Abstract
Background:
Prevalence of Helicobacter pylori infection, the main risk factor for gastric cancer, has been decreasing in the US; however, there remains a substantial racial disparity. Moreover, the time-trends for prevalence of CagA-positive H. pylori infection, the most virulent form, are unknown in the US population. We sought to assess prevalence of CagA-positive Helicobacter pylori infection over time by race, in the US.
Methods:
We utilized multiplex serology to quantify antibody responses to H. pylori antigens in 4,476 participants across 5 cohorts that sampled adults from 1985 to 2009. Using log-binomial regression models, we calculated prevalence ratios (PRs) and 95% confidence intervals (CIs) for the association between H. pylori-CagA sero-prevalence and birth year by race.
Results:
African Americans were 3-times more likely to be H. pylori-CagA sero-positive than whites. After adjustment, H. pylori-CagA sero-prevalence was lower with increasing birth year among whites (Ptrend=0.001), but remained stable for African Americans. When stratified by sex and education separately, the decline in H. pylori-CagA sero-positivity among whites remained only for females (Ptrend<0.001) and was independent of educational attainment. Among African Americans, there was no difference by sex; further, sero-prevalence increased with increasing birth year among those with a high-school education or less (P=0.006).
Conclusions:
Among individuals in the US born from the 1920s to 1960s, H. pylori-CagA sero-prevalence has declined among whites, but not among African Americans.
Impact:
Our findings suggest a widening racial disparity in the prevalence of the most virulent form of Helicobacter pylori, the main cause of gastric cancer.
Keywords: Cytotoxin associated gene A, CagA, Antibodies, Helicobacter pylori, Prevalence, Racial differences
INTRODUCTION
Infection with the gastric bacterium, Helicobacter pylori, is the single greatest risk factor for the development of non-cardia gastric cancer (1). Although nearly half the world’s population carries this bacterium, its prevalence varies strongly by geographical region and ethnicity, coinciding mainly with gastric cancer incidence in the respective populations (2–7). In the United States (US), there are pronounced ethnic and racial disparities in both H. pylori prevalence and gastric cancer incidence whereby non-white populations are at an approximately two-fold increased risk for both (3, 6, 8–10). However, only a minority of H. pylori infected individuals will eventually develop gastric cancer. One determinant for this is the heterogeneity of H. pylori, as not all H. pylori strains have the same level of virulence and consequently carcinogenic properties. One strain-specific H. pylori virulence factor that augments cancer risk is the cytotoxin-associated gene (cag) pathogenicity island (11). The cag pathogenicity island encodes for a type 4 secretion system which translocates the oncoprotein CagA into host cells (11). The CagA protein is highly immunogenic and serum antibody responses to CagA persist throughout the life-time of an infection, and can even persist after H. pylori is eradicated (12, 13). Meta-analyses and observational studies from around the world have consistently demonstrated that infection with CagA-positive H. pylori strains is associated with increased risk of non-atrophic gastritis and non-cardia gastric cancers (12, 14–20). As such, serum antibody responses to CagA have been used as markers for cancer risk in diverse population groups, including African Americans and Asian Americans in the US (3, 4, 9, 21).
Although gastric cancer incidence has been on the decline for decades across the globe, there remains a need to monitor H. pylori infection to help further reduce the burden of this cancer (22–24). In the US alone, it is estimated that there will be 27,600 new gastric cancer cases and 11,010 deaths from gastric cancer in 2020 (25). However, there exist scant data examining racial differences in prevalence and trends over time for infection with H. pylori and, more importantly CagA-positive H. pylori, especially taking into consideration the large disparity in gastric cancer incidence by race previously found in the US. Therefore, we sought to examine trends by race in H. pylori-CagA sero-prevalence over time in a subset of participants from 5 highly diverse prospective cohorts in the US, specifically: the Southern Community Cohort Study (SCCS) (26); NYU Women’s Health Study (NYUWHS) (27); Women’s Health Initiative (WHI) (28) ; the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO) (29); and the Multiethnic Cohort (MEC) (30).
MATERIALS AND METHODS
Study participants
This study was conducted within a larger prospective consortium assembled to examine the associations between H. pylori blood biomarkers and colorectal cancer risk as described previously (31). Briefly, the consortium comprises prospectively ascertained colorectal cancer (CRC) cases and 1:1 controls matched on age, sex, self-reported race, and date of blood collection, nested within ten different US cohort studies. Because the present study is primarily focused on disparities between African Americans and whites (including both non-Latino and Latino whites) in H. pylori prevalence, we included only those cohorts, across the larger consortium of 10 cohorts, with at least 50 African American participants (NYUWHS, PLCO, SCCS, and WHI, and MEC). We did not include participants from the other five cohorts in the consortium as each cohort had specific inclusion criteria and target populations that require within-cohort comparisons for race-specific analyses. For the purposes of this prevalence study, we also supplemented the five cohorts with 177 CRC cases and 351 controls who were from the SCCS but had been included in a previous nested case-control study of the H. pylori and CRC association (32). In total, our analyses included 4,550 participants, 2,188 prospectively ascertained colorectal cancer cases and 2,362 controls. Covariates collected from participating cohorts included case-control status, age, sex, body mass index (BMI, missing n=62), smoking status (missing n=37), and education (missing n=74) as a measure of socioeconomic status. We then removed all participants missing education – who did not differ from the included subjects by age, sex, BMI, or smoking status – as we consider adjustment for socioeconomic status a necessity in these analyses, resulting in a final population of 4,476 individuals.
Institutional Review Board approval was obtained from all participating institutions.
H. pylori multiplex serology
We applied multiplex serology in sera from consortium participants to detect antibodies against 13 recombinantly expressed H. pylori antigens from strains 26695 and G27 as described previously (31, 33). Cutoff values for sero-positivity to each H. pylori antigen were calculated as defined previously (31, 33). Being sero-positive to 4 or more individual H. pylori antigens defined overall H. pylori sero-positivity. Those who were, in addition, sero-positive to H. pylori-CagA defined the CagA sero-positive group.
Statistical analysis
H. pylori-CagA sero-prevalence in our population was not rare (>10%) and, thus, odds ratios are not an appropriate approximation of relative risk. We therefore estimated prevalence ratios (PR) and 95% confidence intervals (CI) for the association of race with H. pylori and H. pylori-CagA sero-prevalence using log-binomial regression models (34). The association was assessed with three models: 1) a crude, unadjusted model; 2) a multivariable-adjusted model including age (continuous), sex, case-control status, and education (≤ high school [HS] or General Education Diploma [GED]; technical school or some college; ≥college degree), to remove the potential mediation of the association by socio-economic status; and 3) model 2 with further adjustment for BMI (<30 kg/m2, ≥30kg/m2) and smoking status (ever, never) as these additional two factors were associated both with race and H. pylori-CagA sero-positivity in our study (Table 1 and 2).
Table 1.
Study Characteristics by Race, in a Subset of the H. pylori-Colorectal Cancer Consortium (NYUWHS, PLCO, SCCS, WHI, and MEC), 1985–2009
| Whitesa (n = 3,292) |
African Americans (n = 1,184) |
|||
|---|---|---|---|---|
| n | % | n | % | |
| Age, yearsb | ||||
| <60 | 784 | 24 | 555 | 47 |
| 60-69 | 1656 | 50 | 406 | 34 |
| ≥70 | 852 | 26 | 223 | 19 |
| Year of birthb, c | ||||
| Before 1925 | 278 | 15 | 94 | 9 |
| 1925 – 1935 | 1020 | 53 | 247 | 24 |
| 1936 – 1945 | 468 | 25 | 270 | 26 |
| 1946 – 1953 | 97 | 5 | 218 | 21 |
| 1954 or later | 45 | 2 | 194 | 19 |
| Missing | 1,384 | 161 | ||
| Cohortb | ||||
| NYUWHS | 412 | 13 | 280 | 24 |
| PLCO | 1098 | 33 | 55 | 5 |
| SCCS | 172 | 5 | 98 | 8 |
| WHI | 1384 | 42 | 590 | 50 |
| MEC | 226 | 7 | 161 | 14 |
| Sexb | ||||
| Female | 2,519 | 77 | 732 | 62 |
| Male | 773 | 23 | 452 | 38 |
| Educationb | ||||
| ≤HS | 998 | 30 | 629 | 53 |
| Technical school or some college | 1,080 | 33 | 339 | 29 |
| ≥College | 1,214 | 37 | 216 | 18 |
| BMI, [kg/m2]b | ||||
| <30 | 2,385 | 73 | 670 | 58 |
| ≥30 | 867 | 27 | 492 | 42 |
| Missing | 40 | 22 | ||
| Smokingb | ||||
| Never | 1,515 | 47 | 443 | 38 |
| Ever | 1,743 | 53 | 738 | 62 |
| Missing | 34 | 3 | ||
Abbreviations: HS, high school:, BMI, body mass index.
Includes non-Latino and Latino whites.
P < 0.05 in Chi-Square test, two-sided.
WHI did not provide data on year of birth.
Table 2.
Study Characteristics by H. pylori and H. pylori-CagA Sero-status, in a Subset of the H. pylori-Colorectal Cancer Consortium (NYUWHS, PLCO, SCCS, WHI, and MEC), 1985–2009
|
H. pylori − (n = 2,550) |
H. pylori + (n = 1,926) |
H. pylori− and/or CagA− (n = 3,106) |
H. pylori+ and CagA+ (n = 1,370) |
|||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Age, yearsa | ||||||||
| <60 | 698 | 27 | 641 | 33 | 838 | 27 | 501 | 37 |
| 60-69 | 1235 | 48 | 827 | 43 | 1490 | 48 | 572 | 42 |
| ≥70 | 617 | 24 | 458 | 24 | 778 | 25 | 297 | 22 |
| Year of birtha, b | ||||||||
| Before 1925 | 171 | 12 | 201 | 14 | 232 | 13 | 140 | 13 |
| 1925 – 1935 | 696 | 48 | 571 | 39 | 871 | 48 | 396 | 36 |
| 1936 – 1945 | 397 | 27 | 341 | 23 | 474 | 26 | 264 | 24 |
| 1946 – 1953 | 121 | 8 | 194 | 13 | 163 | 9 | 152 | 14 |
| 1954 or later | 70 | 5 | 169 | 11 | 88 | 5 | 151 | 14 |
| Missing | 1,095 | 450 | 1,287 | 267 | ||||
| Cohorta | ||||||||
| NYUWHS | 240 | 9 | 227 | 12 | 314 | 10 | 153 | 11 |
| PLCO | 760 | 30 | 436 | 23 | 910 | 29 | 286 | 21 |
| SCCS | 223 | 9 | 539 | 28 | 319 | 10 | 443 | 32 |
| WHI | 1095 | 43 | 450 | 23 | 1278 | 41 | 267 | 19 |
| MEC | 232 | 9 | 274 | 14 | 285 | 9 | 221 | 16 |
| Sexa | ||||||||
| Female | 1,955 | 77 | 1,296 | 67 | 2,364 | 76 | 887 | 65 |
| Male | 595 | 23 | 630 | 33 | 742 | 24 | 483 | 35 |
| Racea | ||||||||
| Whitec | 2,211 | 87 | 1,081 | 56 | 2,654 | 85 | 638 | 47 |
| African American | 339 | 13 | 845 | 44 | 452 | 15 | 732 | 53 |
| Educationa | ||||||||
| ≤HS | 714 | 28 | 913 | 47 | 945 | 30 | 682 | 50 |
| Technical school or some college | 845 | 33 | 574 | 30 | 1,021 | 33 | 398 | 29 |
| ≥College | 991 | 39 | 439 | 23 | 1,140 | 37 | 290 | 21 |
| BMI, [kg/m2]a | ||||||||
| <30 | 1,791 | 71 | 1,264 | 66 | 2,157 | 70 | 898 | 66 |
| ≥30 | 721 | 29 | 638 | 34 | 905 | 30 | 454 | 34 |
| Missing | 38 | 24 | 44 | 18 | ||||
| Smokinga | ||||||||
| Never | 1,157 | 46 | 801 | 42 | 1,407 | 46 | 551 | 41 |
| Ever | 1,371 | 54 | 1,110 | 58 | 1,672 | 54 | 809 | 59 |
| Missing | 22 | 15 | 27 | 10 | ||||
Abbreviations: −, sero-negative; +, sero-positive; HS, high school; BMI, body mass index.
P < 0.05 in Chi-Square test, two-sided, compared to respective sero-negative group.
WHI did not provide data on year of birth.
Includes non-Latino and Latino whites.
Year of birth was categorized into 5 groups (<1925, 1925-1935, 1936-1945, 1946-1953, and ≥1954) based on the distribution among the participants of the cohorts with the exception of WHI (which was not able to provide the variable year of birth) (Supplementary figure S1). We plotted the proportion of H. pylori-CagA sero-positive individuals by year of birth category. To determine the PR, 95% CI and two-sided P-values for trends, year-of-birth categories were treated as a linear continuous variable with the lowest year of birth category considered as reference for each cohort. Log-binomial regression models were adjusted for sex, case-control status, and education. No further adjustment was made for BMI and smoking status in this analysis as the PR and 95% CI were not changed by more than 10%. Separate models were constructed by race with further stratification by sex and education (≤HS, and >HS). We were unable to adjust for age in the year of birth analyses because age and year of birth were highly correlated, r/rho = 0.81, P < 0.001.
As a sensitivity analysis, because disease status might alter H. pylori-CagA sero-status, all models were repeated among controls only, and no qualitative differences were found (see Supplemental Tables S3–S6), so only the results among cases and controls combined are presented here.
In order to expand the scope of our prevalence study beyond differences between whites and African Americans, we secondarily analyzed 995 additional CRC cases and controls from the MEC, in which there were 570 Japanese Americans, 329 Latinos, and 96 Native Hawaiians, in addition to the 226 non-Latino white and 280 African American MEC participants included in the larger study. Analyses in the MEC to calculate PRs and 95% CIs were conducted as described above.
RESULTS
Study characteristics by race and H. pylori and H. pylori-CagA sero-status
In our study population, compared to whites, African Americans were younger and, thus, over-represented in the younger birth cohorts. Moreover, they were more likely to be male, of lower educational achievement, ever smokers, and obese (BMI ≥30 kg/m2) (Table 1). H. pylori, as well as H. pylori-CagA, sero-positive individuals also were more likely to be younger, born in a later year, male, of lower educational achievement, and ever smokers and to have higher BMI than their respective sero-negative groups (Table 2).
H. pylori and H. pylori-CagA sero-prevalence by race and birth cohort
Differences in sero-prevalence of H. pylori were found by race, specifically H. pylori sero-prevalence was 33% among whites and 71% among African Americans. Of those H. pylori sero-positive, 59% of whites and 87% of African Americans were also CagA sero-positive, leading to an overall H. pylori-CagA sero-prevalence of 19% among whites and 62% among African Americans (Table 3). This racial difference in the sero-prevalence of H. pylori and H. pylori-CagA was consistent for each of the cohorts. In crude analyses, African Americans were twice as likely to be H. pylori sero-positive compared to whites (PR: 2.17; 95% CI: 2.05, 2.31) and three times as likely to be H. pylori-CagA sero-positive (PR: 3.19; 95% CI: 3.94, 3.47). After adjusting for age, sex, case-control status, and education, being African American remained associated with an almost two-fold increase in odds of H. pylori sero-prevalence (PR: 1.96; 95% CI: 1.83, 2.09) and an almost three-fold increase in odds of H. pylori-CagA sero-prevalence (PR: 2.83; 95% CI: 2.59, 3.10). This association did not change when additionally adjusting for BMI and smoking (H. pylori: PR: 1.96; 95% CI: 1.84, 2.10; H. pylori-CagA: PR: 2.87; 95% CI: 2.62, 3.14).
Table 3.
H. pylori and H. pylori-CagA Sero-Prevalence by Race and Cohort, in a Subset of the H. pylori-Colorectal Cancer Consortium (NYUWHS, PLCO, SCCS, WHI, and MEC), 1985–2009
| Cohort | H. pylori + | H. pylori + CagA + | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Whitesa |
African Americans |
Whitesa |
African Americans |
|||||||||||||||||
| n | % | n | % | PRb | 95%CI | PRc | 95%CI | PRd | 95%CI | n | % | n | % | PRb | 95%CI | PRc | 95%CI | PRd | 95%CI | |
| Total | 1,081 | 33 | 845 | 71 | 2.17 | 2.05, 2.31 | 1.96 | 1.83, 2.09 | 1.96 | 1.84, 2.10 | 638 | 19 | 732 | 62 | 3.19 | 2.94, 3.47 | 2.83 | 2.59, 3.10 | 2.87 | 2.62, 3.14 |
| NYUWHS | 193 | 47 | 34 | 62 | 1.32 | 1.05, 1.66 | 1.41 | 1.12, 1.77 | 1.34 | 1.04, 1.73 | 125 | 30 | 28 | 51 | 1.68 | 1.25, 2.26 | 1.68 | 1.25, 2.26 | 1.70 | 1.25, 2.32 |
| PLCO | 373 | 34 | 63 | 64 | 1.89 | 1.60, 2.24 | 1.80 | 1.52, 2.13 | 1.85 | 1.55, 2.20 | 233 | 21 | 53 | 54 | 2.55 | 2.06, 3.16 | 2.39 | 1.93, 2.97 | 2.46 | 1.97, 3.07 |
| SCCS | 85 | 49 | 454 | 77 | 1.56 | 1.33, 1.82 | 1.52 | 1.30, 1.78 | 1.51 | 1.29, 1.77 | 41 | 24 | 402 | 68 | 2.86 | 2.18, 3.75 | 2.75 | 2.09, 3.62 | 2.76 | 2.10, 3.63 |
| WHI | 361 | 26 | 89 | 55 | 2.12 | 1.80, 2.50 | 2.10 | 1.78, 2.47 | 2.03 | 1.71, 2.41 | 196 | 14 | 71 | 44 | 3.11 | 2.51, 3.87 | 3.17 | 2.53, 3.96 | 3.16 | 2.51, 3.98 |
| MEC | 69 | 31 | 205 | 73 | 2.40 | 1.95, 2.96 | 2.27 | 1.83, 2.81 | 2.28 | 1.84, 2.82 | 43 | 19 | 178 | 64 | 3.34 | 2.52, 4.44 | 3.23 | 2.43, 4.31 | 3.23 | 2.43, 4.30 |
Abbreviations: +, sero-positive; CagA, Cytotoxin-associated gene A; CI, confidence interval; MEC, Multiethnic cohort study; n, number; NYUWHS, New York University Women’s Health Study; PLCO, Prostate, Lung, Colorectal, and Ovarian Cancer Screening Study; PR, prevalence ratio; SCCS, Southern Community Cohort Study; WHI, Women’s Health Initiative.
Includes non-Latino and Latino whites.
Log-binomial regression models without further adjustment.
Log-binomial regression models with adjustment for age (continuous), sex, education (≤HS, technical school or some college, ≥college), and case-control status.
Log-binomial regression models with adjustment for age (continuous), sex, education (≤HS, technical school or some college, ≥college), case-control status, BMI (non-obese/obese), and smoking status (ever/never).
When examining trends over time, among all cohorts except for WHI, which did not provide year of birth, both H. pylori and H. pylori-CagA sero-prevalence were lower across later years of birth among whites (for a one increment increase in year of birth category: H. pylori: PR: 0.83; 95% CI: 0.77, 0.91; H. pylori-CagA: PR: 0.83; 95% CI: 0.75, 0.93; both Ptrend < 0.001). However, among African Americans, sero-prevalence of both were stable across birth years (H. pylori: PR: 1.01; 95% CI: 0.97, 1.05, Ptrend = 0.625; H. pylori-CagA: PR: 1.04; 95% CI: 1.00, 1.09, Ptrend = 0.078) (Table 4 and Figure 1A). As seen in Table 4, the decline in sero-prevalence across birth years among whites was primarily driven by white study participants from NYUWHS and PLCO, whereas whites in SCCS and MEC did not show such a decline.
Table 4.
Association of H. pylori and H. pylori-CagA Sero-Prevalence with Year of Birth, Overall and by Cohort, in a Subset of the H. pylori-Colorectal Cancer Consortium (NYUWHS, PLCO, SCCS, and MEC), 1985–2009
|
H. pylori + |
H. pylori + CagA + |
||||||
|---|---|---|---|---|---|---|---|
| Cohort | n | PRa | 95% CIa | Ptrenda | PRa | 95% CIa | Ptrenda |
| All | |||||||
| Whitesb | 1,908 | 0.83 | 0.77, 0.91 | <0.001 | 0.83 | 0.75, 0.93 | 0.001 |
| African American | 1,023 | 1.01 | 0.97, 1.05 | 0.625 | 1.04 | 1.00, 1.09 | 0.078 |
| NYUWHS | |||||||
| Whitesb | 412 | 0.79 | 0.69, 0.90 | <0.001 | 0.76 | 0.62, 0.92 | 0.006 |
| African American | 55 | 0.99 | 0.74, 1.31 | 0.920 | 0.92 | 0.66, 1.29 | 0.630 |
| PLCO | |||||||
| Whitesb | 1,098 | 0.85 | 0.74, 0.98 | 0.020 | 0.85 | 0.71, 1.03 | 0.094 |
| African American | 98 | 1.03 | 0.81, 1.30 | 0.815 | 1.13 | 0.82, 1.55 | 0.449 |
| SCCS | |||||||
| Whitesb | 172 | 0.92 | 0.80, 1.06 | 0.258 | 1.07 | 0.83, 1.39 | 0.589 |
| African American | 590 | 1.02 | 0.97, 1.06 | 0.447 | 1.03 | 0.98, 1.09 | 0.231 |
| MEC | |||||||
| Whitesc | 226 | 0.82 | 0.65, 1.05 | 0.120 | 0.92 | 0.67, 1.28 | 0.635 |
| African American | 280 | 0.94 | 0.86, 1.02 | 0.120 | 0.98 | 0.89, 1.07 | 0.609 |
| Japanese American | 570 | 0.78 | 0.70, 0.87 | <0.001 | 0.72 | 0.63, 0.83 | <0.001 |
| Latino | 329 | 0.98 | 0.91, 1.07 | 0.667 | 0.96 | 0.85, 1.09 | 0.538 |
| Native Hawaiian | 96 | 1.04 | 0.77, 1.40 | 0.810 | 1.06 | 0.77, 1.45 | 0.739 |
Abbreviations: +, sero-positive; CagA, Cytotoxin-associated gene A; CI, confidence interval; MEC, Multiethnic cohort study; n, number; NYUWHS, New York University Women’s Health Study; PLCO, Prostate, Lung, Colorectal, and Ovarian Cancer Screening Study; PR, prevalence ratio; SCCS, Southern Community Cohort Study.
PR and 95% CI estimated for one increment increase in year of birth category (<1925, 1925-1935, 1936-1945, 1946-1953, and ≥1954) estimated by Log-binomial regression adjusted for sex, education (≤HS, technical school or some college, ≥college), and case control status; for each cohort the lowest year of birth category was considered as reference.
Includes both non-Latino and Latino whites.
Non-Latino whites only.
Figure 1.
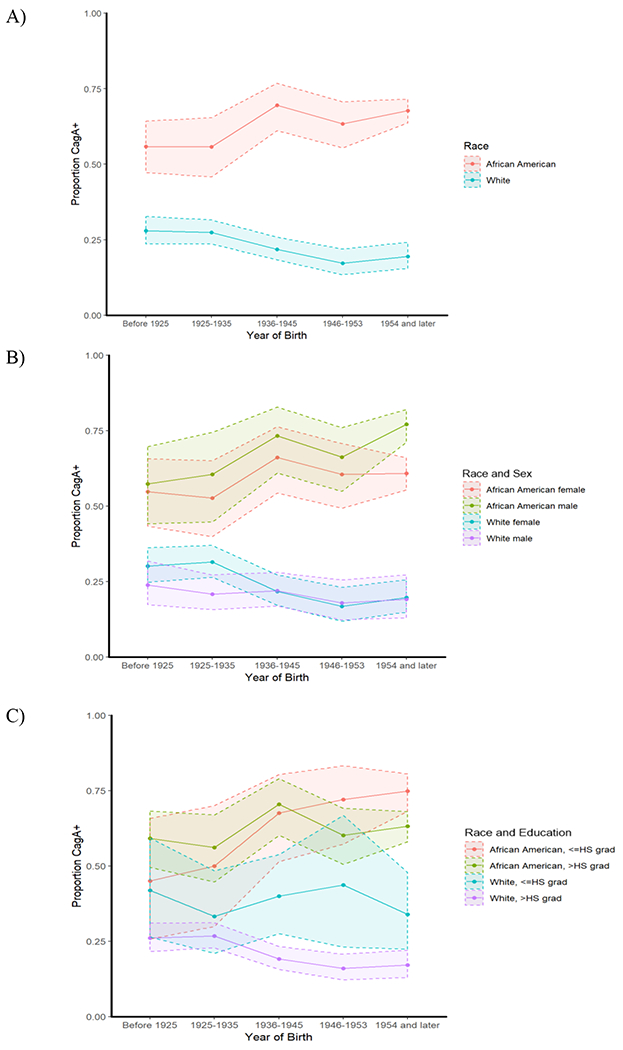
H. pylori-CagA sero-positivity in CRC cases and controls in all cohorts excluding the WHI by year of birth category and stratified by A) Race (whites n=1,908, African American n=1,023); B) Race and sex (female/white n=1,135, female/African American n=571, male/white n=773, male/African American n=452) C) Race and education (≤HS/white n=703, ≤HS/African American n=583, >HS/white n=1,205, >HS/African American n=440)
Stratifying by sex (Figure 1B) revealed that it was only white females who had lower prevalence across later years of birth (H. pylori: PR: 0.81; 95% CI: 0.73, 0.89, Ptrend < 0.001; H. pylori-CagA: PR: 0.79; 95% CI: 0.68, 0.90, Ptrend < 0.001). Stratifying on level of education (≤HS compared to >HS) showed that there was a lower H. pylori and H. pylori-CagA sero-prevalence across later years of birth among whites, regardless of educational level attained. In contrast, the association between H. pylori sero-positivity with birth year remained stable for African Americans independent of their educational level attained and sero-prevalence of H. pylori-CagA was higher in later birth years for African Americans with a high school education or less (PR: 1.08; 95% CI: 1.02, 1.14, Ptrend = 0.006; P for interaction by education among African Americans = 0.04) (Figure 1C).
Secondary analysis of H. pylori and H. pylori-CagA sero-prevalence among the five racial/ethnic groups in the Multiethnic Cohort
In our Multiethnic Cohort study population, compared to non-Latino whites, Native Hawaiians were younger, and Native Hawaiians were likewise more likely to be born in later cohorts, while African Americans and Latinos were more likely to be born in earlier cohorts (Supplemental Table S1). African Americans were also more likely to be obese (BMI ≥30 kg/m2) than non-Latino whites, while Japanese Americans were less likely to be obese than non-Latino whites (Supplemental Table S1). Unlike in the multi-cohort study reported above, H. pylori, as well as H. pylori-CagA, sero-positive individuals were more likely to be older and born in an earlier year than their respective sero-negative groups, but were similarly more likely to have lower educational achievement (Supplemental Table S2).
Similar to our multi-cohort study reported above, ethnic/racial minorities in the MEC were more likely to be H. pylori and H. pylori-CagA sero-positive than non-Latino whites (Table 5). Specifically, compared to non-Hispanic whites, and adjusting for age, sex, case-controls status, and education, African Americans, Latinos, and Native Hawaiians were two- to three-fold more likely to be H. pylori-CagA sero-positive (PR: 3.05; 95% CI: 2.29, 4.06; PR: 2.55; 95% CI: 1.91, 3.41; and PR: 2.11; 95% CI: 1.47, 3.02, respectively), and Japanese Americans were 1.7-fold more likely to be H. pylori-CagA sero-positive (PR: 1.71; 95% CI: 1.28, 2.30) (Table 5). Additionally, the trends in sero-prevalence remained stable by year of birth for all races except for Japanese Americans, for whom there was a lower prevalence of H. pylori-CagA sero-positivity across later years of birth (PR: 0.72; 95% CI: 0.63, 0.83, Ptrend < 0.001) (Table 4).
Table 5.
Secondary Analysis, H. pylori and H. pylori-CagA Sero-Prevalence by Race in the Multiethnic Cohort (MEC), 1995–2006
|
H. pylori− |
H. pylori+ |
H. pylori−/CagA− |
H. pylori+CagA+ |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Race | n | % | n | % | PRa | 95% CIa | PRb | 95% CIb | PRc | 95% CIc | n | % | n | % | PRa | 95% CIa | PRb | 95% CIb | PRc | 95% CIc |
| Whited | 157 | 69 | 69 | 31 | 1.00 | Ref | 1.00 | Ref | 1.00 | Ref | 183 | 81 | 43 | 19 | 1.00 | Ref | 1.00 | Ref | 1.00 | Ref |
| African American | 75 | 27 | 205 | 73 | 2.40 | 1.95, 2.96 | 2.21 | 1.79, 2.73 | 2.21 | 1.79, 2.73 | 102 | 36 | 178 | 64 | 3.34 | 2.52, 4.44 | 3.05 | 2.29, 4.06 | 3.07 | 2.31, 4.08 |
| Japanese American | 334 | 59 | 236 | 41 | 1.36 | 1.09, 1.69 | 1.32 | 1.06, 1.65 | 1.34 | 1.07, 1.66 | 381 | 67 | 189 | 33 | 1.74 | 1.30, 2.34 | 1.71 | 1.28, 2.30 | 1.71 | 1.27, 2.29 |
| Latino | 78 | 24 | 251 | 76 | 2.50 | 2.03, 3.07 | 2.24 | 1.82, 2.76 | 2.25 | 1.82, 2.77 | 153 | 47 | 176 | 53 | 2.81 | 2.11, 3.75 | 2.55 | 1.91, 3.41 | 2.57 | 1.92, 3.43 |
| Native Hawaiian | 52 | 54 | 44 | 46 | 1.50 | 1.12, 2.01 | 1.44 | 1.07, 1.93 | 1.44 | 1.07, 1.93 | 56 | 58 | 40 | 42 | 2.19 | 1.53, 3.13 | 2.11 | 1.47, 3.02 | 2.12 | 1.48, 3.04 |
Abbreviations: −, sero-negative; +, sero-positive; CagA, Cytotoxin-associated gene A; CI, Confidence interval; PR, Prevalence ratio; Ref, Reference.
Log-binomial regression models without further adjustment.
Log-binomial regression models with adjustment for age (continuous), sex, education (≤HS, technical school or some college, ≥college), and case-control status.
Log-binomial regression models with adjustment for age (continuous), sex, education (≤HS, technical school or some college, ≥college), case-control status, BMI (non-obese/obese), and smoking status (ever/never).
Non-Latino whites only.
DISCUSSION
Although the overall prevalence of H. pylori has been declining in the US for several decades (3, 35), this study provides evidence that it persists as a common infection, especially among ethnic/racial minorities. Current estimates of H. pylori prevalence in the US have been based on National Health and Nutrition Examination Surveys (NHANES) collected in 1988–1994 and 1999–2000. The overall age-standardized sero-prevalence of H. pylori has decreased from 34.0% to 30.7% among adults between the surveys; however, this decrease was driven entirely by non-Latino whites (3). Concordantly, small cohort studies of H. pylori prevalence in non-white populations have suggested a stable, or even increasing prevalence (4, 9, 36, 37). Data on sero-positivity to the strongly gastric cancer-associated H. pylori virulence factor CagA in US populations, however, are rare, and trends over time have not been reported previously.
This racially diverse study of 4,476 participants demonstrates that the racial disparities in the prevalence of sero-positivity to H. pylori – and, more importantly, to H. pylori-CagA – infection remain high and, particularly for African Americans, are not changing over time. Our secondary analyses of the MEC show that these same trends also apply to Latinos and Native Hawaiians. These data also confirm our previous, smaller study within the SCCS, where African American individuals were shown to be approximately twice as likely to be sero-positive to H. pylori and H. pylori-CagA than whites (4). The observations regarding prevalence of H. pylori sero-positivity across birth cohorts are concordant with previous reports from NHANES data, in which no declines over time were observed among African Americans or Latinos (3, 35). We here add important information to the literature, namely that prevalence of sero-positivity to H. pylori-CagA is not declining among African Americans, Latinos, or Native Hawaiians in the US.
This study has several strengths and weaknesses. It is composed of a large (4,476 participants in the primary analyses, 995 additional in the MEC), highly diverse population of US adults that encompasses groups not commonly included in large national surveys, notably low-income African Americans in the southeastern US as well as Native Hawaiians and Japanese Americans. We were also able to assess H. pylori sero-positivity through a comprehensive panel of 13 H. pylori antigens, including the cancer-associated CagA toxin, an assay that has been previously used to assess H. pylori in other large national and international cohorts (13, 38–41). Future work, beyond the scope of the current analyses, could delve more deeply into the differences by race in terms of the magnitude of fluorescence intensity of individual antigens.
Socio-economic status is strongly associated with both race and H. pylori sero-prevalence, and a limitation of the current study is that we adjusted for this potentially important variable with educational attainment alone. Although this may not necessarily be sufficient to capture variation in socio-economic status, we have previously conducted studies in the SCCS of H. pylori sero-prevalence in which we examined race, African ancestry, and both individual and neighborhood-level socioeconomic status (SES) factors including employment, education, and home value (from census block groups) (9). All of these SES factors were associated with H. pylori sero-prevalence, even separately among race/ancestry groups. However, once adjusted for, none of these SES factors explained the strong association between race and the prevalence of sero-positivity to the most virulent CagA-positive H. pylori strains. We did not have this level of SES data in the current study but our data, combined with previous studies, suggest that SES alone does not account for the observed racial disparities in H. pylori and H. pylori-CagA sero-prevalence. We hypothesize that other factors such as host genetics, other bacterial factors, and environmental factors, may also contribute to differences in H. pylori prevalence by race.
Another limitation of our study is that the inclusion criteria for the study and the larger consortium was incident CRC diagnosis and controls matched on age, sex, and race. Thus, these individuals do not represent the entire underlying cohort populations, limiting the generalizability of our findings. However, secondary analyses among only the control individuals found no qualitative differences from cases and controls together (fully adjusted PRs for H. pylori and H. pylori-CagA sero-positivity for African Americans, compared to whites, among controls only: PR: 1.91; 95% CI: 1.74, 2.10 and PR: 2.81; 95% CI: 2.47, 3.20, respectively). Additionally, our consortium includes a large proportion of less-educated individuals and, by implication, low SES individuals, thereby potentially overestimating the H. pylori and H. pylori-CagA sero-prevalence compared to the general US population. We were also unable to assess more recent birth cohorts for H. pylori and H. pylori-CagA sero-prevalence, due to the time periods and age ranges from which the cohorts recruited participants. Another limitation of our study is that H. pylori serology cannot distinguish between current and past infection. However, infection typically occurs in childhood and lasts for a lifetime unless the individual is treated with eradication therapy (42–44). Therefore, in this instance, H. pylori serology is a close approximation to a measure of current infection.
H. pylori is the greatest risk factor for the development of gastric cancer and is the leading causative agent of infection-associated cancers in highly developed countries. Although the overall prevalence of this bacterium is decreasing in the US (3), our data reaffirm that H. pylori and H. pylori-CagA sero-prevalence remains high in ethnic/racial minorities and, moreover, in these populations it is not lower among individuals born in later years (3, 35, 36). Simultaneously, trends in gastric cancer incidence mirror those of H. pylori prevalence whereby substantial racial disparities are observed (6, 45–47). H. pylori erradication strategies have been estimated to be cost-effective in reducing the burden of gastric diseases including gastric cancer, even in low-incidence countries such as the US (48). However, nationwide H. pylori screening and eradication to reduce gastric cancer incidence requires a comprehensive understanding of prevalence and associated risk factors to allow for targeted treatment and prevention strategies.
In conclusion, our study highlights the large differences in the prevalence of H. pylori and H. pylori-CagA sero-positivity by race, and suggests that other factors beyond education are responsible for this disparity. Thus, continued surveillance of H. pylori prevalence by race is needed to inform how this treatable infection can be selectively eradicated to benefit high cancer-risk populations and ultimately reduce the widening racial disparity in both infection and gastric cancer incidence in the US.
Supplementary Material
Acknowledgments
Funding
This work was supported by the U.S. National Institutes of Health (R01 CA190428 to M.E., U01 CA202979 to WJB, UM1 CA182934 to A.Z.-J., U01 CA164973 to L.L.M., R01 DK058587 and R01 CA077955 to R.M.P., R01 AI039657, R01 AI118932, and P01 CA116087 to T.L.C., and T32 CA057726 for fellowship support to M.G.V.); contracts to the WHI from the U.S. Department of Health and Human Services (HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C, and HHSN268201600004C); and the Department of Veterans Affairs (1I01BX004447 to T.L.C.). The development of H pylori multiplex serology was funded in part by the Joint Initiative for Innovation and Research of the German Helmholtz Association.
Abbreviations
- −
sero-negative
- +
sero-positive
- BMI
body mass index
- CagA
cytotoxin-associated gene A
- CI
confidence interval
- GED
General Education Diploma
- HS
high school
- H. pylori
Helicobacter pylori
- MEC
Multiethnic Cohort Study
- n
number
- NYUWHS
New York University Women’s Health Study
- PLCO
Prostate, Lung, Colorectal, and Ovarian Cancer Screening Study
- PR
prevalence ratio
- Ref
reference
- SCCS
Southern Community Cohort Study
- SES
socioeconomic status
- US
United States
- WHI
Women’s Health Initiative
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
REFERENCES
- 1.Amieva M, Peek RM Jr. Pathobiology of Helicobacter pylori-Induced Gastric Cancer. Gastroenterology. 2016;150(1):64–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology. 2017;153(2):420–9. [DOI] [PubMed] [Google Scholar]
- 3.Grad YH, Lipsitch M, Aiello AE. Secular Trends in Helicobacter pylori Seroprevalence in Adults in the United States: Evidence for Sustained Race/Ethnic Disparities. American journal of epidemiology. 2012;175(1):54–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Epplein M, Signorello LB, Zheng W, Peek RM Jr., Michel A, Williams SM, et al. Race, African ancestry, and Helicobacter pylori infection in a low-income United States population. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2011;20(5):826–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Correa P Helicobacter pylori and gastric cancer: state of the art. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 1996;5(6):477–81. [PubMed] [Google Scholar]
- 6.Dong E, Duan L, Wu BU. Racial and Ethnic Minorities at Increased Risk for Gastric Cancer in a Regional US Population Study. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2017;15(4):511–7. [DOI] [PubMed] [Google Scholar]
- 7.An international association between Helicobacter pylori infection and gastric cancer. The EUROGAST Study Group. Lancet. 1993;341(8857):1359–62. [PubMed] [Google Scholar]
- 8.Ashktorab H, Kupfer SS, Brim H, Carethers JM. Racial Disparity in Gastrointestinal Cancer Risk. Gastroenterology. 2017;153(4):910–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Epplein M, Cohen SS, Sonderman JS, Zheng W, Williams SM, Blot WJ, et al. Neighborhood socio-economic characteristics, African ancestry, and Helicobacter pylori sero-prevalence. Cancer causes & control : CCC. 2012;23(6):897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malaty HM, Graham DY. Importance of childhood socioeconomic status on the current prevalence of Helicobacter pylori infection. Gut. 1994;35(6):742–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Polk DB, Peek RM Jr. Helicobacter pylori: gastric cancer and beyond. Nat Rev Cancer. 2010;10(6):403–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park JY, Forman D, Waskito LA, Yamaoka Y, Crabtree JE. Epidemiology of Helicobacter pylori and CagA-Positive Infections and Global Variations in Gastric Cancer. Toxins (Basel). 2018;10(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cover TL, Glupczynski Y, Lage AP, Burette A, Tummuru MK, Perez-Perez GI, et al. Serologic detection of infection with cagA+ Helicobacter pylori strains. Journal of clinical microbiology. 1995;33(6):1496–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang JQ, Zheng GF, Sumanac K, Irvine EJ, Hunt RH. Meta-analysis of the relationship between cagA seropositivity and gastric cancer. Gastroenterology. 2003;125(6):1636–44. [DOI] [PubMed] [Google Scholar]
- 15.Parsonnet J, Friedman GD, Orentreich N, Vogelman H. Risk for gastric cancer in people with CagA positive or CagA negative Helicobacter pylori infection. Gut. 1997;40(3):297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pormohammad A, Ghotaslou R, Leylabadlo HE, Nasiri MJ, Dabiri H, Hashemi A. Risk of gastric cancer in association with Helicobacter pylori different virulence factors: A systematic review and meta-analysis. Microb Pathog. 2018;118:214–9. [DOI] [PubMed] [Google Scholar]
- 17.Queiroz DM, Mendes EN, Rocha GA, Oliveira AM, Oliveira CA, Magalhaes PP, et al. cagA-positive Helicobacter pylori and risk for developing gastric carcinoma in Brazil. International journal of cancer Journal international du cancer. 1998;78(2):135–9. [DOI] [PubMed] [Google Scholar]
- 18.Rudi J, Kolb C, Maiwald M, Zuna I, von Herbay A, Galle PR, et al. Serum antibodies against Helicobacter pylori proteins VacA and CagA are associated with increased risk for gastric adenocarcinoma. Digestive diseases and sciences. 1997;42(8):1652–9. [DOI] [PubMed] [Google Scholar]
- 19.Shimoyama T, Fukuda S, Tanaka M, Mikami T, Munakata A, Crabtree JE. CagA seropositivity associated with development of gastric cancer in a Japanese population. J Clin Pathol. 1998;51(3):225–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Torres J, Perez-Perez GI, Leal-Herrera Y, Munoz O. Infection with CagA+ Helicobacter pylori strains as a possible predictor of risk in the development of gastric adenocarcinoma in Mexico. International journal of cancer Journal international du cancer. 1998;78(3):298–300. [DOI] [PubMed] [Google Scholar]
- 21.Nomura AM, Lee J, Stemmermann GN, Nomura RY, Perez-Perez GI, Blaser MJ. Helicobacter pylori CagA seropositivity and gastric carcinoma risk in a Japanese American population. J Infect Dis. 2002;186(8):1138–44. [DOI] [PubMed] [Google Scholar]
- 22.Bertuccio P, Chatenoud L, Levi F, Praud D, Ferlay J, Negri E, et al. Recent patterns in gastric cancer: a global overview. International journal of cancer Journal international du cancer. 2009;125(3):666–73. [DOI] [PubMed] [Google Scholar]
- 23.Colquhoun A, Arnold M, Ferlay J, Goodman KJ, Forman D, Soerjomataram I. Global patterns of cardia and non-cardia gastric cancer incidence in 2012. Gut. 2015;64(12):1881–8. [DOI] [PubMed] [Google Scholar]
- 24.Plummer M, de Martel C, Vignat J, Ferlay J, Bray F, Franceschi S. Global burden of cancers attributable to infections in 2012: a synthetic analysis. The Lancet Global health. 2016;4(9):e609–16. [DOI] [PubMed] [Google Scholar]
- 25.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA: a cancer journal for clinicians. 2020;70(1):7–30. [DOI] [PubMed] [Google Scholar]
- 26.Signorello LB, Hargreaves MK, Steinwandel MD, Zheng W, Cai Q, Schlundt DG, et al. Southern community cohort study: establishing a cohort to investigate health disparities. J Natl Med Assoc. 2005;97(7):972–9. [PMC free article] [PubMed] [Google Scholar]
- 27.Toniolo PG, Levitz M, Zeleniuch-Jacquotte A, Banerjee S, Koenig KL, Shore RE, et al. A prospective study of endogenous estrogens and breast cancer in postmenopausal women. Journal of the National Cancer Institute. 1995;87(3):190–7. [DOI] [PubMed] [Google Scholar]
- 28.Anderson G, Cummings S, Freedman LS, et al. Design of the Women’s Health Initiative clinical trial and observational study. The Women’s Health Initiative Study Group. Control Clin Trials. 1998;19(1):61–109. [DOI] [PubMed] [Google Scholar]
- 29.Hayes RB, Reding D, Kopp W, Subar AF, Bhat N, Rothman N, et al. Etiologic and early marker studies in the prostate, lung, colorectal and ovarian (PLCO) cancer screening trial. Control Clin Trials. 2000;21(6 Suppl):349S–55S. [DOI] [PubMed] [Google Scholar]
- 30.Kolonel LN, Henderson BE, Hankin JH, Nomura AM, Wilkens LR, Pike MC, et al. A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. American journal of epidemiology. 2000;151(4):346–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Butt J, Varga MG, Blot WJ, Teras L, Visvanathan K, Le Marchand L, et al. Serologic Response to Helicobacter pylori Proteins Associated With Risk of Colorectal Cancer Among Diverse Populations in the United States. Gastroenterology. 2019;156(1):175–86 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Epplein M, Pawlita M, Michel A, Peek RM Jr., Cai Q, Blot WJ Helicobacter pylori Protein-Specific Antibodies and Risk of Colorectal Cancer. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2013;22(11):1964–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Michel A, Waterboer T, Kist M, Pawlita M. Helicobacter pylori multiplex serology. Helicobacter. 2009;14(6):525–35. [DOI] [PubMed] [Google Scholar]
- 34.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. American journal of epidemiology. 2005;162(3):199–200. [DOI] [PubMed] [Google Scholar]
- 35.Everhart JE, Kruszon-Moran D, Perez-Perez GI, Tralka TS, McQuillan G. Seroprevalence and ethnic differences in Helicobacter pylori infection among adults in the United States. The Journal of infectious diseases. 2000;181(4):1359–63. [DOI] [PubMed] [Google Scholar]
- 36.Nguyen T, Ramsey D, Graham D, Shaib Y, Shiota S, Velez M, et al. The Prevalence of Helicobacter pylori Remains High in African American and Hispanic Veterans. Helicobacter. 2015;20(4):305–15. [DOI] [PubMed] [Google Scholar]
- 37.Cardenas VM, Mena KD, Ortiz M, Karri S, Variyam E, Behravesh CB, et al. Hyperendemic H. pylori and tapeworm infections in a U.S.-Mexico border population. Public Health Rep. 2010;125(3):441–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lorenzo I, Fernandez-de-Larrea N, Michel A, Romero B, Lope V, Bessa X, et al. Helicobacter pylori seroprevalence in Spain: influence of adult and childhood sociodemographic factors. European journal of cancer prevention : the official journal of the European Cancer Prevention Organisation. 2019;28(4):294–303. [DOI] [PubMed] [Google Scholar]
- 39.Epplein M, Butt J, Zhang Y, Hendrix LH, Abnet CC, Murphy G, et al. Validation of a Blood Biomarker for Identification of Individuals at High Risk for Gastric Cancer. Cancer Epidemiol Biomarkers Prev. 2018;27(12):1472–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cai H, Ye F, Michel A, Murphy G, Sasazuki S, Taylor PR, et al. Helicobacter pylori blood biomarker for gastric cancer risk in East Asia. International journal of epidemiology. 2016;45(3):774–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Varga MG, Wang T, Cai H, Xiang YB, Gao YT, Ji BT, et al. Helicobacter pylori Blood Biomarkers and Gastric Cancer Survival in China. Cancer Epidemiol Biomarkers Prev. 2018;27(3):342–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cover TL, Blaser MJ. Helicobacter pylori in health and disease. Gastroenterology. 2009;136(6):1863–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Atherton JC, Blaser MJ. Coadaptation of Helicobacter pylori and humans: ancient history, modern implications. The Journal of clinical investigation. 2009;119(9):2475–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cover TL, Peek RM Jr. Diet, microbial virulence, and Helicobacter pylori-induced gastric cancer. Gut microbes. 2013;4(6):482–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kamangar F, Dawsey SM, Blaser MJ, Perez-Perez GI, Pietinen P, Newschaffer CJ, et al. Opposing risks of gastric cardia and noncardia gastric adenocarcinomas associated with Helicobacter pylori seropositivity.[see comment]. J Nat Cancer Inst. 2006;98(20):1445–52. [DOI] [PubMed] [Google Scholar]
- 46.Lui FH, Tuan B, Swenson SL, Wong RJ. Ethnic disparities in gastric cancer incidence and survival in the USA: an updated analysis of 1992–2009 SEER data. Dig Dis Sci. 2014;59(12):3027–34. [DOI] [PubMed] [Google Scholar]
- 47.Wu H, Rusiecki JA, Zhu K, Potter J, Devesa SS. Stomach carcinoma incidence patterns in the United States by histologic type and anatomic site. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2009;18(7):1945–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Connor A, O’Morain CA, Ford AC. Population screening and treatment of Helicobacter pylori infection. Nature reviews Gastroenterology & hepatology. 2017;14(4):230–40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


