Abstract
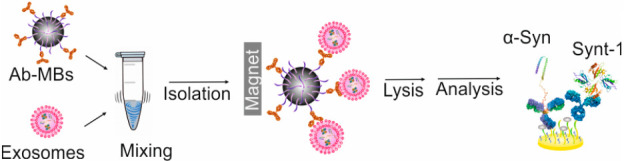
The egress of α-synuclein in neuronally derived exosomes predates the clinical presentation of Parkinson’s disease (PD), offering a means of developing a predictive or prognostic test. Here, we report the reagentless impedimetric assay of two internal exosome markers (α-synuclein and syntenin-1) from neuronal exosomes. Exosomes were efficiently extracted from patient sera using anti-L1CAM conjugated zwitterionic polymer-modified magnetic beads prior to lysis and analyzed by electrochemical impedance spectroscopy. The quantification of α-synuclein level across 40 clinical samples resolved statistically significant differences between PD patients and healthy controls (HC).
Parkinson’s disease (PD) is the second most common neurodegenerative disease with increasing prevalence but, currently, no cure. It is primarily characterized by a movement disorder arising from a loss of dopaminergic neurons in the substantia nigra and intraneuronal α-synuclein (α-Syn) aggregation.1 PD is typically preceded by a long prodromal phase and can progress to dementia in advanced stages associated with diffuse α-Syn aggregation throughout the brain.2,3 Currently, there is no blood or cerebrospinal fluid (CSF) biomarker in clinical practice for predicting PD in the prodromal phase or objectively assessing disease progression. The development of efficient methods to assay key biomarkers in the earliest stages of PD will undoubtedly underpin therapeutic intervention when disease-modifying therapies become available.
Exosomes are endosome-derived vesicles (40–140 nm) secreted by most cell types and detectable in most biological fluids including blood and CSF.4−6 They have been implicated in cell–cell signaling and the egress of unwanted proteins. We have very recently shown that the content of neuronally derived exosomes isolated from serum reflects pathological changes in the brain even at the prodromal stage of PD.4 However, neuron-derived exosomes constitute approximately 15% of total circulating exosomes4 and contain proteins at low concentrations requiring both effective extraction and a sensitive assaying of their contents. Exosomes are most typically isolated by differential ultracentrifugation (UC), a process which is laborious, equipment heavy, and associated with low isolation efficiency.7,8 Moreover, the standard differential ultracentrifugation methods do not discriminate between exosomes and other structures such as larger extracellular vesicles or protein/lipid aggregates.9,10 In recent years, immunoaffinity-based technology has been widely used to improve the efficiency and purity of exosome subtype isolation, and appropriately modified magnetic beads (MBs) have become a valuable part of this toolbox.11,12 They are, however, also readily fouled, and their “magnetic pull down” thus collects an unhelpfully low ratio of specific exosome to fouling material during immunocapture in complex biological fluids like serum, compromising the specificity of any downstream exosomal protein assay.13,14 This is especially troublesome for proteins such as α-Syn which is present in biofluids primarily in a free form and in much lower abundance when exosome bound.2 The use of capture beads with higher levels of selectivity could mitigate this limitation. Of the nonfouling interfaces available, those based on highly hydrated zwitterionic polymers like poly(carboxybetaine methacrylamide)) (pCBMA) are the most potent.15 The most effective high density “brush type” interfaces are only realistically accessible through surface-initiated polymer growth, such as that through an appropriately designed reversible addition–fragmentation chain transfer (RAFT). Once prepared, these films can be further modified with antibodies in generating specifically receptive interfaces.16,17
Conventional lab-based biomarker quantification is by enzyme-linked immunosorbent assay (ELISA); this is laborious, has high cost, and is often insufficiently sensitive.18 Electrochemical assays can be conveniently run with higher sensitivity, enhanced simplicity (single step and/or “reagentless”), and lower cost.19−22 There has been much interest in assessments of α-Syn in PD from serum and CSF, though, thus far, these assessment have been of limited clinical value.23−25 Although a number of analyses of exosomes have been reported by electrochemical methods, these are almost exclusively of exosomes themselves and not internal protein markers.26,27 We have recently reported the analysis of the internal cargo protein syntenin-1 (Synt-1) from UC-isolated serum exosomes.28 In this current study, polymer brush-coated magnetic beads were generated via RAFT and conjugated to anti-L1CAM (i.e., L1 cell adhesion molecule) antibodies for the specific immunocapture of neuronally derived exosomes. It was envisaged that improved levels of capture specificity would enable the use of comparatively simple sensor surfaces without detrimental loss of specificity.29−31 The isolation of exosomes was followed by lysis and a robust impedimetric quantification of two internal markers in a manner that enabled a statistically significant differentiation of PD from control samples.
Magnetic Bead Characterization and Exosome Isolation
As noted, nonspecific adsorption can negatively influence downstream exosomal proteins detection and analysis. To bypass this, magnetic beads (∼2.4 μm) were coated with a zwitterionic polymer pCBMA via the RAFT process and were further modified with the anti-L1CAM antibody (Figure 1). Successful polymerization of pCBMA on beads was resolved by infrared attenuated total reflection spectroscopy (IR-ATR) (Figure S1A).32,33 Zeta potential assessments (Figure 1A) were measured before (Fe3O4, −33.8 ± 3.2 mV) and after (pCBMA@Fe3O4, −2.3 ± 1.2 mV) polymerization, indicating a near-zero overall charge34,35 as desired for optimal performance.36,37 The antifouling properties of the pCBMA@Fe3O4 MBs were confirmed through a markedly reduced (∼90%) nonspecific adsorption of bovine serum albumin (BSA) when compared to native Fe3O4 beads (Figure S1B) It is noteworthy that, even after antibody conjugation (i.e., anti-L1CAM modified pCBMA@Fe3O4 MBs), antifouling performance is not significantly compromised.38,39 We further demonstrated that pCBMA@Fe3O4 MBs, unlike commercially available carboxylate MBs, exhibited good antifouling properties when incubated with soluble recombinant α-synuclein, irrespective of the antibody used (anti-L1CAM or anti-HA as shown in Figure 2 A). This is critically important in supporting the selective and clean isolation of exosomes from serum samples.
Figure 1.
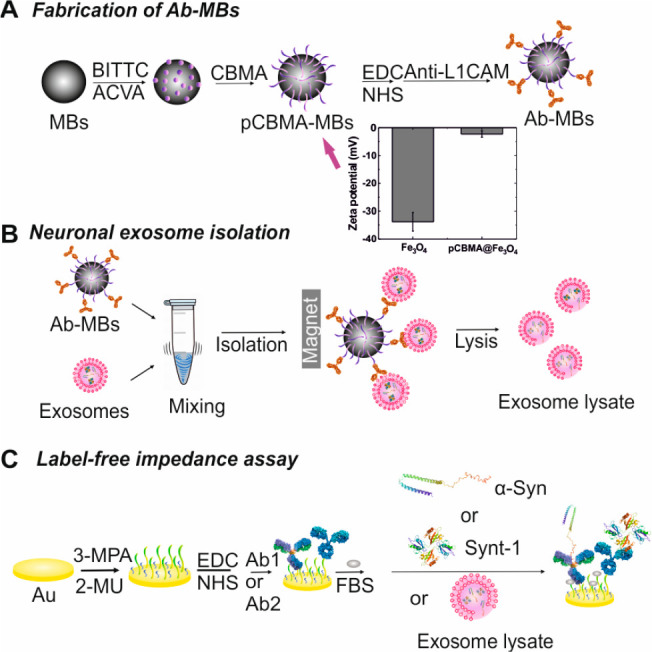
Schematic representation of (A) the synthesis of pCBMA-coated MBs and anti-L1CAM Ab conjugation. Inset shows zeta potential of bare MBs before (marked as Fe3O4) and after polymerization (pCBMA@Fe3O4). (B) Neuronal exosome isolation using anti-L1CAM pCBMA-coated MBs. (C) Label-free impedimetric assays for two internal markers (Ab1 = anti-α-Syn antibody, Ab2 = anti-Synt-1 antibody).
Figure 2.
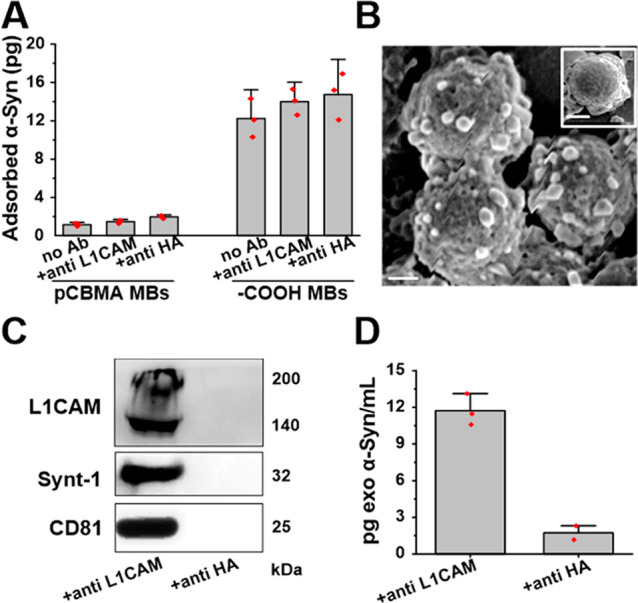
(A) Histogram depicting the quantified adsorption of recombinant α-Syn on different Ab-modified pCBMA@Fe3O4 MBs surfaces. The commercial carboxylic acid-terminated MBs were used as the control. (B) SEM image of serum-captured exosomes on anti-L1CAM-modified MBs versus anti-HA (control)-modified MBs (insert). Scale bar 1 μm. (C) Immunoblotting of lysates of immunocaptured vesicles confirming the detection of both transmembrane proteins (L1CAM, CD81) and internal protein Synt-1 from exosomes. Specific electrochemiluminescence detection of α-Syn (D) in neuronal exosomes immunocaptured from serum with anti-L1CAM vs anti-HA (control)-modified pCBMA@Fe3O4 MBs.
The anti-L1CAM antibody-coated pCMBA were then assessed for immunocapture of neuronal exosomes in serum. SEM image analysis clearly showed exosomes bound to anti-L1CAM conjugated pCBMA@Fe3O4 MBs (Figure 2B) but not control beads (i.e., anti-HA Ab-coated pCBMA@Fe3O4 beads, inset in Figure 2B). Captured exosomes were eluted using a pH 2.9 glycine solution, followed by neutralization with a Tris buffer and negative staining for TEM (Figure S1C). The image resolves double-membrane and cup-shaped vesicles with a diameter of ∼100 nm, typical of exosome morphology and size.40 To further confirm their molecular composition, captured vesicles were lysed and processed for immunoblotting (Figure 2C). The transmembrane markers L1CAM and CD 81 and the internal protein marker Synt-1 were detected in lysates from anti-L1CAM@ pCBMA@Fe3O4 MBs samples but not in control lysates (samples incubated with anti-HA-coated pCBMA@Fe3O4 MBs).
We also confirmed that anti-L1CAM-modified pCBMA@Fe3O4 MBs are effective in isolating from serum neuronal exosomes containing α-Syn (Figure 2D).
It is noteworthy that the ratio of CD81 content in L1CAM+ (neuronal exosomes)/CD9+ (generic exosomes) was calculated to be ∼11% based on a comparative analysis of immunoblot intensities (Figure S2). Taken together, these observations strongly support the selective isolation of serum neuronal exosomes containing α-Syn by anti-L1CAM-modified pCBMA@Fe3O4 beads. The overall immunocapture efficiency was also examined using anti-CD9-modified pCBMA@Fe3O4 (Figure S3) and resolved 81% by nanoparticle tracking analysis.
Subsequent to this analysis of selective exosome capture, we sought to quantify internal markers electrochemically. Mixed, low initial impedance and high impedimetric baseline stability, self-assembled monolayers (SAMs) comprising 3-mercaptopropionic acid (3-MPA), 2-mercaptoethanol (2-MU) were generated on gold electrodes prior to antibody loading (Figure S4). These interfaces were thereafter applied to the analysis of exosomal content (Figure 1C) after controlled lysis; specifically, both α-Syn and Synt-1 were assayed. The former is associated with PD,3 whereas the latter, as prior indicated, is a generic cargo protein.
Label-Free Impedimetric Assays in Spiked Serum
Prior to the analysis of real patient samples, we initially set out to gauge the reliability of biomarker quantification through the repeat analysis of prepared spiked solutions for both α-Syn and Synt-1, including analyses with control proteins (e.g., C-reactive protein (CRP) and BSA) at greater than 106 times excess of the expected marker levels (Figure S5). Reliable triplicate quantifications of both markers (Figure S6) were demonstrable within 30 min with limits of detection (LOD) and quantification (LOQ) at 0.3 and 0.8 pg/mL for α-Syn, respectively (Figure S7A), notably better than most prior exosomal analyses.41 The assays herein are significantly more sensitive than commercial electrochemiluminescence kits (by almost an order of magnitude), much cheaper, much faster, and require markedly less sample input (100 vs 500 μL).
Clinical Sample Analysis
A statistical analysis across 40 clinical samples in a cohort study between PD and HC (n = 20 per group) was conducted to access the applicability of internal neuronal exosomal biomarkers to differentiate between PD and HC using 10 μL of exosome lysate immunocaptured from 100 μL of input serum using anti-L1CAM-coated pCBMA@Fe3O4 MBs. Box plot analyses (Figure 3) confirmed an elevation of α-Syn in neuronal exosomes in PD (p value of 4.3 × 10–4, i.e., <0.0001). There was no difference in the content of the generic exosomal protein Synt-1 (p = 0.34) between the two groups. To further assess the reliability of the electrochemical assay in measuring neuronal exosome-associated α-Syn and Synt-1, we cross referenced the same samples with the electrochemiluminescence kit platform (Figure S9) where the difference between PD vs HC was also observed (p = 1.48 × 10–5 albeit with 500% greater serum volume). Absolute quantifications of α-Syn using EIS were higher than those detected by electrochemiluminescence. This may be a reflection of differences in assay sensitivity and selectivity at the respective surfaces (32 mm2 in 96-well chemiluminescence plate vs 9 mm2 in EIS sensor and different surface chemistries).42−44 To the best of our knowledge, this is the first time that a reagentless electrochemical method has been shown to be of clinical value in analyzing the protein content of exosomes.
Figure 3.
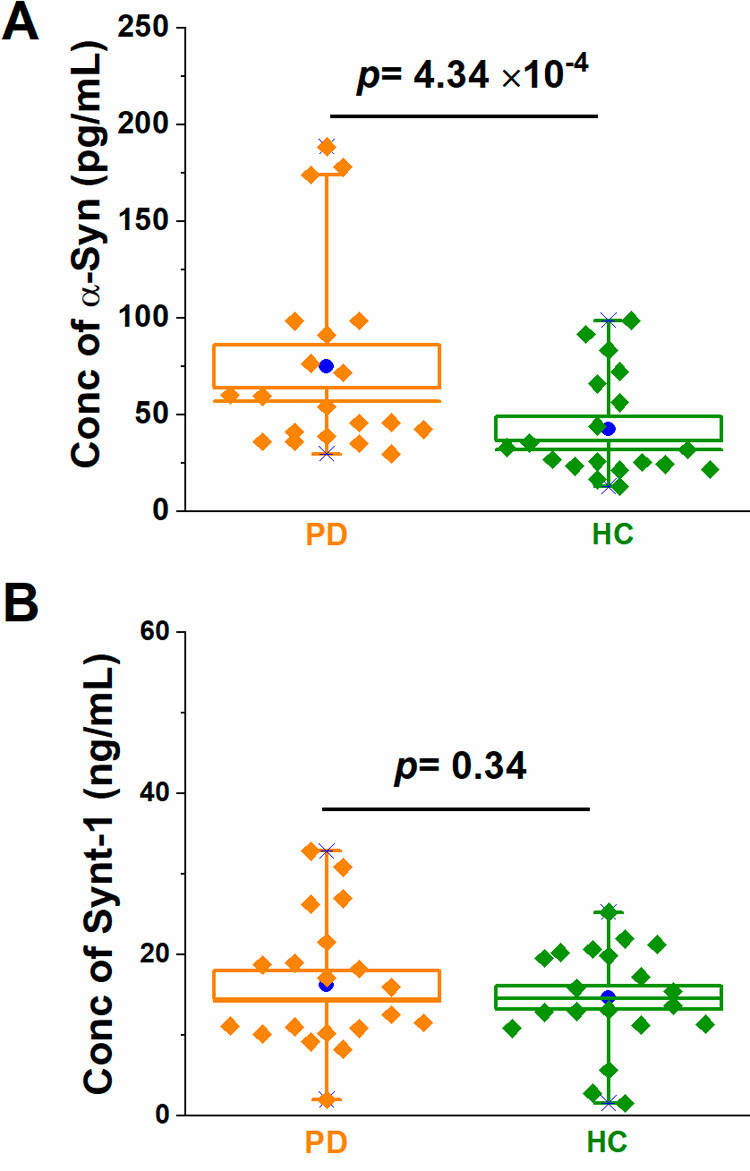
Box plots for impedimetric exosomal quantifications of (A) α-Syn and (B) Synt-1 from 40 clinical samples (PD and HC, n = 20 per group). In the box plots, the lower and upper boundaries indicate the 25th and 75th percentiles, respectively. The line within the box marks the median, and the blue circle within the box marks the mean. Diamonds represent individual patient sample data points (the mean value of nine measurements across three electrodes for each clinical sample).
Conclusions
Anti-L1CAM-modified polymer brush-coated MBs enable the selective immunocapture of neuronal exosome subpopulations from 100 μL of serum prior to a sensitive (sub pg/mL) and reagentless impedimetric assay. The cleanliness of the initial isolation enables subsequent electroanalyses to be performed on a standard and readily scalable monolayer film from much lower levels of serum than required for electrochemiluminescence or ELISA. Significantly, these analyses further confirm the value of exosomal of α-Syn as a relevant biomarker in PD.4 The study herein offers a solution for effectively verifying biomarkers in clinical translation and also lays a foundation for on-chip integration of immunocapture and reagentless analysis of exosome biomarkers in a scalable manner.
Acknowledgments
We thank Dr. Robert Hein for the synthesis of the CBMA monomer and helpful input and Dr. Franziska Hopfner for providing clinical samples. The work was funded by grants from the EPSRC (EP/M006204/1), the Selfridges Group Foundation, and the NIHR Oxford Biomedical Research Centre to G.K.T and J.J.D.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.analchem.0c03092.
Materials and methods, magnetic bead characterization, selectivity assessments, and supplementary data (PDF)
Author Contributions
Y.F. and C.J. performed experiments and analyzed data. G.K.T. and J.J.D. designed research and analyzed data. The manuscript was written with contributions from all authors. All authors have given approval to the final version of the manuscript.
Author Contributions
§ Y. Fu and C. Jiang contributed equally.
The opinions expressed and arguments employed herein do not necessarily reflect the official views of these funding bodies.
The authors declare no competing financial interest.
Supplementary Material
References
- Chaudhuri K. R.; Healy D. G.; Schapira A. H. Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol. 2006, 5, 235–245. 10.1016/S1474-4422(06)70373-8. [DOI] [PubMed] [Google Scholar]
- Tofaris G. K. A critical assessment of exosomes in the pathogenesis and stratification of Parkinson’s disease. J. Parkinson's Dis. 2017, 7, 569–576. 10.3233/JPD-171176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tofaris G. K.; Spillantini M. G. Alpha-synuclein dysfunction in Lewy body diseases. Mov. Disord. 2005, 20, S37–S44. 10.1002/mds.20538. [DOI] [PubMed] [Google Scholar]
- Jiang C.; Hopfner F.; Katsikoudi A.; Hein R.; Catli C.; Evetts S.; Huang Y.; Wang H.; Ryder J. W.; Kuhlenbaeumer G.; Deuschl G.; Padovani A.; Berg D.; Borroni B.; Hu M. T.; Davis J. J.; Tofaris G. K. Serum neuronal exosomes predict and differentiate Parkinson’s disease from atypical parkinsonism. J. Neurol., Neurosurg. Psychiatry 2020, 91, 720–729. 10.1136/jnnp-2019-322588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson P. R.; Zheng Y.; Fischer R.; Heidasch R.; Gardiner C.; Evetts S.; Hu M.; Wade-Martins R.; Turner M. R.; Morris J.; Talbot K.; Kessler B. M.; Tofaris G. K. Identification of distinct circulating exosomes in Parkinson’s disease. Ann. Clin. Transl. Neurol. 2015, 2, 353–361. 10.1002/acn3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuendl A.; Kunadt M.; Kruse N.; Bartels C.; Moebius W.; Danzer K. M.; Mollenhauer B.; Schneider A. Induction of α-synuclein aggregate formation by CSF exosomes from patients with Parkinson’s disease and dementia with Lewy bodies. Brain 2016, 139, 481–494. 10.1093/brain/awv346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théry C.; Amigorena S.; Raposo G.; Clayton A. Isolation and Characterization of Exosomes from Cell Culture Supernatants and Biological Fluids. Curr. Protoc. Cell Biol. 2006, 30, 3.22.1–3.22.29. 10.1002/0471143030.cb0322s30. [DOI] [PubMed] [Google Scholar]
- Li P.; Kaslan M.; Lee S. H.; Yao J.; Gao Z. Progress in exosome isolation techniques. Theranostics 2017, 7, 789. 10.7150/thno.18133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. D.; Zacharias W.; Gercel-Taylor C. In Serum/Plasma Proteomics: Methods and Protocols; Humana Press: Totowa, NJ, 2011; pp 235–246. [Google Scholar]
- Zlotogorski-Hurvitz A.; Dayan D.; Chaushu G.; Korvala J.; Salo T.; Sormunen R.; Vered M. Human saliva-derived exosomes: comparing methods of isolation. J. Histochem. Cytochem. 2015, 63, 181–189. 10.1369/0022155414564219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai S.; Luo B.; Jiang P.; Zhou X.; Lan F.; Yi Q.; Wu Y. Immuno-modified superparamagnetic nanoparticles via host–guest interactions for high-purity capture and mild release of exosomes. Nanoscale 2018, 10, 14280–14289. 10.1039/C8NR02871K. [DOI] [PubMed] [Google Scholar]
- Fan Z.; Yu J.; Lin J.; Liu Y.; Liao Y. Exosome-specific tumor diagnosis via biomedical analysis of exosome-containing microRNA biomarkers. Analyst 2019, 144, 5856–5865. 10.1039/C9AN00777F. [DOI] [PubMed] [Google Scholar]
- Clayton A.; Court J.; Navabi H.; Adams M.; Mason M. D.; Hobot J. A.; Newman G. R.; Jasani B. Analysis of antigen presenting cell derived exosomes, based on immuno-magnetic isolation and flow cytometry. J. Immunol. Methods 2001, 247, 163–174. 10.1016/S0022-1759(00)00321-5. [DOI] [PubMed] [Google Scholar]
- Van Andel E.; De Bus I.; Tijhaar E. J.; Smulders M. M. J.; Savelkoul H. F. J.; Zuilhof H. Highly Specific Binding on Antifouling Zwitterionic Polymer-Coated Microbeads as Measured by Flow Cytometry. ACS Appl. Mater. Interfaces 2017, 9, 38211–38221. 10.1021/acsami.7b09725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C.; Wang G.; Hein R.; Liu N.; Luo X.; Davis J. J. Antifouling Strategies for Selective In Vitro and In Vivo Sensing. Chem. Rev. 2020, 120, 3852–3889. 10.1021/acs.chemrev.9b00739. [DOI] [PubMed] [Google Scholar]
- Kanyong P.; Catli C.; Davis J. J. Ultrasensitive Impedimetric Immunosensor for the Detection of C-Reactive Protein in Blood at Surface-Initiated-Reversible Addition–Fragmentation Chain Transfer Generated Poly(2-hydroxyethyl methacrylate) Brushes. Anal. Chem. 2020, 92, 4707–4710. 10.1021/acs.analchem.9b05030. [DOI] [PubMed] [Google Scholar]
- Kanyong P.; Patil A. V.; Davis J. J. Functional Molecular Interfaces for Impedance-Based Diagnostics. Annu. Rev. Anal. Chem. 2020, 13, 183–200. 10.1146/annurev-anchem-061318-115600. [DOI] [PubMed] [Google Scholar]
- Zangar R. C.; Daly D. S.; White A. M. ELISA microarray technology as a high-throughput system for cancer biomarker validation. Expert Rev. Proteomics 2006, 3, 37–44. 10.1586/14789450.3.1.37. [DOI] [PubMed] [Google Scholar]
- Fu Y.; Wang N.; Yang A.; Law H. K. W.; Li L.; Yan F. Highly sensitive detection of protein biomarkers with organic electrochemical transistors. Adv. Mater. 2017, 29, 1703787. 10.1002/adma.201703787. [DOI] [PubMed] [Google Scholar]
- Chen L.; Fu Y.; Wang N.; Yang A.; Li Y.; Wu J.; Ju H.; Yaen F. Organic electrochemical transistors for the detection of cell surface glycans. ACS Appl. Mater. Interfaces 2018, 10, 18470–18477. 10.1021/acsami.8b01987. [DOI] [PubMed] [Google Scholar]
- Fu Y.; Liu K.; Sun Q.; Lin B.; Lu D.; Xu Z.; Hu C.; Fan G.; Zhang S.; Wang C.; Zhang W. A highly sensitive immunosensor for calmodulin assay based on enhanced biocatalyzed precipitation adopting a dual-layered enzyme strategy. Biosens. Bioelectron. 2014, 56, 258–263. 10.1016/j.bios.2014.01.027. [DOI] [PubMed] [Google Scholar]
- Baradoke A.; Hein R.; Li X.; Davis J. J. Reagentless Redox Capacitive Assaying of C-Reactive Protein at a Polyaniline Interface. Anal. Chem. 2020, 92, 3508–3511. 10.1021/acs.analchem.9b05633. [DOI] [PubMed] [Google Scholar]
- Aerts M.; Esselink R.; Abdo W.; Bloem B.; Verbeek M. CSF α-synuclein does not differentiate between parkinsonian disorders. Neurobiol. Aging 2012, 33, 430. e1–430. e3. 10.1016/j.neurobiolaging.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Chahine L. M.; Stern M. B.; Chen-Plotkin A. Blood-based biomarkers for Parkinson’s disease. Parkinsonism Relat Disord. 2014, 20, S99–S103. 10.1016/S1353-8020(13)70025-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bougea A.; Stefanis L.; Paraskevas G. P.; Emmanouilidou E.; Vekrelis K.; Kapaki E. Plasma alpha-synuclein levels in patients with Parkinson’s disease: a systematic review and meta-analysis. Neurol Sci. 2019, 40, 929–938. 10.1007/s10072-019-03738-1. [DOI] [PubMed] [Google Scholar]
- Jeong S.; Park J.; Pathania D.; Castro C. M.; Weissleder R.; Lee H. Integrated Magneto-Electrochemical Sensor for Exosome Analysis. ACS Nano 2016, 10, 1802–1809. 10.1021/acsnano.5b07584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav S.; Boriachek K.; Islam M. N.; Lobb R.; M?ller A.; Hill M. M.; Al Hossain M. S.; Nguyen N. T.; Shiddiky M. J. A. An Electrochemical Method for the Detection of Disease-Specific Exosomes. ChemElectroChem 2017, 4, 967–971. 10.1002/celc.201600391. [DOI] [Google Scholar]
- Li Q.; Tofaris G. K.; Davis J. J. Concentration-Normalized Electroanalytical Assaying of Exosomal Markers. Anal. Chem. 2017, 89, 3184–3190. 10.1021/acs.analchem.6b05037. [DOI] [PubMed] [Google Scholar]
- Jiang C.; Alam M. T.; Parker S. G.; Darwish N.; Gooding J. J. Strategies To Achieve Control over the Surface Ratio of Two Different Components on Modified Electrodes Using Aryldiazonium Salts. Langmuir 2016, 32, 2509–2517. 10.1021/acs.langmuir.5b04550. [DOI] [PubMed] [Google Scholar]
- Jiang C.; Tanzirul Alam M.; Parker S. G.; Gooding J. J. Zwitterionic Phenyl Phosphorylcholine on Indium Tin Oxide: a Low-Impedance Protein-Resistant Platform for Biosensing. Electroanalysis 2015, 27, 884–889. 10.1002/elan.201400557. [DOI] [Google Scholar]
- Jiang C.; Alam M. T.; Silva S. M.; Taufik S.; Fan S.; Gooding J. J. Unique Sensing Interface That Allows the Development of an Electrochemical Immunosensor for the Detection of Tumor Necrosis Factor α in Whole Blood. ACS Sens 2016, 1, 1432–1438. 10.1021/acssensors.6b00532. [DOI] [Google Scholar]
- Jiang C.; Xu S.; Zhang S.; Jia L. Chitosan functionalized magnetic particle-assisted detection of genetically modified soybeans based on polymerase chain reaction and capillary electrophoresis. Anal. Biochem. 2012, 420, 20–25. 10.1016/j.ab.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Dong C.; Wang H.; Zhang Z.; Zhang T.; Liu B. Carboxybetaine methacrylate oligomer modified nylon for circulating tumor cells capture. J. Colloid Interface Sci. 2014, 432, 135–143. 10.1016/j.jcis.2014.07.003. [DOI] [PubMed] [Google Scholar]
- Chen K.; Hu F.; Gu H.; Xu H. Tuning of surface protein adsorption by spherical mixed charged silica brushes (MCB) with zwitterionic carboxybetaine component. J. Mater. Chem. B 2017, 5, 435–443. 10.1039/C6TB02817A. [DOI] [PubMed] [Google Scholar]
- Ding F.; Yang S.; Gao Z.; Guo J.; Zhang P.; Qiu X.; Li Q.; Dong M.; Hao J.; Yu Q.; Cui J. Antifouling and pH-Responsive Poly(Carboxybetaine)-Based Nanoparticles for Tumor Cell Targeting. Front. Chem. 2019, 7, 770. 10.3389/fchem.2019.00770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Víšová I.; Vrabcová M.; Forinová M.; Zhigunová Y.; Mironov V.; Houska M.; Bittrich E.; Eichhorn K.-J.; Hashim H.; Schovánek P.; Dejneka A.; Vaisocherová-Lísalová H. Surface Preconditioning Influences the Antifouling Capabilities of Zwitterionic and Nonionic Polymer Brushes. Langmuir 2020, 36, 8485–8493. 10.1021/acs.langmuir.0c00996. [DOI] [PubMed] [Google Scholar]
- Keefe A. J.; Jiang S. Poly(zwitterionic)protein conjugates offer increased stability without sacrificing binding affinity or bioactivity. Nat. Chem. 2012, 4, 59–63. 10.1038/nchem.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S.; Jiang S. Zwitterionic polymer-protein conjugates reduce polymer-specific antibody response. Nano Today 2016, 11, 285–291. 10.1016/j.nantod.2016.05.006. [DOI] [Google Scholar]
- Yang W.; Sundaram H. S.; Ella J.-R.; He N.; Jiang S. Low-fouling electrospun PLLA films modified with zwitterionic poly (sulfobetaine methacrylate)-catechol conjugates. Acta Biomater. 2016, 40, 92–99. 10.1016/j.actbio.2016.05.035. [DOI] [PubMed] [Google Scholar]
- Fan Z.; Xiao K.; Lin J.; Liao Y.; Huang X. Functionalized DNA Enables Programming Exosomes/Vesicles for Tumor Imaging and Therapy. Small 2019, 15, 1903761. 10.1002/smll.201903761. [DOI] [PubMed] [Google Scholar]
- An Y.; Tang L.; Jiang X.; Chen H.; Yang M.; Jin L.; Zhang S.; Wang C.; Zhang W. A Photoelectrochemical Immunosensor Based on Au-Doped TiO2 Nanotube Arrays for the Detection of α-Synuclein. Chem. - Eur. J. 2010, 16, 14439–14446. 10.1002/chem.201001654. [DOI] [PubMed] [Google Scholar]
- Noble J.; Knight A.; Reason A.; Di Matola A.; Bailey M. A comparison of protein quantitation assays for biopharmaceutical applications. Mol. Biotechnol. 2007, 37, 99–111. 10.1007/s12033-007-0038-9. [DOI] [PubMed] [Google Scholar]
- Christiansson L.; Mustjoki S.; Simonsson B.; Olsson-Strömberg U.; Loskog A. S.; Mangsbo S. M. The use of multiplex platforms for absolute and relative protein quantification of clinical material. EuPa Open Proteomics 2014, 3, 37–47. 10.1016/j.euprot.2014.02.002. [DOI] [Google Scholar]
- Kinn Rød A. M.; Harkestad N.; Jellestad F. K.; Murison R. Comparison of commercial ELISA assays for quantification of corticosterone in serum. Sci. Rep. 2017, 7, 6748. 10.1038/s41598-017-06006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


