isomer and 71:29 for the Z-isomer. Absolute stereochemistry only tentatively assigned. See supporting information part B for HPLC chromatograms.
S-(4-chlorobenzyl) (S,E)-2-(3-butyl-2,2-dimethylcyclobutylidene)ethanethioate [38]:
HNTf2 (14.0 mg, 0.05 mmol, 0.20 equiv) was added to a flame dried round bottom flask in an argon filled glovebox, capped with a septum, and removed from the glovebox. CH2Cl2 (0.50 mL, 0.50 M) was added and the reaction mixture cooled to −78 °C. Oxazaborolidine 3 (0.25 M in PhMe, 250 μL, 0.06 mmol, 0.25 equiv) was added and allowed to stir for 30 minutes. 1-hexene (313 μL, 2.50 mmol, 10.0 equiv) was added followed quickly by a solution of S-(4-chlorobenzyl) 4-methylpenta-2,3-dienethioate (32)(63.0 mg, 0.25 mmol, 1.00 equiv) in PhMe (0.20 mL) and the reaction was allowed to warm to room temperature. After 48 hours, the reaction was quenched with 100 μL Et3N and the solution was concentrated. Purification by silica gel chromatography (1% Et2O:hexanes) provided 73.9 mg (81% yield, >20:1 E/Z) of the title compound as a colorless oil. IR (film): 2956 (s), 2942 (s), 2864 (w), 1675 (s), 1640 (s), 1492 (m), 1114 (m), 1036 (s), 883 (m) cm-1. 1H NMR (600 MHz, CDCl3) δ 7.26 – 7.22 (m, 4H), 5.91 (app. t, J≈ 2.4 Hz, 1H), 4.10 (s, 2H), 3.25 (ddd, J = 17.9, 8.6, 2.2 Hz, 1H), 2.65 (ddd, J = 17.9, 7.7, 2.6 Hz, 1H), 2.04 (app. tdd, J≈ 9.0, 7.7, 6.1 Hz, 1H), 1.50 (app. ddt, J≈ 13.1, 9.6, 5.8 Hz, 1H), 1.37 – 1.19 (m, 5H), 1.18 (s, 3H), 1.09 (s, 3H), 0.89 (t, J = 7.2 Hz, 3H). 13C NMR (125 MHz, CDCl3) δ 188.2, 173.5, 137.0, 133.0, 130.4, 128.8, 116.9, 47.2, 42.7, 37.0, 32.2, 30.6, 30.2, 27.5, 22.9, 21.3, 14.2. HRMS (APCI): Calcd for C19H26Cl1O1S1 [M+H]+: 337.1387, Found: 337.1390. Optical Rotation: [α]D20 +9.0 (c = 1.00, CHCl3) for an enantiomerically enriched sample of 69:31 er. The enantiomeric purity was established by HPLC analysis using a chiral column (Chiralpak IA-PG024 column, 22 °C, 0.3 mL/min, 99:1 hexane:isopropanol, 254 nm, tmajor = 15.876 min, tminor = 16.613 min). Absolute stereochemistry only tentatively assigned. See supporting information part B for HPLC chromatograms.The reaction was repeated using oxazaborolidine 34 under otherwise unchanged conditions. The isolated yield was 39.5 mg (47% yield of pure E-isomer, ~4:1 E/Z isomeric mixture from crude NMR) and the corresponding er was 67:33 for the E-isomer. Absolute stereochemistry only tentatively assigned. See supporting information part B for HPLC chromatograms.
Supplementary Material
Acknowledgments
We thank Indiana University and the National Institutes of Health (R01GM110131) for generous support of this work. The Deutsche Forschungsgemeinschaft (WI 4933/1-2) is acknowledged for postdoctoral fellowship support to J.M.W. This project was partially funded by the Vice Provost for Research through the Research Equipment Fund and the NSF (CHE1726633).
Footnotes
Supplementary Material
Supplementary material (1H NMR and 13C NMR) is available for all compounds as well as additional experimental details.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.(a) Lee-Ruff E, Mladenova G, Chem. Rev 2003, 103, 1449. [DOI] [PubMed] [Google Scholar]; (b) Namyslo JC, Kaufmann DE, Chem. Rev 2003, 103, 1485. [DOI] [PubMed] [Google Scholar]; (c) N. Hoffmann Chem. Rev 2008, 108, 1052. [DOI] [PubMed] [Google Scholar]; (d) Bach T; Hehn Angew JP. Chem., Int. Ed 2011, 50, 1000. [DOI] [PubMed] [Google Scholar]; (e) Yoon TP, ACS Catal.2013, 3, 895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(a) Xu Y; Conner ML; Brown MK Angew. Chem. Int. Ed 2015, 54 (41), 11918. [DOI] [PubMed] [Google Scholar]; (b) Poplata S; Tröster A; Zou Y-Q; Bach T Chem. Rev 2016, 116 (17), 9748. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Wang N; Lu P Org. Chem. Front 2018, 5, 254. [Google Scholar]
- 3.(a) Brimioulle R; Lenhart D; Maturi MM; Bach T Enantioselective Catalysis of Photochemical Reactions. Angew. Chem. Int. Ed. Engl 2015, 54 (13), 3872–3890. [DOI] [PubMed] [Google Scholar]; (b) Blum TR; Miller ZD; Bates DM; Guzei IA; Yoon TP Enantioselective Photochemistry Through Lewis Acid-Catalyzed Triplet Energy Transfer. Science 2016, 354 (6318), 1391–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Poplata S; Bach T Enantioselective Intermolecular [2+2] Photocycloaddition Reaction of Cyclic Enones and Its Application in a Synthesis of (−)-Grandisol. J. Am. Chem. Soc 2018, 140 (9), 3228–3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Du J; Skubi KL; Schultz DM; Yoon TP A Dual-Catalysis Approach to Enantioselective [2 + 2] Photocycloadditions Using Visible Light. Science 2014, 344, 392–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishihara K; Nakano K Enantioselective [2 + 2] Cycloaddition of Unactivated Alkenes with Α-Acyloxyacroleins Catalyzed by Chiral Organoammonium Salts. J. Am. Chem. Soc 2007, 129 (29), 8930–8931. [DOI] [PubMed] [Google Scholar]
- 6.(a) Conner ML; Xu Y; Brown MK J. Am. Chem. Soc 2015, 137 (10), 3482. [DOI] [PubMed] [Google Scholar]; (b) Xu Y; Hong YJ; Tantillo DJ; Brown MK Org. Lett 2017, 19 (14), 3703. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Wiest JM; Conner ML; Brown MK Angew. Chem. Int. Ed 2018, 57 (17), 4647. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Wiest JM; Conner ML; Brown MK J. Am. Chem. Soc 2018, 140 (46), 15943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.For a chirality transfer [2+2] cycloaddition of an electron deficient allene, see:Line NJ; Witherspoon BP; Hancock EN; Brown MK J. Am. Chem. Soc 2017, 139 (41), 14392..
- 8. For early studies on the development of [2+2] Cycloadditions between electron deficient allenes and alkenes, see:; (a) Snider BB; Spindell DK J. Org. Chem 1980, 45 (25), 5017. [Google Scholar]; (b) Hoffmann H; Ismail ZM; Weber A Tetrahedron Lett 1981, 22 (21), 1953. [Google Scholar]; (c) Snider BB; Ron EJ Org. Chem 1986, 51 (19), 3643. [Google Scholar]; (d) Padwa A; Meske M; Murphree SSJ Am. Chem. Soc 1995, 117, 7071. [Google Scholar]; Padwa A; Lipka H; Watterson SH; Murphree SS J. Org. Chem 2003, 68 (16), 6238. [DOI] [PubMed] [Google Scholar]; (e) Jung ME; Murakami M Org. Lett 2006, 8 (25), 5857. [DOI] [PubMed] [Google Scholar]; (f) Zhao; Loh T-P Angew. Chem. Int. Ed 2009, 48 (39), 7232. [DOI] [PubMed] [Google Scholar]
- 9.For similar observations made in the development of enantioselective [3+2] cycloadditions with N-acyl imines and allenoates, seeCowen BJ; Saunders LB; Miller SJ J. Am. Chem. Soc 2009, 131 (17), 6105..
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.